Introduction
Endometrial cancer (EC), the most common type of
female genital tract malignancy, with substantial cancer related
mortality. It was estimated that there were 52,630 new cases of EC
with 8,590 deaths in 2014 (1).
Approximately 70% of EC patients are histologically
adenocarcinomas, which may arise from complex atypical hyperplasia
and closely link to estrogen overdose, metabolic disturbance and
positive hormone receptors (2,3). The
prognosis of EC patients is affected by several risk factors,
including tumor grade, myometrial invasion, lymphovascular space
invasion, non-endometrioid histology and cervical stromal
involvement (3). Metastasis is a
major cause of mortality and recurrence in endometrial
adenocarcinoma patients. In the process of tumor metastasis,
numerous adhesion molecules, cytoskeleton interacting proteins,
extracellular matrix proteins and angiogenesis related proteins are
involved (4,5). Further elucidating the mechanisms of
tumor aggressiveness is meaningful for endometrial cancer patients
to acquire better progression-free survival (PFS) and overall
survival (OS) chance.
The S100 calcium-binding protein family has been
shown to play an essential role in multiple steps of tumorigenesis
and aggressiveness (6). Frequent
locus gene diversification and protein conformational alteration
facilitate their wide range of functions both intracellular and
extracellular, such as proliferation, apoptosis, invasion,
Ca2+ homeostasis and energy metabolism (7). Accumulating evidence suggest that
S100A4, a member of S100 family, was overexpressed in various
malignancies. Its pivotal role in promoting tumor progression and
metastasis made it a valid predictor for poor outcome (8–11).
Additionally, therapeutic targeting of S100A4 significantly inhibit
tumor progress, indicating its potential value in human cancer
treatment (12). The evidence for
expression and functions of S100A4 in EC remains scant.
Accordingly, S100A4 was overexpressed in high-grade EC which
heralded poor prognosis (13,14).
However, little is known about the exact mechanisms of S100A4 in EC
progression.
Epithelial-mesenchymal transition (EMT) has been
demonstrated to be implicated in tumor progression and metastasis.
During this process epithelial cells convert to a more migratory
and invasive phenotype, which is accompanied with downregulated
expression of epithelial markers, particularly E-cadherin, and
induced expression of mesenchymal markers (15–17).
Numerous studies indicated that S100A4 involved in EMT process and
is considered as a key EMT molecular marker (18–20).
Although the molecular mechanisms causing EMT has been well
established in recent years, the implication of EMT in endometrial
cancer pathogenesis and whether S100A4 is involved remains to be
investigated.
In the present study, augmented cytoplasmic
expression of S100A4 in poorly-differentiated EC was observed which
closely related to EMT markers. We hypothesized that S100A4 promote
EC metastasis via modulating EMT process, mainly affecting
E-cadherin and vimentin expression. To confirm this, the role of
S100A4 in HEC-1B cell migration, invasion, and morphological
transition was studied, and the impact of S100A4 on EMT marker
expression was investigated.
Materials and methods
Patients and samples
Formalin-fixed paraffin-embedded specimens were
obtained from patients diagnosed with EC (n=50) and atypical
endometrial hyperplasia (n=20) and who underwent surgical resection
at Wuhan Union Hospital from 2010 to 2013. Those administered prior
chemotherapy, hormone therapy or radiotherapy were excluded. The
Surgical staging (I–IV) and histological grades (G1–G3) were
established based on the criteria of the International Federation
of Gynecology and Obstetrics (FIGO) for EC. The clinicopathological
characteristics, such as age, pathological grading, stage, lymph
node metastasis and myometrial invasion depth, were also collected.
The present study was approved by The Ethics Committee of Tongji
Medical College, Huazhong University of Science and Technology
(IORG no: IORG0003571) after receiving informed consent from the
patients.
Immunohistochemistry (IHC) and
assessments
The four micrometer-thick tissue slides were
deparaffinized with xylene and rehydrated in graded alcohol. Slides
were then immersed in 0.01 M sodium citrate buffer (pH 6.0) and
heated on microwave for 20 min. This was followed by endogenous
peroxidase activity blocking with 3% hydrogen peroxide (15 min) and
nonspecific staining blocking with 10% normal goat serum (30 min).
Then 200 µl of antibodies against S100A4 (1:800; CST,
#13018), vimentin (1:100, Santa Cruz Biotechnology (Santa Cruz, CA,
USA), sc-7557), and E-cadherin (1:100, Santa Cruz Biotechnology,
sc-7870) were added on each tissue slide and incubated in a
humidified box at 4°C overnight. For negative control, we added
phosphate-buffered saline (PBS) instead of the primary antibody.
Slides were then treated with Envision/HRP, rabbit secondary
antibodies for 30 min. Diaminobenzidine (DAB) substrate was added
on each slide for visualizing, followed by rinsing, and
counterstaining with hematoxylin. Finally, the slides were
dehydrated in 4 different concentrations of alcohol and cleared in
xylene.
The intensity evaluation of IHC staining was
performed independently by two pathologists. Positive S100A4
staining was analyzed according to cytoplasmic and/or nuclear
staining of the tumor cells. The scoring categories take into
account both the intensity of IHC color reaction (none, 0; weak, 1;
moderate, 2; strong, 3) and the percentage of positive tumor cells
(<10%, 1; 10–49%, 2; >50%, 3). The final score was the sum of
the two scores. Histological score ≤1 point was defined as
negative, whereas ≥2 points were considered positive.
