Introduction
Hepatocellular carcinoma (HCC) is the sixth most
common cancer and the second leading cause of cancer-related death
worldwide (1). Even though the
clinical diagnosis and management of HCC have improved
significantly during the last few decades, HCC is still associated
with a poor prognosis (2).
Therefore, considering the current limited therapy options for HCC,
finding a new therapeutic target molecule has become very
important.
Epithelial cell adhesion molecule (EpCAM) is a
transmembrane glycoprotein that functions as a hemophilic,
epithelial-specific intercellular cell-adhesion molecule (3). EpCAM consists of an extracellular
domain (EpEX), a single transmembrane domain, and a short 26-amino
acid intracellular domain (EpICD) (4). EpCAM is expressed in several human
epithelial tissues and cancers, including HCC and progenitor and
stem cells (4,5). The presence of a high amount of
membranous EpCAM in various cancers has rendered EpCAM an ideal
target for immunotherapy (4,6).
However, despite the broad distribution of EpCAM in human
malignancies, results from recent clinical trials of EpCAM-specific
monoclonal antibodies have shown limited efficacy (7,8).
Maetzel et al reported that regulated
intramembrane proteolysis (RIP)-mediated loss of EpCAM from the
tumor cell surface might be one of the reasons for the limited
efficacy of EpCAM-based cancer therapies (9). The cleavage of the EpCAM ectodomain,
EpEx, by protease tumor necrosis factor α converting enzyme (TACE)
and presenilin-2 (PS-2) and its shedding have been shown to release
its intracellular domain (EpICD), which then translocates to the
nucleus and results in the activation of oncogenic signaling
(9). The association of EpICD with
the FHL2 and Wnt pathway components β-catenin and Lef-1 forms a
nuclear complex that binds DNA at Lef-1 consensus sites and induces
gene transcription, leading to increased cell proliferation
(9).
EpICD is frequently detected in various cancers,
including breast, prostate, colon, bladder, thyroid, and ovarian
(10,11). A recent study revealed that the
nuclear expression of EpICD is correlated with cell growth and
proliferation via RIP-mediated cell signaling in extrahepatic
cholangiocarcinoma (12). Nuclear
expression of EpICD is also associated with a poor prognosis in
thyroid and breast cancers (10,13).
However, RIP-mediated cell signaling of EpCAM in HCC has not yet
been studied.
Given the above background, we conducted this study
to evaluate the nuclear EpICD expression in HCC and clarify the
role of EpICD in the progression and prognosis of HCC.
Materials and methods
Patients and tissue samples
Formalin-fixed, paraffin-embedded HCC blocks were
retrieved from the archive maintained at the Department of
Pathology at Chonbuk National University Hospital and also at
Konyang University Hospital from 2002–2009. A total of 100 patients
who underwent surgical resection were eligible, according to the
following criteria: availability of hematoxylin and eosin-stained
glass slides and paraffin blocks for construction of a tissue
microarray, no preoperative treatment, such as transarterial chemo
embolization or radiation.
The clinical and pathological characteristics of the
patients regarding age, gender, tumor size, histologic
differentiation according to the Edmonson-Steiner grade,
multiplicity, underlying etiology, presence of vascular invasion,
and recurrence were obtained by a review of medical records.
Patients were 36–75 years of age (mean age: 56) and consisted of 80
males and 20 females. Sixty-five cases were associated with
hepatitis B, 4 were hepatitis C-related, 14 were alcohol related,
and 18 had an unknown etiology. Overall survival was calculated
from the date of surgery to the date of death or last follow-up
visit. The follow-up period ranged from one to 134 months (median,
68 months). This study was approved by the Institutional Review
Board of Konyang University Hospital (KYUH 2015-05-011).
Tissue microarray and immunohistochemical
analysis
Tissue microarrays were constructed for
immunohistochemical staining. At least two tissue cores (3.0 mm in
diameter) were obtained from the most representative area in all
individual cases. Additionally, normal liver tissues from each
matched HCC were included as the negative controls.
Immunohistochemistry was performed on 4-µm
sections of tissue microarray blocks that included 100 surgically
removed samples. Tissue sections were deparaffinized and rehydrated
following standard procedure. Heat-induced antigen retrieval was
carried out, and the sections were incubated for 30 min along with
primary antibodies. Antibodies were used for EpCAM (Clone VU-1D9,
Calbiochem, La Jolla, CA, USA) for the extracellular domain of
EpCAM (EpEX), EpICD (Clone E144, Abcam, Cambridge, UK) and
β-catenin (Clone 14, Ventana Medical Systems, Tucson, AZ, USA).
Ki-67 (Clone 30-9, Ventana Medical Systems) was performed on whole
tissue sections. All immunohistochemical staining was carried out
using a BenchMark XT autostainer (Ventana Medical Systems).
Immunohistochemical scoring was performed by two
pathologists who were blinded to the clinical outcome. For all
antibodies studied except Ki-67, the results of the immunostaining
were scored based on the size of the positive area and the
intensity. The proportion score was defined as follows: 0, <10%
cells; 1, 10–30% cells; 2, 31–60% cells; 3, >60% cells. The
intensity score was interpreted as follows: 0, none; 1, mild; 2,
moderate; 3, strong. A total score (range: 0–6) was obtained by
adding the scores of proportion and intensity. EpICD, EpEX, and
β-catenin positive staining was defined as a staining score ≥2.
Ki-67 positivity was defined as an expression in ≥10% of tumor
cells. The cut-off score for determining positive expression was
determined by receiver-operating characteristic curve analysis.
HCC cell lines
Human HCC cell lines HLE, HLF, and Huh-7 were
purchased from the Health Science Research Resources Bank (Osaka,
Japan). The HepG2 cell line was obtained from the American Type
Culture Collection (Manassas, VA, USA). In addition, sarcomatoid
HCC cell line (SH-J1) was also used (14). All HCC cell lines were cultured in
Dulbecco's modified Eagle medium supplemented with 10% fetal bovine
serum (FBS), 100 U/ml penicillin, and 100 µg/ml streptomycin
in a 5% CO2 humidified incubator.
