Introduction
Tuberous sclerosis complex (TSC) is a genetic
disease characterized by formation of benign tumors in multiple
organ systems, including the brain, eyes, heart, kidneys, skin and
lungs. There are several typical symptoms in TSC patients,
including epilepsy, mental deterioration, facial angiofibroma,
pulmonary lymphangiomyomatosis (LAM), kidney angiomyolipoma, and
renal cyst. The incidence of TSC is approximately 1:6,000–10,000
(1–3).
Tuberous sclerosis 1 (TSC1) and 2 (TSC2) are two
tumor suppressor genes located upstream of mechanistic target of
rapamycin (mTOR) (4). TSC is caused
by inactivating mutations of either TSC1 or TSC2. Hyperactivation
of mTOR caused by deficiency of either TSC1 or TSC2 is thought to
be the major cause of TSC development (1,5,6).
Therefore, mTOR inhibition is considered to be effective in the
treatment of TSC patients. However, rapamycin (mTOR specific
inhibitor) mediated disruption of the feedback suppression of
phosphatidylinositol 3-kinase (PI3K)/AKT signaling and
extracellular signal-regulated kinase (ERK)/mitogen-activated
protein kinase (MAPK) signaling from mTOR limited the therapeutic
effect of rapamycin on TSC patients (7–9).
Moreover, the effect of rapamycin treatment is not very
satisfactory because of the immunosuppressive property and drug
dependence of rapamycin (10,11).
Thus, to ameliorate the treatment for TSC, it is extremely
important to search for new therapeutic drugs for TSC.
Increasing attention has been paid to antitumor
drugs originating from traditional Chinese medicine (12,13).
As a kind of officinal fungi, Trametes robiniophila Murr.
(Huaier) is applied for the treatment of inflammation and cancer in
China. There are increasing evidence reporting that Huaier exerts
anti-neoplastic activities through inhibition of proliferation,
induction of apoptosis, suppression of angiogenesis, and inhibition
of metastasis of cancer cells (14–18).
However, the underlying mechanisms of anticancer effect of Huaier
remain poorly understood.
In this study, we investigated the effect of Huaier
aqueous extract on Tsc1- or Tsc2-null mouse embryonic fibroblasts
(MEFs), two widely used TSC cell models. Huaier aqueous extract
inhibited the proliferation and metastasis, and promoted cell cycle
arrest and apoptosis of
Tsc1−/− or
Tsc2−/− MEFs.
Interestingly, we have demonstrated that Huaier aqueous extract
inhibited JAK2/signal transducer and activator of transcription 3
(STAT3) and MAPK signaling pathways in these two TSC cell models.
Thus, this study provide new insight into the treatment of TSC.
Materials and methods
Reagents and antibodies
Dulbecco's modified Eagle's medium (DMEM), fetal
bovine serum (FBS), and 0.25% trypsin-EDTA were purchased from
Corning Life Sciences (Corning, NY, USA). Antibodies against JAK2,
p-JAK2 (Y1007/1008), STAT3, p-STAT3 (Y705), ERK, p-ERK, c-Jun
N-terminal kinase (JNK), p-JNK, N-cadherin, β-catenin, TCF8/ZEB1,
claudin-1, Slug, Snail, and MMP9 were purchased from Cell Signaling
Technology, Inc. (Danvers, MA, USA). Anti-MMP9 antibody was
purchased from Abcam (Cambridge, UK). Anti-β-actin antibody was
purchased from Abgent, Inc. (San Diego, CA, USA). Anti-mouse and
rabbit IgG-HRP antibodies were from Santa Cruz Biotechnology, Inc.
(Santa Cruz, CA, USA).
Preparation of Huaier aqueous
extract
The electuary ointment of Huaier (Gaitianli Medicine
Co., Ltd., Jiangsu, China) was dissolved in complete medium and
then sterilized by filtration with a 0.22-mm filter to get the 10
mg/ml stock solution, which was stored at 4°C for short-term
storage.
Cell culture
Tsc1−/− MEFs and
Tsc2−/− MEFs were
previously described (7,19). Cells were maintained in DMEM
containing 10% FBS and 1% penicillin/streptomycin in 5%
CO2 at 37°C.
Cell proliferation assay
Tsc1−/− MEFs and
Tsc2−/− MEFs were
seeded in 96-well plate at a density of 2.0×103
cells/well. Twenty-four hours later, cells were treated with Huaier
aqueous extract at the indicated concentrations of ranging from 0
to 8 mg/ml, and incubated for 12, 24 and 48 h, respectively. Ten
microliters CCK-8 reagent (Dojindo, Kumamoto, Japan) was added to
each well, and then incubated for 2 h at 37°C. The optical density
values were measured at 450 nm using a microplate reader
(Perkin-Elmer, Waltham, MA, USA).
Colony formation
Tsc1−/− MEFs and
Tsc2−/− MEFs were
seeded in a 6-well plate at a density of 1,000 cells/well.
Twenty-four hours later, cells were treated with Huaier aqueous
extract at the indicated concentrations. After 10 days of
treatment, the cells were fixed with methanol for 15 min, and then
colonies were stained with 0.1% crystal violet. The cell colonies
were photographed using a digital camera (Leica Microsystems GmbH,
Wetzlar, Germany).
Hoechst staining
Cells were washed twice with PBS and fixed with 4%
paraformaldehyde for 15 min, and stained with Hoechst 33258
(Biyuntian Biotechnology Co., Ltd., Shanghai, China) for 30 min in
the dark before washed with PBS again. Then cells were observed
under an inverted fluorescence microscope (Leica Microsystems
GmbH).
Apoptosis analysis
Tsc1−/− MEFs and
Tsc2−/− MEFs were
seeded in 12-well plates at 2.5×105 cells/well. The next
day, the cells were treated with Huaier aqueous extract at 4 or 8
mg/ml for 48 h. Apoptosis was analyzed with an Annexin V-FITC
apoptosis detection kit (BD Biosciences, Franklin Lakes, NJ, USA)
according to the manufacturer's instructions. In brief, cells were
collected and washed twice with PBS, then resuspended at a
concentration of 1×106 cells/ml in binding buffer. Five
microliters Annexin V-FITC and 5 µl propidium iodide (PI)
were added and incubated for 15 min at room temperature in the
dark. Finally, 400 µl the binding buffer was added and then
cells were analyzed using a flow cytometer (BD Biosciences).
Cell cycle analysis
Tsc1−/− MEFs and
Tsc2−/− MEFs were
seeded in 6-well plates at 5×105 cells/well. After 12-h
starvation in serum-free medium, cells were treated with Huaier
aqueous extract at the concentrations of 0, 4 and 8 mg/ml for 48 h,
and then cells were collected, washed twice with PBS and fixed
overnight with 70% ethanol at 4°C. The next day, the fixed cells
were centrifuged at 1,000 × g for 5 min and washed twice with PBS,
then cells were resuspended with 500 µl binding buffer
containing RNaseA and PI (Biyuntian Biotechnology Co., Ltd.). After
30-min incubation at 37°C in the dark, the cells were analyzed by
flow cytometry.
