Introduction
Endometrial cancer (EC) is one of the most common
types of cancer in women, and ranks 4th (7.1%) in incidence among
all female cancers. In 2012 in Poland, 5,426 women were diagnosed
with endometrial cancer. The highest incidence was observed in
women between 60 and 64 years. With regard to mortality,
endometrial cancer ranks 11 (2.76%) with 1,162 deaths in 2012. The
highest mortality has been observed in women between 70–74 years
(1). Most endometrial cancer cases
are diagnosed in the early stages and therefore result in
favourable clinical outcomes (2).
On the other hand, a significant number of patients with
early-stage disease develop both localised recurrence and distant
metastases (2). The mechanism of
development of these tumours remains unclear. One of the postulated
key processes in the development of endometrial tumours is
uncontrolled proliferation stimulated by physiological local
factors (3). Therefore, various
biologically active peptides such as angiotensin, affecting cell
proliferation, have become a new area of study in endometrial
cancer research.
Angiotensin II (Ang II) is the major octapeptide of
the renin-angiotensin system (RAS). For many decades Ang II was
mainly perceived as a regulator of the cardiovascular system, an
important factor in the pathogenesis of hypertension,
atherosclerosis, and angiogenesis. Recently, a number of studies
have shown that Ang II plays an important role in proliferation,
invasiveness and migration of tumour cells, alteration of
expression of cancer-related genes, as well as in physiological
tissue remodelling. All components of the RAS (angiotensinogen,
angiotensin converting enzyme, angiotensin receptors) are expressed
in many types of tumours, including endometrial cancer (2,4).
Moreover, both angiotensin receptors (AT1 and AT2) are
overexpressed in different types of cancers, which confirms the
role of local RAS in cancer development and progression (3,5–8).
The aim of the present study was to evaluate the
influence of Ang II on human endometrial cancer cells. Biological
assays were performed on well-[Ishikawa (ISH)] moderately (MFE-296)
and poorly (MFE-280) differentiated human adenocarcinoma cancer
cell lines, representing the G1, G2 and G3 stage of EC. Moreover,
we examined alterations in the expression of genes related to
cancer cell behaviour after Ang II treatment.
Materials and methods
Cell culture
ISH, MFE-296 and MFE-280 were obtained from the
European Collection of Cell Cultures (Sigma-Aldrich, Poznan,
Poland) grown in MEM medium supplemented with 10% fetal bovine
serum (FBS), 1% penicillin-streptomycin-neomycin, 1% HEPES, 1%
sodium pyruvate and 1% L-glutamine [ISH cell line additionally
required 1% MEM non-essential amino acid (NEAA)]. All cell culture
reagents were obtained from Gibco® by Life Technologies™
(Warsaw, Poland). Human endometrial ISH cells were derived from
well-differentiated adenocarcinoma of the endometrium, while the
MFE-296 and MFE-280 cells were obtained from a moderately and
poorly differentiated human endometrial adenocarcinoma,
respectively.
Experimental design
For each subsequent biological assay, cells were
seeded and maintained in the same way. Cells were seeded on 6-well
plates (MFE-296 and ISH, 2–5×105/well; MFE-280,
4–10×105/well) in culture medium with the exception of
gene expression analysis, where cells were seeded on 100-mm Petri
dishes (1.2×106 cells/dish). The next day culture medium
was replaced by experimental medium containing human Ang II
(Sigma-Aldrich) and incubation was continued for 72 h at 37°C. The
following conditions were used during the experiments: control
without Ang II and medium with 0.1, 1 and 10 µg/ml of Ang
II. Experimental media were changed every day.
2-(4-Iodophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium
(WST-1) assay
The effect of Ang II on the proliferative capacity
of endometrial cells was quantified using the WST-1 assay. Briefly,
endometrial cells were seeded in 96-well plates at 4×103
cells/well in growth medium and incubated overnight under standard
culture conditions. Following incubation, the growth medium was
discarded and replaced with fresh experimental media containing
different concentrations of Ang II and control media. WST-1 assay
(Roche diagnostics GmbH, Mannheim, Germany) was carried out
according to the manufacturer's protocols as previously described
(9). Cell viability was calculated
as: viability = (absorbance of experimental wells)/(absorbance of
control wells).
Apoptosis analysis
Analysis of apoptosis was performed using
Muse® Annexin V & Dead Cell kit on a Muse automated
cell analyser (Merck Millipore, Darmstadt, Germany). This test is
based on Annexin V binding to phosphatidylserine (PS), which is
predominantly located in the inner leaflet of the plasma membrane
under physiological conditions, whereas upon initiation of
apoptosis PS is translocated to the extracellular membrane leaflet
marking cells as targets of phagocytosis. One hundred microliters
of cells suspended in culture medium (with FBS) were used for this
analysis. This test was performed according to the manufacturer's
instructions.
Cell cycle analysis
Cell cycle analysis was performed using a
Muse™ Cell Cycle kit on a Muse automated cell analyser
(Merck Millipore). This test is based on nuclear DNA intercalating
stain: propidium iodide (PI), that differentiates cells at various
stages of the cell cycle based on differential DNA content. After
incubation the cells were harvested and suspended in culture medium
(with FBS). Cell cycle assay was performed according to the
manufacturer's instructions.
Monolayer cell migration assay
wound-healing migration assays
Endometrial cancer cells were seeded on 6-well
plates (MFE-296 and ISH, 4×105/well; MFE-280,
1×106/well) and maintained in culture medium until they
reached 90% confluency. Cell monolayers were scraped using a
sterile pipette tip forming a cross in the middle of the well. Each
well was washed with sterile 1X PBS to eliminate the unattached
cells. Subsequently, the cells were maintained in experimental
medium according to the experimental design. Cell cycle blocker,
hydroxyurea (0.5 mM) (Sigma-Aldrich), was added to the scratch
wound in order to exclude the contribution of cell proliferation to
wound closure (10). Images were
collected just after the culture medium was replaced with
experimental medium and after 72 h of incubation. Wound area
measurements were averaged from the same fields of the same wells,
using ImageJ 1.48v program (http://imagej.nih.gov/ij/download.html). The influence
of Ang II was calculated and presented as the percentage of changes
in comparison to the control (100%).
Adhesion assay
After 72 h of incubation with Ang II the cells were
collected from each well using Trypsin-EDTA (0.05%) and counted.
The cells were seeded on 24-well plates coated with collagen I,
collagen IV, fibronectin or laminin (Corning, BioCoat™;
Corning Incorporated, Corning, NY, USA); 1×105
cells/well from each well from a 6-well plate were seeded. Plates
were incubated for 90 min at 37° C, with 5% CO2. After
incubation, each well was washed three times with 200 µl
sterile 1X PBS. The remaining cells adhering to the matrix proteins
were stained with 0.1% crystal violet for 10 min. Subsequently, the
cells were washed three times with 200 µl water. Two hunded
microliters of 10% acetic acid was added to each well to extract
the crystal violet from the cells. Dye/solution mixture was
transferred into a 96-well plate and measured on an El808 plate
reader (BioTek, Winooski, VT, USA) at 550 nm wavelength.
Gene expression analysis
RNA was isolated using TRIzol reagent. RNA from
triplicates was pooled into one sample. The concentration of RNA
was measured using BioDrop DUO spectrophotometer (Biodrop,
Cambridge, UK). RNA was transcribed into CDNA using transcription
First Strand CDNA Synthesis kit (Roche) according to the
manufacturer's instructions. We undertook a two-way approach to
RT-qPCR. Firstly, we performed standard RT-qPCR for genes involved
in cell cycle and proliferation regulation (CCND1,
CCNE1 and MKI67), as well as adhesion (CDH1).
The PCR conditions were designed to omit the unspecific products.
Each reaction was performed in four replicates. Housekeeping genes
(RPS17, RPLP0 and H3F3A) were used as internal
normalization controls and reference RNA (Stratagene, Agilent
Technologies, Inc., Santa Clara, CA, USA) as reference samples. The
relative gene expression was calculated according to the Roche
algorithm. Subsequently, we used TaqMan real-time
microarrays-RealTime ready Custom Panel (Roche). For this assay,
the reaction mix was prepared using LightCycler® 480
Probes Master kit (10 ng of cDNA/well) according to the
manufacturer's instructions. The mix was added to RealTime ready
Custom Panel with pre-plated genes: BAD, TGFB,
SNAI1, ZEB2, ZEB1, VIM and CD44.
