Introduction
As is well known, transforming growth factor-β1
(TGF-β1) is a multifunctional cytokine that regulates cell
proliferation, growth, differentiation as well as cell movement
(1,2). In addition, TGF-β1 has been shown to
play a critical role during tumor progression potentially by
regulating cellular motility and invasive capability. However, the
underlying mechanism remains largely unknown.
Fascin is a 55-kDa globular protein that belongs to
a unique family of actin-bundling proteins (3). There are three isoforms of fascin that
are found in vertebrate cells. Fascin1 is widely expressed in
mesenchymal tissues as well as in the nervous system, while fascin2
is expressed by retinal photoreceptor cells, and fascin3 is a
typical testis-specific protein (4). Fascin (also known as fascin1) is found
to be located in the micro-spikes, membrane ruffles and stress
fibers, and is reported to induce membrane protrusions. Its
principal function is to form the parallel actin bundles that
support lamellipodial and filopodial cell protrusions, which are
the key cellular structures involved in environmental guidance and
cell motility, migration and adhesion (5–7).
Recent studies show that the fascin1 protein level
is significantly increased in transformed epithelial cells and
various types of carcinomas (8–11).
Overexpression of fascin1 has been reported in bladder carcinoma
(12,13). Previously, we reported that fascin1
plays an important role in migration and invasion of urothelial
carcinoma of the bladder (14,15).
Given the fact that the cytoskeleton proteins are
involved in TGF-β1-induced tumor progression and metastasis
(16), and that the expression of
fascin1 was upregulated by TGF-β1 in various types of cancers such
as lung and gastric cancer (17,18),
we hypothesized that fascin1 could be a potential mediator of
TGF-β1-induced cell invasion and tumor metastasis in bladder
cancer. We thus undertook the present study to investigate the
effect of TGF-β1 on the expression of fascin1 as well as the role
of fascin1 in the TGF-β1-induced cellular biological changes in
human bladder urothelial carcinoma cell lines T24 and BIU87.
Materials and methods
Cell culture
The human urothelial carcinoma cell lines T24 and
BIU87 were purchased from the China Type Culture Center (Wuhan,
China) and maintained according to the manufacturer's instructions.
Cells were cultured in a suitable incubator with sufficiently
moisturized conditions (monolayer cells grow in a 37°C environment
with an atmospheric composition of 5% CO2 and 95% air)
in RPMI-1640 medium (Lonza, Verviers, Belgium) supplemented with
10% fetal bovine serum (FBS) (EuroClone, West York, UK), 100 U/ml
penicillin and 100 mg/ml streptomycin (Santa Cruz Biotechnology,
Santa Cruz, CA, USA). When cells grew to 70% confluency, the
routine medium was removed and replaced with FBS-free RPMI-1640
medium without or with 10 ng/ml TGF-β1 (RayBiotech, Atlanta, GA,
USA) for 24 h.
Small interfering RNA (siRNA) preparation
and transfection
T24 or BIU87 cells were seeded to grow for 48 h to
~80% confluency in RPMI-1640 medium containing 10% FBS. One OD
fascin1 siRNA (GenePharma, Shanghai, China) was diluted in 125
μl diethylpyrocarbonate (DEPC) solution. Then, 8 μl
siRNA solution and 8 μl Lipofectamine 2000 (Invitrogen,
Carlsbad, CA, USA) were added to 242 μl of fresh RPMI-1640
medium, incubated for 5 min at room temperature, and mixed for
another 20 min at room temperature. The transfection complex
mixture was added to 1,500 μl RPMI-1640 medium without FBS
to the cells. Scrambled siRNA with Lipofectamine 2000 alone was
used as control. After 6 h, the medium was replaced with RPMI-1640
containing 10% FBS, and the cells were cultured for 24 or 48 h with
or without 10 ng/ml TGF-β1 until ready for further assay.
Real-time RT-PCR
Total RNA was isolated from cultured cells with
ice-cold TRIzol reagent (Invitrogen), according to the
manufacturer's instructions. The concentration of RNA was
determined by Thermo Scientific NanoDrop ND-100 (Wilmington, DE,
USA). Total RNA was used for reverse transcription using
SYBR® PrimeScript® RT-PCR kit (Perfect
Real-Time) (Takara, Kyoto, Japan). Real-time PCR was performed
using Thermal Cycler Dice™ Real-Time system TP800 (Takara). The
reaction system was maintained at 60°C for 2 min and heated to 95°C
for 10 min followed by 45 cycles, denaturation of the mixture at
95°C for 15 sec, annealing at 60°C for 30 sec and extension at 72°C
for 30 sec. The sequence of primers designed for fascin1 and GAPDH
are listed in Table I. The
2−ΔΔCt method was used to calculate mRNA expression of
the target gene.