Cell culture and shRNA transfection
The human endometrial cancer cell lines HEC-1B,
Ishikawa, and KLE were purchased from the American Type Culture
Collection (ATCC), cultured in Dulbecco's modified Eagle's medium
(DMEM, Hyclone, USA) containing 10% fetal bovine serum with 100
U/ml penicillin and 100 µg/ml streptomycin, at 37°C in a
humidified atmosphere containing 5% CO2.
shRNAs for S100A4 and vector was commercially
purchased from GeneChem (Shanghai, China). The target sequence for
synthetic oligonucleotide primers of S100A4 shRNA were: shS100A4-1:
CAGATAAGCAGCCCAGGAA; shS100A4-2: CCATGATGTGTAACGAATT; shS100A4-3:
TCCAAGAGTACTGTGTCTT; shS100A4-4: CTGCTTTCCAGAAGCTGAT; The target
sequence: CTCCGAACGTGTCACGTT was used as a non-silencing control
(vector). The vector plasmid and plasmid carrying S100A4 shRNA were
transfected into HEC-1B cells using Lipofectamine 2000 (Invitrogen,
Carlsbad, CA, USA) following manufacturer's recommendations. The
cells were harvested after 48 h of transfection. Stably transfected
clones of shS100A4-4 and vector HEC-1B cells were selected using
100 µg/ml ampicillin (Sigma, Santa Clara, CA, USA) for 3
days.
RNA isolation and quantitative real-time
PCR (qRT-PCR)
Total RNA was extracted from the transfected cells
using the TRIzol (Invitrogen) and First-strand cDNA was
reverse-transcribed from 2 µg total RNA using the Prime
Script RT reagent kit (Takara, Kyoto, Japan) according to the
manufacturer's protocol. cDNA samples (2 µl) were added to
quantitative PCR in 20 µl reactions using SYBR Premix Ex Taq
(Takara). The primers involved were: h-S100A4 (251 bp):
5′-TACTCGGGCAAAGAGGGTGA-3′ (sense) and 5′-CATTTCTTCCTGGGCTGCTTA-3′
(antisense). h-GAPDH (255 bp): 5′-ACTTTGGTATCGTGGAAGGACTAT-3′
(sense) and 5′-GTTTTTCTAGACGGCAGGTCAGG-3′ (antisense). Values on
the y-axis represent 2(−ΔΔCt), while ΔCt is the
difference between target-gene Ct and GAPDH Ct.
Western blot analysis
The transfected HEC-1B cells were rinsed in ice-cold
PBS twice and lysed in lysis buffer. The protein concentration was
measured using the Bio-Rad assay system. Proteins (20 µg)
were boiled for 10 min and run on 15% SDS-PAGE gels. Then
transferred onto nitrocellulose membrane and the membranes were
blocked with 5% skim milk in 1X TBS buffer containing 0.1% Tween-20
and then probed with antibodies against S100A4 (1:2000, CST,
#13018), GAPDH (1:800, Santa Cruz Biotechnology, sc-25778),
vimentin (1:1000, Santa Cruz Biotechnology, sc-7557), E-cadherin
(1:1000, Santa Cruz Biotechnology, sc-7870) at 4°C; Horseradish
peroxidase (HRP)-conjugated anti-rabbit IgG was used as the
secondary antibody. The bands were detected by enhanced
Chemiluminescence reagents (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). The optical density was quantified using Image
Lab™ analysis software. The relative expression data have been
normalized by GAPDH expression.
Immunofluorescence staining
Cells were cultivated in six-well plates overnight.
Then washed by PBS twice and fixed with 4% paraformaldehyde for 15
min. Cells for vimentin and tubulin staining were treated with 0.1%
Triton X-100 for enhancing permeability and blocked with 5% BSA for
1 h, then incubated with primary antibodies overnight. After two
washes with PBS, incubated with Cy3-conjugated goat anti-mouse or
anti-rabbit IgG antibody (1:100 dilution, Guge, Wuhan, China) in
the dark for 1 h, then counterstained with DAPI. Images were taken
using an Olympus Fluoview IX-300 laser-scanning confocal
microscope.
CCK-8 assay
The CCK-8 assay was adopted to detect the effect of
S100A4 in EC cell viability. Briefly, the shS100A4-4 and control
group HEC-1B cells were plated in a 96-well plate at the density of
5×103/100 µl per well. Cell growth was analyzed
every 24 h using a CCK-8 commercial kit (Sigma-Aldrich) following
manufacturer's instructions.
Wound-healing assay
The shS100A4-4 and control group HEC-1B cells were
plated in a six-well plate, 1×105 per well. When cells
were grown to confluence as a monolayer, a wound was created using
a sterile 200 µl pipette tip followed with serum starvation
for 48 h. Photographs of the wounded area were taken at the time of
wounding and every 12 h afterwards for 2 days to determine the rate
of wound closure. The migration percentage was calculated using the
following formula: [Δ area/area (0 h)] ×100%.
Transwell invasion assay
The shS100A4-4 and control group cells were plated
in the upper chamber coated with Matrigel basement membrane matrix
(BD Biosciences, Bedford, MA, USA) in serum-free medium,
5×104 cells per well. A chemoattractant (10% fetal
bovine serum) was added to the lower chamber and incubated for 48
h. Invasive cells, were fixed in 4% paraformaldehyde and stained in
0.5% crystal violet, were counted in five random fields of each
trans-well filter using a microscope.
Statistical analyses
Statistical analysis was performed using the SPSS
software 21.0 statistical software (Chicago, IL, USA). Chi-square
test and Fisher's exact tests were used when comparing frequencies
between groups. Values represent the mean ± SD of one
representative experiment from a series of independent experiments.
