Introduction
Gastric cancer is one of the most common
aerodigestive tract malignancies and is the third leading cause of
cancer-related deaths in the world (1). Although therapy for cancer has been
greatly improved, the prognosis of advanced gastric cancer is still
poor because of tumor invasion and metastasis (2). Thus, uncovering the molecular
mechanisms of gastric cancer to seek new molecular markers for
early detection of gastric cancer is necessary.
As a member of G protein-coupled receptor family,
CXCR3 binds to ELR-negative CXC chemokines such as CXCL9, CXCL10,
CXCL11 and CXCL4, and participates in various human diseases
including chronic inflammation (3),
immune dysfunction (4) and cancer
(5). CXCR3 has been reported to be
upregulated in many cancers including colon cancer and basal cell
carcinomas, and is closely associated with tumorigenesis and
prognosis (6,7). Three variants of CXCR3 (CXCR3A, CXCR3B
and CXCR3alt) have been identified in human cells. Many studies
have focused on the role of the major CXCR3 isoforms (CXCR3A and
CXCR3B) in cancer progression, and have found that these variants
of CXCR3 may exert different, even opposite, functions in cancer
(8). It has been reported that
CXCR3A is upregulated in clear cell ovarian cancer, but CXCR3B is
downregulated (9). Studies also
found that CXCR3A mRNA level is upregulated while CXCR3B mRNA is
downregulated in prostate cancer specimens, and downregulation of
CXCR3A but upregulation of CXCR3B significantly inhibits prostate
cancer cell proliferation and invasion (10,11).
In gastric cancer, a recent study showed that upregulation of
CXCR3B correlates with favorable prognosis of gastric cancer
patients (12). However, the role
of CXCR3A in gastric cancer remains unclear.
In this study, we examined the expression of CXCR3
variants in gastric cancer tissues and cells, and tried to uncover
the functions of CXCR3A and CXCR3B in gastric cancer cell invasion,
growth and metastasis in vitro and in vivo.
Materials and methods
Cell culture and reagents
All cell lines were purchased from Cell Resource
Center of the Chinese Academy of Medical Science (Beijing, China).
Gastric epithelium immortalized GES-1 cells were cultured in DMEM
medium supplemented with 10% fetal bovine serum (FBS), while
gastric cancer MKN28, SGC-7901 and AGS cells were cultured in
RPMI-1640 medium supplemented with 10% FBS. Cells were incubated in
a humidified incubator (37°C, 5% CO2). CXCL10 was
obtained from Sigma-Aldrich (St. Louis, MO, USA).
Antibodies against CXCR3, ERK1/2 and β-actin were
obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Antibody against phosho-ERK1/2 was obtained from Cell Signaling
Technology (Danvers, MA, USA).
Tumor tissues
Gastric cancer tissues and their corresponding
non-cancerous tissues were obtained from the Department of
Pathology (n=40), Liaocheng People's Hospital. All specimens were
approved by the Committee for Ethical Review of Research in the
hospital, and histopathologically confirmed by the pathologist.
Real-time PCR
Total RNA from the specimens and cancer cell lines
were extracted using TRIzol reagent (Invitrogen, Carlsbad, CA,
USA), according to the manufacturer's instructions. Reverse
transcription PCR was performed with total RNA and First Strand
cDNA Synthesis kit (Fermentas, Glen Burnie, MD, USA). Then the cDNA
was subjected to real-time PCR with ABI Prism 7700 Sequence
Detection System (Applied Biosystems, Foster City, CA, USA).
Primers used in the real-time PCR are as follows: CXCR3A forward,
5′-ACCCAGCAGCCAGAGCACC-3′ and reverse, 5′-TCA
TAGGAAGAGCTGAAGTTCTCCA-3′; CXCR3B forward,
5′-TGCCAGGCCTTTACACAGC-3′ and reverse, 5′-TCGGCGTCATTTAGCACTTG-3′;
CXCR3alt forward, 5′-CCAATACAACTTCCCACAGGGGT-3′ and reverse,
5′-GTCTCAGACCAGGATGAATCCCG-3′; β-actin forward,
5′-CATGTACGTTGCTATCCAGGC-3′ and reverse, 5′-CTCCTTAATGTCACGCACG-3′.
β-actin expression was used as an internal control. The results
were assessed using the 2−ΔΔCT method.
Western blot analysis
After washing with cold PBS, cells were lysed in
RIPA lysis buffer. Total protein concentration was determined using
BCA Protein Assay kit (Pierce Biotechnology, Inc., Rockford, IL,
USA). A total of 40 µg protein was resolved by SDS-PAGE and
then transferred to PVDF membrane. The membrane was blocked in 5%
BSA for 1 h, and incubated with primary antibodies at 4°C
overnight. Next, the membrane was incubated with appropriate
secondary antibodies and developed by enhanced chemiluminescence
(ECL) Plus detection system (Pierce Biotechnology, Inc.).
siRNA and shRNA
CXCR3A siRNA (siCXCR3A) and CXCR3B siRNA (siCXCR3B)
were purchased from Genchem Biotechnology Co. (Shanghai, China) for
transient silence CXCR3A and CXCR3B expression, respectively. A
scramble siRNA was purchased as control siRNA (siNC). CXCR3A shRNA
(shCXCR3A) was also purchased from Genchem Biotechnology Co. to
stably silence CXCR3A expression and a scramble shRNA was used as
control shRNA (shNC). Cells were transfected with siRNA or shRNA
using Lipofectamine 2000 (Invitrogen). Stable shRNA clone was
selected by G418. The knockdown efficiency was determined by
real-time PCR.
In vitro invasion and migration
assays
In vitro invasion and migration assays were
performed with 24-well Transwell plates (Corning Incorporated,
Corning, NY, USA). The upper filters were coated with 50 µl
Matrigel for invasion assay, whereas without Matrigel for migration
assay. Cells were resuspended in RPMI-1640 medium at a density of
5×105 cells/ml. A total of 200 µl of cell
suspension was added into the upper chambers while 500 µl of
RPMI-1640 medium containing 20% FBS was added into the lower
chambers. After 12 h, the cells that passed through the membranes
were fixed with methanol and stained with crystal blue. Cell number
was counted in seven random fields under a light microscope.
In vitro cell counting assay
Cells were seeded into a 24-well plate at a density
of 1×104 cells/well. Further, cells were stimulated with
or without CXCL10 and then incubated in the medium for the
indicated time. Next, the cells were trypsinized, stained with
trypan blue and counted in a hemocytometer.