Nuclear fractionation
To generate nuclear and cytoplasmic lysates, the
Subcellular Protein Fractionation kit (Thermo Scientific, Rockford,
IL, USA) was used. Stepwise lysis of cells generated both
functional cytoplasmic and nuclear protein extracts. The protocol
was performed according to the manufacturer's instructions.
Plasmid cDNA and small interfering RNA
transfection
For EpICD plasmid cDNA transfection, EpICD cDNA
(NCBI accession number: NM_002354.2; EpCAM cytoplasmic domain) was
synthesized by CosmoGeneteck Co., Ltd. (Seoul, Korea) and inserted
into the XhoI and BamHI sites of the pEGFP-NI vector
(Clontech, Palo Alto, CA, USA). Transfection of EpICD plasmid DNA
was performed using Lipofectamine 2000 transfection reagent
(Invitrogen, Carlsbad, CA, USA) following the manufacturer's
protocol. At 48 h after transfection, the cells were collected and
used for further experiments.
Small interfering RNA (siRNA) sequences were
employed to silence EpCAM expression. EpCAM siRNA and negative
controls were purchased from Bioneer Corp. (Daejeon, Korea).
Sequences for EpCAM-specific siRNA and negative control siRNA were
as follows: EpCAM: sense 5′-GUGAGAACCUACUGGAUCA(dTdT)-3′, antisense
5′-UGAUCCAGUAGGUUCUCAC(dTdT)-3′, and negative control: sense
5′-CCUACGCCACCAAUUUCGU (dTdT)-3′, antisense 5′-ACGAAAUUGGUGGCGUA
GG(dTdT)-3′. Transfection of siRNA was performed with Lipofectamine
RNAiMAX transfection reagent (Invitrogen) following the
manufacturer's instructions.
Western blot analysis
Western blot was carried out in order to determine
the effect of forced expression of EpICD and silencing EpCAM on the
protein expression related to proliferation of the HCC cell lines.
Cell lysates were resolved using a 10% polyacrylamide gel in a
sodium dodecyl sulfate buffer and electrophoresis. After transfer
onto a polyvinylidene difluoride membrane, the blots were incubated
with anti-EpCAM (Calbiochem), EpICD (Abcam), active β-catenin
(Merck Millipore, Billerica, MA, USA), c-myc (Abcam), cyclin D1
(Santa Cruz Biotechnology, Santa Cruz, CA, USA), β-catenin (BD
Biosciences), and E-cadherin (BD Biosciences). The blots were
developed using secondary antibody, and immune complexes were
visualized using an enhanced chemiluminescence detection system
(Amersham Biosciences, Buckinghamshire, UK). They were then
analyzed with a LAS-3000 luminescent image analyzer (FujiFilm,
Tokyo, Japan).
Cell proliferation assay
To evaluate the effect of EpICD gene transfection
and silencing of EpCAM on the proliferation of HCC cell lines, an
MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-di-phenyltetrazolium bromide)
assay was conducted. Briefly, the cells of each group were seeded
into 96-well plates at 3000 cells per well. After 24 and 48 h of
incubation, the MTT substrate (Sigma, St. Louis, MO, USA) was added
to each well, and the cells were incubated at 37°C for 4 h.
Following elimination of the culture medium, the cells were
dissolved in 0.2 ml of dimethyl sulfoxide. The optical density was
measured using a microplate reader (Bio-Rad, Hercules, CA, USA) at
a wavelength of 560 nm. Each experiment was repeated three
times.
In vitro migration and invasion
assays
The cell migration assay was performed using a
24-Transwell migration chamber (Corning Life Sciences, Acton, MA,
USA), and the cell invasion assay was performed in a 24-Transwell
BioCoat Matrigel invasion chamber (BD Biosciences) according to the
manufacturer's instructions. For migration assay, 2×105
HepG2 and HLE cells were seeded in serum-free medium in the upper
chamber. For migration assay, 2×103 HepG2 and HLE cells
were seeded. The cells, which either migrated to or invaded the
lower surface of the membrane, were fixed with methanol and stained
with a dye for 10 min. Their numbers were counted from 10 random
microscopic fields at ×100 magnification.
Statistical analysis