 | Table ISequences of primer pairs for
real-time RT-PCR analysis. |
Table I
Sequences of primer pairs for
real-time RT-PCR analysis.
| Gene | Nucleotide sequence
(5′-3′) | Length (bp) |
|---|
| Fascin1 | F
GGCAAGTTTGTGACCTCCAAGAA | 136 |
| R
AGCCGATGAAGCCATGCTC | |
| β-actin | F
CTCCATCCTGGCCTCGCTGT | 268 |
| R
GCTGTCACCTTCACCGTTCC | |
Western blotting
Western blot analysis was carried out to investigate
the expression of fascin1 before and after TGF-β1 treatment in T24
and BIU87 cells. Cells were lysed in lysis buffer with a cocktail
of protease inhibitors (both from Sigma, St. Louis, MO, USA). Total
proteins were measured using the BCA protein assay kit (Sigma)
according to the manufacturer's protocol. A sample consisting of 30
μg of total protein was analyzed by 10% SDS-PAGE and
transferred to polyvinylidene fluoride membranes (Sigma). The
membranes were subsequently blocked with 5% skim milk for 2 h at
room temperature and incubated overnight at 4°C with a specific
primary antibody against fascin1 (1:5,000; Abcam, Hong Kong, China)
or β-actin (1:2,000; Santa Cruz Biotechnology). β-actin was used as
an internal control. Next, the membranes were incubated at room
temperature for 1 h with horseradish peroxidase-conjugated goat
anti-mouse IgG (1:2,500; Santa Cruz Biotechnology), and signals
were developed using Western Blotting Luminol Reagent (Gene Company
Ltd., Hong Kong, China).
Cell proliferation assay
The MTT assay was used for measuring cell
proliferation with and without TGF-β1 treatment. T24 and BIU87
cells were seeded at a density of 1×104 cells/well into
96-well plates. After 24 h, the culture medium was removed and
fresh medium contained 0 or 10 ng/ml TGF-β1 was added. After a 48-h
treatment, MTT working solution (Sangon Biological Company, China)
(20 μl, 5 mg/ml) was added to each well, and the cells were
incubated at 37°C for 4 h. Then, the medium in each well was
completely removed, 150 μl of dimethyl sulfoxide (DMSO)
solution (Sigma) was added into each well and the plate was shaken
for 10 min. The 490 nm absorbance of the dissolved chemical
crystals was measured by a plate reader (model 680; Bio-Rad,
Hertfordshire, UK). All assays were performed three times in sets
of six replicate wells.
Cell migration assay
The migration activities of the T24 and BIU87 cells
were evaluated by wound-healing assay. Cells were plated in a
24-well plate and cultured to reach a confluency before the assay.
The wound was made by scraping using a conventional pipette tip
across the monolayer. The monolayer was softly washed with
phosphate-buffered saline (PBS), and then the cells were continued
to be cultured in the medium supplemented with 0 or 10 ng/ml
TGF-β1. The initial gap length (0 h) and the residual gap length 24
and 48 h after wounding was measured from photomicrographs.
Cell invasion assay
Cell invasion was determined by an invasion chamber
assay. Cells (1×104) in RPMI-1640 medium containing 5%
FBS were seeded onto the top chamber of a 24-well Matrigel-coated
micropore membrane filter with 8-μm pores (Corning, Corning,
NY, USA), and the bottom chamber was filled with RPMI-1640 medium
containing 10% FBS as a chemoattractant. After 36 h of incubation
in a 5% CO2 humidified incubator at 37°C, the cells on
the upper surface were carefully removed with a cotton swab, and
the membranes were fixed with 4% paraformaldehyde and stained with
crystal violet staining solution. Invasion was quantified by
counting all of the cells that had migrated through the membrane in
five random fields under a light microscope (magnification, ×200).
The mean value was calculated from data obtained from three
separate chambers.
Statistical analysis
All values are expressed as means ± standard
deviation (SD), and were analyzed using SPSS for windows, version
16.0 (SPSS, Inc., Chicago, IL, USA). The independent and paired
t-tests were used and the values were considered to be significant
at p<0.05.
