Introduction
Gastric cancer (GC) is one of the most common
cancers worldwide and 42% of the cases are reported in China alone
(1). Although surgical treatment
and chemotherapy have greatly progressed in recent years, the
prognosis of advanced GC patients remains poor (2). According to statistics, 10,900 GC
deaths were projected to occur in the US in 2013, accounting for 2%
of the estimated total cancer deaths (3). Adhesion of GC cells at a distant site
is closely related to the metastasis of GC, which is the leading
cause of mortality for GC patients. It is vital for tumor cells to
adapt to extracellular environments. The slight increase in
extracellular pressure has been found to stimulate tumor cell
adhesion in several types of human cancers, such as colon, tongue
and breast cancer (4–6).
However, to the best of our knowledge, this is the
first study evaluating the influence of high extracellular pressure
on gene expression and the biological behavior of GC cells. This is
important since clarifying the mechanism of the effect of high
extracellular pressure on tumor cells may ultimately provide new
therapeutics to suppress tumor growth. In the present study,
SGC7901 cells were incubated in RPMI-1640 medium for 30 min at 37°C
under ambient and increased pressure (760 and 1,520 mmHg)
conditions. Thus, we investigated the effects of high pressure on
SGC7901 cell proliferation, apoptosis, adhesion, invasion,
migration, differentiation and examined the expression of matrix
metalloproteinase-2 (MMP-2), inhibitor of DNA binding-1 (ID1),
sonic Hedgehog (SHH) and E-cadherin in the three groups.
Materials and methods
Cells and reagents
Human GC cell line SGC7901 was cultured in RPMI-1640
medium (Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal
bovine serum (FBS) and 1% penicillin/streptomycin. The primary
antibodies used included anti-ID1 (1:1,000), anti-SHH (1:1,000),
anti-E-cadherin (1:1,000), anti-MMP-2 (1:500) (all from ProteinTech
Group, Chicago, IL, USA) and anti-β-actin (1:1,000; Boster
Bioengineering, Wuhan, China).
Western blotting
We studied the expression of ID1, SHH, E-cadherin
and MMP-2 under increased or ambient pressure for 30 min in SGC7901
cells after lysing by standard methods. Fifteen milliliters of each
sample was separated by sodium dodecyl sulfate-polyacrylamide gel
electrophoresis before transfer to nitrocellulose membranes. The
membranes were then blocked with buffer [Tris-buffered saline
(TBS), 0.1% Tween-20 and 5% non-fat dry milk] for 60 min at room
temperature and incubated with the primary antibody overnight at
4°C. After washing three times with Tris-buffered solution with
Tween-20 (TBST), the membranes underwent hybridization with the
horseradish peroxidase-conjugated secondary antibody for 60 min at
room temperature. Membrane-bound secondary antibody was washed
three times with TBST and detected by chemiluminescence with the
Western Blotting Luminal reagent (Santa Cruz Biotechnology, Santa
Cruz, CA, USA) according to the manufacturer's instructions.
Pressure regulation
Ambient pressure was controlled using an airtight
pressure tank with inlet and outlet valves, an anthracometer, a
thermometer and a manometer for pressure measurement. The carbon
dioxide concentration was regulated to within ± 0.5%, temperature
within ± 1°C and pressure within ± 1.5 mmHg.
Matrix pre-coating
Matrigel matrix (BD Biosciences, Bedford, MA, USA)
was pre-coated at saturating densities onto 96-well plates or
Transwell inserts using serum-free RPMI-1640 medium for 60 min at
37°C.
Cell adhesion assay
For the adhesion experiments, the SGC7901 cells were
suspended in RPMI-1640 medium and seeded into 96-well plates
(104 cells/well) coated with Matrigel. The cells were
then incubated for 30 min at 37°C under ambient and increased
pressure (760 and 1,520 mmHg) conditions. Next, non-adherent cells
were gently removed by two washes with warmed phosphate-buffered
saline (PBS). After washing, 100 µl of RPMI-1640 medium and
10 µl of Cell Counting Kit-8 (CCK-8; Boster Bioengineering)
were added to each well, and the fluorescence intensity was
measured using Tecan EVOlyzer at 60 min after CCK-8 administration
(excitation, 450 nm; emission, 630 nm). After fluorescence
measurement, attached cells were ethanol-fixed, crystal
violet-stained and observed by an Olympus microscope.
Cell cycle analysis
SGC7901 cells were incubated for 30 min at 37°C
under ambient and increased pressure (760 and 1,520 mmHg)
conditions. Twenty-four hours after incubation under increased
pressure, the cells were then harvested, washed and fixed with
ethanol. Cell cycle distribution was analyzed by FACSCalibur flow
cytometry (BD Biosciences).
Transwell invasion assay
Cell invasion assays were performed using Transwell
inserts (Corning Costar, Cambridge, MA, USA), which consist of
12-well Transwell tissue culture plates with 8-µm pore size
membranes coated with Matrigel (upper chamber). After 24 h of serum
starvation, the SGC7901 cells were incubated for 30 min at 37°C
under ambient and increased pressure (760 and 1,520 mmHg)
conditions. Cells (10,000 cells/well) in serum-free medium were
then added to the upper chambers of the Transwell inserts, and the
lower chambers were filled with medium containing 10% FBS. After
incubation for another 24 h at 37°C, the cells on the upper chamber
were scraped with a cotton swab. The invasive cells that passed
through the filter were measured according to the manufacturer's
instructions. All of the experiments were performed in triplicate
and independently repeated three times.
Transwell migration assay
Cell migration assays were performed using Transwell
inserts (Corning Costar), which consisted of 12-well Transwell
tissue culture plates with 8-µm pore size membranes not
coated with Matrigel. After 24 h of serum starvation, the SGC7901
cells were incubated for 30 min at 37°C under ambient and increased
pressure (760 and 1,520 mmHg) conditions. Cells (10,000 cells/well)
in serum-free medium were then added to the upper chambers of the
Transwell inserts, and the lower chambers were filled with medium
containing 10% FBS. After incubation for another 24 h at 37°C, the
cells on the upper chamber were scraped with a cotton swab. The
invasive cells that passed through the filter were measured
according to the manufacturer's instructions. All of the
experiments were performed in triplicate and independently repeated
three times.
