Introduction
Melanoma is a malignant tumor which originates from
melanocytes or cells evolved from melanocytes. Malignant melanoma
is the most common cancer located on the back in men and on the
legs in women. Each year in the world, the number of estimated new
cases of malignant melanoma is 132,000 and approximately 48,000
patients die from malignant melanoma. Rapid growth, early and
multiple metastases and low susceptibility to treatment determine
the significant malignant potential of melanoma. The prognosis for
patients with advanced melanoma is grim, with a 1-year survival
rate of 25% and a median overall survival of 6.2 months (1,2).
For advanced melanoma, systemic therapy is usually
needed; however, therapeutic options for unresectable or metastatic
melanoma are limited. Many patients are resistant to conventional
chemotherapies with alkylating agents, dacarbazine or interferon
(IFN)-α (3,4). In recent years, due to the low
effectiveness of chemotherapy and radiotherapy in anti-melanoma
treatment, alternatively, inducers of differentiation warrant
therapeutic importance. Some studies have demonstrated that
malignant cancer cells could be transformed into mature cells by
induction of differentiation (5,6).
Several compounds including dimethyl sulfoxide, retinoic acid,
phorbolester and 1,25-dihydroxy vitamin D3 are known to induce
acute promyelocytic leukemia (AML) cells to differentiate toward
mature cells (7,8). More intriguingly, induction of
differentiation can enhance bortezomib efficacy and overcome drug
resistance in multiple myeloma (9).
The B16F0 cell line, which was derived from C57BL/6
mice, provides a useful cellular differentiation model. The
terminal differentiation of B16F0 cells can be monitored by changes
in morphology, upregulation of melanin biosynthesis, and induction
of dendrite outgrowths. The differentiation of melanoma cells into
a terminal stage may be an effective strategy for the treatment of
melanoma.
Alternol, a novel compound purified from microbial
fermentation products obtained from the bark of the yew tree,
exhibits a variety of antitumor activities, including proliferation
inhibition, cell cycle arrest, apoptosis, and suppression of
migration and invasion (10–15).
However, the anticancer effect and molecular mechanisms of alternol
have not yet been established in B16F0 cells. Here, we report the
effects of alternol on the proliferation and differentiation
potential of B16F0 cells.
Materials and methods
Materials and reagents
Alternol with 99.5% purity was acquired from Shantou
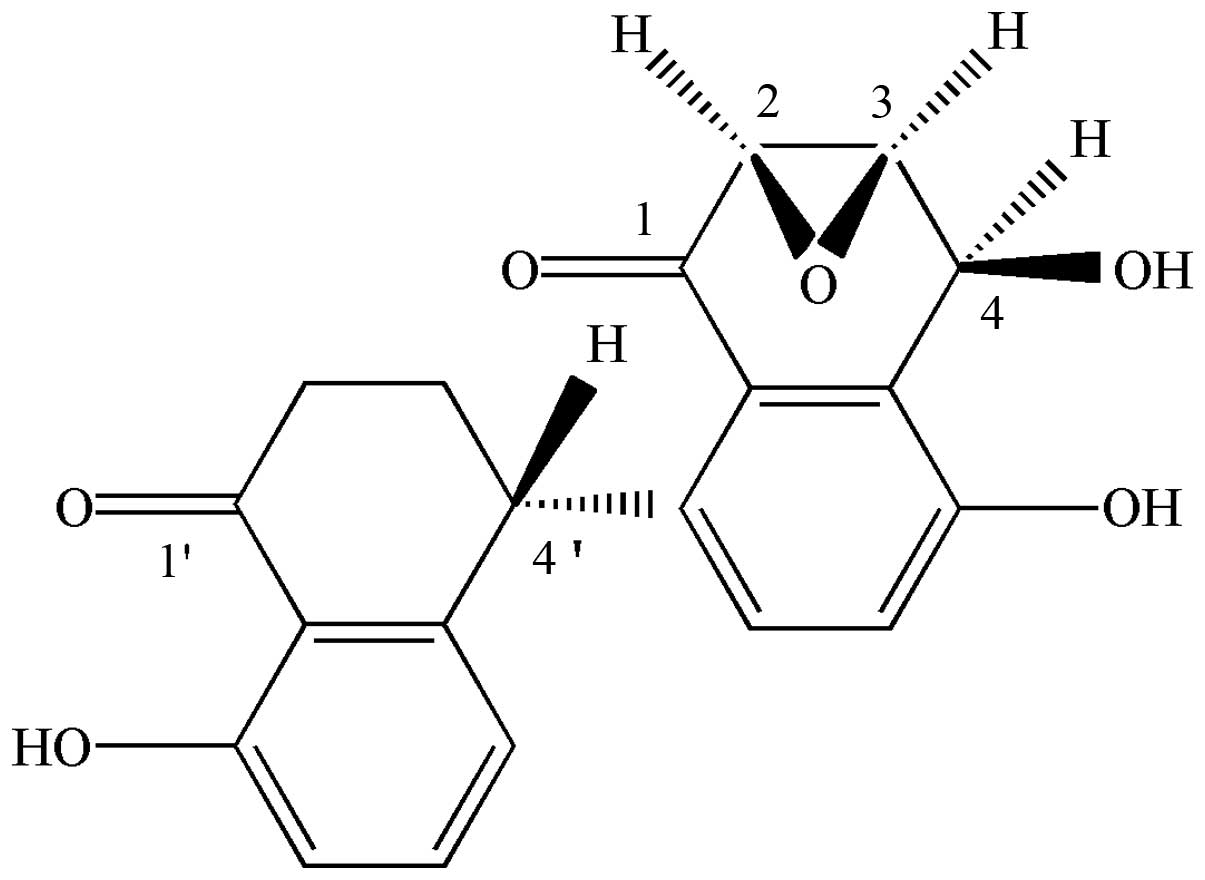
Strand Biotech Co., Ltd. The chemical structure of alternol is
shown in Fig. 1. The alternol stock
solution at 10 mmol/l was made in dimethyl sulfoxide (DMSO) and
stored in the dark at −20°C. Dulbecco's modified Eagle's medium
(DMEM) and fetal bovine serum (FBS) were purchased from Gibco
Laboratories (Grand Island, NY, USA). L-DOPA, thiazolyl blue (MTT)
and Triton X-100 were purchased from Sigma Chemical Co. (St. Louis,
MO, USA). Antibiotics such as penicillin and streptomycin were
obtained from Shandong Lukang Pharmaceutical Co., Ltd. (Shandong,
China). All other chemicals were of analytical grade and
commercially available.
Cell culture
B16F0 cells were obtained from the China Center for
Type Culture Collection (Wuhan, China). The cells were cultured in
DMEM supplemented with 10% FBS, 100 U/ml penicillin and 100
µg/ml streptomycin. The cells were maintained at 37°C in a
humidified incubator with 5% CO2 atmosphere.
Logarithmically growing B16F0 cells were used for each experiment,
and the density of inoculation was ~1×105 cells/ml. In
order to avoid changes in cell characteristics that are caused by
prolonged cell culture time, the cells were used between passages
15 and 25.