Data were analyzed using the Student's t-test. P-values <0.05
were statistically significant.
Results
S100A4 is overexpressed in the cytoplasm
of cancer cells in EC
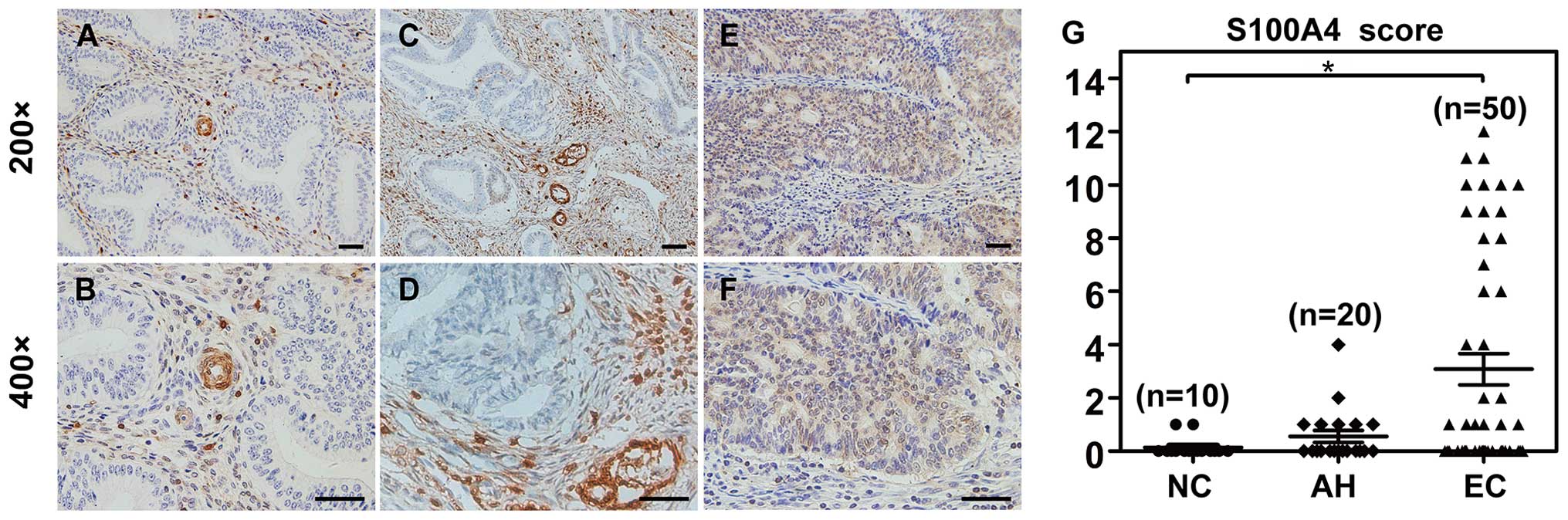
Immunohistochemistry was performed to characterize
the quantity and localization of S100A4 protein. In all normal
endometrial tissues (10/10) and the majority of atypical
endometrial hyperplasia tissues (18/20), the expression of S100A4
was confined in the mesenchymal sections, particularly in
endometrial stromal cells and vascular structures. None of the
glandular epithelial cells expressed S100A4 protein (Fig. 1A–D). In contrast, 32% (16/50) of the
endometrial cancer tissues overexpressed S100A4 both in mesenchymal
sections and tumor epithelial cells (Fig. 1E and F). Statistically, cytoplasmic
expression of S100A4 protein in endometrial cancer epithelial cells
was significantly higher than normal group (P=0.014). However,
there was no difference between endometrial cancer and atypical
hyperplasia endometrium (P=0.057) (Fig.
1G).
Positive cytoplasmic S100A4 protein
indicates EC progression
To investigate the clinical significance of S100A4
in EC patients, correlation between cytoplasmic S100A4 protein
expression and clinicopathological parameters was analyzed.
Positive staining for S100A4 in tumor cells was significantly
associated with cell differentiation, more frequent incidence was
observed in high-grade cases (G2/G3, 14/24) compared with the
low-grade cases (G1, 2/26). Age and lymph node metastasis also
showed remarkable correlation with expression of S100A4. No
significant correlation between S100A4 expression and FIGO stage or
myometrial invasion depth was found, though a related trend was
observed (Table I).
 | Table IClinicopathological characteristics
and cytoplasmic S100A4 expression in patients with endometrial
cancer. |
Table I
Clinicopathological characteristics
and cytoplasmic S100A4 expression in patients with endometrial
cancer.
