Introduction
Colorectal cancer (CRC) is one of the most common
carcinomas in the world and the incidence is increasing in Asia
(1). Despite many advances in the
treatment of CRC, metastasis remains a difficult challenge
(2). Although the exact molecular
mechanisms are not completely understood, it is well established
that epithelial-mesenchymal transition (EMT) is indispensable for
carcinoma aggression, development and drug resistance (3). Some researches have demonstrated that
tumors undergoing EMT are associated with poor prognosis (4–6).
Traditionally, the mechanism(s) controlling the development of
cancer are proliferation and apoptosis, Yet, efforts to inhibit the
growth and spread of CRC has focused on the effective mechanism(s)
of EMT by which carcinoma can rapidly migrate.
EMT refers to the process by which epithelial cells
forfeit their polarity, change into interstitial cells and can move
easily in the extracellular matrix (7,8).
Consequently, epithelial cell markers such as E-cadherin are
downregulated, while mesenchymal cell markers such as N-cadherin,
vimentin, Snail, and Twist are downregulated (9). The loss or reduction of epithelial
marker E-cadherin expression is the paramount feature of EMT
(10). E-cadherin expression may be
suppressed by transcription factors Snail, Twist and Slug, causing
the metastasis of colon tumors (11–13).
Overexpression of Snail or Twist and loss of E-cadherin expression
play a significant role in the malignant progression of a multitude
of epithelial carcinomas, such as gastric, breast and prostate
cancer (4–6).
However, recent studies have found that oxymatrine,
a traditional Chinese herb extracted from Sophora flavescens
Ait., exhibits various anticancer activities. It is effectively
used for the medical therapy of liver fibrosis, viral hepatitis and
autoimmune disease (14–18). In addition, previous studies have
demonstrated that oxymatrine has antitumor efficacy in human
malignant cancer, associated with the stimulation of cell cycle
arrest, apoptosis and downregulation of the Wnt/β-catenin signaling
pathway (19–23). However, the possible mechanisms of
the antitumor activity of oxymatrine in human CRC are not well
elucidated.
In addition, recent studies have shown that
oxymatrine can prevent NF-κB nuclear translocation (14), suppress the transcriptional
activation of NF-κB in the VEGF signaling pathway and improve
intestinal epithelial barrier function via the NF-κB-mediated
signaling pathway (24,25). In short, oxymatrine has been shown
to be closely associated with the NF-κB signaling pathway. The
present study aimed to explore the relationship between the
antitumor efficacy of oxymatrine and EMT markers in CRC. We found
that oxymatrine impeded EMT by inhibiting NF-κB signaling pathway
activation.
Materials and methods
Cell culture and transfection
Human colon cancer RKO, HCT116 and SW480 cell lines
were acquired from the American Type Culture Collection (ATCC;
Manassas, VA, USA) and were cultured in Dulbecco's modified Eagle's
medium (DMEM) with 10% fetal bovine serum (FBS) and antibiotics
under a 5% CO2 humidified atmosphere at 37°C. The shRNAs
targeting p65 (Shanghai GenePharma, China) were diluted in OPTI-MEM
(Gibco-BRL, Gaithersburg, MD, USA). According to the manufacturer's
instructions, the cells were transfected with a complex of
Lipofectamine 2000 (Life Technologies Co. Carlsbad, CA, USA) and
shRNA oligo nucleotides. The cells were cultured in 6-well plates
at a density of 3×104 cells/well.
MTT assay to measure cell
proliferation
The cytotoxicity of oxymatrine was evaluated by an
MTT assay (Sigma-Aldrich). The cells were cultivated into 96-well
plates (1×104/well) and treated with distinct
concentrations of oxymatrine (0, 0.25, 0.5, 0.75 mg/ml) for 24 or
48 h. Cells treated with 0.9% NaCl were the control group. Then MTT
(20 µg;2 mg/ml) was added to each well and then incubation
was carried out for 4 h at 37°C, and 100 µl DMSO was added
to each well. Finally, cell viability was analyzed by utilizing a
microplate reader (Thermo Multiskan MK3; Fermentas, Glen Burnie,
MD, USA) at a 490-nm wavelength. The cell growth inhibition rate
(%) was calculated using the subsequent equation: Inhibitory rate
(%) = (1 − Atreatment/Acontrol) × 100%.