Cell proliferation assay
For the cell proliferation assay, B16F0 cells were
innoculated into 96-well plates at ~5×104 cells/well.
Subsequently, the cells were treated with alternol in a range of
concentrations: 0, 0.4, 1.6, 2.4, 3.2, 4.0 µg/ml. The effect
of alternol on the growth of B16F0 cells was assessed by MTT assay
as previously described (16,17).
The absorbance at 490 nm was measured using a microplate reader
(Thermo Varioskan Flash 3001; Thermo Scientific).
Morphological changes of B16F0 cells
Logarithmically growing B16F0 cells were inoculated
into 6-well plates at a concentration of 1×105
cells/well. After cells were grown on the glass slides, different
concentrations of alternol 0–2.4 µg/ml were added at 37°C
for 24 h (18). Then the cells were
observed using a phase-contrast microscopy (Zeiss).
Trypan blue exclusion test
The survival rate of B16F0 cells was assessed by the
trypan blue exclusion test as previously described (19,20).
Cells in the exponential growth phase were seeded in 6-well plates
at 5×104 cells/well. After a 24-h growth period, the
cells were treated with different concentrations of alternol at
37°C for 24 and 48 h. After being digested with trypsin, the viable
and dead cells were collected for counting using an optical
microscope with a hemacytometer. Trypan blue exclusion test was
used to record the number of living cells for each group. Cell
survival rate (%) = (total number of viable cells per ml of
aliquot)/(total number of cells per ml of aliquot) × 100%.
Soft agar colony formation assay
Anchorage-independent cell growth of B16F0 cells was
assessed by the soft agar formation assay. Cells for each group
were harvested by trypsinization, and then a single-cell suspension
in DMEM was plated into 6-well plates containing 0.35% low melting
agarose and solidified 0.6% agarose. Cells in agar were incubated
at 37°C in a humidified environment for ~2 weeks. The colonies were
counted directly and photographed by an imaging system as
previously described (21,22).
Determination of melanin content
The melanin content was measured as described in
previous studies with slight modifications (23,24).
Melanoma B16F0 cells were seeded at a density of 1×105
cells/well in 6-well plates. After incubation for 24 h at 37°C, the
cells were treated with different concentrations of alternol for 48
h. The supernatant was collected separately. Extracellular melanin
content was measured as previously described. Upon the
determination of intracellular melanin content, adhered cells were
washed with PBS and digested with 0.25% trypsin. The cells were
then centrifugated at 12,000 rpm for 10 min. Then the mixture
consisting of 0.4 M HEPES buffer (pH 6.8) and EtOH (9:1, v/v) was
added to the cells. Melanin was dissolved in the mixture after
incubation at 42°C for 16 h. Then the solution was transferred into
96-well plates and observed at 475 nm using a microplate
reader.
Cellular tyrosinase activity assay
For the determination of tyrosinase activity, we
used a previously reported method (25,26).
According to the description of Dopa oxidation method, B16F0 cells
were seeded in 6-well plates at a concentration of 1×105
cells/well. The cells were collected and washed with ice-cold PBS
prior to centrifugation. After incubation at −80°C for 30 min, the
cells were lysed with buffer containing 1% Triton X-100 and PMSF
(0.1 mM). The cell lysate was thawed, mixed and centrifuged at
12,000 rpm for 30 min to obtain the supernatant. The mixture of 80
µl of supernatant and 20 µl of L-DOPA were placed in
a 96-well plate and incubated at 37°C for 40 min. The absorbance
was measured at 475 nm.
Reverse transcription-polymerase chain
reaction (RT-PCR)
B16F0 cells were pretreated with alternol (0, 0.4,
0.8, 1.6, 2.4, 3.2 µg/ml) as protocols planned in advance.
Total RNA was isolated using TRIzol reagent as previously described
(27). Reverse transcription of
cDNA was accomplished using a cDNA synthesis kit (Promega, Madison,
WI, USA). cDNA was synthesized in a 25-µl reaction system by
adding 3 µl total RNA primed with oligo(dT)
(deoxy-thymidine). The sequences of the primers were as follows:
tyrosinase upstream, 5′-GGCCAGCTTTCAGGCAGAGGT-3′ and downstream,
5′-TGGTGCTTCATGGGCAAAATC-3′; TRP-1 upstream,
5′-GCTGCAGGAGCCTTCTTTCTC-3′ and downstream,
5′-AAGACGCTGCACTGCTGGTCT-3′; and TRP-2 upstream,
5′-GGATGACCGTGAGCAATGGCC-3′ and downstream,
5′-CGGTTGTGACCAATGGGTGCC-3′. The level of GAPDH (upstream,
5′-CAAGGTCATCCATGACAACTTTG-3′ and downstream,
5′-GTCCACCACCCTGTTGCTGTA G-3′) was added as a control. PCR was
performed in a mixture containing cDNA, 10X PCR buffer, 2.5 mM
dNTPs, 10 mM forward and reverse primers, DNA polymerase and
sterile water.
Statistical analysis
All data are presented as the mean ± SE from at
least three independent experiments and were evaluated by analysis
of variance (ANOVA) followed by the Student's t-test. Values of
P<0.05 were considered to indicate statistically significant
results.