| Parameters | S100A4 expression
| P-value |
|---|
| (n=50) | + (%) | − (%) |
|---|
| Age | | | | |
| <50 | 14 | 1 (7.1) | 13 (92.9) | 0.021 |
| ≥50 | 36 | 15 (41.7) | 21 (58.3) | |
| FIGO stage | | | | |
| I/II | 39 | 10 (25.6) | 29 (74.4) | 0.147 |
| III/IV | 11 | 6 (54.5) | 5 (45.5) | |
| Histologic grade | | | | |
| G1 | 26 | 2 (7.7) | 24 (92.3) | <0.001 |
| G2/G3 | 24 | 14 (58.3) | 10 (41.7) | |
| Myometrial
invasion | | | | |
| <1/2 of
myometrium | 34 | 8 (23.5) | 26 (76.5) | 0.061 |
| ≥1/2 of
myometrium | 16 | 8 (50) | 8 (50) | |
| LN metastasis | | | | |
| Absent | 44 | 2 (4.5) | 42 (95.5) | <0.001 |
| Present | 6 | 4 (66.7) | 2 (33.3) | |
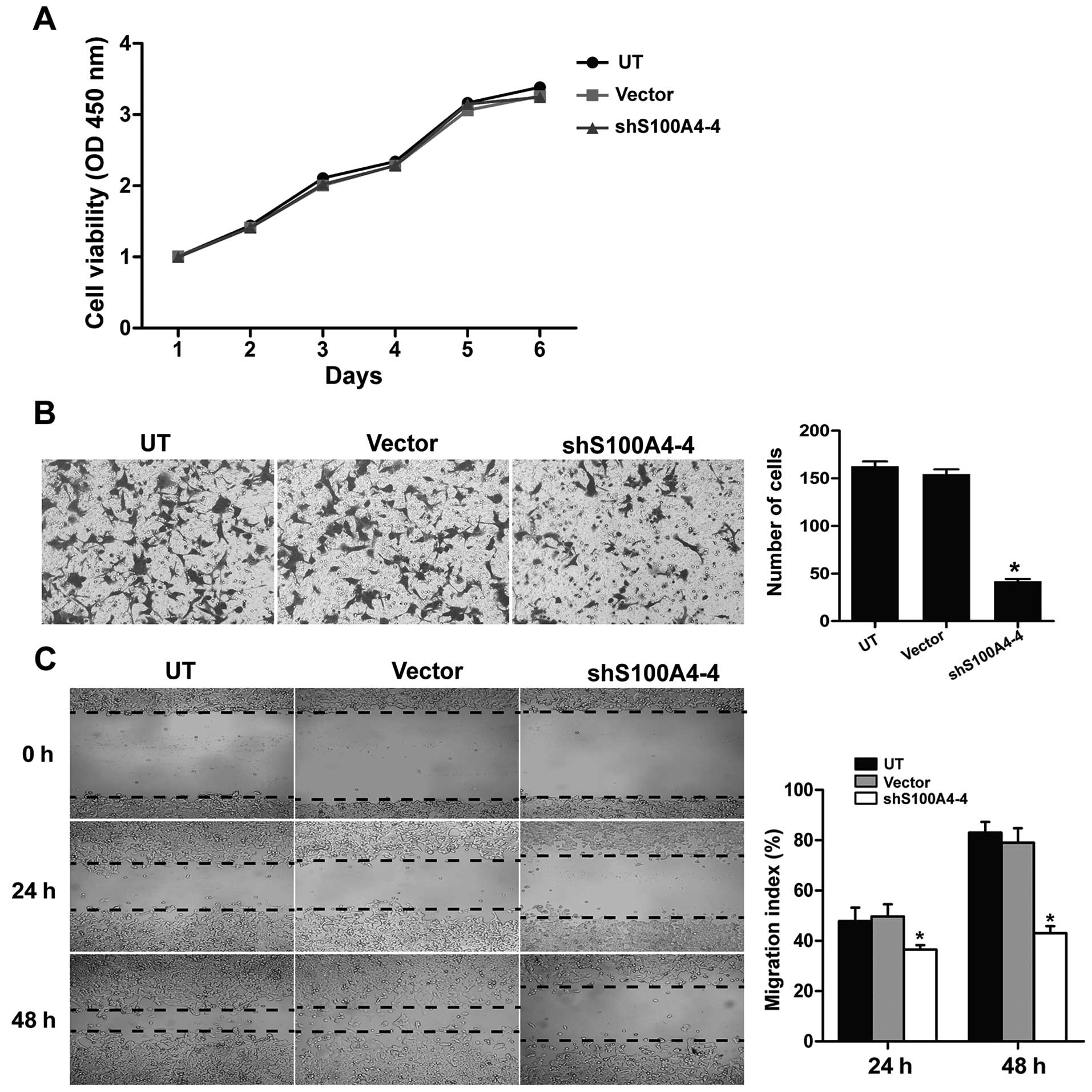
S100A4 silencing suppresses migration and
invasion capability of HEC-1B cells
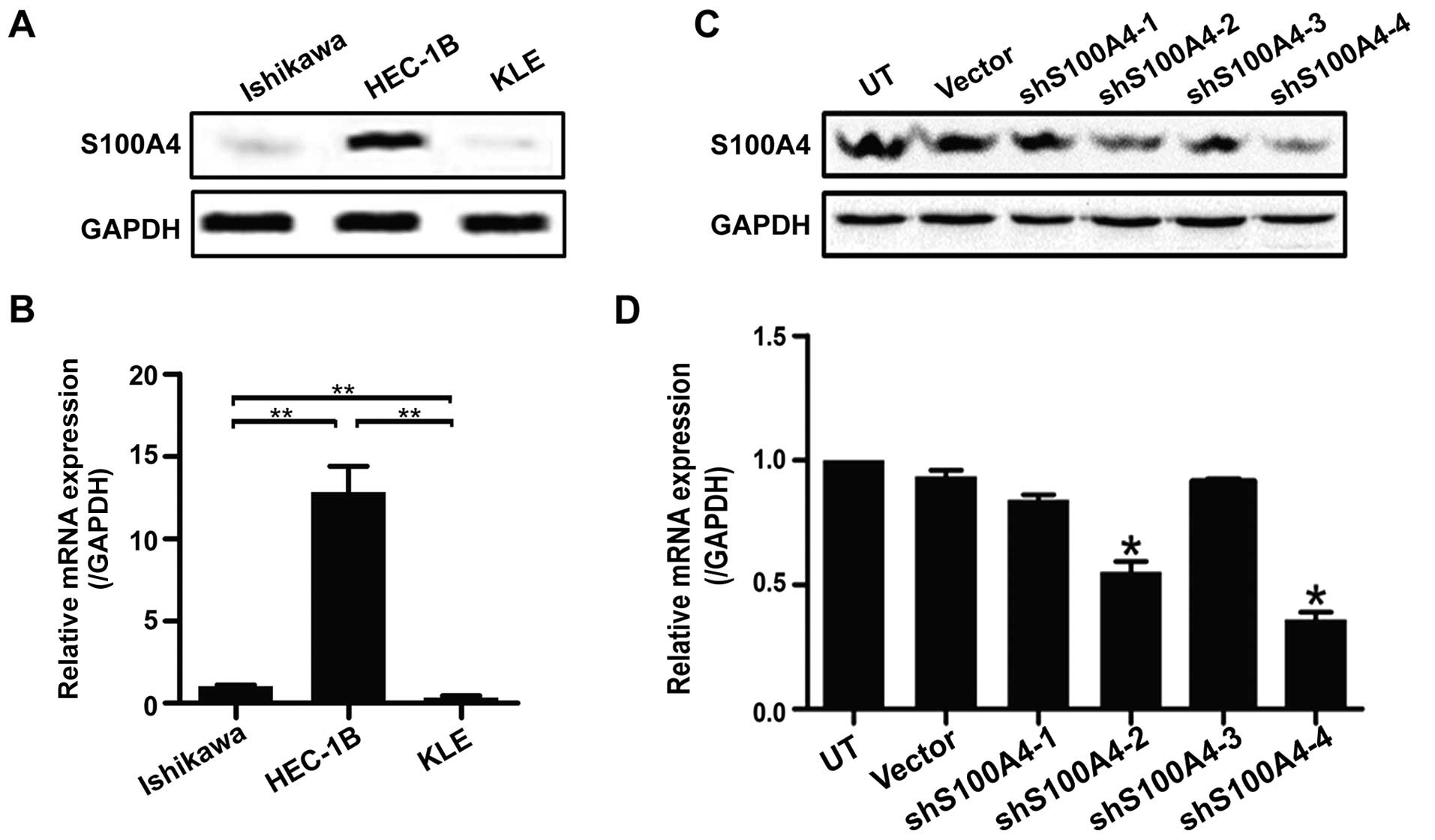
The high invasive EC cell line HEC-1B possessed high
endogenous S100A4 when compared to low invasive EC cell lines
Ishikawa and KLE (Fig. 2A and B).
To study the effects of S100A4 on migration and invasion of HEC-1B
cells, four shRNAs were constructed and transfected into HEC-1B
cells. Transient transfection of all the four groups, except for
shS100A4-3 group, exhibited an inhibitory effect on S100A4
expression in HEC-1B cells (Fig. 2C and
D). The shS100A4-4 group provided the strongest efficacy.
Further, the inhibitory effect in shS100A4-4 group was maximal
after stable screening with puromycin confirmed by western blotting
(Fig. 4C). Functional experiments
demonstrated that the migration and invasion of HEC-1B cells were
significantly suppressed in shS100A4-4 group compared with control
group (Fig. 3B and C). However, no
differences were detected in viability of HEC-1B cells (Fig. 3A). Of note, marked morphological
change was also observed in shS100A4-4 group with attenuated
EMT-like phenotype.
Downregulation of S100A4 reverses
EMT
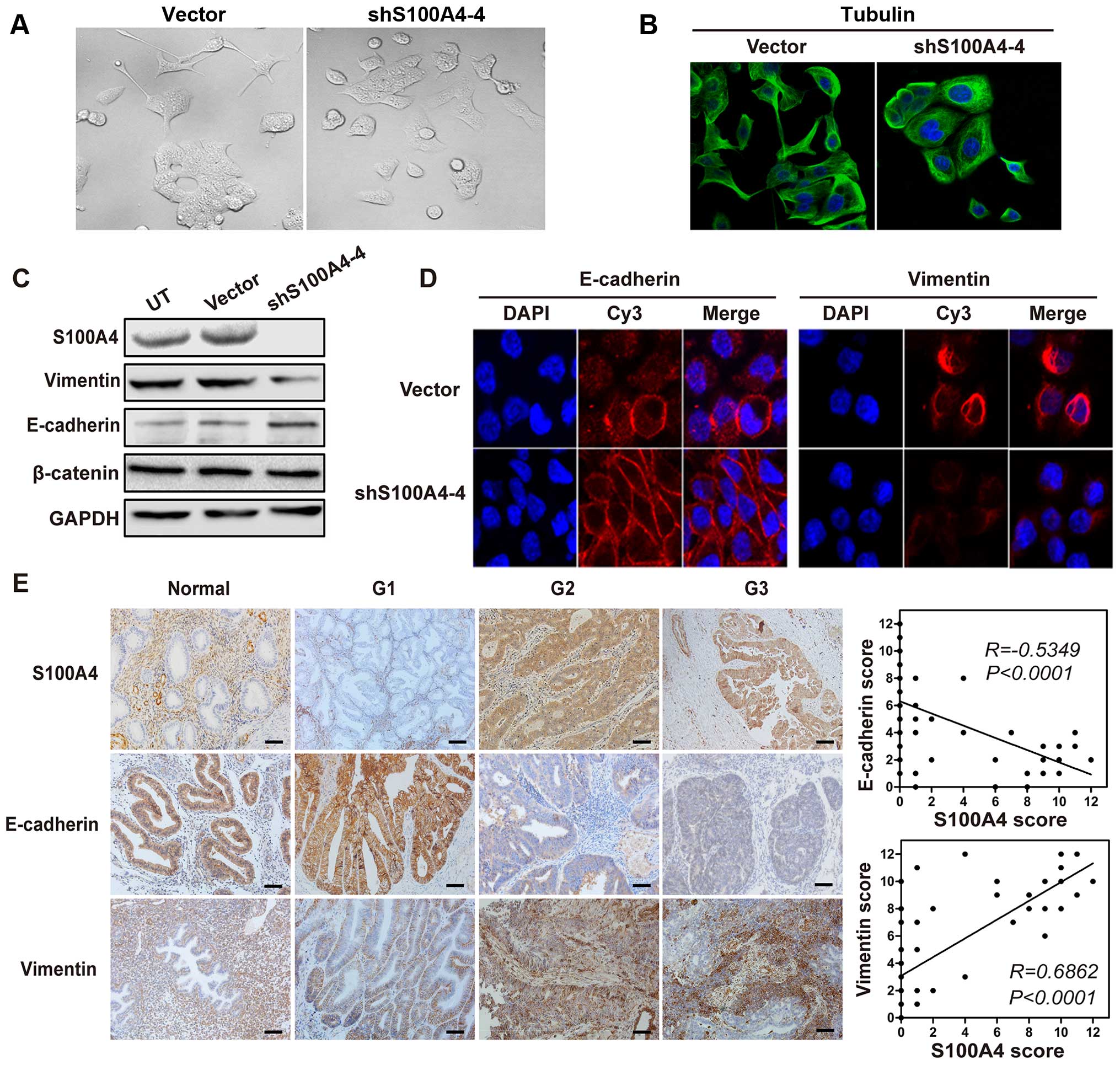
To study the effects of S100A4 on EC cell
