Introduction
Gastric cancer (GC) is the second leading cause of
cancer-related death and the fourth most common malignant tumor in
the world (1). There are
approximately 876,000 new cases diagnosed (8.7% of the total cancer
cases), and 647,000 people die from the disease (10.4% of cancer
deaths) every year (2). However,
the mechanisms underlying the development of GC remain largely
unclear, and the genetic and molecular alterations in this
malignant disease are not fully understood. Thus, good
understanding of the molecular mechanisms underlying GC development
and progression is urgently needed.
Tetraspanins are a large family of proteins (33 in
mammals). These proteins have two small and large extracellular
loops, four putative hydrophobic membrane-spanning domains and
short amino- and carboxy-terminal cytoplasmic domains. The
hypervariable region in the large extracellular domain of each
tetraspanin can distinguish it from other members in this family
(3). Extracellular domains allow
tetraspanins to associate with a wide range of proteins by
homo/heterotypic interactions (4).
Tetraspanins form complexes termed tetraspanin-enriched membrane
microdomains (TEMs) by interacting with other tetraspanins and with
a variety of transmembrane and cytosolic proteins that are required
for their function (5,6). A multitude of biological processes are
associated with tetraspanins, such as fertilization, parasite and
viral infection, synaptic contacts at neuromuscular junctions,
platelet aggregation, maintenance of skin integrity, immune
response induction, metastasis suppression and tumor progression
(7–9). Furthermore, changing the composition
and localization of TEMs in response to external or internal
stimuli reveals that TEMs are dynamic and flexible structures
(10).
Tspan9, as a member of the tetraspanins, is a
component of TEMs that include the collagen receptor GPVI
(glycoprotein VI) and integrin α6β1, which suggest a role for
Tspan9 in regulating platelet function in concert with other
platelet tetraspanins and their associated proteins (11). However, the role of Tspan9 in GC
cannot be predicted as tetraspanins own different abilities to
promote or suppress carcinogenesis and cancer metastasis. For
example, tetraspanins CD82 and CD9 mostly suppress tumor
progression as they suppress motility and promote adherence to the
surrounding matrix. Their expression is often reduced in late-stage
human tumors. In contrast, tetraspanin CD151 and tetraspanin 8 are
overexpressed in several tumors and appear to support tumor
progression. CD151 regulates post-adhesion events, that is, cell
spreading, migration and invasion including subsequent
intravasation and formation of metastasis. Tetraspanin 8 regulates
cell motility and survival and is involved in the promotion of
angiogenesis (9,12,13).
Matrix metalloproteinases (MMPs) are highly
expressed in almost all human cancers and so is urokinase
plasminogen activator (uPA) (14,15).
The matrix metalloproteinase-9 (MMP-9) and uPA are responsible for
the degradation of extracellular matrix components and play
important roles in the process of cancer invasion and metastasis
(16–18). In addition, many tetraspanins
directly or indirectly regulate the expression and activity of
MMP-9 or uPA. Thus, their regulation of MMP-9 or uPA indicates an
anticancer therapeutic strategy (9,19).
In the present study, we established a stable cell
line with overexpressed Tspan9 to investigate the influence of
Tspan9 on GC in vitro, looking at the functional effects and
the possible involvement of Tspan9 in GC proliferation, migration
and invasion by regulating the expression of MMP-9 and uPA through
the ERK1/2 pathway.
Materials and methods
Cell culture and construction of the
stable cell line overexpressing Tspan9
Human GC SGC7901 cells were obtained from the
Central Laboratory of the Affiliated Hospital of Qingdao University
and were cultured in Roswell Park Memorial Institute-1640 medium
(RPMI-1640; Hyclone, Logan, UT, USA) supplemented with 10% fetal
bovine serum (FBS; Gibco, Grand Island, NY, USA) at 37°C under 95%
humidity and 5% CO2 content. Cells were harvested in the
logarithmic growth phase for use in the experiments described
below. The day before transfection, the SGC7901 cells were seeded
into 6-well plates to ensure 60% confluency at the time of