The reaction was carried out using Light Cycler® 480 II
(Roche) according to the manufacturer's instructions. The results
from RT-qPCR microarrays are presented as fold change (compared to
the control untreated cells). The results from standard RT-qPCR are
presented as the mean mRNA expression level.
Statistical analysis
All data are presented as mean ± SE and were
analysed with GraphPad Prism 5 (GraphPad Software, Inc., La Jolla,
USA). Student's t-test was used to compare two groups; the ANOVA
test was used to compare differences among multiple groups followed
by Bonferroni's multiple comparison tests as applicable. P<0.05
was considered statistically significant.
Results
Ang II regulates the proliferation of
endometrial cancer cells
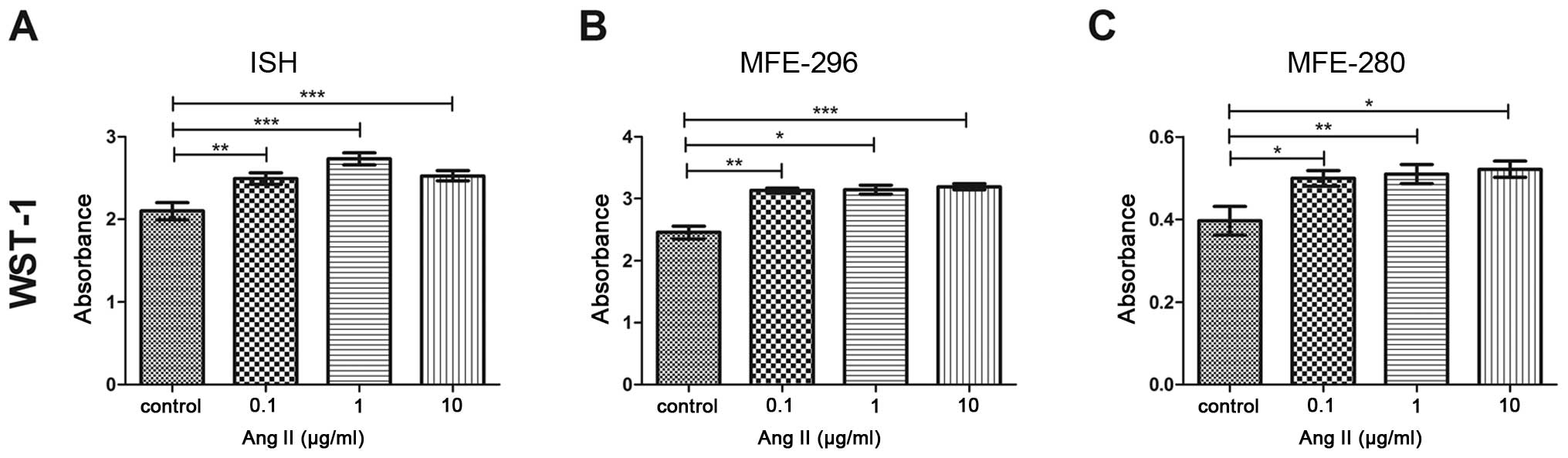
We first investigated the effect of Ang II on the
proliferation of endometrial cancer cells. After a 72-h incubation
with Ang II, the proliferative capacity was altered in all tested
cell lines in a dose-dependent manner (Fig. 1). The highest increase in
proliferation in comparison to the control was observed in the
MFE-280 (0.52±0.02) (Fig. 1C) and
MFE-296 cells (3.2±0.05) (Fig. 1B)
following treatment with 10 µg/ml of Ang II. In the case of
the ISH cell line, the highest increase was noted in cells treated
with 1 µg/ml of Ang II (2.7±0.07) (Fig. 1A).
Anti-apoptotic activity of Ang II
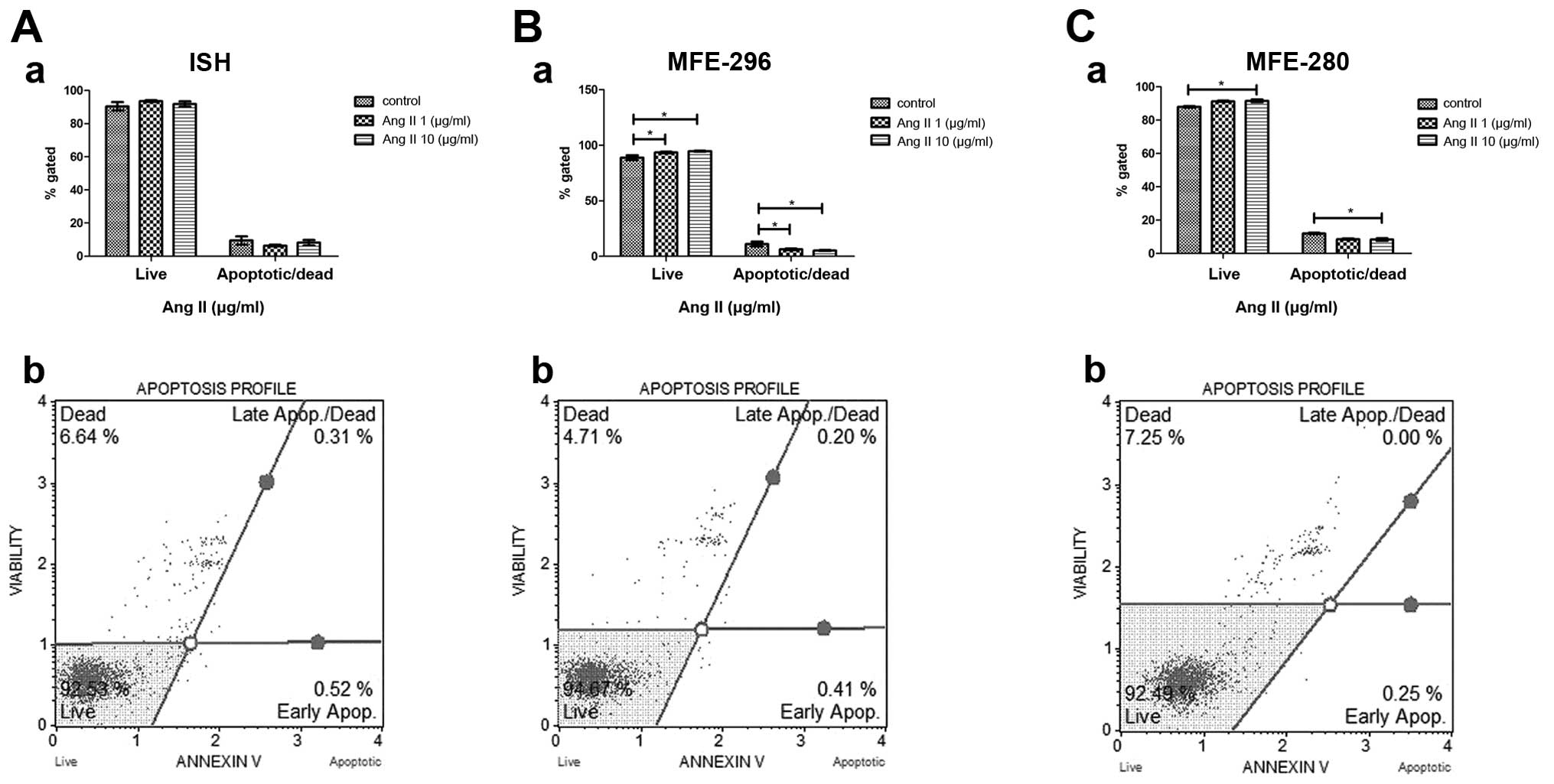
In order to verify the possible anti-apoptotic
activity of Ang II, we performed the Annexin V assay on endometrial
cancer cells treated with Ang II for 72 h. In all endometrial
adenocarcinoma cell lines Ang II treatment decreased the percentage
of apoptotic/dead cells in comparison to the control (untreated
cells). In the case of cells derived from poorly and moderately
differentiated human endometrial adenocarcinoma, the number of
apoptotic/dead cells decreased proportionally to the Ang II dose.
In the untreated MFE-280 cells, 12% of the population was
characterized as apoptotic/dead cells, while after incubation with
1 µg/ml of Ang II the percentage of apoptotic cells amounted
to 8.7% and the dose of 10 µg/ml decreased the percentage of
apoptotic cells to 8.3% (Fig.