Statistical analysis was performed using the
Statistical Package for the Social Sciences (SPSS) and assessed
using the Chi-square test and Student's t-test. Overall survival
was calculated using the Kaplan-Meier method. Statistical
significance was assumed at p<0.05.
Results
EpEx, EpICD, and β-catenin expression in
HCC and clinicopathologic correlations
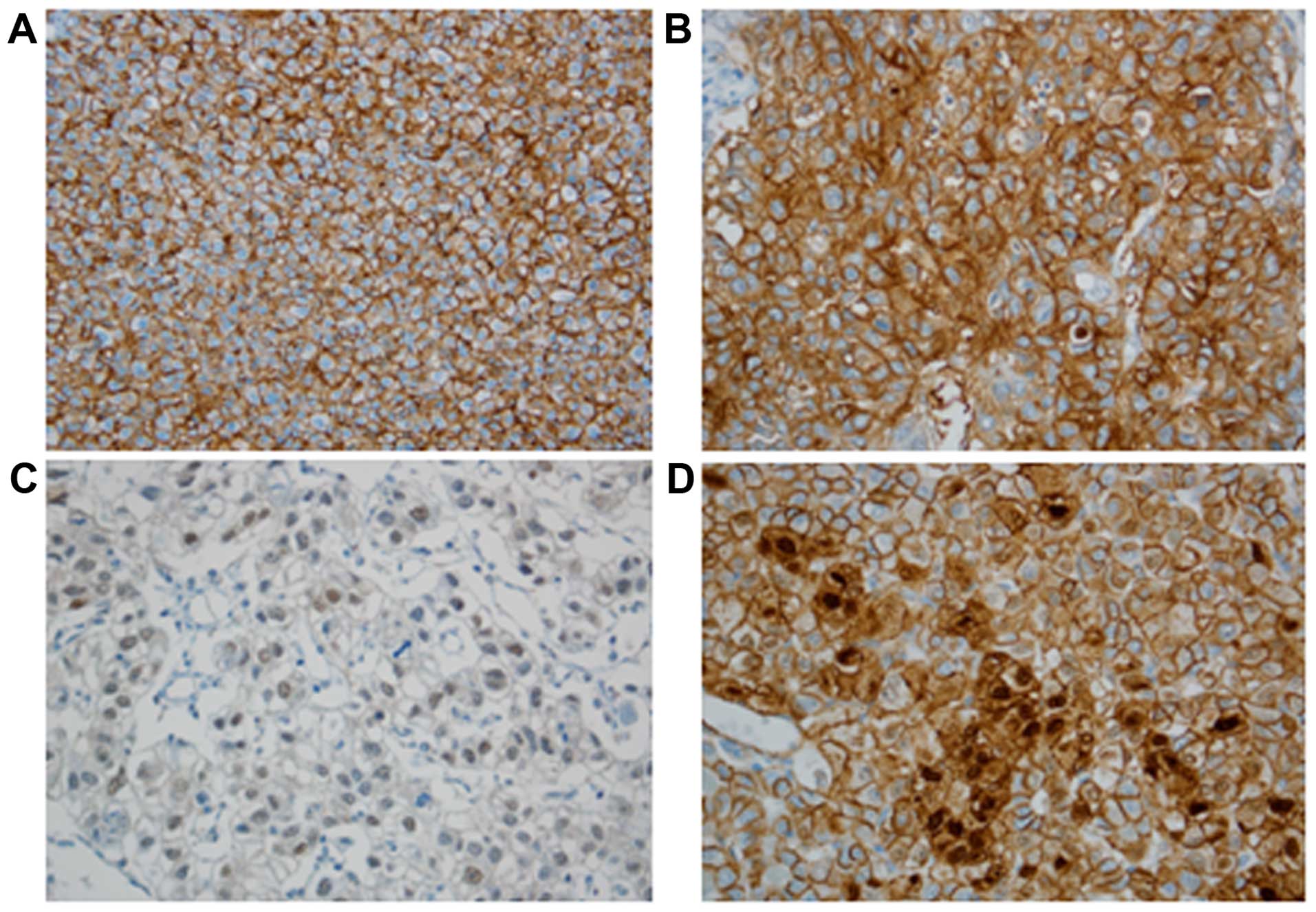
EpEx expression was detected in 37 out of 100 HCC
cases (37%), showing predominantly membrane staining. EpICD
immunoreactivity was observed as nuclear, cytoplasmic, and
membranous immunostaining. Nuclear EpICD expression was seen in 19
of 100 (19%) cases; cytoplasmic and membranous expression were
observed in 49% (49/100) and 17% (17/100) of cases, respectively.
No nuclear EpICD expression was present in non-neoplastic
hepatocytes. Nuclear expression of β-catenin was noted in 10%
(10/100) of HCC cases (Fig. 1).
The correlations between nuclear translocation of
EpICD and clinicopathological variables are summarized in Table I. The nuclear translocation of EpICD
was significantly higher in grades 3–4 (Edmondson-Steiner's grade)
as compared with grades 1–2 (p=0.030). There was a significant
correlation between the nuclear translocation of EpICD and a high T
category (T1-2 vs. T3-4, p=0.044). The nuclear translocation of
EpICD was significantly correlated with the expression of nuclear
β-catenin (p=0.02), and Ki-67 (p=0.027). Moreover, it was also
correlated with a loss of E-cadherin expression (p<0.001). No
significant correlation was found between the nuclear translocation
of EpICD and other clinicopathological variables, including tumor
size (p=0.161), tumor recurrence (p=0.249), vascular invasion
(p=0.395), and EpCAM expression (p=0.309).
 | Table ICorrelation between nuclear expression
of EpICD or EpCAM and clinicopathological factors. |
Table I
Correlation between nuclear expression
of EpICD or EpCAM and clinicopathological factors.
| Factors | Total | EpICD nuclear
expression
| EpCAM
|
|---|
| Negative | Positive | p-value | Negative | Positive | p-value |
|---|
| Differentiation |
| G1-2 | 52 | 48 (92.3) | 4 (7.7) | 0.03 | 30 (57.7) | 22 (42.3) | 0.174 |
| G3-4 | 48 | 33 (68.8) | 15 (31.2) | | 33 (68.8) | 15 (31.3) | |
| Tumor size |
| <5 cm | 65 | 55 (84.6) | 10 (14.4) | 0.161 | 41 (63.1) | 24 (36.9) | 0.575 |
| ≥5 cm | 35 | 26 (74.3) | 9 (25.7) | | 22 (62.9) | 13 (37.1) | |
| T
classification |
| T1,2 | 76 | 65 (85.5) | 11 (14.5) | 0.044 | 61 (80.3) | 15 (19.7) | 0.136 |
| T3,4 | 24 | 16 (66.7) | 8 (33.3) | | 16 (66.7) | 8 (33.3) | |
| Recurrence |
| Negative | 67 | 56 (83.6) | 11 (16.4) | 0.249 | 42 (62.7) | 25 (37.3) | 0.553 |
| Positive | 33 | 25 (75.8) | 8 (24.2) | | 21 (63.6) | 12 (36.4) | |
| Vascular
invasion |
| Negative | 37 | 31 (83.8) | 6 (16.2) | 0.395 | 25 (67.6) | 12 (32.4) | 0.306 |
| Positive | 63 | 50 (79.4) | 13 (20.6) | | 38 (60.3) | 25 (39.7) | |
| β-catenin nuclear
expression |
| Negative | 90 | 76 (84.4) | 14 (15.6) | 0.02 | 55 (61.1) | 35 (38.9) | 0.207 |
| Positive | 10 | 5 (50) | 5 (50) | | 8 (80.0) | 2 (20.0) | |
| Ki-67 |
| Negative | 54 | 48 (88.9) | 6 (11.1) | 0.027 | 34 (63.0) | 20 (37.0) | 0.580 |
| Positive | 46 | 33 (71.7) | 13 (28.3) | | 29 (63.0) | 17 (37.0) | |
| E-cadherin |
| Loss | 22 | 11 (50) | 11 (50) | <0.001 | 16 (72.7) | 6 (27.3) | 0.208 |
| Preserved | 78 | 70 (89.7) | 8 (10.3) | | 47 (60.3) | 31 (39.7) | |
| EpCAM |
| Negative | 77 | 61 (79.2) | 16 (20.8) | 0.309 | | | |
| Positive | 23 | 20 (87.0) | 3 (13.0) | | | | |
In addition, we also analyzed the correlation
between EpCAM and clinicopathologic variables. However, none of the
clinicopathologic variables had a statistically significant
correlation with the EpCAM expression. The overall survival
analysis showed no significant difference in survival based on the
nuclear expression of EpICD (p=0.586).
