Introduction
Gastric cancer (GC) is one of the most common
cancers worldwide, and GC is the 2nd leading cause of cancer death
(1,2). Currently surgery is the main treatment
for early GC; however recurrence is very common in GC patients
after surgery (3,4). Therefore, the combination of surgery
and chemotherapy is a new strategy for the treatment of GC
(5,6). Although chemotherapy has developed
rapidly in recent years, cisplatin (DDP) still remains the most
widely used for GC treatment (7–9).
However, the strategy of DDP-based chemotherapy and surgery
combination is limited by drug resistance, on the other hand
continuous and multiple DDP administration often caused serious
side effects (10). Therefore,
improving the sensitivity of human GC cell to DDP is a critical
point for resolving the challenge. Previous studies have indicated
that cell proliferation, cell apoptosis and DNA repair may play
important roles in drug resistance (11,12),
and the drug resistance always associated with the abnormal
activation of some signal pathways (13–16).
It is well known that microRNAs (miRs) are a class
of short noncoding RNAs. miRs consist of approximately 19–24
nucleotides, and they were involved in post-transcriptional gene
regulation or degradation by direct regulation of target messenger
RNA or translational repression (17,18).
Previous studies reported that aberrant miRs were found in many
types of human tumors (19–23), and some miRs were related to
chemoresistance of DDP in human tumor cells (24–29).
Therefore, the combination of miRs and chemotherapy may play
important roles in the treatment of human cancer. Previous study
indicated that miR-34a was significantly decreased in human gastric
cancer (30); however, whether the
regulation of miR-34a was associated with DDP drug resistance is
still unknown.
In this study, we found the expression of miR-34a
was significantly downregulated in DDP-resistant human GC tissue
samples and cell line. Moreover, upregulation of miR-34a could
inhibit the cell proliferation of DDP resistant SGC7901/DDP cells
by the induction of cell apoptosis through targeting the MET gene.
These finds could help to resolve the DDP resistance in the
treatment of human GC.
Materials and methods
Cell culture
SGC7901 cells were purchased from ATCC. SGC7901/DDP
cells were purchased from Nanjing KeyGEN Biotech. Cells were
cultured in Roswell Park Memorial Institute (RPMI)-1640 medium
supplemented with FBS (10%), penicillin (100 IU/ml) in and
streptomycin (100 IU/ml). RPMI-1640, FBS, penicillin and
streptomycin were all purchased from Invitrogen. To maintain the
DDP resistant phenotype of SGC7901/DDP, SGC7901/DDP cells were
cultured with DDP, the concentration of DDP is 1 μg/ml.
Before our study, SGC7901/DDP cells were cultured without DDP for 1
week.
Clinical samples
A total of 38 histopathologically confirmed GC
patient samples were obtained from the Affiliated Tumor Hospital of
Zhengzhou University. All these patients received DDP-based
chemotherapy before. The GC tissue samples were collected from
these advanced patients who underwent 2 cycles of DDP chemotherapy.
The study was approved by the institutional review boards of the
Affiliated Tumor Hospital of Zhengzhou University, and written
informed consent was obtained from each patient.
Cell viability assay
The cell viability was evaluated by methyl thiazolyl
tetrazolium (MTT) assay. First ~5000 cells were seeded into 96-well
plate for 12 h, and then the cells were treated with serial
concentrations of DDP. At 48 h after DDP treatment, MTT solution
was added to each well (1:10), the final concentration of MTT was
0.5 mg/ml, then the cells were incubated for 4 h. The absorbance
was read at 570 nm with a microplate reader (MD). Each experiment
was performed in triplicate and the data are presented as mean ±
SD.
Transfections
miR-34a mimic, miR-34a inhibitor and control were
all purchased from Invitrogen. Cells were cultured in RPMI-1640
medium with 10% FBS for 24 h, then miR-34a or miR-34a inhibitor
were transfected with Lipofectamine 2000 in antibiotic-free
Opti-MEM medium according to the manufacturer's instruction. The
final concentration of miR-34a mimic and miR-34a inhibitor was 10
nM. At 6 h after transfection, the medium was changed to RPMI-1640
with 10% FBS. MET siRNA was purchased from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA). The protocol used for
MET knockdown has been previously described (30).
RNA extraction and miR-34a
examination
SGC-7901 or SGC-7901/DDP cells were treated and
cultured, total RNA was extracted from the cells using TRIzol
reagent as described by the manufacturer. TaqMan miRNA assays
(Applied Biosystems) was performed to examine the expression of
miRNA-34a. Briefly, total RNA was reverse transcribed by using a
miRNA-specific looped RT primer (Applied Biosystems). Each TaqMan
miRNA Reverse Transcription kit was used for a corresponding miRNA.
Then qPCR was performed to evaluate the level of miR-34a using
TaqMan Universal PCR Master Mix with miRNA-specific TaqMan minor
groove binder probes. In the present study RNA U6 was used as an
internal control. The relative expression of miR-34a was calculated
using the comparative Cycle threshold method. Each experiment was
performed in triplicate and the data are presented as mean ±
SD.
Flow cytometric analysis of
apoptosis
SGC-7901 or SGC-7901/DDP cells were transfected with
inhibitor or mimic and treated with DDP for 48 h. The DDP
concentration of SGC7901 was 0.5 μg/ml and for SGC7901/DDP
was 5 μg/ml. Cells were harvested and submitted to FACS
analysis. The cells were double stained with FITC-Annexin V and
Propidium iodide (PI), and then the stained cells were analyzed
with flow cytometry equipped with CellQuest software. Each
experiment was performed in triplicate and the data are presented
as mean ± SD.
Luciferase assay
The 3′-untranslated region (3′UTR) sequences of MET
was cloned into the downstream region of the firefly luciferase
gene, mutant (Mut) MET was used as control. Human gastric cancer
SGC7901 cells were cultured for 24 h, pGL3-MET-3′-UTR (WT or Mut)
and miR-34a mimic or miRNA mimic control were co-transfected by
Lipofectamine 2000. Cells were cultured for another 48 h, and then
the cells were harvested for luciferase assay. The Dual-Luciferase
Reporter Assay System was used to measure luciferase activity as
described by the manufacturer (Promega). The relative luciferase
activity was normalized to the renilla luciferase expression. Each
experiment was performed in triplicate and the data are presented
as mean ± SD.
Western blot analysis
Human GC tissues and cells were harvested and
protein was extracted by using RIPA lysis buffer. Total protein (40
μg) was separated on 15% SDS-PAGE gels and
electrophoretically transferred onto a PVDF membrane (GE). The PVDF
membrane was blocked with 5% non-fat dry milk for ~2 h, and then
the PVDF membrane was incubated with specific primer antibodies
MET, and GAPDH for 2 h. The PVDF membrane was washed with TBST 3
times, and incubated with horseradish peroxidase-linked second
antibody for another 1 h. The PVDF membranes were washed and the
proteins were visualized using ECL chemiluminescence and exposed to
X-ray film. Each experiment was performed in triplicate.