Western blot analysis
RKO cells were cultured overnight, and treated with
distinct concentrations of oxymatrine or ammonium pyrrolidine
dithiocarbamate (PDTC; Sigma-Aldrich, USA) for 24 h. RKO cells
treated with 0.9% NaCl were used as the control group. Cells were
collected and lysed in cell lysis buffer [150 mM NaCl, 1% sodium
deoxycholate, 50 mM Tris (pH 7.5), SDS (sodium dodecyl sulfate
0.1%), 1 mM PMSF, 1% Triton X-100, 1 mM EDTA and 1 mM
Na3VO4]. Then centrifugation was carried out
at 12,000 × g for 15 min at 4°C, and the protein was collected and
evaluated using the BCA protein assay kit (Pierce, Rockford, IL,
USA). Protein (35 µg) from each sample was separated with
5–10% SDS-PAGE. After addition of 5% nonfat milk, each membrane was
incubated overnight at 4°C with the antibody against GAPDH, P65,
E-cadherin, N-cadherin or Snail, and then incubated with
peroxidase-conjugated secondary antibody (all from Cell Signaling
Technology, Inc., USA). The protein semaphores were detected using
an enhanced chemiluminescence kit (Pierce) and exposed to X-ray
film.
Wound healing assay
RKO cells were put into a 6-well cell culture plate
and grown to 70–80% confluency. Then the cells were scratched using
a 1-mm tip. RKO cells were cultured in medium containing 2% serum
and oxymatrine (0, 0.25, 0.5, 0.75 mg/ml) for 24 h. Images were
acquired at 0 and 24 h after the addition of oxymatrine. The
migration distance of the RKO cells was measured under a
microscope.
Invasion assay
The invasion of the cells was evaluated with 24-well
Transwell chambers (Corning, Tewksbury, MA, USA). Cells
(1.0×105) in 0.2 ml serum-free medium were placed into
the upper wells, and 0.6 ml medium with 10% FBS was put in the
lower wells. The upper wells were coated with 100 µl 5
µg/ml Matrigel (Corning Costar, Corning, NY, USA). The
chambers were incubated in 5% CO2 air for 24 h at 37°C.
Cells penetrating through the porous membrane were detected by
crystal violet staining, and then observed with a light microscope.
The numbers of cells were counted in four random fields.
Statistical analyses
The experimental data are expressed as mean ± SD,
and were statistically analyzed by the t-test method (two-tailed).
Statistical data were processed by SPSS software (SPSS Inc.,
Chicago, IL, USA).
Results
Oxymatrine inhibits the proliferation of
CRC cells
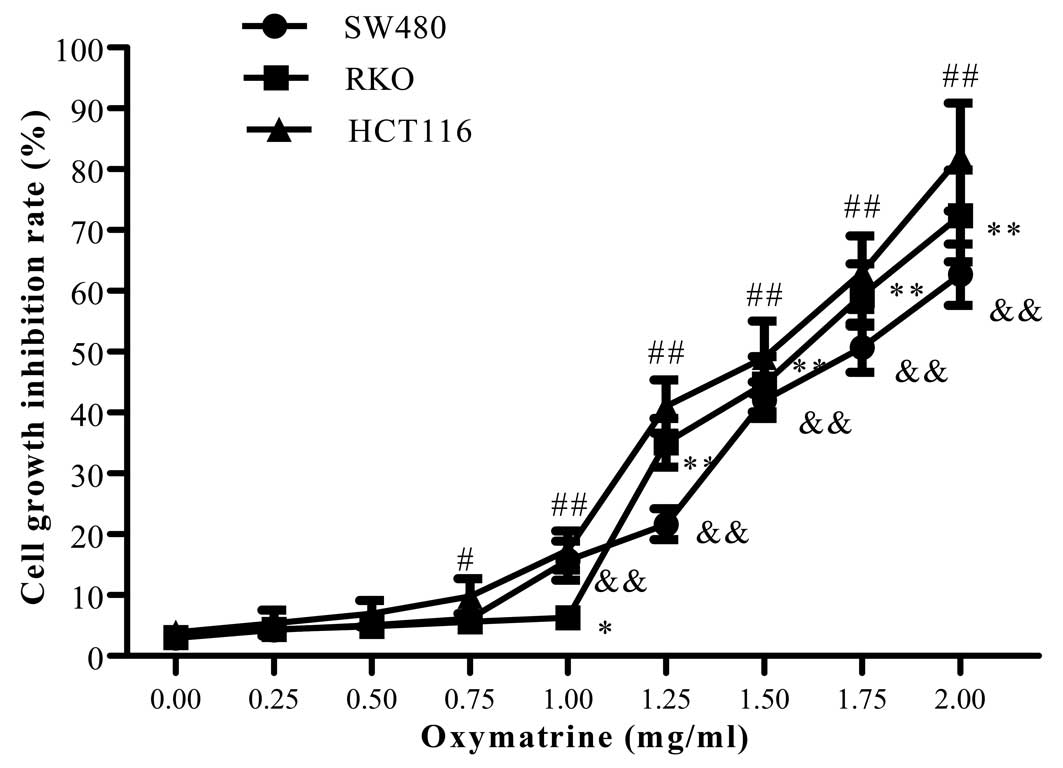
To determine the effect of oxymatrine on CRC cell
proliferation, SW480, RKO and HCT116 cells were simultaneously
treated with various concentrations of oxymatrine (0, 0.25, 0.5,
0.75, 1, 1.25, 1.5, 1.75, 2 mg/ml) for 24 h. Oxymatrine had no
significant effects on the proliferation of CRC cells at a
concentration <1 mg/ml. When the concentration was increased to
1 mg/ml, oxymatrine obviously decreased the proliferation of CRC
cells in a dose and time-dependent manner (Fig. 1). To exclude the an effect of
oxymatrine on cell proliferation, we chose a concentration <1
mg/ml to study the efficacy of oxymatrine on CRC cell invasion in
all subsequent experiments.
