Introduction
Lung cancer is the leading cause of cancer death all
over the world. It is classified into two main histological groups:
non-small cell lung cancer (NSCLC, 85%) and small cell lung cancer
(SCLC, 15%) (1). The 5-year
survival rate for lung cancer is only 16% (2). Therefore, it is necessary and critical
to find a novel approach to increase the survival rate of lung
cancer.
The phosphatidylinositol-3kinase (PI3K)/Akt
signaling pathway is vital to cell growth and apoptosis (3). Many studies have reported that the
PI3K/Akt signaling pathway was aberrantly activated in lung cancer
(4–6). Some anticancer-drugs downregulated the
expression of Akt and induced G2/M phase arrest. The G2/M
checkpoint is regulated by cdc25B and cyclin B1 (7). For example, knocking down Sox2 induced
G2/M arrest by decreasing expression levels of cyclin B1 and cdc2
in lung squamous cell carcinomas (8). Genistein induced cell cycle arrest in
the G2/M phase by downregulating expression levels of cyclin B1 and
cdc25B in H446 NSCLC (9).
Carthamus tinctorius L. (C.
tinctorus), commonly named safflower, is a herbal plant in the
family compositae. Safflower is well known for its function in the
promotion of blood flow, removal of blood stasis, promotion of
menstruation and alleviation of pain (10). The active components of safflower
are quinochalones, flavonoids, alkaloids, and safflower
polysaccharide (SPS). In recent years, many pharmacological
experiments have demonstrated that safflower has a wide variety of
biological activities, including the improvement of acute ischemic
stroke (11–13) and the enhancement of
antiinflammation (14), antioxidant
(15), antitumor (16), and antibacterial activities
(17). SPS is one of the most
important active components and is used for modulating the immune
system and cancer prevention. However, the effect of SPS on lung
cancer progression is rarely reported and the underlying mechanisms
remain unknown. In this study, we investigated the effect of SPS on
the proliferation of A549 and YTMLC-90 cell lines. We also focused
on the underlying mechanisms of SPS on the cell cycle and apoptosis
in A549 and YTMLC-90 cells. We observed that SPS induced NSCLC cell
apoptosis by enhancing immunomodulatory activities and blocking the
PI3K/Akt signaling pathway. This study provides new insights into
the anticancer mechanism of SPS.
Materials and methods
Chemicals and reagents
RPMI-1640 medium, heat-inactivated fetal bovine
serum (FBS), penicillin and streptomycin, and
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
were obtained from Sigma Chemical Co. (St. Louis, MO, USA). ELISA
kits were from Shanghai Enzme-linked Biotechnology Co., Ltd.
(Shanghai, China). BALB/c nude mice were from Vital River
Laboratory Animal Technology Co. Ltd., Beijing, China. Antibodies
to cyclin B1, Cdc25B, PI3K, Akt and p-Akt were purchased from Abcam
(Cambridge, MA, USA). The PrimeScript™ RT-PCR kit and SYBR Premix
Ex Taq™ kit were obtained from Takara (Takara, Kusatsu, Japan).
Ethical standard
All procedures performed in studies involving
animals were in accordance with the ethical standards of the
Authors' institution.
Preparation of SPS
SPS (AR, 90%) was purchased from Xi'an Reain
Biotechnology Co. Ltd, Xi'an, China. The SPS was dissolved with
DMSO to different concentrations: 0.04, 0.08, 0.16, 0.32, 0.64,
1.28, and 2.56 mg/ml.
Cell culture and treatment
Human lung cancer cell lines, A549 and YTMLC-90 were
purchased from the Cell Library Committee on Type Culture
Collection of the Chinese Academy of Sciences, Beijing. Cells were
cultured in RPMI-1640 supplemented with 10% fetal bovine serum
(FBS), 100 U/ml penicillin and 100 µg/ml streptomycin in a
humidified, 5% CO2 atmosphere at 37°C. The cells were
plated at a density of 105 cells per well and grown for
24 h.
MTT assay
The MTT assay was used to evaluate cell viability.
Cells were seeded in 96-well plates at a density of
5×103 cells per well and grown for 24 h. Cells were then
treated with various concentrations of SPS ranging from 0.04 to
2.56 mg/ml for 24, 48, and 72 h. Cells treated with an equivalent
volume of DMSO were regarded as the control groups. Each group was
treated in triplicate. Following treatment, 10 µl MTT was
added to each well and incubated for 4 h. Finally, blue formazan
crystals of viable cells were solubilized in 100 µl DMSO.
The absorbance was measured at 450 nm using a microplate
reader.
Flow cytometric analysis of
apoptosis
Apoptosis was assessed in A549 and YTMLC-90 cells
using an Annexin V-FITC/propidium iodide (PI) staining assay. Cells
were cultured in 6-well plates at a density of 2×105/ml
per well overnight. Cells were then treated with various
concentrations of SPS ranging from 0.04 to 2.56 mg/ml for 48 h.
Control cells were treated with culture medium containing DMSO.
Each group was treated in triplicate. After treatment, cells were
washed with cold PBS and resuspended in binding buffer (100 mM
HEPES, pH 7.4, 100 mM NaCl, 25 mM CaCl2). Cells were
stained with Annexin V-FITC/PI at 4°C for 30 min. Apoptotic cells
were analyzed using a fluorescence-activated cell-sorting (FACS)
flow cytometer.
Real-time PCR
The expression levels of bax, caspase-3 and
cdc25B were measured using real-time PCR. Cells were treated
with various concentrations of SPS ranging from 0.04 to 2.56 mg/ml
for 48 h. Following treatment, cells were collected and their total
RNA extracted using TRIzol reagent. cDNA was synthesized using the
PrimeScript RT-PCR kit (Takara). Real-time PCR was performed using
SYBR Premix Ex Taq kit (Takara). Primer sequences were as follows:
cdc25B: 5′-TTC ATC AGG GAA CGA GA CCG TG-3′, 5′-TTC ACA GAA GTT CGG
GTG CTG AG-3′; bax: 5′-GGA GCT GCA GAG GAT GAT TG-3′, 5′-CCT CCC
AGA AAA ATG CCA TA-3′; caspase-3: 5′-ATG GAG AAC ACT GAA AAC
TCAG-3′, 5′-GAC CGA GAT GTC ATT CCA GTG-3′; GAPDH: 5′-GAA GGT GAA
GGT CGG AGT C-3′, 5′-GAA GAT GGT GAT GGG ATT TC-3′. The reaction
was repeated 3 times and carried out in an ABI7500 Real-time PCR
System (Applied Biosystems, Carlsbad, CA, USA). Templates were
initially denatured at 95°C for 5 min followed by 40 cycles at 95°C
for 5 sec and 60°C for 34 sec. The relative expression levels of
tested genes were calculated using the 2−ΔΔCT
method.