Real-time PCR to detect the mRNA of
MET
Total RNA was isolated from treated gastric cancer
tissues and cells using TRIzol reagent as described by the
manufacturer (Invitrogen). cDNA was synthesized from total RNA
using a Reverse Transcription kit (Applied Biosystems). The primer
and probe sequences were: GAPDH primer (F:
5′-TCGACAGTCAGCCGCATCTTCTTT-3′; R: 5′-ACCAAATCCGTTGACTCCGACCTT-3′;
and probe: 5′-6FAM-AGCCACATCGCTCAGACACCATGGG-TAMRA-3′); MET primer
(F: 5′-TGCAGCGCGTTGACTTATTCATGG-3′; R:
5′-GAAACCACAACCTGCATGAAGCGA-3′; and probe:
5′-6FAM-AGGAGACCTCACCATAGCTAATCTTGGG-TAMRA-3′); Real-time PCR was
performed as follows, 95°C for 10 min followed by 40 cycles at 95°C
for 15 sec and at 56°C for 60 sec. GAPDH was used as an internal
control. Each experiment was performed in triplicate and the data
are presented as mean ± SD.
Statistical analysis
The data are expressed as the mean ± SD from at
least 3 separate experiments. Statistical analysis was performed by
two-tailed Student's t-tests using SPSS 13.0 (SPSS Inc., Chicago,
IL, USA) to evaluate the significance of differences between
groups. p<0.05 was defined as statistically significant.
Results
DDP resistance of SGC7901/DDP cells
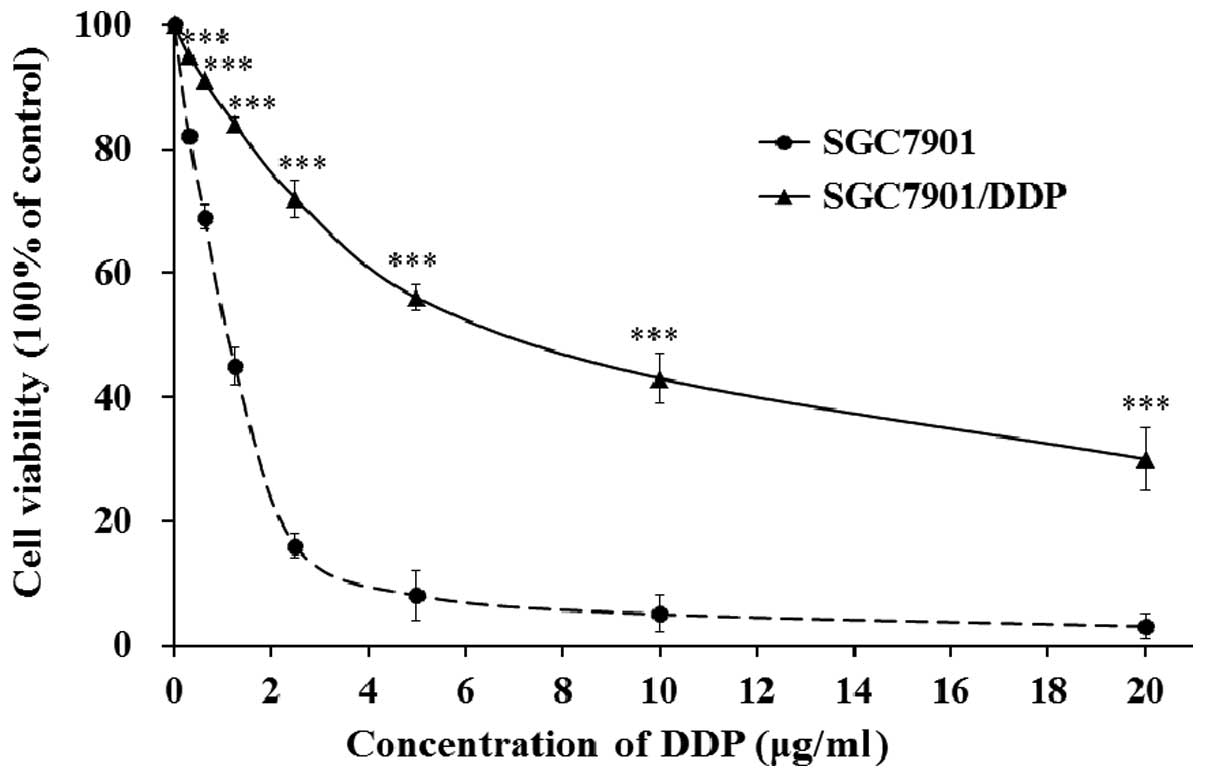
In order to check the DDP resistance of SGC7901/DDP
cells, we evaluated the cell viability of parental SGC7901 cells
and DDP resistant SGC7901/DDP cells when exposed to different
concentrations of DDP. SGC7901 and SGC7901/DDP cells were cultured
and treated with different concentrations of DDP for 48 h; the cell
viability was evaluated by MTT assay. As shown in Fig. 1, when exposed to DDP the cell
viability of both SGC7901 cells and SGC7901/DDP cells decreased
dose-dependently. The results also indicated that SGC7910/DDP cell
lines showed an acquired resistance to DDP compared with the
parental SGC7901 cell lines.