CCK-8 assay
Cells were seeded in a 96-well plate at a
concentration of 1×103 cells/well. Further, cells were
stimulated with or without CXCL10 and then incubated in the medium
for the indicated time. Next, CCK-8 was added into the plate and
incubated for 2 h. Optical density (OD) was measured by microplate
reader (Bio-Rad Model 680) at 490 nm.
ELISA assay
After the cell supernatant or tumor tissues in the
mice were collected, matrix metalloproteinase (MMP)-13 and IL-6
ELISA kits (Invitrogen) were used to measure the protein level of
MMP-13 and IL-6, respectively, according to the manufacturer's
instructions.
In vivo growth and metastasis assays
The male BABL/c nude mice (4-week old) were
maintained in the pathogen-free conditions. All procedures were
conducted following the Animal Care and Use Committee guidelines of
Liaocheng People's Hospital. Cells at a density of 3×105
cells/100 µl were subcutaneously injected in the back of the
mice (n=8). The tumor formed in ~3 days. Then the length (L) and
width (W) of tumors in mice were measured every week. Tumor volume
was calculated with the formula of (L × W2)/2. Eight
weeks later, the mice were sacrificed and tumors were lysed to
detect MMP-13 and IL-6 expression and ERK1/2 activation. The livers
of all mice were fixed in 4% paraformaldehyde and sectioned into
slices. Each slice was stained with haematoxylin and eosin
(H&E) and then the number of micrometastasis in the livers was
observed and counted under a light microscope.
Statistical analysis
Experiments were performed at least three times.
Statistical analysis was performed using SPSS 17.0 (SPSS, Inc.,
Chicago, IL, USA). Student's t-test was used for comparison between
two groups whereas one-way analysis of variance (ANOVA) was used
for comparison among multiple groups. P<0.05 was considered as
statistically significant.
Results
CXCR3A is overexpressed in gastric cancer
cells and tissues
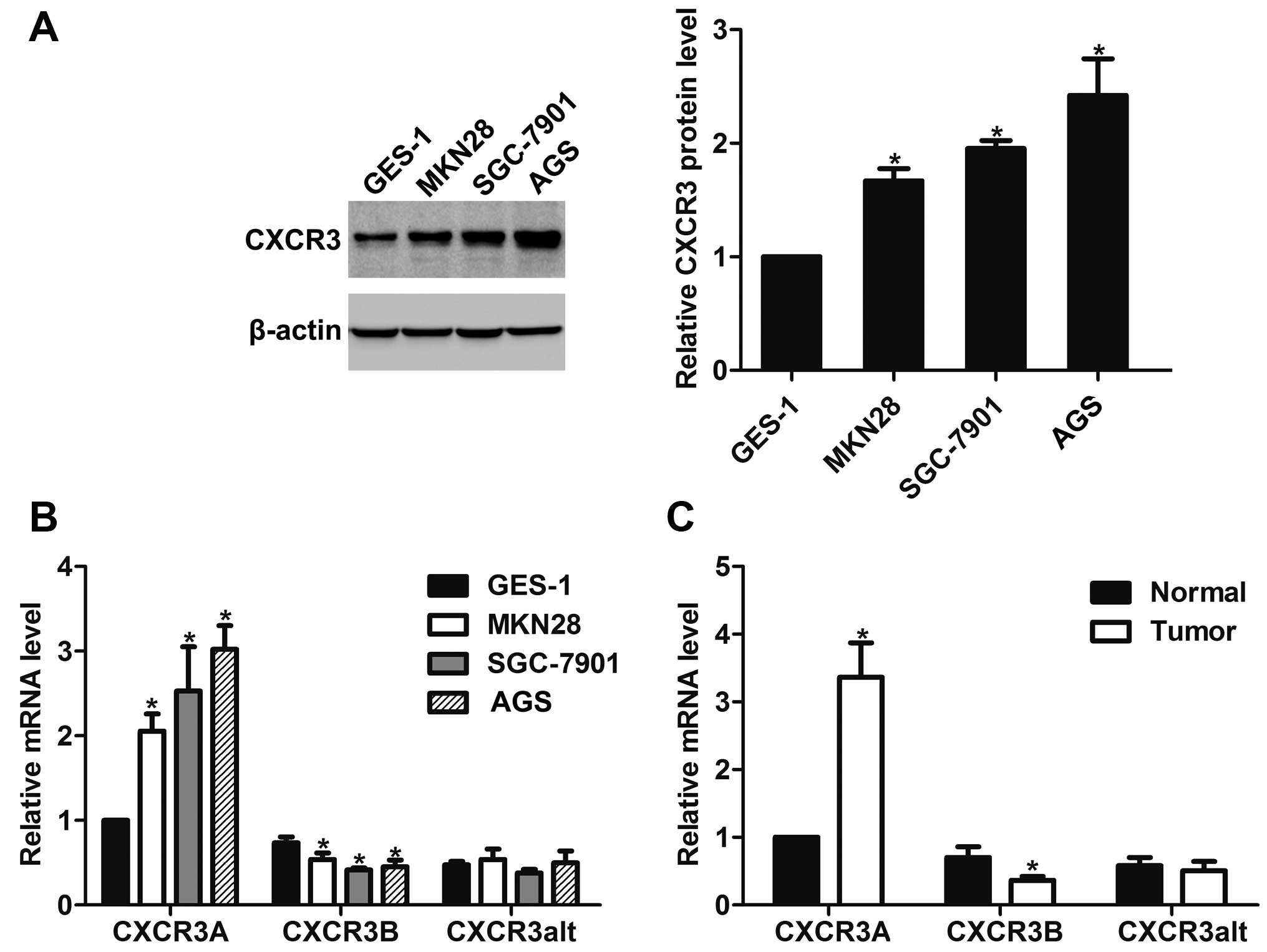
By western blot analysis, we found that CXCR3 was
overexpressed in gastric cancer MKN28, SGC-7901 and AGS cells as
compared to gastric epithelium immortalized GES-1 cells (Fig. 1A). The mRNA expression of its three
variants in gastric cancer cells were further assessed by real-time
PCR. The results showed that CXCR3A expression was upregulated but
CXCR3B expression was downregulated in gastric cancer cells.
CXCR3alt mRNA expression showed no significant change in any of the
detected gastric cancer cells compared with GES-1 cells (Fig. 1B). In addition, CXCR3A expression
was increased but CXCR3B expression was decreased in gastric cancer
tissues as compared to the corresponding gastric tissues, whereas
no significant change was observed for CXCR3alt expression
(Fig. 1C).