transfection. Then the cells were transfected with lentivirus
particles (Genechem Co., Ltd., Shanghai, China) coding for Tspan9
or the negative control (NC), respectively, at a multiplicity of
infection of 10. Six hours later, the medium was replaced, and
cells were cultured for an additional 24 h and processed for
further experiments. Transduction efficiency, as determined by
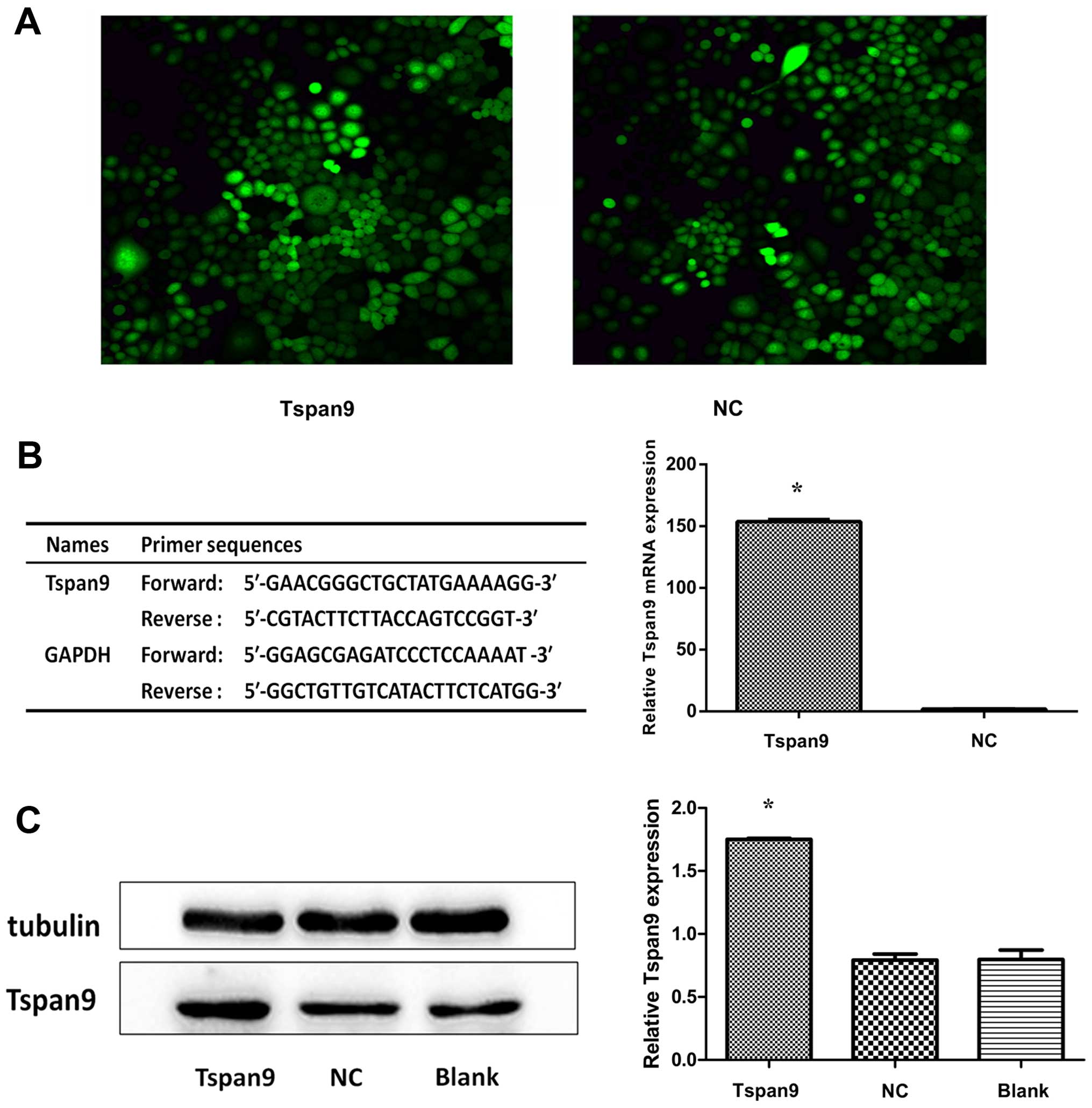
electron microscopy (GFP, × 200 magnification) ranged between 80
and 90%. The clones transfected with LV-Tspan9 were defined as the
Tspan9 group, cells transfected with the negative control vectors
were considered as the NC group, and the blank group included cells
without transfection. Experiments were conducted with cell passages
3–20.
RNA extraction and real-time quantitative
reverse transcription-PCR (qRT-PCR)
Primer sequences (Invitrogen, Carlsbad, CA, USA) for
human Tspan9 were forward, 5′-GAACGGGCTGCTATGAAAAGG-3′ and reverse,
5′-CGTACTTCTTACCAGTCCGGT-3′. Total RNA was extracted from the
cultured cells using mirVana™ PARIS™ kit (Ambion, Carlsbad, CA,
USA). The quality and quantity of the RNA were assessed with a
spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA) at
260 and 280 nm (A260/280). Then the RNA samples were
reverse-transcribed with Transcriptor First Strand cDNA Synthesis
kit (Roche, Mannheim, Germany). PCR conditions were as follows: 10
min at 95°C; 45 cycles of 10 sec at 95°C, 20 sec at 60°C and 10 sec
at 72°C. According to the manufacturer's protocol, each
20-μl reaction system included 5 μl of the cDNA
reverse-transcribed as described above, 10 μl of 2X
FastStart Essential DNA Green Master (Roche), 2 μl of 10X
forward and reverse primers and 3 μl of PCR grade water.
Real-time PCR was performed on LightCycler® 96
instrument (Roche), as fluorescence was detected at the end of each
cycle. The relative amount of target genes was carried out using
the 2−ΔΔCt method while GAPDH was used as internal
control.
Western blot analysis
Cells were lysed on ice in RIPA buffer containing
PMSF (Solarbio, Beijing, China) for 30 min vortexing every 10 min,
followed by centrifugation at 14,000 × g for 5 min at 4°C to remove
cell debris. Then the supernatant was collected for determination
of total protein concentration using the BCA protein assay kit
(CWBIO, Beijing, China). Approximately 25–30 μg of protein
was resolved by 12% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis and was transferred onto polyvinylidene difluoride
membranes (Millipore, USA). The membranes were blocked in 5% bovine
serum albumin (BSA), and then incubated with primary antibodies
targeting Tspan9 (1:1,000; Abcam, Cambridge, UK), ERK1/2 (1:1,000),
p-ERK1/2 (1:2,000) (both from Cell Signaling Technology, Danvers,
MA, USA) or tubulin (1:1,000; Beyotime, Jiangsu, China) in TBST
containing 5% BSA overnight at 4°C. Subsequently, incubation was
conducted with the appropriate secondary antibodies for 1 h at room
temperature. Reactive protein bands were visualized with BeyoECL
Plus (Beyotime) and were detected using the Vilber enhanced
chemiluminescence system (Vilber Lourmat, Marne la Vallée,
France).
Cell viability assay
After trypsinization, the cells were seeded into
96-well plates at a density of 0.2 × 104/well for
culture, and cell proliferation was measured by Cell Counting Kit-8
(CCK-8; Dojindo, Tokyo, Japan) at 24, 48 and 72 h. Briefly, 10
μl of CCK-8 solution was added to each well and incubation
was performed for 2 h at 37°C. Then the optical density (OD) was
measured at 450 nm with a microplate spectrophotometer (BioTek
Instruments, Winooski, VT, USA).
Cell cycle analysis
Cell cycle analysis was performed by a FACSCalibur
cytometer (BD Biosciences, Franklin Lakes, NJ, USA) after staining
the cells with propidium iodide (PI; Abcam). Cells were harvested
by trypsinization, washed with phosphate-buffered saline (PBS), and
fixed in 70% ethanol at 4°C overnight. On the following day, the
fixed cells were washed with PBS, treated with RNase A (50
μg/ml) in PBS at 37°C for 20 min and then mixed with PI (50
μg/ml) for 30 min in the dark. After staining with PI had
been completed, a minimum of 10,000 cells were counted for each
sample by flow cytometry and the cell cycle profile was analyzed
with FlowJo software (Tree Star, Inc., USA).