miR-34a was downregulated in GC patients
with DDP resistance and DDP-resistant SGC7901/DDP cells
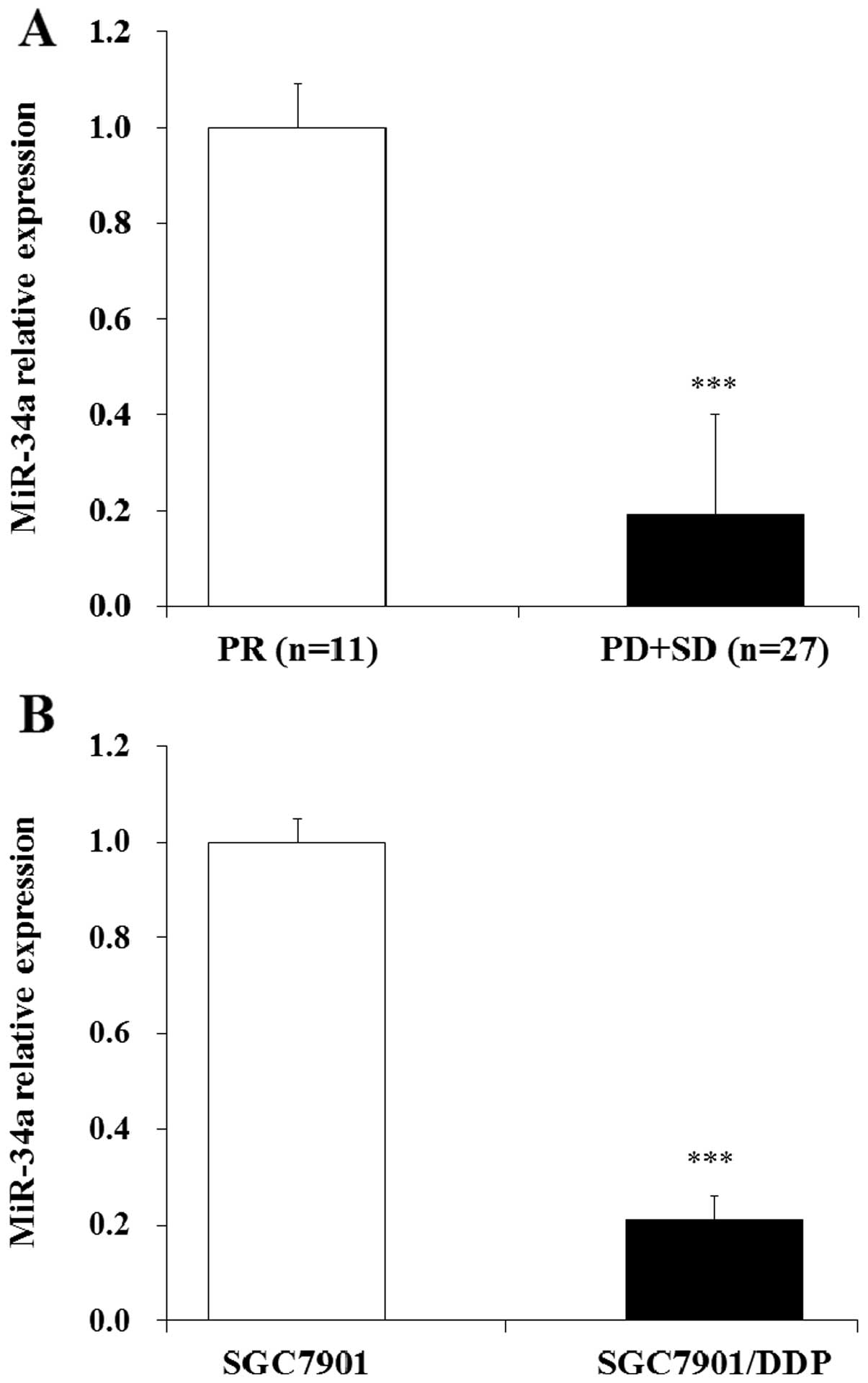
Previous study indicated that the expression of
miR-34a was down regulated in human gastric cancer tissues and
SGC7901 cell lines (30). To
further investigate whether miR-34a was associated with human
gastric cancer DDP resistance, we measured the expression of
miR-34a in 38 GC tissues from advanced patients who underwent 2
cycles of DDP chemotherapy. Response to chemotherapy was evaluated
as previous described (31);
defined as complete remission (CR), partial remission (PR), stable
disease (SD), and progressive disease (PD). In these patients, 11
patients acquired PR, 12 patient acquired SD, 15 patients acquired
PD and no patients acquired CR. The expression of miR-34a in these
tissue samples was evaluated by qRT-PCR assay, in our study we
found that the expression of miR-34a was downregulated in SD, PD
group compared with the PR group (Fig.
2A). Then we tested the level of miR-34a in DDP resistant GC
SGC7901/DDP cells, the results indicated that the expression of
miR-34a was also downregulated in SGC7901/DDP cells compared with
SGC7901 cells (Fig. 2B). These
results showed that miR-34a was downregulated both in clinical GC
tissues and DDP-resistant GC cells. We hypothesized that the
regulation of miR-34a may play important roles in DDP resistant
human GC cells.
Manipulation of miR-34a expression in
human gastric cancer cells
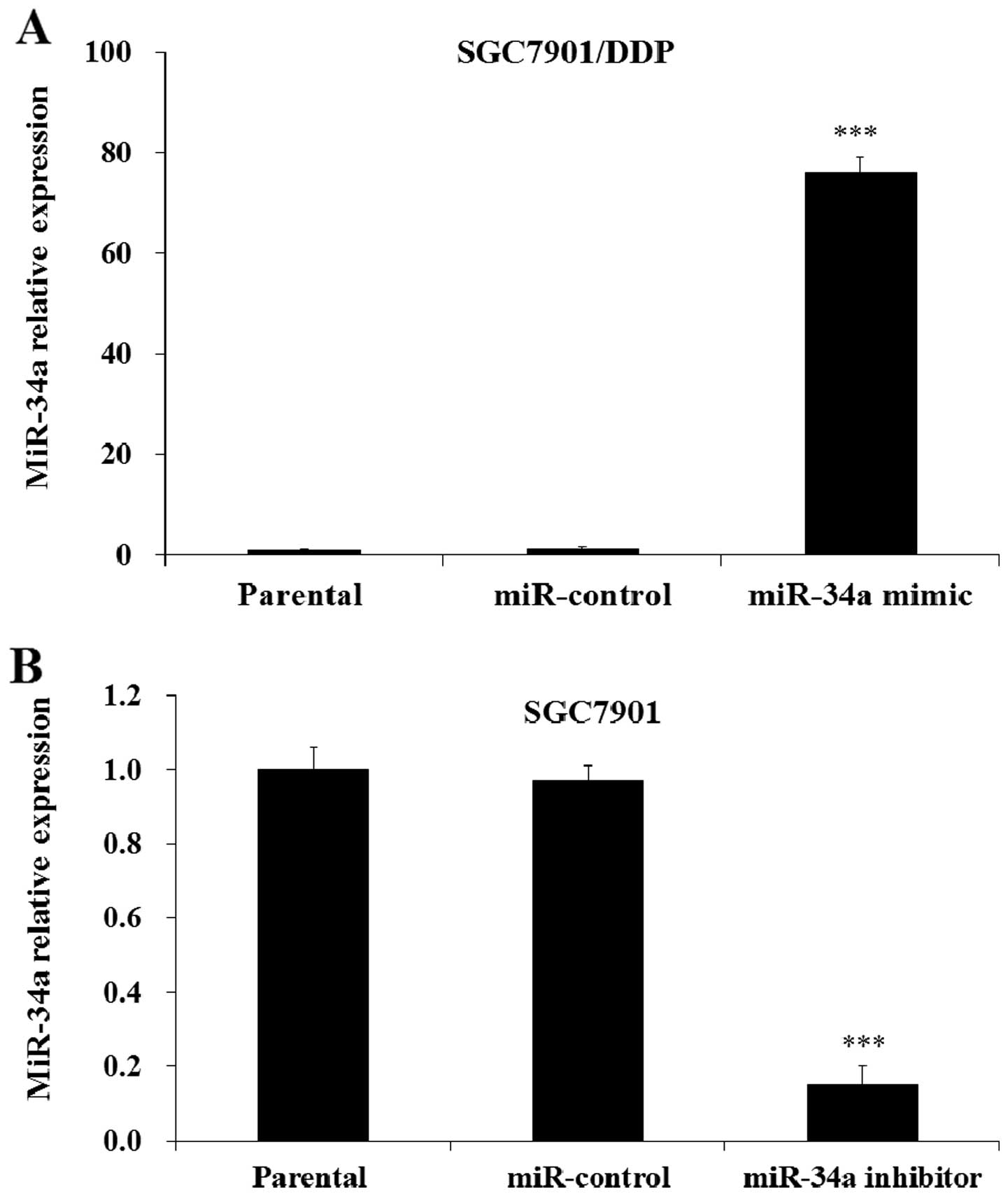
In order to investigate the biological roles of
miR-34a in human gastric cancer cells, we selectively regulated the
expression of miR-34a by miR-34a mimic or inhibitor transfection.