Flow cytometry analysis of the cell
cycle
PI staining followed by flow cytometry was used to
assess the effect of SPS on the cell cycle of A549 and YTMLC-90
cells. Cells were treated with different concentrations of SPS for
48 h. Cells were then washed with cold PBS with 75% ethanol for 1 h
at 4°C. The protocol was then followed as previously described
(18). Cells were suspended in 1 ml
of PBS that contained 1 mg/ml RNase and 50 µg/ml PI,
followed by 30 min of shaking at 37°C in the dark. DNA content was
detected using a flow cytometer (BD FACSCalibur System, San Jose,
CA, USA).
Western blotting
Protein expression levels of cyclin B1, Cdc25B,
PI3K, Akt and p-Akt were measured by western blot according to a
previous study (19). After
treatment, cells were lysed in RIPA buffer. Protein concentration
was determined using the Bradford assay (Bio-Rad, Hercules, CA,
USA). Twenty micrograms of protein from each sample were separated
by 12% SDS-PAGE and transferred to PVDF membranes. Primary
antibodies (rabbit monoclonal anti-human cyclin B1, 1:3000
dilution; rabbit polyclonal anti-human Cdc25B, 1:1000 dilution;
rabbit monoclonal anti-human Akt, 1:1000 dilution; rabbit
monoclonal anti-human p-Akt, 1:1000 dilution) were then applied and
incubated at 4°C overnight, after which the appropriate
HRP-conjugated secondary antibody was added and incubated for 1 h
at room temperature. Proteins were detected using the ChemiDoc XRS
imaging system and analysis software Quantity One (Bio-Rad).
The effect of SPS injection in vivo
To further analyze the effect of SPS injection in
vivo, BALB/c nude mice (7 weeks old) were purchased from Vital
River Laboratory Animal Technology Co. Ltd. Murine tumor models
were induced by subcutaneous injection of A549 cells
(5×106 cells in 0.2 ml of PBS) at one site in the right
flank, and tumors allowed to develop for 20 days. When tumors
reached ~100 mm3 in volume, 40 animals were divided
randomly into 4 groups (n=10 for each group): low dose (15 mg/kg),
middle dose (45 mg/kg) and high dose (135 mg/kg) injection groups,
and a control group that was treated with an equal volume of normal
saline (NS). All injections were administered intraperitoneally
every day. At 15, 20, 25, and 30 days post-injection, tumor size
was measured using calipers and tumor volume was estimated
according to a previous study (18). Blood samples were taken from a tail
vein to measure the contents of TNF-α and IL-6 using ELISA
kits.
Statistical analysis
Data were expressed as mean ± SD and considered
significant at P<0.05. Statistical analysis was performed using
a Student's unpaired t-test (SPSS release 19.0; SPSS Inc.).
Results
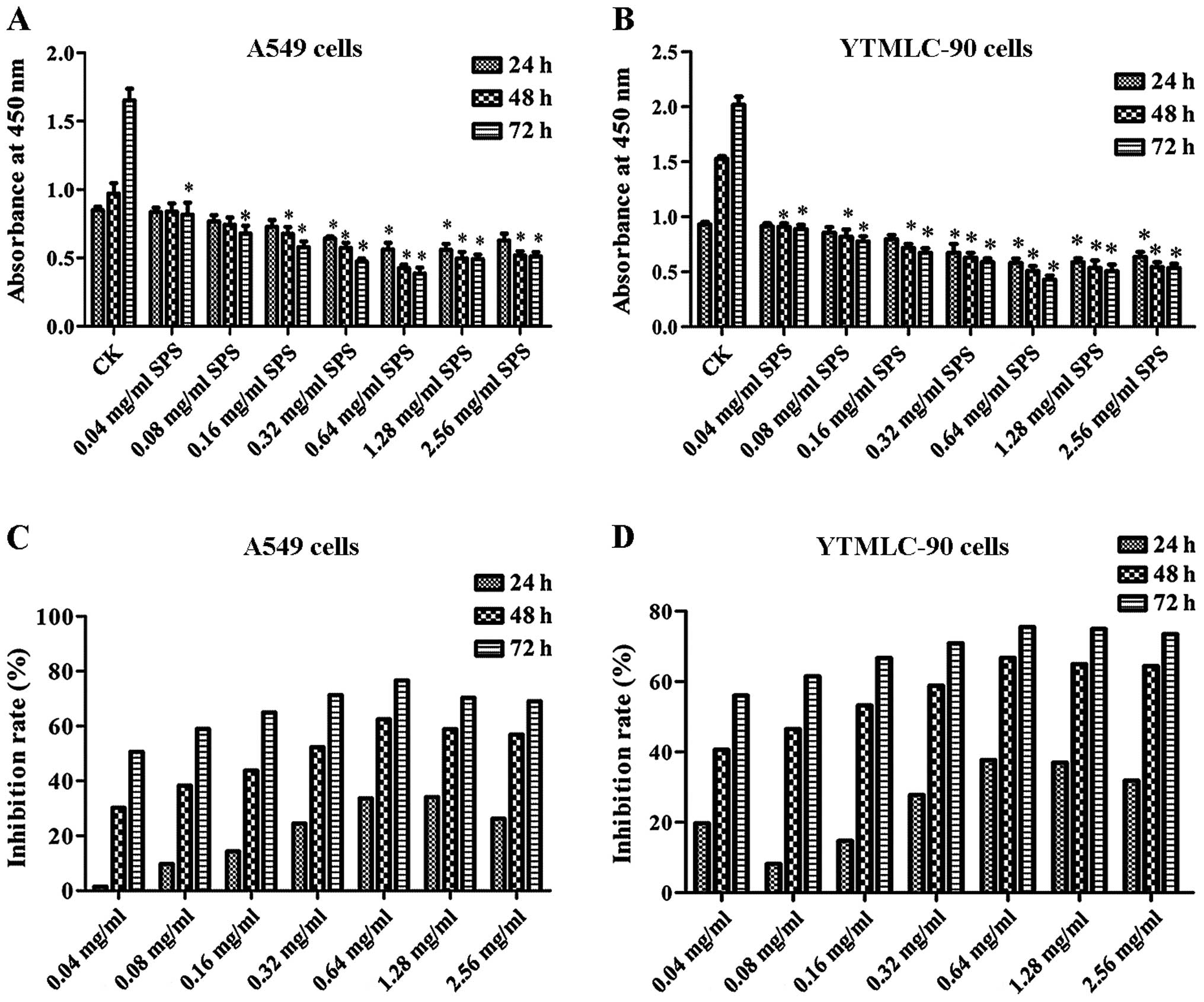
SPS treatment inhibited A549 and YTMLC-90
cell proliferation
To evaluate the effect of SPS on A549 and YTMLC-90
cell proliferation activity in vitro, an MTT assay was
conducted. Results of the MTT assay are shown in Fig. 1A and B. These results demonstrated
that the proliferation of A549 and YTMLC-90 cells was inhibited by
different concentrations of SPS. Compared with the CK group,
concentrations of 0.04 to 2.56 mg/ml of SPS suppressed the
viability of A549 and YTMLC-90 cells, with the exception of 0.04
mg/ml at 24 h (P<0.05). According to Fig. 1C and D, a time-dependent inhibitory
effect of SPS on cell survival was found in A549 and YTMLC-90
cells. At 0.64 mg/ml of SPS, the inhibition rate peaked after 72 h
of treatment, being 76.66 and 75.47% in A549 and YTMLC-90 cells,
respectively. These results suggested that SPS inhibited A549 and
YTMLC-90 cell proliferation.