Extraction of total RNA and polymerase
chain reaction (PCR)
TRIzol reagent (Invitrogen) was used to lyse cells
and extract total RNA according to the manufacturer's instructions.
GAPDH was used as an internal control. For semi-quantitative
reverse transcription PCR (RT-PCR), the primer sequences were as
follows: forward, 5′-CCCATTCTGTTTCAGCCAGT-3′ and reverse,
5′-TTGCTCACCTTGCGGTTC-3′ for ID1; forward, 5′-GAGTGAAACTGCGGGTGA-3′
and reverse, 5′-CCAGGAAAGTGAGGAAGTCG for SHH; forward, 5′-CC
GTGTGAAGTATGGGAACG-3′ and reverse, 5′-CGGTCGTAGTCCTCAGTGGT-3′ for
MMP-2; forward, 5′-GTGAAGGTCGGAGTCAACGG-3′ and reverse,
5′-CTCCTGGAAGATGGTGATGGG-3′ for GAPDH. Following RT-PCR, the
amplified samples were stained with ethidium bromide and separated
using electrophoresis in 2% agarose gels. The agarose gels were
then viewed and photographed under ultraviolet illumination.
Transmission electron microscopy
(TEM)
SGC7901 cells were incubated for 30 min at 37°C
under ambient and increased pressure (760 and 1,520 mmHg)
conditions. Twenty-four hours after incubation under increased
pressure, the cells were then harvested, washed and fixed in
mixtures of 2.5% ice-cold glutaraldehyde in 0.1 M sodium cacodylate
buffer for 24 h. Next, the cells were rinsed with PBS, post-fixed
in 2% osmium tetraoxide (in the same buffer) for 60 min, and
dehydrated in a series of graded ethanol. The cells were then
incubated in propylene oxide and embedded in TAAB epoxy resin.
Following counterstaining with lead citrate and uranyl acetate,
ultrathin sections (50 nm) were placed under 200 mesh standard
copper grids and visualized using a Hitachi H-7500 transmission
electron microscope.
Cell proliferation assay
For the cell proliferation assay, the SGC7901 cells
were seeded into 96-well plates (104 cells/well) and
allowed to attach overnight. The cells were then incubated for 30
min at 37°C under ambient and increased pressure (760 and 1,520
mmHg) conditions. Seventy-two hours after incubation under
increased pressure, the cells were washed twice with PBS. Next, 100
µl of RPMI-1640 medium and 10 µl of CCK-8 were added
to each well, and the fluorescence intensity was measured using
Tecan EVOlyzer at 60 min after CCK-8 administration (excitation,
450 nm; emission, 630 nm).
Apoptosis assay
SGC7901 cells were incubated for 30 min at 37°C
under ambient and increased pressure (760 and 1,520 mmHg)
conditions. Twenty-four hours after incubation under increased
pressure, the cells were harvested and washed twice with PBS. The
cells were then labeled with Annexin V-fluorescein isothiocyanate
and propidium iodide according to the manufacturer's instructions
(BD Biosciences) and were analyzed by FACSCalibur flow
cytometry.
Statistical analyses
Statistical analyses were performed with SPSS
software package version 15.0 for Windows. To analyze the
statistical significance between groups, the Student's t-test,
Spearman rank or ANOVA tests were used. All statistical tests were
two-sided. A P-value <0.05 was considered to indicate a
statistically significant result.
Results
Effect of extracellular pressure on
solution pH
Since the ambient pressure increase may exert
effects on the pH of the solutions, we examined cell culture medium
pH in each study. There was no significant difference between the
pH of the cell culture solutions maintained at ambient and
increased pressure at 37°C in room air for 30 min.
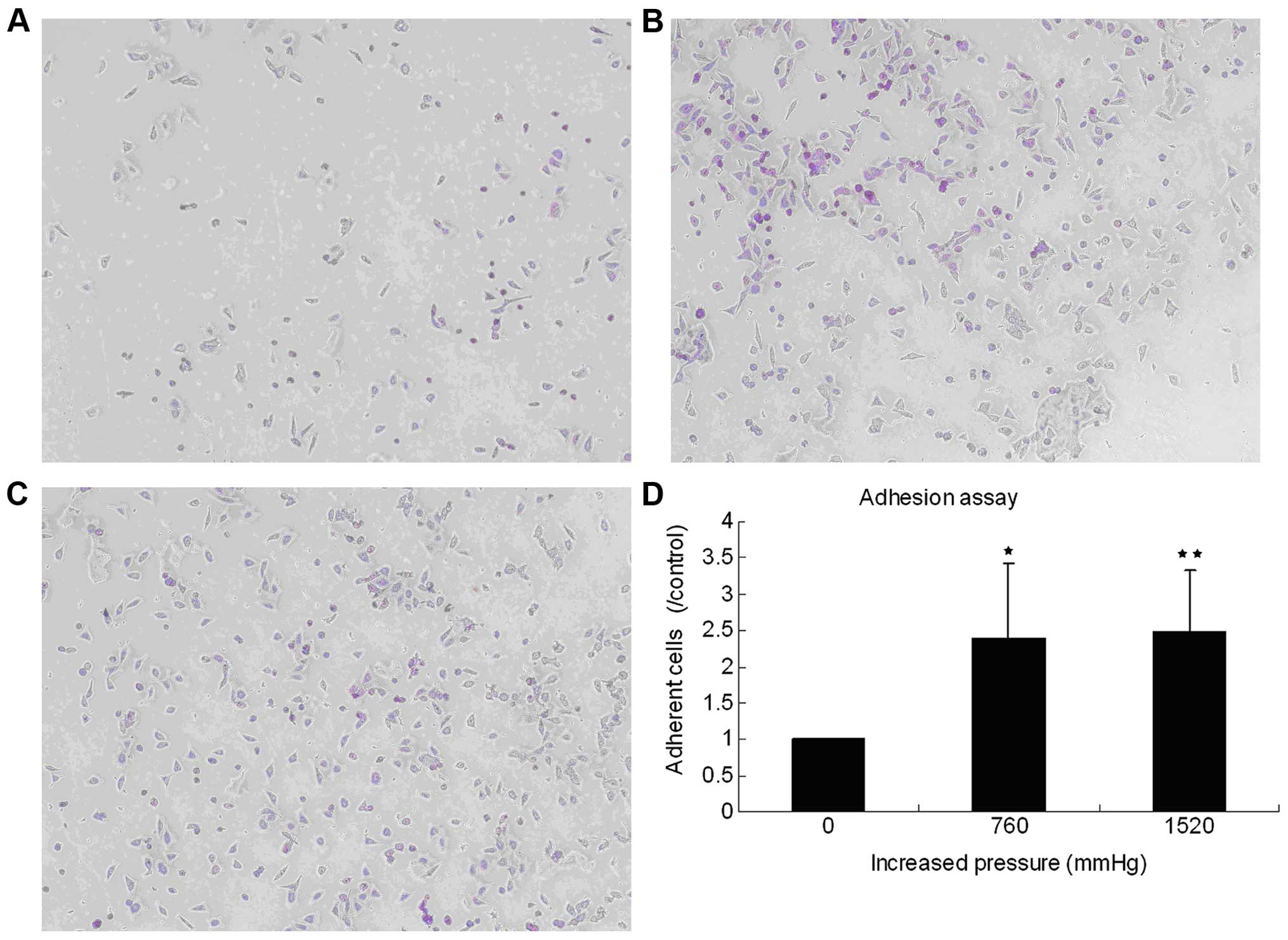
High extracellular pressure promotes GC
cell adhesion
To determine the influence of high extracellular
pressure on cell adhesion, human SGC7901 cells were incubated at
37°C under ambient and increased pressure (760 and 1,520 mmHg).