The qRT-PCR results indicated that the level of miR-34a in
SGC7901/DDP cells was significantly increased after the
transfection of miR-34a mimic compared with miR-control or parental
SGC7901/DDP cells (Fig. 3A). The
results also indicated that the level of miR-34a in SGC7901 cells
was significantly decreased with transfection of the cells with
miR-34a inhibitor compared with the control and parental cells
(Fig. 3B). Therefore, we
selectively manipulated the expression of miR-34a by mimic or
inhibitor transfection in this study.
miR-34a modulates DDP resistance of human
gastric cancer cells
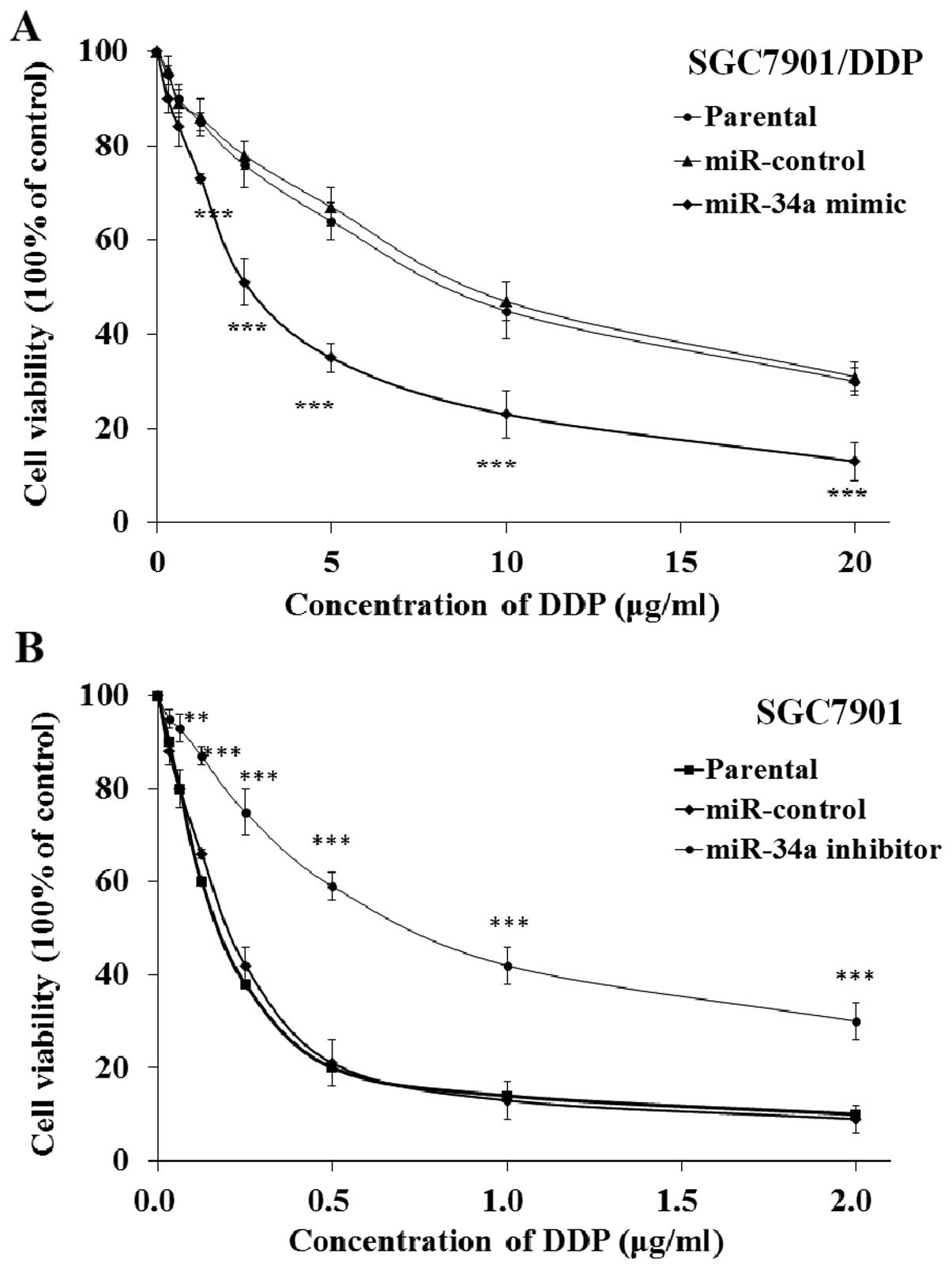
To study the biological activity of miR-34a, miR-34a
mimic or inhibitor transfected SGC7901/DDP or SGC7901 cells were
cultured with DDP; the cell viability was evaluated by MTT assay.
The results indicated that transfection of miR-34a mimic
significantly decreased the cell viability of SGC7901/DDP cells,
while the transfection of mimic control showed no influence
compared with parental SGC7901/DDP cells (Fig. 4A). The results also indicated that
transfection of miR-34a inhibitor significantly increased the cell
viability of SGC7901 cells compared with the control and the
parental SGC7901 cells (Fig. 4B).
These results demonstrated that miR-34a could modulate the DDP
resistance of human gastric cancer cells.
