Introduction
Gastric cancer is an aggressive disease that still
has a daunting impact on global health. Seventy-three percent of
gastric cancer cases are diagnosed in Asia, with almost 50% of the
world's cases diagnosed in China (1,2).
Despite an overall decline in incidence and mortality over the past
decade, gastric cancer remains the fourth most common type of
cancer and is the second leading cause of tumor-related death
worldwide (3). Currently, diagnosis
of gastric cancer is made by gastroscopic biopsy. Patients often
evade detection until they have obvious symptoms, resulting in
diagnosis at the middle or advanced stage. Due to the poor
prognosis of gastric cancer, early diagnosis is essential. Surgical
resection, chemotherapy and radiotherapy, the main methods of
treatment for gastric cancer, have improved survival (4). Unfortunately, treatment of advanced or
metastatic gastric cancer has seen little progress, and the median
overall survival (OS) in this group remains <1 year (5). The mechanisms of occurrence and
development of gastric cancer remain unclear.
Tumor biomarkers are the ̔hot spot̓ of cancer
research. Tumor markers are often involved in the development of
cancer, and perform an important function in the process of tumor
evolution. Therefore, they have potential as targets for tumor
therapy. CA19-9 and CEA are common serum biomarkers for
gastrointestinal tumors, but low sensitivity and specificity limit
their clinical usefulness (6).
Therefore, it is important to find molecular markers with high
sensitivity and specificity to diagnose gastric cancer earlier.
In our previous study, heterogeneous nuclear
ribonucleoprotein K (hnRNP K), a potential human gastric
carcinoma-associated antigen, was found in gastric cancer using
serologic proteome analysis (SERPA) (7). hnRNP K was found to be upregulated in
various types of human tumors, including colon (8), lung (9), breast, liver (10), esophageal (11), oral squamous cell (12) and nasopharyngeal cancers (13). Barboro et al reported that
the association between androgen receptor (AR) and hnRNP K
expression plays an important role in the progression of prostate
cancer and has potential prognostic value (14). Very little is known concerning the
behavior of hnRNP K in gastric cancer. Only Zhao et al has
reported that hnRNP K is expressed at a higher level in gastric
carcinoma with H. pylori L-form (Hp-L) infection than in
gastric cancer without Hp-L infection (15). In the present study, we examined the
expression of hnRNP K in gastric cancer tissue microarrays,
cultured cell lines and serum samples, and evaluated the
relationship between the survival rate of gastric cancer patients
and hnRNP K expression.
Materials and methods
Ethics statement
The present study was approved by the Ethics
Committee of the University of South China. The patients and
healthy volunteers provided signed informed consent. The present
study was conducted in accordance with the Declaration of
Helsinki.
Cell culture and cell immunochemical
staining
Gastric cancer cell lines MGC-803 and SGC-7901 and
normal gastric mucosal epithelial GES-1 cells were cultured in
RPMI-1640 medium containing heat-inactivated 15% fetal bovine serum
(FBS) on 6-well plate with a cover slide. Cells were cultured for
three days at 37°C in 5% CO2.
Cells were washed three times in phosphate-buffered
saline (PBS), fixed for 15 min in 10% formaldehyde, and then again
washed three times with PBS. The S-P immunohistochemical kit was
purchased from Fujian Maixin Biological Technology Ltd. (Fujian,
China). The cells were then incubated 10 min with peroxidase
blocking solution and washed three times with PBS. Cells were
incubated at 37°C for 10 min with enough non-immunologic animal
serum to block non-specific binding sites, and then anti-hnRNP K
antibody (diluted 1:1,500; ab-32969; Abcam Trade Company) was added
and incubated at 4°C overnight. Next, the cells were incubated at
37°C for 10 min with the secondary antibody marked by biotin, and
then incubated for 10 min with Streptomyces avidin-peroxidase. The
reaction was visualized using DAB substrate chromogen (Fujian
Maixin Biological Technology Ltd.).
Tissue microarrays and
immunohistochemistry
Human gastric cancer tissue microarrays (TMA) were
obtained from Dr Yongjun Wu (Department of Pathology, The First
People's Hospital of Xiangtan, Hunan, China). The specimens were
biopsy or gastric cancer resection specimens collected from year
2003 to 2006, including 199 gastric cancer, 31 tumor-adjacent
gastric mucosal and 98 normal gastric mucosal specimens. None of
the patients received preoperative radiotherapy or
chemotherapy.
We placed the paraffin sections at 55°C constant
temperature in an incubator for one night, and then they were
dewaxed with xylene, and washed three times with PBS. After that an
antigen retrieval step was performed. Immunohistochemistry (IHC)
was performed according to S-P kit instructions.
Immunohistochemical reactions were developed in freshly prepared
3,3′-diamino-benzidine tetrahydrochloride (DAB kit; Fujian Maixin
Biological Technology Ltd.) for immune complex visualization.
Finally, the TMA IHC was evaluated by light microscopic
examination, and the intensity of immunostaining was assessed by
two independent professors of pathology. We selected five different
high magnifications, counting the total number of cells and hnRNP
K-positive cells. The results were evaluated based on both the
intensity of immunostaining and the positive cell percentage. The
intensity of immunostaining in each core was scored 0–3 (0,
negative; 1, weak, 2, moderate; and 3, strong). In addition, the
samples were scored into four groups based on the percentage of
positively stained cells: 0–25% positivity scored as 1, 26–50% as
2, 51–75% as 3, and 76–100% as 4. The staining intensity score and
the percentage immunoreactivity score were then calculated to
obtain a composite score (composite score = intensity score ×
percentage score); 0–5 was defined as low expression, and 6–12 was
defined as high expression (16).