Wound-healing assay
Cells were seeded into 6-well plates at 90%
confluency and incubated overnight for adherence. Then a wound was
made along the center of each well by scratching the cell layer
with the tip of a 200-μl pipette (Axygen Scientific, Inc.,
Union City, CA, USA). Next, the wells were washed twice with PBS to
remove loose cells and fresh medium was added. Images (× 100
magnification) were captured at 0 and 24 h to assess cell migration
into the wound.
Transwell assay for cell invasion and
migration
Respectively, cell invasion and migration assays
were conducted with or without Matrigel (Corning Inc., Corning, NY,
USA). Cell invasion was measured using Matrigel-coated Transwell
cell culture chambers (8-μm pore size; Corning Inc.). After
being maintained for 24 h in serum-free medium, the cells were
trypsinized and resuspended in serum-free RPMI-1640 and placed in
the upper chamber of the Transwell insert (5 × 104
cells/well), as RPMI-1640 containing 10% FBS was added to the lower
chamber. The plates were incubated in a humidified atmosphere with
95% air and 5% CO2 at 37°C for 24 or 48 h. Non-invasive
cells in the upper chamber were removed by wiping with a cotton
swab, and invasive cells were fixed with 4% formaldehyde in PBS and
stained with 2% crystal violet in ethanol. Cells in the lower
surface of the filter that penetrated through the Matrigel were
counted under a light microscope at × 200 magnification. Cell
migration was determined as described for cell invasion assay
except that the filter membrane was not coated with Matrigel. Cells
located on the underside of the filter were counted under a light
microscope at × 200 magnification.
ELISA assay
The protein level of MMP-9 or uPA in the supernatant
of the cells was assessed by MMP-9 or uPA ELISA kit (Neo
Scientific, Cambridge, MA, USA). Cell supernatant was collected and
centrifuged at 10,000 × g for 10 min at 4°C to remove the cell
debris. Then, 100 μl of supernatant and standard were added
to the wells in the supplied Neoplate while 100 μl of PBS
was added to the blank well, following the manufacturer's protocol.
After adding 50 μl of enzyme solution to each well (not
blank well) and washed 5 times, substrate A (50 μl) and
substrate B (50 μl) were added to each well in order. Gentle
mixing and incubation for 15 min at 37°C in dark was carried out
and then 50 μl of stop solution was added. Finally, optical
density at 450 nm was read within 15 min. The relative protein