Results
Alternol inhibits the proliferation of
B16F0 cells
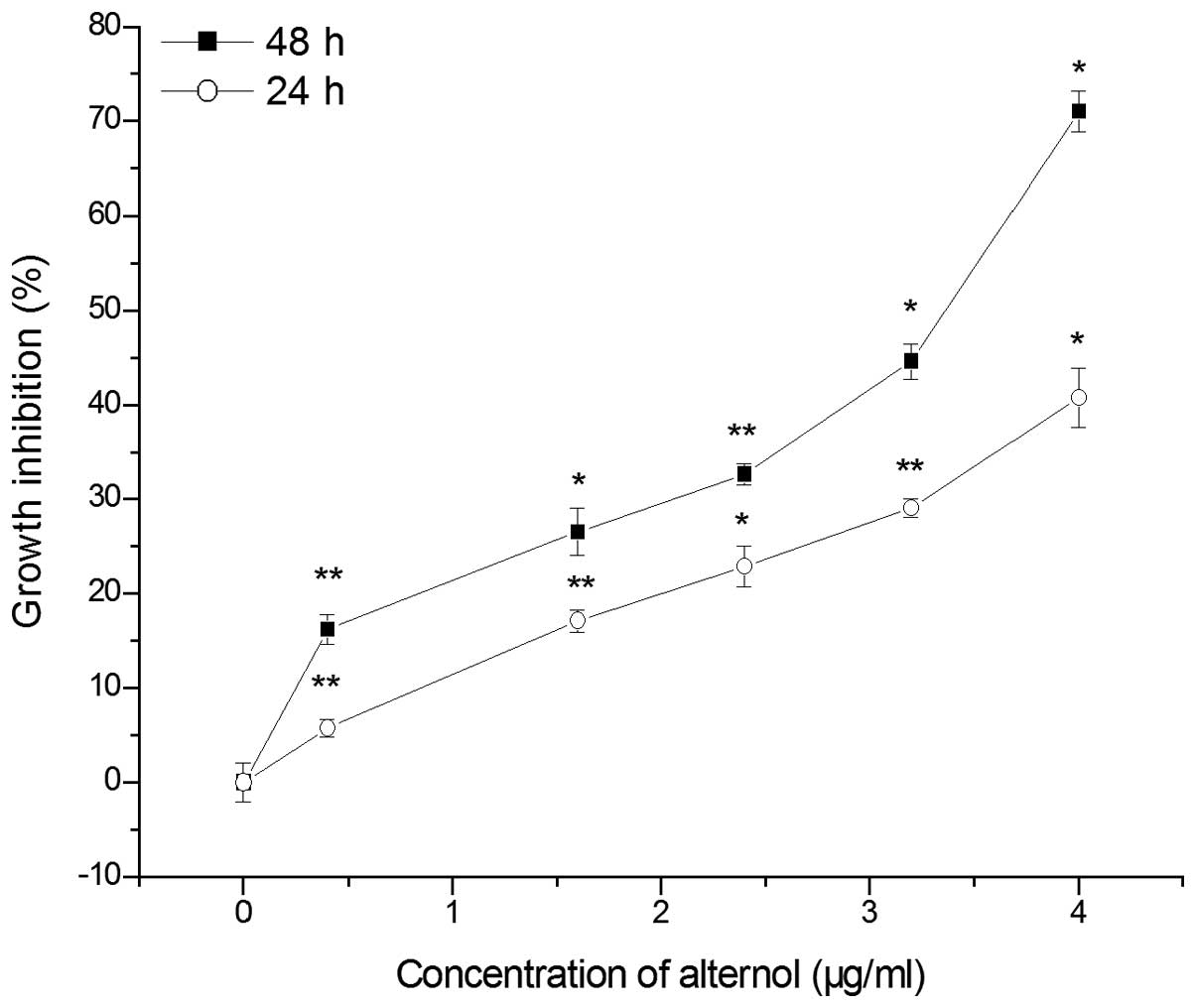
The anti-tumor effect of alternol on B16F0 cells was
monitored by MTT method. As shown in Fig. 2, alternol significantly inhibited
the proliferation of B16F0 cells. When cells were exposed to
alternol at 4 µg/ml for 48 and 24 h, the inhibitory growth
rate of alternol in the B16F0 cells was 71.03±2.17 and 40.74±3.16%,
respectively. Moreover, the cell proliferation of the B16F0 cells
was reduced by alternol in a dose-and time-dependent manner.
Morphological changes in the B16F0 cells
after alternol treatment
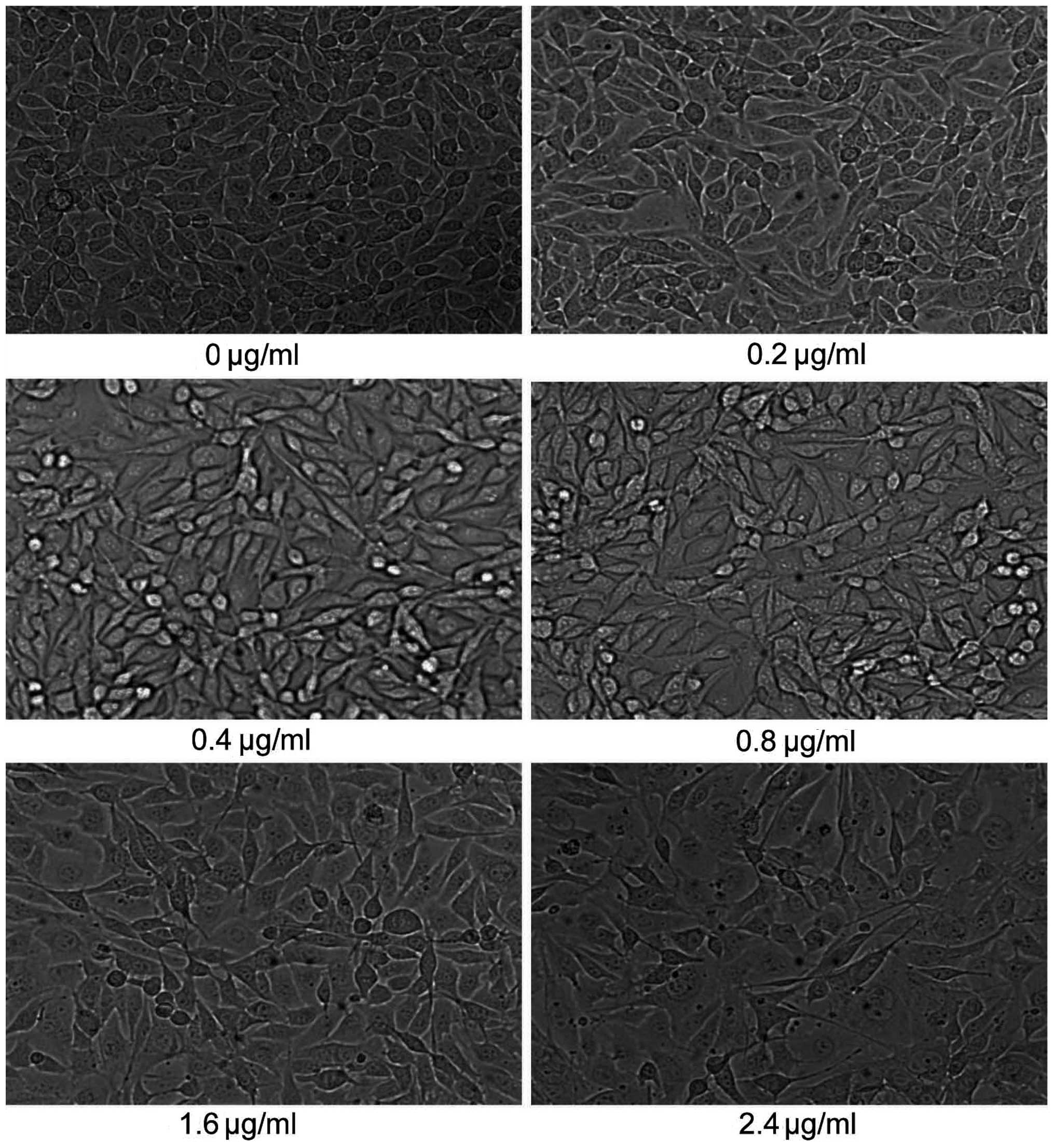
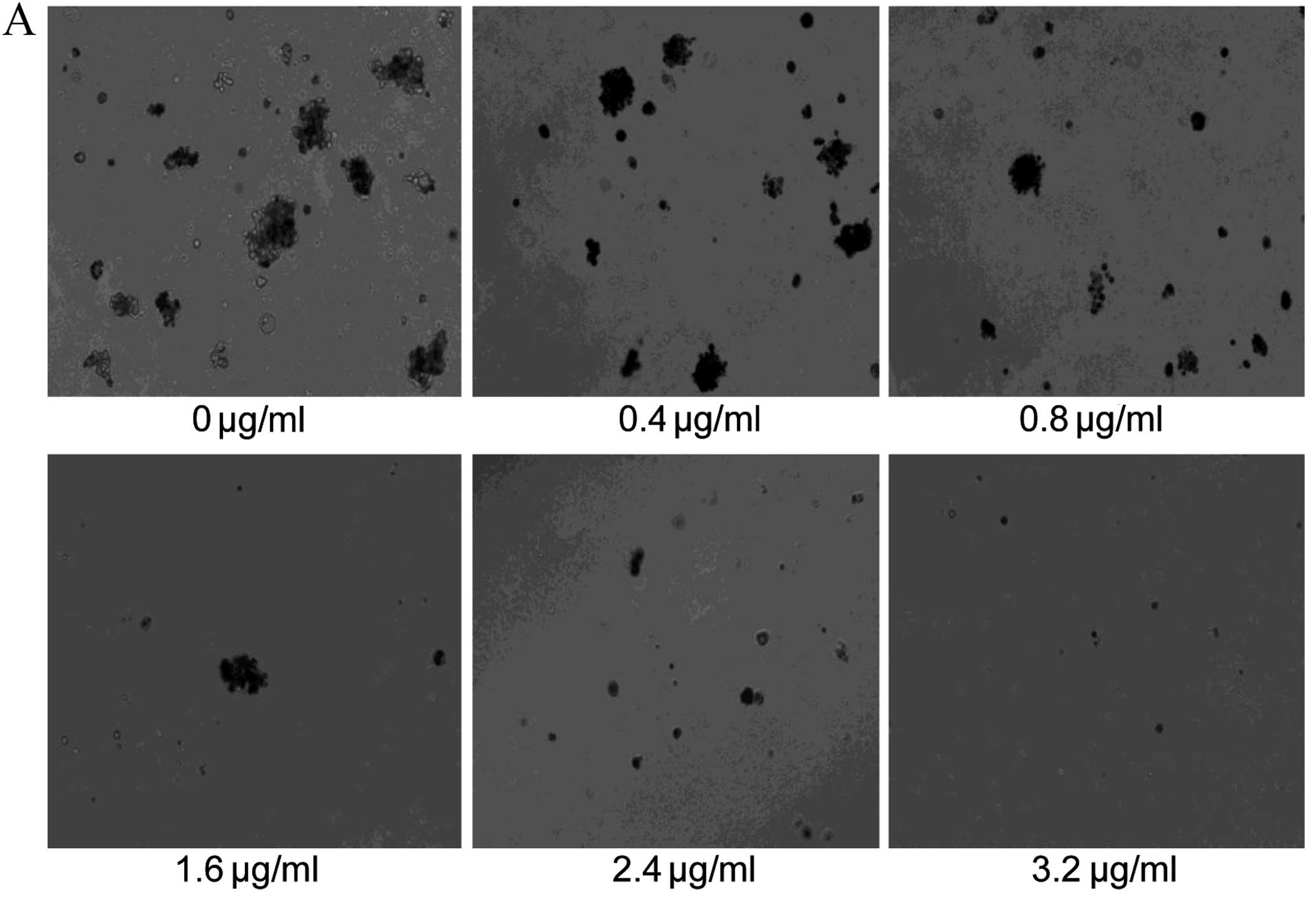
Morphological changes in the B16F0 cells were
observed after treatment with different concentrations of alternol
(0, 0.2, 0.4, 0.8, 1.6 and 2.4 µg/ml) for 24 h (Fig. 3). With the increase in alternol
concentration, cells exhibited dendritic-like projections which
gave a star-like shape to the cells compared with the rounded