Oxymatrine suppresses the migratory
ability of RKO cells
In order to determine whether oxymatrine suppresses
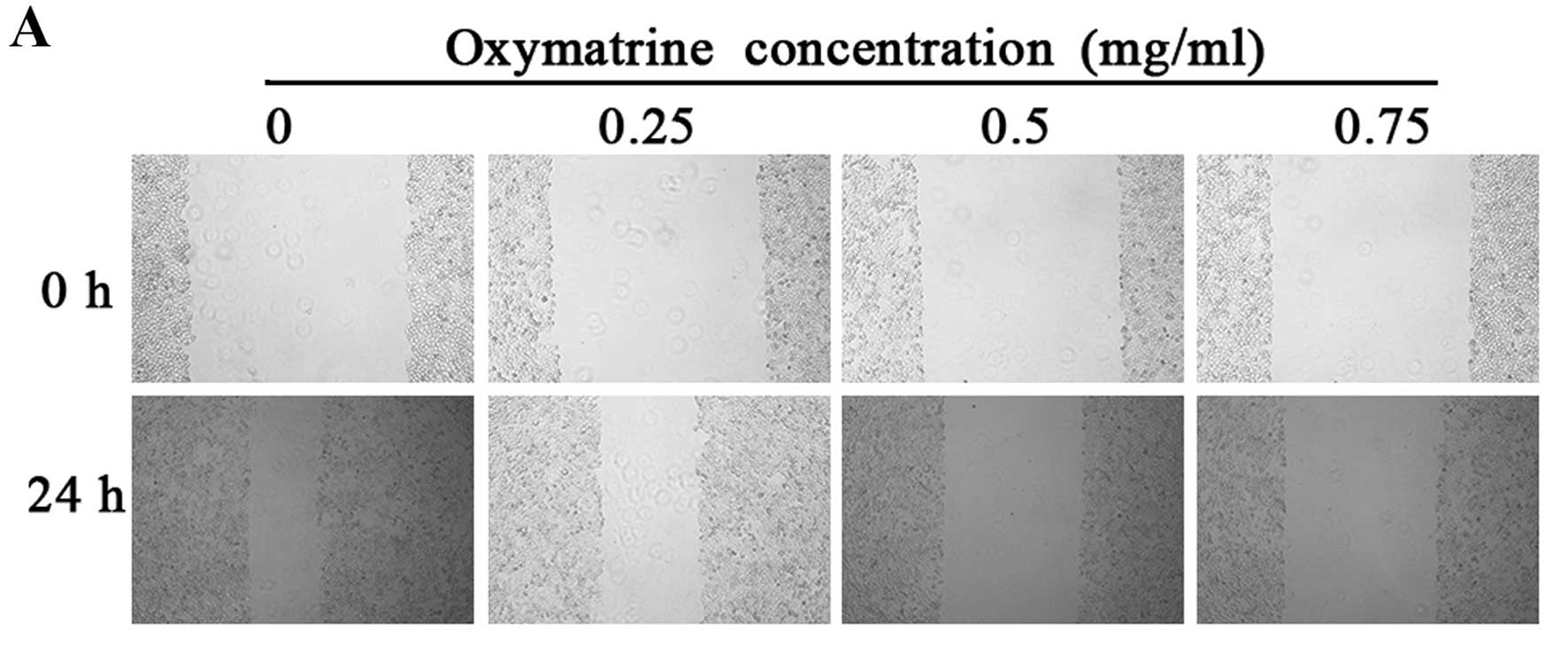
the migratory ability of RKO cells, we performed a wound healing
assay. Compared with the control group, the scratch wound in the
oxymatrine-treated group healed much slower (Fig. 2A). At 24 h after wounding, the
scratch width was 64.00, 39.33 and 28.87% of the control group in
the RKO cells following treatment with 0.25, 0.5 and 0.75 mg/ml
oxymatrine, respectively (Fig. 2B).
These data demonstrated that oxymatrine had the ability to inhibit
the migration of RKO cells in a concentration-dependent manner.