Results
Effects of TGF-β1 and fascin1 siRNA on
fascin1 expression in the T24 and BIU87 cells
TGF-β1 increases the expression of
fascin1
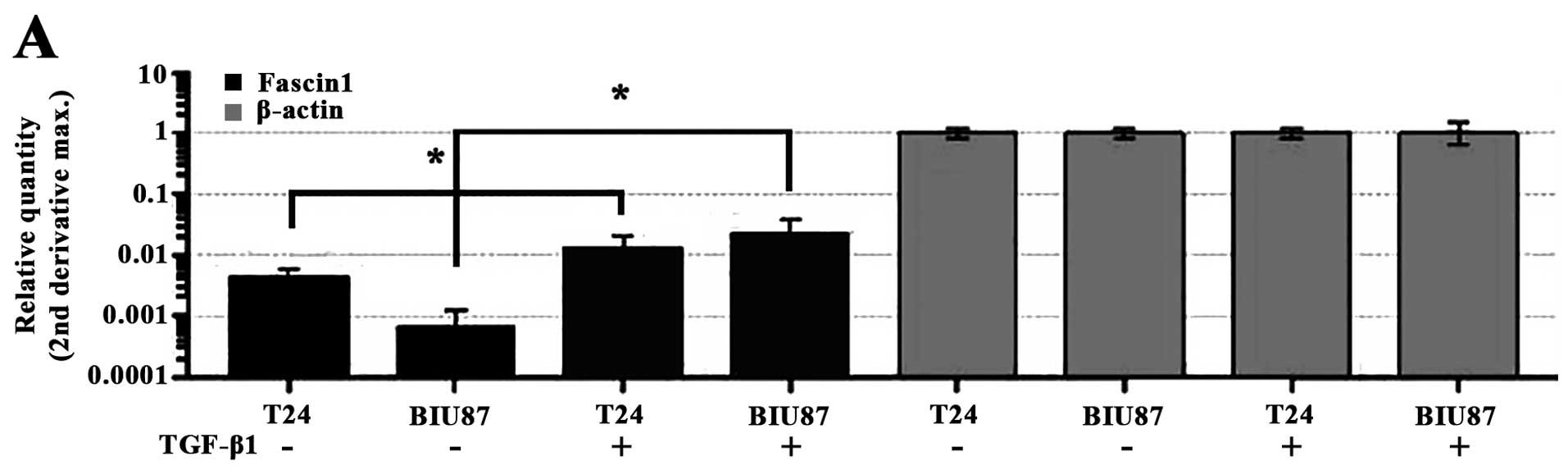
Expression of fascin1 in the T24 and BIU87 cells
after treatment with TGF-β1 is shown in Fig. 1A and B. As shown in Fig. 1A, compared with the corresponding
control, the mRNA levels of fascin1 in the T24 and BIU87 cells were
significantly increased after a 24-h treatment with TGF-β1
(p<0.05). The protein levels of fascin1 in the T24 and BIU87
cells were significantly increased after a 48-h treatment with
TGF-β1 (p<0.05) (Fig. 1B).
Fascin1 siRNA effectively suppresses
fascin1 expression
The inhibitory efficiency of fascin1 siRNA on
fascin1 expression was assessed at both the mRNA and protein levels
(Fig. 1C–E). As shown, compared
with the si-control cells, fascin1 mRNA levels were significantly
reduced by 78.7±2.3 and 73.8±2.1% following transfection with
fascin1 siRNA in the T24 and BIU87 cells, respectively (Fig. 1C), while fascin1 protein level was
significantly reduced by 56.3±1.7% in the T24 and 72.4±2.2% in the
BIU87 cells, respectively (p<0.05). Moreover, after treatment