EpCAM expression in HCC cell lines
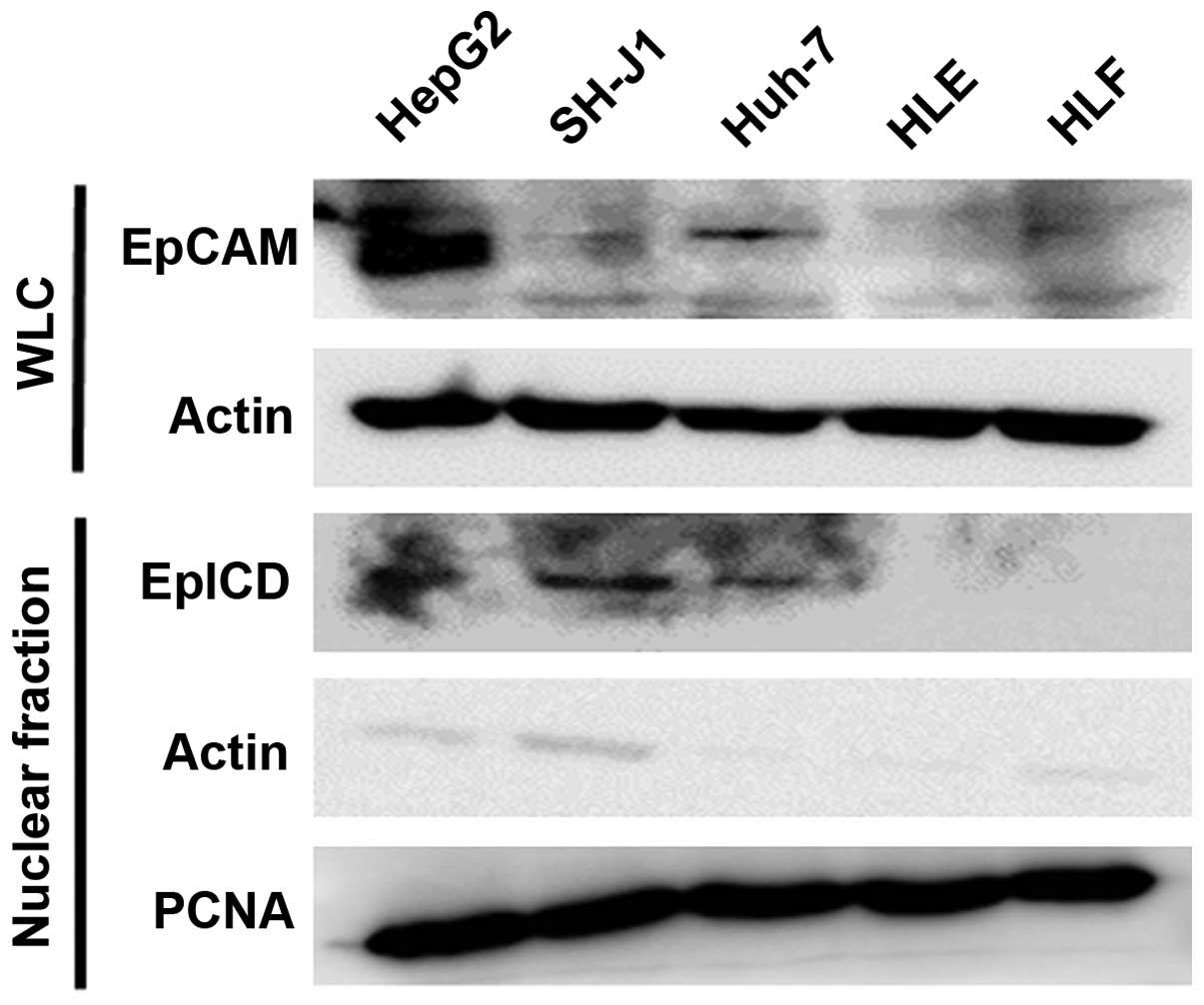
The expression level of the EpCAM protein was higher
in the HepG2 and Huh-7 cell lines than in other cell lines. To
demonstrate the presence of EpICD in the nuclei, we performed a
western blot analysis of the nuclear fraction of HCC cell lines.
Nuclear translocation of EpICD was detected in the nuclear fraction
of the HepG2, SH-J1, and Huh-7 cell lines (Fig. 2).