untreated cells. These branches in cells become increasingly
evident. The exposure of B16F0 cells to alternol resulted in a
marked differentiation phenomenon.
Alternol reduces the cell survival
rate
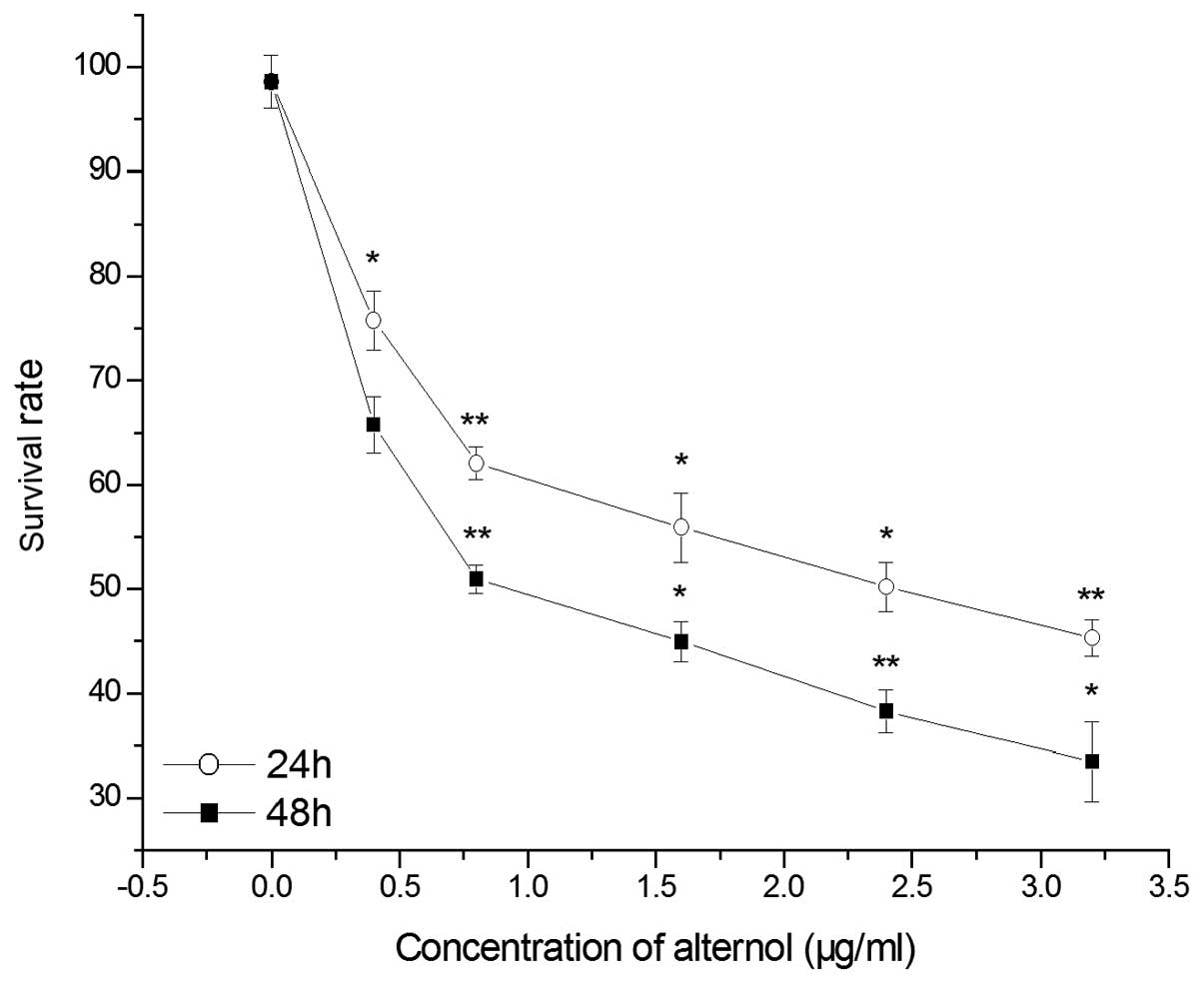
The dye exclusion test is based on the principle
that live cells possess intact cell membranes that exclude certain
dyes, such as trypan blue, eosin and propidium, whereas dead cells
do not. This method was used to determine the number of viable
cells present in the cell suspension. In this experiment, we used
trypan blue as a target dye. The results showed that after 24 and
48 h of exposure to alternol (0–2.4 µg/ml), the survival
rate of the B16F0 cells was decreased in a dose-dependent manner
(Fig. 4). The survival rate of the
B16F0 cells was only 46% after 1.6 µg/ml alternol
stimulation. Together these results confirmed that alternol
significantly affected cell survival even at low concentrations
(0.2–0.8 µg/ml).