Activation of CXCR3 by CXCL10 promotes
the invasion and migration of gastric cancer cells in vitro
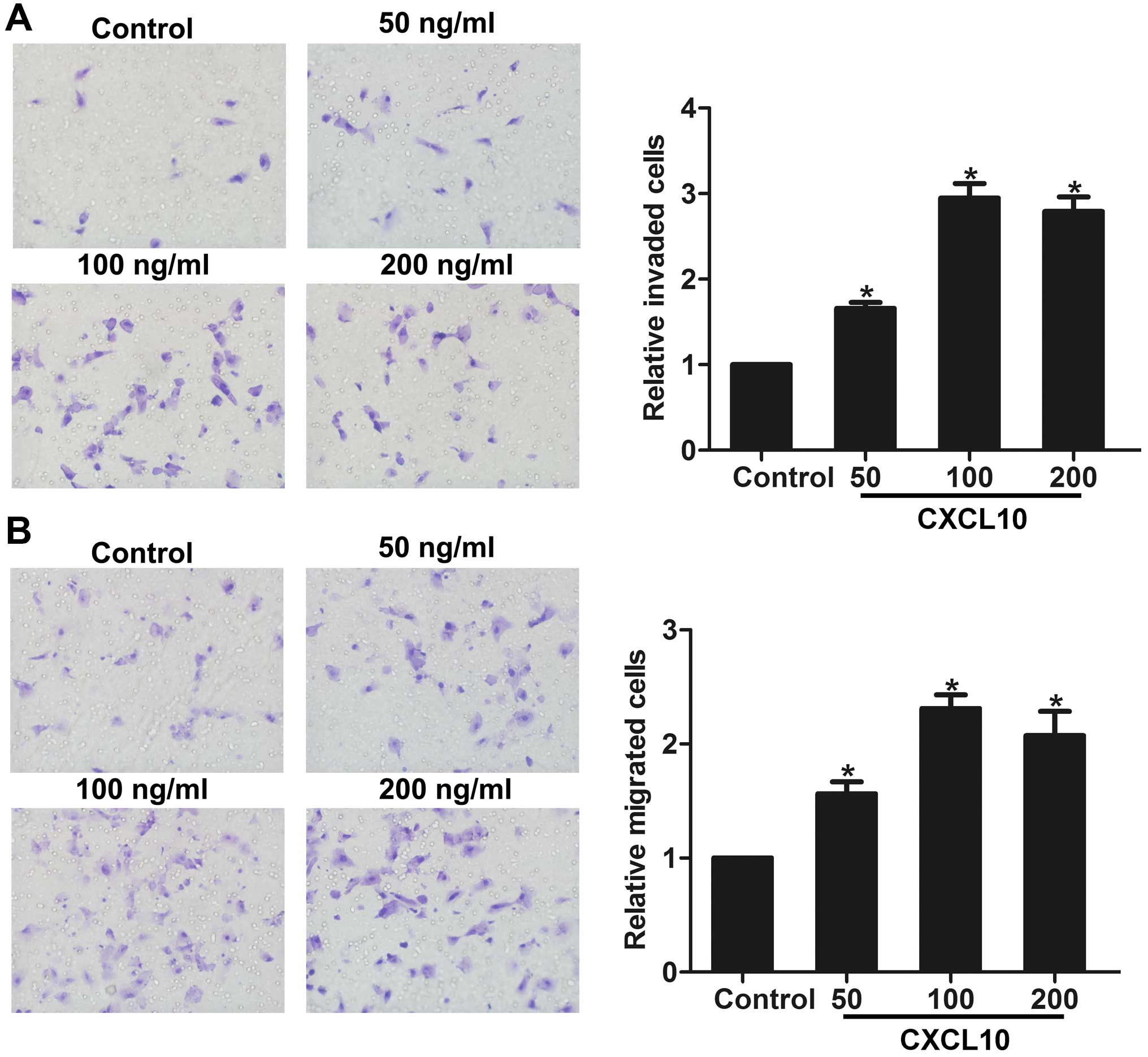
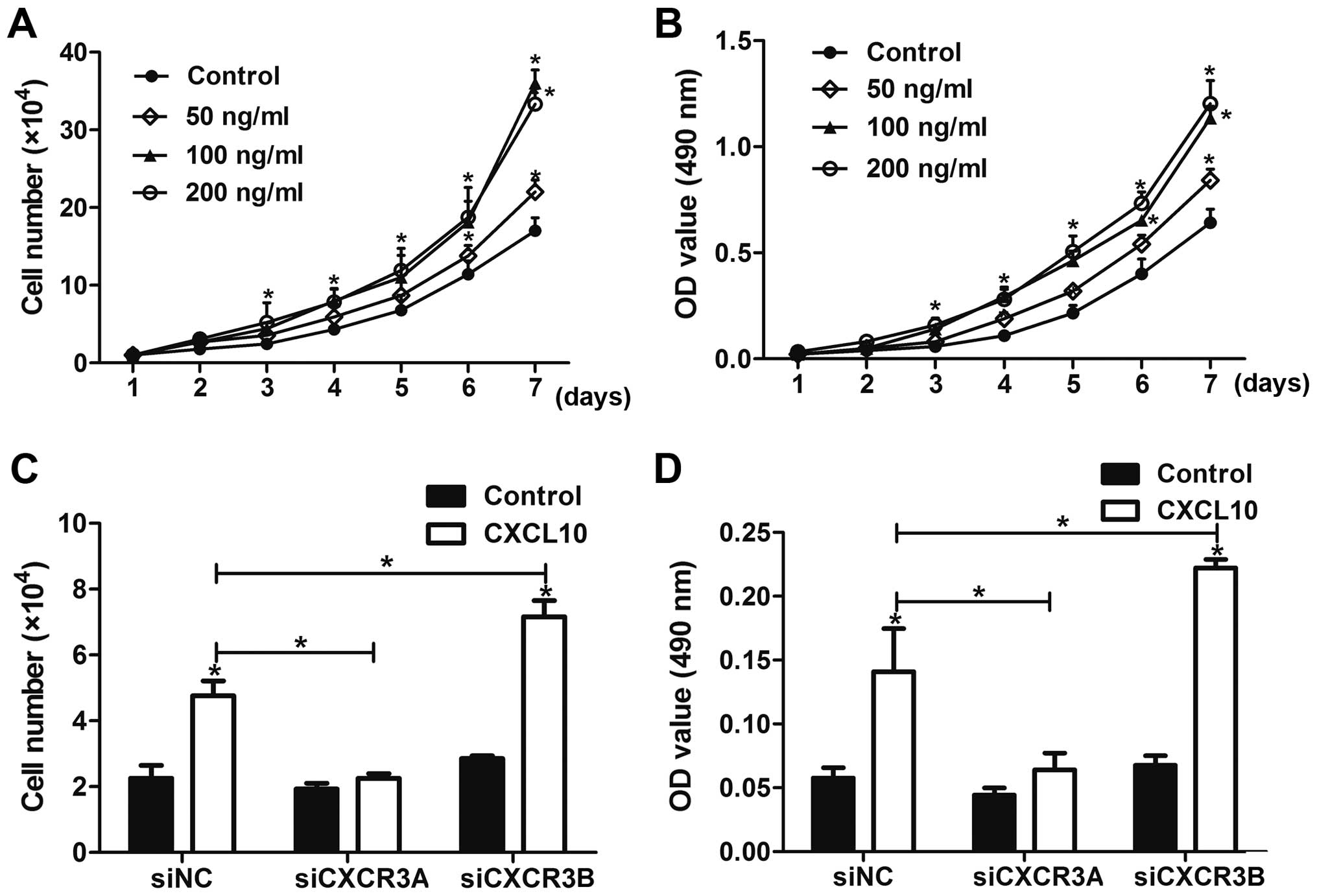
To investigate the role of CXCR3 in gastric cancer
cell invasion and migration in vitro, we stimulated AGS
cells with different concentrations of CXCL10 (CXCR3 ligand) to
activate CXCR3. Invasion and migration assays showed that CXCL10
promoted the invasion and migration of gastric cancer cells, in a
dose-dependent manner (Fig. 2A and
B), indicating that activation of CXCR3 may be involved in
gastric cancer cell invasion and migration in vitro.