level of MMP-9 or uPA in each sample was determined by the standard
curve.
Statistical analysis
SPSS 21 software (IBM, USA) was employed for
statistical analysis. One-way ANOVA was conducted with Bonferroni's
multiple comparison exact probability test, and the Student's
t-test was used to compare continuous variables between two groups.
Experiments with cell cultures were carried out at least in
triplicate. Data are expressed as mean ± standard deviation (SD).
Statistical significance was accepted at p<0.05.
Results
Tspan9 inhibits the proliferation of
gastric cancer SGC7901 cells
We found that Tspan9 expression was lower in the
SGC7901 cells (a poorly differentiated cell line) than that noted
in the other cell lines. Accordingly, we chose this cell line for
transfection with LV-Tspan9 (Fig.
1A) to better understand the role of Tspan9 in the development
of GC. The effect of LV-Tspan9 was confirmed by real-time PCR
(Fig. 1B) and western blot analysis
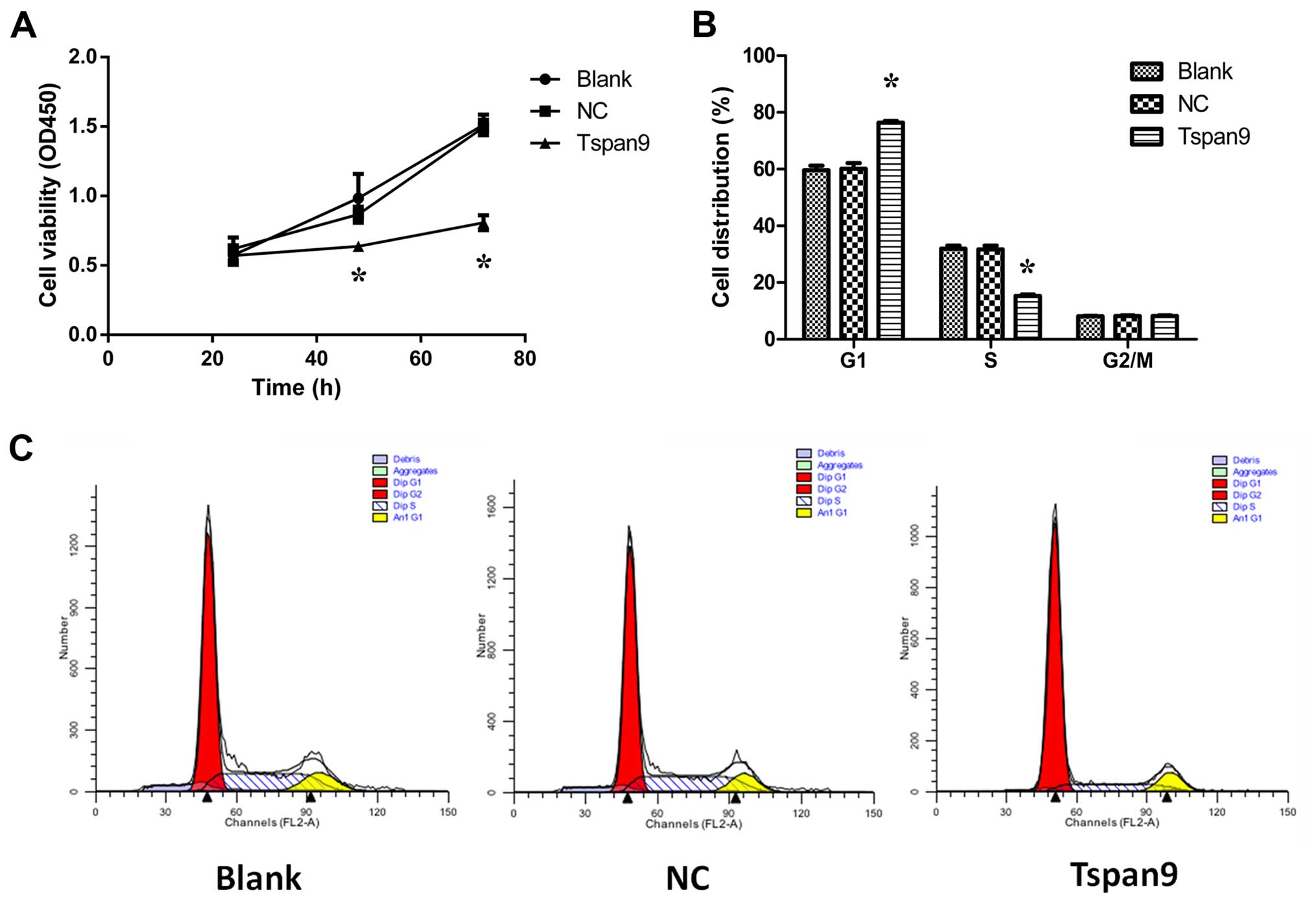
(Fig. 1C). When the CCK-8 assay was
used to assess cell viability over a period of 3 days, we found
that cell viability was significantly lower in the Tspan9 group
than that noted in the NC and blank groups, indicating that cell
viability was suppressed by overexpression of Tspan9 (Fig. 2A). To analyze the mechanisms by
which ectopic Tspan9 expression inhibited cell proliferation, flow
cytometric analysis of the cell cycle was applied. As shown in
Fig. 2B and C, the cells of the
Tspan9 group were arrested in the G1 phase, accompanied by a
significant reduction of cells in the S phase, compared with the
other two groups. These results indicate that cell proliferation
was markedly inhibited by high expression of Tspan9.
Tspan9 inhibits the migration and
invasion of gastric cancer SGC7901 cells
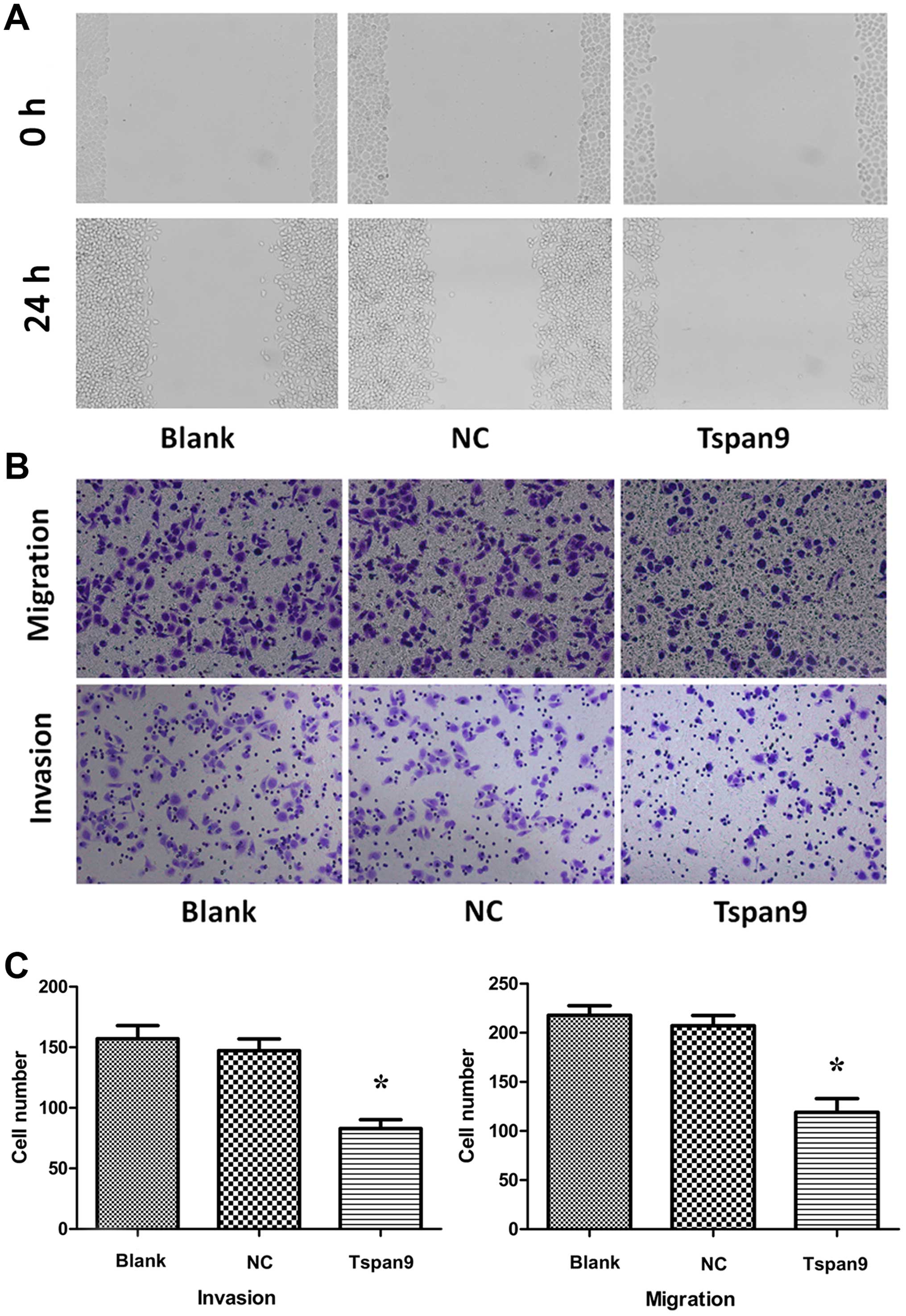
To assess the effect of the upregulation of Tspan9
on cell motility, wound-healing assay and Transwell assays were
performed. We found that fewer cells of the Tspan9 group migrated
to the center of the wound in the wound-healing assay (Fig. 3A) compared to the NC and blank
groups. In addition, overexpression of Tspan9 obviously suppressed
both the cell migration and invasion through Matrigel into the
lower chamber in the Transwell assay (Fig. 3B and C). These findings support the
notion that Tspan9 is involved in the modulation of the cell
migration and invasion of GC cells.