In vitro scratch assay
Cells were seeded in 12-well plates. The complete
medium was replaced with serum-free medium when the cells grew to a
subconfluent state. After 12-h starvation, a straight cell-free
wound was created with a 10-µl pipette tip and washed twice
with PBS, then the cells were maintained in serum-free medium
containing Huaier aqueous extract at the concentrations of 0, 2 and
4 mg/ml. The cells were observed and the scratch width was measured
at 0, 12 and 24 h. The migration distances were analyzed
quantitatively.
Cell invasion assay
Tsc1−/− MEFs and
Tsc2−/− MEFs were
maintained in serum-free medium for 12 h to reduce the interference
of serum. Transwell chambers were placed in 24-well plates to
constitute the Transwell system. The Matrigel (BD Biosciences, San
Jose, CA, USA) was dissolved in serum-free medium at a ratio of
1:12, and then 60 µl mixture was put to the upper chamber
gently. Next the Transwell system was incubated at 37°C for 4 h.
The cells were collected and resuspended in serum-free medium
containing different concentrations of Huaier aqueous extract. In
brief, 1×105 cells suspended in 200 µl serum-free
medium containing drug were added to the upper chamber, and 750
µl complete medium containing 10% FBS was added to the lower
chamber. After incubation for 12 or 24 h, the cells on the upper
surface of the membrane were wiped off with cotton swabs, and the
cells on the lower surface of the membrane were fixed with ethanol
for 15 min, then stained with crystal violet for 10 min and washed
twice with PBS. The successfully invaded cells were observed and
photographed on 5 random fields with an inverted microscope.
Western blotting
Cells were washed twice with PBS and harvested with
cell lysis solution as previously described (20). The cell lysate was boiled at 98°C
for 10 min. The proteins were separated by 4–12% Bis-Tris Nu-PAGE
(Invitrogen, Carlsbad, CA, USA) and then transferred onto PVDF
membrane. The membrane was blocked with 3–5% skim milk in TBST at
room temperature for 1 h, and then incubated with the primary
antibodies at 4°C overnight, followed by the incubation with the
HRP-conjugated secondary antibody at room temperature for 2 h.
Finally, the membrane was washed three times in TBST and detected
by chemiluminescence.
Statistical analysis
The data groups were compared with the two-tailed
Student's t-test using GraphPad Prism 5.0 software. The data are
presented as mean ± SD. P<0.05 was considered to be
statistically significant.
Results
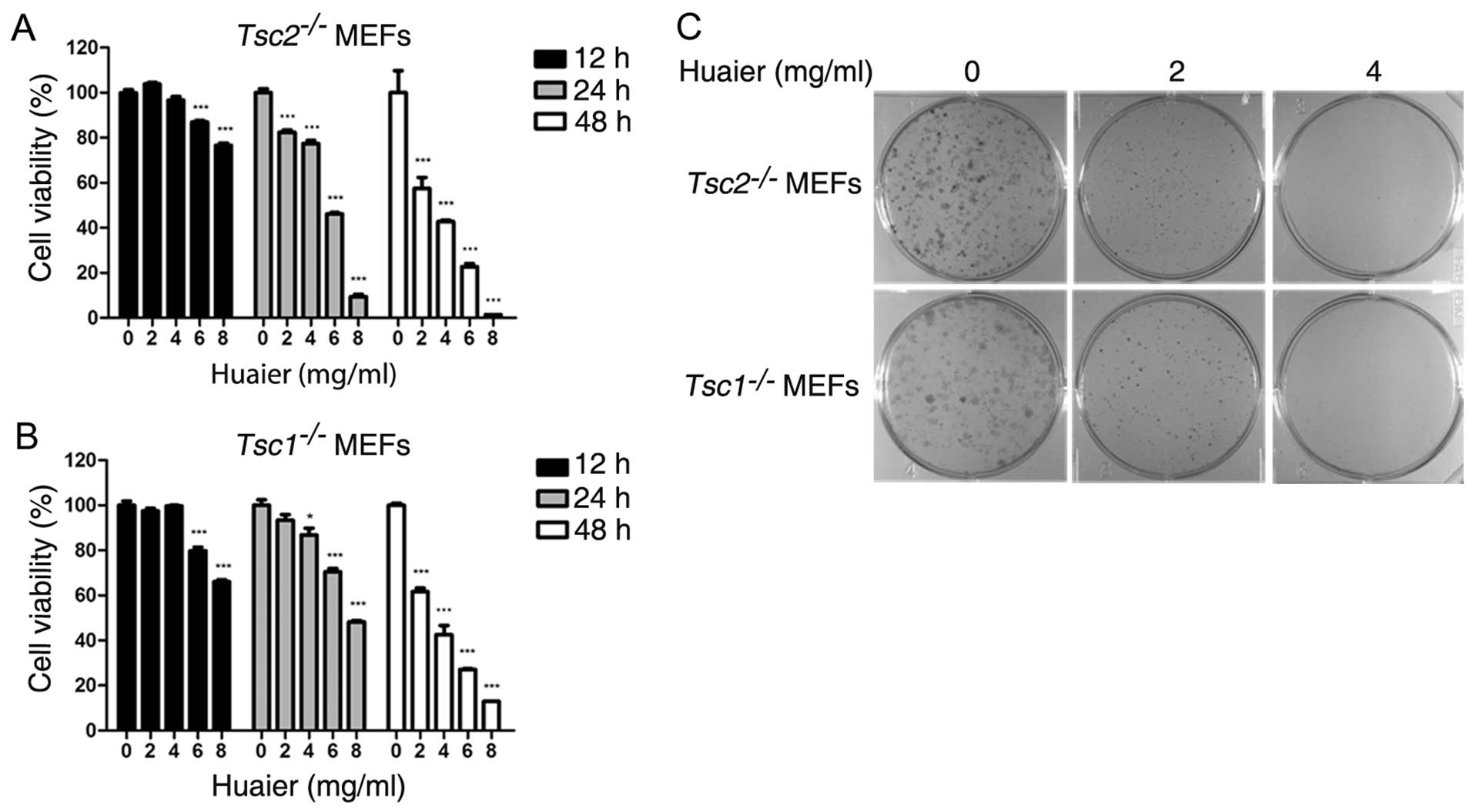
Huaier inhibits proliferation of
Tsc1−/− or Tsc2−/− MEFs
To evaluate the effect of Huaier aqueous extract on
Tsc1−/− or Tsc2−/− MEFs, we
examined cell viability with the CCK-8 assay.
Tsc1−/− or Tsc2−/− MEFs were
treated with Huaier at the indicated concentrations (0, 2, 4, 6 and
8 mg/ml) for 12, 24 and 48 h, respectively. As shown in Fig. 1A and B, Huaier significantly
suppressed the proliferation of Tsc2−/− or
Tsc1−/− MEFs in a time- and dose-dependent
manner. The IC50 values of Tsc1−/− or
Tsc2−/− MEFs exposed to Huaier aqueous extract of
various concentrations for 48 h were 3.89 and 3.56 mg/ml,
respectively. Furthermore, we performed colony formation assay to
assess the effect of Huaier aqueous extract on cell proliferation.