Oxymatrine inhibits EMT of RKO cells
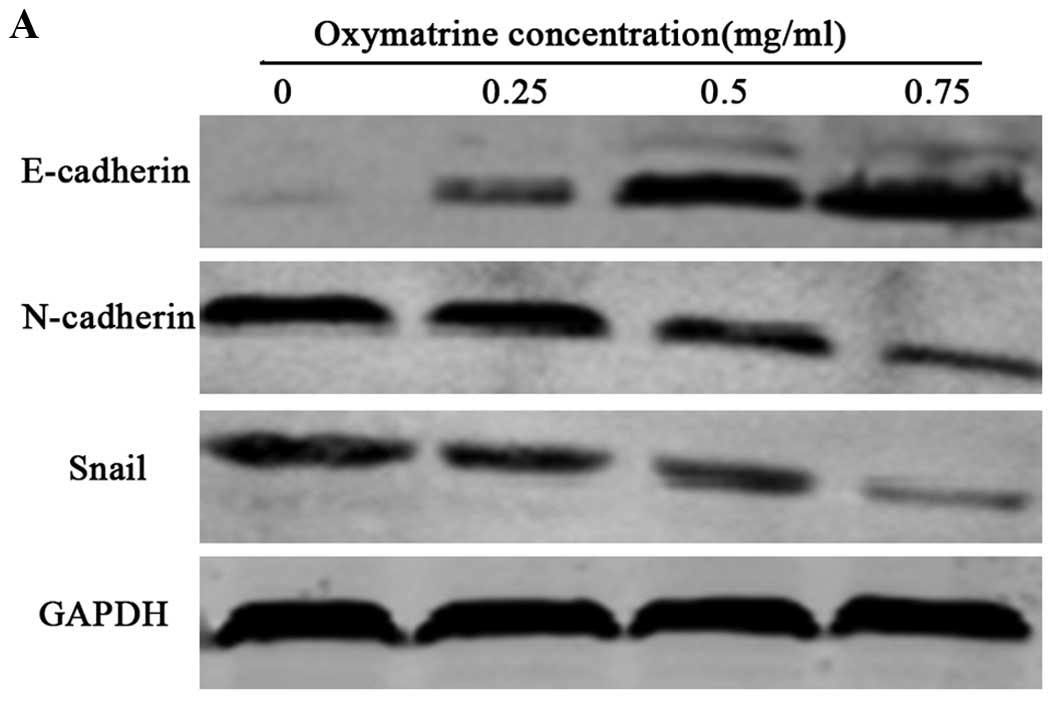
To verify whether oxymatrine can inhibit the EMT
process in RKO cells, we assessed the protein expression of EMT
markers, such as E-cadherin, Snail and N-cadherin, in the RKO cells
following treatment with 0.25, 0.5, and 0.75 mg/ml oxymatrine.
After 24 h of treatment, the protein levels of N-cadherin and Snail
were obviously decreased, however the protein level of epithelial
marker E-cadherin was obviously increased in a dose-dependent
manner in the RKO cells (Fig.
3).
Oxymatrine inhibits the protein level of
p65 in RKO cells
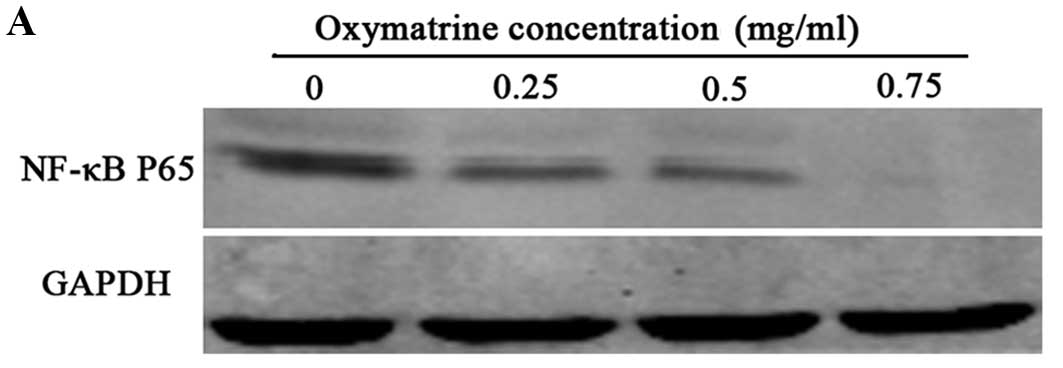
In order to explain the possible mechanism by which
oxymatrine inhibits EMT in RKO cells, we further aimed to ascertain
which upstream pathway is involved in mediating the effects of
oxymatrine. Western blot analysis demonstrated that oxymatrine
decreased the protein level of p65 in the RKO cells in a
dose-dependent manner (Fig. 4).
These data suggest that the NF-κB signaling pathway may mediate the
effect of oxymatrine in RKO cells.