Western blot analysis
Total cell proteins from the MGC-803, SGC-7901 and
GES-1 cells were quantified using a BCA protein assay kit. Then,
the samples were separated by 10% SDS-PAGE, transferred to
polyvinylidene difluoride (PVDF) membranes, and then blocked with
Tris-buffered saline Tween-20 (TBST) containing 5% non-fat milk for
1 h, and washed with TBST three times. Then, the samples were
probed at 4°C overnight with rabbit anti-hnRNP K antibody (diluted
1:2,000; ab-32969), washed with TBST three times 5 min each time,
and incubated with the appropriate secondary antibody (goat
anti-rabbit IgG; diluted 1:3,000) for 1 h. The samples were then
washed with TBST three times again for 5 min each time and enhanced
chemiluminescence (ECL) western blotting detection reagents were
used to visualize the target proteins (both from KeyGen Biotech,
Nanjing, China).
Patients and sample collection for
ELISA
We used a total of 96 serum samples including 37
paired samples from gastric cancer patients (preoperative and
postoperative) and 22 samples from healthy volunteers with no
evidence of cancer and other disease. All samples were collected
from the Affiliated First Hospital of the University of South China
according to our previously published protocol (17). The serum samples were analyzed for
hnRNP K using a commercially available ELISA (USCN Life Science
Inc., Houston, TX, USA).
Statistical analysis
Data are reported as mean ± SD. The differences
between two groups were compared using Chi-square tests, t-test,
one-way ANOVA. Kaplan-Meier survival analysis and log-rank test
were performed to determine survival differences between the
different groups. Factors associated with the outcomes were
evaluated by Cox multivariate regression analysis. Statistical
analysis was carried out using SPSS version 17.0 software program
(SPSS, Inc., Chicago, IL, USA). All analyses were regarded as
statistically significant at the P<0.05 level, and all P-values
were two-tailed.
Results
Higher expression of hnRNP K in human
gastric cancer cell lines
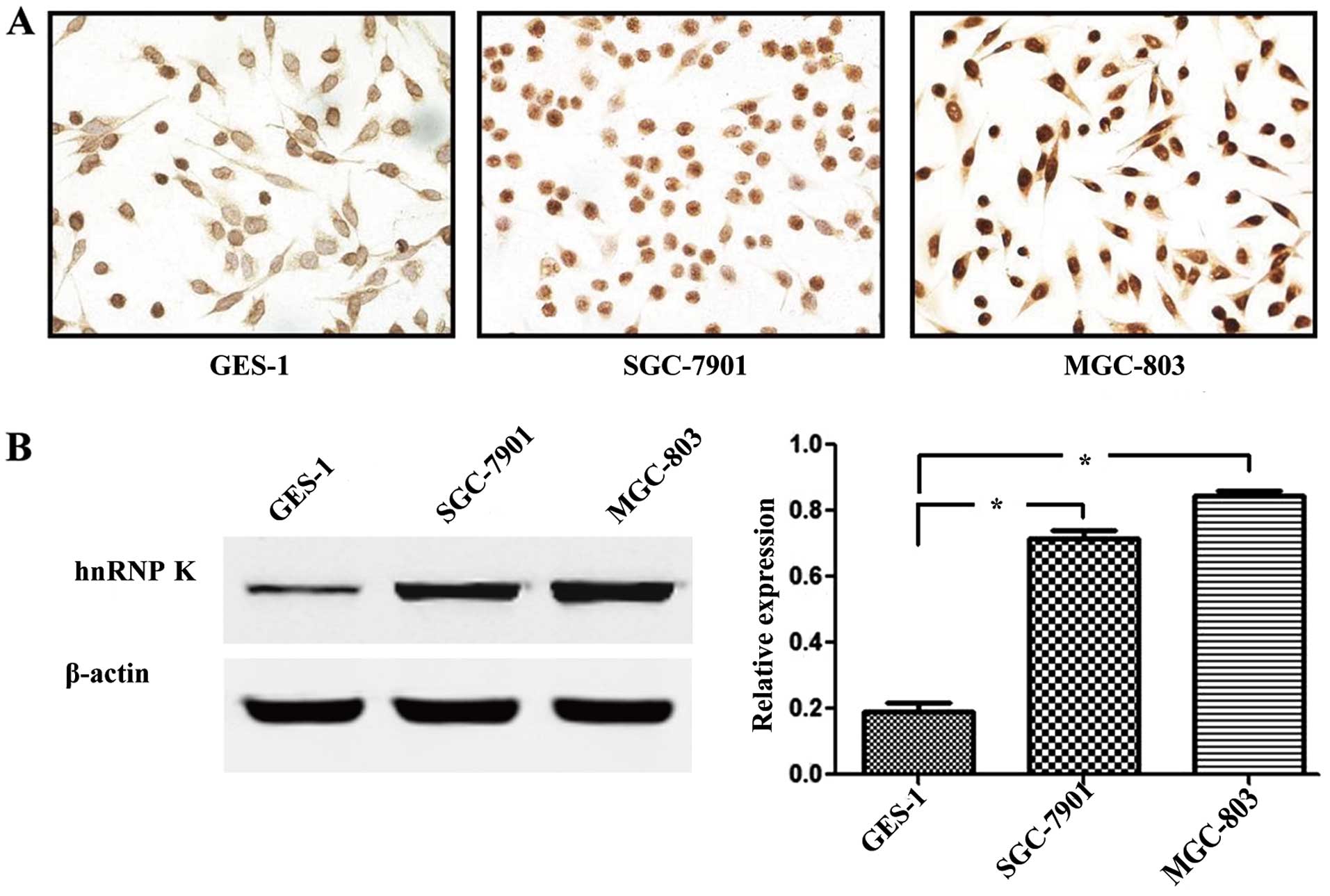
To evaluate the expression of hnRNP K between
gastric cancer and normal gastric mucosal epithelial cells, we
performed cell immunochemical staining analysis in two gastric
cancer cell lines (MGC-803 and SGC-7901) and a normal gastric
mucosal epithelial cell line (GES-1). Obviously, the intensity of
immunostaining in the MGC-803 and SGC-7901 cells was stronger than
that found in the GES-1 cells (Fig.
