1. Introduction
Osteosarcoma (OS) is the most common primary bone
tumor. It affects both children and adolescents (with a second peak
incidence in the middle aged population (1). Of note, a higher frequency in males as
compared to females has been reported (2). The most common presenting site for OS
is the metaphysis of long bone (3)
The lesion is defined as a mass composed of transformed osteoblasts
secreting various mineralized components of the extracellular
matrix (ECM) and is associated with local bone and soft tissue
destruction. Conventional OS exhibits 7 distinct pathological
subtypes (4). The most commonly
diagnosed subtype of OS (approximately 60% of cases) is the
osteoblastic subtype with osteoid matrix as its dominant feature
(5). The outcomes for patients do
not vary significantly among the subtypes, with the cohort size
being a limiting factor, as the numbers of patients with the more
rare subtypes are not many in number, and thus patients with
different subtypes cannot be effectively compared (6). In the past, the disease prognosis was
extremely dismal with the mortality rate being >90%, mostly due
to pulmonary metastases (3). During
the past decades, more effective treatment regimes have been
developed, with the tumor recurrence, however, remaining as high as
30–40% for all patients (7).
Importantly, for the subgroup of patients with established
metastatic disease, there is no therapeutic strategy able to
establish long-term tumor control (5). The genetic drivers of OS initiation
and progression are not conclusively defined. Thus, mutations at
the both level of mesenchymal stem cells (MSCs), as well as in
committed osteoprogenitors have been implicated in the development
of OS (8). Some data obtained from
murine models support the hypothesis that the committed osteoblast
pool gives rise to the precursor OS cell (9). Furthermore, it has been suggested that
the stage of differentiation of the transformed osteoprogenitor
predetermines the OS subtype (10).
However, as OS is a relatively rare tumor, it is impossible to
correlate these subtypes with specific mutations due to the
extensive heterogeneity of OS (11)
and to the fact that the variety of its subtypes occur in different
frequencies (8). On the other hand,
it has been suggested that OS originates from MSCs as a consequence
of aneuploidization and the genomic loss of Cdkn2 (12). That said, taking into account all of
the above, the weight of evidence seems to favor an osteoblastic
population as the cell of origin (8).
2. Parathyroid hormone (PTH) and parathyroid
hormone-related peptide (PTHrP)
PTH is an 84-amino-acid polypeptide hormone (PTH
1-84) which is, along with its fragments (e.g., PTH 1-34 and PTH
7-84), secreted by the chief cells of the parathyroid glands. The
function of PTH is perpetrated through the activation of PTH
receptor 1 (PTHR1) and respective downstream intracellular pathways
(13,14). PTHR1 is a G-protein-coupled receptor
(GPCR) that regulates skeletal development, bone turnover and
mineral ion homeostasis (15). The
PTHR1 receptor can also be activated through PTH-related protein
(PTHrP) binding to induce target cells to promote diverse
biochemical responses. The most important function of PTH is to
increase blood calcium and decrease blood phosphate levels, with
principal target organs being the skeleton and the kidneys
(13). The feedback between the
parathyroid glands and the blood calcium levels regulates the
secretion of PTH in an inverse manner (13), and specifically modulates the
relative abundance of various PTH-derived peptides that are
released into the circulation (16).
The effects of PTH are focused on the osteoid, the
highly specialized bone ECM. Of note, PTH inhibits the bone
growth-promoting activity of osteoblasts and induces osteoclasts to
resorb bone and release calcium and phosphate ions into the blood.
Simultaneously, the mobilized phosphate ions are excreted by the
kidneys in a PTH-regulated manner (13).
In humans, PTHrP may be comprised of 139, 141 or 173
amino acids, which is dependent on alternative mRNA splicing
(17). Importantly, this peptide
exhibits considerate N-terminal amino acid sequence homology with
PTH and functions likewise through the activation of PTHR1. PTHrP
exhibits both paracrine and autocrine function, and has an
important role in the regulation of differentiation in the growth
plates of developing long bones (18).
3. Parathyroid hormone/parathyroid
hormone-related protein receptor (PTH/PTHrP type 1 receptor)
PTHR1 is highly expressed in the skeleton and the
kidneys (15,18,19).
Agonist binding to PTHR1 is coupled with the activation of
phospholipase C, adenylyl cyclase, or cyclic adenosine
monophosphate (cAMP)/protein kinase A (20–22).
Alternatively, Rey et al (23) demonstrated that 'PTH can stimulate
p38 in early differentiating osteoblastic cells, probably by a
cAMP-PKA-dependent mechanism and that activation of p38 by PTH is
important for the stimulation of ALP and matrix calcification
induced by this peptide hormone'.
Moreover, it has been determined that upon PTH
binding to PTHR1, the resulting ligand-receptor complex is
internalized through the involvement of clathrin-coated pits via an
arrestin-dependent mechanism (24,25).
Furthermore, it has been suggested that the regulation of GPCR
endocytosis is a complex multistep process that involves the
catalytic action of several lipid-modifying enzymes (26). Intriguing results came to light upon
utilizing structurally modified ligands to analyze PTHR1 downstream
signaling. Thus, PTHR1 was found to continue to signal through a
G-protein-mediated pathway within endosomes, challenging the
established ground rule in GPCR biology of transient membrane
receptor activation with subsequent rapid deactivation and receptor
internalization (19).