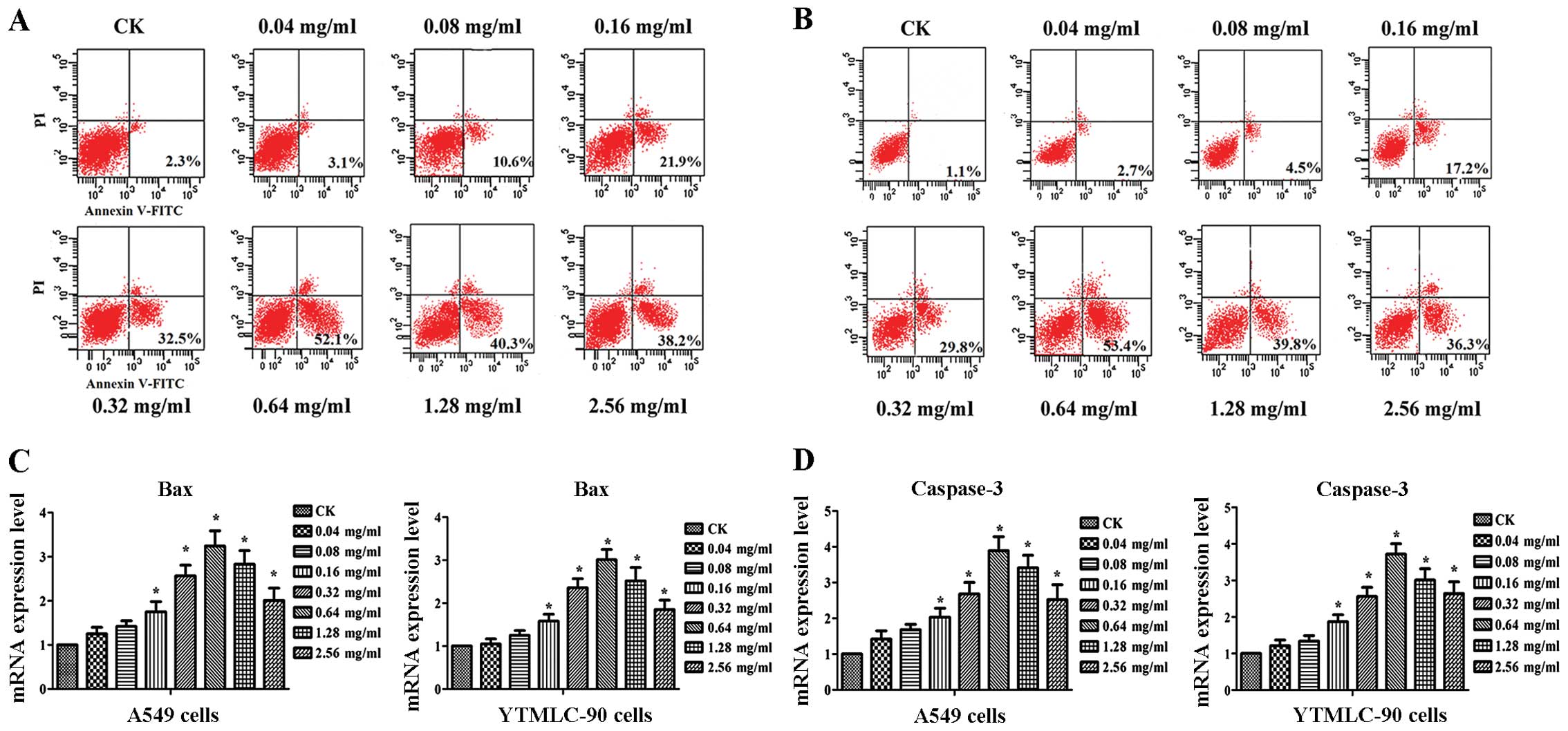
SPS treatment induced A549 and YTMLC-90
apoptosis
Since a significant decrease in cell viability was
found after treatment with SPS, we further tested the effect of SPS
treatment on apoptosis. The results of Annexin V-FITC/PI staining
showed a dose-dependent effect of SPS on apoptosis in A549 and
YTMLC-90 cells (Fig. 2A, B).
Compared with CK, apoptosis in A549 and YTMLC-90 cells was
significantly increased (P<0.05). Even at 0.64 mg/ml, SPS
treatment induced the highest apoptosis rate in both cell lines
(P<0.05). When the treatment concentration was >0.64 mg/ml,
the level of increase in the apoptosis rate was decreased. To
further analyze the mechanism of apoptosis, we also investigated
expression levels of bax and capase-3. For the two cell
lines, expression levels of bax and capase-3 were both
notably induced after treatment with various concentrations of SPS.
The expression level of bax peaked at 0.64 mg/ml of SPS
treatment, increasing 2.4- and 3.01-fold in A549 and YTMLC-90
cells, respectively, and the transcription level of capase-3 was
increased 3.89- and 3.72-fold, respectively (Fig. 2C and D). This result was consistent
with the apoptosis rate. In brief, SPS induced apoptosis in A549
and YTMLC-90 cells.