1A). We also performed western blot analysis in the three cell
lines. Compared with the normal gastric mucosal epithelial cell
line GES-1, hnRNP K protein expression was significantly higher in
the gastric cancer cell lines MGC-803 and SGC-7901 (P<0.05;
Fig. 1B).
hnRNP K is overexpressed in gastric
cancer tissues
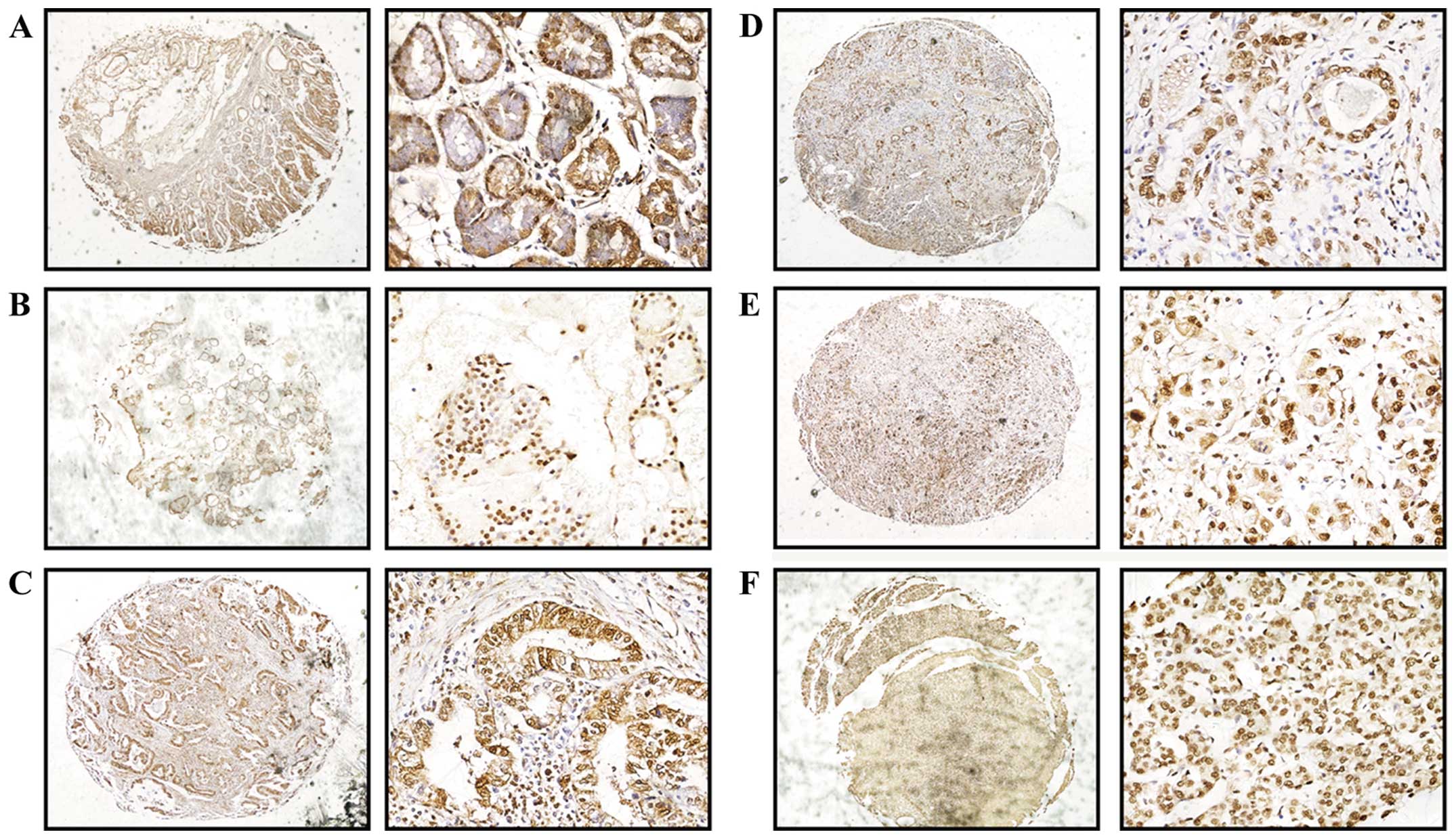
To confirm whether hnRNP K expression is elevated in
human gastric cancer tissues, we analyzed the level of hnRNP K
protein by IHC in the human gastric carcinoma tissue microarrays
(TMAs). The hnRNP K expression in gastric cancer tissues was
significantly higher compared with that found in the tumor-adjacent
gastric mucosal and normal gastric mucosal specimens. Both in the
gastric cancer and non-gastric carcinoma tissues, hnRNP K was
localized in the nucleus and showed low staining in the cytoplasm.