Alternol inhibits the colony formation of
B16F0 cells
Colony-forming ability in soft agar provides strong
evidence for the tendency of tumor cells to undergo neoplastic
transformation. The colony-forming efficiency of the B16F0 melanoma
cells is exhibited in Fig. 5A. The
numbers of colonies observed within a field of vision under a
microscope are shown in Fig. 5B.
Results revealed that alternol inhibited both the number and the
size of colonies, which indicates that alternol can effectively
suppress tumorigenicity in vitro.
Alternol pretreatment induces
melanogenesis of B16F0 cells
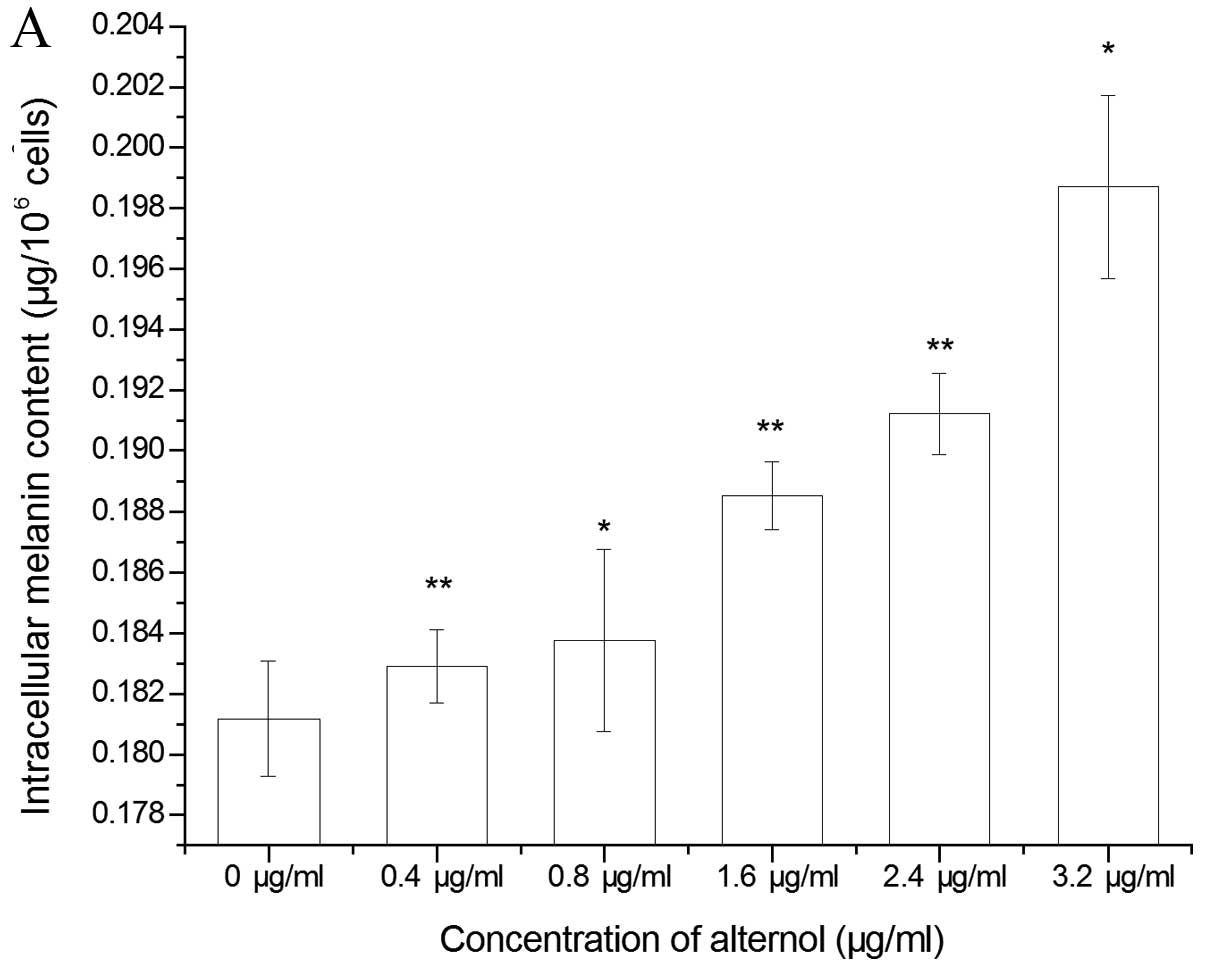
The effect of alternol on the melanogenesis of B16F0
cells was determined by the process of melanin synthesis. Both the
intracellular and the extracellular melanin content are shown in
Fig. 6. Alternol treatment
significantly increased the melanin content in the B16F0 cells in a
concentration-dependent manner. These results also demonstrated
that alternol induced cell differentiation by increasing the
melanin content as in a previous study (28) which indicates that melanogenesis is
a well-known marker of melanoma cell differentiation.