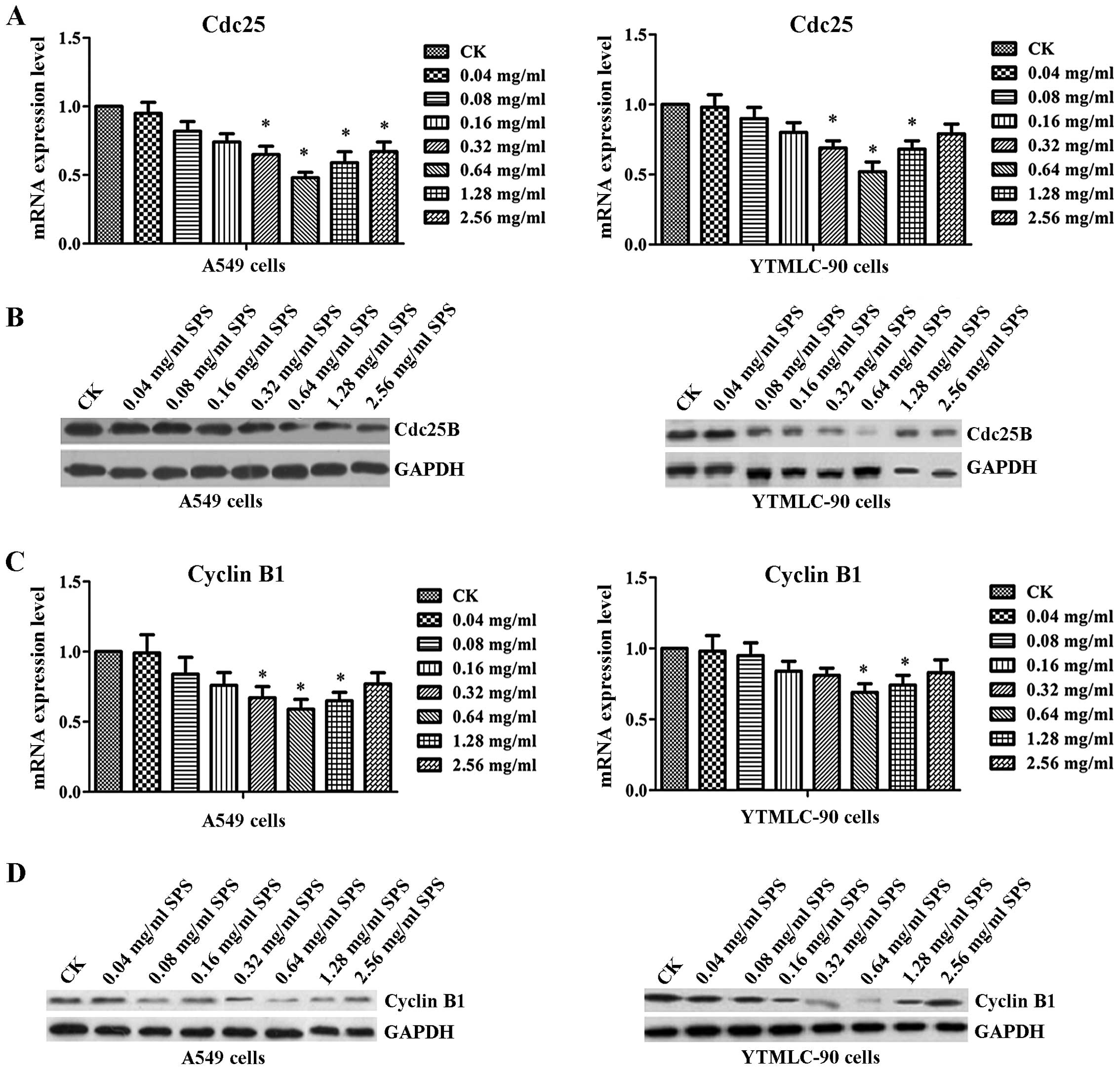
SPS treatment induced A549 and YTMLC-90
cell cycle arrest in the G2/M phase
To further analyze the effect of SPS treatment on
the cell cycle, we examined both mRNA and protein expression levels
of cdc25B and cyclin B1 (Fig. 3).
The results of real-time PCR showed that after treatment with SPS,
the expression levels of cdc25B and cyclin B1 were both decreased
with increasing concentration of SPS when compared with the CK
group in A549 and YTMLC-90 cells, reaching their lowest levels at
0.64 mg/ml SPS (Fig. 3A and C).
Western blot results also confirmed that the activity of cdc25B and
cyclin B1 were both decreased, especially after treatment with 0.64
mg/ml of SPS in A549 or TYMLC-90 cells (Fig. 3B and D). As shown in Table I and Table II, when cells were treated with SPS
for 48 h, their DNA contents were significantly increased in the
G2/M phase. In the 0.64 mg/ml SPS treatment group, DNA content was
markedly increased from 11.1 to 60.3% in A549 cells, and from 4.2
to 61.8% in YTMLC-90 cells. In contrast, the G0/G1 phase population
in the SPS treatment group decreased from 65.7 to 29.3% in A549
cells, and from 68.2 to 28.9% in YTMLC-90 cells. These results
suggested that SPS induced cell cycle arrest in the G2/M phase.
 | Table ISPS induced A549 cell cycle arrest in
G2/M phase. |
Table I
SPS induced A549 cell cycle arrest in
G2/M phase.
| A549
cells/Groups | Cell cycle
distribution (%)
|
|---|
| G1/G0 | S | G2/M |
|---|
| CK | 65.7 | 23.2 | 11.1 |
| 0.04 mg/ml SPS | 62.3 | 24.5 | 13.2 |
| 0.08 mg/ml SPS | 59.2 | 20.9 | 19.9 |
| 0.16 mg/ml SPS | 53.2 | 15.3 | 31.5 |
| 0.32 mg/ml SPS | 42.9 | 12.6 | 44.5 |
| 0.64 mg/ml SPS | 29.3 | 10.4 | 60.3 |
| 1.28 mg/ml SPS | 32.6 | 12.7 | 54.7 |
| 2.56 mg/ml SPS | 39.8 | 16.9 | 43.3 |
 | Table IISPS induced YTMLC-90 cell cycle
arrest in G2/M phase. |
Table II
SPS induced YTMLC-90 cell cycle
arrest in G2/M phase.