Knockdown of CXCR3A inhibits gastric
cancer cell invasion and migration in vitro
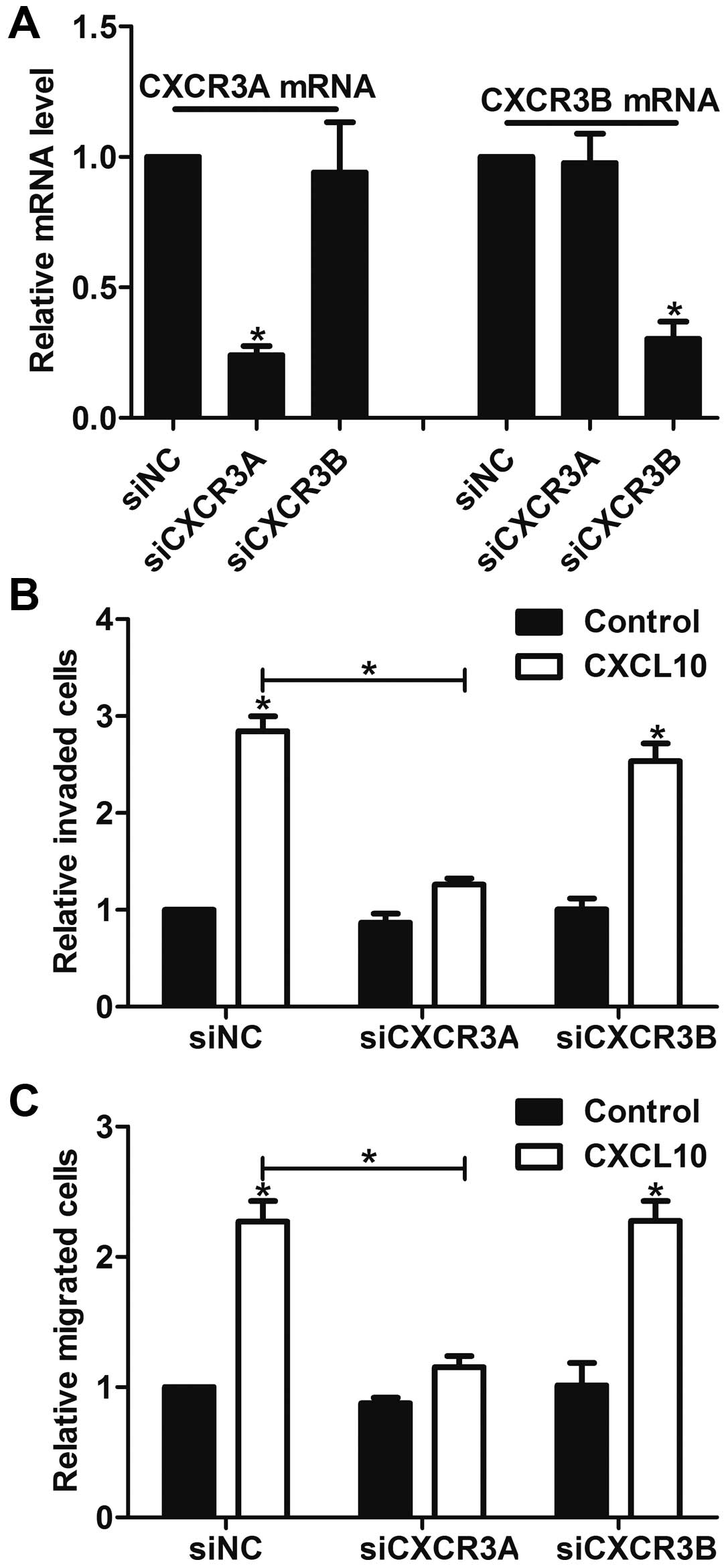
We then focused on the role of CXCR3A and CXCR3B in
gastric cancer cells, as they both showed significant changes in
gastric cancer tissues and cells. After confirming the specific
knockdown efficiency of CXCR3A and CXCR3B in AGS cells (Fig. 3A), the effects of CXCR3A and CXCR3B
on cell invasion and migration were detected by in vitro
invasion and migration assay. We found that CXCL10 (100 ng/ml)
stimulated the invasion and migration in siNC cells. In contrast,
knockdown of CXCR3A inhibited CXCL10-induced cell invasion and
migration, whereas knockdown of CXCR3B had little effect on the
invasion and migration (Fig. 3B and
C). These results confirm that it is CXCR3A, but not CXCR3B,
that participates in gastric cancer cell invasion and migration
in vitro.