cytoskeleton, optical microscope observation and immunofluorescence
staining against tubulin were utilized. We found morphological
changes and cytoskeleton reorganization when S100A4 was
downregulated (Fig. 4A and B). The
HEC-1B cells lost their spindle-shaped morphology and showed
suppressed spreading. Besides, E-cadherin and vimentin, which
account for major epithelial and mesenchymal markers were also
detected in shS100A4-4 cells and EC specimens. E-cadherin was
mainly expressed in the membrane whereas vimentin mostly in the
cytosol. In addition, concurrent upregulation of E-cadherin and
downregulation of vimentin upon ablation of S100A4 were observed
(Fig. 4D) and further confirmed by
western blotting (Fig. 4C).
Moreover, in high-grade EC samples, high S100A4 expression closely
correlated with decreased E-cadherin and elevated vimentin
expression (Fig. 4E). These results
suggested a crucial role for S100A4 in EMT-mediated metastasis of
EC.
Discussion
Elevated S100A4 protein expression has been detected
in endometrial carcinoma and other tumors, and higher S100A4
protein levels concordant with advanced stages of cancer (13,21).
Thus, a positive correlation was established between upregulation
of S100A4 and tumor malignancy progression. In agreement with
previous study, S100A4 was abundantly expressed in higher grade EC
patients (14). Besides, a
transitional expression pattern of S100A4 was observed in which
S100A4 was undetectable in epithelial cells of low-grade EC, normal
endometrium and atypical endometrial hyperplasia while widely
expressed in mesenchymal origins, especially in stromal cells and
vascular structures. This kind of expression pattern was also found
in prostate cancer which may imply an epithelial to mesenchymal
transition (EMT) derived from gene hypomethylation (22). Since EMT has been recognized as a
core molecular mechanism underlying cancer invasion and metastasis,
the role of S100A4 was focussed on in EC progression. Additionally,