| YTMLC-90
cells/Groups | Cell cycle
distribution (%)
|
|---|
| G1/G0 | S | G2/M |
|---|
| CK | 68.2 | 27.6 | 4.2 |
| 0.04 mg/ml SPS | 65.9 | 25.7 | 8.4 |
| 0.08 mg/ml SPS | 60.3 | 20.9 | 18.8 |
| 0.16 mg/ml SPS | 53.6 | 15.7 | 30.7 |
| 0.32 mg/ml SPS | 42.1 | 12.3 | 45.6 |
| 0.64 mg/ml SPS | 28.9 | 9.3 | 61.8 |
| 1.28 mg/ml SPS | 34.6 | 14.9 | 50.5 |
| 2.56 mg/ml SPS | 40.2 | 18.3 | 41.5 |
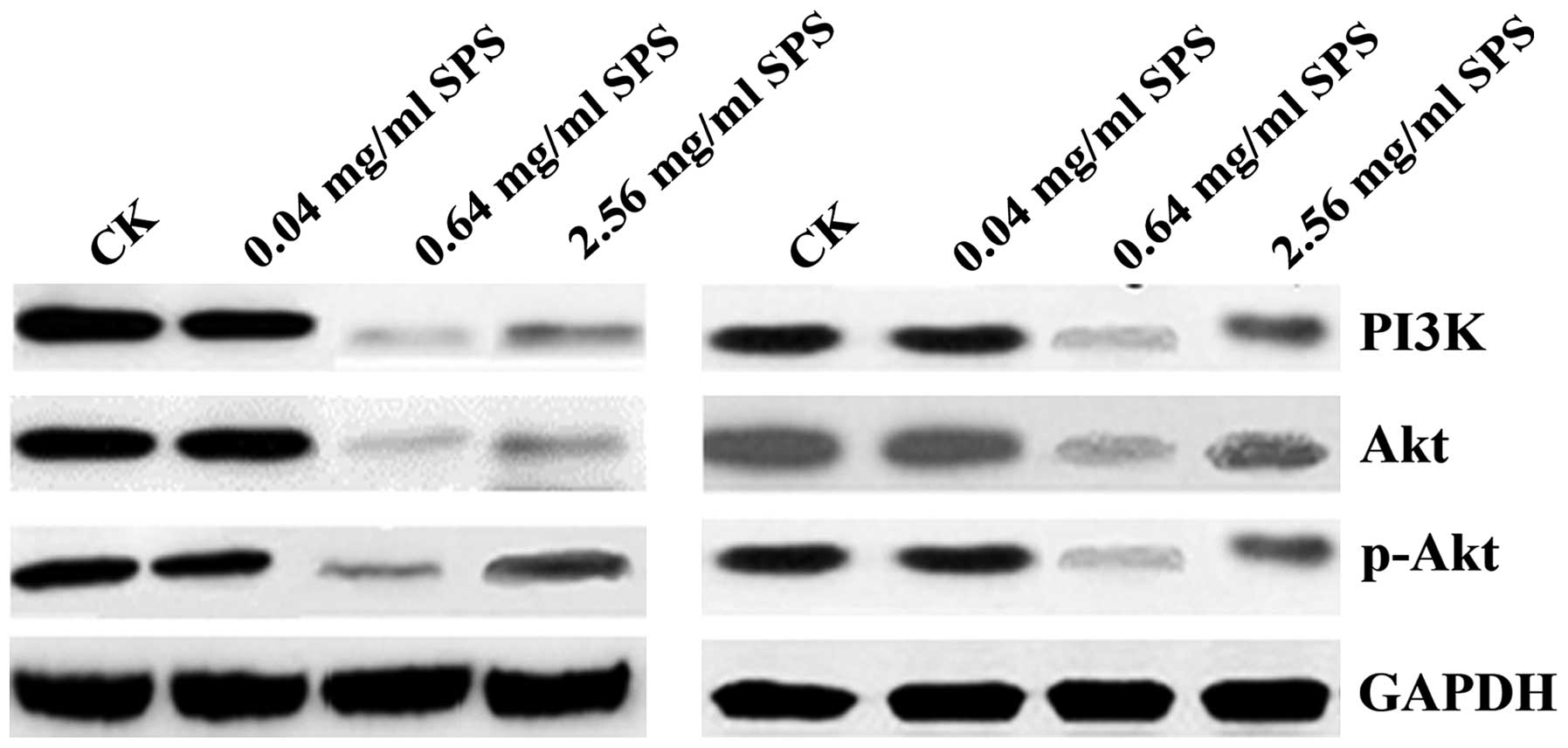
SPS treatment inhibited the Akt
pathway
To investigate whether the inhibitory effect of SPS
treatment on NSCLC cell proliferation was mediated by the PI3K/Akt
pathway, we analyzed protein expression levels of the PI3K/Akt
pathway. In these experiments, cells were treated with SPS (0.04,
0.64, and 2.56 mg/ml) for 48 h. As seen in Fig. 4, we found that compared with the CK
group, 0.04 mg/ml of SPS treatment had no effect on the expression
of Akt, p-Akt, and PI3K, whereas 0.64 mg/ml of SPS treatment
markedly decreased the expression of Akt, p-Akt and PI3K; 2.56
mg/ml of SPS treatment also decreased the expression of these four
proteins, but the decreased level was lower than in the 0.64 mg/ml
group. This result suggested that SPS inhibited NSCLC cell
proliferation by decreasing protein expression levels of the
PI3K/Akt pathway.
SPS injection inhibited tumor growth and
improved immunomodulatory activities in BALB/c nude mice
BALB/c nude mice were used to determine the
anti-lung cancer effect of SPS in vivo. Mice were injected
with different concentrations of SPS (15, 45, and 135 mg/kg). As
shown in Fig. 5A, compared with the
control group, SPS injection significantly decreased tumor volume
(P<0.05). SPS injection of 45 mg/kg and 135 mg/kg dramatically
suppressed tumor growth, with the inhibition rate reaching 75 and
65% 25 days after injection (Fig.
5B). This result indicated that SPS inhibited tumor growth in
mice. TNF-α and IL-6 levels were measured to evaluate the
immunomodulatory activities of SPS injection in mice. According to
Fig. 5C and D, SPS significantly
increased TNF-α and IL-6 levels when compared with the control
group (P<0.05). For instance, 30 days after injection of SPS (45
mg/kg) in mice, TNF-α was increased by 33.55%, relative to the
control group. The level of IL-6 in the 45 mg/kg SPS injection
group was increased by 39.50% relative to the control group. These
results indicated that SPS injection increased immunomodulatory
activities in mice in vivo, suggesting a potential antitumor
application in tumor-bearing mice.