Early toxicological studies using rat models
suggested that long-term PTH treatment may increase the risk of
developing OS (27). Experiments on
both Fisher 344 and Sprague-Dawley rats indicated that the
occurence of OS depends on the level of the PTH 1-34 dose and the
duration of treatment (27–29). Importantly however, the doses tested
in the rat models were a 100-fold higher as compared to the human
dose approved by the Food and Drug Administration (30). Up-to-date clinical trials and
patient follow-up have not not shown a correlation between PTH 1-34
use and the incidence of OS (31–33).
Moreover, in the US post-marketing surveillance study of adult OS
and teriparatide treatment, it was determined that after 7 years,
there were no patients with OS who had a prior history of
teriparatide treatment (33).
4. PTH/PTHR1 signaling in OS
PTHR1 was found to be expressed in various tumor
tissues, including OS. Importantly, the expression of PTHR1 affects
OS cell functions, as it has been reported that the knockdown of
PTHR1 decreases invasion and growth, and increases tumor
differentiation in vivo (34). In the same study, upon
overexpressing PHTR1, an increase in proliferation, motility and in
the invasion ability of OS cells was evident. Indeed, this was
perpetrated without the addition of exogenous PTHr, thus indicating
the existence of autocrine signaling (34). Moreover, the overexpression of PTHR1
in OS leads to increased proliferation and motility, delayed
osteoblastic differentiation, as well as in the upregulation of
genes that are involved in the production of ECM (34). The function of PTHR1 is regulated at
several levels in bone tissue (35). Furthermore, in OS cells, it was
demonstrated that the Na/H exchanger regulatory factor-1 (NHERF1),
which is a cytoplasmic PDZ protein, binds PTHR1 through its PDZ
motif to anchor it to the actin skeleton, and thus mediates the
downstream signaling of this receptor (36). A feedback mechanism is suggested for
PTH/PTHR1, as PTH was shown to transcriptionally downregulate
PTH/PTHrP receptor gene expression in osteoblast-like cells via a
cAMP-dependent, PKA-independent pathway (37).
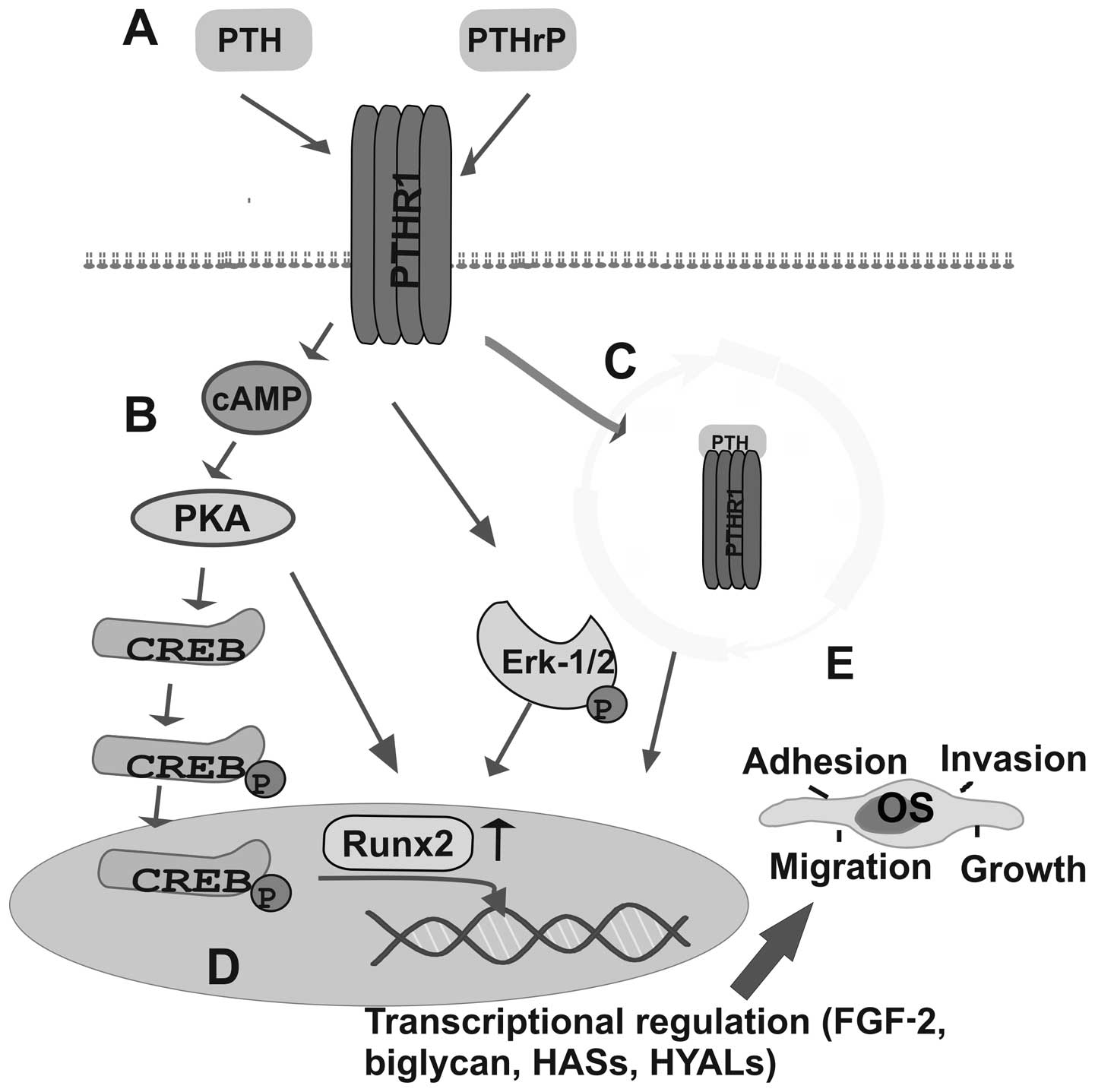
In an early study on OS cells, it was demonstrated
that PTH induces c-fos transcription, which is important to bone
metabolism. Indeed, the induction of c-fos transcription by PTH
appears to occur principally through the activation of PKA, that
then targets CREB and the c-fos calcium/cAMP response element
(38). Thus, in PTH-treated SaOS2
OS cells, CREB was shown to be phosphorylated at Ser133, leading to
the decreased motility of the CREB:CRE complex. The phosphorylated
CREB:CRE complex recruits an adaptor protein that enhances c-fos
transcription (39).