with 10 ng/ml TGF-β1, the fascin1 protein levels did not increase
in the fascin1 siRNA-transfected cells compared with the
non-transfected (NT) cells (p>0.05).
Effect of TGF-β1 on the proliferation of
the T24 and BIU87 cells
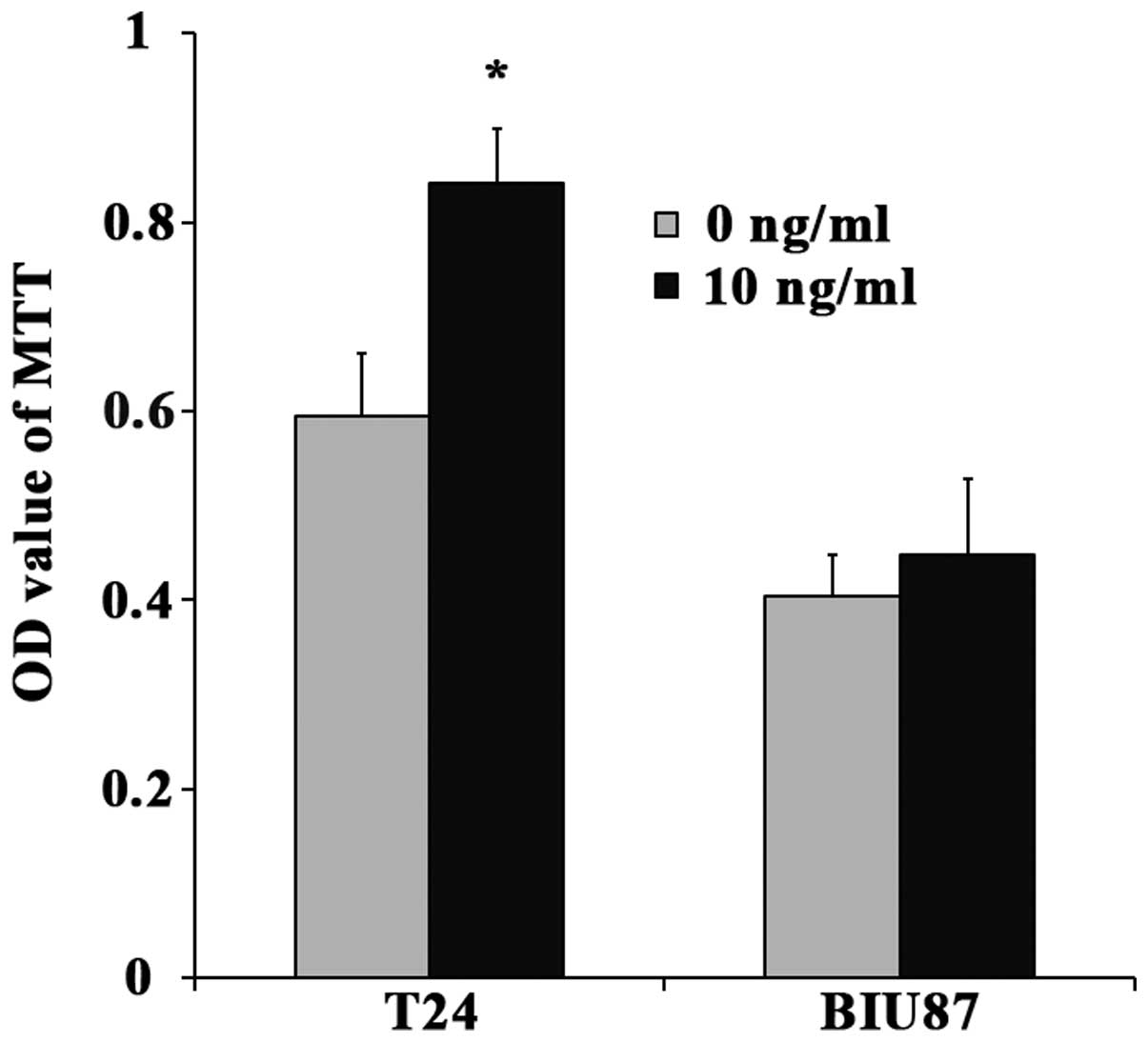
Changes in the proliferation of T24 and BIU87 cells
after TGF-β1 treatment are summarized in Fig. 2. As shown in this figure, the
proliferation of T24 cells was significantly increased after
treatment with 10 ng/ml TGF-β1 (p=0.005). However, the
proliferation of the BIU87 cells treated with 10 ng/ml TGF-β1
failed to show significant difference compared with the
TGF-β1-untreated cells (p=0.318).
Effects of TGF-β1 and fascin1 siRNA on
the migratory abilities of the T24 and BIU87 cells
TGF-β1 promotes the migration
activities of T24 and BIU87 cells
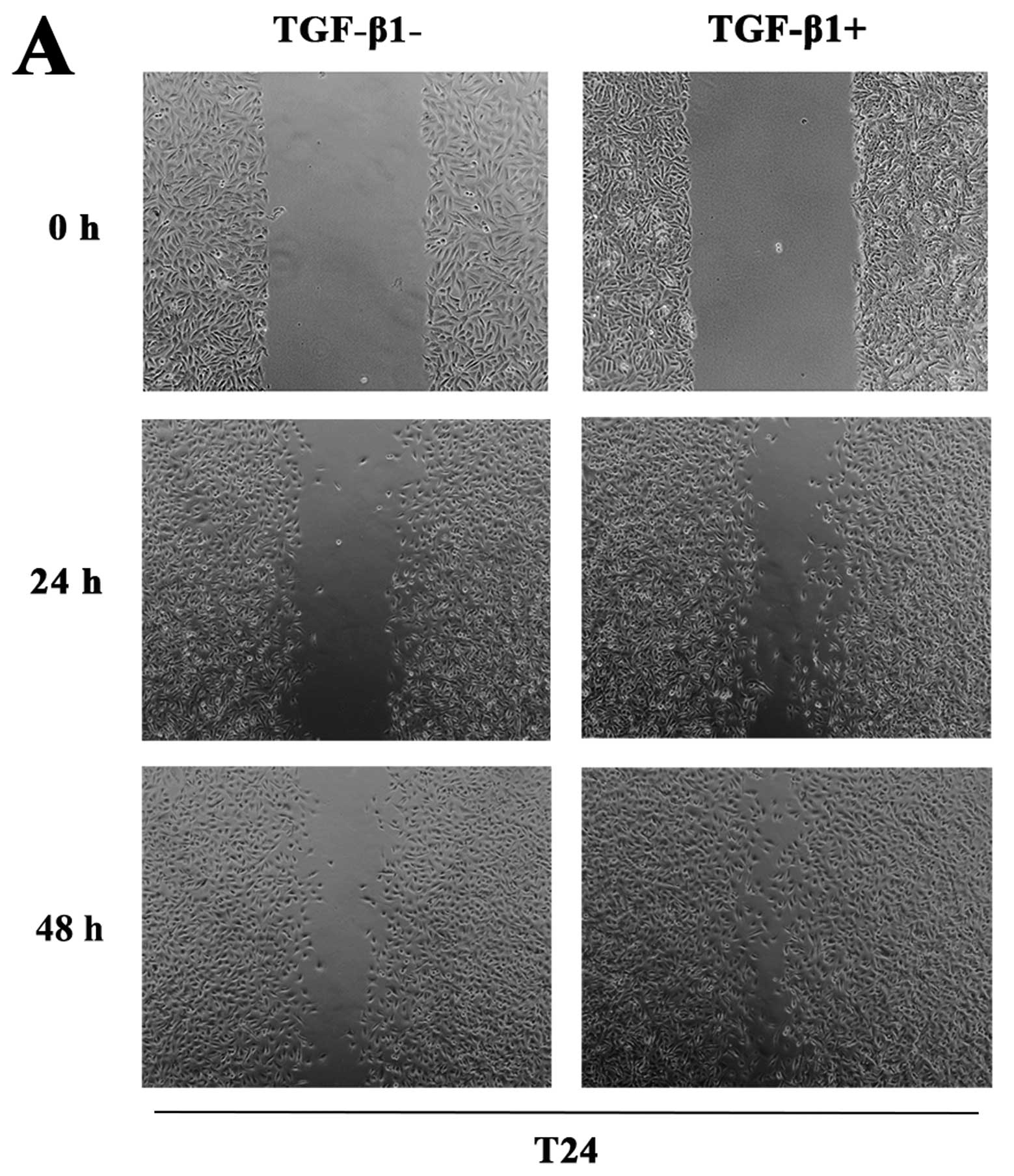
Changes in the migration activities of T24 and BIU87
cells after TGF-β1 treatments are shown in Fig. 3A and B. As shown in Fig. 3A, after the wound was made at 24 h,
the initial width of the wound was closed by 40% in the
TGF-β1-untreated T24 cells, while it was closed by 60% in the
TGF-β1-treated T24 cells, which was a significant increase compared
with the TGF-β1-untreated cells. After the wound was made at 48 h,
there still existed a significant ̔unhealed̓ wound in the
TGF-β1-untreated cells; however, the TGF-β1-treated cells almost
̔healed̓ the wound by 90%, which also increased significantly
compared with the TGF-β1-untreated cells. The similar response of
̔wound healing̓ to TGF-β1 was also found in another bladder cancer
cell line BIU87 (Fig. 3B).
Fascin1 siRNA inhibits the cell migratory
abilities induced by TGF-β1
Changes in the migration activities of T24 and BIU87
cells after fascin1 siRNA and TGF-β1 treatment are summarized in
Fig. 3C and D. As shown in this
figure, compared with the si-control cells, the fascin1 siRNA
treatment decreased the cell migration in both the TGF-β1-untreated
and TGF-β1-treated cells in the T24 and BIU87 cell lines,
respectively. Importantly, the treatment of TGF-β1 significantly
promoted the migration of both cell lines, and this effect was
largely attenuated by lowering the expression of fascin1 using
fascin1 siRNA in these cells, as the migration of cells in the
fascin1 siRNA treatment group was significantly lower than that in
the si-control group.