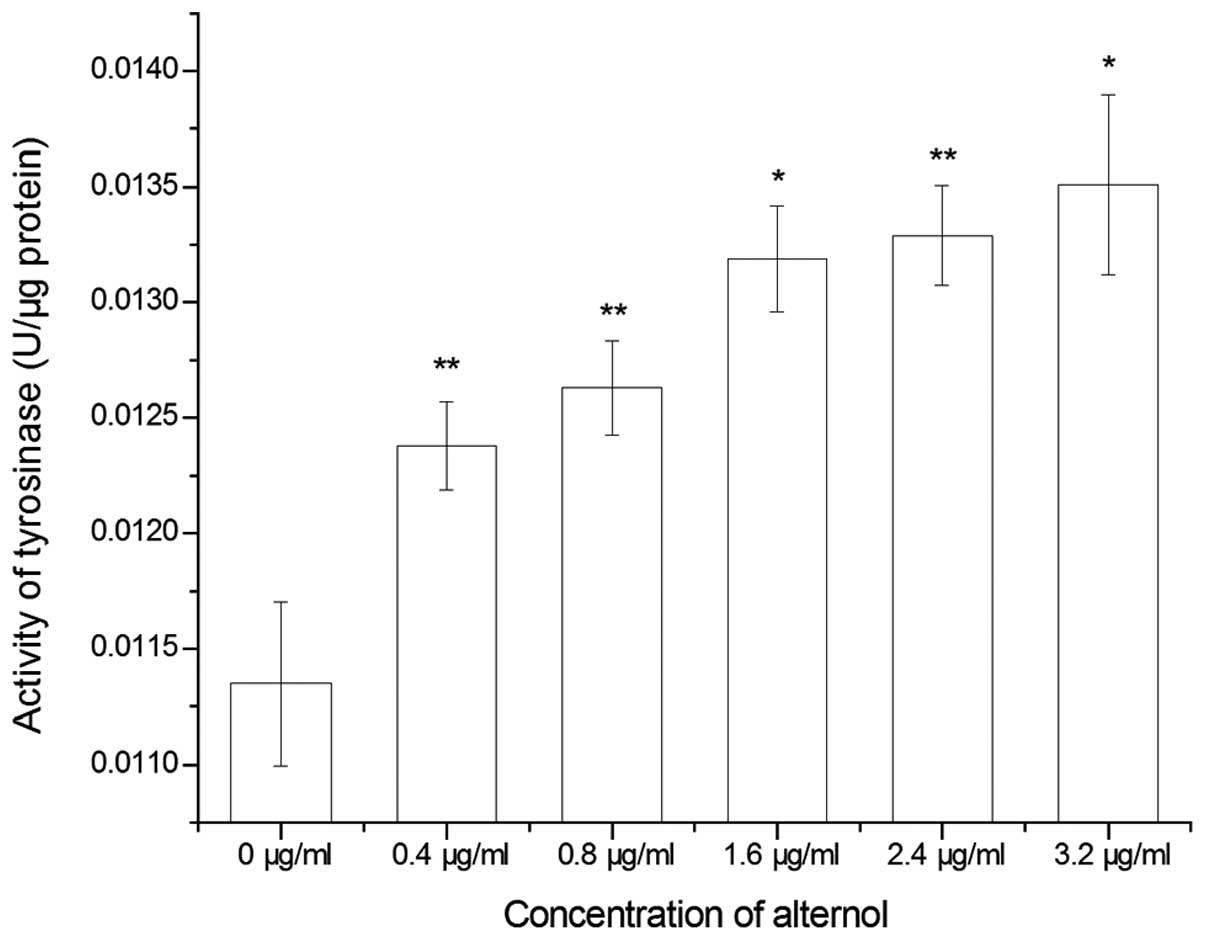
Alternol increases tyrosinase activity in
B16F0 cells
Tyrosinase activity in the B16F0 cells was assessed
by a protocol named as DOPA oxidation. As shown in Fig. 7, 0.4 µg/ml alternol
apparently increased the activity of tyrosinase in the
alternol-treated cells compared with the control group. Alternol
treatment also increased tyrosinase activity in a dose-dependent
manner, which was consistent with the increase in melanin content
in the alternol-treated cells.
Alternol increases the mRNA level of
genes related to melanogenesis
There are many complex networks in the process of
melanin synthesis. It is well known that tyrosinase, TRP-1 and
TRP-2 are all key factors during the process of melanogenesis. As
shown in Fig. 8, alternol
significantly enhanced the mRNA levels of tyrosinase, TRP-1
and TRP-2. The results are consistent with the increase in
tyrosinase activity and melanogenesis induced by alternol.
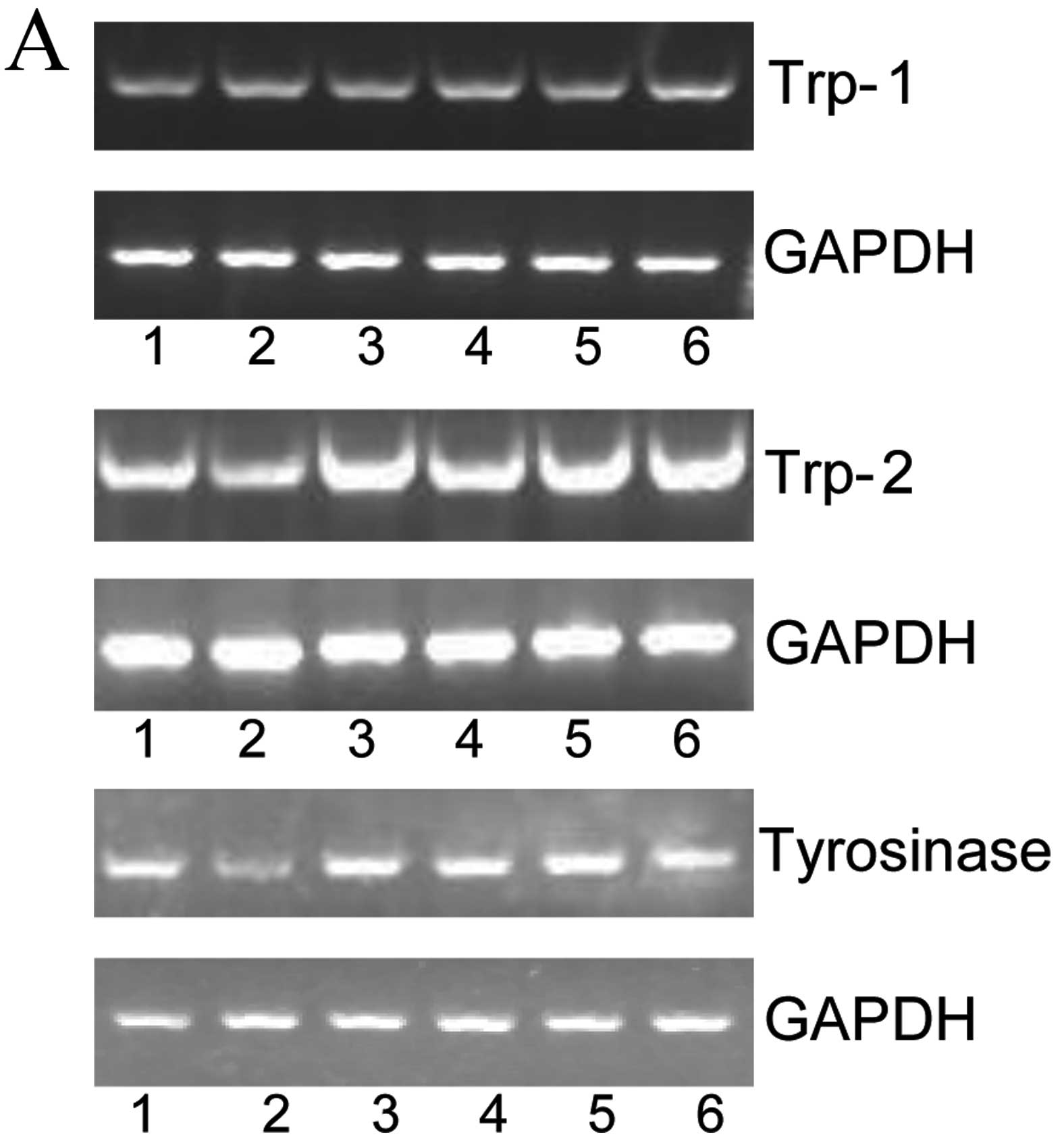 | Figure 8Effects of alternol (lane 1, 0
µg/ml; lane 2, 0.4 µg/ml; lane 3, 0.8 µg/ml;
lane 4, 1.6 µg/ml; lane 5, 2.4 µg/ml; lane 6, 3.2
µg/ml) on the expression of tyrosinase, TRP-1 and
TRP-2. (A) The expression levels of tyrosinase, Trp1
and Trp2 mRNA were analyzed by RT-PCR. (B) Relative
expression is shown as normalized to GAPDH in all cells. Bars
represent means ± SEM of three independent experiments
*P<0.05, **P<0.01, vs. the control. |
Discussion
Malignant melanoma accounts for 80% of all deaths
from skin cancer (29). At present,
an increased proliferation capacity to metastasize and broad
spectrum of associated genetic and epigenetic changes contribute to
the resistance of melanoma therapy. The inhibitory effects of
multiplication and tumorigenicity are a significant marker of
induced differentiation. Although surgical resection and
chemotherapy play a key role in improving the survival rate, drug
resistance, relapse and metastasis remain the main obstacles to the
success of cancer treatments. Lack of effectiveness of
anti-melanoma therapies makes it necessary to search for new drugs
that improve or replace standard chemotherapy. Defect in cell
differentiation is the main factor that causes malignant
proliferation of tumor cells (30).