PTHrP is widely expressed in fetal and adult
tissues, and is able to regulate cellular calcium transport, as
well as cell proliferation, development and differentiation. The
dysregulated expression of PTHrP has shown to correlate with
cancer-related pathologies, as the key inducer of
malignancy-associated hypercalcemia; however, new developments
suggest its crucial participation in the progression of skeletal
metastasis (40). In a previous
study, it was demonstrated that increased autocrine secretion of,
and responsiveness to, PTHrP results in the inhibition of the
growth kinetics of the rat osteoblastic OS cell line, UMR 106-01,
both in vitro and in vivo (41). Moreover, the osteostatin domain of
C-terminal PTHrP induces the activation of Src, extracellular
signal-regulated kinase (ERK) and Akt, resulting in the
phosphorylation of vascular endothelial growth factor receptor 2
(VEGFR2) in rat osteoblastic OS UMR-106 cells thus, regulating the
functions of these cells (42). Two
human osteoblast-like OS cell lines show distinct expression and
differential regulation of PTHrP expression (43). Furthermore, PTHrP expression
possibly mediates the function of bone microenvironment-related
growth factors in MG-63 OS cells. It is of clinical relevance that
PTHrP and anticancer drugs show opposing interactions on death
receptor-triggered, as well as on mitochondrial apoptotic pathways.
Thus, stimulation experiments of the CD95-, the TNF-R- and the
TRAIL-R-death receptor systems in Saos2 human OS cells revealed
that PTHrP can block signaling via each of these death receptors.
In addition, PTHrP induces chemoresistance by interference with p53
family-dependent apoptotic signaling pathways, and the p53-mediated
transactivation of apoptosis target genes (44).
5. PTH/PTHrP-induced ECM remodeling in OS:
Novel insights
The ECM is a network of molecules secreted by cells,
with an inherent self-assembly ability, that provides structure and
organization to tissues. This extensive network is mainly composed
of collagens, elastin, proteoglycans (PGs), glycosaminoglycans
(GAGs), fibronectins and laminins (45,46).
Similar to the cell membrane, the ECM forms an active interface,
which is the basis of both out-in and in-out signaling. The
constant interactions between cells and ECM components are mostly
perpetrated through cell membrane receptors (47,48),
whereupon the binding of a ligand results in the activation of a
cascade of signaling pathways which in turn induce several cellular
responses (49), including
proliferation, adhesion, invasion, cell motility and metastasis
(50,51).
The OS ECM is extensively altered as compared to
physiological bone tissue. Indeed, the main characteristic of the
most common osteoblastic subtype of OS is the non-mineralized
osteoid production (52,53). Normally, osteoblasts produce a
highly specialized ECM, osteoid, which is a complex of mineralized
proteins. The changes in the ECM of OS have been directly
correlated to disease progression (54–57).
Importantly, ECM is a pool of growth factors, hormones, cytokines
and other active mediators, whose bioavailability (45) is altered in OS, and may contribute
to disease progression (56).
Extensive feedback exists among these mediators and the OS ECM
content and organization which ultimately regulate OS biological
function (54–57). Importantly, a significant facet of
PTH function is the regulation of bone ECM, osteoid (58).
Recently, the anabolic effect of PTH 1-34 on bone
metabolism has been shown to correlate with changes in fibroblast
growth factor-2 (FGF-2) expression. These FGF fluctuations have
been shown to modify the nuclear accumulation and subsequent action
of runt-related transcription factor 2 (Runx-2) and CREB
transcription factors, key in the regulation of osteoblast growth
and differentiation (59). Indeed,
in a FGF-2-dependent manner, Runx2 modifies the transcription of
genes related to signaling mechanisms perpetrated by PGs, including
syndecans, glypicans and versican. Moreover, it has been proposed
that Runx2/FGF-2/PG downstream signaling constitutes an
ECM-mediated feedback loop that regulates the growth of
osteoblastic lineage cells (60).
The existence of this signaling axis was recently confirmed in OS
cells (61). Thus, PTH 1-34
intermittent treatment induces a significant increase in FGF-2
transcripts. Enhanced FGF-2 signaling decreases the expression of
the small leucine-rich repeat proteoglycan (SLRP), biglycan, in a
manner positively correlated to MG63 OS cell migration (61). Indeed, Datsis et al
speculated that 'the PTH 1-34-FGF-2 axis, by causing strong
downregulation of biglycan expression, dramatically changes the
ratio among the SLRP members, resulting in altered bioavailability
of growth factors involved in OS cell migration' (61). Importantly, the family of SLRPs has
shown to be associated with the progression of OS (51,56,57).