Tspan9 suppresses ERK1/2 activity and
downregulates the secretion levels of MMP-9 and uPA
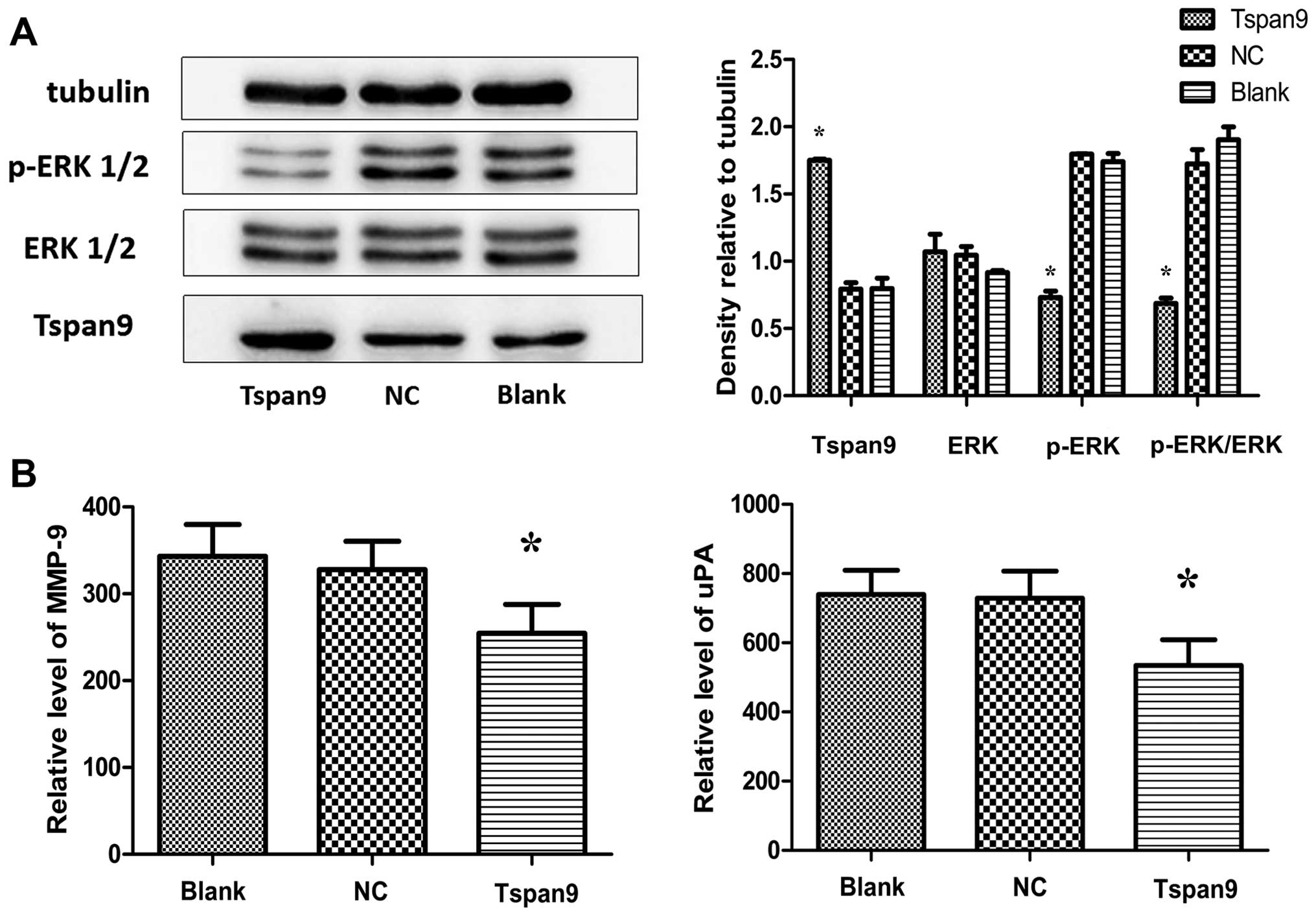
The ERK1/2 pathways are known to play crucial roles
in the promotion of cell survival. In order to elucidate whether
the ERK1/2 pathway is involved in our experiments, we determined
the levels of phosphorylated ERK1/2 (p-ERK1/2) and ERK1/2 by
western blotting. Our results showed that overexpression of Tspan9
inhibited p-ERK1/2 in the SGC7901 cells but had no influence on
total ERK1/2 (Fig. 4A). As for
metastasis-associated proteins, the secretion levels of MMP-9 and
uPA were significantly lower in the Tspan9 group than levels in the
other groups (Fig. 4B). These
results indicate that Tspan9 may inhibit the migration and invasion
of gastric cancer SGC7901 cells by downregulating MMP-9 and uPA
through the ERK1/2 pathway.
Discussion
The present study provides initial evidence
concerning the role of Tspan9 in GC, showing that i) Tspan9
inhibits SGC7901 cell proliferation, migration and invasion, ii)
Tspan9 is involved in the downregulation of the secretion of MMP-9
and uPA via the p-ERK1/2 pathway, and iii) Tspan9 is a potential
tumor suppressor in patients with GC.
Tetraspanins are components of distinct multiprotein
membrane microdomains where they interact with other tetraspanins
and other membrane proteins such as integrins (particularly α3β1,
α4β1, α6β1 and α6β4 isoforms), intracellular associated
heterotrimeric G proteins, proteases, major histocompatibility
complex (MHC) and immunoglobulin superfamily members depending on
tetraspanin isoform, cellular context and other parameters
(8,20). And different tetraspanins
interacting with various partners produce the variability and
specificity among cell types (21).