Increasing extracellular pressure for 30 min promoted cell adhesion
to Matrigel (BD Biosciences). At increased pressure of 760 and
1,520 mmHg, the adherent cells were 238.719±105.0972% (P=0.025) and
247.3855±85.3597% (P=0.003) of those at the ambient pressure,
respectively (Fig. 1).
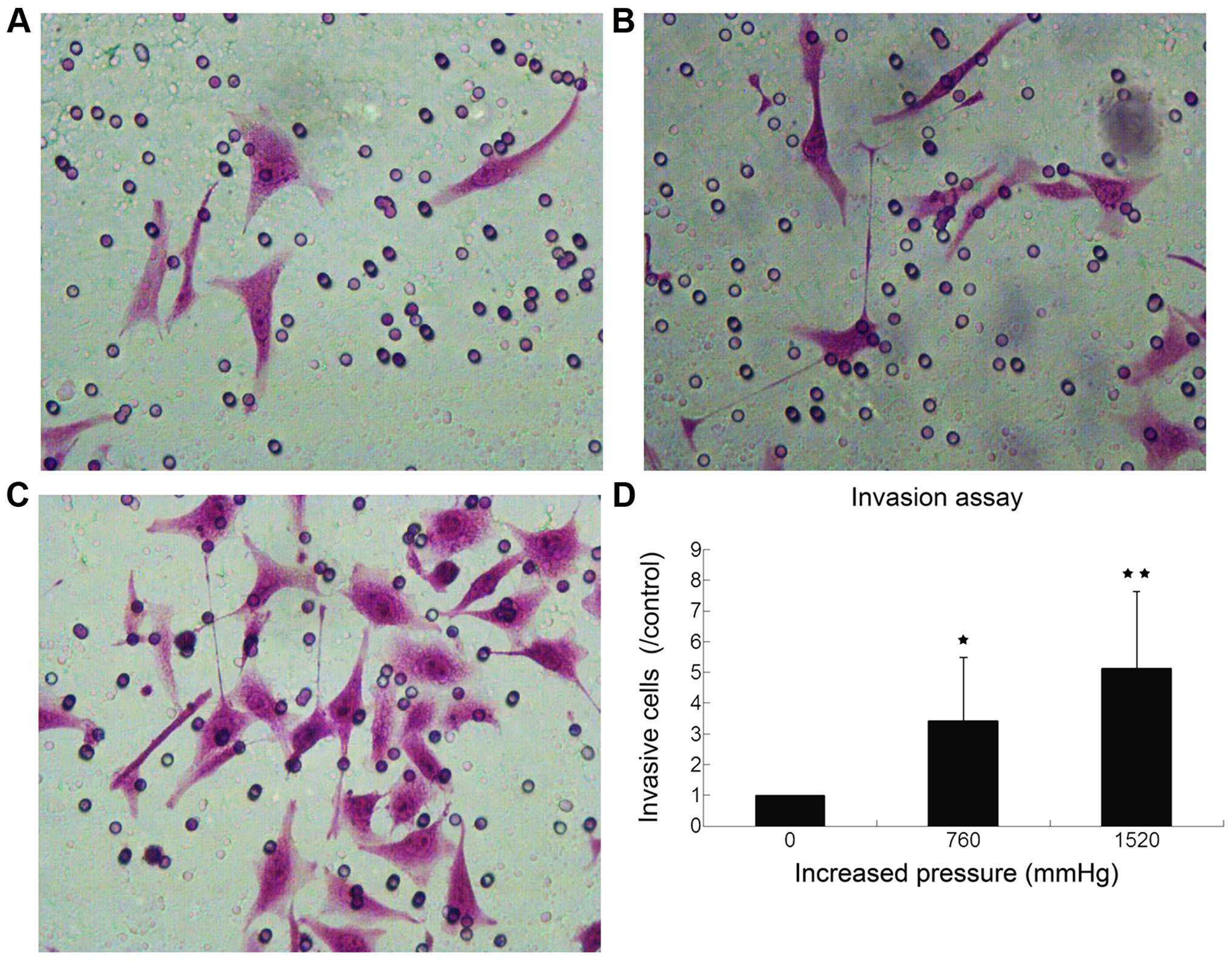
High extracellular pressure enhances GC
cell invasion and migration
To investigate whether the pressure has a role in
cancer metastasis, we evaluated the effect of high extracellular
pressure on cell invasion and migration in the SGC7901 cells. At
increased pressure of 760 and 1,520 mmHg, the invasive cells were
342.08±206.429% (P<0.05), and 513.409±249.235% (P<0.01) of
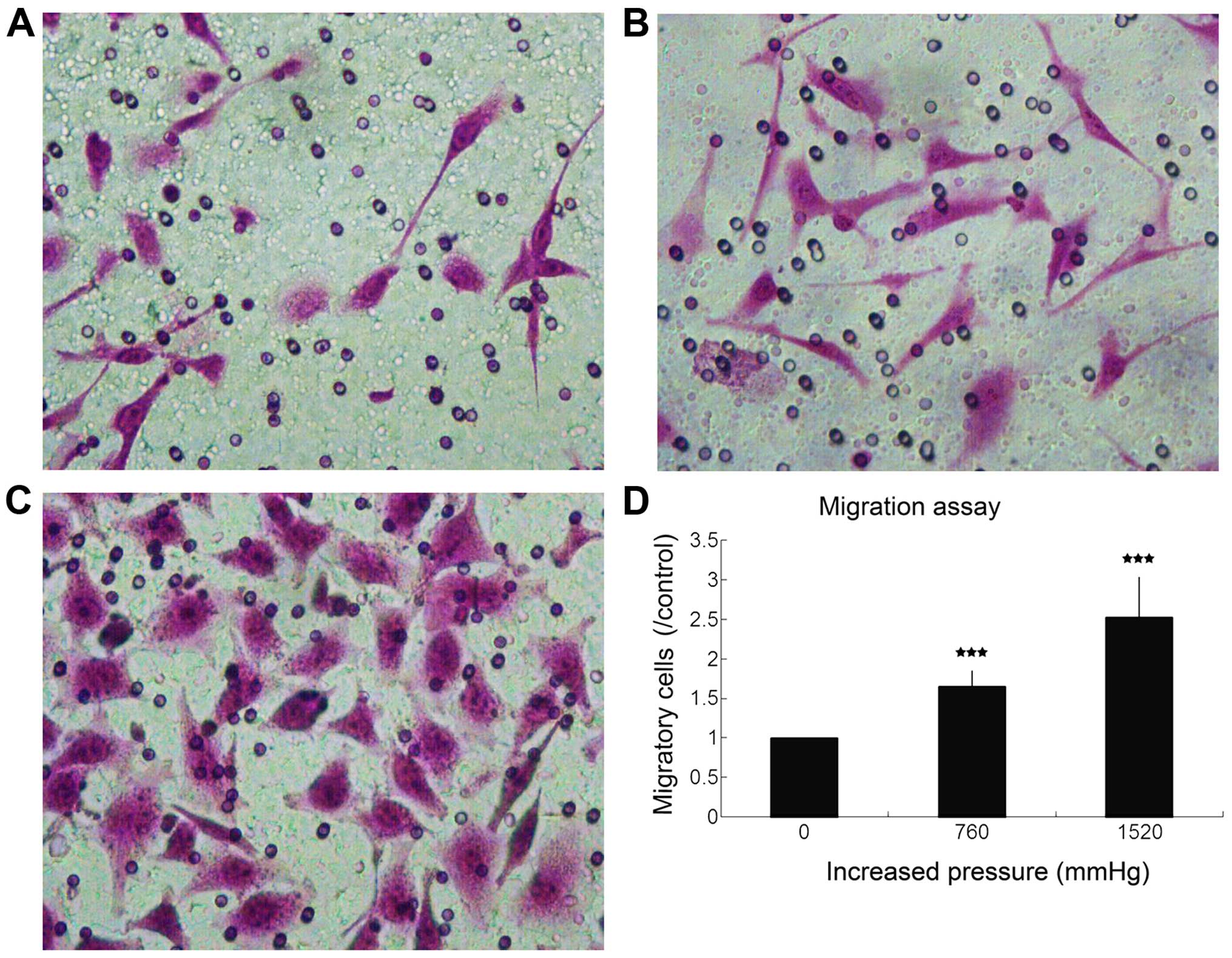
those at the ambient pressure, respectively (Fig. 2). Compared with normal air-pressure
control, the fold change of migration was 1.65±0.20 (P<0.001)
and 2.53±0.50 (P<0.001), respectively (Fig. 3).