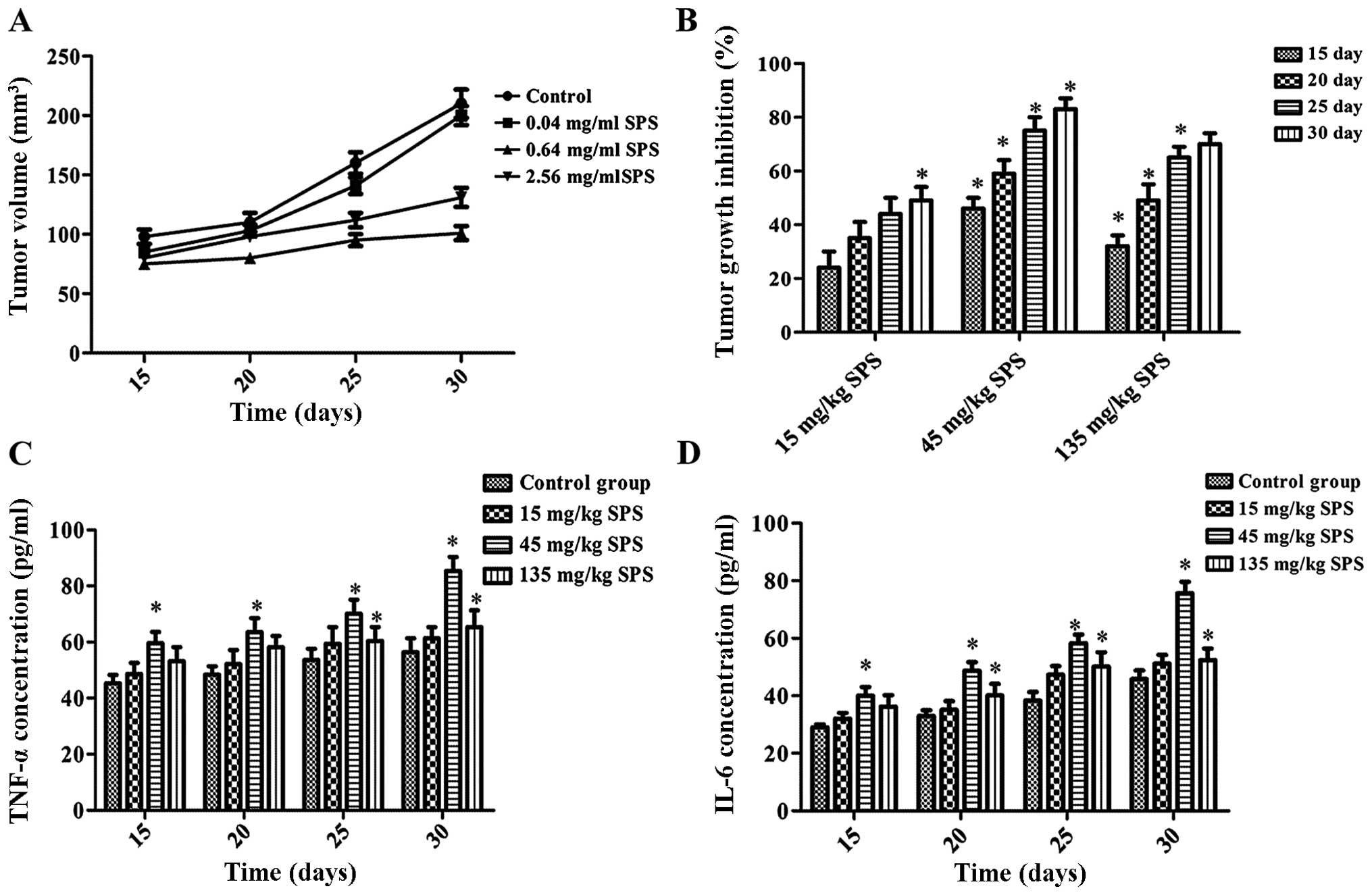 | Figure 5SPS treatment increased
immunomodulatory activities in tumor bearing mice. Murine tumor
models were induced by subcutaneous injection of A549 cells
(5×106 cells in 0.2 ml of PBS) at one site in the right
flank. When the tumor reached ~100 mm3 in volume, 40
animals were divided randomly into 4 groups (n=10 for each group):
low (15 mg/kg), middle (45 mg/kg) and high (135 mg/kg) dose groups,
and a control group that was treated with an equal volume of normal
saline (NS). All injections were administered intraperitoneally
every day. (A) Tumor volume was measured 15, 20, 25, and 30 days
after SPS injection. (B) The inhibition rate of tumor growth. (C)
TNF-α concentration (pg/ml) was assayed by ELISA 15, 20, 25, and 30
days after SPS injection. (D) IL-6 concentration (pg/ml) was
assayed by ELISA 15, 20, 25, and 30 days after SPS injection. SPS
concentrations: 15, 45, and 135 mg/kg. All data are expressed as
mean ± SD (n=10). *P<0.05 vs. CK group. |
Discussion
Safflower is a herb medicine famous for its ability
to improve blood circulation and blood stasis and relieve pain. So
far, the anticancer effects of safflower have been focused on
safflower yellow, which is a kind of flavonoids (11,20).
The antitumor effect of SPS is rarely reported, apart from one
study indicating that SPS inhibited the proliferation and
metastasis of MCF-7 breast cancer cells (16). In this study, the effect of SPS in
human NSCLC was investigated. Results of the MTT assay suggested
that SPS suppressed the proliferation of A549 and YTMLC-90 cells
and exhibited a dose-dependent effect; in particular, the
inhibition rate in the 0.64 mg/ml SPS group reached 76.66 and
75.47% in A549 and YTMLC-90 cells, respectively. Results of the
apoptosis assay also showed that SPS induced cell apoptosis and
exhibited a dose-dependent effect. Moreover, expression levels of
bax and capase-3 were increased after treatment with SPS. In
recent years, more and more plant polysaccharides have been used in
antitumor studies. For example, cactus polysaccharides induced
growth arrest and apoptosis in lung squamous carcinoma cells
(21). Pleurotus nebrodensis
polysaccharide induced apoptosis in A549 cells (22). Scutellaria Barbata D.
polysaccharides showed anti-tumor growth activity on human lung
cancer 95-D (23). In our study,
SPS induced apoptosis in NSCLC cells and increased expression
levels of bax and capase-3.
Cell viability and apoptosis were dose- and
time-dependent within the range of 0.04 to 0.64 mg/ml of SPS
treatment. When the dose of SPS was higher than 0.64 mg/ml, the
rate of increase declined. It is well known that
Na+/K+-ATPase activity is an important
indicator of erythrocyte viability and is downregulated in tumor
cells (24). For example, L.
barbarum polysaccharides prevented the development of
cardiovascular disease by increasing the activity of
Na+-K+-ATPase in heart ischemia reperfusion
(IR) in rats (25). Sargassum
fusiforme polysaccharides (SFPS) could restore some biochemical
functions of erythrocyte membranes in S180 mice by
increasing Na+-K+-ATPase activity (26). SPS may play a similar role to that
of L. barbarum polysaccharides and SFPS.
