Introduction
Triple-negative breast cancers (TNBC) account for
approximately 15–25% of all breast cancer. They are characterized
by the lack of expression of estrogen, progesterone receptors
(ER/PgR) and human epidermal growth factor receptor 2 (HER2) and by
an aggressive clinical course with higher rates of relapse and poor
overall survival in metastatic disease (1–3).
Treatment options for patients with TNBC are limited due to the
absence of hormone receptors and HER2; therefore, cytotoxic
chemotherapy currently remains the only available treatment
(4).
Given these characteristics, TNBC is a challenge in
clinical practice.
Several studies demonstrated that a subgroup of TNBC
patients displayed a remarkable sensitivity to chemotherapeutic
agents. Between 17 and 58% of TNBC patients have been shown to
achieve pathological complete response (pCR) after
anthracycline/platinum-based neoadjuvant chemotherapy and these
patients had an excellent prognosis. On the contrary, those who
failed to achieve a pCR had an exceptionally poor outcome (5,6). Over
the past decades there have been several attempts to use genomic
data in order to explain the highly variable responses to therapy
and clinical outcome of this setting of patients. Recently, genomic
analyses have provided additional insights, showing a wide
heterogeneity of molecular characteristics of TNBCs by gene
expression profile. On this basis, Lehmann et al (7) identified six different TNBC subtypes
including basal-like (type 1 and 2), immunomodulatory, mesenchymal,
mesenchymal stem like and luminal androgen receptor subtype
demonstrating the heterogeneity of TNBC.
Ongoing research into the molecular and genetic
mechanisms of TNBC tumorigenesis are helping to find out processes
involved in local tumor progression and distant metastases.
One of the most important mechanisms involved in the
control of neoplastic transformation is the PI3K/Akt/mTOR pathway.
The aberrant activation of this cascade seems to be of great
importance in breast cancer. In addition, a high activation level
of the PI3K/Akt/mTOR pathway has been related to worse prognosis
and resistance to conventional chemotherapy.
The mammalian target of rapamycin (mTOR) is an
important serine/threonine protein kinase of the phosphoinositide
3-kinase (PI3K)-related kinase family, which functions as an
environmental sensor and regulates organismal growth, cell
physiology and homeostasis. mTOR is the catalytic subunit of two
distinct complexes, mTOR complex 1 and mTOR complex 2 (mTORC1 and
mTORC2), which consist of several additional regulatory proteins.
The subunit composition of each mTORC dictates its substrate
specificity. Main substrates of mTORC1 are S6 kinase 1 (S6KB1) and
eIF4E-binding protein 1 (4E-BP1), both implicated in the regulation
of mRNA and protein synthesis. mTORC2 controls several members of
kinases including Akt and is thereby implicated in the regulation
of cell survival, cell cycle progression and anabolism (8,9).
Previous in vitro studies showed that
phosphorylated mTOR (p-mTOR), the activate form is closely related
with the active status of mTOR and the cell proliferative capacity
(10,11).
p-mTOR was found more frequently expressed in
triple-negative than non-TNBC, suggesting that mTOR may play a
crucial role in the molecular biology of TNBC. Besides, emerging
preclinical evidence suggested that TNBC cells seem particularly
sensitive to mTOR inhibitors, especially the androgen
receptor-positive (AR+) TNBC cell lines, opening the
possibility for the incorporation of target agents in treatments
(12–15).
The aims of the present study were to assess the
expression of the activated form p-mTOR in early TNBC and to
evaluate possible correlations between immunohistochemical AR
expression, clinicopathological parameters and disease outcome.
Patients and methods
Eligibility criteria
Between January 2009 and December 2013, all
consecutive patients who were diagnosed and had completed the
treatment of invasive TNBC at our institution were eligible for
this analysis.
The study obtained the necessary approval by the
Department of Medical Oncology, AO Ospedali Riuniti, Ancona.
According to Italian legislation, since it was a retrospective
study, with no direct patient involvement, ethical approval and
patients consent for the study were not required (Official Gazette
no. 72 of March 26, 2012). Patients with stage IV disease or with
ductal carcinoma in situ with or without micro-invasion and
patients with lack of information on pathologic or laboratory
results were excluded. We analysed several parameters: clinical
(age, performance status, type of surgery and adjuvant
chemotherapy), pathological (tumor size, grading, necrosis, lymph
nodes status, tumor histology, Ki-67 and lymphovascular invasion)
and molecular (AR and p-mTOR).
Immunohistochemistry
IHC analysis was performed on formalin-fixed,
paraffin-embedded breast cancer tissue. The detection of antigens
occurred automatically with Dako PT Link using EnVision™ FLEX
Target Retrieval Solution High and Low pH (50x) at 98°C.
After treatment with 3% hydrogen peroxide solution
for 10 min to block endogenous peroxidase, the sections were
incubated with primary antibody: ER (clone 1D5, 1:30; Dako,
Carpinteria, CA, USA), PR (clone PgR636, 1:50; Dako), Ki-67 (clone
MIB-1, 1:80; Dako), HER2/neu (HercepTest RTU; Dako), AR (clone
F39.4.1, 1:60; BioGenex, San Ramon, CA, USA) and phospho-mTOR
(Ser2448) (clone 49F9, 1:50; Cell Signaling Technology Inc.,
Beverly, MA, USA). The staining was completed using EnVision
FLEX™/HRP (Dako) as detection system; 3,3-diaminobenzidine-hydrogen
peroxide was used as chromogen. IHC was performed using an
autostaining system (Autostainer Link 48; Dako).
For ER, PR, Ki-67 and AR the percentage of positive
nuclei was evaluated by counting 5,000 neoplastic cells in
different areas of the neoplasia (16).