However, we found that hnRNP K expression was significantly
elevated in the gastric glandular neck epithelium when compared to
that in the other glandular epithelium (Fig. 2). According to various studies,
gastric gland stem cells localize in the gastric neck glands, where
they have the ability to proliferate and differentiate. This
indicates that elevated expression of hnRNP K promotes the ability
of gastric cancer cells to grow and proliferate.
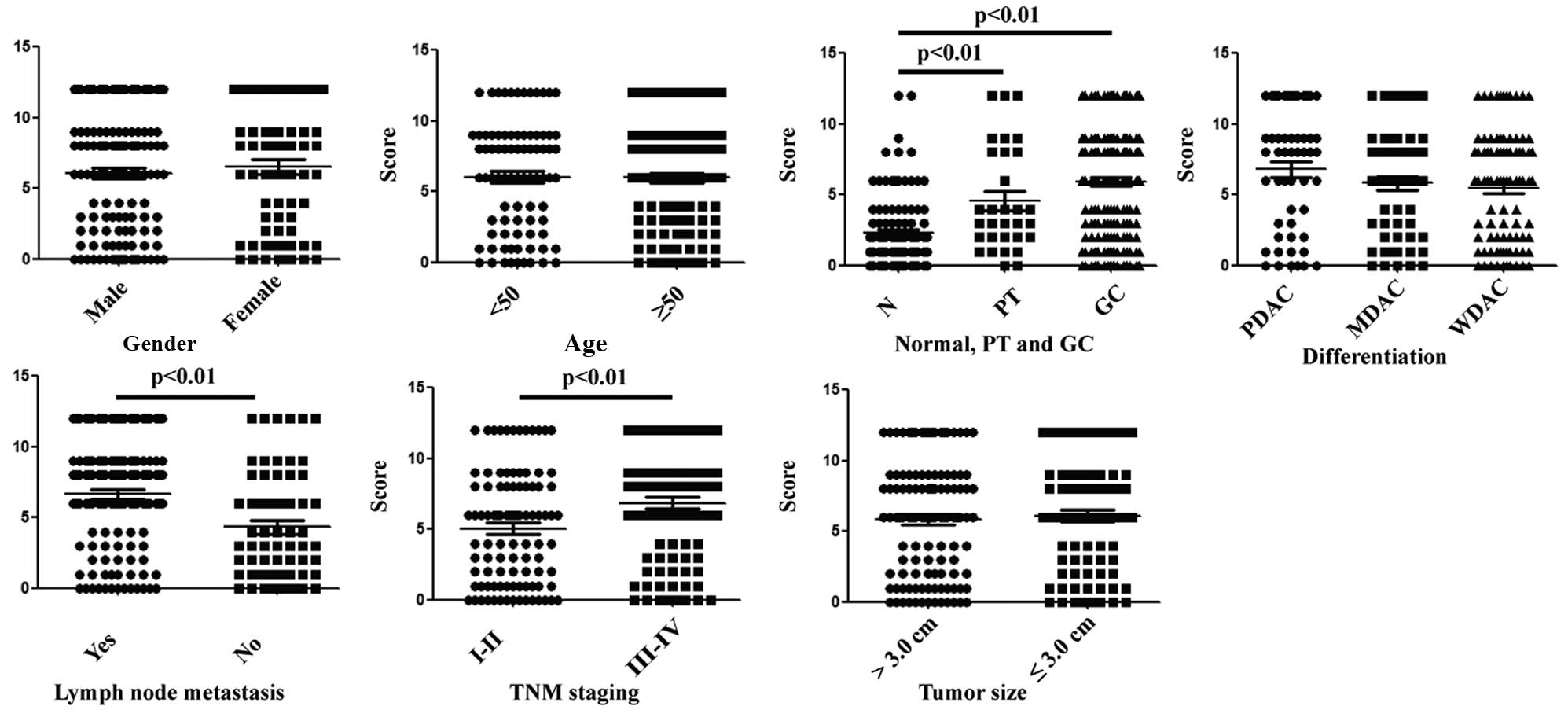
We also evaluated the relationship between the
clinicopathological parameters of the gastric cancer patients and
the nRNP K expression level. We found that there was no correlation
between the protein expression level and gender, age, degree of
differentiation or tumor size. However, hnRNP K expression was
significantly elevated in the group with lymph node metastasis than
that found in the group without lymph node metastasis, and was also
higher in stage III and IV than stage I and II samples (Table I; Fig.