Result indicated that Tsc1−/− or
Tsc2−/− MEFs treated with Huaier aqueous extract
formed smaller and fewer colonies in a dose-dependent manner
(Fig. 1C).
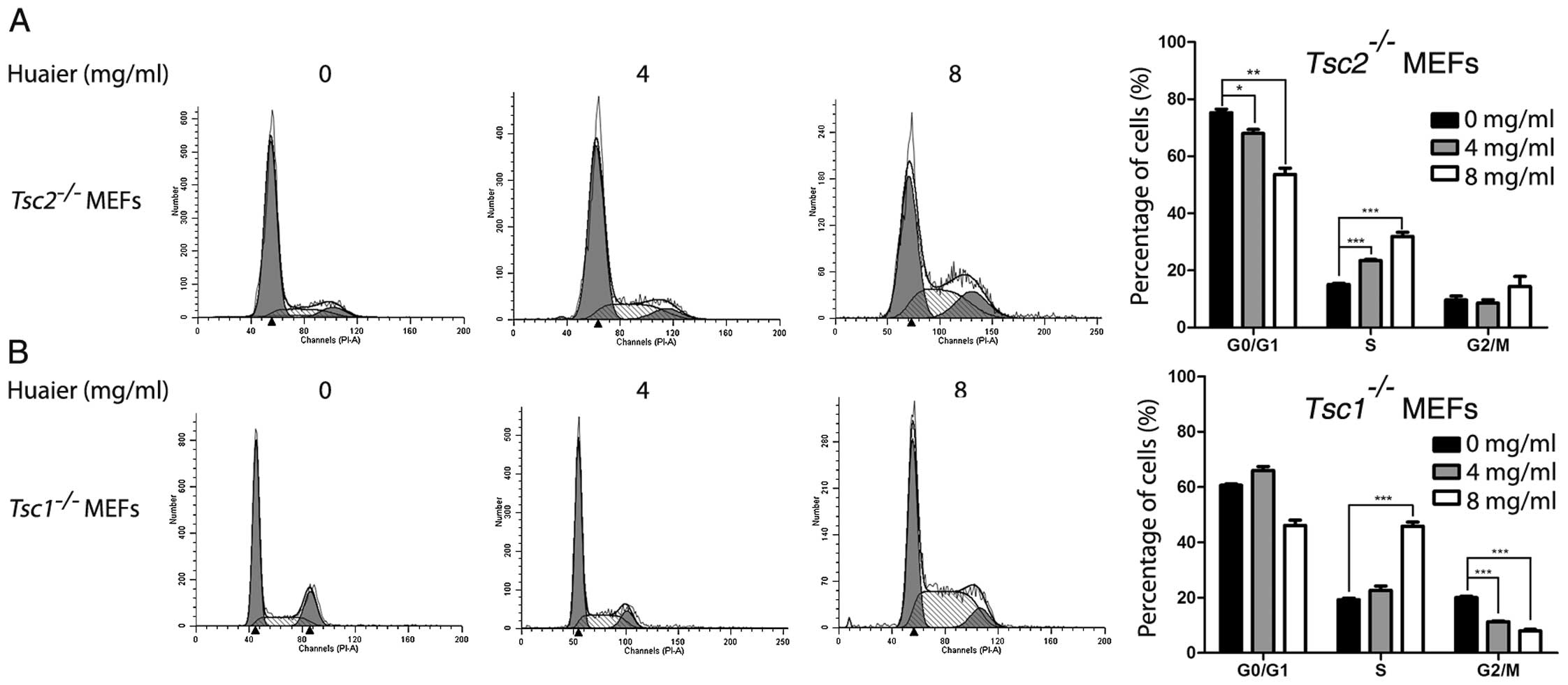
Huaier induced cell cycle arrest of
Tsc1−/− or Tsc2−/− MEFs
To investigate whether the inhibitory effect of
Huaier aqueous extract was attributed to cell cycle arrest, we
performed flow cytometry assay to evaluate cell cycle distribution
of Tsc1−/− or Tsc2−/− MEFs
exposed to Huaier. Tsc1−/− or
Tsc2−/− MEFs were treated with Huaier aqueous
extract for 48 h. As shown in Fig.
2A, Tsc2−/− MEFs exposed to Huaier showed
significantly decreased fraction of the G0/G1 phase and increased
fraction of the S phase in a dose-dependent manner. In addition,
Tsc1−/− MEFs treated with Huaier exhibited
significantly decreased fraction of the G2/M phase and increased
fraction of the S phase (Fig. 2B).
Taken together, these results revealed that Huaier-induced S-phase
arrest was partially responsible for the proliferation inhibition
of Tsc1−/− or Tsc2−/− MEFs.
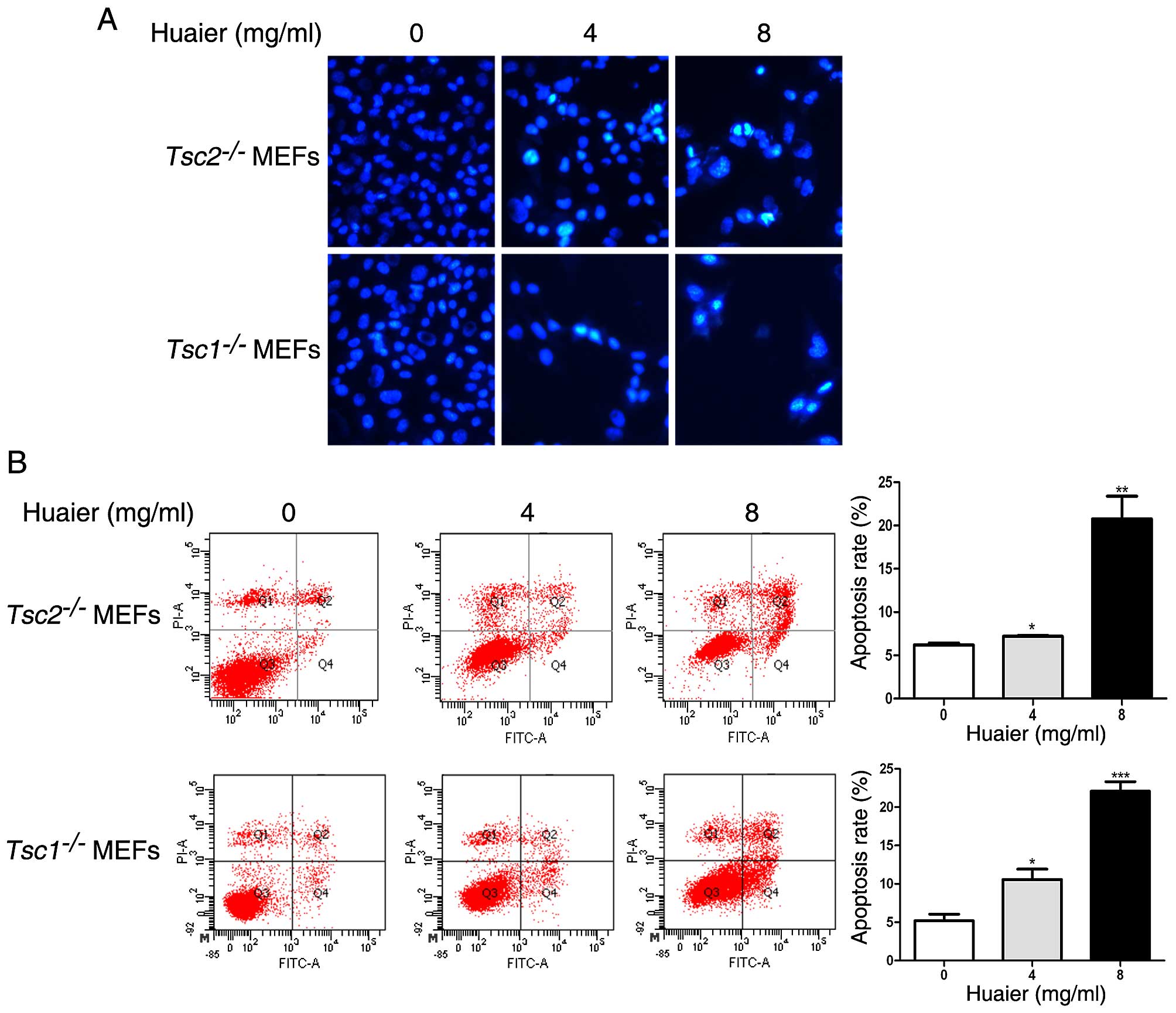
Huaier induces apoptosis in
Tsc1−/− or Tsc2−/− MEFs
Next we performed Hoechst staining assay to
determine the effect of Huaier aqueous extract on apoptosis.