For Ki-67, the count was performed in the peripheral
part of the neoplasia (i.e. the most proliferating part). The
staining intensity was not considered. The values were expressed as
continuous variable, and ranging from 0 to 100%. Immunostaining for
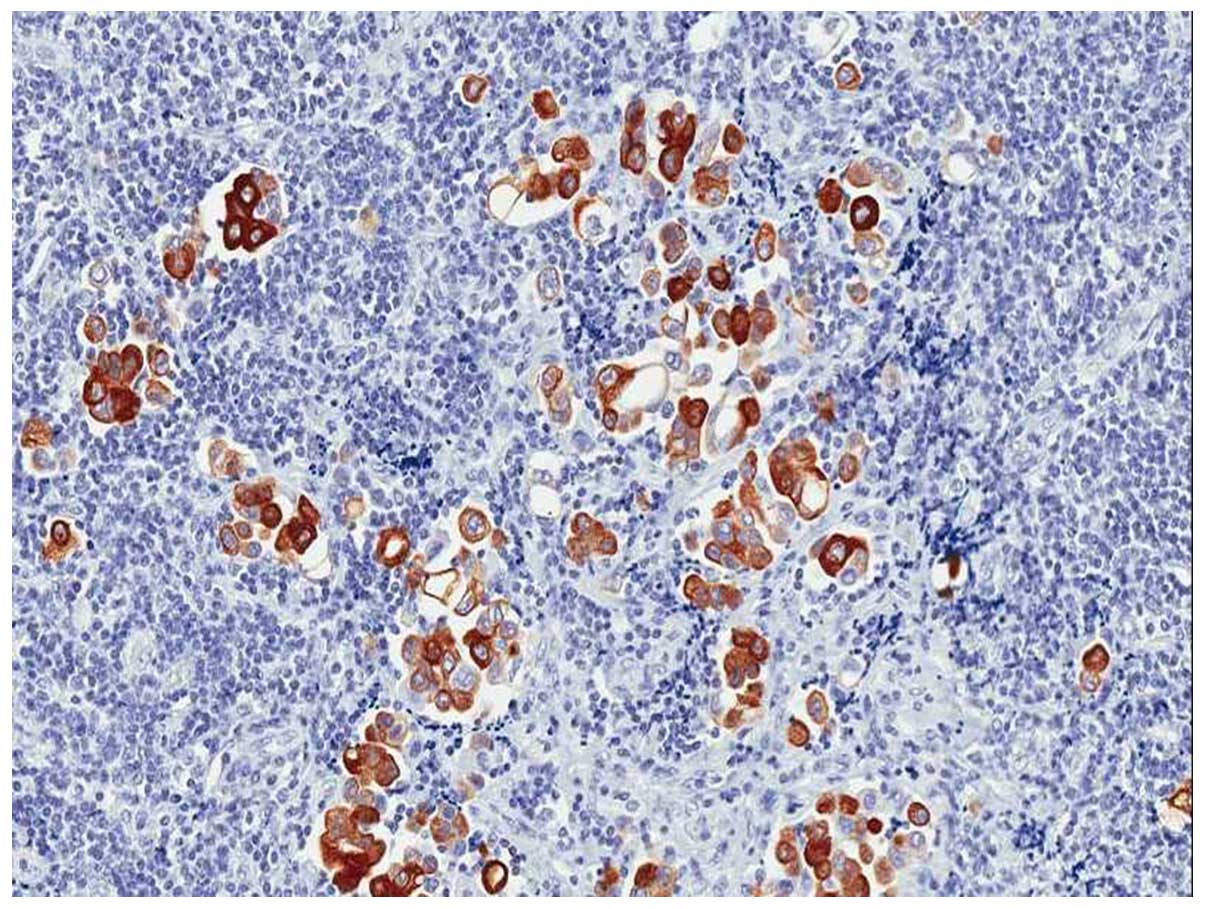
p-mTOR was semi-quantitatively assessed by considering both the
percentage of positive neoplastic cells (range, 0–100%), and the
strongest staining intensity (range, 0–3+; 0, no staining, 1+,
weak, 2+, moderate; 3+, strong) (Fig.
1). Also a 'score of positivity' was calculated by multiplying
the value of the percentage of positive neoplastic cells for the
value of staining intensity (range, 0–300). HER-2 status was
evaluated using a semi-quantitative score (0–3+); patients with 2+
IHC staining for HER2 underwent fluorescence in situ
hybridization to determine HER2 status (17). The evaluation of the above
immunohistochemical staining was carried out, with a double-blind
method, by two experienced pathologists, they were not aware of any
clinical data of patients, including follow-up and status.
Statistical analysis
Disease-free survival (DFS) was defined as the
interval between the date of diagnosis of TNBC to the first failure
(including loco-regional and/or distant relapse, second primary or
death). Overall survival (OS) was calculated from the date of
diagnosis to the date of the last follow-up visit or death.
Patients who were not reported to be dead at the time of the
analysis were censored at the date they were last known to be
alive. Survival distribution was estimated by the Kaplan-Meier
method. Subgroup differences were estimated by Chi-square test. The
Cox multivariate proportional hazard regression model was used to
evaluate the prognostic factors on disease-free survival (DFS) and
overall survival (OS). Significant differences in probability of
surviving between the data were evaluated by log-rank test. Hazard
ratios and 95% confidence intervals (CIs) were estimated from
regression coefficients. A significance level of 0.05 was chosen to
assess the statistical significance. Statistical analysis was
performed with the MedCalc package (MedCalc® v9.4.2.0;
MedCalc Software, Ostend, Belgium).
Results
Clinicopathological characteristics
Ninety-eight TNBC patients were included in our
analysis. Clinicopathological characteristics are summarized in
Table I. Median age was 52 years
(range 26–83 years) and the majority of patients (79.6%) underwent
breast conservative surgery.
 | Table IBaseline characteristics of 98 TNBC
patients based on p-mTOR expression. |
Table I
Baseline characteristics of 98 TNBC
patients based on p-mTOR expression.