In particular, decorin seems to affect differentiation of OS cells
(62), whereas biglycan, is related
to the growth, proliferation and migration of OS cells (3,57). The
expression of another SLRP, lumican, was found to be 'positively
correlated with the differentiation and negatively with the growth
of human OS cells' (51). The GAG
chains bound in to PG cores participate in the fine modulation of
osteoblastic cell functions (63).
It has been shown that under estrogen deficiency,
resulting from bilateral ovariectomy (OVX), ECM proteins, including
biglycan, tenascin and fibronectin have an altered expression and
distribution in OVX as compared to control animals (64). The increase in biglycan expression
has been shown to correlate with the regulation of bone formation
and matrix mineralization by facilitating osteoblast
differentiation through Erk activation and increased Runx2
transcriptional activity (65).
Periostin, a conserved ECM protein, is crucial for the process of
tumorigenesis (66). Notably, the
expression of this bone matrix protein is regulated by PTH
(67). Importantly, periostin was
found to be overexpressed in various types of human cancer, where
it interacts with multiple cell-surface receptors, most notably
integrins, and signals mainly via the PI3-K/Akt to promote cancer
cell survival, and metastasis (66).
Another ECM component, the glycosaminoglycan,
hyaluronan (HA), was established to regulate cancer cell function
(50,68). In an early study, PTH was
demonstrated to strongly increase HA synthesis in UMR 106-01 BSP OS
cells (69). Moreover, periosteal
and endosteal osteoblastic cell populations exhibited metabolic
differences in their HA synthesis responses to PTH (70). Interestingly, Berdiaki et al
(71) suggest that there is a
regulatory effect of PTH 1-34, in an administration mode-dependent
manner, on HA metabolism that is essential for OS cell migration.
The effects of PTH were shown to correlate with OS cell
differentiation and behavior. Specifically, intermittent PTH 1-34
treatment of poorly differentiated and aggressive MG-63 cells
increased their HA-synthase-2 expression, which resulted in
enhanced high-molecular size HA deposition in the pericellular
matrix, in association with the increased ability of these cells to
migrate. Interestingly, continuous PTH 1-34 treatment stimulated
well-differentiated Saos2 cell HA production and modestly enhanced
their migration (71). Recently,
PTH intermittent treatment was shown to increase HA deposition in
rat long bones (72).
OS pathogenesis is, additionally, characterized by
the differential expression of matrix metalloproteinases (MMPs)
which results in ECM integrity disruption (73). MMPs are proteolytic enzymes
responsible for ECM remodeling under both pathological and
physiological conditions (73–78).
Importantly, the overexpression of MMPs has been observed in many
types of cancer (77), indicating
that their expression may be utilized as a possible prognostic
marker (78). Furthermore, it has
been suggested, that the increased expression of MMP-1 and -9 may,
in OS, predict an adverse outcome such as invasion or metastasis
(79–81). Indeed, Husmann et al
(76) based on in vivo and
in vitro experiments, suggested that MMP-1 overexpression in
OS plays an important role in tumor burden and pulmonary
metastasis. In a recent study, PTH was shown to increase MMP-13
expression in UMR 106-01 OS cells, as well as in normal osteoblasts
(82). On the other hand, the daily
injection of rhPTH 1-34 has been shown to result in a decrease in
serum Runx2 and MMP-13 levels in post-menopausal women (83). The PTH/PTHrP-dependent ECM signaling
on OS cell functions is shown in Fig.
1.
6. Conclusions
In conclusion, this review has highlighted the
evolving roles that PTH/PTRrP signaling play in the progression of
OS. PTH/PTRrP are established to specifically regulate bone
remodeling, as well as to participate in the progression of
commonly debilitating and degenerative bone diseases. Further
progress in discerning the specific signaling pathways of PTH/PTRrP
in the pathogenesis of OS is warranted.
Abbreviations:
|
OS
|
osteosarcoma
|
|
ECM
|
extracellular matrix
|
|
MSCs
|
mesenchymal stem cells
|
|
PTH
|
parathyroid hormone
|
|
PTHR1
|
parathyroid hormone receptor 1
|
|
GPCR
|
G-protein-coupled receptor
|
|
PTHrP
|
parathyroid hormone-related
peptide
|
|
CREB
|
cAMP response element-binding
protein
|
|
PGs
|
proteoglycans
|
|
GAGs
|
glycosaminoglycans
|
|
FGF
|
fibroblast growth factor
|
|
Runx-2
|
runt-related transcription factor
2
|
|
SLRPs
|
small leucine-rich repeat
proteoglycans
|
|
HA
|
hyaluronan
|
|
MMPs
|
matrix metalloproteinases
|
References
|
1
|
Mirabello L, Troisi RJ and Savage SA:
International osteosarcoma incidence patterns in children and
adolescents, middle ages and elderly persons. Int J Cancer.