Thus, these tetraspanin-enriched microdomains (TEMs) contribute to
the organization of cell surface proteins and can influence cell
signal transduction (22,23). It has been reported that Tspan9 is
relatively highly expressed in the megakaryocyte/platelet lineage
and is a component of tetraspanin microdomains on the platelet
surface. Tspan9 is markedly highly conserved during evolution, even
relative to most other tetraspanins (11). As many tetraspanins (e.g., CD9,
CD82, CD151 and tetraspanin 8) play crucial roles in carcinogenesis
and cancer progression (9), we have
reason to assume that Tspan9 may also participate in these
processes.
In the present study, we firstly constructed a
stable cell line overexpressing Tspan9 and then investigated the
role of Tspan9 in GC cells. Since tetraspanins have also been
implicated in the control of cell proliferation directly or
indirectly (24,25), we wished to understand to what
extent Tspan9 plays a role in cell proliferation of GC cells. The
results presented in Fig. 2
indicate that Tspan9 is a potential driver of aberrant cell
proliferation in GC. High expression of Tspan9 suppressed cell
proliferation of SGC7901 cells compared with its controls.
Furthermore, overexpression of Tspan9 resulted in the arrest of the
cell cycle progression as indicated by accumulation of cells in the
G1 phase and reduced progression into the S phase compared with its
negative control. Our data confirmed the regulatory function of
Tspan9 in cancer development and progression. However, the
downstream signaling pathways or specific factors which act on
these biological functions of tumor cells, warrant further
investigation. Identification and elucidation of the possible
molecular mechanisms underlying them, also need further
investigation.
Cancer cells can operate different migration
programs under diverse environmental conditions (26). Cancer cell metastases, based on
cancer cell migration and their invasion to surrounding tissues and
vessels, require changes in locomotion-related genes and upstream
signaling pathways (27,28). In addition, different tetraspanin
variants are involved in the regulation of cell motility and
invasion. For instance, highly expressed CD151 was found to
stimulate the migration of HeLa cells and promote migration and
invasion in prostate cancer cell lines LNCaP and PC3 (12,29).
In contrast, tetraspanin CD9 inhibited cell growth and motility of
the human lung adenocarcinoma cell line MAC10 and the myeloma cell
line ARH77 (25,30). In our study, high expression of
Tspan9 depressed cell migration and invasion of the GC cell line
SGC7901 by wound-healing and Transwell assays (Fig. 3). Thus, we speculated that distinct
combinations of tetraspanins act in concert in distinct cell types
to regulate and govern various aspects related to cancer cell
motility, invasion and migration. Therefore, a comprehensive
understanding of the effect of Tspan9 on cancer cell migration and
invasion is needed.
In order to understand the mechanisms by which
Tspan9 regulates the invasive properties of SGC7901 cells we
focused on the expression of MMP-9 and uPA. On one hand, MMP-9 is a
member of the matrix metalloproteinase family, which binds to zinc
and acts on the extracellular matrix (ECM) to degrade type IV
collagen in the basement membrane. Activation of MMPs has been
detected in almost all types of human cancers, and are closely
correlated to advanced tumor stage, increasing tumor invasion and
metastasis. Metastasis occurs and the survival rate markedly
decreases in GC patients as a result of loss of basement membrane
integrity (31–34). Numerous studies indicate that
inhibition of MMP expression or enzyme activity can be used as
early targets for preventing cancer metastasis (19,35,36).
As a member of the serine protease family, uPA plays a crucial role
in the decomposition of basement membranes, and the activation of
the uPA/uPAR/plasmin proteolytic network plays a key role in tumor
invasion and dissemination of various malignancies (37–39).
The levels of uPA and uPAR expression serve as prognostic markers
in various malignancies, and high levels of expression are often
associated with a poor prognosis (40). Furthermore, many tetraspanins
participate in the regulation of MMPs and uPA by recruitment of
specific signals and by activating or inhibiting diverse signaling
pathways (e.g., PI3K and Ras-Raf-MAPK pathway) (9). Based on the above investigations,
MMP-9 and uPA, two metastasis-associated proteins, were selected in
our study. Our result showed that Tspan9 downregulated the
secretion levels of MMP-9 and uPA in the SGC7901 cells
significantly, confirming that Tspan9 may be a tumor suppressor
reducing the motility and invasiveness of GC cells.
Numerous studies have shown that MAPKs (JNK1/2,
ERK1/2, and p38) are involved in cancer cell proliferation,
migration, invasion, and changes in MMP or uPA activity (19,41–43).
The ERK1/2 pathway plays an important role in the invasion or
metastasis of a number of tumors, such as oral cancer,
hepatocellular carcinoma, prostate cancer and lung cancer (28,44,45).
In the present study, we observed that the ERK1/2 signaling pathway
mediated expression of MMP-9 and uPA in response to Tspan9, which
further contributed to cellular motility of the human GC SGC7901
cells. In conclusion, the anti-proliferation, anti-migration, and
anti-invasion effects of Tspan9 are likely based on its
inactivation of the ERK1/2 pathway in SGC7901 cells, as shown in
our study. Our evidence suggests that downregulation of ERK1/2
signaling pathway by Tspan9 is an important mechanism underlying
cancer progression, and may serve as a potential treatment for
modulating this pathway in cancers. In addition, the specific
molecular mechanisms of Tspan9 and corresponding TEMs require
elucidation. Much research is needed before Tspan9 can be used as a
molecular-target in the therapy for GC.
In summary, Tspan9 may be an important tumor
suppressor of GC. As shown in our research, high expression of
Tspan9 significantly inhibited the proliferation, migration and
invasion of human gastric cancer SGC7901 cells via inactivation of
the ERK1/2 pathway. Better understanding of the precise molecular
mechanism in the Tspan9-mediated signaling pathway may help design
an effective therapeutic modality to control GC.
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (81472338) and A Project of Shandong
Province Higher Educational Science and Technology Program
(J15LL58).