2C–a). In the control MFE-296 cells, the percentage of
apoptotic cells amounted to 11%; whereas after treatment with 1
µg/ml of Ang II this percentage decreased to 6.4%, and the
highest dose of 10 µg/ml resulted in the most pronounced
decrease in the percentage of apoptotic cells, 5.3% (Fig. 2B–a). ISH cells, which represented
the well-differentiated adenocarcinoma model, also responded to Ang
II treatment by the reduction of apoptotic cell percentage from the
control value of 9.5 to 6.4 and 8.2% after a 72 h-incubation with 1
µg/ml and 10 µg/ml of Ang II, respectively (Fig. 2A–a).
Ang II regulates the cell cycle of
endometrial cancer cells
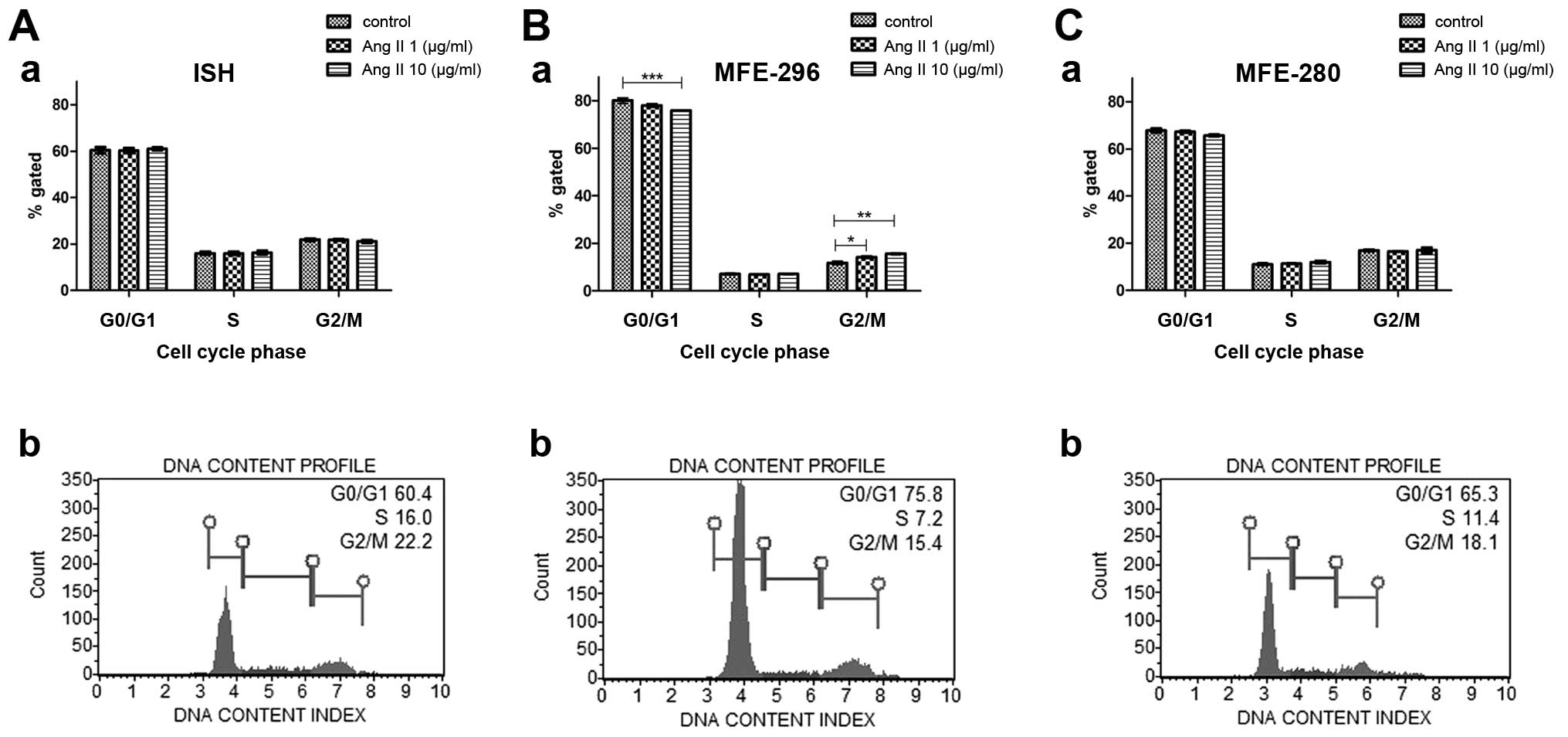
To verify the role of Ang II in the regulation of
the cell cycle, we performed flow cytometric analysis of the
percentage of cells in different stages of the cell cycle under
control conditions and after a 72-h treatment with Ang II (1 or 10
µg/ml). In most cases, incubation with Ang II did not cause
any significant changes in cell cycle progression in comparison to
the control conditions. However, in the MFE-296 cells, Ang II
induced transition from the G0/G1 to the G2/M phase (Fig. 3B-b). For this cell line, the
percentage of cells in the G0/G1 phase was statistically
significantly lower (75.85±0.06%) after treatment with 10
µg/ml of Ang II in comparison to the control (80.05±1.1%);
whereas the percentage of cells in G2/M phase was significantly
higher after treatment with 1 µg/ml Ang II (14.22±0.55%) and
10 µg/ml Ang II (15.6±0.32%) when compared with control
conditions (11.77±0.68%) (Fig.
3).
Ang II modulates endometrial cancer cell
migration and adhesion
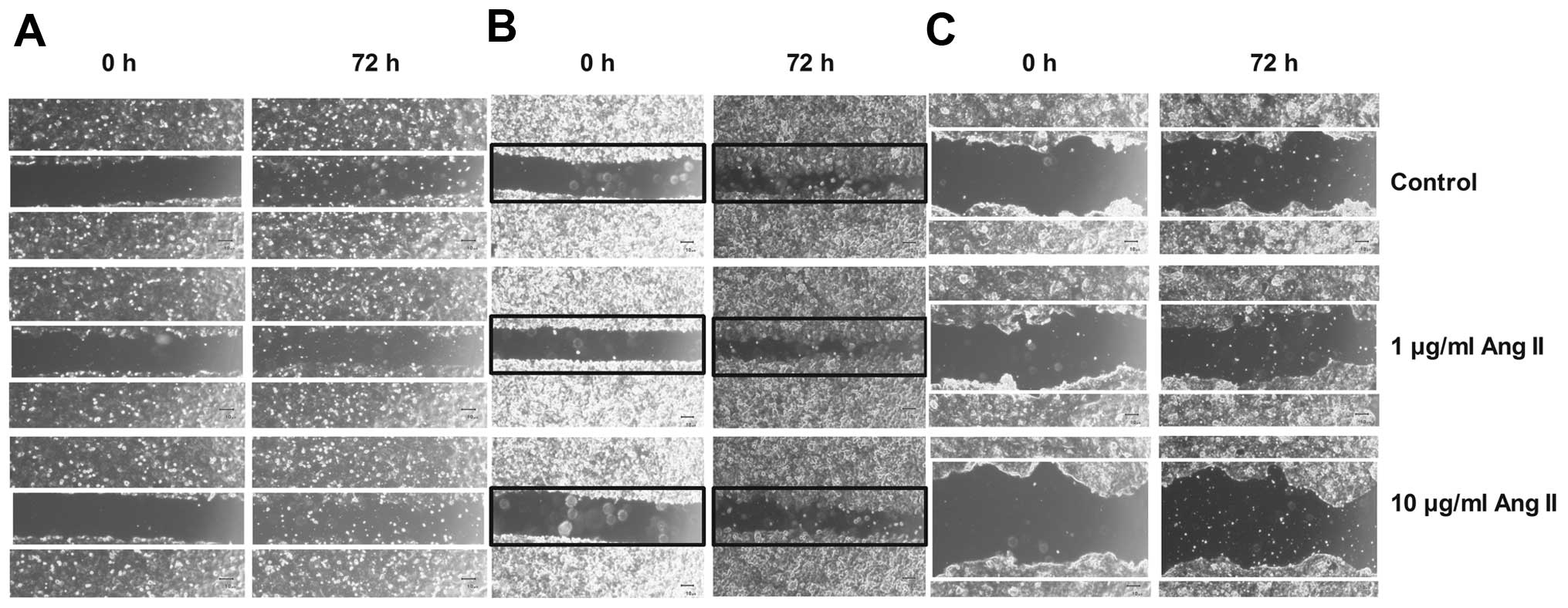
Our previous results as well as data presented by
others indicate that Ang II modulates cell mobility in breast
cancer cells (10,11). Thus, we performed a monolayer cell
migration assay to investigate the influence of Ang II on migration
of endometrial cancer cell lines. In the case of moderately and
poorly differentiated human endometrial adenocarcinoma cell lines
(MFE-296 and MFE-280, respectively), there was a strong tendency
towards enhancement of cell migration ability after treatment with
10 µg/ml Ang II (MFE-296, 6±2.36%; MFE-280, 3.6±0.88%) in
comparison to the untreated cells (control, 100% of wound surface)
(Fig. 4B and C). In the case of the
well-differentiated adenocarcinoma ISH cell line, high migration
potential was also observed in cells treated with 1 µg/ml
Ang II (3.6±6.96%) in comparison to the control (100%) (Fig. 4A).