3)
 | Table IRelationship between the expression
levels of hnRNP K and clinicopathological characteristics of the
patients. |
Table I
Relationship between the expression
levels of hnRNP K and clinicopathological characteristics of the
patients.
| Clinical
parameters | N | Expression of hnRNP K
| P-value
(two-sided) |
|---|
| Low n (%) | High n (%) |
|---|
| Gender | | | | 0.790 |
| Male | 126 | 49 (38.9) | 77 (61.1) | |
| Female | 73 | 27 (40.0) | 46 (60.0) | |
| Age (years) | | | | 0.836 |
| <50 | 83 | 31 (37.3) | 52 (62.7) | |
| ≥50 | 116 | 45 (38.8) | 71 (61.2) | |
| Normal gastric
mucosa | 98 | 82 (83.7) | 16 (16.3) | 0.001a |
| Tumor-adjacent
gastric mucosa | 31 | 21 (67.7) | 10 (32.3) | |
| Differentiation | | | | 0.498 |
| WDAC | 88 | 37 (42.0) | 51 (58.0) | |
| MDAC | 58 | 22 (37.9) | 36 (62.1) | |
| PDAC | 53 | 17 (32.1) | 36 (67.9) | |
| Lymph node
metastasis | | | | 0.000 |
| No | 65 | 39 (60.0) | 26 (40.0) | |
| Yes | 134 | 37 (27.6) | 97 (72.4) | |
| TNM stage | | | | 0.003 |
| I–II | 96 | 47 (49.0) | 49 (51.0) | |
| III–IV | 103 | 29 (28.2) | 74 (71.8) | |
| Tumor size
(cm) | | | | 0.666 |
| >3.0 | 114 | 45 (39.5) | 69 (60.5) | |
| ≤3.0 | 85 | 31 (36.5) | 54 (63.5) | |
Relationship between the survival rate of
gastric cancer patients and hnRNP K expression and
clinicopathological characteristics
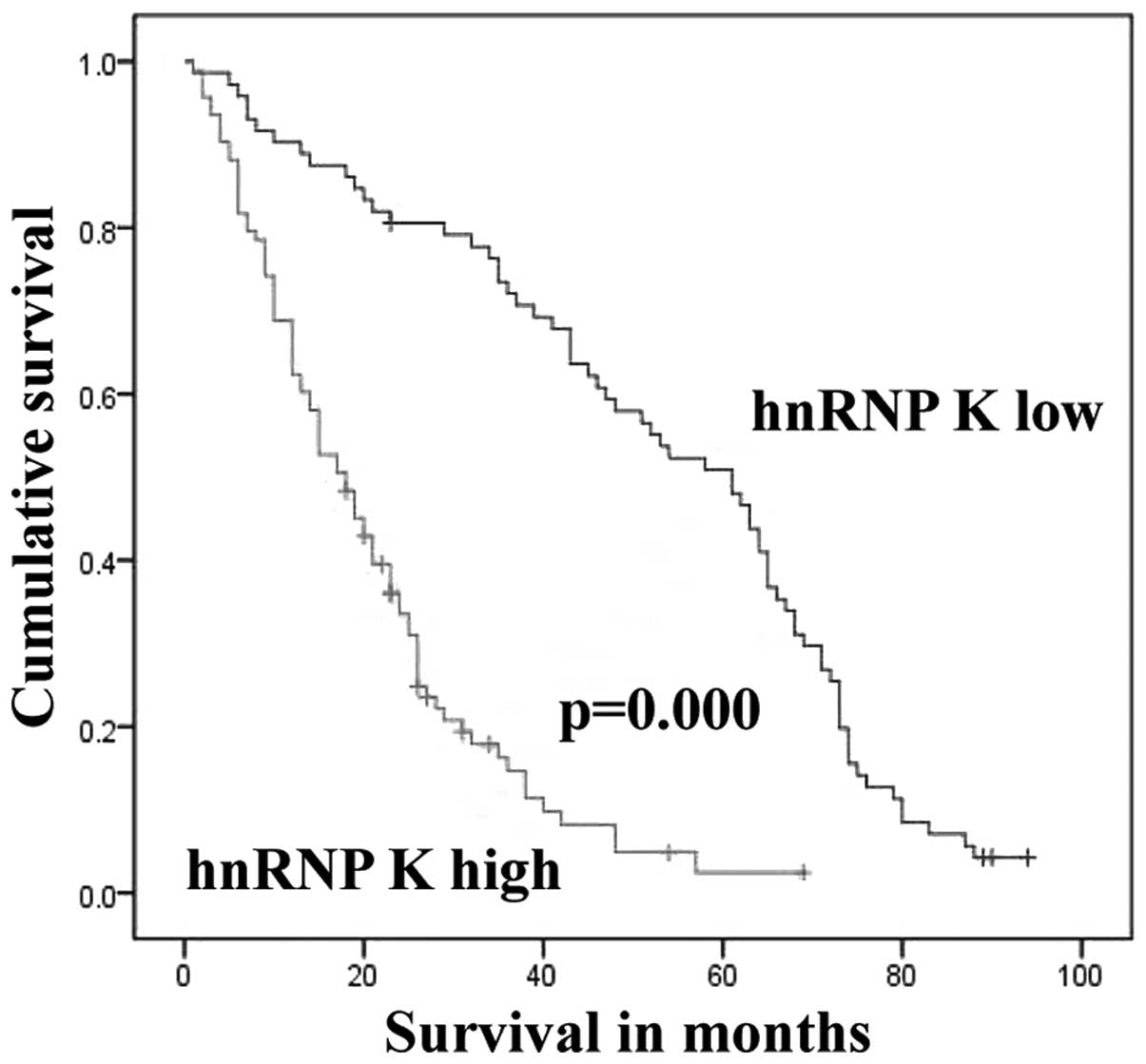
One hundred ninety-nine patients with gastric cancer
were followed-up for 8 years. There were 34 cases lost to follow-up
and 121 patients died during this time; overall survival (OS) was
26.7%. Twenty-eight of the 53 (52.8%) patients with low expression
of hnRNP K died; while 93 of 112 (83.0%) patients with high hnRNP K
died. The death rate of the high hnRNP K group was 1.57 times
higher than that in the low hnRNP K group. We found that patients
with elevated hnRNP K expression in cancer cells had poorer
survival compared with those patients with low hnRNP K expression
(log-rank=62.339, P=0.000; Fig. 4).