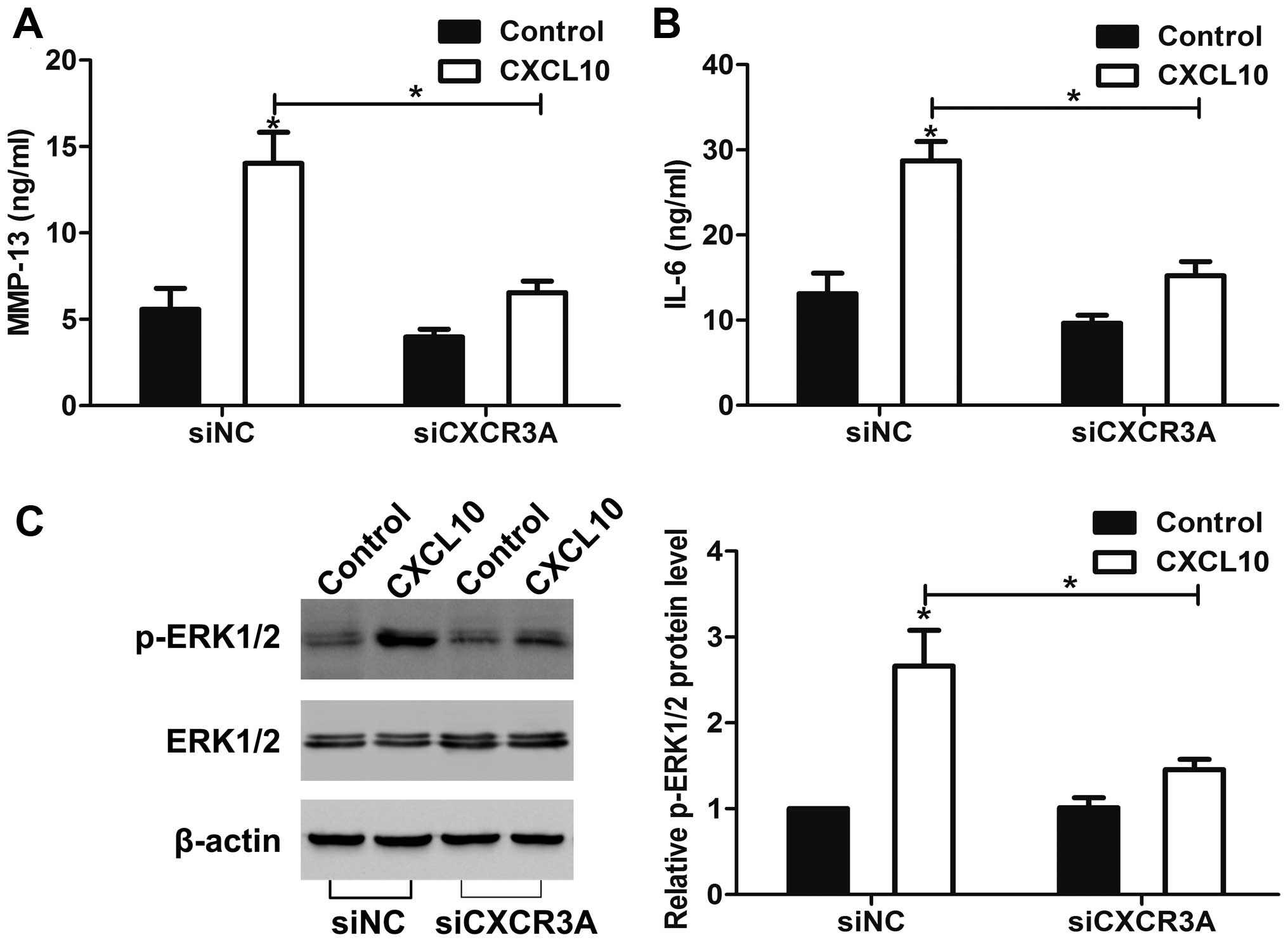
CXCR3A regulates MMP-13 and IL-6
secretion and ERK1/2 activation in vitro
We then found that MMP-13 and IL-6 secretion was
increased in siNC cells after CXCL10 stimulation for 12 h. However,
knockdown of CXCR3A greatly suppressed the CXCL10-induced MMP-13
and IL-6 secretion (Fig. 4A and B).
Moreover, after stimulated with CXCL10 for 30 min, ERK1/2 kinases
were activated in siNC cells. In contrast, the CXCL10-mediated
ERK1/2 activation was markedly attenuated in siCXCR3A cells
(Fig. 4C).
CXCR3A promotes the growth of gastric
cancer in vitro
Next, we found that CXCL10 treatment
dose-dependently promoted the growth of AGS cells (Fig. 5A and B). To test the role of CXCR3A
and CXCR3B in the growth of gastric cancer cells in vitro,
cells were transfected with siNC, siCXCR3A and siCXCR3B,
respectively, and then stimulated with 100 ng/ml CXCL10 for 72 h.
The results showed that knockdown of CXCR3A inhibited the
CXCL10-mediated growth of AGS cells, whereas knockdown of CXCR3B
promoted cell growth in vitro (Fig. 5C and D).