|
Characteristics | Total no. of
patients (%) | p-mTOR negativity
no. of patients (%) | p-mTOR positivity
no. of patients (%) | P-value |
|---|
| Age (years) |
| ≤50 | 46 (46.9) | 31 (31.6) | 15 (15.3) | 0.83 |
| >50 | 52 (53.1) | 35 (35.8) | 17 (17.3) | |
| Menopausal
status |
| Yes | 45 (45.9) | 30 (30.6) | 15 (15.3) | 0.93 |
| No | 53 (54.1) | 36 (36.8) | 17 (17.3) | |
| Tumor size |
| pT1 | 56 (57.1) | 33 (33.7) | 23 (23.4) | 0.03 |
| pT2 | 41 (41.8) | 32 (32.6) | 9 (9.2) | |
| pT3-T4 | 1 (1.1) | 1 (1.1) | 0 (0) | |
| Lymph node status
(pN) |
| pN0 | 58 (59.1) | 41 (41.8) | 17 (17.3) | 0.52 |
|
pN+ | 40 (40.9) | 25 (25.6) | 15 (15.3) | |
| Histological
type |
| Ductal
carcinoma | 95 (96.9) | 64 (65.4) | 31 (31.5) | 0.21 |
| Lobular
carcinoma | 1 (1.1) | 0 | 1 (1.1) | |
| Other | 2 (2.0) | 2 (2.0) | 0 (0) | |
| Lymphovascular
invasion |
| No | 72 (73.5) | 52 (53.1) | 20 (20.4) | 0.14 |
| Yes | 26 (26.5) | 14 (14.3) | 12 (12.2) | |
| Necrosis |
| No | 81 (82.7) | 55 (56.2) | 26 (26.5) | 0.97 |
| Yes | 17 (17.3) | 11 (11.2) | 6 (6.1) | |
| AR |
| Negative | 80 (81.6) | 58 (59.2) | 22 (22.4) | 0.04 |
| Positive | 18 (18.4) | 8 (8.2) | 10 (10.2) | |
| Recurrences |
| No | 76 (77.6) | 52 (53.1) | 24 (24.5) | 0.87 |
| Yes | 22 (22.4) | 14 (14.3) | 8 (8.1) | |
| Deaths |
| No | 81 (82.7) | 55 (56.2) | 26 (26.5) | 0.97 |
| Yes | 17 (17.3) | 11 (11.2) | 6 (6.1) | |
| Total | 98 (100) | 66 (67.4) | 32 (32.6) | |
Most patients (57.1%) presented pT1 tumors (up to 2
cm in size). Lymph nodes were disease-positive in 40.9% of cases.
Patients (95.9%) received an adjuvant chemotherapy while 12.2% of
them underwent neo-adjuvant treatment.
The mean follow-up time was 4.7 years (0.65–8.3
years). The median DFS was 4.9 years (range, 0.18–8.35) and the
median OS was 5.1 years (range, 0.65–8.3). All tumors were grade 3
and with a high proliferating index (Ki-67 >20%). Lymphovascular
invasion and necrosis were reported in 26 (26.5%) and 17 cases
(17.3), respectively. The androgen receptor expression was reported
in 18 cases (18.4%) and the p-mTOR was positive in 32 cases
(32.6%). P-mTOR was located exclusively in the cytoplasm and its
expression did not correlate with any of the following
clinicopathological features investigated (Table I). Notably, p-mTOR positivity was
associated with small tumor size (P=0.03) and AR expression
(P=0.04).
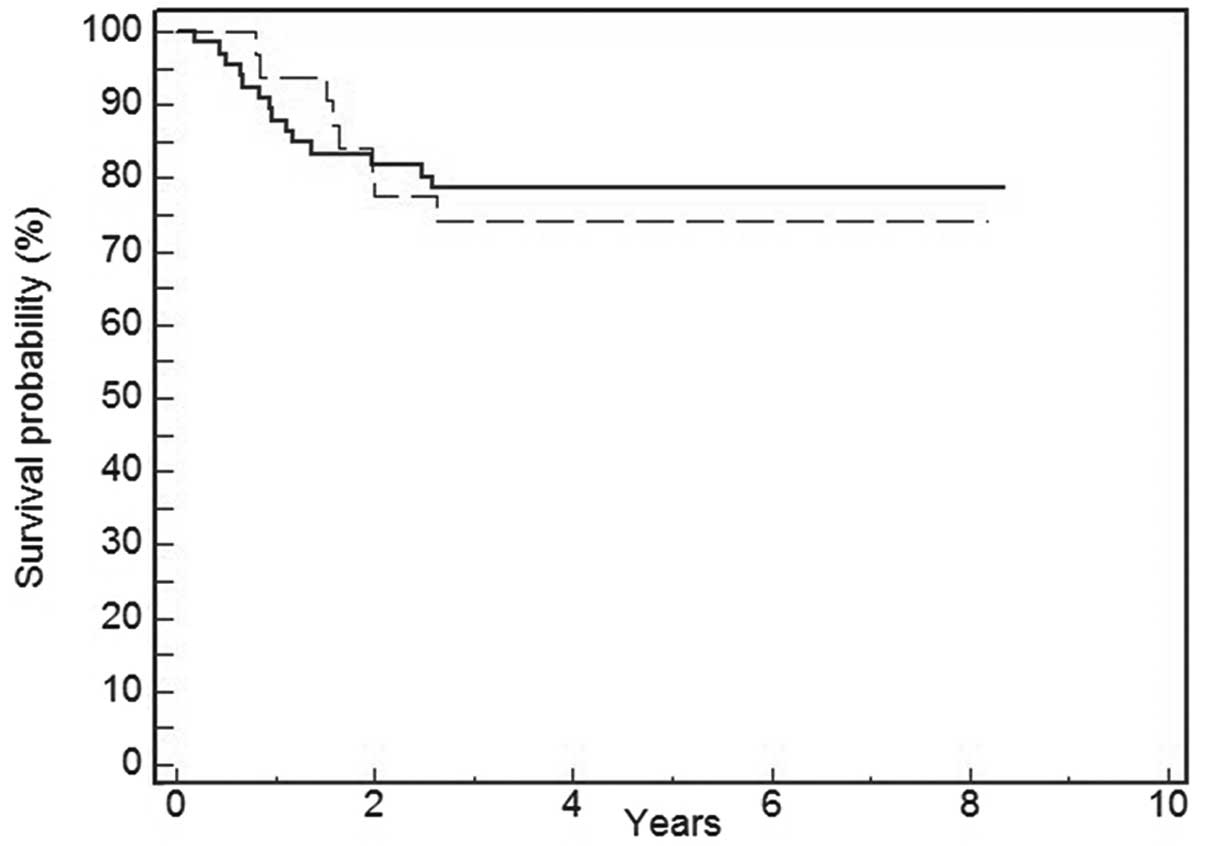
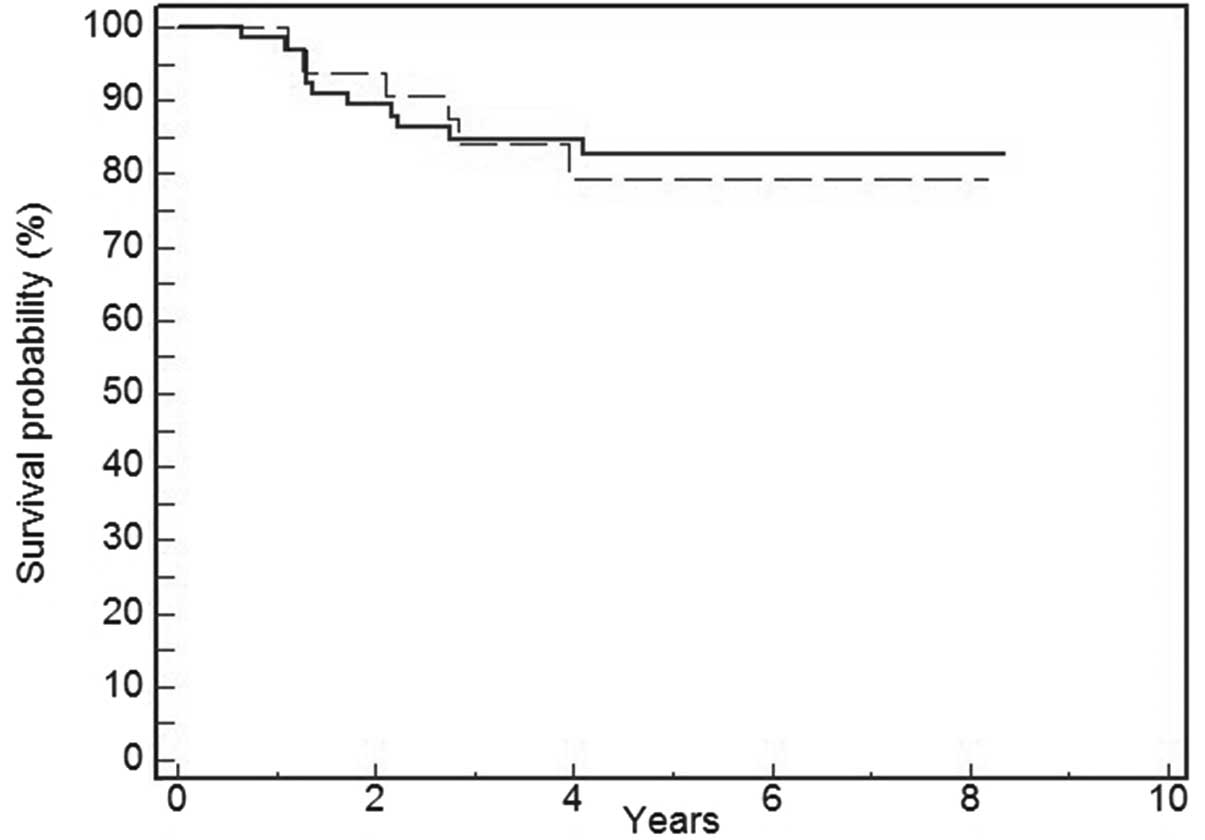
Univariate survival analysis revealed that positive
immunostaining for p-mTOR was not associated with DFS (P=0.74)
(Fig. 2) and OS (P=0.81) (Fig. 3). Tumor size (P=0.03) and lymph node
involvement (P=0.03) were significantly related to worse DFS and OS
(Tables II and III).
 | Table IIUnivariate and multivariate analysis
of sample features and DFS. |
Table II
Univariate and multivariate analysis
of sample features and DFS.