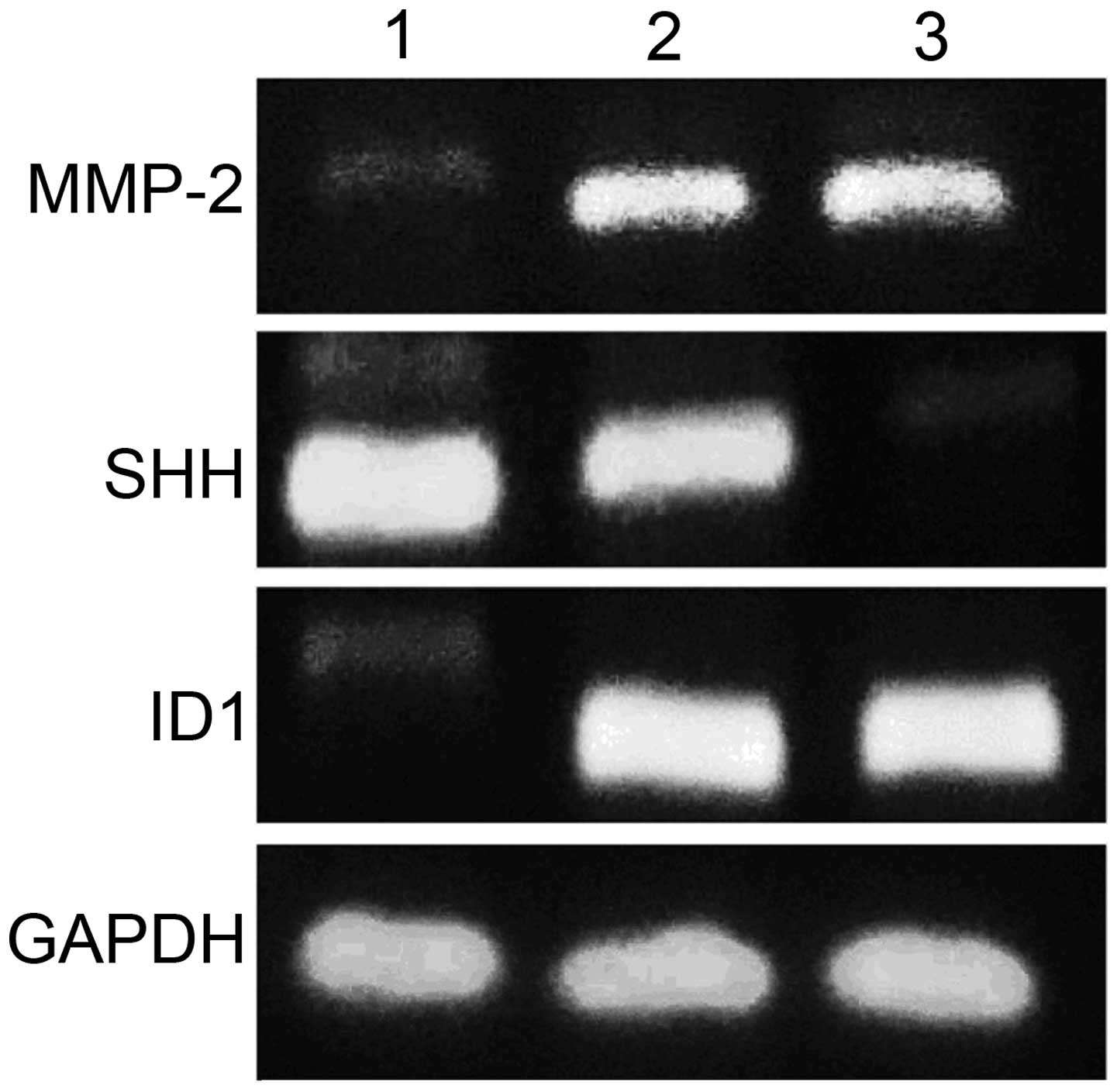
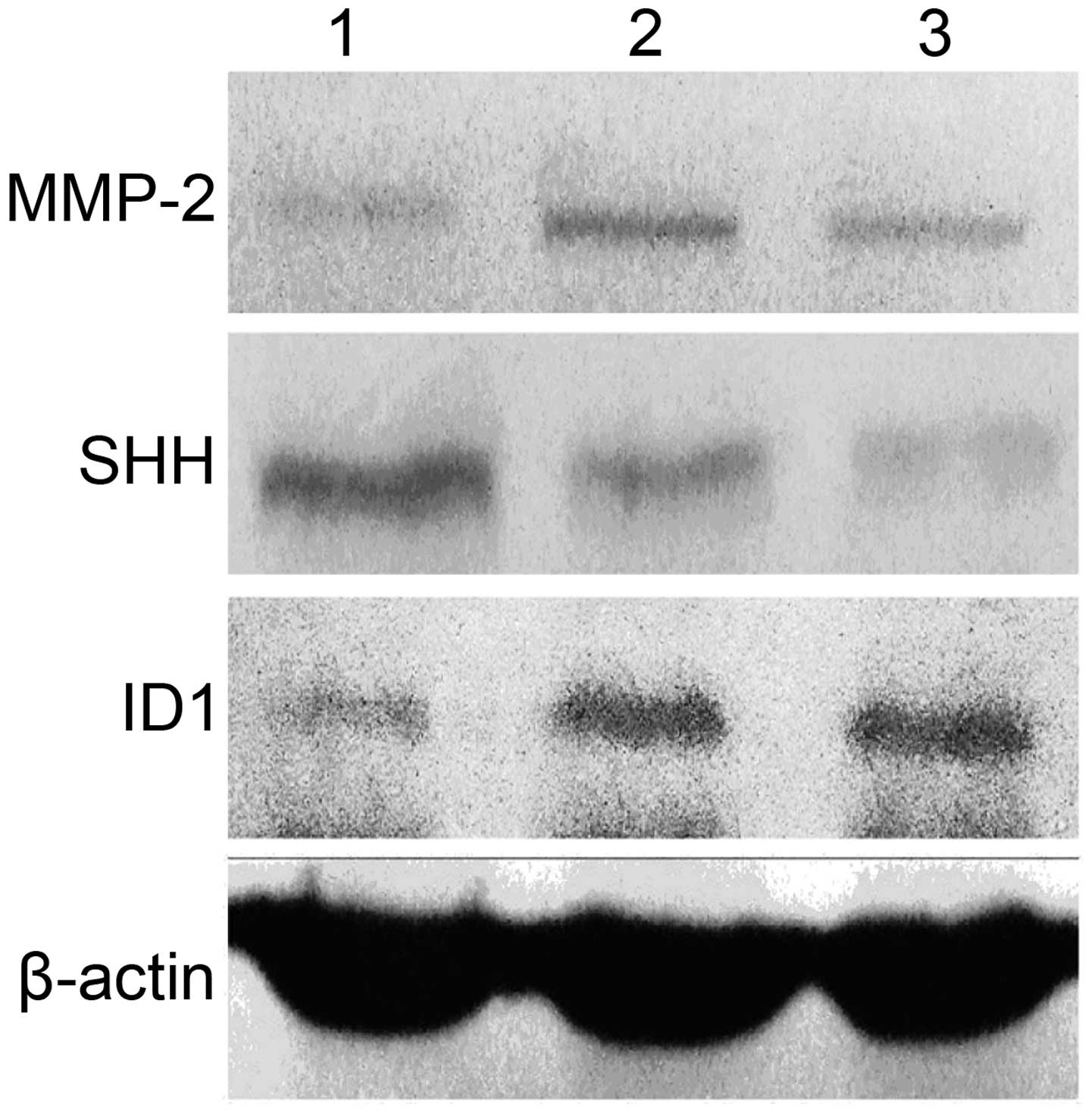
Effect of high extracellular pressure on
MMP-2, ID1 and SHH expression in SGC7901 cells
To further clarify the underlying molecular
mechanisms of high extracellular pressure in cancer progression, we
examined the gene and protein expression levels of MMP-2 ID1 and
SHH in the SGC7901 cells, maintained for 30 min under ambient or
increased pressure (760 and 1,520 mmHg) conditions. Pressure of 760
mmHg above ambient stimulated MMP-2 gene expression (P<0.01) and
protein expression (P<0.05), meanwhile 1,520 mmHg pressure
increased MMP-2 gene expression (P<0.05) and protein expression
(P<0.05). However, there was no significant difference in MMP-2
expression between increased pressure of 760 and 1,520 mmHg
(P>0.05) (Figs. 4 and 5). Pressure of 1,520 mmHg above ambient
suppressed SHH gene expression (P<0.01) and protein expression
(P<0.05), whereas 760 mmHg pressure did not influence SHH gene
expression (P>0.05) and protein expression (P>0.05) (Figs. 4 and 5). Pressure of 760 mmHg above ambient
stimulated ID1 gene expression (P<0.05) and protein expression
(P<0.05), meanwhile 1,520 mmHg pressure increased ID1 gene
(P<0.01) and protein expression (P<0.01). Moreover, there was
significant difference in ID1 protein expression between increased
pressure of 760 and 1,520 mmHg (P<0.05) (Figs. 4 and 5).