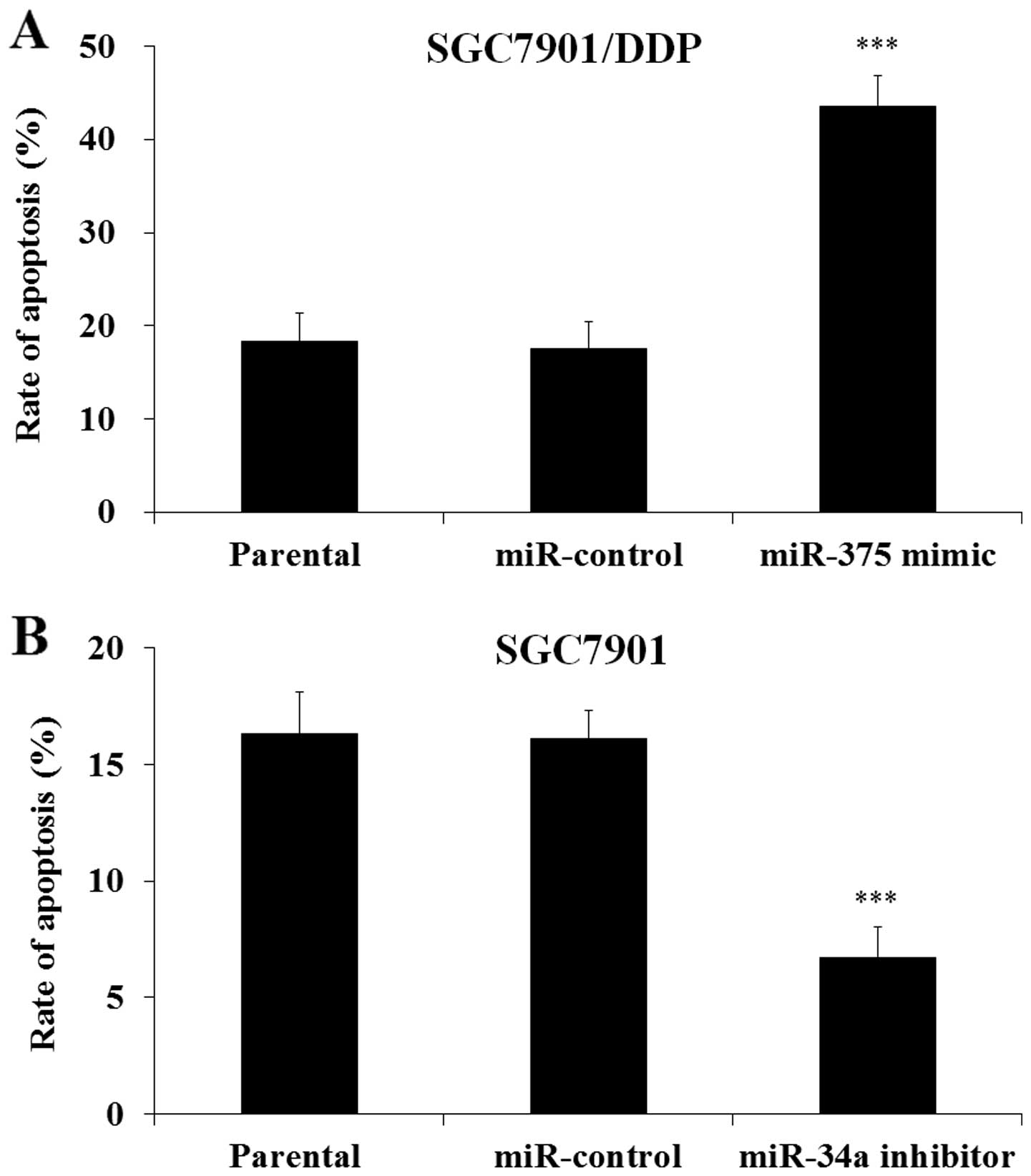
miR-34a modulated the DDP-induced
apoptosis in human gastric cancer cells
To investigate whether the regulation of cell
viability was through the regulation of cell apoptosis, miR-34a
mimic or inhibitor transfected SGC7901/DDP or SGC7901 cells were
cultured with DDP, and then the cells were harvested. FACS assay
was performed to evaluate cell apoptosis. As shown in Fig. 5 the transfection of miR-34a mimic
significantly increased cell apoptosis of SGC7901/DDP cells
(Fig. 5A), while the transfection
of miR-34a inhibitor decreased apoptosis of SGC7901 cells (Fig. 5B). These results demonstrated that
miR-34a modulated the DDP induced human gastric cancer cell
apoptosis.
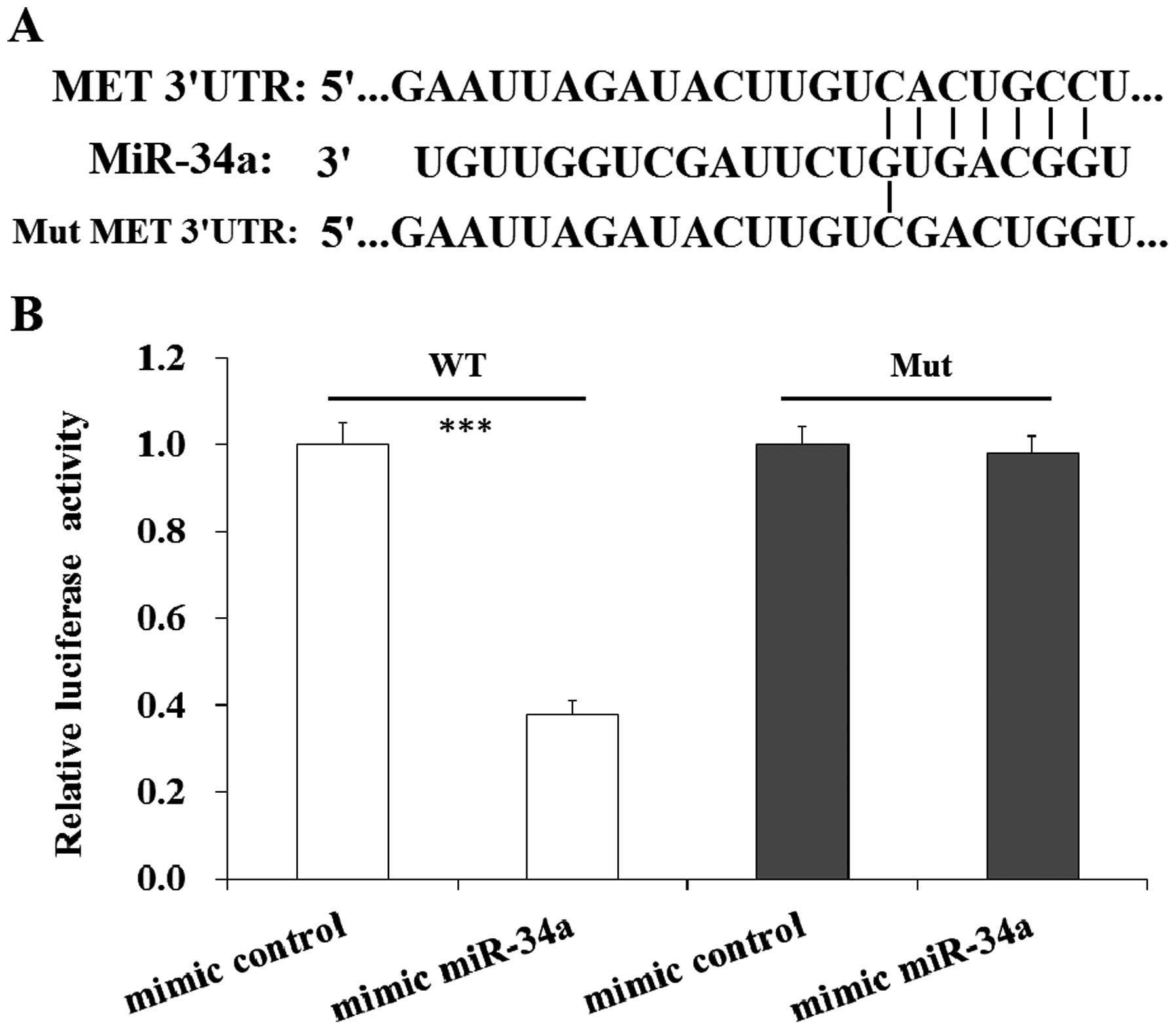
MET is the target gene of miR-34a
Previous study predicted that MET gene was the
target gene of miR-34a (30). In
our study we searched the potential target genes of miR-34a by
using TargetScan, PicTar and miRanda program. The predicted results
also indicated that MET was a direct target gene of miR-34a
(Fig. 6A). To confirm MET was the
direct gene of miR-34a, dual luciferase assay was performed. Human
gastric cancer SGC7901 cells were co-transfected with pGL3-MET and
mimic miR-34a and cultured, then luciferase was tested. The results
indicated that the induction of miR-34a led to a reduction of
luciferase in SGC7901 cells co-transfected with MET WT, while there
was no impact of luciferase in SGC7901 cells co-transfect with MET
Mut (Fig. 6B). The result indicated
MET is a direct target of miR-34a.