| Parameters | Univariate analysis
| Multivariate
analysis
|
|---|
| P-value | HR | 95% CI | P-value |
|---|
| Age (years) | 0.91 | | | |
| ≤50 vs.
>50 | | | | |
| Tumor size
(cm) | 0.03 | | | |
| ≤2 vs. >2 | | 2.39 | 0.97–5.86 | 0.05 |
| Lymph node
status | 0.04 | | | |
| pN0 vs.
pN+ | | 2.25 | 0.96–5.25 | 0.06 |
| Lympho-vascular
invasion | 0.18 | | | |
| Negative vs.
positive | | | | |
| Necrosis | 0.52 | | | |
| Negative vs.
positive | | | | |
| AR expression | 0.49 | | | |
| ≤10 vs.
>10 | | | | |
| p-mTOR | 0.74 | | | |
| Negative vs.
positive | | | | |
 | Table IIIUnivariate and multivariate analysis
of sample features and OS. |
Table III
Univariate and multivariate analysis
of sample features and OS.
| Parameters | Univariate analysis
| Multivariate
analysis
|
|---|
| P-value | HR | 95% CI | P-value |
|---|
| Age (years) | 0.64 | | | |
| ≤50 vs.
>50 | | | | |
| Tumor size
(cm) | 0.01 | | | |
| ≤2 vs. >2 | | 3.48 | 1.13–10.67 | 0.03 |
| Lymph node
status | 0.02 | 2.63 | 0.97–7.12 | 0.05 |
| pN0 vs.
pN+ | | | | |
| Lympho-vascular
invasion | 0.11 | | | |
| Negative vs.
positive | | | | |
| Necrosis | 0.14 | | | |
| Negative vs.
positive | | | | |
| AR expression | 0.39 | | | |
| ≤10 vs.
>10 | | | | |
| p-mTOR | 0.95 | | | |
| Negative vs.
positive | | | | |
Multivariate analysis confirmed that tumor size was
the only significant independent prognostic variable influencing
both DFS and OS (P=0.05 and P=0.03, respectively) while lymph node
involvement influenced only OS (P=0.05).
Discussion
The PI3K/Akt/mTOR pathway regulates several cellular
functions such as cell growth, survival and proliferation,
characterizing tumorigenesis as well as tumor progression (18). In breast cancer, a high activation
level of the PI3K/Akt/mTOR pathway has been related to resistance
to conventional chemo and endocrine therapy. The recent BOLERO-2
trial comparing everolimus plus exemestane vs. placebo plus
exemestane in women with resistance to no-steroidal aromatase
inhibitors demonstrated a 6-month improvement in progression-free
survival leading to the approval for the treatment of ER-positive
metastatic breast cancer.
Previous in vitro studies showed that p-mTOR
correlated with the activation of mTOR and an increase in
proliferation (10,19). In the present study, the high
expression of the active form of mTOR (p-mTOR) was present in
almost one third of TNBC, suggesting that aberrant activation of
the PI3K/Akt/mTOR may drive tumor proliferation in this subtype of
breast cancer. Although there are no therapeutic evidence using
mTOR inhibitors in TNBC, these data may open new therapeutic
scenarios, also suggested by in vitro and in vivo
assays, in which mTOR inhibitors demonstrated anti-tumor activity
in TNBC respectively in cell lines and mouse xenograft models
(18,20,21)
indicating mTOR as the potential target.