Effect of high extracellular pressure on
proliferation and apoptosis of SGC7901 cells
Although the slight increase in extracellular
pressure is reported to promote proliferation of colon cancer cells
(7), high extracellular pressure
neither significantly influenced cell proliferation as analyzed by
CCK-8 assay (data not shown) nor influenced cell cycle distribution
(data not shown), indicating that high extracellular pressure could
promote the invasion and migration of the SGC7901 cells without
affecting proliferation. In addition, apoptosis analysis of cells
24 h after treatment with increased pressure demonstrated that the
apoptosis level was not statistically significant compared with the
normal air-pressure control.
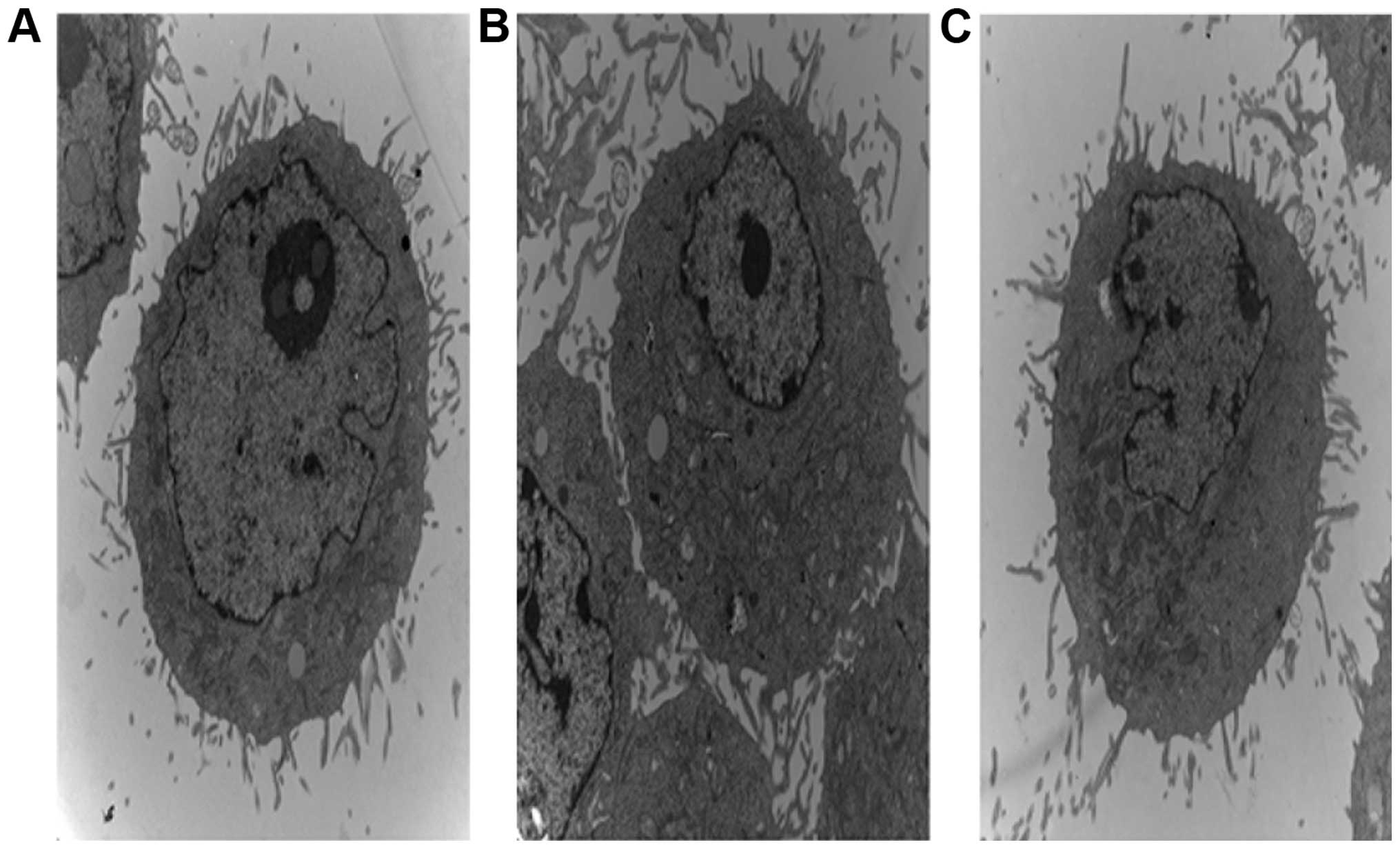
Effect of high extracellular pressure on
ultrastructure of SGC7901 cells
SGC7901 cells had large deformed nuclei and
prominent nucleoli with abundant rough endoplasmic reticulum,
ribosomes and mitochondria in the cytoplasm. Microvilli were also
noted in the cytoplasmic membrane. However, there were no prominent
changes in cells that were incubated at 37°C under increased
pressure (Fig. 6).