Cell differentiation is usually accompanied by a low proliferation
rate. Thus, differentiation therapy opens a new area in the field
of tumor treatment. At present, although the induction of
differentiation therapy is an effective method with low damage to
the body, most differentiation-inducing agents are toxic. For
example, antitumor drugs such as phorbol esters (TPA) and dimethyl
sulfoxide (DMSO) have good effects on differentiation induction
(31–33), yet they have toxic effects which
makes it difficult for clinical application. The aim of our study
was to clarify the differentiation-inducing activity of alternol.
Alternol obviously induced cancer cell differentiation, but also
had a cytotoxic effect. In future research, we will alter the
structure of alternol to obtain analogues with a superior inducing
effect and low toxicity. It is imperative to identify novel
antitumor drugs which have low toxicity and superior
tumor-suppressing effects.
Alternol as a novel compound plays a potential role
in the treatment of many types of cancer. In the present study, we
treated B16F0 cells with different concentrations of alternol. The
results revealed that alternol significantly inhibited the growth
of B16F0 cells and the colony formation rate. Marked changes in
cell morphology were observed in the B16F0 cells after treatment
with alternol. In addition, long outgrowth and dendritic structure
became increasingly visible. Both intracellular and extracellular
melanin content were evidently increased along with alternol in a
dose-dependent manner. Moreover tyrosinase is one of the key
enzymes in the melanin biosynthesis of B16F0 cells (34). In addition, tyrosinase-related
protein such as TRP-1 and TRP-2 play extremely essential roles in
the process of melanin synthesis (35,36).
Tyrosinase can catalyze tyrosine into DOPA, and then DOPA is
oxidized into dopaquinone; thus the accumulation of melanin
pigments is accomplished (37,38).
Alternol upregulated the mRNA level of tyrosinase, TRP-1 and
TRP-2. All these results indicated that alternol can
obviously increase the content of differentiation markers in
melanoma cells (39). The mechanism
of the proliferation inhibition effect of alternol is mediated by
induction of the differentiation of melanoma cells.
Overall, the obtained results suggest that alternol
is a potent anti-melanoma agent, reducing proliferation and
inducing differentiation of the mouse melanoma B16F0 cell line in a
dose-dependent manner. Alternol has good prospects for clinical
application. The possibility of synergistic action of alternol with
known anticancer drugs warrents further testing. Currently, our
findings provide further support for the clinical application of
alternol in the inhibition and therapy of cancer.
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (no. 31471338), and funding by Binzhou
Medical University (BY2014KYQD01), and the Xinjiang Production and
Construction Corps Funds for Innovation Team in Key Areas
(2015BD005) to Q. Z.
References
|
1
|
Balch CM, Gershenwald JE, Soong SJ,
Thompson JF, Atkins MB, Byrd DR, Buzaid AC, Cochran AJ, Coit DG,
Ding S, et al: Final version of 2009 AJCC melanoma staging and
classification. J Clin Oncol. 27:6199–6206. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Korn EL, Liu PY, Lee SJ, Chapman JA,
Niedzwiecki D, Suman VJ, Moon J, Sondak VK, Atkins MB, Eisenhauer
EA, et al: Meta-analysis of phase II cooperative group trials in
metastatic stage IV melanoma to determine progression-free and
overall survival benchmarks for future phase II trials. J Clin
Oncol. 26:527–534. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Velho TR: Metastatic melanoma - a review
of current and future drugs. Drugs Context.
2012:2122422012.PubMed/NCBI
|
|
4
|
Hagen B and Trinh VA: Managing side
effects of vemurafenib therapy for advanced melanoma. J Adv Pract
Oncol. 5:400–410. 2014.
|
|
5
|
Pierce GB and Wallace C: Differentiation
of malignant to benign cells. Cancer Res. 31:127–134.
1971.PubMed/NCBI
|
|
6
|
Pierce GB: The cancer cell and its control
by the embryo. Rous-Whipple Award lecture. Am J Pathol.
113:117–124. 1983.PubMed/NCBI
|
|
7
|
Wang ZY and Chen Z: Acute promyelocytic
leukemia: From highly fatal to highly curable. Blood.
111:2505–2515. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Marcucci G, Haferlach T and Döhner H:
Molecular genetics of adult acute myeloid leukemia: Prognostic and
therapeutic implications. J Clin Oncol. 29:475–486. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gu JL, Li J, Zhou ZH, Liu JR, Huang BH,
Zheng D and Su C: Differentiation induction enhances bortezomib
efficacy and overcomes drug resistance in multiple myeloma. Biochem
Biophys Res Commun. 420:644–650. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu ZZ, Zhu J, Sun B, Liu S, Geng S, Liu X
and Li CL: Alternol inhibits proliferation and induces apoptosis in
mouse lymphocyte leukemia (L1210) cells. Mol Cell Biochem.
306:115–122. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhu XL, Wang YL, Chen JP, Duan LL, Cong
PF, Qu YC, Li-Ling J and Zhang MX: Alternol inhibits migration and
invasion of human hepatocellular carcinoma cells by targeting
epithelial-to-mesenchymal transition. Tumour Biol. 35:1627–1635.