S100A4 has also been shown to possess the ability of activating or
integrating pathways to promote migratory phenotype, angiogenesis,
extracellular matrix components (ECM) remodeling process (23–25).
The crucial role of S100A4 in EC cell invasion and metastasis were
confirmed by in vitro studies which elucidated that
overexpression of S100A4 was associated with metastatic properties
while targeting knockdown of S100A4 markedly reduced the migratory
ability.
The clinical significance of S100A4 in cancer is
still controversial. In cases of cancers, S100A4 was universally
overexpressed and the excessive expression of S100A4 was an
independent predictor for tumor progression, metastasis, and poor
prognosis. For example, increased S100A4 expression was detected in
the metastatic sections of breast cancer (26). Enhanced expression of S100A4
underlies lymph node metastasis and predicts recurrence in
colorectal cancer (27). However,
some studies obtained contradictive results (28). Even so, the majority of the
investigators believed that S100A4 indeed closely related to some
clinical and pathological parameters. In the study of Chong et
al, partly consistent with us, indicated that cytoplasmic
expression of S100A4 was significantly related to the
clinicopathological parameters of EC patients, including FIGO
stage, histological grade, loss of progesterone receptor (PR) and
lymph node metastasis. Kaplan-Meier survival analysis suggested a
significant correlation between cytoplasmic expression of S100A4
and poorer outcome (13). The
present study also showed relevance between S100A4 expression and
age. Age represents an important risk factor for EC occurrence.
Most EC are diagnosed after menopause and older women are more
likely to have poorly differentiated endometrioid histology and
higher stage disease. These results led to a hypothesis that S100A4
may be an effective indicator for EC progression.