Discussion
It has been reported that mildly increasing ambient
pressure stimulates adhesion in a variety of human cancer cells
(4–6). However, the effect of high
extracellular pressure on cell gene expression and the biological
behavior in gastric cancer (GC) has not been evaluated. The present
study presents the first evidence to assess the impact of highly
increased extracellular pressure on cell biological behavior and
gene expression in human GC. We found that pressure of 760 and
1,520 mmHg above ambient stimulated cell adhesion to Matrigel. This
result is consistent with previous studies in other cell types that
increasing ambient pressure to a small degree promoted adhesion
(4–6). Studies have shown that the activation
of the phosphatidylinositol 3′ kinase (PI3K)/Akt signaling pathway
is required for pressure-induced tumor cell adhesion (8–10).
Pressure results in cytoskeletal independent Src activation that
induces PI3K/FAK/Akt pathway activation (10,11).
Then, associated with p85, FAK translocates to the cell membrane
and interacts with β1 integrin heterodimers, which increases
integrin-binding affinity through β1 integrin T788/9
phosphorylation and thereby facilitates cancer cell adhesion
(10,12). Perry et al (13) found that pressure stimulated FAK and
Akt phosphorylation, which was responsible for the
pressure-augmented adhesion in sarcoma cell lines. Wang et
al (14) identified the AKT1 PH
domain and hinge region as structural determinants in permitting
AKT1 translocation and phosphorylation and mediating
pressure-induced cancer cell adhesion. However, due to diversified
ways of regulating tumor cell adhesion (6,15–19),
the mechanisms of pressure-stimulated adhesion await further
studies.
Since high extracellular pressure was correlated
with the adhesion of SGC7901 cells, it is likely that pressure has
a role in cancer metastasis. We, therefore, tested the effect of
high extracellular pressure on cell invasion and migration in
SGC7901 cells. As expected, increasing extracellular pressure for
30 min promoted the invasion and migration of the SGC7901 cells
without affecting proliferation and apoptosis. MMP-2 is a
zinc-dependent endopeptidase involved in tumor invasion and
migration (20). The overexpression
of MMP-2 promotes tumor cell invasion and migration by degrading
type IV collagen of the basement membrane and affecting tumor cell
adhesion to the basement membrane (20–23).
Increasing pressure stimulated MMP-2 expression in the GC cells
that we studied. Pressure of 760 mmHg above ambient stimulated
MMP-2 gene expression (P<0.01) and protein expression
(P<0.05), meanwhile 1,520 mmHg pressure increased MMP-2 gene
expression (P<0.05) and protein expression (P<0.05). This
indicates that the high extracellular pressure may promote invasion
and migration of SGC7901 cells by enhancing MMP-2 expression.
ID1 protein, belonging to the helix-loop-helix (HLH)
protein superfamily, has a highly conserved HLH dimerization
domain. Ubiquitously expressed bHLH transcription factors such as
the E12/E47 family members or tissue-specific bHLH transcription
factors such as Tal-1 and MyoD bind to DNA by means of basic DNA
binding regions, and therefore activate the process of cell
differentiation. ID1 protein lacks the basic DNA binding domain
that is necessary for DNA binding. By forming nonfunctional Id-bHLH
heterodimers and sequestering bHLH transcription factors, ID1
proteins serve as negative regulators of bHLH-dependent gene
expression and, consequently, of cell differentiation in numerous
different cell lineages (24,25).
SHH gene plays a key role in the regulation of cell
differentiation, cell proliferation and gastric acid secretion. SHH
expression is regulated by gastric acid secretion, pepsin and
gastrin (26). By mobilizing
intracellular calcium release and protein kinase C (PKC), SHH
activates JNK (c-Jun N-terminal kinases) and extracellular
signal-regulated kinase (ERK)/mitogen-activated protein kinase
(MAPK) signaling pathway, which regulates cell proliferation and
differentiation (23–28). SHH, in turn, promotes a negative
feedback loop to control gastric acid and gastrin concentration by
inducing somatostatin secretion (31). E-cadherin gene, acting as a
regulator of cell differentiation, may be a downstream target of
the SHH signaling pathway (29,30).
However, our data indicate that the high extracellular pressure
suppressed SHH expression without affecting E-cadherin
expression.
At increased pressure of 760 and 1,520 mmHg, we
found that the expression of ID1 was significantly increased, while
the expression of SHH was significantly decreased. Notably, our
data showed that high extracellular pressure neither significantly
influenced cell proliferation nor cell apoptosis. In addition,
transmission electric microscopy showed that SGC7901 cell
ultrastructure did not change significantly under increased
pressure conditions. Due to the relationship between tumor
differentiation and expression of ID1 and SHH, we speculate that
high extracellular pressure may suppress SGC7901 cell
differentiation by influencing gene and protein expression.
In conclusion, high extracellular pressure promotes
adhesion, invasion and migration of SGC7901 cells, and may suppress
SGC7901 cell differentiation. Moreover, the present study suggests
that the increase in SGC7901 cell invasion and migration was
associated with increased MMP-2 expression. The inhibition of
SGC7901 cell differentiation was correlated with the change in SHH
and ID1 expression.
Acknowledgments
We thank Professor Yuru Xu, of the Chinese Academy
of Engineering (Harbin Engineering University) for the technical
assistance. The present study was financially supported by the
Natural Science Foundation of Heilongjiang Province, China (grant
no. ZD200920).