The next step was to investigate the alterations in
the ability of endometrial adenocarcinoma cells to adhere to
various matrix proteins in the presence of Ang II. Poorly
differentiated human endometrial adenocarcinoma MFE-280 cells
exhibited reduced adhesion ability to matrix proteins after 72 h of
Ang II treatment. After exposure to 10 µg/ml of Ang II a
statistically significant reduction in adhesion to fibronectin
(−32.22±1.73%), collagen IV (−28.02±1.33%) and collagen I
(−27.4±3.98%) was observed in comparison to the control cells
(control, 100%) (Fig. 5). In
contrast in the MFE-296 cell line, representing the moderately
differentiated model of endometrial adenocarcinoma, no
statistically significant changes in adhesion to all tested matrix
proteins was noted. In the case of the well-differentiated
adenocarcinoma ISH cells, a statistically significant influence of
Ang II on cell adhesion was observed. However, the Ang II-induced
effect was divergent to that observed for the MFE-280 cell line.
The ISH cells treated with 10 µg/ml of Ang II showed
enhanced adhesion to fibronectin (+43.3±2.62%), collagen IV
(+35±7.24%), collagen I (+54.4%±3.46) and laminin (+11.3%±1.13) in
comparison to the untreated cells (control, 100%) (Fig. 5).
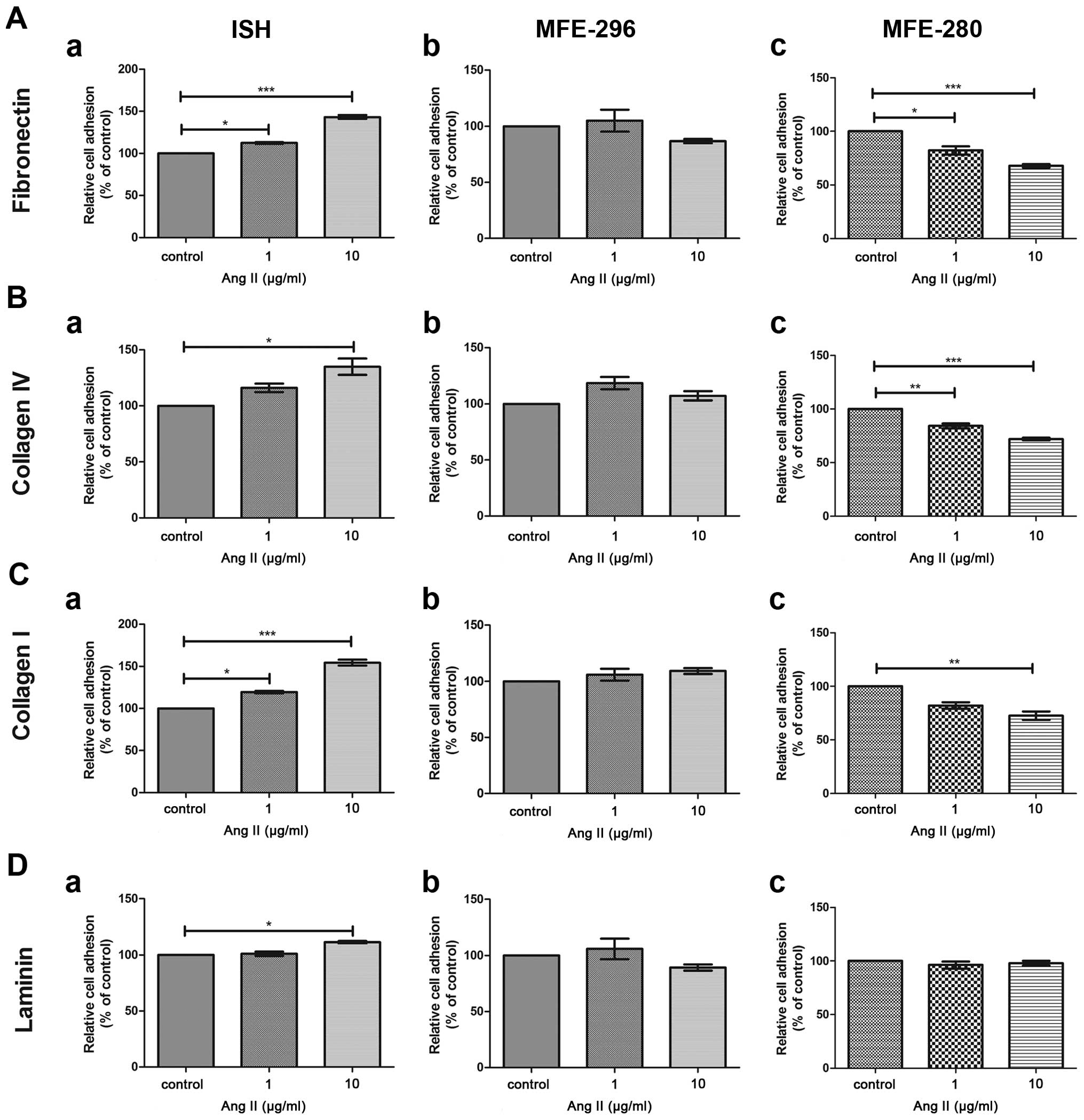 | Figure 5Adhesion to ECM proteins: (A)
fibronectin, (B) collagen IV, (C) collagen I, (d) laminin of the
endometrial cancer cell lines: (A-a, B-a, C-a and d-a) ISH, (A-b,
B-b, C-b, d-b) MFE-296 and (A-c, B-c, C-c, d-c) MFE-280 after
treatment with Ang II (1 or 10 µg/ml) in comparison to
control, untreated cells (100%). *p<0.05,
**p<0.01 and ***p<0.001 compared to the
control. |
Association between Ang II biological
activity and changes in gene expression
Next, we verified the association between the
observed changes in cellular processes induced by Ang II and the
potential changes in the expression of genes involved in the
regulation of cell cycle progression, proliferation, motility and
programmed cell death. For this analysis we chose the following
genes connected with cell cycle regulation: CCND1 and
CCNE1, proliferation: MKI67, apoptosis: BAD,
cell adhesion: CDH1, motility and induction of epithelial to
mesenchymal transition (EMT): TGFβ, SNAI1,
VIM, ZEB1 and ZEB2.