Apoptotic cells could be stained with Hoechst 33258 dye. As
observed in Fig. 3A, the nuclei in
the untreated Tsc1−/− or
Tsc2−/− MEFs were stained with a less bright blue
fluorescence and these two cells appeared to be intact oval shape.
Whereas, more condensed or fragmented chromatin were observed in
Tsc1−/− or Tsc2−/− MEFs exposed
to Huaier aqueous extract. To further explore the effect of Huaier
on apoptosis in Tsc1−/− or
Tsc2−/− MEFs, we conducted flow cytometry
analysis with Annexin V-FITC/PI staining. As shown in Fig. 3B, the apoptosis rate of
Tsc1−/− or Tsc2−/− MEFs
increased in a dose-dependent manner in response to Huaier.
Collectively, Huaier aqueous extract promotes apoptosis of
Tsc1−/− or Tsc2−/− MEFs.
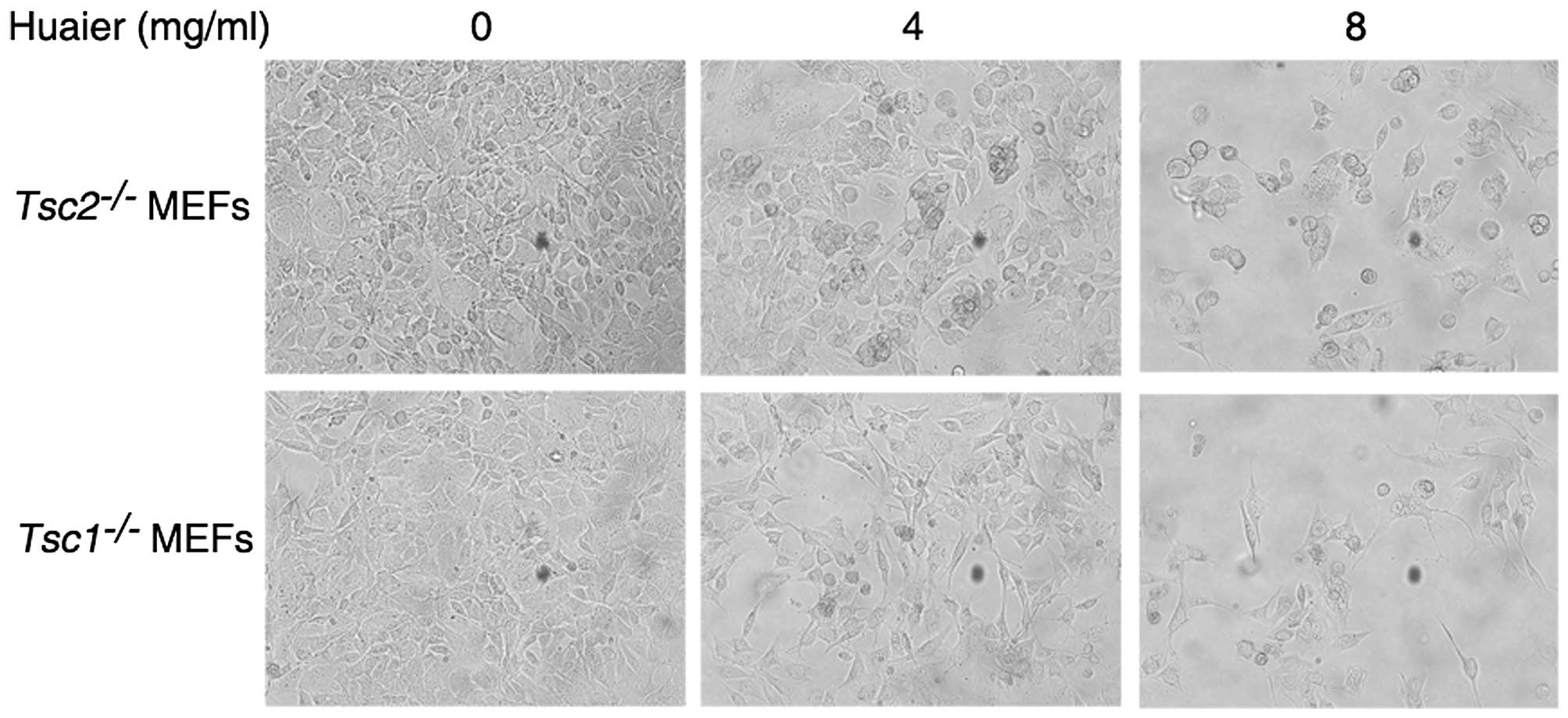
Effect of Huaier treatment on the
morphology of Tsc1−/− or Tsc2−/− MEFs
Next we determined the effect of Huaier aqueous
extract on cell morphology. Tsc1−/− or
Tsc2−/− MEFs were treated with Huaier aqueous
extract at a concentration of 4 or 8 mg/ml for 24 h. As shown in
Fig. 4, the untreated cells showed
plump cell body, homogeneous cytoplasm, and good refraction.
However, Huaier treatment led to shrinked cell body and weakened
refraction. Moreover, more cells became round and died, which was
concomitant with an increase in the concentration of Huaier.
Huaier inhibits cell motility of
Tsc1−/− or Tsc2−/− MEFs
Migration and invasion play a critical role in
metastasis of cancer cells (21).