References
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Catalano V, Labianca R, Beretta GD, Gatta
G, de Braud F and Van Cutsem E: Gastric cancer. Crit Rev Oncol
Hematol. 71:127–164. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Basson MD, Yu CF, Herden-Kirchoff O,
Ellermeier M, Sanders MA, Merrell RC and Sumpio BE: Effects of
increased ambient pressure on colon cancer cell adhesion. J Cell
Biochem. 78:47–61. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Conway WC, Van der Voort van Zyp J,
Thamilselvan V, Walsh MF, Crowe DL and Basson MD: Paxillin
modulates squamous cancer cell adhesion and is important in
pressure-augmented adhesion. J Cell Biochem. 98:1507–1516. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Downey C, Alwan K, Thamilselvan V, Zhang
L, Jiang Y, Rishi AK and Basson MD: Pressure stimulates breast
cancer cell adhesion independently of cell cycle and apoptosis
regulatory protein (CARP)-1 regulation of focal adhesion kinase. Am
J Surg. 192:631–635. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Walsh MF, Woo RK, Gomez R and Basson MD:
Extracellular pressure stimulates colon cancer cell proliferation
via a mechanism requiring PKC and tyrosine kinase signals. Cell
Prolif. 37:427–441. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hirabayashi Y, Yamaguchi K, Shiraishi N,
Adachi Y, Saiki I and Kitano S: Port-site metastasis after
CO2 pneumoperitoneum: Role of adhesion molecules and
prevention with antiadhesion molecules. Surg Endosc. 18:1113–1117.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vanhaesebroeck B, Leevers SJ, Ahmadi K,
Timms J, Katso R, Driscoll PC, Woscholski R, Parker PJ and
Waterfield MD: Synthesis and function of 3-phosphorylated inositol
lipids. Annu Rev Biochem. 70:535–602. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Thamilselvan V, Craig DH and Basson MD:
FAK association with multiple signal proteins mediates
pressure-induced colon cancer cell adhesion via a Src-dependent
PI3K/Akt pathway. FASEB J. 21:1730–1741. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Thamilselvan V and Basson MD: The role of
the cytoskeleton in differentially regulating pressure-mediated
effects on malignant colonocyte focal adhesion signaling and cell
adhesion. Carcinogenesis. 26:1687–1697. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Craig DH, Gayer CP, Schaubert KL, Wei Y,
Li J, Laouar Y and Basson MD: Increased extracellular pressure
enhances cancer cell integrin-binding affinity through
phosphorylation of beta1-integrin at threonine 788/789. Am J
Physiol Cell Physiol. 296:C193–C204. 2009. View Article : Google Scholar
|
|
13
|
Perry BC, Wang S and Basson MD:
Extracellular pressure stimulates adhesion of sarcoma cells via
activation of focal adhesion kinase and Akt. Am J Surg.
200:610–614. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang S and Basson MD: Identification of
functional domains in AKT responsible for distinct roles of AKT
isoforms in pressure-stimulated cancer cell adhesion. Exp Cell Res.
314:286–296. 2008. View Article : Google Scholar
|
|
15
|
van Zyp J, Conway WC, Craig DH, van Zyp N,
Thamilselvan V and Basson MD: Extracellular pressure stimulates
tumor cell adhesion in vitro by paxillin activation. Cancer Biol
Ther. 5:1169–1178. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Downey C, Craig DH and Basson MD: Pressure
activates colon cancer cell adhesion via paxillin phosphorylation,
Crk, Cas, and Rac1. Cell Mol Life Sci. 65:1446–1457. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Downey C, Craig DH and Basson MD:
Isoform-specific modulation of pressure-stimulated cancer cell
proliferation and adhesion by α-actinin. Am J Surg. 202:520–523.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang S and Basson MD: Integrin-linked
kinase: A multi-functional regulator modulating extracellular
pressure-stimulated cancer cell adhesion through focal adhesion
kinase and AKT. Cell Oncol. 31:273–289. 2009.PubMed/NCBI
|
|
19
|
Li XD, Ji M, Wu J, Jiang JT and Wu CP:
Clinical significance of CD44 variants expression in colorectal
cancer. Tumori. 99:88–92. 2013.PubMed/NCBI
|
|
20
|
Tester AM, Waltham M, Oh SJ, Bae SN, Bills
MM, Walker EC, Kern FG, Stetler-Stevenson WG, Lippman ME and
Thompson EW: Pro-matrix metalloproteinase-2 transfection increases
orthotopic primary growth and experimental metastasis of MDA-MB-231
human breast cancer cells in nude mice. Cancer Res. 64:652–658.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kubben FJ, Sier CF, van Duijn W, Griffioen
G, Hanemaaijer R, van de Velde CJ, van Krieken JH, Lamers CB and
Verspaget HW: Matrix metalloproteinase-2 is a consistent prognostic
factor in gastric cancer. Br J Cancer. 94:1035–1040. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Curran S and Murray GI: Matrix
metalloproteinases: Molecular aspects of their roles in tumour
invasion and metastasis. Eur J Cancer. 36:1621–1630. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ray JM and Stetler-Stevenson WG:
Gelatinase A activity directly modulates melanoma cell adhesion and
spreading. EMBO J. 14:908–917. 1995.PubMed/NCBI
|
|
24
|
Norton JD and Atherton GT: Coupling of
cell growth control and apoptosis functions of Id proteins. Mol
Cell Biol. 18:2371–2381. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fong S, Debs RJ and Desprez PY: Id genes
and proteins as promising targets in cancer therapy. Trends Mol
Med. 10:387–392. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zavros Y, Waghray M, Tessier A, Bai L,
Todisco A, L Gumucio D, Samuelson LC, Dlugosz A and Merchant JL:
Reduced pepsin A processing of sonic Hedgehog in parietal cells
precedes gastric atrophy and transformation. J Biol Chem.
282:33265–33274. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Daulhac L, Kowalski-Chauvel A, Pradayrol
L, Vaysse N and Seva C: Gastrin stimulates the formation of a
p60Src/p125FAK complex upstream of the
phosphatidylinositol 3-kinase signaling pathway. FEBS Lett.
445:251–255. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dockray G, Dimaline R and Varro A:
Gastrin: Old hormone, new functions. Pflugers Arch. 449:344–355.
2005. View Article : Google Scholar
|
|
29
|
Feng R, Xiao C and Zavros Y: The role of
Sonic Hedgehog as a regulator of gastric function and
differentiation. Vitam Horm. 88:473–489. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li X, Deng W, Nail CD, Bailey SK, Kraus
MH, Ruppert JM and Lobo-Ruppert SM: Snail induction is an early
response to Gli1 that determines the efficiency of epithelial
transformation. Oncogene. 25:609–621. 2006.
|