References
|
1
|
Carcas LP: Gastric cancer review. J
Carcinog. 13:142014. View Article : Google Scholar
|
|
2
|
Parkin DM, Ferlay J, Curado MP, Bray F,
Edwards B, Shin HR and Forman D: Fifty years of cancer incidence:
CI5 I-IX. Int J Cancer. 127:2918–2927. 2010. View Article : Google Scholar
|
|
3
|
Lee AJ, Endesfelder D, Rowan AJ, Walther
A, Birkbak NJ, Futreal PA, Downward J, Szallasi Z, Tomlinson IP,
Howell M, et al: Chromosomal instability confers intrinsic
multidrug resistance. Cancer Res. 71:1858–1870. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Simons K and Toomre D: Lipid rafts and
signal transduction. Nat Rev Mol Cell Biol. 1:31–39. 2000.
View Article : Google Scholar
|
|
5
|
Hemler ME: Targeting of tetraspanin
proteins - potential benefits and strategies. Nat Rev Drug Discov.
7:747–758. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Claas C, Stipp CS and Hemler ME:
Evaluation of prototype transmembrane 4 superfamily protein
complexes and their relation to lipid rafts. J Biol Chem.
276:7974–7984. 2001. View Article : Google Scholar
|
|
7
|
Yáñez-Mó M, Barreiro O, Gordon-Alonso M,
Sala-Valdés M and Sánchez-Madrid F: Tetraspanin-enriched
microdomains: A functional unit in cell plasma membranes. Trends
Cell Biol. 19:434–446. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mazzocca A, Birgani MT, Sabbà C and
Carloni V: Tetraspanin-enriched microdomains and hepatocellular
carcinoma progression. Cancer Lett. 351:23–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zöller M: Tetraspanins: Push and pull in
suppressing and promoting metastasis. Nat Rev Cancer. 9:40–55.
2009. View
Article : Google Scholar
|
|
10
|
Kovalenko OV, Metcalf DG, DeGrado WF and
Hemler ME: Structural organization and interactions of
transmembrane domains in tetraspanin proteins. BMC Struct Biol.
5:112005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Protty MB, Watkins NA, Colombo D, Thomas
SG, Heath VL, Herbert JM, Bicknell R, Senis YA, Ashman LK,
Berditchevski F, et al: Identification of Tspan9 as a novel
platelet tetraspanin and the collagen receptor GPVI as a component
of tetraspanin microdomains. Biochem J. 417:391–400. 2009.
View Article : Google Scholar :
|
|
12
|
Sadej R, Grudowska A, Turczyk L, Kordek R
and Romanska HM: CD151 in cancer progression and metastasis: A
complex scenario. Lab Invest. 94:41–51. 2014. View Article : Google Scholar
|
|
13
|
Murayama Y, Oritani K and Tsutsui S: Novel
CD9-targeted therapies in gastric cancer. World J Gastroenterol.
21:3206–3213. 2015.PubMed/NCBI
|
|
14
|
Cakarovski K, Leung JY, Restall C,
Carin-Carlson A, Yang E, Perlmutter P, Anderson R, Medcalf R and
Dear AE: Novel inhibitors of urokinase-type plasminogen activator
and matrix metalloproteinase expression in metastatic cancer cell
lines. Int J Cancer. 110:610–616. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Toda D, Ota T, Tsukuda K, Watanabe K,
Fujiyama T, Murakami M, Naito M and Shimizu N: Gefitinib decreases
the synthesis of matrix metalloproteinase and the adhesion to
extracellular matrix proteins of colon cancer cells. Anticancer
Res. 26:129–134. 2006.PubMed/NCBI
|
|
16
|
Yang SF, Hsieh YS, Lin CL, Hsu NY, Chiou
HL, Chou FP and Chu SC: Increased plasma levels of urokinase
plasminogen activator and matrix metalloproteinase-9 in nonsmall
cell lung cancer patients. Clin Chim Acta. 354:91–99. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mook OR, Frederiks WM and Van Noorden CJ:
The role of gelatinases in colorectal cancer progression and
metastasis. Biochim Biophys Acta. 1705:69–89. 2004.PubMed/NCBI
|
|
18
|
Hwang ES and Lee HJ: Benzyl isothiocyanate
inhibits metalloproteinase-2/-9 expression by suppressing the
mitogen-activated protein kinase in SK-Hep1 human hepatoma cells.
Food Chem Toxicol. 46:2358–2364. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lai KC, Huang AC, Hsu SC, Kuo CL, Yang JS,
Wu SH and Chung JG: Benzyl isothiocyanate (BITC) inhibits migration
and invasion of human colon cancer HT29 cells by inhibiting matrix
metalloproteinase-2/-9 and urokinase plasminogen (uPA) through PKC
and MAPK signaling pathway. J Agric Food Chem. 58:2935–2942. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hölters S, Anacker J, Jansen L,
Beer-Grondke K, Dürst M and Rubio I: Tetraspanin 1 promotes
invasiveness of cervical cancer cells. Int J Oncol. 43:503–512.
2013.PubMed/NCBI
|
|
21
|
Carloni V, Mazzocca A, Mello T, Galli A
and Capaccioli S: Cell fusion promotes chemoresistance in
metastatic colon carcinoma. Oncogene. 32:2649–2660. 2013.
View Article : Google Scholar
|
|
22
|
Rubinstein E, Le Naour F,
Lagaudrière-Gesbert C, Billard M, Conjeaud H and Boucheix C: CD9,
CD63, CD81, and CD82 are components of a surface tetraspan network
connected to HLA-DR and VLA integrins. Eur J Immunol. 26:2657–2665.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Berditchevski F, Odintsova E, Sawada S and
Gilbert E: Expression of the palmitoylation-deficient CD151 weakens
the association of alpha 3 beta 1 integrin with the
tetraspanin-enriched microdomains and affects integrin-dependent
signaling. J Biol Chem. 277:36991–37000. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen L, Yuan D, Zhao R, Li H and Zhu J:
Suppression of TSPAN1 by RNA interference inhibits proliferation
and invasion of colon cancer cells in vitro. Tumori. 96:744–750.