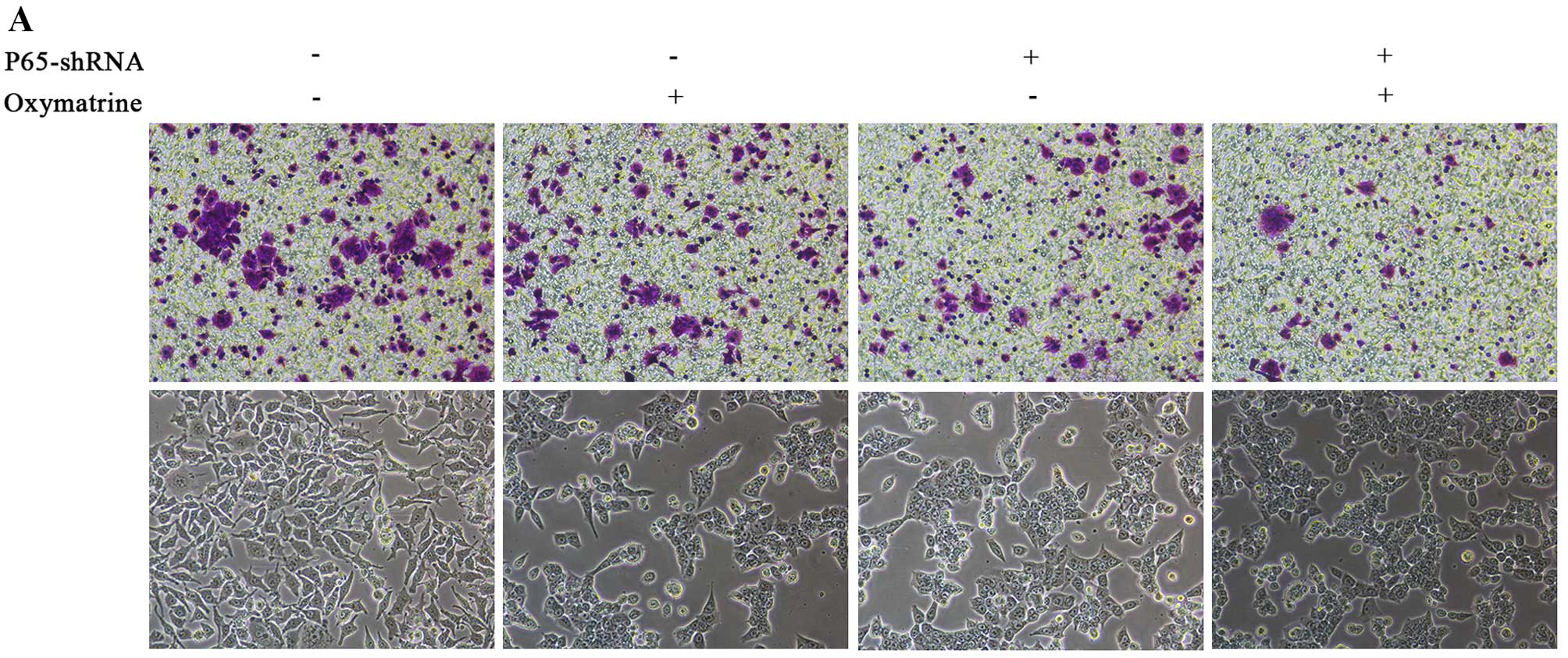
Oxymatrine inhibits the invasion of RKO
cells via NF-κB signaling
NF-κB signaling is closely correlated with cancer
growth and progression. To confirm whether NF-κB signaling mediates
the effects of oxymatrine on CRC cell invasion, we inhibited
endogenous p65 expression by shRNA method. p65 shRNA significantly
inhibited cell invasion. Moreover, p65 knockdown in the RKO cells
or the oxymatrine-treated RKO cells caused cellular morphological
changes, with the transformation from a long shuttle to
cobblestone-like shape and disappearance of tentacles (Fig. 5).