Influence of EpICD silencing and
overexpression on expression of nuclear β-catenin, c-myc and cyclin
D1
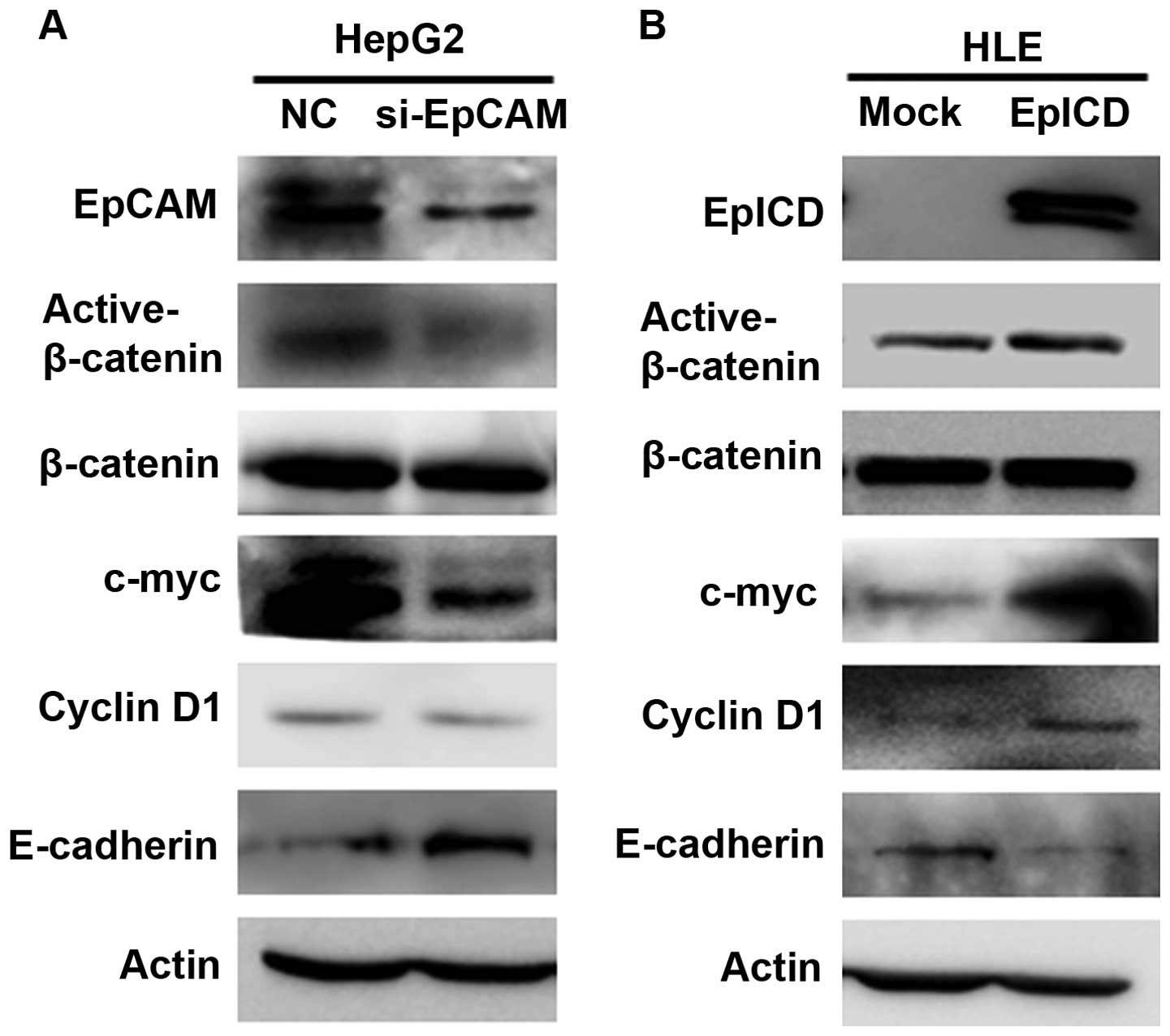
EpCAM siRNA was used to silence EpCAM, and the
results showed that EpCAM siRNA lead to a marked decrease in EPCAM
expression. Western blot analysis revealed that the down-regulation
of EpCAM decreased the expression of the active form β-catenin in
HepG2 cells. Downregulation of EpCAM also induced a diminished
expression level of EpCAM target genes, such as c-myc and cyclin
D1. In contrast, E-cadherin was significantly increased in the
EpCAM siRNA group compared to the controls (Fig. 3A).
To investigate the mechanism by which the
overexpression of EpICD induces cell proliferation, the expression
of nuclear β-catenin and EpCAM target genes was analyzed using
western blot. The result revealed that the overexpression of EpICD
increases the expression of the active form of β-catenin in HLE
cells. In addition, c-myc and cyclin D1 were also highly expressed
in the EpICD-transfected HLE cells, while the expression of
E-cadherin was decreased (Fig.
3B).
Effect of EpICD silencing and
overexpression on cell proliferation, migration, and invasion
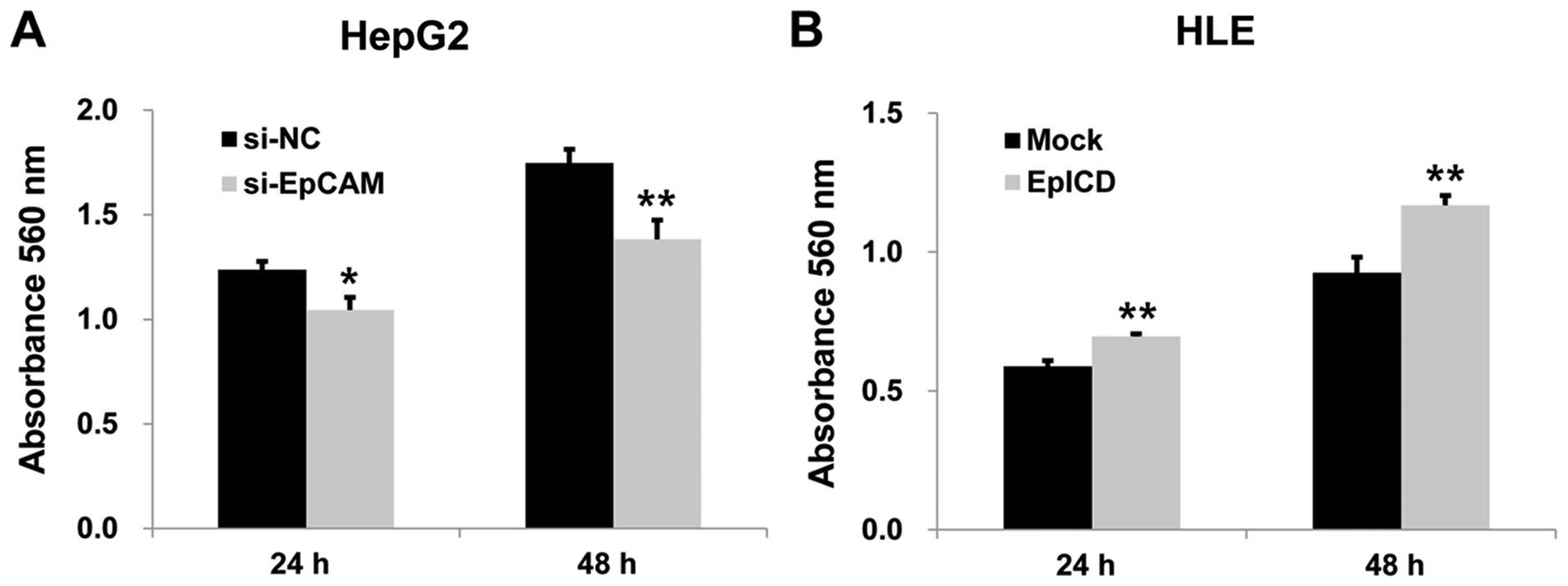
To investigate the effect of silencing EpCAM on the
proliferation of the HepG2 cell line, MTT assay was performed.