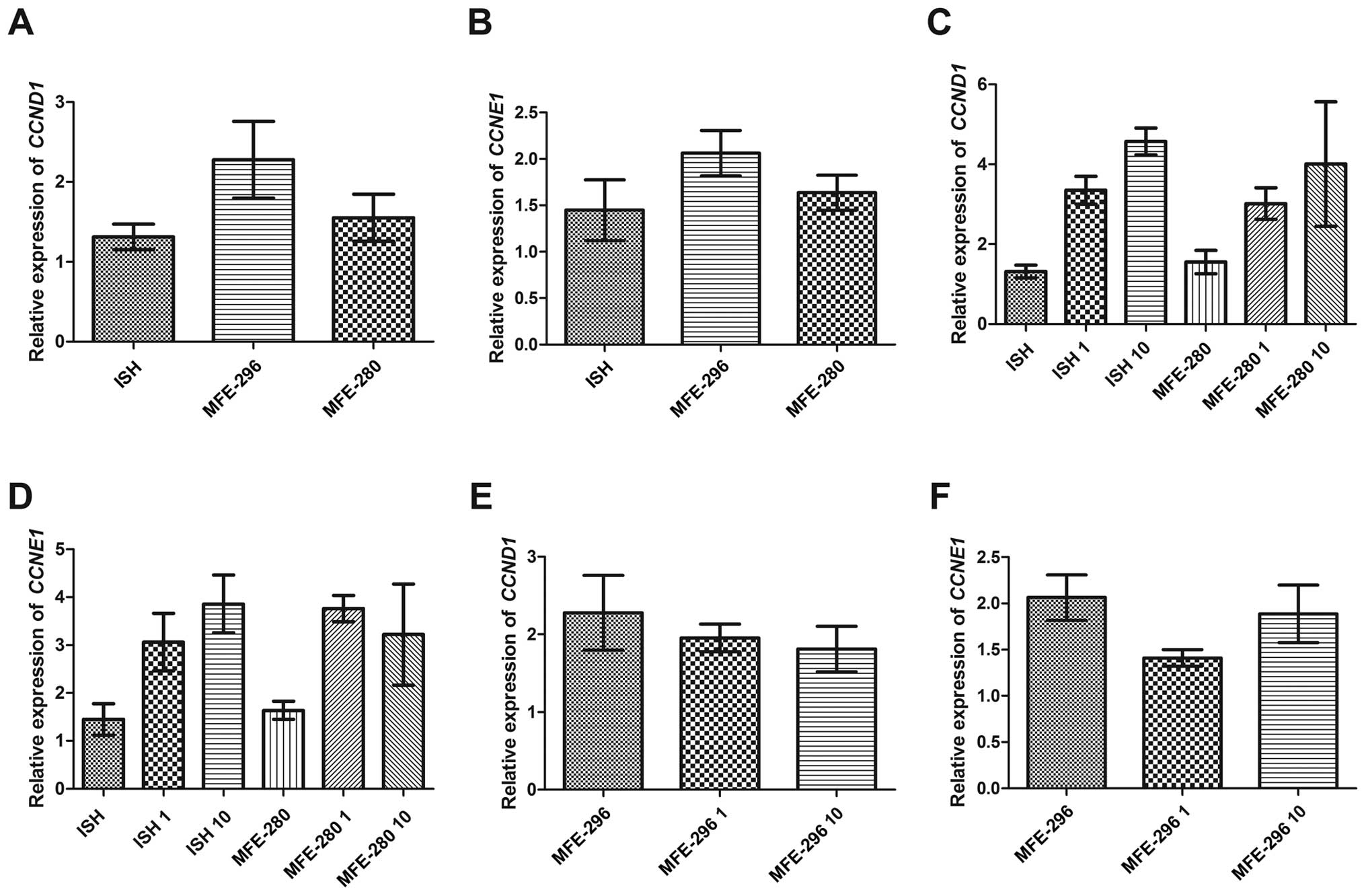
Since we noted that Ang II increased the
proliferative activity of the investigated cell lines, we firstly
focused on the expression of genes related to cell cycle
regulation. The basal level of mRNA expression in the case of both
cyclins i.e., CCND1 and CCNE1 was the lowest in the
well-differentiated endometrial adenocarcinoma cell line (1.3±0.16
and 1.4±0.33 for CCND1, CCNE1, respectively) (Fig. 6A and B) and higher in the two other
cancer cell lines, characterized by lower status of differentiation
(Fig. 6A and B). However, when
comparing the moderately and poorly differentiated cell lines, we
noted that the MFE-296 cells had higher basal mRNA expression of
both cyclins than the MFE-280 cells (2.3±0.48 and 2.1±0.24 for
CCND1 and CCNE1 in MFE-296, respectively vs. 1.6±0.3/±0.19 for both
cyclins in MFE-280) (Fig. 6A and
B). Furthermore, Ang II increased the expression of both
cyclins in a dose-dependent manner only in the well- and poorly
differentiated cell lines. The post-Ang II treatment expression of
CCND1 in these cell lines was as follows: 3.4±0.35 and
3.0±0.4 for ISH and MFE-280 cells, respectively, treated with 1
µg/ml of Ang II, and 4.6±0.33 and 4.0±1.6 for ISH and
MFE-280 cell lines incubated with 10 µg/ml of Ang II
(Fig. 6C). The mRNA level of
CCNE1 was 3.1±0.6 and 3.8±0.27 for ISH and MFE-280 cells,
respectively, after treatment with 1 µg/ml of Ang II, and
3.9±0.6 and 3.2±1.1 for ISH and MFE-280 cells, respectively,
treated with 10 µg/ml of Ang II (Fig. 6D).
In the case of moderately differentiated MFE-296
cells Ang II caused downregulation of mRNA expression of both
cyclins in a dose-dependent manner. The values of CCND1
expression were 2.0±0.18 and 1.8±0.29, respectively for 1
µg/ml and 10 µg/ml of Ang II (Fig. 6E). A similar tendency was observed
in the case of CCNE1, although a slight increase in mRNA
expression was higher after the 10 µg/ml Ang II dose than in
the presence of 1 µg/ml (1.9±0.31 and 1.4±0.09,
respectively) (Fig. 6F). This
result is quite surprising as the BrdU and WST assays showed
increased values after Ang II treatment; however, the proliferation
potential was not only regulated by cyclins but also by the main
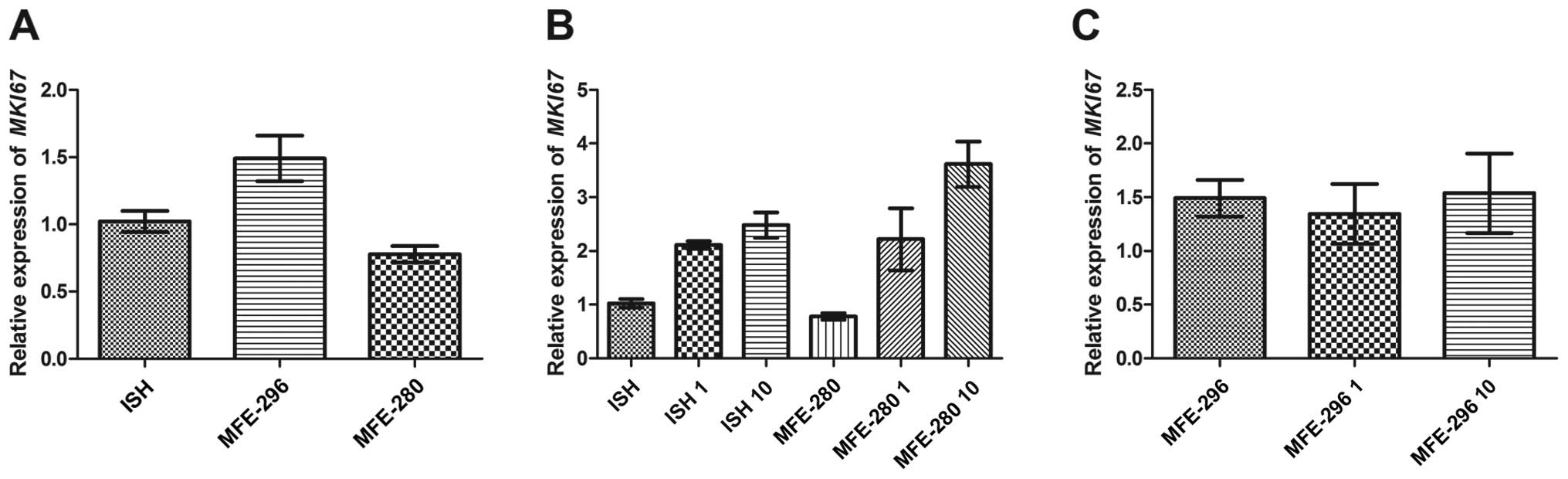
proliferation marker i.e., MKI67.
The basal expression of MKI67 was the highest
in the moderately differentiated human endometrial adenocarcinoma
MFE-296 cells (1.5±0.17) and the lowest in the poorly
differentiated MFE-280 cell line (0.78±0.06) (Fig. 7A). However, in the case of well- and
poorly differentiated cancer cells, the MKI67 mRNA
expression was upregulated by Ang II in a dose-dependent manner and
reached values of 2.1±0.071 and 2.2±0.58, respectively for the ISH
and MFE-280 cells treated with 1 µg/ml of Ang II and
2.5±0.24 and 3.6±0.42, respectively for 10 µg/ml of Ang II
(Fig. 7B). In the moderately
differentiated MFE-296 cells, the changes in MKI67
expression were similar to those observed in the case of cyclin E1
(CCNE1). MKI67 expression was higher after incubation
with 10 µg/ml of Ang II than after treatment with the 1
µg/ml dose (1.5±0.37 and 1.3±0.28, respectively) (Fig. 7C). The observed changes in
expression of proliferation-related genes appear to be consistent
with the results of the biological assays, but it is worth noting
that the most pronounced effect of Ang II treatment was observed
for cancer cell lines showing opposite status of differentiation,
i.e., well- and poorly differentiated cell lines: ISH and MFE-280,
respectively. This may suggest that Ang II exerts the highest
stimulatory actions in cells at the beginning and in the last steps
of cancer transformation.