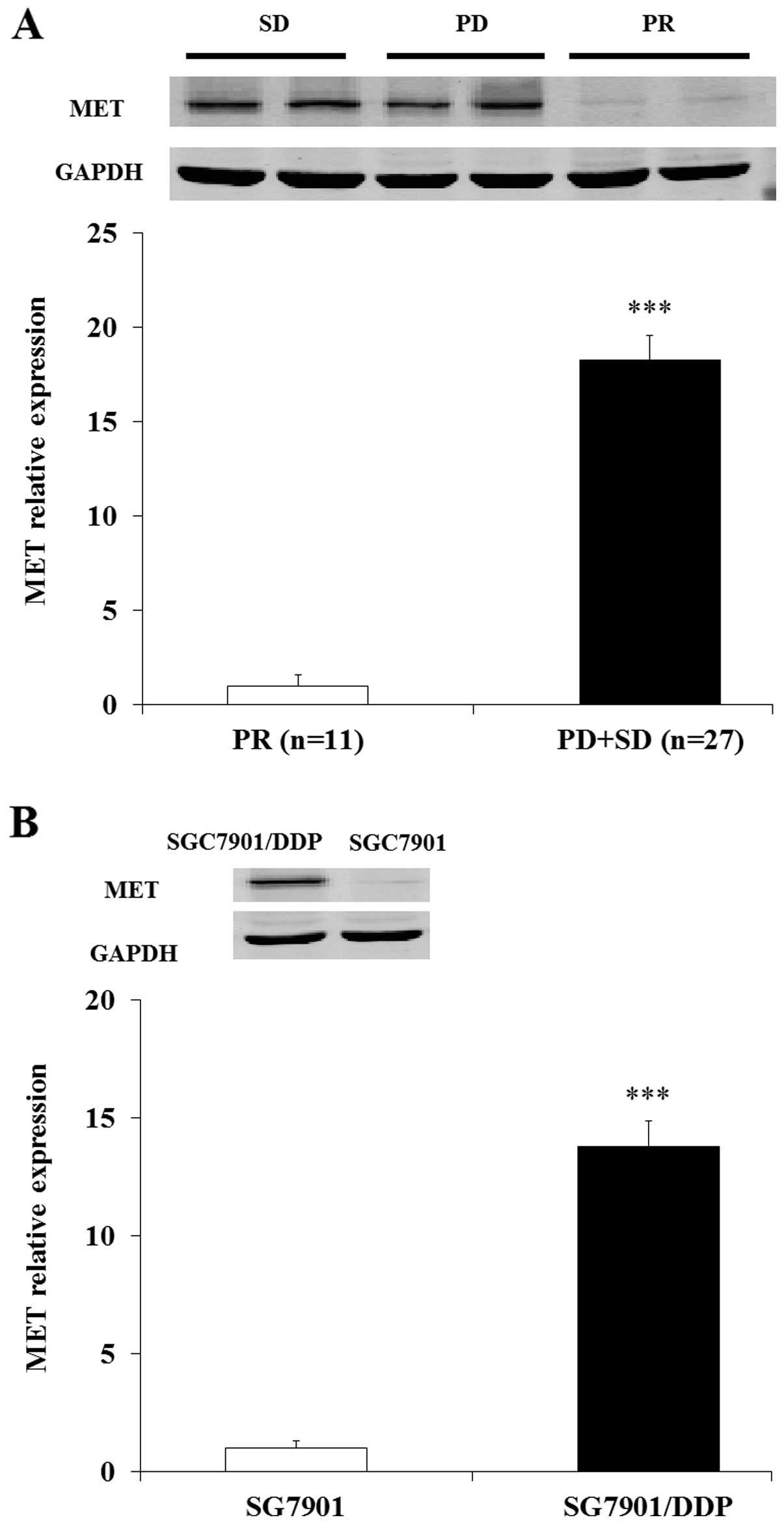
MET was differently expressed in GC
patients with DDP resistance and DDP-resistant SGC7901/DDP
cells
Then we examined the expression of MET in human GC
patients and GC patients with DDP-resistance. Both the western and
RT-PCR results demonstrated that the MET was overexpressed in DDP
resistance patients (PD+SD) compared with human GC patients (PR)
(Fig. 7A). Further study indicated
the level of MET was also upregulated in SGC7901/DDP cells compared
with SGC7901 cells (Fig. 7B). These
results demonstrated that MET was overexpressed in DDP resistant GC
patients and DDP resistant SGC7901/DDP cells.