Na+-K+-ATPase activity may be increased when
cells are treated with various concentrations of SPS within the
range of 0.04 to 0.64 mg/ml, but when the concentration is greater
than 0.64 mg/ml, Na+-K+-ATPase activity may
be decreased. Na+-K+-ATPase is very sensitive
and regulated in a dose-dependent manner by some drugs, such as
ouabain. In this study, SPS within a certain range of
concentrations may also have influenced
Na+-K+-ATPase activity in a dose-dependent
manner.
SPS induced A549 and YTMLC-90 cell cycle arrest at
the G2/M phase. Cyclin B1 and cdc25B are upregulated in tumor cells
and are crucial for the cell cycle. In recent years, many studies
have demonstrated that plant polysaccharides induced cell cycle
arrest at the G2/M phase by regulating cell cycle-related protein
expression. Polysaccharides from Masson pine pollen induced cell
cycle arrest at the G2/M phase by downregulating expression levels
of CDK1 and Cyclin B (27).
Wolfberry (Lycium barbarum) polysaccharide induced cell
cycle arrest at the G0/G1 phase and the expression of cyclins and
CDKs was consistent with changes in cell cycle distribution
(28). In this study, we also found
that both transcription and protein levels of cdc25B and cyclin B1
were decreased with increasing concentration of SPS treatment. This
result indicated that SPS induced cell cycle arrest at the G2/M
phase by downregulating expression levels of cdc25B and cyclin
B1.
SPS inhibited the Akt pathway. SPS treatment at 0.64
mg/ml markedly decreased the expression of Akt, p-Akt and PI3K.
Inhibition of the PI3K/Akt pathway prevented tumor cell
proliferation. Such as Astragalus polysaccharide could
ameliorate doxorubicin-mediated cardiotoxicity via regulation of
the PI3k/Akt pathway (29).
Glycyrrhiza polysaccharide induced apoptosis and inhibited
proliferation in human hepatocellular carcinoma cells by blocking
the PI3K/Akt pathway (30). Our
study also showed that SPS suppressed the PI3K/Akt pathway by
decreasing expression levels of PI3K, Akt and p-Akt. To summarize,
SPS inhibited NSCLC cell proliferation and induced apoptosis by
suppressing the PI3K/Akt pathway.
SPS increased immunomodulatory activities by raising
the levels of TNF-α and IL-6. The improvement of immunomodulatory
activities in cancer by medicinal plant polysaccharides has been
widely investigated. For example, a water-soluble polysaccharide
from Chaenomeles speciosa increased antitumor and
immunomodulatory activities in tumor-bearing mice (31). Polysaccharides from Cymbopogon
citratus increased immunomodulatory activities by raising
levels of TNF-α, IL-2, and IL-6 in transplanted S180 tumors
(32). Polysaccharide fractions
from safflower petals stimulated the production of IL-1, IL-6 and
TNF-α and increased immunomodulatory activity (33). Our results also showed that SPS
significantly increased levels of TNF-α and IL-6. We posit that the
antitumor activity of SPS may be due to its positive influence on
TNF-α and IL-6 expression.
Taken together, our results suggest that, on the one
hand, SPS induces cell cycle arrest at the G2/M phase by decreasing
expression levels of cyclin B1 and cdc25B. On the other hand, SPS
induces A549 and YTMLC-90 apoptosis by decreasing expression levels
of capase-3 and bax. The underlying mechanism of apoptosis
may involve blocking the PI3K/Akt pathway or increasing
immunomodulatory activities. Therefore, SPS may have therapeutic
implications for the clinical management of lung cancer.
Acknowledgments
This work was supported by the Natural Science
Foundation of Shaanxi Province (2012JC2-06) and Shaanxi Science and
Technology Plan Projects Fund (2014K11-01-02-15).
References
|
1
|
Lev-Ari S, Starr A, Katzburg S, Berkovich
L, Rimmon A, Ben-Yosef R, Vexler A, Ron I and Earon G: Curcumin
induces apoptosis and inhibits growth of orthotopic human non-small
cell lung cancer xenografts. J Nutr Biochem. 25:843–850. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liloglou T, Bediaga NG, Brown BR, Field JK
and Davies MP: Epigenetic biomarkers in lung cancer. Cancer Lett.
342:200–212. 2014. View Article : Google Scholar
|
|
3
|
Hennessy BT, Smith DL, Ram PT, Lu Y and
Mills GB: Exploiting the PI3K/AKT pathway for cancer drug
discovery. Nat Rev Drug Discov. 4:988–1004. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhu Z, Sun H, Ma G, Wang Z, Li E and Liu Y
and Liu Y: Bufalin induces lung cancer cell apoptosis via the
inhibition of PI3K/Akt pathway. Int J Mol Sci. 13:2025–2035. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang X-Y, Kuang J-L, Yan C-S, Tu XY, Zhao
JH, Cheng XS and Ye XQ: NRSN2 promotes non-small cell lung cancer
cell growth through PI3K/Akt/mTOR pathway. Int J Clin Exp Pathol.
8:2574–2581. 2015.PubMed/NCBI
|
|
6
|
Chew CL, Lunardi A, Gulluni F, Ruan DT,
Chen M, Salmena L, Nishino M, Papa A, Ng C, Fung J, et al: In vivo
role of INPP4B in tumor and metastasis suppression through
regulation of PI3K/AKT signaling at endosomes. Cancer Discov.
5:740–751. 2015. View Article : Google Scholar :
|
|
7
|
Xu X, Zhang Y, Qu D, Jiang T and Li S:
Osthole induces G2/M arrest and apoptosis in lung cancer A549 cells
by modulating PI3K/Akt pathway. J Exp Clin Cancer Res. 30:332011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hou Z, Zhao W, Zhou J, Shen L, Zhan P, Xu
C, Chang C, Bi H, Zou J, Yao X, et al: A long noncoding RNA Sox2ot
regulates lung cancer cell proliferation and is a prognostic
indicator of poor survival. Int J Biochem Cell Biol. 53:380–388.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tian T, Li J, Li B, Wang Y, Li M, Ma D and
Wang X: Genistein exhibits anti-cancer effects via down-regulating
FoxM1 in H446 small-cell lung cancer cells. Tumour Biol.
35:4137–4145. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhou X, Tang L, Xu Y, Zhou G and Wang Z:
Towards a better understanding of medicinal uses of Carthamus
tinctorius L. in traditional Chinese medicine: A phytochemical and
pharmacological review. J Ethnopharmacol. 151:27–43. 2014.