To evaluate the migration ability of cells in vitro, we
performed in vitro scratch assay. We treated
Tsc1−/− or Tsc2−/− MEFs with
Huaier aqueous extract at the concentrations of 0, 2 and 4 mg/ml,
and then measured the scratch width at time-points of 0, 12 and 24
h. As shown in Fig. 5A, the
migration distance was decreased in response to Huaier in a
dose-dependent manner.
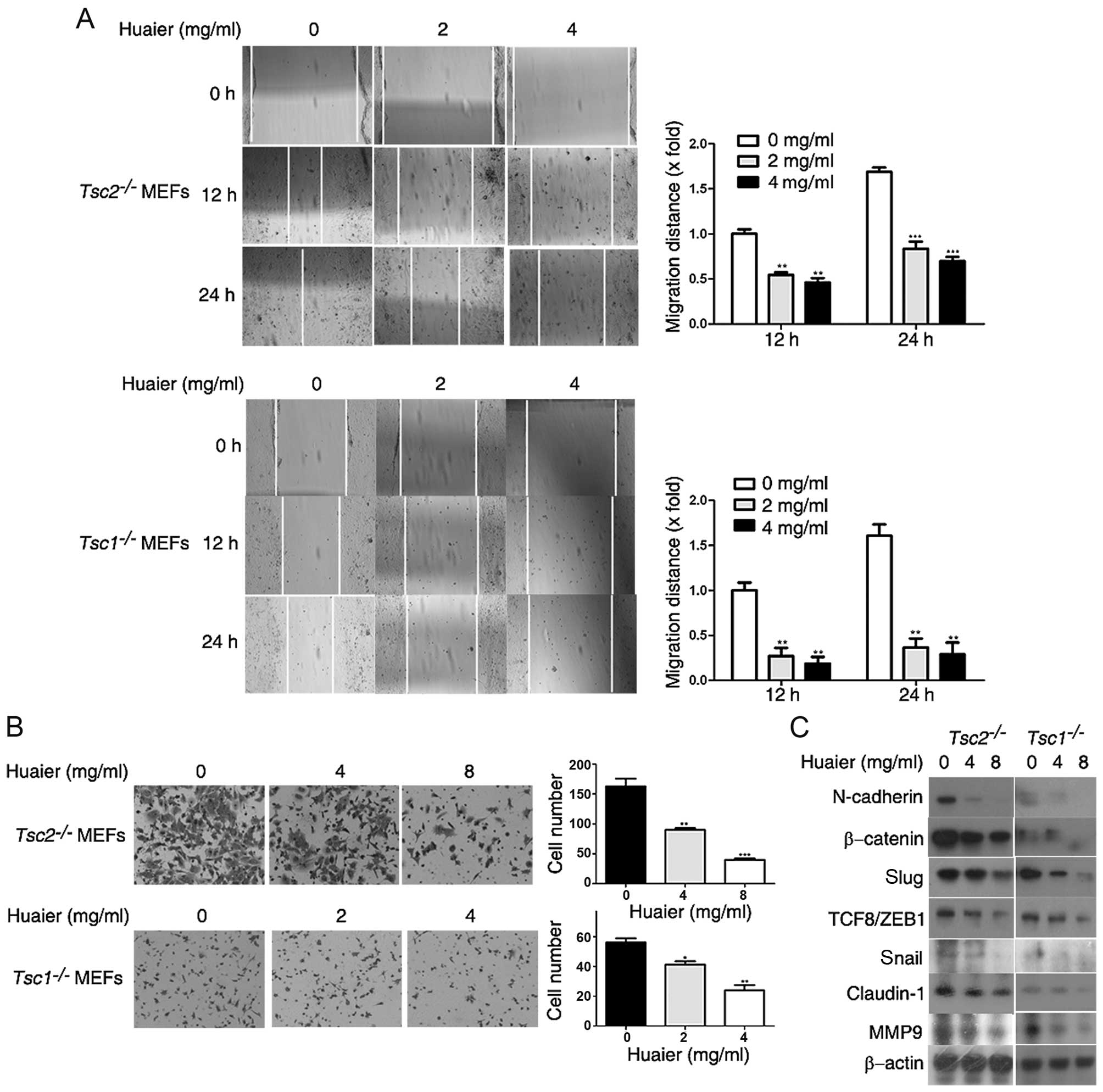 | Figure 5Huaier inhibits cell motility of
Tsc1−/− or Tsc2−/− mouse
embryonic fibroblasts (MEFs). (A) Tsc2−/− MEFs
and Tsc1−/− MEFs treated with Huaier aqueous
extract at the concentrations of 0, 2 and 4 mg/ml were subjected to
in vitro scratch assay and observed at 0, 12 and 24 h,
respectively. Left panel, representative images (×100); right
panel, quantitative data. **P<0.01,
***P<0.001. (B) Tsc2−/− MEFs or
Tsc1−/− MEFs treated with Huaier aqueous extract
at the concentrations of 0, 4 and 8 or 0, 2 and 4 mg/ml for 12 or
24 h, respectively, were subjected to cell invasion assay.
Representative images are presented (×200). (C)
Tsc2−/− MEFs and Tsc1−/− MEFs
treated with Huaier aqueous extract at the concentrations of 0, 4
and 8 mg/ml for 48 h were subjected to immunoblotting.
*P<0.05, **P<0.01,
***P<0.001. |
The scratch healing inhibition rates of
Tsc2−/− MEFs and Tsc1−/− MEFs
were 58.57±4.52 and 82.01±13.97% after treatment with Huaier
aqueous extract at a concentration of 4 mg/ml for 24 h. Thus,
Huaier significantly inhibits the migration of
Tsc1−/− or Tsc2−/− MEFs.
Furthermore, we performed Transwell assay to assess the invasive
capacity of Tsc1−/− or Tsc2−/−
MEFs in vitro. Tsc2−/− MEFs were treated
with Huaier aqueous extract at the concentrations of 4 and 8 mg/ml
for 12 h. As observed in Fig. 5B,
the number of Tsc2−/− MEFs invading through the
Matrigel-coated membrane was drastically decreased. In addition,
the invasive capacity of Tsc1−/− MEFs was also
reduced by treatment with Huaier (Fig.
5B).
The epithelial-mesenchymal transition (EMT) is a
critical process in cancer development, which enhances the
metastatic capacity of cancer cells (22,23).
We therefore investigated the effects of Huaier on EMT of
Tsc1−/− or Tsc2−/− MEFs. We
examined the expression levels of EMT markers by western blotting.
As shown in Fig. 5C, Huaier
treatment downregulated the protein levels of N-cadherin,
β-catenin, Slug, TCF8/ZEB1, Snail, and claudin-1 (24–26) in
Tsc1−/− or Tsc2−/− MEFs,
indicating that Huaier inhibited EMT of Tsc1−/−
or Tsc2−/− MEFs. MMP-9 has been reported to be
important in cancer metastasis (27,28).
Here we found that MMP-9 expression was reduced by Huaier treatment
in a dose-dependent manner (Fig.