125:229–234. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stiller CA, Bielack SS, Jundt G and
Steliarova-Foucher E: Bone tumours in European children and
adolescents, 1978–1997. Report from the Automated Childhood Cancer
Information System project. Eur J Cancer. 42:2124–2135. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fletcher CDM, Unni KK and Mertens F: World
Health Organization Classification of Tumours. Pathology and
Genetics of Tumours of Soft Tissue and Bone. IARC Press; Lyon:
2002
|
|
4
|
Ragland BD, Bell WC, Lopez RR and Siegal
GP: Cytogenetics and molecular biology of osteosarcoma. Lab Invest.
82:365–373. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bacci G, Longhi A, Fagioli F, Briccoli A,
Versari M and Picci P: Adjuvant and neoadjuvant chemotherapy for
osteosarcoma of the extremities: 27 year experience at Rizzoli
Institute, Italy. Eur J Cancer. 41:2836–2845. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Weiss A, Khoury JD, Hoffer FA, Wu J,
Billups CA, Heck RK, Quintana J, Poe D, Rao BN and Daw NC:
Telangiectatic osteosarcoma: The St. Jude Children's Research
Hospital's experience. Cancer. 109:1627–1637. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jaffe N, Carrasco H, Raymond K, Ayala A
and Eftekhari F: Can cure in patients with osteosarcoma be achieved
exclusively with chemotherapy and abrogation of surgery? Cancer.
95:2202–2210. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mutsaers AJ and Walkley CR: Cells of
origin in osteosarcoma: Mesenchymal stem cells or osteoblast
committed cells? Bone. 62:56–63. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lin PP, Pandey MK, Jin F, Raymond AK,
Akiyama H and Lozano G: Targeted mutation of p53 and Rb in
mesenchymal cells of the limb bud produces sarcomas in mice.
Carcinogenesis. 30:1789–1795. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mutsaers AJ, Ng AJ, Baker EK, Russell MR,
Chalk AM, Wall M, Liddicoat BJ, Ho PW, Slavin JL, Goradia A, et al:
Modeling distinct osteosarcoma subtypes in vivo using Cre:lox and
lineage-restricted transgenic shRNA. Bone. 55:166–178. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Overholtzer M, Rao PH, Favis R, Lu XY,
Elowitz MB, Barany F, Ladanyi M, Gorlick R and Levine AJ: The
presence of p53 mutations in human osteosarcomas correlates with
high levels of genomic instability. Proc Natl Acad Sci USA.
100:11547–11552. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mohseny AB, Szuhai K, Romeo S, Buddingh
EP, Briaire-de Bruijn I, de Jong D, van Pel M, Cleton-Jansen AM and
Hogendoorn PC: Osteosarcoma originates from mesenchymal stem cells
in consequence of aneuploidization and genomic loss of Cdkn2. J
Pathol. 219:294–305. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vilardaga JP, Romero G, Friedman PA and
Gardella TJ: Molecular basis of parathyroid hormone receptor
signaling and trafficking: A family B GPCR paradigm. Cell Mol Life
Sci. 68:1–13. 2011. View Article : Google Scholar
|
|
14
|
Huan J, Olgaard K, Nielsen LB and Lewin E:
Parathyroid hormone 7–84 induces hypocalcemia and inhibits the
parathyroid hormone 1–84 secretory response to hypocalcemia in rats
with intact parathyroid glands. J Am Soc Nephrol. 17:1923–1930.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cheloha RW, Gellman SH, Vilardaga JP and
Gardella TJ: PTH receptor-1 signalling-mechanistic insights and
therapeutic prospects. Nat Rev Endocrinol. 11:712–724. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Friedman PA and Goodman WG:
PTH(1-84)/PTH(7-84): A balance of power. Am J Physiol Renal
Physiol. 290:F975–F984. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Burton DW, Brandt DW and Deftos LJ:
Parathyroid hormone-related protein in the cardiovascular system.
Endocrinology. 135:253–261. 1994.PubMed/NCBI
|
|
18
|
Kronenberg HM: Developmental regulation of
the growth plate. Nature. 423:332–336. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Abou-Samra AB, Jüppner H, Force T, Freeman
MW, Kong XF, Schipani E, Urena P, Richards J, Bonventre JV and
Potts JT Jr: Expression cloning of a common receptor for
parathyroid hormone and parathyroid hormone-related peptide from
rat osteoblast-like cells: A single receptor stimulates
intracellular accumulation of both cAMP and inositol trisphosphates
and increases intracellular free calcium. Proc Natl Acad Sci USA.
89:2732–2736. 1992. View Article : Google Scholar
|
|
20
|
Mannstadt M, Jüppner H and Gardella TJ:
Receptors for PTH and PTHrP: Their biological importance and
functional properties. Am J Physiol. 277:F665–F675. 1999.PubMed/NCBI
|
|
21
|
Kondo H, Guo J and Bringhurst FR: Cyclic
adenosine monophosphate/protein kinase A mediates parathyroid
hormone/parathyroid hormone-related protein receptor regulation of
osteoclastogenesis and expression of RANKL and osteoprotegerin
mRNAs by marrow stromal cells. J Bone Miner Res. 17:1667–1679.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cheung R, Erclik MS and Mitchell J:
Increased expression of G11alpha in osteoblastic cells enhances
parathyroid hormone activation of phospholipase C and AP-1
regulation of matrix metalloproteinase-13 mRNA. J Cell Physiol.
204:336–343. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rey A, Manen D, Rizzoli R, Ferrari SL and
Caverzasio J: Evidences for a role of p38 MAP kinase in the
stimulation of alkaline phosphatase and matrix mineralization
induced by parathyroid hormone in osteoblastic cells. Bone.