View Article : Google Scholar
|
|
11
|
Fan S, Lin N, Shan G, Zuo P and Cui L:
Safflower yellow for acute ischemic stroke: A systematic review of
randomized controlled trials. Complement Ther Med. 22:354–361.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li LJ, Li YM, Qiao BY, Jiang S, Li X, Du
HM, Han PC and Shi J: The value of safflower yellow injection for
the treatment of acute cerebral infarction: A randomized controlled
trial. Evid Based Complement Alternat Med.
2015:4787932015.PubMed/NCBI
|
|
13
|
Liu Y, Tian X, Cui M and Zhao S: Safflower
yellow inhibits angiotensin II-induced adventitial fibroblast
proliferation and migration. J Pharmacol Sci. 126:107–114. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Toma W, Guimarães LL, Brito AR, Santos AR,
Cortez FS, Pusceddu FH, Cesar A, Júnior LS, Pacheco MTT and Pereira
CDS: Safflower oil: An integrated assessment of phytochemistry,
antiulcerogenic activity, and rodent and environmental toxicity.
Rev Bras Farmacogn. 24:538–544. 2014. View Article : Google Scholar
|
|
15
|
Ali Sahari M, Morovati N, Barzegar M and
Asgari S: Physicochemical and antioxidant characteristics of
safflower seed oil. Curr Nutr Food Sci. 10:268–274. 2014.
View Article : Google Scholar
|
|
16
|
Luo Z, Zeng H, Ye Y, Liu L, Li S, Zhang J
and Luo R: Safflower polysaccharide inhibits the proliferation and
metastasis of MCF-7 breast cancer cell. Mol Med Rep. 11:4611–4616.
2015.PubMed/NCBI
|
|
17
|
Sabah FS and Saleh AA: Evaluation of
antibacterial activity of flavonoid and oil extracts from safflower
(Carthamus tinctorius L.). J Nat Sci Res. 5:41–44. 2015.
|
|
18
|
Yu J, Sun R, Zhao Z and Wang Y:
Auricularia polytricha polysaccharides induce cell cycle arrest and
apoptosis in human lung cancer A549 cells. Int J Biol Macromol.
68:67–71. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim Y-J, Choi W-I, Jeon B-N, Choi KC, Kim
K, Kim TJ, Ham J, Jang HJ, Kang KS and Ko H: Stereospecific effects
of ginsenoside 20-Rg3 inhibits TGF-β1-induced
epithelial-mesenchymal transition and suppresses lung cancer
migration, invasion and anoikis resistance. Toxicology. 322:23–33.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xi SY, Zhang Q, Liu CY, Xie H, Yue LF and
Gao XM: Effects of hydroxy safflower yellow-A on tumor capillary
angiogenesis in transplanted human gastric adenocarcinoma BGC-823
tumors in nude mice. J Tradit Chin Med. 32:243–248. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li W, Wu D, Wei B, Wang S, Sun H, Li X,
Zhang F, Zhang C and Xin Y: Anti-tumor effect of cactus
polysaccharides on lung squamous carcinoma cells (SK-MES-1). Afr J
Tradit Complement Altern Med. 11:99–104. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cui H, Wang C, Wang Y, Li Z, Zhang Y, Chen
M and Li F: Pleurotus nebrodensis polysaccharide induces apoptosis
in human non-small cell lung cancer A549 cells. Carbohydr Polym.
104:246–252. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang X, Yang Y, Tang S, Tang H, Yang G, Xu
Q and Wu J: Anti-tumor effect of polysaccharides from Scutellaria
barbata D. Don on the 95-D xenograft model via inhibition of the
C-met pathway. J Pharmacol Sci. 125:255–263. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sousa L, Garcia IJ, Costa TG, Silva LN,
Renó CO, Oliveira ES, Tilelli CQ, Santos LL, Cortes VF, Santos HL,
et al: Effects of iron overload on the activity of Na,K-ATPase and
lipid profile of the human erythrocyte membrane. PLoS One.
10:e01328522015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lu S-P and Zhao P-T: Chemical
characterization of Lycium barbarum polysaccharides and their
reducing myocardial injury in ischemia/reperfusion of rat heart.
Int J Biol Macromol. 47:681–684. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ji Y, Ji C and Wang C: Study on S180 Tumor
Mice Erythrocyte Membrance Function of Sargassum Fusiform
Polysaccharides. 7th Asian-Pacific Conference on Medical and
Biological Engineering; Springer; pp. 531–533. 2008, https://www.researchgate.net/publication/226856649_Study_on_S180_Tumor_Mice_erythrocyte_Membrance_Function_of_Sargassum_Fusiform_Polysaccharides.
|
|
27
|
Chu H-L, Mao H, Feng W, Liu JW and Geng Y:
Effects of sulfated polysaccharide from Masson pine (Pinus
massoniana) pollen on the proliferation and cell cycle of HepG2
cells. Int J Biol Macromol. 55:104–108. 2013. View Article : Google Scholar
|
|
28
|
Mao F, Xiao B, Jiang Z, Zhao J, Huang X
and Guo J: Anticancer effect of Lycium barbarum polysaccharides on
colon cancer cells involves G0/G1 phase arrest. Med Oncol.
28:121–126. 2011. View Article : Google Scholar
|
|
29
|
Cao Y, Ruan Y, Shen T, Huang X, Li M, Yu
W, Zhu Y, Man Y, Wang S and Li J: Astragalus polysaccharide
suppresses doxorubicin-induced cardiotoxicity by regulating the
PI3k/Akt and p38MAPK pathways. Oxid Med Cell Longev.
2014:6742192014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen J, Jin X, Chen J and Liu C:
Glycyrrhiza polysaccharide induces apoptosis and inhibits
proliferation of human hepatocellular carcinoma cells by blocking
PI3K/AKT signal pathway. Tumour Biol. 34:1381–1389. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xie X, Zou G and Li C: Antitumor and
immunomodulatory activities of a water-soluble polysaccharide from
Chaenomeles speciosa. Carbohydr Polym. 132:323–329. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bao XL, Yuan HH, Wang CZ, Fan W and Lan
MB: Polysaccharides from Cymbopogon citratus with antitumor and
immunomodulatory activity. Pharm Biol. 53:117–124. 2015. View Article : Google Scholar
|
|
33
|
Wakabayashi T, Hirokawa S, Yamauchi N,
Kataoka T, Woo JT and Nagai K: Immunomodulating activities of
polysaccharide fractions from dried safflower petals.
Cytotechnology. 25:205–211. 1997. View Article : Google Scholar : PubMed/NCBI
|