As all treated cell lines responded to Ang II in
terms of reduced apoptosis, increased motility and adhesion in a
dose-dependent manner, we also evaluated the changes in expression
of genes connected with these cellular processes.
First of all, we noted a slight reduction in the
expression of proapoptotic gene BAD after treatment with Ang
II, and this change was dose-dependent (data not shown). In all
investigated cell lines, the highest decrease in BAD
expression was observed for 10 µg/ml of Ang II, even though
in the biological assays the best results for ISH cells were
obtained for lower Ang II dose. Nevertheless, the changes in
BAD expression may partially explain the observed reduction
in apoptosis obtained in all experimental conditions.
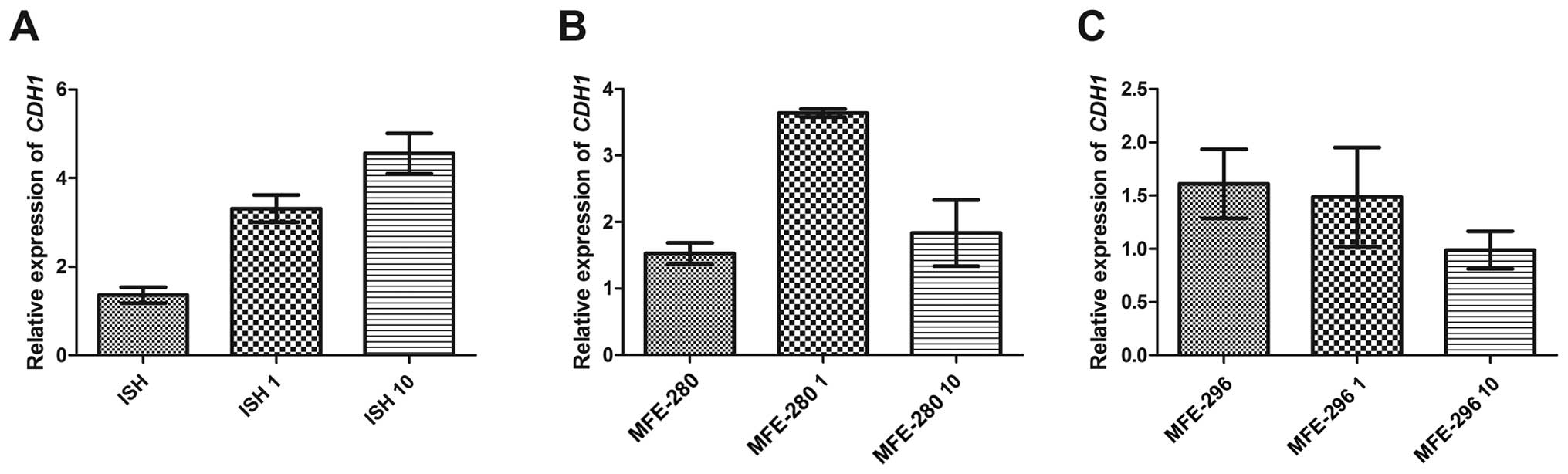
The changes in motility and adhesion induced by Ang
II were also reflected by the changes in expression of genes
associated with these processes. First of all, we noted that the
relative expression of the main epithelial marker CDH1
varied among the investigated cell lines. In well differentiated
ISH cells, the basic CDH1 expression level in control cells
was 1.4 and increased after Ang II treatment in a dose-dependent
manner, i.e., 3.3 for 1 µg/ml and 4.6 for 10 µg/ml of
Ang II (Fig. 8A). In contrast, in
the poorly differentiated MFE-280 cells we observed a higher
increase in CDH1 relative expression (from the basic level
of 1.5 to 3.6) after incubation with 1 µg/ml of Ang II, but
after treatment with the dose of 10 µg/ml the level changed
significantly (CDH1 expression level of 1.8) (Fig. 8B). In the moderately differentiated
cell line MFE-296, both Ang II doses caused downregulation of
CDH1 expression (basic level 1.6, 1.5 after 1 µg/ml
of Ang II and 1.0 after 10 µg/ml of Ang II) (Fig. 8C).
Moreover, we also observed contradictory effects of
Ang II treatment on expression of genes connected with the EMT
process in two cell lines representing two opposite phenotypes:
well- and poorly differentiated (data not shown). Taq-Man real time
microarrays revealed that in the ISH cell line there was a slight
reduction in TGFβ expression after Ang II treatment. The
same situation was observed for SNAI1 and ZEB2 genes,
whose downregulation was stronger after incubation with 10
µg/ml of Ang II (1.2-fold decrease and no detected
expression for SNAI1 and ZEB2, respectively) (data
not shown). On the other hand, there was no change in expression of
the main mesenchymal marker i.e., VIM, and a 1.3-fold
decrease in the level of CD44 mRNA, which is also a known
EMT-related mesenchymal marker. In contrast, in the poorly
differentiated MFE-280 cells, we observed adverse changes in gene
expression to those seen in the ISH cell line. We noted a
dose-dependent upregulation of TGFβ after Ang II treatment.
The higher expression was observed for the 10 µg/ml Ang II
dose (a 2.3-fold change increase in comparison to the control; a
2-fold increase for the lower experimental dose). Moreover, Ang II
at a dose of 10 µg/ml also elevated the expression of other
EMT markers, i.e., a 1.3-fold change for SNAI1, a 1.1-fold
increase for ZEB1, and induction of expression for ZEB2.
VIM and CD44 expression was also higher than in the
control samples (a 1.3- and 1.1-fold increase for VIM and
CD44, respectively). The moderately differentiated MFE-296
cells showed decreased expression of TGFβ, SNAI1,
VIM, ZEB1 and ZEB2 (fold change of -1.1, -1.6,
-1.2, -1.7, -1.1 for listed genes, respectively) also after
treatment with 10 µg/ml; whereas the expression of Cd44 was
not influenced by Ang II treatment.
Discussion
Previous studies have shown that the local
renin-angiotensin system (RAS) has higher activity in various
malignant tumour tissues. Moreover, our previous results and data
in other studies confirmed that Ang II promotes tumour cell
proliferation, migration, and has anti-apoptotic potential
(4,5,9,10,12,13).
In terms of endometrial cancer, our research demonstrated a
correlation between the expression of angiotensin and its receptors
and the clinicopathological characteristics of primary endometrial
adenocarcinoma (2).
In the present in vitro study on three
endometrial adenocarcinoma cell lines representing three stages of
endometrial cancer, we performed various biological assays in order
to further investigate the specific effects of Ang II on tumour
cell behaviour. Firstly, we demonstrated that Ang II influenced the
proliferation of well- (ISH), moderately (MFE-296) and poorly
differentiated (MFE-280) human endometrial adenocarcinoma cell
lines. This response to Ang II was dose-dependent and was
previously observed in other cancer models (4,9,13,14).
Thus, we confirmed that Ang II promotes cell proliferation also in
endometrial cancer. Additionally, in this study we noted that the
cellular response to Ang II may be associated with cancer cell
differentiation status. In the case of moderately and poorly
differentiated cells, the most pronounced response was observed in
the dose of 10 µg/ml of Ang II; whereas the
well-differentiated ISH cancer cell line showed a significant
response in the presence of the lower dose of Ang II (1
µg/ml). This may indicate that at the beginning of cancer
cell transformation Ang II may induce abnormal proliferation at
lower doses than at the later stages, when the cancer cells have
more genomic, transcriptomic and proteomic changes and loose the
differentiated phenotype. This hypothesis is also based on the
results of real-time PCR analysis, which showed that the
dose-dependent augmentation of cell proliferation was connected
with increased MKI67, CCND1 and CCNE1
expression in the well- and poorly differentiated cancer cells.
All in all, our present observations, as well as
results from previous studies conducted on EC tumour samples,
indicate a specific link between angiotensin effectiveness and cell
differentiation status (2). Our
observations are consistent with data presented by Shan et
al, showing that Ang II is capable of promoting endometrial
epithelium cell proliferation (6).
Similar results on the effect of Ang II on cell proliferation and
survival were obtained for breast cancer and prostate cancer
(12,13). Moreover, in pancreatic cancer cells,
blockage of Ang II type 1 receptor resulted in growth suppression
(15). A study by kinoshita et
al (16) also demonstrated that
Ang II stimulated gastric cancer cell proliferation (the AT1
receptor-positive OCUM2MD3 cell line) and this proliferative effect
of Ang II was mediated by the AT1 receptor.