Three reports have previously been published on the
expression of p-mTOR in breast cancer; Bose et al (22) found increased levels of p-mTOR in
high grade vs. low grade cancers. However, Zhou et al
(13) found no relationship between
p-mTOR and tumor grade. Recently Walsh et al (12) showed a high expression of p-mTOR in
~36% of triple-negative breast carcinomas; this result is
consistent with the present study. Furthermore, they revealed that
p-mTOR was significantly more frequently expressed in
triple-negative than non-triple negative diseases, suggesting that
inhibitors directed against this protein may be effective in at
least some patients affected by this subtype of breast cancer.
In our results, 32.6% of cases were p-mTOR positive
and its high expression was not related to the considered
clinicopathological features neither to DFS and OS at the
univariate and multivariate survival analysis. However, in patients
who had small tumor size and early stage, p-mTOR positivity was
significantly higher and that is consistent with a previous study
(13). Of interest, the present
investigation is the first clinical retrospective study showing the
strong correlation between p-mTOR immunostaining and AR positivity
in a subgroup of TNBC. It is consistent with other reports and it
confirms microarray analysis recently conducted on TNBC (23).
A spectrum of somatic mutations have been discovered
in TNBC; mutations in PIK3CA (10.2%), the gene that encodes the
p110α catalytic subunit of phosphatidylinositol-3 kinase (PI3K) are
the most common. Lehmann et al (23,24)
observed that all AR-positive (AR+) TNBC cell lines
contain the PIK3CA mutation (H1047R) and are highly sensitive to
the PI3K/mTOR inhibitor NVP-BEZ235, suggesting that combination of
AR antagonism and PI3K inhibition may have a synergistic effect on
AR+ TNBC cell growth. Collectively, these findings are
similar with other reports (1) and
consistent with observations that hormonally responsive cancers,
such as those expressing ER and AR are more likely to acquire
PIK3CA mutations (24).
Up to date, in BC AR expression and relation with
the PI3K pathway were studied in cell lines or xenograft models and
the exact mechanism of action of AR in TNBC is still controversial
(1,24–26).
Due to the uncertain biological significance of the relationship
between mTOR and AR, we defined the regulation pathway linking mTOR
with AR by means of literature analysis and referring to Reactome
(www.reactome.org) and KEGG databases (http://www.genome.jp/kegg/pathway.html).
The relationship between AR expression and the mTOR
pathway could be explained by inherent biological data from
literature analysis (Fig. 4). It
should be noted that miR-21 and miR-34a, key elements of our
pathway, were shown to be expressed in TNBC (20,26,27,28).
In fact, there are many modifier pathways, specifically GTPase
activating proteins TSC1 and TSC2 are negative regulators of mTORC1
and positive regulators of mTORC2 (29). These proteins act on Rheb GTPase
hydrolyzing the GTP converting it to Rheb-GDP complex. Rheb is
activated bound to GTP and turned off when bound with GDP, so
respectively triggering or defusing mTORC1. Activated mTORC1, by
phosphorylating some downstream effectors, gives rise to an
increased protein translation and autophagy inhibition. In
particular, it activates RPS6KB1 kinase that, by phosphorylating
RPS6, induces cell growth and proliferation. Moreover, mTORC1
phosphorylates EIF4EBP1 that releases eIF4E so activating the
translation of various mRNAs including HIF1A. This is the α subunit
of a transcription factor that, in response to hypoxia, activates
transcription of genes involved in regulation of erythropoiesis,
angiogenesis, vascular tone, matrix metabolism, glucose metabolism,
cell proliferation and survival, apoptosis (VEDF, IGF-2 and EPO)
(30). Moreover, HIF1A induces
DDIT4 transcription that, through the dissociation of 14-3-3
inhibitory protein from TSC2 (31),
activates TSC2 that in turn inhibits mTORC1. eIF4E not only plays a
key role in translation, but also in the nucleus where it promotes
the export of specific mRNAs, as c-myc, Mdm2, NBN, ornithine
decarboxylase (ODC1), and cyclin D1, that support proliferative and
survival signalling pathways (32–34).
MDM2, by ubiquitination, leads to p53 transcription factor
degradation by the proteasome and, in turn, to miR-34 family
repression, the latter is a direct transcriptional target of p53
(35) and the miR-34 silencing is
frequent in several tumors, including breast cancer, and correlates
with metastasis and poor survival (36). Since miR-34a/c can regulate AR
(37), miR-34 repression causes
lack of AR inhibition. AR promotes miR-21 transcription by some
androgen responsive elements (AREs) in miR-21 promoter (in
particular, three binding sites are known: ARE1 and ARE2/3
(38,39). The oncogene miR-21 indirectly
suppresses p53 with a feedback loop mechanism (40) and downregulates some tumor
suppressor genes, including PIAS3 (41), giving rise also to STAT3
upregulation. Furthermore, miR-21 blocks PTEN (42–44),
the phosphatidylinositol-3,4,5-trisphosphate 3-phosphatase that
dephosphorylates PIP3 to PIP2 negatively regulating AKT/mTORC1
signalling pathway. This pathway analyses demonstrates a
correlation between activated mTORC1 and AR expression, of which
the key elements are p53, miR-21 and miR-34. Therefore, low level
of activated mTORC1 yields low AR expression.