41:59–67. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ferrari SL, Behar V, Chorev M, Rosenblatt
M and Bisello A: Endocytosis of ligand-human parathyroid hormone
receptor 1 complexes is protein kinase C-dependent and involves
beta-arrestin2. Real-time monitoring by fluorescence microscopy. J
Biol Chem. 274:29968–29975. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vilardaga JP, Krasel C, Chauvin S, Bambino
T, Lohse MJ and Nissenson RA: Internalization determinants of the
parathyroid hormone receptor differentially regulate
beta-arrestin/receptor association. J Biol Chem. 277:8121–8129.
2002. View Article : Google Scholar
|
|
26
|
De Camilli P, Emr SD, McPherson PS and
Novick P: Phosphoinositides as regulators in membrane traffic.
Science. 271:1533–1539. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vahle JL, Sato M, Long GG, Young JK,
Francis PC, Engelhardt JA, Westmore MS, Linda Y and Nold JB:
Skeletal changes in rats given daily subcutaneous injections of
recombinant human parathyroid hormone (1-34) for 2 years and
relevance to human safety. Toxicol Pathol. 30:312–321. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tashjian AH Jr and Gagel RF: Teriparatide
[human PTH(1-34)]: 2.5 years of experience on the use and safety of
the drug for the treatment of osteoporosis. J Bone Miner Res.
21:354–365. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Watanabe A, Yoneyama S, Nakajima M, Sato
N, Takao-Kawabata R, Isogai Y, Sakurai-Tanikawa A, Higuchi K,
Shimoi A, Yamatoya H, et al: Osteosarcoma in Sprague-Dawley rats
after long-term treatment with teriparatide (human parathyroid
hormone (1-34)). J Toxicol Sci. 37:617–629. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Subbiah V, Madsen VS, Raymond AK, Benjamin
RS and Ludwig JA: Of mice and men: Divergent risks of
teriparatide-induced osteosarcoma. Osteoporos Int. 21:1041–1045.
2010. View Article : Google Scholar
|
|
31
|
Harper KD, Krege JH, Marcus R and Mitlak
BH: Comments on Initial experience with teriparatide in the United
States. Curr Med Res Opin. 22:19272006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Harper KD, Krege JH, Marcus R and Mitlak
BH: Osteosarcoma and teriparatide? J Bone Miner Res. 22:3342007.
View Article : Google Scholar
|
|
33
|
Andrews EB, Gilsenan AW, Midkiff K,
Sherrill B, Wu Y, Mann BH and Masica D: The US postmarketing
surveillance study of adult osteosarcoma and teriparatide: Study
design and findings from the first 7 years. J Bone Miner Res.
27:2429–2437. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ho PW, Goradia A, Russell MR, Chalk AM,
Milley KM, Baker EK, Danks JA, Slavin JL, Walia M, Crimeen-Irwin B,
et al: Knockdown of PTHR1 in osteosarcoma cells decreases invasion
and growth and increases tumor differentiation in vivo. Oncogene.
34:2922–2933. 2015. View Article : Google Scholar
|
|
35
|
Yang D, Singh R, Divieti P, Guo J,
Bouxsein ML and Bringhurst FR: Contributions of parathyroid hormone
(PTH)/PTH-related peptide receptor signaling pathways to the
anabolic effect of PTH on bone. Bone. 40:1453–1461. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ardura JA, Wang B, Watkins SC, Vilardaga
JP and Friedman PA: Dynamic Na+–H+ exchanger regulatory factor-1
association and dissociation regulate parathyroid hormone receptor
trafficking at membrane microdomains. J Biol Chem. 286:35020–35029.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kawane T, Mimura J, Yanagawa T,
Fujii-Kuriyama Y and Horiuchi N: Parathyroid hormone (PTH)
down-regulates PTH/PTH-related protein receptor gene expression in
UMR-106 osteoblast-like cells via a 3′,5′-cyclic adenosine
mono-phosphate-dependent, protein kinase A-independent pathway. J
Endocrinol. 178:247–256. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Evans DB, Hipskind RA and Bilbe G:
Analysis of signaling pathways used by parathyroid hormone to
activate the c-fos gene in human SaOS2 osteoblast-like cells. J
Bone Miner Res. 11:1066–1074. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Pearman AT, Chou WY, Bergman KD, Pulumati
MR and Partridge NC: Parathyroid hormone induces c-fos promoter
activity in osteoblastic cells through phosphorylated cAMP response
element (CRE)-binding protein binding to the major CRE. J Biol
Chem. 271:25715–25721. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liao J and McCauley LK: Skeletal
metastasis: Established and emerging roles of parathyroid hormone
related protein (PTHrP). Cancer Metastasis Rev. 25:559–571. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Pasquini GM, Davey RA, Ho PW, Michelangeli
VP, Grill V, Kaczmarczyk SJ and Zajac JD: Local secretion of
parathyroid hormone-related protein by an osteoblastic osteosarcoma
(UMR 106-01) cell line results in growth inhibition. Bone.