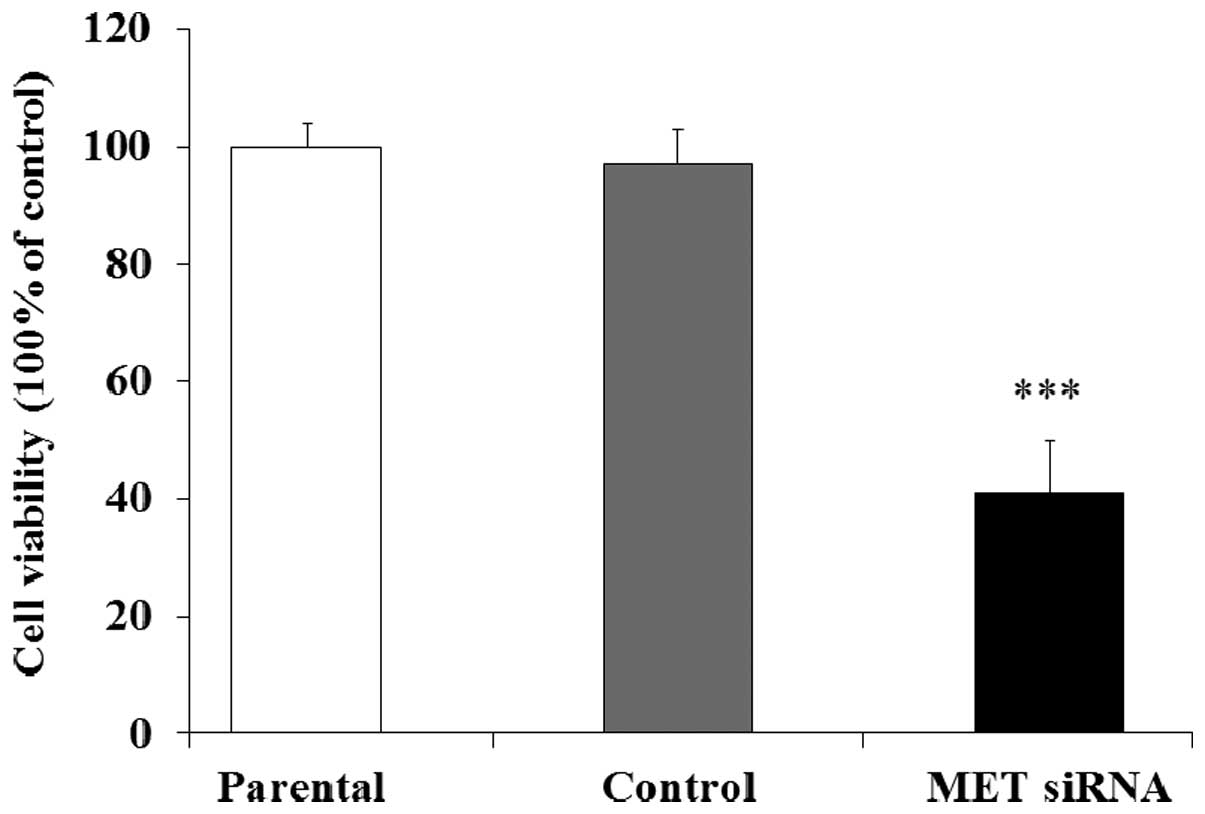
MET is related to DDP resistance in GC
cells
Then we investigated the relationship between the
expression of MET and the DDP resistance in human GC cells.
SGC7901/DDP cells transfected with MET siRNA were co-cultured with
DDP, the cell viability was evaluated by MTT assay. The results
indicated that the cell viability in MET siRNA group was
significantly decreased compared with the control and parental
group (Fig. 8). Therefore, the
expression of MET was associated with the DDP resistance in human
GC SGC7901/DDP cells.
miR-34a modulates DDP resistance by
repressing MET
Finally we investigated the DDP resistance mechanism
of miR-34a in human GC cells. Our study demonstrated that
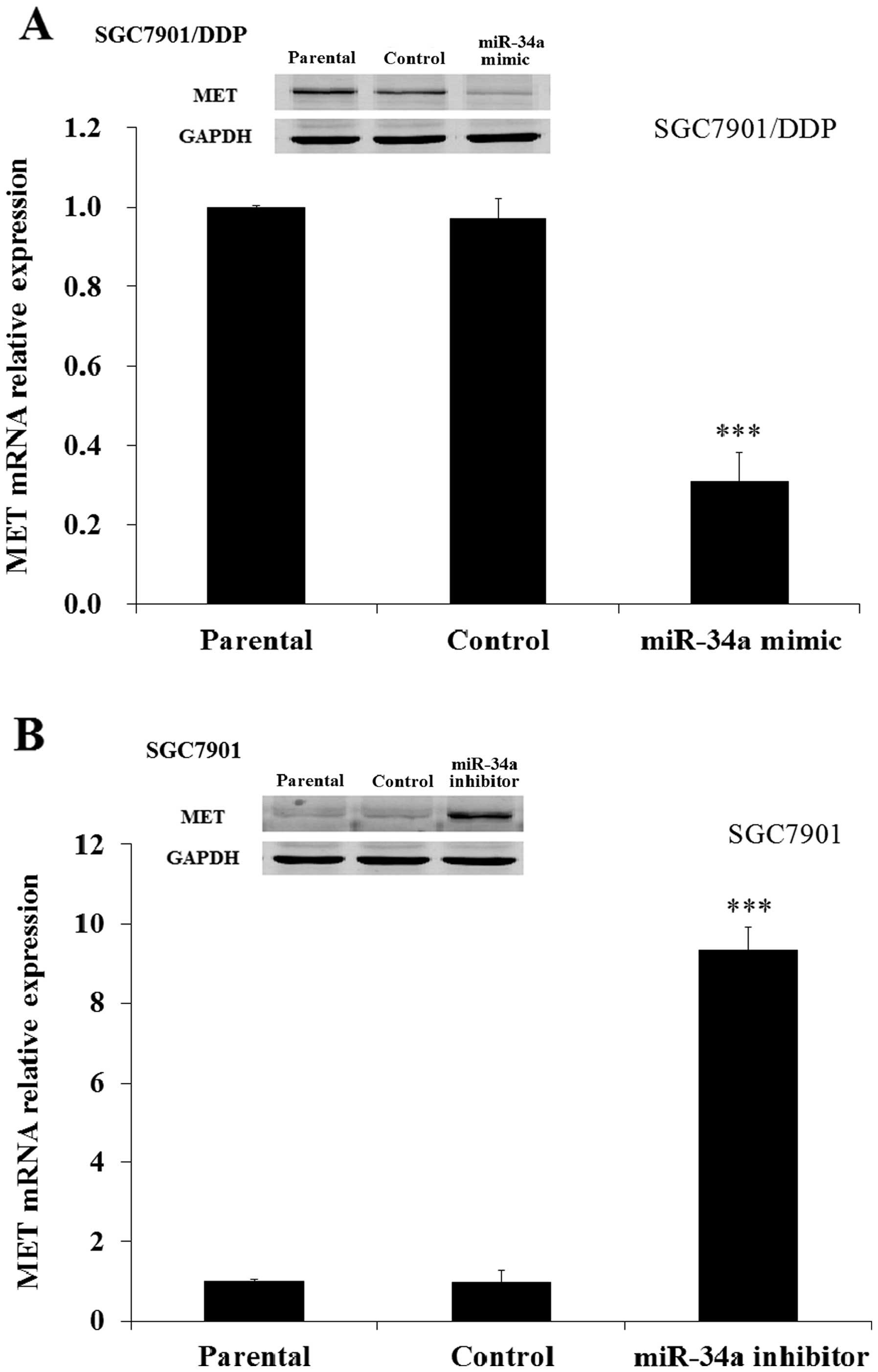
transfection of miR-34a mimic significantly decreased the level of
MET in SGC7901/DDP cells (Fig. 9A)
while transfection of miR-34a inhibitor significantly increased the
level of MET in SGC7901 cells compared with mimic control and
parental cells (Fig. 9B).