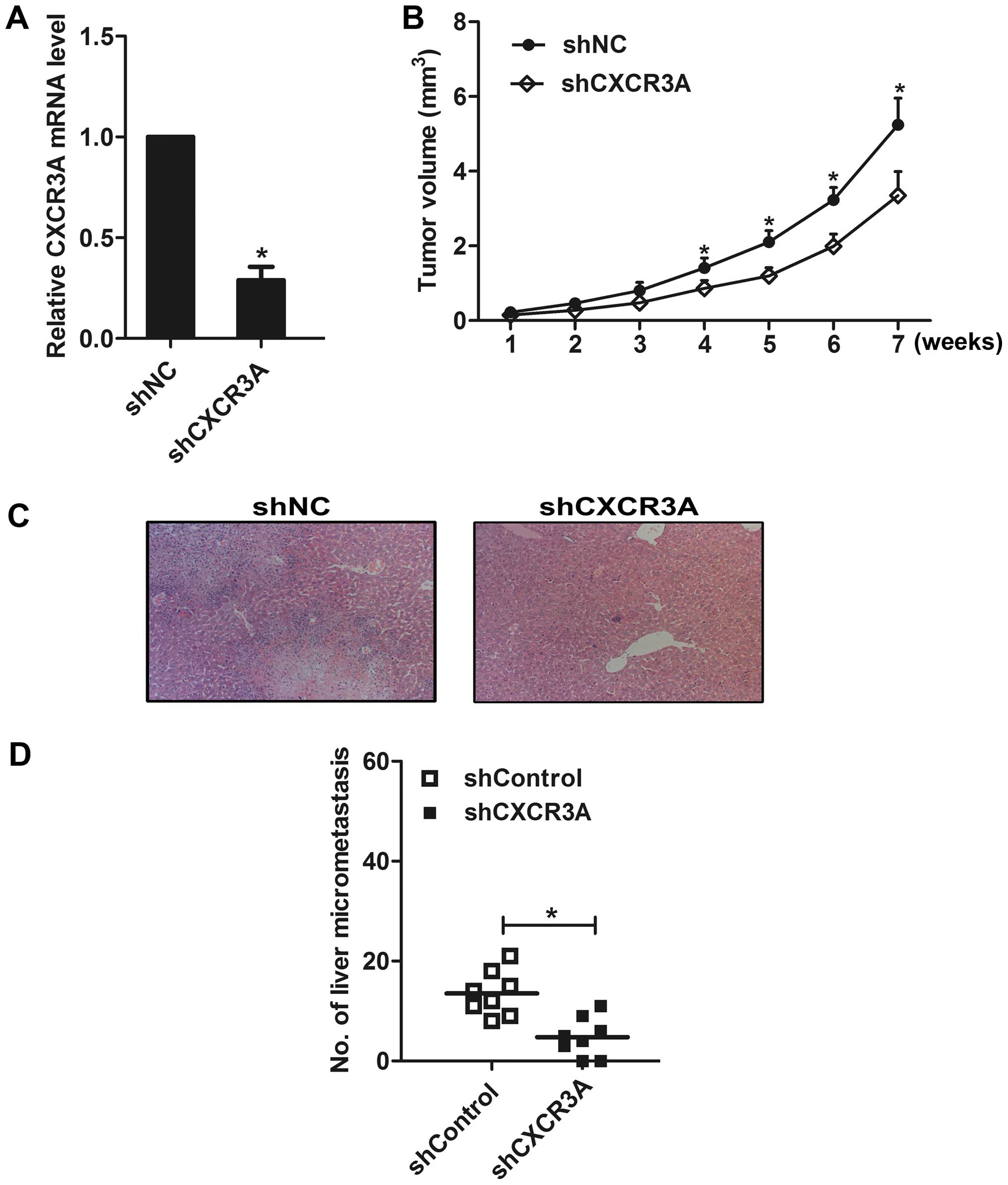
CXCR3A is involved in the growth and
metastasis of gastric cancer cells in vivo
Further, an AGS cell clone that stably silenced the
expression of CXCR3A was established and confirmed by real-time PCR
(Fig. 6A). Then the shCXCR3A and
shNC cells were injected subcutaneously at the back of the mice,
respectively. The tumor volume was assessed through measuring the
length and width of the tumor in mice each week. The results showed
that the tumor size in shCXCR3A group was much smaller than that in
shNC group (Fig. 6B). Eight weeks
later, the mice were sacrificed, micrometastasis in the liver
section was counted under a microscope (Fig. 6C). We found that liver metastasis
was observed in all eight mice in the shNC group, whereas it was
observed in six mice in the shCXCR3A group. We counted the number
of liver micrometastasis in all H&E slices, and found that
knockdown of CXCR3A decreased the number of micrometastasis in the
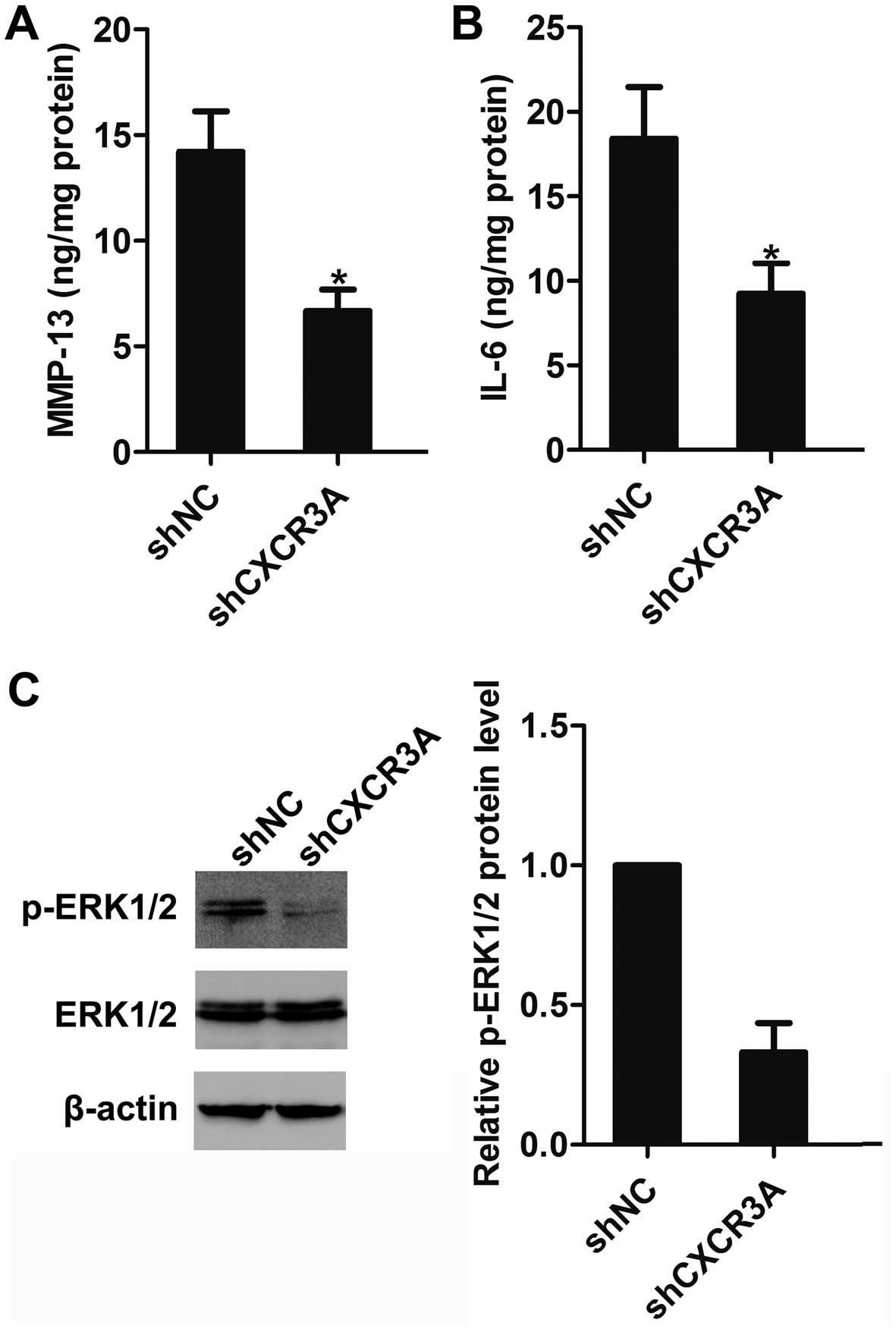
liver (Fig. 6D). In addition, we
found that the expression of MMP-13 and IL-6 in tumor tissues of
shCXCR3A group were significantly decreased (Fig. 7A and B), and the activation of
ERK1/2 was greatly inhibited (Fig.