31:598–605. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
García-Martín A, Acitores A, Maycas M,
Villanueva-Peñacarrillo ML and Esbrit P: Src kinases mediate VEGFR2
transactivation by the osteostatin domain of PTHrP to modulate
osteoblastic function. J Cell Biochem. 114:1404–1413. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Jemtland R, Rian E, Olstad OK, Haug E,
Bruland OS, Bucht E and Gautvik KM: Two human osteoblast-like
osteosarcoma cell lines show distinct expression and differential
regulation of parathyroid hormone-related protein. J Bone Miner
Res. 14:904–914. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Gagiannis S, Müller M, Uhlemann S, Koch A,
Melino G, Krammer PH, Nawroth PP, Brune M and Schilling T:
Parathyroid hormone-related protein confers chemoresistance by
blocking apoptosis signaling via death receptors and mitochondria.
Int J Cancer. 125:1551–1557. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kresse H and Schönherr E: Proteoglycans of
the extracellular matrix and growth control. J Cell Physiol.
189:266–274. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Afratis N, Gialeli C, Nikitovic D,
Tsegenidis T, Karousou E, Theocharis AD, Pavão MS, Tzanakakis GN
and Karamanos NK: Glycosaminoglycans: Key players in cancer cell
biology and treatment. FEBS J. 279:1177–1197. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Sanderson RD, Yang Y, Suva LJ and Kelly T:
Heparan sulfate proteoglycans and heparanase - partners in
osteolytic tumor growth and metastasis. Matrix Biol. 23:341–352.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Maxwell CA, McCarthy J and Turley E:
Cell-surface and mitotic-spindle RHAMM: Moonlighting or dual
oncogenic functions? J Cell Sci. 121:925–932. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Xu R, Boudreau A and Bissell MJ: Tissue
architecture and function: Dynamic reciprocity via extra- and
intra-cellular matrices. Cancer Metastasis Rev. 28:167–176. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kouvidi K, Nikitovic D, Berdiaki A and
Tzanakakis GN: Hyaluronan/RHAMM interactions in mesenchymal tumor
pathogenesis: Role of growth factors. Adv Cancer Res. 123:319–349.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Nikitovic D, Chalkiadaki G, Berdiaki A,
Aggelidakis J, Katonis P, Karamanos NK and Tzanakakis GN: Lumican
regulates osteosarcoma cell adhesion by modulating TGFβ2 activity.
Int J Biochem Cell Biol. 43:928–935. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Unni KK: Osteosarcoma of bone. J Orthop
Sci. 3:287–294. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Benayahu D, Shur I, Marom R, Meller I and
Issakov J: Cellular and molecular properties associated with
osteosarcoma cells. J Cell Biochem. 84:108–114. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Nikitovic D, Tsatsakis AM, Karamanos NK
and Tzanakakis GN: The effects of genistein on the synthesis and
distribution of glycosaminoglycans/proteoglycans by two
osteosarcoma cell lines depends on tyrosine kinase and the estrogen
receptor density. Anticancer Res. 23(1A): 459–464. 2003.PubMed/NCBI
|
|
55
|
Nikitovic D, Zafiropoulos A, Katonis P,
Tsatsakis A, Theocharis AD, Karamanos NK and Tzanakakis GN:
Transforming growth factor-beta as a key molecule triggering the
expression of versican isoforms v0 and v1, hyaluronan synthase-2
and synthesis of hyaluronan in malignant osteosarcoma cells. IUBMB
Life. 58:47–53. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Nikitovic D, Berdiaki K, Chalkiadaki G,
Karamanos N and Tzanakakis G: The role of SLRP-proteoglycans in
osteosarcoma pathogenesis. Connect Tissue Res. 49:235–238. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Nikitovic D, Aggelidakis J, Young MF,
Iozzo RV, Karamanos NK and Tzanakakis GN: The biology of small
leucine-rich proteoglycans in bone pathophysiology. J Biol Chem.
287:33926–33933. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Datta NS and Abou-Samra AB: PTH and PTHrP
signaling in osteoblasts. Cell Signal. 21:1245–1254. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Sabbieti MG, Agas D, Xiao L, Marchetti L,
Coffin JD, Doetschman T and Hurley MM: Endogenous FGF-2 is
critically important in PTH anabolic effects on bone. J Cell
Physiol. 219:143–151. 2009. View Article : Google Scholar :
|
|
60
|
Teplyuk NM, Haupt LM, Ling L, Dombrowski
C, Mun FK, Nathan SS, Lian JB, Stein JL, Stein GS, Cool SM, et al:
The osteogenic transcription factor Runx2 regulates components of
the fibroblast growth factor/proteoglycan signaling axis in
osteoblasts. J Cell Biochem. 107:144–154. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Datsis GA, Berdiaki A, Nikitovic D,
Mytilineou M, Katonis P, Karamanos NK and Tzanakakis GN:
Parathyroid hormone affects the fibroblast growth
factor-proteoglycan signaling axis to regulate osteosarcoma cell
migration. FEBS J. 278:3782–3792. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Zafiropoulos A, Nikitovic D, Katonis P,
Tsatsakis A, Karamanos NK and Tzanakakis GN: Decorin-induced growth
inhibition is overcome through protracted expression and activation
of epidermal growth factor receptors in osteosarcoma cells. Mol
Cancer Res. 6:785–794. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Nikitovic D, Zafiropoulos A, Tzanakakis
GN, Karamanos NK and Tsatsakis AM: Effects of glycosaminoglycans on
cell proliferation of normal osteoblasts and human osteosarcoma
cells depend on their type and fine chemical compositions.