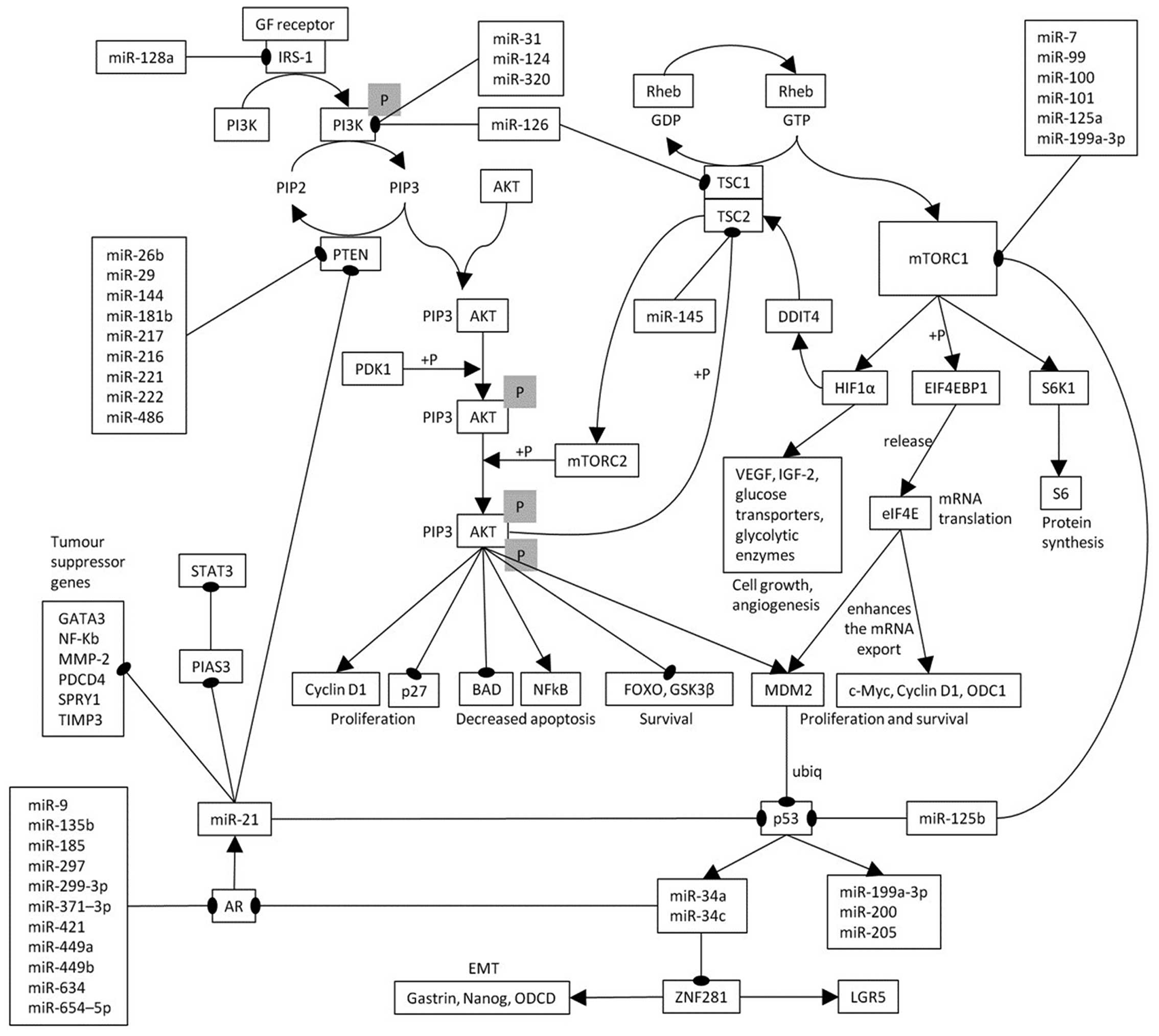 | Figure 4The pathway that links mTORC1 to AR
is shown. Arrows, activation, the connectors ending with thick
point represent inhibition. RPS6KB1, ribosomal protein S6 kinase,
70 kDa, polypeptide; 1 RPS6, ribosomal protein S6; EIF4EBP1,
eukaryotic translation initiation factor 4E binding protein 1;
EIF4E, eukaryotic translation initiation factor 4E; HIF1A, hypoxia
inducible factor 1, α subunit; VEGF, vascular endothelial growth
factor genes; DDIT4, DNA-damage-inducible transcript 4; 14-3-3;
tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation
protein; TSC1, tuberous sclerosis 1; TSC2, tuberous sclerosis 2;
NBN, nibrin; MDM2, MDM2 proto-oncogene; E3, ubiquitin protein
ligase; ODC1, ornithine decarboxylase 1; AR, androgen receptor;
PIAS3, protein inhibitor of activated STAT3; PTEN, phosphatase and
tensin homolog; PIP3, phosphatidylinositol (3,4,5)-trisphosphate;
ubiq, ubiquitination; +P, phosphorylation. |
It seems that some microRNAs are deregulated in
TNBC. Particularly, miR-21 and miR-34a were significantly
overexpressed in breast cancer, but miR-21 was significantly
overexpressed in TNBC vs. non-TNBC, moreover, it seems to be
associated to occurrence of lymph node metastases (27).
Other authors showed that miR-185 was strongly
downregulated in TNBC tissues and its ectopic expression suppressed
tumor proliferation, directly targeting DNMT1 and E2F6 (45). We can suggest that miR-185 acts also
by suppressing AR. Another study showed miR-126 downregulation
(46) and, according to our
pattern, this should increase AKT/mTORC1 activation by targeting
PI3K but, on the contrary, reducing mTORC1 by its target TSC2.
Instead miR-145 downregulation (46) should ameliorate prognosis by TSC2
restoration and so mTORC1 reduction. It is interesting that miR-101
and miR-125a were associated to metastasis (46) instead we show that they should block
mTORC1. The evidence that miR-31 downregulation causes an
enhancement in metastasis (47) can
be explained because it is an AKT inhibitor. The highly migratory
and metastatic characteristics of TNBC with low miR-200 family
expression (48) can be due to the
inferred p53 low levels. The fact that miR-205 is downregulated but
miR-200a/b/c is upregulated (46)
cannot be realized by our pathway. Certainly their regulation is
much more complex than that shown here and much remains to be
clarified. However, this pathway can be useful also to plan new
experiments.
All these complex results and analyses suggested
that a high expression of the active form of mTOR (p-mTOR) and
consequently an aberrant activation of the PI3K/Akt/mTOR pathway
may drive tumor proliferation and that is true for almost one third
of TNBC. Currently, TNBC is a subset of breast cancer with no
available targeted therapies and hence adverse clinical outcome.
Those findings could provide important information as to the
potential opportunity for novel targeted and personalized treatment
for these women but further translational investigations regarding
the therapeutic efficacy of mTOR inhibitors in TNBC are required.
Our analysis also confirms the biological association between mTOR
activation and AR pathway, suggesting that may exist a subgroup of
TNBC in which the combination of both AR antagonism and mTOR
inhibition should have a synergistic effect on cell growth and
tumor progression.
References
|
1
|
Cuenca-López MD, Montero JC, Morales JC,
Prat A, Pandiella A and Ocana A: Phospho-kinase profile of triple
negative breast cancer and androgen receptor signaling. BMC Cancer.