Clinical and experimental evidence indicate a
critical role of EMT interrelated process in progression and
metastasis of EC. EMT is a complex and stepwise phenomenon which is
indispensable for morphogenesis in embryonic development and is
reinitiated during cancer progression forming a more invasive
phenotype. The molecular mechanisms underlying EMT can comprise
multiple extracellular signals and numerous secreted soluble
factors that ultimately activate different transcription factors
and signaling pathways (15).
Disassembly of cell-cell and cell-ECM junction together with
downregulation of epithelial marker E-cadherin is recognized as
hallmark of EMT. Simultaneously, progressive loss of E-cadherin
expression is always coupled with augmented expression of
non-epithelial cadherin, e.g. N-cadherin, cadherin-11 and acquired
mesenchymal markers, such as fibronectin, smooth muscle actin, or
vimentin (29). Changes of these
molecules were also found in human EC, as a steady decrease of
E-cadherin and increase of vimentin was observed in high grade EC
specimens. These observations conformed to the findings of Yang
et al (30).
S100A4 has been considered as a crucial EMT mediator
since directly controlled by Wnt/β-catenin signaling pathway
(31). While the role of S100A4 in
regulating EMT process in EC is still unclear. The findings of Xie
et al revealed a pivotal role of S100A4 in mediating EC
invasion and clarified the regulation effect of TGF-β1 on S100A4
expression (32). TGF-β1 pathways
could mediate EMT in various epithelial cell types. Given the
explicit role of E-cadherin and vimentin in EMT, the current study
found direct correlation between expression of S100A4 and EMT
markers in EC for the first time, S100A4 is an upstream regulator
of E-cadherin and vimentin. EC cells transfected with an S100A4
inhibition vector showed increased cell-cell adhesion morphology,
plus downregulation of E-cadherin and upregulation of vimentin.
Besides, S100A4 induced E-cadherin expression from membranous to
membrano-cytoplasmic, indicated its pivotal role in malignant
transformation of EC cells through EMT regulation. EMT molecules
are more than merely cementing substances but also regulate cell
polarity, differentiation, migration and invasion. These cellular
events may be mediated via their intimate connection to the actin
cytoskeletal network (33). S100A4
has been demonstrated to interact with components of cytoskeleton,
such as F-actin, non-muscle tropomyosin, and non-muscle myosin,
further regulating the cytoskeletal dynamics and cell motility
(34). Conformational changes of
tubulin in transfected cells provided clues for this effect.
In conclusion, our study demonstrates that positive
cytoplasmic S100A4 was observed in high-grade EC as compared with
the negative counterparts. S100A4 promoted migration and invasion
capacity of EC cells via EMT modification. Thus, S100A4 might be a
potential predictor for EC patient progress and targeting S100A4
could be a promising therapeutic target for EC treatment.
Acknowledgments
This work was supported by Natural Science
Foundation of Hubei Province, China (grant no. 2015CFB237).
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kandoth C, Schultz N, Cherniack AD, Akbani
R, Liu Y, Shen H, Robertson AG, Pashtan I, Shen R, Benz CC, et al
Cancer Genome Atlas Research Network: Integrated genomic
characterization of endometrial carcinoma. Nature. 497:67–73. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Amant F, Mirza MR, Koskas M and Creutzberg
CL: Cancer of the corpus uteri. Int J Gynaecol Obstet. 131(Suppl
2): S96–S104. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Psaila B and Lyden D: The metastatic
niche: Adapting the foreign soil. Nat Rev Cancer. 9:285–293. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Valastyan S and Weinberg RA: Tumor
metastasis: Molecular insights and evolving paradigms. Cell.
147:275–292. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen H, Xu C, Jin Q and Liu Z: S100
protein family in human cancer. Am J Cancer Res. 4:89–115.
2014.PubMed/NCBI
|
|
7
|
Donato R, Cannon BR, Sorci G, Riuzzi F,
Hsu K, Weber DJ and Geczy CL: Functions of S100 proteins. Curr Mol
Med. 13:24–57. 2013. View Article : Google Scholar :
|
|
8
|
Lee SJ, Choi SY, Kim WJ, Ji M, Lee TG, Son
BR, Yoon SM, Sung R, Lee EJ, Youn SJ, et al: Combined aberrant
expression of E-cadherin and S100A4, but not β-catenin is
associated with disease-free survival and overall survival in
colorectal cancer patients. Diagn Pathol. 8:992013. View Article : Google Scholar
|
|
9
|
Dahlmann M, Okhrimenko A, Marcinkowski P,
Osterland M, Herrmann P, Smith J, Heizmann CW, Schlag PM and Stein
U: RAGE mediates S100A4-induced cell motility via MAPK/ERK and
hypoxia signaling and is a prognostic biomarker for human
colorectal cancer metastasis. Oncotarget. 5:3220–3233. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee SH, Kim H, Hwang JH, Shin E, Lee HS,
Hwang DW, Cho JY, Yoon YS, Han HS and Cha BH: CD24 and S100A4
expression in resectable pancreatic cancers with earlier disease
recurrence and poor survival. Pancreas. 43:380–388. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Burock S, Herrmann P, Wendler I,
Niederstrasser M, Wernecke KD and Stein U: Circulating metastasis
associated in colon cancer 1 transcripts in gastric cancer patient
plasma as diagnostic and prognostic biomarker. World J
Gastroenterol. 21:333–341. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hernández JL, Padilla L, Dakhel S, Coll T,
Hervas R, Adan J, Masa M, Mitjans F, Martinez JM, Coma S, et al:
Therapeutic targeting of tumor growth and angiogenesis with a novel
anti-S100A4 monoclonal antibody. PLoS One. 8:e724802013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chong HI, Lee JH, Yoon MS, Suh DS, Kim K,
Kim JY and Choi KU: Prognostic value of cytoplasmic expression of
S100A4 protein in endometrial carcinoma. Oncol Rep. 31:2701–2707.