2014. View Article : Google Scholar
|
|
12
|
Liu X, Wang J, Sun B, Zhang Y, Zhu J and
Li C: Cell growth inhibition, G2M cell cycle arrest, and apoptosis
induced by the novel compound Alternol in human gastric carcinoma
cell line MGC803. Invest New Drugs. 25:505–517. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yeung ED, Morrison A, Plumeri D, Wang J,
Tong C, Yan X and Li J: Alternol exerts prostate-selective
antitumor effects through modulations of the AMPK signaling
pathway. Prostate. 72:165–172. 2012. View Article : Google Scholar
|
|
14
|
Liu L, Zhang B, Yuan X, Wang P, Sun X and
Zheng Q: Alternol induces an S-phase arrest of melanoma B16F0
cells. Cell Biol Int. 38:374–380. 2014. View Article : Google Scholar
|
|
15
|
Tang Y, Chen R, Huang Y, Li G, Huang Y,
Chen J, Duan L, Zhu BT, Thrasher JB, Zhang X, et al: Natural
compound Alternol induces oxidative stress-dependent apoptotic cell
death preferentially in prostate cancer cells. Mol Cancer Ther.
13:1526–1536. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Edward M, Gold JA and MacKie RM: Different
susceptibilities of melanoma cells to retinoic acid-induced changes
in melanotic expression. Biochem Biophys Res Commun. 155:773–778.
1988. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ghattas S, Howle J, Wang W, Kefford R and
Gruenewald S: Intravascular metastatic melanoma: A difficult
diagnosis. Australas J Dermatol. 54:141–143. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nakaya K, Nabata Y, Ichiyanagi T and An
WW: Stimulation of dendritic cell maturation and induction of
apoptosis in leukemia cells by a heat-stable extract from azuki
bean (Vigna angularis), a promising immunopotentiating food and
dietary supplement for cancer prevention. Asian Pac J Cancer Prev.
13:607–611. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Macfarlane DE and Manzel L: Activation of
beta-isozyme of protein kinase C (PKC beta) is necessary and
sufficient for phorbol ester-induced differentiation of HL-60
promyelocytes. Studies with PKC beta-defective PET mutant. J Biol
Chem. 269:4327–4331. 1994.PubMed/NCBI
|
|
20
|
Liu YH, Gao XM, Ge FM, Wang Z, Wang WQ and
Li XY: PBK/TOPK expression during TPA-induced HL-60 leukemic cell
differentiation. Asian Pac J Cancer Prev. 13:2145–2148. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Deng R, Wang SM, Yin T, Ye TH, Shen GB, Li
L, Zhao JY, Sang YX, Duan XG and Wei YQ: Dimethyl sulfoxide
suppresses mouse 4T1 breast cancer growth by modulating
tumor-associated macrophage differentiation. J Breast Cancer.
17:25–32. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sulaimon SS and Kitchell BE: The biology
of melanocytes. Vet Dermatol. 14:57–65. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lin JY and Fisher DE: Melanocyte biology
and skin pigmentation. Nature. 445:843–850. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Costin GE and Hearing VJ: Human skin
pigmentation: Melanocytes modulate skin color in response to
stress. FASEB J. 21:976–994. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hearing VJ and Jiménez M: Analysis of
mammalian pigmentation at the molecular level. Pigment Cell Res.
2:75–85. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hearing VJ and Tsukamoto K: Enzymatic
control of pigmentation in mammals. FASEB J. 5:2902–2909.
1991.PubMed/NCBI
|
|
27
|
Valverde P, Garcia-Borron JC,
Jimenez-Cervantes C, Solano F and Lozano JA: Tyrosinase isoenzymes
in mammalian melanocytes. 2. Differential activation by
α-melanocyte-stimulating hormone. Eur J Biochem. 217:541–548. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mosmann T: Rapid colorimetric assay for
cellular growth and survival: Application to proliferation and
cytotoxicity assays. J Immunol Methods. 65:55–63. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang CX, Zhang B, Chen N, Liu LL, Liu JL,
Wang Q, Wang ZH, Sun XL and Zheng QS: Alteronol induces
differentiation of melanoma B16F0 cells. Recent Patents Anticancer
Drug Discov. 210:116–117. 2015.
|
|
30
|
Yao J, Wu J, Yang X, Yang J, Zhang Y and
Du L: Oleuropein induced apoptosis in HeLa cells via a
mitochondrial apoptotic cascade associated with activation of the
c-Jun NH2-terminal kinase. J Pharmacol Sci. 125:300–311. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li J, Huang CY, Zheng RL, Cui KR and Li
JF: Hydrogen peroxide induces apoptosis in human hepatoma cells and
alters cell redox status. Cell Biol Int. 24:9–23. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu L, Li T, Tan J, Fu J, Guo Q, Ji H and
Zhang Y: NG as a novel nitric oxide donor induces apoptosis by
increasing reactive oxygen species and inhibiting mitochondrial
function in MGC803 cells. Int Immunopharmacol. 23:27–36. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kim SJ, Jun S, Cho HY, Lee DC, Yeom YI,
Kim JH and Kang D: Knockdown of anterior gradient 2 expression
extenuates tumor-associated phenotypes of SNU-478 ampulla of Vater
cancer cells. BMC Cancer. 14:8042014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Guo H, Wang Y, Song T, Xin T, Zheng Z,
Zhong P and Zhang X: Silencing of survivin using YM155 inhibits
invasion and suppresses proliferation in glioma cells. Cell Biochem
Biophys. 71:587–593. 2015. View Article : Google Scholar
|
|
35
|
Meyskens FL Jr and Fuller BB:
Characterization of the effects of different retinoids on the
growth and differentiation of a human melanoma cell line and
selected subclones. Cancer Res. 40:2194–2196. 1980.PubMed/NCBI
|
|
36
|
Hosoi J, Abe E, Suda T and Kuroki T:
Regulation of melanin synthesis of B16 mouse melanoma cells by 1
alpha, 25-dihydroxyvitamin D3 and retinoic acid. Cancer Res.
45:1474–1478. 1985.PubMed/NCBI
|
|
37
|
Tomita Y, Maeda K and Tagami H:
Melanocyte-stimulating properties of arachidonic acid metabolites:
Possible role in postinflammatory pigmentation. Pigment Cell Res.
5:357–361. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ko HH, Tsai YT, Yen MH, Lin CC, Liang CJ,
Yang TH, Lee CW and Yen FL: Norartocarpetin from a folk medicine
Artocarpus communis plays a melanogenesis inhibitor without
cytotoxicity in B16F10 cell and skin irritation in mice. BMC
Complement Altern Med. 13:3482013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Choi YM, Jun HJ, Dawson K, Rodriguez RL,
Roh MR, Jun J, Choi CH, Shim JH, Lee CH, Lee SJ, et al: Effects of
the isoflavone puerarin and its glycosides on melanogenesis in B16
melanocytes. Eur Food Res Technol. 231:75–83. 2010. View Article : Google Scholar
|