2010.
|
|
25
|
Ovalle S, Gutiérrez-López MD, Olmo N,
Turnay J, Lizarbe MA, Majano P, Molina-Jiménez F, López-Cabrera M,
Yáñez-Mó M, Sánchez-Madrid F, et al: The tetraspanin CD9 inhibits
the proliferation and tumorigenicity of human colon carcinoma
cells. Int J Cancer. 121:2140–2152. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Friedl P and Alexander S: Cancer invasion
and the microenvironment: Plasticity and reciprocity. Cell.
147:992–1009. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sahai E: Mechanisms of cancer cell
invasion. Curr Opin Genet Dev. 15:87–96. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang J, Kuang XR, Lv PT and Yan XX:
Thymoquinone inhibits proliferation and invasion of human
nonsmall-cell lung cancer cells via ERK pathway. Tumour Biol.
36:259–269. 2015. View Article : Google Scholar
|
|
29
|
Ang J, Fang BL, Ashman LK and Frauman AG:
The migration and invasion of human prostate cancer cell lines
involves CD151 expression. Oncol Rep. 24:1593–1597. 2010.PubMed/NCBI
|
|
30
|
Longo N, Yáñez-Mó M, Mittelbrunn M, de la
Rosa G, Muñoz ML, Sánchez-Madrid F and Sánchez-Mateos P: Regulatory
role of tetraspanin CD9 in tumor-endothelial cell interaction
during transendothelial invasion of melanoma cells. Blood.
98:3717–3726. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kubben FJ, Sier CF, van Duijn W, Griffioen
G, Hanemaaijer R, van de Velde CJ, van Krieken JH, Lamers CB and
Verspaget HW: Matrix metalloproteinase-2 is a consistent prognostic
factor in gastric cancer. Br J Cancer. 94:1035–1040. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chu D, Zhang Z, Li Y, Zheng J, Dong G,
Wang W and Ji G: Matrix metalloproteinase-9 is associated with
disease-free survival and overall survival in patients with gastric
cancer. Int J Cancer. 129:887–895. 2011. View Article : Google Scholar
|
|
33
|
Tang Y, Zhu J, Chen L, Chen L, Zhang S and
Lin J: Associations of matrix metalloproteinase-9 protein
polymorphisms with lymph node metastasis but not invasion of
gastric cancer. Clin Cancer Res. 14:2870–2877. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Verma S, Kesh K, Gupta A and Swarnakar S:
An overview of matrix metalloproteinase 9 polymorphism and gastric
cancer risk. Asian Pac J Cancer Prev. 16:7393–7400. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Akter H, Park M, Kwon OS, Song EJ, Park WS
and Kang MJ: Activation of matrix metalloproteinase-9 (MMP-9) by
neurotensin promotes cell invasion and migration through ERK
pathway in gastric cancer. Tumour Biol. 36:6053–6062. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Guruvayoorappan C and Kuttan G:
Amentoflavone inhibits experimental tumor metastasis through a
regulatory mechanism involving MMP-2, MMP-9, prolyl hydroxylase,
lysyl oxidase, VEGF, ERK-1, ERK-2, STAT-1, NM23 and cytokines in
lung tissues of C57BL/6 mice. Immunopharmacol Immunotoxicol.
30:711–727. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Alfano D, Votta G, Schulze A, Downward J,
Caputi M, Stoppelli MP and Iaccarino I: Modulation of cellular
migration and survival by c-Myc through the downregulation of
urokinase (uPA) and uPA receptor. Mol Cell Biol. 30:1838–1851.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ahmad A, Kong D, Wang Z, Sarkar SH,
Banerjee S and Sarkar FH: Down-regulation of uPA and uPAR by
3,3′-diindolylmethane contributes to the inhibition of cell growth
and migration of breast cancer cells. J Cell Biochem. 108:916–925.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ahmad A, Kong D, Sarkar SH, Wang Z,
Banerjee S and Sarkar FH: Inactivation of uPA and its receptor uPAR
by 3,3′-diindolylmethane (DIM) leads to the inhibition of prostate
cancer cell growth and migration. J Cell Biochem. 107:516–527.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Duffy MJ, Maguire TM, McDermott EW and
O'Higgins N: Urokinase plasminogen activator: A prognostic marker
in multiple types of cancer. J Surg Oncol. 71:130–135. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chou YC, Chang MY, Wang MJ, Yu FS, Liu HC,
Harnod T, Hung CH, Lee HT and Chung JG: PEITC inhibits human brain
glioblastoma GBM 8401 cell migration and invasion through the
inhibition of uPA, Rho A, and Ras with inhibition of MMP-2, -7 and
-9 gene expression. Oncol Rep. 34:2489–2496. 2015.PubMed/NCBI
|
|
42
|
Ma CY, Ji WT, Chueh FS, Yang JS, Chen PY,
Yu CC and Chung JG: Butein inhibits the migration and invasion of
SK-HEP-1 human hepatocarcinoma cells through suppressing the ERK,
JNK, p38, and uPA signaling multiple pathways. J Agric Food Chem.
59:9032–9038. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hsu HH, Hu WS, Lin YM, Kuo WW, Chen LM,
Chen WK, Hwang JM, Tsai FJ, Liu CJ and Huang CY: JNK suppression is
essential for 17β-estradiol inhibits prostaglandin E2-Induced uPA
and MMP-9 expressions and cell migration in human LoVo colon cancer
cells. J Biomed Sci. 18:612011. View Article : Google Scholar
|
|
44
|
Lin CW, Chen PN, Chen MK, Yang WE, Tang
CH, Yang SF and Hsieh YS: Kaempferol reduces matrix
metalloproteinase-2 expression by down-regulating ERK1/2 and the
activator protein-1 signaling pathways in oral cancer cells. PLoS
One. 8:e808832013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lee SH, Jaganath IB, Manikam R and Sekaran
SD: Inhibition of Raf-MEK-ERK and hypoxia pathways by Phyllanthus
prevents metastasis in human lung (A549) cancer cell line. BMC
Complement Altern Med. 13:2712013. View Article : Google Scholar : PubMed/NCBI
|