After 24 and 48 h, the absorbance of the silencing EpCAM group was
significantly lower than those of the controls (p<0.05,
p<0.01, Fig. 4A). Silencing
EpCAM gene expression inhibited the migration and invasion capacity
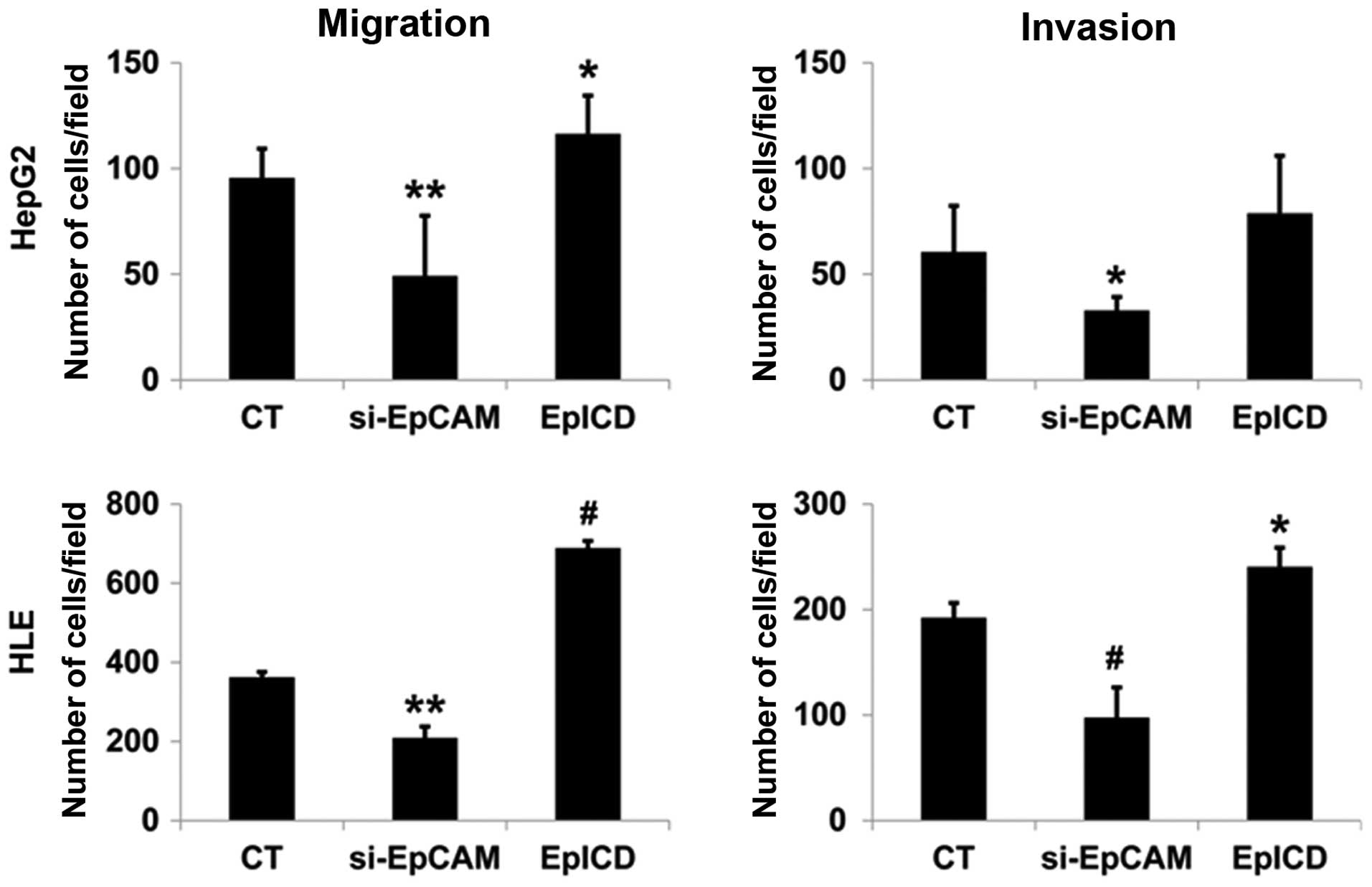
of both the HepG2 and HLE cell lines. These results indicated that
the downregulation of EpCAM expression inhibited the proliferation,
migration, and invasion of tumor cells (Fig. 5).
EpICD overexpression in HLE cells by EpICD cDNA
resulted in significantly increased cell proliferation when
compared to that of the control (p<0.01, p<0.01, Fig. 4B). Overexpression of EpICD in HLE
cells leads to a significant increase in cell migration and
invasion when compared to control cells (p<0.001, p<0.05,
Fig. 5).
Discussion
We determined the role of EpICD and the clinical
significance of nuclear expression of EpICD in HCC. The main
findings of this study were: i) nuclear expression of EpICD and
β-catenin was detected in 19% and 10% of HCC patients,
respectively; ii) nuclear expression of EpICD was significantly
correlated with the nuclear expression of β-catenin, high tumor
grade, high T category, and high Ki-67 index; iii) forced
overexpression of EpICD in HCC cells increased the expression
levels of the active form of β-catenin, c-myc and cyclin D1,
whereas silencing EpCAM decreased the expression of the active form
of β-catenin, c-myc, and cyclin D1; iv) EpICD overexpression
increased cell proliferation, migration, and invasion. Silencing
EpCAM gene expression inhibited proliferation, migration, and
invasion in HCC cells.
EpCAM is a transmembrane glycoprotein that plays an
important role in cell adhesion, proliferation, differentiation,
migration, cell cycle regulation, and stem cell signaling (15). In HCC, EpCAM is known as a stemness
marker, which is associated with aggressive behavior,
chemoresistance, and poor prognosis (16). Although several studies have
investigated the expression of the full length of EpCAM, the
expression of EpICD and its role in tumorigenesis have not been
studied in HCC.
EpCAM predominantly contributes to proliferation,
invasion, and metastasis by regulating β-catenin signaling and
E-cadherin mediated-adhesion (9,17).