Effects of TGF-β1 and fascin1 siRNA on
the invasive abilities of the T24 and BIU87 cells
TGF-β1 promotes the invasive abilities
of the T24 and BIU87 cells
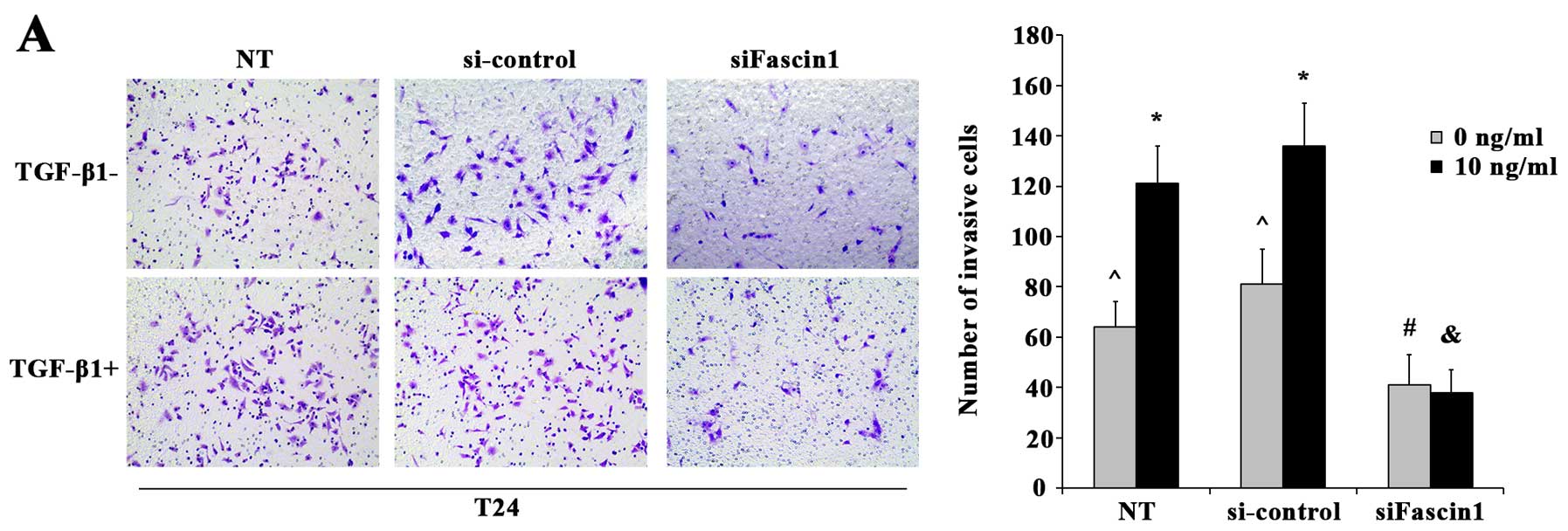
Changes in the invasive abilities of the T24 and
BIU87 cells after TGF-β1 treatments are illustrated in Fig. 4A and B. As shown in this figure, the
numbers of invasive cells penetrating through the membrane in the
10 ng/ml TGF-β1 treatment cells were significantly increased in the
T24 (p=0.005) and BIU87 cells (p=0.001), compared with the NT T24
and BIU87 cells.
Fascin1 siRNA attenuates the invasiveness
induced by TGF-β1
Changes in the invasive abilities of the T24 and
BIU87 cells after fascin1 siRNA and TGF-β1 treatment are summarized
in Fig. 4A and B. Compared with
si-control cells in TGF-β1-untreated group, less cells revealed
invasiveness in the sifascin1 group (T24, p=0.014; BIU87, p=0.003).
When compared with the TGF-β1-untreated cells, treatment with 10
ng/ml TGF-β1 significantly promoted the invasiveness of the
si-control cells (T24, p=0.000; BIU87, p=0.000), however, this
failed to have significant effects on the invasiveness of the
fascin1 siRNA cells (T24, p=0.746; BIU87, p=0.100).
Discussion
TGF-β1 is a multifunctional cytokine that is known
to induce G1 arrest during the cell cycle in order to end
proliferation, induce differentiation, or promote apoptosis in
normal cells, thus being a natural tumor-suppressive agent
(19). In the early stages of
cancer development, cells respond to the antimitotic effect of
TGF-β1 (20). However, at the entry
of tumor cells into the phase of uncontrollable growth, most of
them lose sensitivity to the inhibitory effect of TGF-β1. It is
surprising that this occurs despite the presence on the tumor cell
surface of the receptors for TGF-β1. Moreover, these cancer cells
begin to secrete TGF-β1 themselves. The TGF-β1-dependent
immunosuppressive activity increases the affinity of cancer cells
to cell adhesion molecules (21),
creates a microenvironment favorable to tumor growth and
metastasis, and increases invasiveness of cancer cells (20). Sun et al (22) found that TGF-β1 induced invasion and
filopodia formation in spindle-shaped tumor cells, and Fu et
al (18) found that TGF-β1
promoted invasion and metastasis of gastric carcinoma cells. In
agreement with our previous data, increased migration and
invasiveness potential in TGF-β1-treated bladder cancer cells were
confirmed in the present study.
Fascin1 has been found to play an important role in
migration and invasion of various tumor cells (11,12,15),
and fascin1 overexpression has been previously reported in many
cancer tissues (13,23,24).
Thus, fascin1 was recently used as an identifier of metastatic
carcinoma to differentiate carcinoma metastasis, particularly in
urothelial carcinoma (25,26).