14:3022014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dent R, Trudeau M, Pritchard KI, Hanna WM,
Kahn HK, Sawka CA, Lickley LA, Rawlinson E, Sun P and Narod SA:
Triple-negative breast cancer: Clinical features and patterns of
recurrence. Clin Cancer Res. 13:4429–4434. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liedtke C, Mazouni C, Hess KR, André F,
Tordai A, Mejia JA, Symmans WF, Gonzalez-Angulo AM, Hennessy B,
Green M, et al: Response to neoadjuvant therapy and long-term
survival in patients with triple-negative breast cancer. J Clin
Oncol. 26:1275–1281. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Craig DW, O'Shaughnessy JA, Kiefer JA,
Aldrich J, Sinari S, Moses TM, Wong S, Dinh J, Christoforides A,
Blum JL, et al: Genome and transcriptome sequencing in prospective
metastatic triple-negative breast cancer uncovers therapeutic
vulnerabilities. Mol Cancer Ther. 12:104–116. 2013. View Article : Google Scholar
|
|
5
|
Pal SK, Childs BH and Pegram M: Triple
negative breast cancer: Unmet medical needs. Breast Cancer Res
Treat. 125:627–636. 2011. View Article : Google Scholar :
|
|
6
|
André F and Zielinski CC: Optimal
strategies for the treatment of metastatic triple-negative breast
cancer with currently approved agents. Ann Oncol. 23(Suppl 6):
vi46–vi51. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lehmann BD and Pietenpol JA:
Identification and use of biomarkers in treatment strategies for
triple-negative breast cancer subtypes. J Pathol. 232:142–150.
2014. View Article : Google Scholar :
|
|
8
|
Laplante M and Sabatini DM: mTOR signaling
in growth control and disease. Cell. 149:274–293. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dann SG, Selvaraj A and Thomas G: mTOR
Complex1-S6K1 signaling: At the crossroads of obesity, diabetes and
cancer. Trends Mol Med. 13:252–259. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yaba A, Bianchi V, Borini A and Johnson J:
A putative mitotic checkpoint dependent on mTOR function controls
cell proliferation and survival in ovarian granulosa cells. Reprod
Sci. 15:128–138. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vazquez-Martin A, Oliveras-Ferraros C,
Bernadó L, López-Bonet E and Menendez JA: The serine
2481-autophosphorylated form of mammalian target of rapamycin
(mTOR) is localized to midzone and midbody in dividing cancer
cells. Biochem Biophys Res Commun. 380:638–643. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Walsh S, Flanagan L, Quinn C, Evoy D,
McDermott EW, Pierce A and Duffy MJ: mTOR in breast cancer:
Differential expression in triple-negative and non-triple-negative
tumors. Breast. 21:178–182. 2012. View Article : Google Scholar
|
|
13
|
Zhou X, Tan M, Stone Hawthorne V, Klos KS,
Lan KH, Yang Y, Yang W, Smith TL, Shi D and Yu D: Activation of the
Akt/mammalian target of rapamycin/4E-BP1 pathway by ErbB2
overexpression predicts tumor progression in breast cancers. Clin
Cancer Res. 10:6779–6788. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ueng SH, Chen SC, Chang YS, Hsueh S, Lin
YC, Chien HP, Lo YF, Shen SC and Hsueh C: Phosphorylated mTOR
expression correlates with poor outcome in early-stage triple
negative breast carcinomas. Int J Clin Exp Pathol. 5:806–813.
2012.PubMed/NCBI
|
|
15
|
Shaw RJ and Cantley LC: Ras, PI(3)K and
mTOR signalling controls tumour cell growth. Nature. 441:424–430.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hammond ME, Hayes DF, Dowsett M, Allred
DC, Hagerty KL, Badve S, Fitzgibbons PL, Francis G, Goldstein NS,
Hayes M, et al: American Society of Clinical Oncology/College Of
American Pathologists guideline recommendations for
immunohistochemical testing of estrogen and progesterone receptors
in breast cancer. J Clin Oncol. 28:2784–2795. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wolff AC, Hammond ME, Hicks DG, Dowsett M,
McShane LM, Allison KH, Allred DC, Bartlett JM, Bilous M,
Fitzgibbons P, et al American Society of Clinical Oncology; College
of American Pathologists: Recommendations for human epidermal
growth factor receptor 2 testing in breast cancer: American Society
of Clinical Oncology/College of American Pathologists clinical
practice guideline update. J Clin Oncol. 31:3997–4013. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yunokawa M, Koizumi F, Kitamura Y,
Katanasaka Y, Okamoto N, Kodaira M, Yonemori K, Shimizu C, Ando M,
Masutomi K, et al: Efficacy of everolimus, a novel mTOR inhibitor,
against basal-like triple-negative breast cancer cells. Cancer Sci.
103:1665–1671. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vazquez-Martin A, Oliveras-Ferraros C, Del
Barco S, Martin-Castillo B and Menendez JA: If mammalian target of
metformin indirectly is mammalian target of rapamycin, then the
insulin-like growth factor-1 receptor axis will audit the efficacy
of metformin in cancer clinical trials. J Clin Oncol. 27:e207–209;
author reply e210. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang H, Cohen AL, Krishnakumar S, Wapnir
IL, Veeriah S, Deng G, Coram MA, Piskun CM, Longacre TA, Herrler M,
et al: Patient-derived xenografts of triple-negative breast cancer
reproduce molecular features of patient tumors and respond to mTOR
inhibition. Breast Cancer Res. 16:R362014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xu S, Li S, Guo Z, Luo J, Ellis MJ and Ma
CX: Combined targeting of mTOR and AKT is an effective strategy for
basal-like breast cancer in patient-derived xenograft models. Mol
Cancer Ther. 12:1665–1675. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bose S, Chandran S, Mirocha JM and Bose N:
The Akt pathway in human breast cancer: a tissue-array-based
analysis. Mod Pathol. 19:238–245. 2006. View Article : Google Scholar
|
|
23
|
Lehmann BD, Bauer JA, Schafer JM,
Pendleton CS, Tang L, Johnson KC, Chen X, Balko JM, Gómez H,
Arteaga CL, et al: PIK3CA mutations in androgen receptor-positive
triple negative breast cancer confer sensitivity to the combination
of PI3K and androgen receptor inhibitors. Breast Cancer Res.