7C), as compared to tumor tissues of shNC group.
Discussion
Chemokines are well known to modulate tumor
progression via activation of chemokine receptors in the membrane
of tumor cells (13). Belonging to
the CXC chemokine receptor subfamily, CXCR3 has three variants in
human cells (CXCR3A, CXCR3B and CXCR3alt) (14). Until now little is known about the
role of CXCR3 in gastric cancer. In our study, we found that CXCR3A
expression was increased, while CXCR3B expression was decreased in
gastric cancer tissues and cells. We also demonstrated that CXCR3A
participated in the growth, migration, invasion and metastasis of
gastric cancer cells in vitro and in vivo, supporting
the notion that CXCR3A acts as a positive mediator in gastric
cancer progression.
The level of CXCR3 has been found to be elevated in
many cancer tissues and cells (15,16).
However, the three mRNA splice variants of CXCR3 (CXCR3A, CXCR3B
and CXCR3alt) may show different level in the same tumor. Furuya
et al found that the mRNA levels of CXCR3A and CXCR3alt are
upregulated, while the mRNA expression of CXCR3B is downregulated
in clear cell ovarian cancer (9).
Here, we found that the mRNA expression of CXCR3A was upregulated
in gastric cancer tissues and cells, but the level of CXCR3B was
downregulated compared with normal control (corresponding gastric
tissues or GES-1 cells). Moreover, CXCR3alt expression was not
altered. The results suggest that these splice variants may play
different role in gastric cancer.
It is reported that CXCR3 signaling promotes the
growth of liver tumor (17).
Further studies found that CXCR3B expression correlates with tumor
necrosis and can mediate growth-inhibitory signals in human renal
cancer cells (18,19), whereas downregulation of CXCR3A
inhibits prostate cancer PC-3 cell proliferation (10). However, the role of CXCR3 in gastric
cancer growth remains unclear. In our study, we found that
knockdown of CXCR3A suppressed the growth of gastric cancer cells,
whereas knockdown of CXCR3B promoted gastric cancer growth,
confirming the conclusion that CXCR3A and CXCR3B display converse
roles in regulating gastric cancer cell growth.
Pu et al found that high expression of CXCR3
is an independent prognostic factor in glioblastoma patients that
promotes an invasive phenotype (20). However, Wu et al reported
that overexpression of CXCR3B in prostate cancer DU-145 cells
decreases cell invasion (11). The
function of CXCR3A and CXCR3B in gastric cancer cell invasion is
elusive. Here, we found that activation of CXCR3 by CXCL10
stimulated the invasion and migration of gastric cancer cells.
Further, knockdown of CXCR3A inhibited the CXCL10-mediated cell
invasion and migration, but knockdown of CXCR3B did not affect the
invasion and migration of gastric cancer cells. Studies have
reported that CXCR3 can promote the metastasis of breast and
osteosarcoma metastasis (21,22).
In renal cell carcinoma, CXCR3 and CXCR3A expression is found to be
significantly higher in metastatic than in non-metastatic carcinoma
samples (23). In our study, we
found that knockdown of CXCR3A suppressed the metastasis of gastric
cancer cells in vivo.
The MMP family plays a critical role in tumor
progression through ECM turnover and cancer cell migration. Shen
and Cao have reported that overexpression of CXCR3 increases the
expressions of MMP-1 and -3 in prostate cancer cells (10). Here, we found that CXCL10 increased
the expression of MMP-13 in gastric cancer cells, whereas knockdown
of CXCR3A decreased the MMP-13 expression in vitro.
Interleukins are a group of secreted proteins and signaling
molecules, and are involved in cancer progression (24,25).
As a member of interleukin family, IL-6 is reported to be required
for cancer invasion and migration (26,27).
Jenkins et al reported that CXCR3 signaling increases IL-8
expression in melanoma (28), but
the effect of CXCR3A on IL-6 expression in gastric cancer cells is
unclear. Here, we found that knockdown of CXCR3A downregulated the
CXCL10-mediated secretion of IL-6 in vitro. Moreover,
knockdown of CXCR3A inhibited the expression of MMP-13 and IL-6
in vivo. ERK1/2 pathway plays an important role in
regulating tumorigenicity and tumor development (29). In this study, we found that CXCR3A
could induce the activation of ERK1/2 in vitro and in
vivo.
In conclusion, our study demonstrates that CXCR3A is
overexpressed in gastric cancer tissues and cells. CXCL10
stimulation promotes the growth of gastric cancer cells via CXCR3A.
Moreover, CXCR3A contributes to the invasion and metastasis of
gastric cancer cells in vitro and in vivo, probably
via regulating MMP-13 and IL-6 expression and ERK1/2 activation.
Thus, CXCR3A could be a biomarker for gastric cancer diagnosis and
treatment.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
De Vita F, Di Martino N, Fabozzi A,
Laterza MM, Ventriglia J, Savastano B, Petrillo A, Gambardella V,
Sforza V, Marano L, et al: Clinical management of advanced gastric
cancer: The role of new molecular drugs. World J Gastroenterol.
20:14537–14558. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Singh UP, Venkataraman C, Singh R and
Lillard JW Jr: CXCR3 axis: Role in inflammatory bowel disease and
its therapeutic implication. Endocr Metab Immune Disord Drug
Targets. 7:111–123. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lacotte S, Brun S, Muller S and Dumortier
H: CXCR3, inflammation, and autoimmune diseases. Ann N Y Acad Sci.
1173:310–317. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Billottet C, Quemener C and Bikfalvi A:
CXCR3, a double-edged sword in tumor progression and angiogenesis.