Recent studies have indicated that upregulation of
fascin1 may be the key point in the process of TGF-β1-promoted
invasion and migration of tumor cells (18,22),
and we obtained a similar conclusion in bladder cancer cells
according to the data in the present study. After exposure to
TGF-β1 (10 ng/ml), fascin1 expression was increased in the T24 and
BIU87 bladder cancer cells. Meanwhile, the migration and
invasiveness of these cells were enhanced. However, we did not
observe the same impact of TGF-β1 when the expression of fascin1
was decreased by specific siRNA. We could speculate that fascin1 is
an important mediator of the response of bladder cancer cells to
TGF-β1.
However, in our previous study, we observed that
fascin1 did not influence cell proliferation of 5637 or BIU87 cells
(15). In the present study, we
observed that the cell proliferation of T24 was promoted after
TGF-β1, and there was no significant increase on cellular growth in
the BIU87 cells. These results suggested that the effect of TGF-β1
on cell proliferation was cell line-dependent. Lee et al
(27) recently reported that six
cell lines showed growth inhibition after TGF-β1 treatment and
proved that TGF-β1 did not stimulate cellular proliferation but was
a growth inhibitory factor in bladder cancer cells. Thus, the
effects of TGF-β1 on promoting cell proliferation still need
further research for confirmation.
Moreover, the signaling pathways through which
TGF-β1 upregulates fascin1 expression and increases migration and
invasion potential in cancer cells have not been fully defined. One
study speculated that fascin is regulated by TGF-β through the
canonical TβRI-Smad pathway (22).
In contrast to this conclusion, another study showed that TGF-β1
increased fascin1 expression via the ERK and JNK signaling pathways
in gastric cancer cells (18). In
summary, to fully understand the effect of TGF-β1 on the
proliferation, motility and invasive potential of bladder carcinoma
cells, further studies are needed.
In conclusion, the present study demonstrated for
the first time that TGF-β1 can promote the invasion and migration
abilities of T24 and BIU87 bladder carcinoma cells and an increase
in fascin1 expression may be a key factor for this impact of
TGF-β1.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (81372722), and the Natural
Science Foundation of Liaoning Province (2013225021).
References
|
1
|
Kajdaniuk D, Marek B, Borgiel-Marek H and
Kos-Kudła B: Transforming growth factor β1 (TGFβ1) in physiology
and pathology. Endokrynol Pol. 64:384–396. 2013. View Article : Google Scholar
|
|
2
|
Hirose A, Tajima H, Ohta T, Tsukada T,
Okamoto K, Nakanuma S, Sakai S, Kinoshita J, Makino I, Furukawa H,
et al: Low-dose paclitaxel inhibits the induction of
epidermal-mesenchymal transition in the human cholangiocarcinoma
CCKS-1 cell line. Oncol Lett. 6:915–920. 2013.PubMed/NCBI
|
|
3
|
Adams JC: Roles of fascin in cell adhesion
and motility. Curr Opin Cell Biol. 16:590–596. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kureishy N, Sapountzi V, Prag S, Anilkumar
N and Adams JC: Fascins, and their roles in cell structure and
function. BioEssays. 24:350–361. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Anilkumar N, Parsons M, Monk R, Ng T and
Adams JC: Interaction of fascin and protein kinase Calpha: A novel
intersection in cell adhesion and motility. EMBO J. 22:5390–5402.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Takikita M, Hu N, Shou JZ, Giffen C, Wang
QH, Wang C, Hewitt SM and Taylor PR: Fascin and CK4 as biomarkers
for esophageal squamous cell carcinoma. Anticancer Res. 31:945–952.
2011.PubMed/NCBI
|
|
7
|
Buda A and Pignatelli M: E-cadherin and
the cytoskeletal network in colorectal cancer development and
metastasis. Cell Commun Adhes. 18:133–143. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tong GX, Yee H, Chiriboga L, Hernandez O
and Waisman J: Fascin-1 expression in papillary and invasive
urothelial carcinomas of the urinary bladder. Hum Pathol.
36:741–746. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Durmaz A, Kurt B, Ongoru O, Karahatay S,
Gerek M and Yalcin S: Significance of fascin expression in
laryngeal squamous cell carcinoma. J Laryngol Otol. 124:194–198.
2010. View Article : Google Scholar
|
|
10
|
Arjonen A, Kaukonen R and Ivaska J:
Filopodia and adhesion in cancer cell motility. Cell Adhes Migr.
5:421–430. 2011. View Article : Google Scholar
|
|
11
|
Park SH, Song JY, Kim YK, Heo JH, Kang H,
Kim G, An HJ and Kim TH: Fascin1 expression in high-grade serous
ovarian carcinoma is a prognostic marker and knockdown of fascin1
suppresses the proliferation of ovarian cancer cells. Int J Oncol.