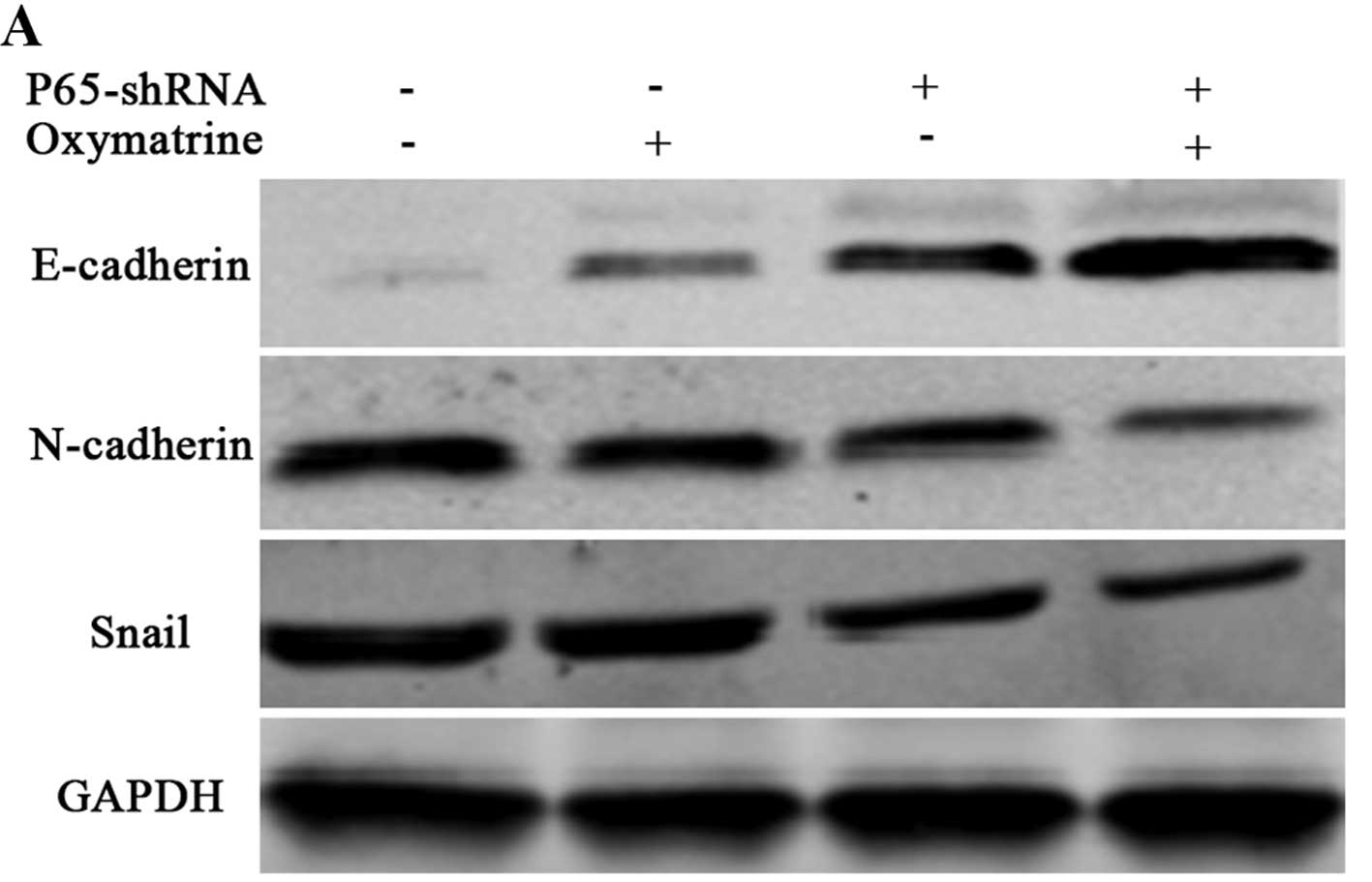
Oxymatrine regulates EMT markers via the
NF-κB signaling pathway in RKO cells
To ascertain whether NF-κB signaling is essential in
oxymatrine-mediated expression of EMT markers, we inhibited
endogenous p65 expression by shRNA method. E-cadherin expression
was obviously increased, however, N-cadherin and Snail were
decreased in the p65-shRNA cells (Fig.
6), indicating that p65 is essential for regulation of the EMT
process. Simultaneously, we discovered that inhibition of p65
increased E-cadherin expression and decreased expression of
N-cadherin and Snail. These data indicate that p65 is essential for
the EMT process and EMT inhibition by oxymatrine is partly through
the reduction of the activation of the NF-κB signaling pathway.
Discussion
EMT plays a crucial role in carcinoma progression
and metastasis. In the present study, we demonstrated that
oxymatrine inhibited the EMT process in RKO cells by decreasing
N-cadherin and Snail levels and by increasing the E-cadherin level.
The mechanism underlying the efficacy of oxymatrine on EMT in CRC
cells was further investigated. We discovered that oxymatrine
inhibited the expression of p65, which is important in regulating
the expression levels of EMT markers.
Oxymatrine exerts anticancer effects on several
types of cancer cells (20–23), but its effects on colorectal
carcinoma and its underlying molecular mechanisms remain
undetermined. In the present study, we investigated whether a low
concentration of oxymatrine exerted an anti-invasive effect on CRC
cells. Our results indicated that oxymatrine had the ability to
suppress the proliferation of CRC cells at high concentrations
(>1 mg/ml). Notably, a low concentration of oxymatrine (<1
mg/ml) exhibited anti-invasive efficacy in the CRC cells. These
results indicated that the inhibition of invasion by oxymatrine in
RKO cells was not due to cytotoxicity. In addition, we discovered
that oxymatrine increased the protein expression level of
E-cadherin, an important EMT marker. EMT is a complex process
characterized by the loss of epithelial cell-cell adhesion, an
important phenotype change in the enhanced invasive ability of
cancer cells (26). Snail is
considered as one of the major transcription factors which modulate
EMT in numerous carcinomas by suppressing E-cadherin (11,27).