Biochim Biophys Acta. 1836:287–295. 2013.PubMed/NCBI
|
|
6
|
Lo BK, Yu M, Zloty D, Cowan B, Shapiro J
and McElwee KJ: CXCR3/ligands are significantly involved in the
tumorigenesis of basal cell carcinomas. Am J Pathol. 176:2435–2446.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wu Z, Han X, Yan J, Pan Y, Gong J, Di J,
Cheng Z, Jin Z, Wang Z, Zheng Q, et al: The prognostic significance
of chemokine receptor CXCR3 expression in colorectal carcinoma.
Biomed Pharmacother. 66:373–377. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ma B, Khazali A and Wells A: CXCR3 in
carcinoma progression. Histol Histopathol. 30:781–792. 2015.
|
|
9
|
Furuya M, Yoneyama T, Miyagi E, Tanaka R,
Nagahama K, Miyagi Y, Nagashima Y, Hirahara F, Inayama Y and Aoki
I: Differential expression patterns of CXCR3 variants and
corresponding CXC chemokines in clear cell ovarian cancers and
endometriosis. Gynecol Oncol. 122:648–655. 2011. View Article : Google Scholar
|
|
10
|
Shen D and Cao X: Potential role of CXCR3
in proliferation and invasion of prostate cancer cells. Int J Clin
Exp Pathol. 8:8091–8098. 2015.
|
|
11
|
Wu Q, Dhir R and Wells A: Altered CXCR3
isoform expression regulates prostate cancer cell migration and
invasion. Mol Cancer. 11:32012. View Article : Google Scholar
|
|
12
|
Hu M, Li K, Maskey N, Xu Z, Yu F, Peng C,
Li Y and Yang G: Overexpression of the chemokine receptor CXCR3 and
its correlation with favorable prognosis in gastric cancer. Hum
Pathol. 46:1872–1880. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Salazar N, Castellan M, Shirodkar SS and
Lokeshwar BL: Chemokines and chemokine receptors as promoters of
prostate cancer growth and progression. Crit Rev Eukaryot Gene
Expr. 23:77–91. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Van Raemdonck K, Van den Steen PE, Liekens
S, Van Damme J and Struyf S: CXCR3 ligands in disease and therapy.
Cytokine Growth Factor Rev. 26:311–327. 2015. View Article : Google Scholar
|
|
15
|
Murakami T, Kawada K, Iwamoto M, Akagami
M, Hida K, Nakanishi Y, Kanda K, Kawada M, Seno H, Taketo MM, et
al: The role of CXCR3 and CXCR4 in colorectal cancer metastasis.
Int J Cancer. 132:276–287. 2013. View Article : Google Scholar
|
|
16
|
Goldberg-Bittman L, Neumark E, Sagi-Assif
O, Azenshtein E, Meshel T, Witz IP and Ben-Baruch A: The expression
of the chemokine receptor CXCR3 and its ligand, CXCL10, in human
breast adenocarcinoma cell lines. Immunol Lett. 92:171–178. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ling CC, Ng KT, Shao Y, Geng W, Xiao JW,
Liu H, Li CX, Liu XB, Ma YY, Yeung WH, et al: Post-transplant
endothelial progenitor cell mobilization via CXCL10/CXCR3 signaling
promotes liver tumor growth. J Hepatol. 60:103–109. 2014.
View Article : Google Scholar
|
|
18
|
Datta D, Banerjee P, Gasser M,
Waaga-Gasser AM and Pal S: CXCR3-B can mediate growth-inhibitory
signals in human renal cancer cells by down-regulating the
expression of heme oxygenase-1. J Biol Chem. 285:36842–36848. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gacci M, Serni S, Lapini A, Vittori G,
Alessandrini M, Nesi G, Palli D and Carini M: CXCR3-B expression
correlates with tumor necrosis extension in renal cell carcinoma. J
Urol. 181:843–848. 2009. View Article : Google Scholar
|
|
20
|
Pu Y, Li S, Zhang C, Bao Z, Yang Z and Sun
L: High expression of CXCR3 is an independent prognostic factor in
glioblastoma patients that promotes an invasive phenotype. J
Neurooncol. 122:43–51. 2015. View Article : Google Scholar
|
|
21
|
Ma X, Norsworthy K, Kundu N, Rodgers WH,
Gimotty PA, Goloubeva O, Lipsky M, Li Y, Holt D and Fulton A: CXCR3
expression is associated with poor survival in breast cancer and
promotes metastasis in a murine model. Mol Cancer Ther. 8:490–498.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pradelli E, Karimdjee-Soilihi B, Michiels
JF, Ricci JE, Millet MA, Vandenbos F, Sullivan TJ, Collins TL,
Johnson MG, Medina JC, et al: Antagonism of chemokine receptor
CXCR3 inhibits osteosarcoma metastasis to lungs. Int J Cancer.
125:2586–2594. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Utsumi T, Suyama T, Imamura Y, Fuse M,
Sakamoto S, Nihei N, Ueda T, Suzuki H, Seki N and Ichikawa T: The
association of CXCR3 and renal cell carcinoma metastasis. J Urol.
192:567–574. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Anestakis D, Petanidis S, Kalyvas S, Nday
CM, Tsave O, Kioseoglou E and Salifoglou A: Mechanisms and
applications of interleukins in cancer immunotherapy. Int J Mol
Sci. 16:1691–1710. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lippitz BE: Cytokine patterns in patients
with cancer: A systematic review. Lancet Oncol. 14:e218–e228. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang G, Ye Y, Zhang X and Song J:
Bradykinin stimulates IL-6 production and cell invasion in
colorectal cancer cells. Oncol Rep. 32:1709–1714. 2014.PubMed/NCBI
|
|
27
|
Dehai C, Bo P, Qiang T, Lihua S, Fang L,
Shi J, Jingyan C, Yan Y, Guangbin W and Zhenjun Y: Enhanced
invasion of lung adenocarcinoma cells after co-culture with
THP-1-derived macrophages via the induction of EMT by IL-6. Immunol
Lett. 160:1–10. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jenkins MH, Brinckerhoff CE and Mullins
DW: CXCR3 signaling in BRAFWT melanoma increases IL-8 expression
and tumorigenicity. PLoS One. 10:e01211402015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Burotto M, Chiou VL, Lee JM and Kohn EC:
The MAPK pathway across different malignancies: A new perspective.
Cancer. 120:3446–3456. 2014. View Article : Google Scholar : PubMed/NCBI
|