Next we investigated the influence of Ang II on
inhibition of programmed cell death (PCD) - apoptosis. Apoptosis is
an evolutionarily conserved process that plays an important role in
tissue homeostasis (17).
Nevertheless, in some conditions, such as cancer, cells lose their
ability to undergo induced PCD leading to uncontrolled
proliferation (17). Recent studies
have shown that excessive proliferation is connected with
inhibition of apoptosis. Herein, we showed that the percentage of
apoptotic/dead cells decreased after treatment with Ang II in
comparison to the untreated cells. In the case of moderately and
poorly differentiated human endometrial adenocarcinoma cells, the
smallest percentage of apoptotic/dead cells was noted after
treatment with 10 µg/ml dose of Ang II; whereas, in the
well-differentiated endometrial adenocarcinoma ISH cell line this
percentage was decreased after incubation with 1 µg/ml of
Ang II. The observed dose-dependent influence of Ang II on
apoptosis further confirms our hypothesis on the connection between
Ang II and the differentiation status of endometrial cancer cells.
Our results are in agreement with a study of Zhao et al who
demonstrated that Ang II significantly prevented apoptosis in human
breast cancer cells (MCF-7) and Ang II type 1 receptor was
responsible for these effects (18). Moreover, the inhibitory effect of
Ang II on apoptosis was also described in endothelial progenitor
cells (7).
Aside from the biological assays measuring the
changes in cell cycle, proliferation and apoptosis, we also
analysed the expression of the main genes connected with the
aforementioned processes. Three genes regulating cell cycle
progression: CCND1, CCNE1 and MKI67 were
analysed as markers of cell proliferation. CCND1, encoding
cyclin D1 is responsible for the growth factor-induced transition
from the G0 into the G1 phase (19); whereas CCNE1, whose
expression occurs in the late G1 phase, plays a pivotal role in
G1/S transition (19). Zapiecki
et al showed that CCND1 expression does not correlate
with the number of cells in the S+G2M phase but cyclin E expression
was directly correlated with an increased population of cells in
the S+G2M phase (20). Our study
revealed that expression of both cyclins only in the case of well-
and poorly differentiated human endometrial adenocarcinoma cells
progressively increased after Ang II treatment. This tendency was
also observed in the case of MKI67 mRNA expression. In terms
of the role of the aforementioned genes in endometrial cancer,
recently Zapiecki et al showed that significantly higher
expression of cyclin D1 and E was detected in patients dying from
endometrial cancer. Moreover, Shevra et al (21) observed increased expression of CCND1
and MKI67 in patients with endometrial carcinoma in relation to
proliferative endometrium and simple hyperplasia, and they showed
that CCND1 expression had a positive correlation with MKI67
expression. These findings are in agreement with our results,
demonstrating that CCND1, CCNE1 and MKI67
increased in parallel with the loss of differentiation of
endometrial adenocarcinoma.
The process of cancerogenesis not only changes the
proliferation potential and apoptosis, but also influences the cell
mobility and adhesion. Both processes are important as they
contribute to cancer progression and participate in the detachment
of cancer cells from the primary origin leading to metastasis
(22,23). EMT is closely connected with
metastasis. During our research we observed increased
mobility/migration and changes in adhesion potential after Ang II
treatment, as well as changes in the expression of EMT-related
genes: CDH1, TGF-β, VIM, CD44,
SNAI1, ZEB1 and ZEB2 (data not shown).
Furthermore, we previously showed that Ang II modulates EMT in
prostate cancer as well as noncancerous cell lines (9,10),
while other research groups demonstrated this effect using other
cell models (11,24–27).
Results from biological assays testing the mobility and adhesion
processes in endometrial adenocarcinoma cells after Ang II
treatment did not give us a clear answer regarding the role of
angiotensin in the modulation of EMT progression, adhesion or
motility. We observed an opposite response of well- vs. Poorly
differentiated cell lines. Gene expression analysis gave additional
information on Ang II activity, which clearly depended on the
differentiation status of these cancer cells. CDH1 was among
the analysed genes showing changes in expression after Ang II
treatment. CDH1 is regarded as the main epithelial marker and loss
of its expression is tightly connected with EMT. We noted that the
relative expression of CDH1 varied between cell lines. In
the ISH cells, incubation with Ang II, administered at a dose of 10
µg/ml, resulted in an increase in CDH1 expression.
The same type of response in poorly differentiated MFE-280 cells
was observed after treatment with the lower dose of Ang II, whereas
the higher one decreased CDH1 expression. Similar tendencies
were noted for other analysed genes tightly connected with CDH1
regulation and acquisition of mesenchymal phenotype Ang II
treatment caused downregulation of TGF-β expression in ISH
cells, but in the MFE-280 cell line Ang II increased the expression
of this gene. TGF-β has a dual role in the process of cancer
formation. In the early stages of neoplastic transformation it
plays a suppressive role; however in later stages of cancer
progression it becomes a tumour promoter. TGF-β has been shown to
regulate cell proliferation, angiogenesis, metastasis, and is an
inducer of EMT (28,29). It has been demonstrated that TGFβ1
synthesis can be induced by Ang II, while blockade of the RAS
results in a decrease in TGFβ1-induced production of matrix
proteins (30). In our endometrial
cancer model this dual modulation of TGF-β by Ang II was confirmed
in both biological assays, as well as analysis of expression of
EMT-related genes. In the case of the ISH cells, even though there
was an increase in cell mobility and adhesion to particular ECM
proteins, this cell line did not express the genes characteristic
of the mesenchymal phenotype. All tested EMT markers were
downregulated with accompanied increase of CDH1 expression.
This observation could explain the increased adhesion, as the cells
were more epithelial than mesenchymal in phenotype and behaviour.
In contrast, the poorly differentiated endometrial cancer cell line
MFE-280 after angiotensin treatment acquired a mesenchymal
phenotype, which was characterized by induced expression of
EMT-related genes, including VIM, CD44, SNAI1,
ZEB1 and ZEB2 (data not shown). Changes in expression
of these genes coincided with increased mobility and decreased
adhesion of MFE-280 cells treated with Ang II. The decreased
adhesion was quite surprising, but it may have resulted from
acquisition of a strong mesenchymal phenotype, in which cells
detaching from the basal membrane do not undergo cell death but
survive these stressful conditions. Ang II-induced EMT observed in
our study was also described by other research groups (6,30).
Shan et al recently showed that Ang II promotes activation
of endometrial epithelium cells and significantly decreases the
expression of E-cadherin, increasing the expression of SMA
(6). Furthermore, Ang II together
with TGF-β1 was shown to play a central role in initiating EMT in
in vitro models of metastatic tumour development (6). This interaction between Ang II and
TGF-β1 in the progression of EMT was also confirmed in other in
vitro and in vivo models (31–33).
Our study demonstrated that only in poorly
differentiated endometrial cancer cells was the expression of
TGF-β1 directly associated with Ang II treatment in a
dose-dependent manner. Moreover, upregulation of TGF-β1 mRNA
was connected with increased SNAI1 expression. On the other
hand, we showed that there is a connection between the basal level
of cell proliferation and TGF-β mRNA expression. Loss of
TGF-β-induced growth inhibition has been associated with disruption
and/or dysregulation of the TGF-β signalling pathway, which may
facilitate invasion, metastasis, and angiogenesis (29). In conclusion, our study revealed
that Ang II influences endometrial cancer cells in terms of
cancer-related processes, i.e., increased proliferation, reduction
of apoptosis, increased mobility and modulation in adhesion
potential. However, its effect and effectiveness seems to be highly
associated with the differentiation status of the cancer cells. Ang
II appears to play a crucial role in the early and late stages of
malignant transformation.
Acknowledgments
This study was supported by the National Science
Centre grant no. UMO-2013/09/B/NZ4/01365.
References
|
1
|
Wojciechowska U and Didkowska J: Illness
and deaths from malignant tumors in Poland. National Cancer
Registry, Cancer Centre. Institute for them. Maria Sklodowska -
Curie. ISSN: 0867-8251http://onkologia.org.pl/wp-content/uploads/BIUL2013.pdf.
|
|
2
|
Piastowska-Ciesielska AW, Płuciennik E,
Wójcik-Krowiranda K, Bieńkiewicz A, Bednarek A and Ochędalski T:
Analysis of the expression of angiotensin II type 1 receptor and
VEGF in endometrial adenocarcinoma with different
clinicopathological characteristics. Tumour Biol. 33:767–774. 2012.