The mean survival time of patients with low hnRNP K expression was
52.277 months and the median survival time was 61 months, while the
mean survival time in the cohort with higher hnRNP K expression was
20.657 months and the median survival time was 18 months.
Cox multivariate analysis indicated that the degree
of differentiation and lymph node metastasis were associated with
poor survival. Moreover, the risk of death in the poorly
differentiated group was 2.203 times (death ratio=1/HR) higher than
that of the moderately and well differentiated groups. The
mortality risk in the lymph node metastasis group was 1.976 times
higher than that in the patients with no lymph node metastasis
(Table II).
 | Table IICorrelation between survival time and
clinicopathological characteristics of the gastric cancer patients
using COX multivariate analysis. |
Table II
Correlation between survival time and
clinicopathological characteristics of the gastric cancer patients
using COX multivariate analysis.
| Variables | HR | P-value | 95% CI |
|---|
| Age (<50 vs. ≥50
years) | 1.001 | 0.96 | 0.980–1.022 |
| gender (male vs.
female) | 0.823 | 0.45 | 0.491–1.378 |
| Differentiation
degree (well + moderate vs. poor) | 0.454 | 0.002 | 0.279–0.741 |
| Lymph node
metastasis (no vs. yes) | 0.506 | 0.023 | 0.281–0.910 |
| TNM stage (I+II vs.
III+IV) | 0.722 | 0.417 | 0.329–1.585 |
3hnRNP K expression in the serum of
gastric cancer patients and healthy volunteers
To evaluate the expression level of serum hnRNP K
between gastric cancer patients and normal controls, we measured
the levels of hnRNP K in serum samples by ELISA. Most serum samples
showed low expression of hnRNP K. There was no significant
difference between the gastric cancer patients and the healthy
volunteers or between the preoperative and postoperative groups
(Table III). We believe that
hnRNP K is not a suitable circulating tumor biomarker for detecting
gastric cancer.
 | Table IIISerum hnRNP K levels in controls,
preoperative gastric cancer patients and paired postoperative
gastric cancer patients. |
Table III
Serum hnRNP K levels in controls,
preoperative gastric cancer patients and paired postoperative
gastric cancer patients.
| Groups | N | Serum hnRNP K level
(mean ± SD) | P-value
(two-sided) |
|---|
| Healthy
volunteers | 22 | 0.984±0.358 | 0.237a |
| Preoperative
group | 37 | 0.908±0.353 | 0.096b |
| Postoperative
group | 37 | 0.783±0.306 | |
Discussion
hnRNP K is a RNA-binding protein of the large hnRNP
family. hnRNP K was mainly found localized in the nucleus, but
recently Thompson et al found it also exists in the
cytoplasm and mitochondria (18).
hnRNP K contains three repeats of a motif termed the KH domain (for
K homology) (19), which could
recognize single-stranded DNA or RNA. In addition, hnRNP K has a
nuclear-localization signal (NLS) and a nuclear shuttling domain
(KNS) which allow transport from the cytoplasm to the nucleus
(20). Due to these different
structures, hnRNP K is involved in translational modifications,
including methylation, sumoylation and phosphorylation. These
regulate its interactions with different molecules and influence
its functions, such as DNA splicing, chromosome remolding,
transcriptional regulation, mRNA stability, splicing and
translation (21). In particular,
hnRNP K plays an important role in carcinogenesis. Researchers have
shown that hnRNP K activates important genes associated with human
tumors, including c-src and c-myc, indicating that hnRNP K
participates in cancer development and progression (22). It is active at the chromatin level,
and present in greater density near transcribed genes with respect
to silent ones. hnRNP K directly binds to the promoter region of
the human c-myc gene (23) and was
found to promote neoplastic transformation in an eIF4E-dependent
manner (24). The expression level
of hnRNP K was higher in melanoma, breast and prostate cancers than
that in normal control groups (14,25,26).
hnRNP K can also suppress apoptosis independent of p53 status by
maintaining high levels of endogenous caspase inhibitors (27). Recent studies found that hnRNP K was
closely related to non-coding RNA, including lncRNA and miRNA.
Moreover, studies have shown that both long (>200 nucleotides)
and short ncRNAs have critical regulatory roles in several human
diseases, including cancer development and progression (28). Carpenter et al reported that
the expression level of hnRNP K is often associated with colorectal
cancer staging (29). Wu et
al detected elevated protein levels in the cytoplasm of oral
squamous cell carcinoma, suggesting that hnRNP K may be an
independent prognostic predictor (30). They showed that increased
cytoplasmic expression of hnRNP K was associated with poor patient
prognosis by multivariate analysis. Even Inoue et al found
that the cytoplasmic accumulation of hnRNP K was crucial for its
role in the metastasis of fibrosarcoma (31).