Maetzel et al (9) revealed
that signaling by EpCAM requires regulated intra-membrane
proteolysis to release its intracellular domain EpICD. The
sequential proteolysis of EpCAM by TACE and PS-2 produces EpEX and
EpICD. The released EpICD forms a nuclear complex with β-catenin,
Lef-1, and FHL2, and this transcription complex binds the DNA to
activate the target genes (9).
Expression of EpCAM is associated with upregulation of target
genes, including c-myc and cyclins, which enhance
proliferation (18,19). We observed that overexpression of
EpICD in HCC cells increased the expression of the active form of
β-catenin, c-myc, and cyclin D1 and significantly increased the
rate of cell proliferation. We found that nuclear EpICD expression
was significantly correlated with a high Ki-67 proliferation index.
These findings suggest that EpICD may be acting as an oncogenic
signal transducer in HCC by activating β-catenin to promote
proliferation.
EpCAM can inhibit E-cadherin-mediated cell-to-cell
adhesion by disrupting the link between α-catenin and F-actin
(17). It is known that the
cytoplasmic domain of EpCAM is required for its negative effect on
cadherins (20). In addition,
dissociation of cadherin adhesion leads to the accumulation of
intracellular β-catenin and enhances cell proliferation (21). The results of this study are in
agreement with these findings. Overexpression of EpICD decreased
the expression of E-cadherin, whereas downregulation of EpCAM
increased the expression of E-cadherin in HCC cells. The loss of
E-cadherin function is a crucial step in epithelial-mesenchymal
transition (EMT) (22). EMT is a
biologic process in which polarized epithelial cells acquire the
motile and invasive characteristics of mesenchymal cells, resulting
in invasion and metastasis (22).
EpICD regulates reprogramming and EMT genes, including c-Myc,
Oct4, SOX2 and Nanog (21). Downregulation of EpICD suppresses
the expression of EMT-related transcription factors Snail, Slug,
Twist, and TCF4, thus reducing tumor invasiveness (21,23).
These transcriptional factors are well-known repressors of
E-cadherin expression (24,25). Jachin et al (12) demonstrated that the nuclear
expression of EpICD, which paralleled the nuclear expression of
β-catenin, was increased in tumor buds in invasive front
extrahepatic cholangiocarcinoma. Forced overexpression of EpICD
enhanced the cell motility and invasiveness of cholangiocarcinoma
cells (12). These findings were
consistent with the results of our study in that forced EpICD
overexpression increased migration and invasion, while silencing
EpCAM expression suppressed migration and invasion in HCC
cells.
Recently, there is increasing interest in the role
of EpICD in cancer progression, aggressive behavior, and poor
prognosis. Nuclear EpICD staining was observed in undifferentiated
and poorly differentiated thyroid cancer, but not in
well-differentiated thyroid cancer (10). It has also been demonstrated that
the expression of nuclear EpICD is associated with a high tumor
grade in colon cancer and extrahepatic cholangiocarcinoma (12,21).
Our results showed that the nuclear expression of EpICD was
correlated with a high tumor grade and a high T category in HCC.
This finding suggests that EpICD might be associated with HCC
progression. There were no significant relationships between
survival and nuclear EpICD expression in our study. However,
nuclear EpICD expression has been correlated with a poor prognosis
in thyroid and breast cancers and is significantly associated with
tumor recurrence in breast cancer (10,13).
Further studies with a larger number of cases will be needed to
confirm the prognostic significance of nuclear EpICD expression in
HCC.
EpCAM is a well-known therapeutic target antibody
against epithelial tumors. The European Medicines Agency approved
the use of catumaxomab (Removab®), a trifunctional
bispecific antibody targeting EpCAM, for the intraperitoneal
treatment of malignant ascites (26). Clinical trials of EpCAM have already
been performed in head and neck squamous cell carcinoma and bladder
cancer patients (27,28). A recent study attempted to verify
the usefulness of EpCAM inhibitors for the treatment of HCC
(29). Based on our results, EpCAM
signaling pathway-associated RIP and EpICD can be considered a
therapeutic target in HCC. Extensive further studies and
verification will be necessary in order to confirm the potential of
the EpICD inhibitor for use in the treatment of patients with
HCC.
In conclusion, this study demonstrated an
association between the nuclear expression of EpICD and β-catenin
in HCC. The overexpression of EpICD in HCC cells induced a
concomitant nuclear expression of the β-catenin and EpCAM target
genes. The overexpression of EpICD significantly enhanced cell
proliferation, migration, and invasion, while silenced EpCAM
suppressed the proliferation, migration, and invasion in HCC cells
in vitro. These findings support that the RIP-mediated EpCAM
signaling pathway is involved in HCC, and EpICD plays important
roles in HCC progression by modulating the expression of target
genes of EpCAM. Therefore, the RIP-mediated EpCAM signaling
pathway, including EpICD, may be a possible candidate for molecular
targeting in future treatments of HCC.
Acknowledgments
This work was supported by the National Research
Foundation of Korea (NRF) grant funded by the Korean Government
(MSIP) (no. 2008-0062279).
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar
|
|
2
|
Graf D, Vallbohmer D, Knoefel WT, Kröpil
P, Antoch G, Sagir A and Häussinger D: Multimodal treatment of
hepatocellular carcinoma. Eur J Intern Med. 25:430–437. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Litvinov SV, Velders MP, Bakker HA,
Fleuren GJ and Warnaar SO: Ep-CAM: A human epithelial antigen is a
homophilic cell-cell adhesion molecule. J Cell Biol. 125:437–446.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Baeuerle PA and Gires O: EpCAM (CD326)
finding its role in cancer. Br J Cancer. 96:417–423. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
van der Gun BT, Melchers LJ, Ruiters MH,
de Leij LF, McLaughlin PM and Rots MG: EpCAM in carcinogenesis: The
good, the bad or the ugly. Carcinogenesis. 31:1913–1921. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Went PT, Lugli A, Meier S, Bundi M,
Mirlacher M, Sauter G and Dirnhofer S: Frequent EpCam protein
expression in human carcinomas. Hum Pathol. 35:122–128. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schmidt M, Scheulen ME, Dittrich C, Obrist
P, Marschner N, Dirix L, Schmidt M, Ruttinger D, Schuler M,
Reinhardt C, et al: An open-label, randomized phase II study of
adecatumumab, a fully human anti-EpCAM antibody, as monotherapy in
patients with metastatic breast cancer. Ann Oncol. 21:275–282.