2014.PubMed/NCBI
|
|
14
|
Xie R, Loose DS, Shipley GL, Xie S,
Bassett RL Jr and Broaddus RR: Hypomethylation-induced expression
of S100A4 in endometrial carcinoma. Mod Pathol. 20:1045–1054. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lamouille S, Xu J and Derynck R: Molecular
mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell
Biol. 15:178–196. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zheng H and Kang Y: Multilayer control of
the EMT master regulators. Oncogene. 33:1755–1763. 2014. View Article : Google Scholar
|
|
17
|
Kidd ME, Shumaker DK and Ridge KM: The
role of vimentin intermediate filaments in the progression of lung
cancer. Am J Respir Cell Mol Biol. 50:1–6. 2014.
|
|
18
|
Wang H, Shi J, Luo Y, Liao Q, Niu Y, Zhang
F, Shao Z, Ding Y and Zhao L: LIM and SH3 protein 1 induces
TGFβ-mediated epithelial-mesenchymal transition in human colorectal
cancer by regulating S100A4 expression. Clin Cancer Res.
20:5835–5847. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lo JF, Yu CC, Chiou SH, Huang CY, Jan CI,
Lin SC, Liu CJ, Hu WY and Yu YH: The epithelial-mesenchymal
transition mediator S100A4 maintains cancer-initiating cells in
head and neck cancers. Cancer Res. 71:1912–1923. 2011. View Article : Google Scholar
|
|
20
|
Nitta T, Mitsuhashi T, Hatanaka Y,
Miyamoto M, Oba K, Tsuchikawa T, Suzuki Y, Hatanaka KC, Hirano S
and Matsuno Y: Prognostic significance of epithelial-mesenchymal
transition-related markers in extrahepatic cholangiocarcinoma:
Comprehensive immunohistochemical study using a tissue microarray.
Br J Cancer. 111:1363–1372. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
McKiernan E, McDermott EW, Evoy D, Crown J
and Duffy MJ: The role of S100 genes in breast cancer progression.
Tumour Biol. 32:441–450. 2011. View Article : Google Scholar
|
|
22
|
Rosty C, Ueki T, Argani P, Jansen M, Yeo
CJ, Cameron JL, Hruban RH and Goggins M: Overexpression of S100A4
in pancreatic ductal adenocarcinomas is associated with poor
differentiation and DNA hypomethylation. Am J Pathol. 160:45–50.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Buetti-Dinh A, Pivkin IV and Friedman R:
S100A4 and its role in metastasis - computational integration of
data on biological networks. Mol Biosyst. 11:2238–2246. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Miranda KJ, Loeser RF and Yammani RR:
Sumoylation and nuclear translocation of S100A4 regulate
IL-1beta-mediated production of matrix metalloproteinase-13. J Biol
Chem. 285:31517–31524. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
O'Connell JT, Sugimoto H, Cooke VG,
MacDonald BA, Mehta AI, LeBleu VS, Dewar R, Rocha RM, Brentani RR,
Resnick MB, et al: VEGF-A and Tenascin-C produced by
S100A4+ stromal cells are important for metastatic
colonization. Proc Natl Acad Sci USA. 108:16002–16007. 2011.
View Article : Google Scholar
|
|
26
|
Kim HM, Jung WH and Koo JS: Expression of
cancer-associated fibroblast related proteins in metastatic breast
cancer: An immunohistochemical analysis. J Transl Med. 13:2222015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kang YG, Jung CK, Lee A, Kang WK, Oh ST
and Kang CS: Prognostic significance of S100A4 mRNA and protein
expression in colorectal cancer. J Surg Oncol. 105:119–124. 2012.
View Article : Google Scholar
|
|
28
|
Jung EA, Cho HD, Lee JH and Oh MH:
Clinicopathological significance of S100A4 expression in non-small
cell lung carcinomas. Korean J Pathol. 44:477–482. 2010. View Article : Google Scholar
|
|
29
|
Thiery JP and Sleeman JP: Complex networks
orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell
Biol. 7:131–142. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang WN, Ai ZH, Wang J, Xu YL and Teng YC:
Correlation between the overexpression of epidermal growth factor
receptor and mesenchymal makers in endometrial carcinoma. J Gynecol
Oncol. 25:36–42. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sack U, Walther W, Scudiero D, Selby M,
Aumann J, Lemos C, Fichtner I, Schlag PM, Shoemaker RH and Stein U:
S100A4-induced cell motility and metastasis is restricted by the
Wnt/β-catenin pathway inhibitor calcimycin in colon cancer cells.
Mol Biol Cell. 22:3344–3354. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xie R, Schlumbrecht MP, Shipley GL, Xie S,
Bassett RL Jr and Broaddus RR: S100A4 mediates endometrial cancer
invasion and is a target of TGF-beta1 signaling. Lab Invest.
89:937–947. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Knights AJ, Funnell AP, Crossley M and
Pearson RC: Holding tight: Cell junctions and cancer spread. Trends
Cancer Res. 8:61–69. 2012.PubMed/NCBI
|
|
34
|
Goh Then Sin C, Hersch N, Rudland PS,
Barraclough R, Hoffmann B and Gross SR: S100A4 downregulates
filopodia formation through increased dynamic instability. Cell
Adhes Migr. 5:439–447. 2011. View Article : Google Scholar
|