There has been little investigation concerning the
relationship between hnRNP K and gastric cancer. In the present
study, we demonstrated that expression of hnRNP K was higher in
gastric cancer cells (MGC-803 and SGC-7901) than that in normal
gastric mucosal epithelial cells (GES-1) and it was localized in
the nucleus. Tissue array immunohistochemistry showed that levels
of hnRNP K were significantly elevated in gastric carcinoma tissues
than that in tumor-adjacent gastric mucosal or normal gastric
mucosal specimens. We evaluated the correlation between the
expression of hnRNP K and clinical pathology, and found that it was
correlated with lymph node metastasis and tumor stage. Moreover,
the survival rate of patients with gastric cancer was associated
with the degree of tumor differentiation and lymph node metastasis.
Thus, the results supported the observation that patients with
elevated expression of hnRNP K have poorer survival compared to
those with low hnRNP K expression.
ELISA results showed low expression of hnRNP K in
serum of the gastric cancer patients. This suggests that hnRNP K is
mainly transported into the nucleus rather than secreted out of
cells. There was no significant difference between the gastric
cancer patients and healthy volunteers. Comparing the preoperative
groups with the postoperative groups, we also found no difference
between these two groups. Therefore, hnRNP K is unsuitable as a
serum marker in gastric cancer.
We conclude that hnRNP K is upregulated in gastric
cancer and is associated with patient survival. Therefore, hnRNP
has the potential as a key biomarker for detection of gastric
cancer and is of prognostic value to patients with gastric cancer.
However, according to the present study, serum levels may not be
useful for measuring this tumor-associated biomarker. We may
continue to study the role of hnRNP K in gastric cancer to
determine whether it is a promising target for anticancer
therapies.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (81101643), the Foundation of
the Construct Program of the Key Discipline in Hunan Province of
China (no. 2011-76), Hunan Provincial Education Department document
(approval no. 2014-405), and the Foundation of the Department of
Science and Technology of Hunan Province (2012SK3152). The authors
wish to thank Dr Chun Wang of Washington University and Carol
Gordon of Southern Illinois University for modification to language
of the present study.
References
|
1
|
Luyimbazi D, Nelson RA, Choi AH, Li L,
Chao J, Sun V, Hamner JB and Kim J: Estimates of conditional
survival in gastric cancer reveal a reduction of racial disparities
with long-term follow-up. J Gastrointest Surg. 19:251–257. 2015.
View Article : Google Scholar
|
|
2
|
Ferlay J, Steliarova-Foucher E,
Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, Forman D and
Bray F: Cancer incidence and mortality patterns in Europe:
Estimates for 40 countries in 2012. Eur J Cancer. 49:1374–1403.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ferro A, Peleteiro B, Malvezzi M, Bosetti
C, Bertuccio P, Levi F, Negri E, La Vecchia C and Lunet N:
Worldwide trends in gastric cancer mortality (1980–2011), with
predictions to 2015, and incidence by subtype. Eur J Cancer.
50:1330–1344. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Deng X, Jin X, Xue S, Zhang X, Su H, Zhang
P and Xie C: Postoperative chemoradiotherapy for advanced gastric
cancer after D2 gastrectomy. Hepatogastroenterology. 61:1472–1477.
2014.PubMed/NCBI
|
|
5
|
Zheng CH, Lu J, Huang CM, Li P, Xie JW,
Wang JB and Lin JX: Treatment of locally advanced gastric cancer
with the XELOX program of neoadjuvantchemotherapy combined with
laparoscopic surgery: The experience in China.
Hepatogastroenterology. 61:1876–1882. 2014.
|
|
6
|
Jiang X, Du L, Wang L, Li J, Liu Y, Zheng
G, Qu A, Zhang X, Pan H, Yang Y, et al: Serum microRNA expression
signatures identified from genome-wide microRNA profiling serve as
novel noninvasive biomarkers for diagnosis and recurrence of
bladder cancer. Int J Cancer. 136:854–862. 2015. View Article : Google Scholar
|
|
7
|
Zeng X, Liao AJ, Tang HL, Yi L, Xie N and
Su Q: Screening human gastric carcinoma-associated antigens by
serologic proteome analysis. Ai Zheng. 26:1080–1084. 2007.In
Chinese. PubMed/NCBI
|
|
8
|
Wang F, Zhang P, Shi C, Yang Y and Qin H:
Immunohistochemical detection of HSP27 and hnRNP K as prognostic
and predictive biomarkers for colorectal cancer. Med Oncol.
29:1780–1788. 2012. View Article : Google Scholar
|
|
9
|
Chen Y, Li W and Zhang S: hnRNP K
expression and its clinical significance in human lung cancer
tissues. Zhongguo Fei Ai Za Zhi. 11:241–245. 2008.In Chinese.
PubMed/NCBI
|
|
10
|
Guo Y, Zhao J, Bi J, Wu Q, Wang X and Lai
Q: Heterogeneous nuclear ribonucleoprotein K (hnRNP K) is a tissue
biomarker for detection of early hepatocellular carcinoma in
patients with cirrhosis. J Hematol Oncol. 5:372012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Namikawa T, Kobayashi M and Hanazaki K:
Esophageal tumor after radical surgery for gastric cancer.
Gastroenterology. 148:e9–e10. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Don KR, Ramani P, Ramshankar V, Sherlin
HJ, Premkumar P and Natesan A: Promoter hypermethylation patterns
of P16, DAPK and MGMT in oral squamous cell carcinoma: A systematic
review and meta-analysis. Indian J Dent Res. 25:797–805. 2014.