We further found that oxymatrine inhibited the expression of Snail
in RKO cells, suggesting that oxymatrine exerts its anti-invasive
effect by inhibiting EMT. To the best of our knowledge, this is the
first demonstration of the anti-invasive efficacy of oxymatrine on
CRC.
p50/p105, p52/p100, p65, c-Rel, and RelB are 5
members of the NF-κB family, and the NF-κB complex (mainly p65/p50)
is maintained in an inactive state by inhibitory IκB protein in the
cytoplasm (28). After stimulation,
IκB is quickly phosphorylated and degraded via the ubiquitin
proteosome pathway. Thus, NF-κB p65 is released into the nucleus
and activates a variety of biological processes (29). Oxymatrine prevents NF-κB nuclear
translocation (14), suppresses the
transcriptional activation of NF-κB in the VEGF signaling pathway
and improves intestinal epithelial barrier function via the
NF-κB-mediated signaling pathway (24,25).
In the present study, we discovered that oxymatrine inhibited the
level of p65 in RKO cells, consistent with the above reports.
Emerging evidence indicates that NF-κB plays an indispensable role
in carcinoma invasion and metastasis (30–32).
Moreover, NF-κB can regulate invasion-related genes and is closely
linked to invasion and metastasis in CRC (33). Recent studies connect its activity
with the regulation of the EMT process. Anoxia/reoxygenation
induces EMT in human CRC which may be linked to NF-κB activation
(34). We inhibited endogenous p65
expression by shRNA method and demonstrated that oxymatrine
inhibited the invasion and modulated EMT in RKO cells at least
partially in an NF-κB-dependent manner. These results indicate that
oxymatrine may inhibit EMT and invasion via NF-κB signaling in RKO
cells. The mechanism underlying the effects of oxymatrine on the
NF-κB signaling pathway requires further investigation.
In conclusion, our results indicate that oxymatrine
reduces the activation of the NF-κB signaling pathway and inhibits
CRC invasion by modulating EMT. Oxymatrine is a promising agent for
CRC therapy.
References
|
1
|
Sung JJ, Lau JY, Goh KL and Leung WK; Asia
Pacific Working Group on Colorectal Cancer: Increasing incidence of
colorectal cancer in Asia: Implications for screening. Lancet
Oncol. 6:871–876. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Thomson S, Petti F, Sujka-Kwok I, Mercado
P, Bean J, Monaghan M, Seymour SL, Argast GM, Epstein DM and Haley
JD: A systems view of epithelial-mesenchymal transition signaling
states. Clin Exp Metastasis. 28:137–155. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rosivatz E, Becker I, Specht K, Fricke E,
Luber B, Busch R, Höfler H and Becker KF: Differential expression
of the epithelial-mesenchymal transition regulators snail, SIP1,
and twist in gastric cancer. Am J Pathol. 161:1881–1891. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Martin TA, Goyal A, Watkins G and Jiang
WG: Expression of the transcription factors snail, slug, and twist
and their clinical significance in human breast cancer. Ann Surg
Oncol. 12:488–496. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yuen HF, Chua CW, Chan YP, Wong YC, Wang X
and Chan KW: Significance of TWIST and E-cadherin expression in the
metastatic progression of prostatic cancer. Histopathology.
50:648–658. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Arias AM: Epithelial mesenchymal
interactions in cancer and development. Cell. 105:425–431. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Thiery JP: Epithelial-mesenchymal
transitions in tumour progression. Nat Rev Cancer. 2:442–454. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gheldof A and Berx G: Cadherins and
epithelial-to-mesenchymal transition. Prog Mol Biol Transl Sci.
116:317–336. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Peinado H, Olmeda D and Cano A: Snail, Zeb
and bHLH factors in tumour progression: An alliance against the
epithelial phenotype? Nat Rev Cancer. 7:415–428. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Celesti G, Di Caro G, Bianchi P, Grizzi F,
Basso G, Marchesi F, Doni A, Marra G, Roncalli M, Mantovani A, et
al: Presence of Twist1-positive neoplastic cells in the stroma of
chromosome-unstable colorectal tumors. Gastroenterology.
145:647–657.e15. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Toiyama Y, Yasuda H, Saigusa S, Tanaka K,
Inoue Y, Goel A and Kusunoki M: Increased expression of Slug and
Vimentin as novel predictive biomarkers for lymph node metastasis
and poor prognosis in colorectal cancer. Carcinogenesis.
34:2548–2557. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guzman JR, Koo JS, Goldsmith JR, Mühlbauer
M, Narula A and Jobin C: Oxymatrine prevents NF-κB nuclear
translocation and ameliorates acute intestinal inflammation. Sci
Rep. 3:16292013. View Article : Google Scholar
|
|
15
|
Chen XS, Wang GJ, Cai X, Yu HY and Hu YP:
Inhibition of hepatitis B virus by oxymatrine in vivo. World J
Gastroenterol. 7:49–52. 2001. View Article : Google Scholar
|
|
16
|
Chai NL, Fu Q, Shi H, Cai CH, Wan J, Xu SP
and Wu BY: Oxymatrine liposome attenuates hepatic fibrosis via
targeting hepatic stellate cells. World J Gastroenterol.