2010. View Article : Google Scholar
|
|
8
|
Niedzwiecki D, Bertagnolli MM, Warren RS,
Compton CC, Kemeny NE, Benson AB, Eckhardt SG, Alberts S, Porjosh
GN, Kerr DJ, et al: Documenting the natural history of patients
with resected stage II adenocarcinoma of the colon after random
assignment to adjuvant treatment with edrecolomab or observation:
Results from CALGB 9581. J Clin Oncol. 29:3146–3152. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Maetzel D, Denzel S, Mack B, Canis M, Went
P, Benk M, Kieu C, Papior P, Baeuerle PA, Munz M, et al: Nuclear
signalling by tumour-associated antigen EpCAM. Nat Cell Biol.
11:162–171. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ralhan R, Cao J, Lim T, Macmillan C,
Freeman JL and Walfish PG: EpCAM nuclear localization identifies
aggressive thyroid cancer and is a marker for poor prognosis. BMC
Cancer. 10:3312010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ralhan R, He HC, So AK, Tripathi SC, Kumar
M, Hasan MR, Kaur J, Kashat L, MacMillan C, Chauhan SS, et al:
Nuclear and cytoplasmic accumulation of Ep-ICD is frequently
detected in human epithelial cancers. PLoS One. 5:e141302010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jachin S, Bae JS, Sung JJ, Park HS, Jang
KY, Chung MJ, Kim DG and Moon WS: The role of nuclear EpICD in
extrahepatic cholangiocarcinoma: Association with β-catenin. Int J
Oncol. 45:691–698. 2014.PubMed/NCBI
|
|
13
|
Srivastava G, Assi J, Kashat L, Matta A,
Chang M, Walfish PG and Ralhan R: Nuclear Ep-ICD accumulation
predicts aggressive clinical course in early stage breast cancer
patients. BMC Cancer. 14:7262014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim DG, Park SY, Kim H, Chun YH, Moon WS
and Park SH: A comprehensive karyotypic analysis on a newly
established sarcomatoid hepatocellular carcinoma cell line SH-J1 by
comparative genomic hybridization and chromosome painting. Cancer
Genet Cytogenet. 132:120–124. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dollé L, Theise ND, Schmelzer E, Boulter
L, Gires O and van Grunsven LA: EpCAM and the biology of hepatic
stem/progenitor cells. Am J Physiol Gastrointest Liver Physiol.
308:G233–G250. 2015. View Article : Google Scholar :
|
|
16
|
Chan AW, Tong JH, Chan SL, Lai PB and To
KF: Expression of stemness markers (CD133 and EpCAM) in
prognostication of hepatocellular carcinoma. Histopathology.
64:935–950. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Winter MJ, Nagelkerken B, Mertens AE,
Rees-Bakker HA, Briaire-de Bruijn IH and Litvinov SV: Expression of
Ep-CAM shifts the state of cadherin-mediated adhesions from strong
to weak. Exp Cell Res. 285:50–58. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chaves-Pérez A, Mack B, Maetzel D,
Kremling H, Eggert C, Harréus U and Gires O: EpCAM regulates cell
cycle progression via control of cyclin D1 expression. Oncogene.
32:641–650. 2013. View Article : Google Scholar
|
|
19
|
Münz M, Kieu C, Mack B, Schmitt B, Zeidler
R and Gires O: The carcinoma-associated antigen EpCAM upregulates
c-myc and induces cell proliferation. Oncogene. 23:5748–5758. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Litvinov SV, Balzar M, Winter MJ, Bakker
HA, Bruijn IH, Prins F, Fleuren GJ and Warnaar SO: Epithelial cell
adhesion molecule (Ep-CAM) modulates cell-cell interactions
mediated by classic cadherins. J Cell Biol. 139:1337–1348. 1997.
View Article : Google Scholar
|
|
21
|
Lin CW, Liao MY, Lin WW, Wang YP, Lu TY
and Wu HC: Epithelial cell adhesion molecule regulates tumor
initiation and tumorigenesis via activating reprogramming factors
and epithelial-mesenchymal transition gene expression in colon
cancer. J Biol Chem. 287:39449–39459. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Philip R, Heiler S, Mu W, Buchler MW,
Zoller M and Thuma F: Claudin-7 promotes the epithelial-mesenchymal
transition in human colorectal cancer. Oncotarget. 6:2046–2063.
2015. View Article : Google Scholar
|
|
24
|
Medici D, Hay ED and Olsen BR: Snail and
Slug promote epithelial-mesenchymal transition through
beta-catenin-T-cell factor-4-dependent expression of transforming
growth factor-beta3. Mol Biol Cell. 19:4875–4887. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Garg M: Epithelial-mesenchymal transition
- activating transcription factors - multifunctional regulators in
cancer. World J Stem Cells. 5:188–195. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bokemeyer C: Catumaxomab - trifunctional
anti-EpCAM antibody used to treat malignant ascites. Expert Opin
Biol Ther. 10:1259–1269. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
MacDonald GC, Rasamoelisolo M, Entwistle
J, Cuthbert W, Kowalski M, Spearman MA and Glover N: A phase I
clinical study of intratumorally administered VB4-845, an
anti-epithelial cell adhesion molecule recombinant fusion protein,
in patients with squamous cell carcinoma of the head and neck. Med
Oncol. 26:257–264. 2009. View Article : Google Scholar
|
|
28
|
Kowalski M, Guindon J, Brazas L, Moore C,
Entwistle J, Cizeau J, Jewett MAS and MacDonald GC: A phase II
study of oportuzumab monatox: An immunotoxin therapy for patients
with noninvasive urothelial carcinoma in situ previously treated
with bacillus Calmette-Guerin. J Urol. 188:1712–1718. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ogawa K, Tanaka S, Matsumura S, Murakata
A, Ban D, Ochiai T, Irie T, Kudo A, Nakamura N, Tanabe M, et al:
EpCAM-targeted therapy for human hepatocellular carcinoma. Ann Surg
Oncol. 21:1314–1322. 2014. View Article : Google Scholar
|