View Article : Google Scholar
|
|
13
|
Gross H, Hennard C, Masouris I, Cassel C,
Barth S, Stober-Grässer U, Mamiani A, Moritz B, Ostareck D,
Ostareck-Lederer A, et al: Binding of the heterogeneous
ribonucleoprotein K (hnRNP K) to the Epstein-Barr virus nuclear
antigen 2 (EBNA2) enhances viral LMP2A expression. PLoS One.
7:e421062012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Barboro P, Salvi S, Rubagotti A, Boccardo
S, Spina B, Truini M, Carmignani G, Introini C, Ferrari N, Boccardo
F, et al: Prostate cancer: Prognostic significance of the
association of heterogeneous nuclear ribonucleoprotein K and
androgen receptor expression. Int J Oncol. 44:1589–1598.
2014.PubMed/NCBI
|
|
15
|
Zhao Y, Jin X, Tian T and Yu DH:
Expression of hnRNPK in gastric carcinoma and its relationship with
Helicobacter pylori L-form infection. Zhonghua Zhong Liu Za Zhi.
33:759–763. 2011.In Chinese.
|
|
16
|
Xia YJ, Ma YY, He XJ, Wang HJ, Ye ZY and
Tao HQ: Suppression of selenium-binding protein 1 in gastric cancer
is associated with poor survival. Hum Pathol. 42:1620–1628. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xiang M, Zeng Y, Yang R, Xu H, Chen Z,
Zhong J, Xie H, Xu Y and Zeng X: u6 is not a suitable endogenous
control for the quantification of circulating microRNAs. Biochem
Biophys Res Commun. 454:210–214. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Thompson PJ, Dulberg V, Moon KM, Foster
LJ, Chen C, Karimi MM and Lorincz MC: hnRNP K coordinates
transcriptional silencing by SETDB1 in embryonic stem cells. PLoS
genet. 11:e10049332015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wojtuszkiewicz A, Assaraf YG, Maas MJ,
Kaspers GJ, Jansen G and Cloos J: Pre-mRNA splicing in cancer: The
relevance in oncogenesis, treatment and drug resistance. Expert
Opin Drug Metab Toxicol. 11:673–689. 2015. View Article : Google Scholar
|
|
20
|
Boisvert M, Bouchard-Lévesque V, Fernandes
S and Tijssen P: Classic nuclear localization signals and a novel
nuclear localization motif are required for nuclear transport of
porcine parvovirus capsid proteins. J Virol. 88:11748–11759. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cao W, Razanau A, Feng D, Lobo VG and Xie
J: Control of alternative splicing by Forskolin through hnRNP K
during neuronal differentiation. Nucleic Acids Res. 40:8059–8071.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Adolph D, Flach N, Mueller K, Ostareck DH
and Ostareck-Lederer A: Deciphering the cross talk between hnRNP K
and c-Src: The c-Src activation domain in hnRNP K is distinct from
a second interaction site. Mol Cell Biol. 27:1758–1770. 2007.
View Article : Google Scholar :
|
|
23
|
Moritz B, Lilie H, Naarmann-de Vries IS,
Urlaub H, Wahle E, Ostareck-Lederer A and Ostareck DH: Biophysical
and biochemical analysis of hnRNP K: Arginine methylation,
reversible aggregation and combinatorial binding to nucleic acids.
Biol Chem. 395:837–853. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Osborne MJ and Borden KL: The eukaryotic
translation initiation factor eIF4E in the nucleus: Taking the road
less traveled. Immunol Rev. 263:210–223. 2015. View Article : Google Scholar
|
|
25
|
Wen F, Shen A, Shanas R, Bhattacharyya A,
Lian F, Hostetter G and Shi J: Higher expression of the
heterogeneous nuclear ribonucleoprotein k in melanoma. Ann Surg
Oncol. 17:2619–2627. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Belli AK, Elibol F and Ozcan O: Other
factors related to the completion of treatment after breast cancer.
Breast. 24:2832015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xiao Z, Ko HL, Goh EH, Wang B and Ren EC:
hnRNP K suppresses apoptosis independent of p53 status by
maintaining high levels of endogenous caspase inhibitors.
Carcinogenesis. 34:1458–1467. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ling H, Fabbri M and Calin GA: MicroRNAs
and other non-coding RNAs as targets for anticancer drug
development. Nat Rev Drug Discov. 12:847–865. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Carpenter B, McKay M, Dundas SR, Lawrie
LC, Telfer C and Murray GI: Heterogeneous nuclear ribonucleoprotein
K is over expressed, aberrantly localised and is associated with
poor prognosis in colorectal cancer. Br J Cancer. 95:921–927. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wu CS, Chang KP, Chen LC, Chen CC, Liang
Y, Hseuh C and Chang YS: Heterogeneous ribonucleoprotein K and
thymidine phosphorylase are independent prognostic and therapeutic
markers for oral squamous cell carcinoma. Oral Oncol. 48:516–522.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Inoue A, Sawata SY, Taira K and Wadhwa R:
Loss-of-function screening by randomized intracellular antibodies:
identification of hnRNP-K as a potential target for metastasis.
Proc Natl Acad Sci USA. 104:8983–8988. 2007. View Article : Google Scholar : PubMed/NCBI
|