View Article : Google Scholar
|
|
3
|
Matysiak ZE, Ochędalski T and
Piastowska-Ciesielska AW: The evaluation of involvement of
angiotensin II, its receptors, and androgen receptor in endometrial
cancer. Gynecol Endocrinol. 31:1–6. 2015. View Article : Google Scholar
|
|
4
|
Rodrigues-Ferreira S, Abdelkarim M,
Dillenburg-Pilla P, Luissint AC, di-Tommaso A, Deshayes F, Pontes
CL, Molina A, Cagnard N, Letourneur F, et al: Angiotensin II
facilitates breast cancer cell migration and metastasis. PloS One.
7:e356672012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Deshayes F and Nahmias C: Angiotensin
receptors: A new role in cancer? Trends Endocrinol Metab.
16:293–299. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shan T, Zhang L, Zhao C, Chen W, Zhang Y
and Li G: Angiotensin-(1–7) and angiotensin II induce the
transdifferentiation of human endometrial epithelial cells in
vitro. Mol Med Rep. 9:2180–2186. 2014.PubMed/NCBI
|
|
7
|
Yin T, Ma X, Zhao L, Cheng K and Wang H:
Angiotensin II promotes NO production, inhibits apoptosis and
enhances adhesion potential of bone marrow-derived endothelial
progenitor cells. Cell Res. 18:792–799. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tokinaga Y, Kimoto Y, Ogawa K, Mizumoto K,
Tange K and Hatano Y: Reduction of adhesion formation by an
angiotensin type 1 receptor antagonist. Langenbecks Arch Surg.
396:127–132. 2011. View Article : Google Scholar
|
|
9
|
Domińska K, Piastowska-Ciesielska AW,
Lachowicz-Ochędalska A and Ochędalski T: Similarities and
differences between effects of angiotensin III and angiotensin II
on human prostate cancer cell migration and proliferation.
Peptides. 37:200–206. 2012. View Article : Google Scholar
|
|
10
|
Piastowska-Ciesielska AW, Domińska K,
Nowakowska M, Gajewska M, Gajos-Michniewicz A and Ochędalski T:
Angiotensin modulates human mammary epithelial cell motility. J
Renin Angiotensin Aldosterone Syst. 15:419–429. 2014. View Article : Google Scholar
|
|
11
|
Zhao Y, Wang H, Li X, Cao M, Lu H, Meng Q,
Pang H, Li H, Nadolny C, Dong X, et al: Ang II-AT1R increases cell
migration through PI3K/AKT and NF-κB pathways in breast cancer. J
Cell Physiol. 229:1855–1862. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhao Y, Chen X, Cai L, Yang Y, Sui G and
Fu S: Angiotensin II/angiotensin II type I receptor (AT1R)
signaling promotes MCF-7 breast cancer cells survival via
PI3-kinase/Akt pathway. J Cell Physiol. 225:168–173. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Uemura H, Ishiguro H, Nakaigawa N,
Nagashima Y, Miyoshi Y, Fujinami K, Sakaguchi A and Kubota Y:
Angiotensin II receptor blocker shows antiproliferative activity in
prostate cancer cells: A possibility of tyrosine kinase inhibitor
of growth factor. Mol Cancer Ther. 2:1139–1147. 2003.PubMed/NCBI
|
|
14
|
De Paepe B, Verstraeten VM, De Potter CR
and Bullock GR: Increased angiotensin II type-2 receptor density in
hyperplasia, DCIS and invasive carcinoma of the breast is
paralleled with increased iNOS expression. Histochem Cell Biol.
117:13–19. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Guo R, Gu J, Zhang Z, Wang Y and Gu C:
MicroRNA-410 functions as a tumor suppressor by targeting
angiotensin II type 1 receptor in pancreatic cancer. IUBMB life.
67:42–53. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kinoshita J, Fushida S, Harada S, Yagi Y,
Fujita H, Kinami S, Ninomiya I, Fujimura T, Kayahara M, Yashiro M,
et al: Local angiotensin II-generation in human gastric cancer:
Correlation with tumor progression through the activation of
ERk1/2, NF-κB and survivin. Int J Oncol. 34:1573–1582. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mohammad RM, Muqbil I, Lowe L, Yedjou C,
Hsu HY, Lin LT, Siegelin MD, Fimognari C, Kumar NB, Dou QP, et al:
Broad targeting of resistance to apoptosis in cancer. Semin Cancer
Biol. 35(Suppl): S78–S103. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhao Y, Chen X, Cai L, Yang Y, Sui G and
Wu J: Angiotensin II suppresses adriamycin-induced apoptosis
through activation of phosphatidylinositol 3-kinase/Akt signaling
in human breast cancer cells. Acta Biochim Biophys Sin (Shanghai).
40:304–310. 2008. View Article : Google Scholar
|
|
19
|
Wolf G and Wenzel UO: Angiotensin II and
cell cycle regulation. Hypertension. 43:693–698. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zapiecki K, Manahan KJ, Miller GA and
Geisler JP: Cyclin E is overexpressed by clear cell carcinomas of
the endometrium and is a prognostic indicator of survival. Eur J
Gynaecol Oncol. 36:114–116. 2015.PubMed/NCBI
|
|
21
|
Shevra CR, Ghosh A and Kumar M: Cyclin D1
and KI-67 expression in normal, hyperplastic and neoplastic
endometrium. J Postgrad Med. 61:15–20. 2015. View Article : Google Scholar
|
|
22
|
Okegawa T, Pong RC, Li Y and Hsieh JT: The
role of cell adhesion molecule in cancer progression and its
application in cancer therapy. Acta Biochim Pol. 51:445–457.
2004.PubMed/NCBI
|
|
23
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Takeda H, Katagata Y, Hozumi Y and Kondo
S: Effects of angiotensin II receptor signaling during skin wound
healing. Am J Pathol. 165:1653–1662. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sakai H, Matsuura K, Tanaka Y, Honda T,
Nishida T and Inui M: Signaling mechanism underlying the promotion
of keratinocyte migration by angiotensin II. Mol Pharmacol.
87:277–285. 2015. View Article : Google Scholar
|
|
26
|
Liu Y, Tian X, Cui M and Zhao S: Safflower
yellow inhibits angiotensin II-induced adventitial fibroblast
proliferation and migration. J Pharmacol Sci. 126:107–114. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Puddefoot JR, Udeozo UK, Barker S and
Vinson GP: The role of angiotensin II in the regulation of breast
cancer cell adhesion and invasion. Endocr Relat Cancer. 13:895–903.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bokhari AA and Syed V: Inhibition of
transforming growth factor-β (TGF-β) signaling by Scutellaria
baicalensis and Fritillaria cirrhosa extracts in endometrial
cancer. J Cell Biochem. 116:1797–1805. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Piestrzeniewicz-Ulanska D, Brys M, Semczuk
A, Rechberger T, Jakowicki JA and Krajewska WM: TGF-β signaling is
disrupted in endometrioid-type endometrial carcinomas. Gynecol
Oncol. 95:173–180. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Saad S, Stanners SR, Yong R, Tang O and
Pollock CA: Notch mediated epithelial to mesenchymal transformation
is associated with increased expression of the Snail transcription
factor. Int J Biochem Cell Biol. 42:1115–1122. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Okazaki M, Fushida S, Harada S, Tsukada T,
Kinoshita J, Oyama K, Tajima H, Ninomiya I, Fujimura T and Ohta T:
The angiotensin II type 1 receptor blocker candesartan suppresses
proliferation and fibrosis in gastric cancer. Cancer Lett.
355:46–53. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Qian YR, Guo Y, Wan HY, Fan L, Feng Y, Ni
L, Xiang Y and Li QY: Angiotensin-converting enzyme 2 attenuates
the metastasis of non-small cell lung cancer through inhibition of
epithelial-mesenchymal transition. Oncol Rep. 29:2408–2414.
2013.PubMed/NCBI
|
|
33
|
Okamoto K, Tajima H, Nakanuma S, Sakai S,
Makino I, Kinoshita J, Hayashi H, Nakamura K, Oyama K, Nakagawara
H, et al: Angiotensin II enhances epithelial-to-mesenchymal
transition through the interaction between activated hepatic
stellate cells and the stromal cell-derived factor-1/CXCR4 axis in
intrahepatic cholangiocarcinoma. Int J Oncol. 41:573–582.
2012.PubMed/NCBI
|