16:4062014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lehmann BD, Bauer JA, Chen X, Sanders ME,
Chakravarthy AB, Shyr Y and Pietenpol JA: Identification of human
triple-negative breast cancer subtypes and preclinical models for
selection of targeted therapies. J Clin Invest. 121:2750–2767.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gucalp A and Traina TA: Triple-negative
breast cancer: Role of the androgen receptor. Cancer J. 16:62–65.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Edlind MP and Hsieh AC: PI3K-AKT-mTOR
signaling in prostate cancer progression and androgen deprivation
therapy resistance. Asian J Androl. 16:378–386. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dong G, Liang X, Wang D, Gao H, Wang L,
Wang L, Liu J and Du Z: High expression of miR-21 in
triple-negative breast cancers was correlated with a poor prognosis
and promoted tumor cell in vitro proliferation. Med Oncol.
31:572014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Medimegh I, Omrane I, Privat M, Uhrhummer
N, Ayari H, Belaiba F, Benayed F, Benromdhan K, Mader S, Bignon IJ,
et al: MicroRNAs expression in triple negative vs non triple
negative breast cancer in Tunisia: Interaction with clinical
outcome. PLoS One. 9:e1118772014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Huang J, Dibble CC, Matsuzaki M and
Manning BD: The TSC1-TSC2 complex is required for proper activation
of mTOR complex 2. Mol Cell Biol. 28:4104–4115. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ke Q and Costa M: Hypoxia-inducible
factor-1 (HIF-1). Mol Pharmacol. 70:1469–1480. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
DeYoung MP, Horak P, Sofer A, Sgroi D and
Ellisen LW: Hypoxia regulates TSC1/2-mTOR signaling and tumor
suppression through REDD1-mediated 14-3-3 shuttling. Genes Dev.
22:239–251. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Culjkovic B, Topisirovic I, Skrabanek L,
Ruiz-Gutierrez M and Borden KL: eIF4E is a central node of an RNA
regulon that governs cellular proliferation. J Cell Biol.
175:415–426. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Culjkovic-Kraljacic B, Baguet A, Volpon L,
Amri A and Borden KL: The oncogene eIF4E reprograms the nuclear
pore complex to promote mRNA export and oncogenic transformation.
Cell Rep. 2:207–215. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Giulietti M, Milantoni SA, Armeni T,
Principato G and Piva F: ExportAid: Database of RNA elements
regulating nuclear RNA export in mammals. Bioinformatics.
31:246–251. 2015. View Article : Google Scholar
|
|
35
|
Rokavec M, Li H, Jiang L and Hermeking H:
The p53/miR-34 axis in development and disease. J Mol Cell Biol.
6:214–230. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hermeking H: MicroRNAs in the p53 network:
Micromanagement of tumour suppression. Nat Rev Cancer. 12:613–626.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Östling P, Leivonen SK, Aakula A, Kohonen
P, Mäkelä R, Hagman Z, Edsjö A, Kangaspeska S, Edgren H, Nicorici
D, et al: Systematic analysis of microRNAs targeting the androgen
receptor in prostate cancer cells. Cancer Res. 71:1956–1967. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ribas J, Ni X, Haffner M, Wentzel EA,
Salmasi AH, Chowdhury WH, Kudrolli TA, Yegnasubramanian S, Luo J,
Rodriguez R, et al: miR-21: An androgen receptor-regulated microRNA
that promotes hormone-dependent and hormone-independent prostate
cancer growth. Cancer Res. 69:7165–7169. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Teng Y, Litchfield LM, Ivanova MM, Prough
RA, Clark BJ and Klinge CM: Dehydroepiandrosterone-induces miR-21
transcription in HepG2 cells through estrogen receptor β and
androgen receptor. Mol Cell Endocrinol. 392:23–36. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ma X, Choudhury SN, Hua X, Dai Z and Li Y:
Interaction of the oncogenic miR-21 microRNA and the p53 tumor
suppressor pathway. Carcinogenesis. 34:1216–1223. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Xiong Q, Zhong Q, Zhang J, Yang M, Li C,
Zheng P, Bi LJ and Ge F: Identification of novel miR-21 target
proteins in multiple myeloma cells by quantitative proteomics. J
Proteome Res. 11:2078–2090. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Meng F, Henson R, Wehbe-Janek H, Ghoshal
K, Jacob ST and Patel T: MicroRNA-21 regulates expression of the
PTEN tumor suppressor gene in human hepatocellular cancer.
Gastroenterology. 133:647–658. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Bao B, Ali S, Kong D, Sarkar SH, Wang Z,
Banerjee S, Aboukameel A, Padhye S, Philip PA and Sarkar FH:
Anti-tumor activity of a novel compound-CDF is mediated by
regulating miR-21, miR-200, and PTEN in pancreatic cancer. PLoS
One. 6:e178502011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Darido C, Georgy SR, Wilanowski T, Dworkin
S, Auden A, Zhao Q, Rank G, Srivastava S, Finlay MJ, Papenfuss AT,
et al: Targeting of the tumor suppressor GRHL3 by a
miR-21-dependent proto-oncogenic network results in PTEN loss and
tumorigenesis. Cancer Cell. 20:635–648. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Tang H, Liu P, Yang L and Xie X, Ye F, Wu
M, Liu X, Chen B, Zhang L and Xie X: miR-185 suppresses tumor
proliferation by directly targeting E2F6 and DNMT1 and indirectly
upregulating BRCA1 in triple-negative breast cancer. Mol Cancer
Ther. 13:3185–3197. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Cascione L, Gasparini P, Lovat F, Carasi
S, Pulvirenti A, Ferro A, Alder H, He G, Vecchione A, Croce CM, et
al: Integrated microRNA and mRNA signatures associated with
survival in triple negative breast cancer. PLoS One. 8:e559102013.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Augoff K, McCue B, Plow EF and
Sossey-Alaoui K: miR-31 and its host gene lncRNA LOC554202 are
regulated by promoter hypermethylation in triple-negative breast
cancer. Mol Cancer. 11:52012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Humphries B, Wang Z, Oom AL, Fisher T, Tan
D, Cui Y, Jiang Y and Yang C: MicroRNA-200b targets protein kinase
Cα and suppresses triple-negative breast cancer metastasis.
Carcinogenesis. 35:2254–2263. 2014. View Article : Google Scholar : PubMed/NCBI
|