5C). Taken together, Huaier suppressed the metastatic ability
of Tsc1−/− or Tsc2−/− MEFs
in vitro.
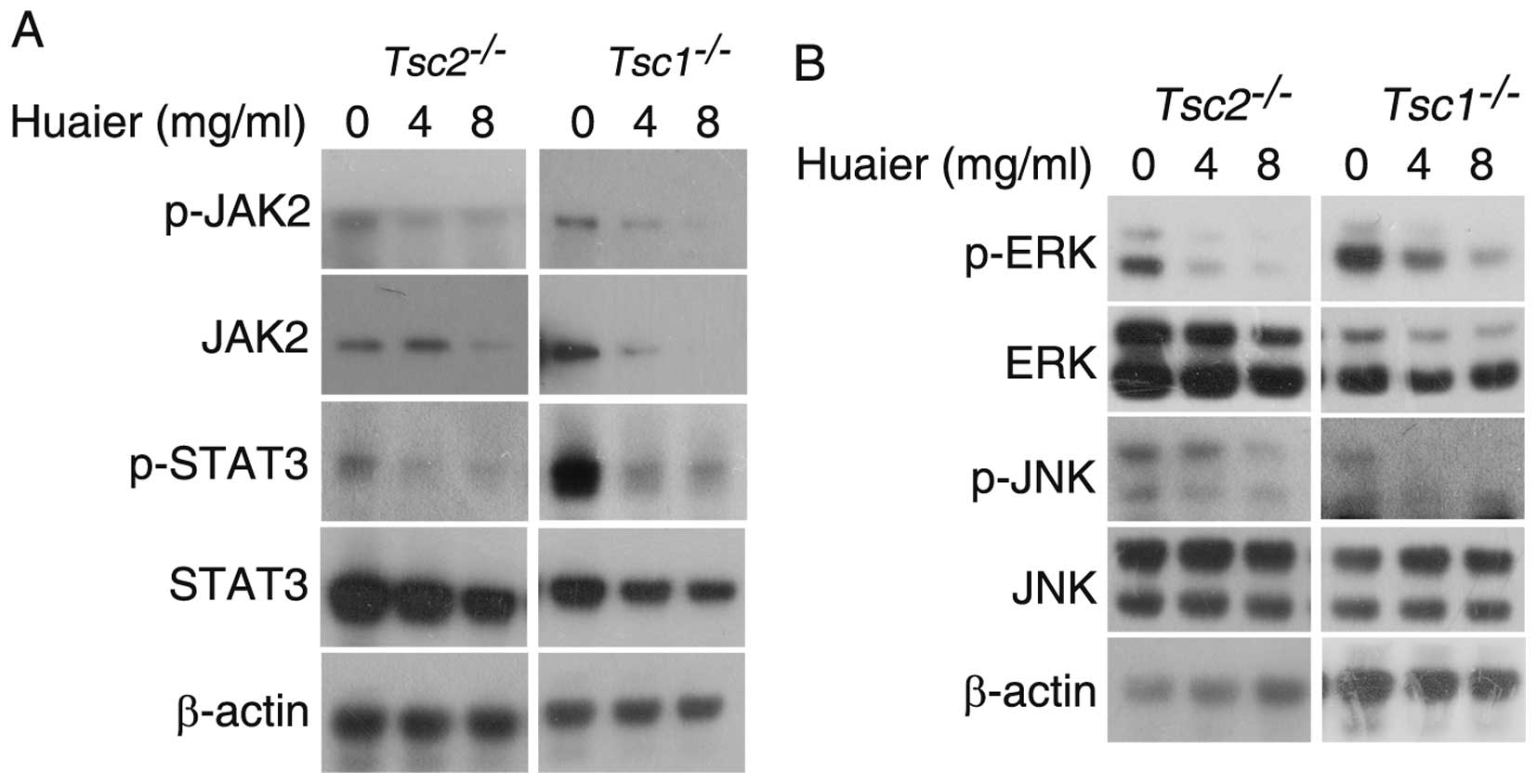
Huaier inhibits JAK2/STAT3 and MAPK
signaling pathways in Tsc1−/− or Tsc2−/−
MEFs
JAK2/STAT3 pathway plays an important role in
tumorigenesis and metastasis (29,30).
Inhibition of JAK2/STAT3 signaling pathway constrains cancer cell
growth and induces apoptosis (31,32).
As shown in Fig. 6A, Huaier
treatment downregulated the protein levels of JAK2, p-JAK2, STAT3,
and p-STAT3 in Tsc1−/− or
Tsc2−/− MEFs, suggesting that Huaier markedly
inhibits JAK2/STAT3 signaling pathway in Tsc1−/−
or Tsc2−/− MEFs. The MAPK pathway plays a crucial
role in multiple cellular processes, such as cell proliferation,
apoptosis, migration and invasion (33,34).
The MAPK super-family consists of three mammalian MAP kinases, ERK,
JNK, and p38 MAPK (35). As shown
in Fig. 6B, we found that the
phosphorylation of ERK and JNK was inhibited by Huaier treatment in
these two cell lines, indicating that Huaier restrains the MAPK
signaling pathway in Tsc1−/− or
Tsc2−/− MEFs.
Discussion
Tumorigenesis is a multiple-step process involving
aberrant genetic alterations. These complicated mechanisms may
confer resistance to drugs which were used for targeted therapy of
cancers. Therefore, it plays an important role in the treatment of
cancers to target multiple pro-survival signaling pathways.
Traditional Chinese medicine (TCM) has become an important source
for developing new antitumor drugs based on some advantages
including low toxicity, low cost, and the multi-targets (13). Huaier has been applied in TCM for
approximately 1,600 years in China and has good clinical effects.
As a single drug or adjuvant drug in the treatment of cancers,
Huaier has attracted increasing attention in recent years.
The dysregulation of cell growth and cell death
signals are main characteristics of tumors, so the strategy of
inhibition of cell proliferation and induction of apoptosis could
be exploited to treat tumors. Tsc1−/− MEFs and
Tsc2−/− MEFs, two widely used TSC cell models
(7,36–38),
were applied in this study. We have demonstrated that Huaier could
inhibit cell proliferation of Tsc1−/− MEFs and
Tsc2−/− MEFs in vitro by CCK-8 assay,
colony formation, and cell cycle analysis (Figs. 1 and 2). Moreover, we also showed that Huaier
induced apoptosis in Tsc1−/− MEFs and
Tsc2−/− MEFs by flow cytometry and Hoechst
staining (Fig. 3). These data
provide potent in vitro evidence for treatment of TSC with
Huaier.
Tumor metastasis is an extremely complicated
multi-step process and play a critical role in tumorigenesis.
Migration and invasion are two important cellular processes in
tumor metastasis. In this study, we performed in vitro
scratch assay and Transwell assay to evaluate the effects of Huaier
on migratory and invasive ability in vitro of
Tsc1−/− MEFs and Tsc2−/− MEFs.