Anticancer Res. 25:2851–2856. 2005.PubMed/NCBI
|
|
64
|
El Khassawna T, Böcker W, Brodsky K,
Weisweiler D, Govindarajan P, Kampschulte M, Thormann U, Henss A,
Rohnke M and Bauer N: Impaired extracellular matrix structure
resulting from malnutrition in ovariectomized mature rats.
Histochem Cell Biol. 144:491–507. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Wang X, Harimoto K, Xie S, Cheng H, Liu J
and Wang Z: Matrix protein biglycan induces osteoblast
differentiation through extracellular signal-regulated kinase and
Smad pathways. Biol Pharm Bull. 33:1891–1897. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Ruan K, Bao S and Ouyang G: The
multifaceted role of periostin in tumorigenesis. Cell Mol Life Sci.
66:2219–2230. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Fortunati D, Reppe S, Fjeldheim AK,
Nielsen M, Gautvik VT and Gautvik KM: Periostin is a collagen
associated bone matrix protein regulated by parathyroid hormone.
Matrix Biol. 29:594–601. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Nikitovic D, Kouvidi K, Kavasi RM,
Berdiaki A and Tzanakakis GN: Hyaluronan/Hyaladherins - a Promising
Axis for Targeted Drug Delivery in Cancer. Curr Drug Deliv.
13:500–511. 2016. View Article : Google Scholar
|
|
69
|
Midura RJ, Evanko SP and Hascall VC:
Parathyroid hormone stimulates hyaluronan synthesis in an
osteoblast-like cell line. J Biol Chem. 269:13200–13206.
1994.PubMed/NCBI
|
|
70
|
Midura RJ, Su X, Morcuende JA, Tammi M and
Tammi R: Parathyroid hormone rapidly stimulates hyaluronan
synthesis by periosteal osteoblasts in the tibial diaphysis of the
growing rat. J Biol Chem. 278:51462–51468. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Berdiaki A, Datsis GA, Nikitovic D,
Tsatsakis A, Katonis P, Karamanos NK and Tzanakakis GN: Parathyroid
hormone (PTH) peptides through the regulation of hyaluronan
metabolism affect osteosarcoma cell migration. IUBMB Life.
62:377–386. 2010.PubMed/NCBI
|
|
72
|
Pacheco-Costa R, Campos JF, Katchburian E,
de Medeiros VP, Nader HB, Nonaka KO, Plotkin LI and Reginato RD:
Modifications in bone matrix of estrogen-deficient rats treated
with intermittent PTH. BioMed Res Int. 2015:4541622015. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Kido A, Tsutsumi M, Iki K, Takahama M,
Tsujiuchi T, Morishita T, Tamai S and Konishi Y: Overexpression of
matrix metalloproteinase (MMP)-9 correlates with metastatic potency
of spontaneous and 4-hydroxyaminoquinoline 1-oxide (4-HAQO)-induced
transplantable osteosarcomas in rats. Cancer Lett. 137:209–216.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Wang J, Shi Q, Yuan TX, Song QL, Zhang Y,
Wei Q, Zhou L, Luo J, Zuo G, Tang M, et al: Matrix
metalloproteinase 9 (MMP-9) in osteosarcoma: Review and
meta-analysis. Clin Chim Acta. 433:225–231. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Deryugina EI and Quigley JP: Matrix
metalloproteinases and tumor metastasis. Cancer Metastasis Rev.
25:9–34. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Husmann K, Arlt MJ, Muff R, Langsam B,
Bertz J, Born W and Fuchs B: Matrix Metalloproteinase 1 promotes
tumor formation and lung metastasis in an intratibial injection
osteosarcoma mouse model. Biochim Biophys Acta. 1832:347–354. 2013.
View Article : Google Scholar
|
|
77
|
Egeblad M and Werb Z: New functions for
the matrix metal-loproteinases in cancer progression. Nat Rev
Cancer. 2:161–174. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
78
|
Shuman Moss LA, Jensen-Taubman S and
Stetler-Stevenson WG: Matrix metalloproteinases: Changing roles in
tumor progression and metastasis. Am J Pathol. 181:1895–1899. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Uchibori M, Nishida Y, Nagasaka T, Yamada
Y, Nakanishi K and Ishiguro N: Increased expression of
membrane-type matrix metalloproteinase-1 is correlated with poor
prognosis in patients with osteosarcoma. Int J Oncol. 28:33–42.
2006.
|
|
80
|
Himelstein BP, Asada N, Carlton MR and
Collins MH: Matrix metalloproteinase-9 (MMP-9) expression in
childhood osseous osteosarcoma. Med Pediatr Oncol. 31:471–474.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Kimura R, Ishikawa C, Rokkaku T, Janknecht
R and Mori N: Phosphorylated c-Jun and Fra-1 induce matrix
metallopro-teinase-1 and thereby regulate invasion activity of 143B
osteosarcoma cells. Biochim Biophys Acta. 1813:1543–1553. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Lee M and Partridge NC: Parathyroid
hormone activation of matrix metalloproteinase-13 transcription
requires the histone acetyltransferase activity of p300 and PCAF
and p300-dependent acetylation of PCAF. J Biol Chem.
285:38014–38022. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Zhu W, Yang ML, Yang GY, Boden G and Li L:
Changes in serum runt-related transcription factor 2 levels after a
6-month treatment with recombinant human parathyroid hormone in
patients with osteoporosis. J Endocrinol Invest. 35:602–606.
2012.
|