44:637–646. 2014.PubMed/NCBI
|
|
12
|
Karasavvidou F, Barbanis S, Pappa D,
Moutzouris G, Tzortzis V, Melekos MD and Koukoulis G: Fascin
determination in urothelial carcinomas of the urinary bladder: A
marker of invasiveness. Arch Pathol Lab Med. 132:1912–1915.
2008.PubMed/NCBI
|
|
13
|
Soukup V, Babjuk M, Dusková J, Pesl M,
Szakáczová M, Zámecník L and Dvorácek J: Does the expression of
fascin-1 and tumor subclassification help to assess the risk of
recurrence and progression in t1 urothelial urinary bladder
carcinoma? Urol Int. 80:413–418. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bi J, Chen X, Zhang Y, Li B, Sun J, Shen H
and Kong C: Fascin is a predictor for invasiveness and recurrence
of urothelial carcinoma of bladder. Urol Oncol. 30:688–694. 2012.
View Article : Google Scholar
|
|
15
|
Bi JB, Zhu Y, Chen XL, Yu M, Zhang YX, Li
BX, Sun JW, Shen HL and Kong CZ: The role of fascin in migration
and invasion of urothelial carcinoma of the bladder. Urol Int.
91:227–235. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bakin AV, Safina A, Rinehart C, Daroqui C,
Darbary H and Helfman DM: A critical role of tropomyosins in TGF-β
regulation of the actin cytoskeleton and cell motility in
epithelial cells. Mol Biol Cell. 15:4682–4694. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Keshamouni VG, Jagtap P, Michailidis G,
Strahler JR, Kuick R, Reka AK, Papoulias P, Krishnapuram R,
Srirangam A, Standiford TJ, et al: Temporal quantitative proteomics
by iTRAQ 2D-LC-MS/MS and corresponding mRNA expression analysis
identify post-transcriptional modulation of actin-cytoskeleton
regulators during TGF-beta-Induced epithelial-mesenchymal
transition. J Proteome Res. 8:35–47. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fu H, Hu Z, Wen J, Wang K and Liu Y:
TGF-beta promotes invasion and metastasis of gastric cancer cells
by increasing fascin1 expression via ERK and JNK signal pathways.
Acta Biochim Biophys Sin. 41:648–656. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Valkov A, Sorbye SW, Kilvaer TK, Donnem T,
Smeland E, Bremnes RM and Busund LT: The prognostic impact of
TGF-β1, fascin, NF-κB and PKC-ζ expression in soft tissue sarcomas.
PLoS One. 6:e175072011. View Article : Google Scholar
|
|
20
|
Heldin CH, Miyazono K and ten Dijke P:
TGF-beta signalling from cell membrane to nucleus through SMAD
proteins. Nature. 390:465–471. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Blobe GC, Schiemann WP and Lodish HF: Role
of transforming growth factor beta in human disease. N Engl J Med.
342:1350–1358. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sun J, He H, Xiong Y, Lu S, Shen J, Cheng
A, Chang WC, Hou MF, Lancaster JM, Kim M, et al: Fascin protein is
critical for transforming growth factor β protein-induced invasion
and filopodia formation in spindle-shaped tumor cells. J Biol Chem.
286:38865–38875. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zou J, Yang H, Chen F, Zhao H, Lin P,
Zhang J, Ye H, Wang L and Liu S: Prognostic significance of
fascin-1 and E-cadherin expression in laryngeal squamous cell
carcinoma. Eur J Cancer Prev. 19:11–17. 2010. View Article : Google Scholar
|
|
24
|
Kefeli M, Yildiz L, Kaya FC, Aydin O and
Kandemir B: Fascin expression in uterine smooth muscle tumors. Int
J Gynecol Pathol. 28:328–333. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
McKnight R, Cohen C and Siddiqui MT:
Fascin stain as a potential marker of invasiveness in carcinomas of
the urinary bladder: A retrospective study with biopsy and cytology
correlation. Diagn Cytopathol. 39:635–640. 2011. View Article : Google Scholar
|
|
26
|
Vogt AP, Cohen C and Siddiqui MT: Fascin
as an identifier of metastatic urothelial carcinoma: A
retrospective study of fine-needle aspiration cell blocks and
histologic tissue microarrays. Diagn Cytopathol. 40:882–886. 2012.
View Article : Google Scholar
|
|
27
|
Lee C, Lee SH, Kim DS, Jeon YS, Lee NK and
Lee SE: Growth inhibition after exposure to transforming growth
factor-β1 in human bladder cancer cell lines. Korean J Urol.
55:487–492. 2014. View Article : Google Scholar : PubMed/NCBI
|