We showed that migration and invasion of Tsc1−/−
or Tsc2−/− MEFs were markedly impaired by Huaier
treatment in a dose-dependent manner (Fig. 5A and B). In addition, EMT is a
process characterized with the conversion of epithelial cells into
motile mesenchymal cells and the increase in cellular migration and
invasion. EMT has been reported to occur in tumor metastasis
(23,33). Our study revealed that Huaier
compromised the process of EMT in Tsc1−/− or
Tsc2−/− MEFs (Fig.
5C). As LAM is considered to be initiated by cells with
mutations of TSC1 or TSC2, which metastasized from renal
angiomyolipomas (39,40), Huaier may be exploited as a
potential drug targeting metastasis in the treatment of LAM and
TSC.
JAK2/STAT3 signaling pathway has a critical role in
cell proliferation, migration, apoptosis, and differentiation
(41,42). JAK2/STAT3 signaling pathway has been
considered as a novel target for drugs against cancers (43,44).
STAT3 is an oncoprotein highly overexpressed in many cancers
(45). STAT3 is required for
aberrant proliferation and survival of TSC2-null cells (46). In addition, the role of MAPK
signaling pathway in cell proliferation, apoptosis, motility, and
morphogenesis has been extensively investigated (33). Suppression of MAPK signaling
inhibited Tsc2−/− cell proliferation (47). In this study, we reported that
Huaier inhibited JAK2/STAT3 signaling through down-regulation of
the total expression and phosphorylation of JAK2 and STAT3.
Additionally, Huaier inhibited the phosphorylation/activation of
ERK and JNK in a dose-dependent manner in both
Tsc1−/− MEFs and Tsc2−/− MEFs
(Fig. 6). Collectively, we propose
that Huaier inhibits the proliferation and metastasis of Tsc1- or
Tsc2-null cells partially via downregulation of JAK2/STAT3 and MAPK
signaling pathways.
In conclusion, we have clarified that the
attenuation of JAK2/STAT3 and MAPK signaling are partially
responsible for the inhibition of proliferation and metastasis of
two TSC cell models induced by Huaier. Therefore, based on the low
toxicity and multi-targets of Huaier treatment, Huaier may be a
candidate drug for TSC treatment.
Abbreviations:
|
TSC
|
tuberous sclerosis complex
|
|
LAM
|
lymphangiomyomatosis
|
|
TCM
|
traditional Chinese medicine
|
|
TSC1
|
tuberous sclerosis 1
|
|
TSC2
|
tuberous sclerosis 2
|
|
mTOR
|
mechanistic target of rapamycin
|
|
PI3K
|
phosphatidylinositol 3-kinase
|
|
ERK
|
extracellular signal-regulated
kinase
|
|
MAPK
|
mitogen-activated protein kinase
|
|
MEFs
|
mouse embryonic fibroblasts
|
|
STAT3
|
signal transducer and activator of
transcription 3
|
Acknowledgments
This study was financially supported by the
National Natural Science Foundation of China (81403147) and grants
from the Beijing University of Chinese Medicine (2014-JYBZZ-JS-024,
2015-JYB-XYQ-004).
References
|
1
|
Crino PB, Nathanson KL and Henske EP: The
tuberous sclerosis complex. N Engl J Med. 355:1345–1356. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Weiner DM, Ewalt DH, Roach ES and Hensle
TW: The tuberous sclerosis complex: A comprehensive review. J Am
Coll Surg. 187:548–561. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Islam MP and Roach ES: Tuberous sclerosis
complex. Handb Clin Neurol. 132:97–109. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Inoki K, Corradetti MN and Guan KL:
Dysregulation of the TSC-mTOR pathway in human disease. Nat Genet.
37:19–24. 2005. View
Article : Google Scholar
|
|
5
|
Curatolo P and Moavero R: mTOR Inhibitors
in Tuberous Sclerosis Complex. Curr Neuropharmacol. 10:404–415.
2012. View Article : Google Scholar :
|
|
6
|
Orlova KA and Crino PB: The tuberous
sclerosis complex. Ann N Y Acad Sci. 1184:87–105. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang H, Cicchetti G, Onda H, Koon HB,
Asrican K, Bajraszewski N, Vazquez F, Carpenter CL and Kwiatkowski
DJ: Loss of Tsc1/Tsc2 activates mTOR and disrupts PI3K-Akt
signaling through downregulation of PDGFR. J Clin Invest.
112:1223–1233. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang H, Bajraszewski N, Wu E, Wang H,
Moseman AP, Dabora SL, Griffin JD and Kwiatkowski DJ: PDGFRs are
critical for PI3K/Akt activation and negatively regulated by mTOR.
J Clin Invest. 117:730–738. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Carracedo A, Ma L, Teruya-Feldstein J,
Rojo F, Salmena L, Alimonti A, Egia A, Sasaki AT, Thomas G, Kozma
SC, et al: Inhibition of mTORC1 leads to MAPK pathway activation
through a PI3K-dependent feedback loop in human cancer. J Clin
Invest. 118:3065–3074. 2008.PubMed/NCBI
|
|
10
|
Bissler JJ, McCormack FX, Young LR, Elwing
JM, Chuck G, Leonard JM, Schmithorst VJ, Laor T, Brody AS, Bean J,
et al: Sirolimus for angiomyolipoma in tuberous sclerosis complex
or lymphangioleiomyomatosis. N Engl J Med. 358:140–151. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Franz DN, Leonard J, Tudor C, Chuck G,
Care M, Sethuraman G, Dinopoulos A, Thomas G and Crone KR:
Rapamycin causes regression of astrocytomas in tuberous sclerosis
complex. Ann Neurol. 59:490–498. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nie J, Zhao C, Deng LI, Chen J, Yu B, Wu
X, Pang P and Chen X: Efficacy of traditional Chinese medicine in
treating cancer. Biomed Rep. 4:3–14. 2016.PubMed/NCBI
|
|
13
|
Liu J, Wang S, Zhang Y, Fan HT and Lin HS:
Traditional Chinese medicine and cancer: History, present
situation, and development. Thorac Cancer. 6:561–569. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang N, Kong X, Yan S, Yuan C and Yang Q:
Huaier aqueous extract inhibits proliferation of breast cancer
cells by inducing apoptosis. Cancer Sci. 101:2375–2383. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang X, Zhang N, Huo Q and Yang Q:
Anti-angiogenic and antitumor activities of Huaier aqueous extract.
Oncol Rep. 28:1167–1175. 2012.PubMed/NCBI
|
|
16
|
Wang X, Zhang N, Huo Q, Sun M, Lv S and
Yang Q: Huaier aqueous extract suppresses human breast cancer cell
proliferation through inhibition of estrogen receptor α signaling.
Int J Oncol. 43:321–328. 2013.PubMed/NCBI
|
|
17
|
Wu T, Chen W, Liu S, Lu H, Wang H, Kong D,
Huang X, Kong Q, Ning Y and Lu Z: Huaier suppresses proliferation
and induces apoptosis in human pulmonary cancer cells via
upregulation of miR-26b-5p. FEBS Lett. 588:2107–2114. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Song X, Li Y, Zhang H and Yang Q: The
anticancer effect of Huaier (Review). Oncol Rep. 34:12–21.