18:4199–4206. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li XM and Brown L: Efficacy and mechanisms
of action of traditional Chinese medicines for treating asthma and
allergy. In: J Allergy Clin Immunol. 123. pp. 297–306; quiz
307–308. 2009
|
|
18
|
Liu H, Sun Y, Gao Y, Chen F, Xu M and Liu
Z: The analgesic effect and mechanism of the combination of sodium
ferulate and oxymatrine. Neurochem Res. 35:1368–1375. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Song G, Luo Q, Qin J, Wang L, Shi Y and
Sun C: Effects of oxymatrine on proliferation and apoptosis in
human hepatoma cells. Colloids Surf B Biointerfaces. 48:1–5. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang Y, Liu H, Jin J, Zhu X, Lu L and
Jiang H: The role of endogenous reactive oxygen species in
oxymatrine-induced caspase-3-dependent apoptosis in human melanoma
A375 cells. Anticancer Drugs. 21:494–501. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ling Q, Xu X, Wei X, Wang W, Zhou B, Wang
B and Zheng S: Oxymatrine induces human pancreatic cancer PANC-1
cells apoptosis via regulating expression of Bcl-2 and IAP
families, and releasing of cytochrome c. J Exp Clin Cancer Res.
30:662011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Song MQ, Zhu JS, Chen JL, Wang L, Da W,
Zhu L and Zhang WP: Synergistic effect of oxymatrine and
angiogenesis inhibitor NM-3 on modulating apoptosis in human
gastric cancer cells. World J Gastroenterol. 13:1788–1793. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang Y, Piao B, Zhang Y, Hua B, Hou W, Xu
W, Qi X, Zhu X, Pei Y and Lin H: Oxymatrine diminishes the side
population and inhibits the expression of β-catenin in MCF-7 breast
cancer cells. Med Oncol. 28(Suppl 1): S99–S107. 2011. View Article : Google Scholar
|
|
24
|
Chen H, Zhang J, Luo J, Lai F, Wang Z,
Tong H, Lu D, Bu H, Zhang R and Lin S: Antiangiogenic effects of
oxymatrine on pancreatic cancer by inhibition of the NF-κB-mediated
VEGF signaling pathway. Oncol Rep. 30:589–595. 2013.PubMed/NCBI
|
|
25
|
Wen JB, Zhu FQ, Chen WG, Jiang LP, Chen J,
Hu ZP, Huang YJ, Zhou ZW, Wang GL, Lin H, et al: Oxymatrine
improves intestinal epithelial barrier function involving
NF-κB-mediated signaling pathway in CCl4-induced cirrhotic rats.
PLoS One. 9:e1060822014. View Article : Google Scholar
|
|
26
|
Iwatsuki M, Mimori K, Yokobori T, Ishi H,
Beppu T, Nakamori S, Baba H and Mori M: Epithelial-mesenchymal
transition in cancer development and its clinical significance.
Cancer Sci. 101:293–299. 2010. View Article : Google Scholar
|
|
27
|
Cano A, Pérez-Moreno MA, Rodrigo I,
Locascio A, Blanco MJ, del Barrio MG, Portillo F and Nieto MA: The
transcription factor snail controls epithelial-mesenchymal
transitions by repressing E-cadherin expression. Nat Cell Biol.
2:76–83. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Baldwin AS: Control of oncogenesis and
cancer therapy resistance by the transcription factor NF-kappaB. J
Clin Invest. 107:241–246. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hayden MS and Ghosh S: Shared principles
in NF-kappaB signaling. Cell. 132:344–362. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dolcet X, Llobet D, Pallares J and
Matias-Guiu X: NF-kB in development and progression of human
cancer. Virchows Arch. 446:475–482. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wu Y and Zhou BP: TNF-α/NF-kappaB/Snail
pathway in cancer cell migration and invasion. Br J Cancer.
102:639–644. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li CW, Xia W, Huo L, Lim SO, Wu Y, Hsu JL,
Chao CH, Yamaguchi H, Yang NK, Ding Q, et al:
Epithelial-mesenchymal transition induced by TNF-α requires
NF-κB-mediated transcriptional upregulation of Twist1. Cancer Res.
72:1290–1300. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang S, Liu Z, Wang L and Zhang X:
NF-kappaB signaling pathway, inflammation and colorectal cancer.
Cell Mol Immunol. 6:327–334. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Okajima M, Kokura S, Ishikawa T, Mizushima
K, Tsuchiya R, Matsuyama T, Adachi S, Okayama T, Sakamoto N, Kamada
K, et al: Anoxia/reoxygenation induces epithelial-mesenchymal
transition in human colon cancer cell lines. Oncol Rep.
29:2311–2317. 2013.PubMed/NCBI
|