2015.PubMed/NCBI
|
|
19
|
Kwiatkowski DJ, Zhang H, Bandura JL,
Heiberger KM, Glogauer M, el-Hashemite N and Onda H: A mouse model
of TSC1 reveals sex-dependent lethality from liver hemangiomas, and
up-regulation of p70S6 kinase activity in Tsc1 null cells. Hum Mol
Genet. 11:525–534. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hu Z, Wang Y, Huang F, Chen R, Li C, Wang
F, Goto J, Kwiatkowski DJ, Wdzieczak-Bakala J, Tu P, et al:
Brain-expressed X-linked 2 is pivotal for hyperactive mechanistic
target of Rapamycin (mTOR)-mediated tumorigenesis. J Biol Chem.
290:25756–25765. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bravo-Cordero JJ, Hodgson L and Condeelis
J: Directed cell invasion and migration during metastasis. Curr
Opin Cell Biol. 24:277–283. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tsai JH and Yang J: Epithelial-mesenchymal
plasticity in carcinoma metastasis. Genes Dev. 27:2192–2206. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zeisberg M and Neilson EG: Biomarkers for
epithelial-mesenchymal transitions. J Clin Invest. 119:1429–1437.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee JM, Dedhar S, Kalluri R and Thompson
EW: The epithelial-mesenchymal transition: New insights in
signaling, development, and disease. J Cell Biol. 172:973–981.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Suh Y, Yoon CH, Kim RK, Lim EJ, Oh YS,
Hwang SG, An S, Yoon G, Gye MC, Yi JM, et al: Claudin-1 induces
epithelial-mesenchymal transition through activation of the
c-Abl-ERK signaling pathway in human liver cells. Oncogene.
32:4873–4882. 2013. View Article : Google Scholar
|
|
27
|
van Kempen LC and Coussens LM: MMP9
potentiates pulmonary metastasis formation. Cancer Cell. 2:251–252.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Merdad A, Karim S, Schulten HJ, Dallol A,
Buhmeida A, Al-Thubaity F, Gari MA, Chaudhary AG, Abuzenadah AM and
Al-Qahtani MH: Expression of matrix metalloproteinases (MMPs) in
primary human breast cancer: MMP-9 as a potential biomarker for
cancer invasion and metastasis. Anticancer Res. 34:1355–1366.
2014.PubMed/NCBI
|
|
29
|
Teng Y, Ross JL and Cowell JK: The
involvement of JAK-STAT3 in cell motility, invasion, and
metastasis. JAKSTAT. 3:e280862014.PubMed/NCBI
|
|
30
|
Yu H, Lee H, Herrmann A, Buettner R and
Jove R: Revisiting STAT3 signalling in cancer: New and unexpected
biological functions. Nat Rev Cancer. 14:736–746. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Du W, Hong J, Wang YC, Zhang YJ, Wang P,
Su WY, Lin YW, Lu R, Zou WP, Xiong H, et al: Inhibition of
JAK2/STAT3 signalling induces colorectal cancer cell apoptosis via
mitochondrial pathway. J Cell Mol Med. 16:1878–1888. 2012.
View Article : Google Scholar
|
|
32
|
Judd LM, Menheniott TR, Ling H, Jackson
CB, Howlett M, Kalantzis A, Priebe W and Giraud AS: Inhibition of
the JAK2/STAT3 pathway reduces gastric cancer growth in vitro and
in vivo. PLoS One. 9:e959932014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dhillon AS, Hagan S, Rath O and Kolch W:
MAP kinase signalling pathways in cancer. Oncogene. 26:3279–3290.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kim EK and Choi EJ: Pathological roles of
MAPK signaling pathways in human diseases. Biochim Biophys Acta.
1802:396–405. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Johnson GL and Lapadat R:
Mitogen-activated protein kinase pathways mediated by ERK, JNK, and
p38 protein kinases. Science. 298:1911–1912. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Uhlmann EJ, Apicelli AJ, Baldwin RL, Burke
SP, Bajenaru ML, Onda H, Kwiatkowski D and Gutmann DH:
Heterozygosity for the tuberous sclerosis complex (TSC) gene
products results in increased astrocyte numbers and decreased
p27-Kip1 expression in TSC2+/− cells.
Oncogene. 21:4050–4059. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ghosh S, Tergaonkar V, Rothlin CV, Correa
RG, Bottero V, Bist P, Verma IM and Hunter T: Essential role of
tuberous sclerosis genes TSC1 and TSC2 in NF-kappaB activation and
cell survival. Cancer Cell. 10:215–226. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zha X, Hu Z, Ji S, Jin F, Jiang K, Li C,
Zhao P, Tu Z, Chen X, Di L, et al: NFκB up-regulation of glucose
transporter 3 is essential for hyperactive mammalian target of
rapamycin-induced aerobic glycolysis and tumor growth. Cancer Lett.
359:97–106. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bittmann I, Rolf B, Amann G and Löhrs U:
Recurrence of lymphangioleiomyomatosis after single lung
transplantation: New insights into pathogenesis. Hum Pathol.
34:95–98. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang F, Chen X, Li C, Sun Q, Chen Y, Wang
Y, Peng H, Liu Z, Chen R, Liu K, et al: Pivotal role of augmented
αB-crystallin in tumor development induced by deficient TSC1/2
complex. Oncogene. 33:4352–4358. 2014. View Article : Google Scholar
|
|
41
|
O'Shea JJ, Gadina M and Schreiber RD:
Cytokine signaling in 2002: New surprises in the Jak/Stat pathway.
Cell. 109(Suppl): S121–S131. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kiu H and Nicholson SE: Biology and
significance of the JAK/STAT signalling pathways. Growth Factors.
30:88–106. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Nam S, Xie J, Perkins A, Ma Y, Yang F, Wu
J, Wang Y, Xu RZ, Huang W, Horne DA, et al: Novel synthetic
derivatives of the natural product berbamine inhibit Jak2/Stat3
signaling and induce apoptosis of human melanoma cells. Mol Oncol.
6:484–493. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Um HJ, Min KJ, Kim DE and Kwon TK:
Withaferin A inhibits JAK/STAT3 signaling and induces apoptosis of
human renal carcinoma Caki cells. Biochem Biophys Res Commun.
427:24–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Johnston PA and Grandis JR: STAT3
signaling: Anticancer strategies and challenges. Mol Interv.
11:18–26. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Goncharova EA, Goncharov DA, Damera G,
Tliba O, Amrani Y, Panettieri RA Jr and Krymskaya VP: Signal
transducer and activator of transcription 3 is required for
abnormal proliferation and survival of TSC2-deficient cells:
Relevance to pulmonary lymphangioleiomyomatosis. Mol Pharmacol.
76:766–777. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Mi R, Ma J, Zhang D, Li L and Zhang H:
Efficacy of combined inhibition of mTOR and ERK/MAPK pathways in
treating a tuberous sclerosis complex cell model. J Genet Genomics.
36:355–361. 2009. View Article : Google Scholar : PubMed/NCBI
|