Therefore, upregulation of miR-34a led to a decreased expression of
MET in human gastric cancer cells.
Discussion
miR-34 is a class of conserved miRs widespread in
mammals (32). miR-34 family
consists of miR-34a, miR-34b and miR-34c; the expression of miR-34a
is much higher than miR-34b and miR-34c in human organs (33). Previous studies reported that the
level of miR-34a was downregulated in many human cancers (34,35).
Wei et al reported that miR-34a was also downregulated in
human gastric cancer (30), it is
reported that miR-34a inhibited proliferation invasion and
progression in colon cancer and glioblastoma (36,37).
These findings demonstrated that miR-34a plays important roles in
human cancers; however the relationship between miR-34a and the DDP
sensitivity in human gastric cancer is still unknown.
In order to investigate the relationship between
miR-34a and DDP resistance in human gastric cancer, we examined the
expression of miR-34a in the DDP resistant GC patient tissue and
DDP resistant cell line SGC7901/DDP. The results indicated that the
expression of miR-34a was significantly decreased in DDP resistance
GC patients and DDP resistant SGC7901/DDP. Further study indicated
that upregulation of miR-34a could inhibit the proliferation of
SGC7901/DDP cells and induce SGC7901/DDP cell apoptosis when
treated with DDP. On the other hand, downregulation of miR-34a
increased the cell proliferation of SGC7901 cells and decreased
SGC7901 cell apoptosis when treated with DDP. Therefore, the
downregulation of miR-34a contributed to the decreased sensitivity
of SGC-7901 cells to DDP, and upregulation of miR-34 could increase
the sensitivity of SGC-7901/DDP cells to DDP.
By using miR target tools we confirmed MET was a
target gene of miR-34a. The dual luciferase assay study indicated
that MET is a direct target gene of miR-34a. We also found that the
expression level of MET was significantly increased in DDP
resistant GC patient tissues and cell lines. MET is an oncogene, it
was associated with tumor progress and metastasis (38). A previous study indicated that the
level of MET was a predictive marker for human colorectal cancer
(39). Downregulation of MET lead
to the regulation of PI3K signal pathway in human ovarian cancer
(40). Dang et al reported
that the regulation of miR-34a contributed to hepatocellular cancer
malignancy through targeting MET (41). However, there are few studies
reporting on the relationship between MET and DDP resistance. We
investigated whether the regulation of MET was associated with the
DDP resistance by using siRNA knockdown assay, and the results
indicated that knockout MET could inhibit the SGC7901/DDP cell
proliferation when treated with DDP. These findings suggest
upregulation of miR-34 could be sensitive to the DDP resistance of
human gastric cancer SGC7901/DDP cells through targeting MET. As a
potential concern miR-34a could also non-specifically bind to other
mRNAs, which would cause unwanted side effects. To resolve the
potential unwanted side effects more studies of miR-34a function
are needed.
In conclusion, the miR-34a expression was
significantly downregulated in DDP resistant GC patients and GC
cell lines. The upregulation of miR-34a enhanced the sensitivity of
human GC cells to DDP treatment through regulation of cell
proliferation and apoptosis via the regulation of the MET gene.
These results suggested that the combination of miR-34a
transfection with DDP treatment might be a new strategy for the
treatment of human GC.
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar
|
|
2
|
Roder DM: The epidemiology of gastric
cancer. Gastric Cancer. 5(Suppl 1): 5–11. 2002. View Article : Google Scholar
|
|
3
|
Gallo A and Cha C: Updates on esophageal
and gastric cancers. World J Gastroenterol. 12:3237–3242. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gunderson LL: Gastric cancer - patterns of
relapse after surgical resection. Semin Radiat Oncol. 12:150–161.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cunningham D, Allum WH, Stenning SP,
Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ,
Falk SJ, Iveson TJ, et al MAGIC Trial Participants: Perioperative
chemotherapy versus surgery alone for resectable gastroesophageal
cancer. N Engl J Med. 355:11–20. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bang YJ, Kim YW, Yang HK, Chung HC, Park
YK, Lee KH, Lee KW, Kim YH, Noh SI, Cho JY, et al CLASSIC trial
investigators: Adjuvant capecitabine and oxaliplatin for gastric
cancer after D2 gastrectomy (CLASSIC): A phase 3 open-label,
randomised controlled trial. Lancet. 379:315–321. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mesner PW Jr, Budihardjo II and Kaufmann
SH: Chemotherapy-induced apoptosis. Adv Pharmacol. 41:461–499.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kaufmann SH and Earnshaw WC: Induction of
apoptosis by cancer chemotherapy. Exp Cell Res. 256:42–49. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hannun YA: Apoptosis and the dilemma of
cancer chemotherapy. Blood. 89:1845–1853. 1997.PubMed/NCBI
|
|
10
|
Kostova I: Platinum complexes as
anticancer agents. Recent Patents Anticancer Drug Discov. 1:1–22.
2006. View Article : Google Scholar
|
|
11
|
Holohan C, Van Schaeybroeck S, Longley DB
and Johnston PG: Cancer drug resistance: An evolving paradigm. Nat
Rev Cancer. 13:714–726. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Longley DB and Johnston PG: Molecular
mechanisms of drug resistance. J Pathol. 205:275–292. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nakagawa Y, Sedukhina AS, Okamoto N,
Nagasawa S, Suzuki N, Ohta T, Hattori H, Roche-Molina M, Narváez
AJ, Jeyasekharan AD, et al: NF-κB signaling mediates acquired
resistance after PARP inhibition. Oncotarget. 6:3825–3839. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Halilovic E, She QB, Ye Q, Pagliarini R,
Sellers WR, Solit DB and Rosen N: PIK3CA mutation uncouples tumor
growth and cyclin D1 regulation from MEK/ERK and mutant KRAS
signaling. Cancer Res. 70:6804–6814. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Michaelis M, Rothweiler F, Barth S, Cinatl
J, van Rikxoort M, Löschmann N, Voges Y, Breitling R, von Deimling
A, Rödel F, et al: Adaptation of cancer cells from different
entities to the MDM2 inhibitor nutlin-3 results in the emergence of
p53-mutated multi-drug-resistant cancer cells. Cell Death Dis.
2:e2432011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lin X, Zhang X, Wang Q, Li J, Zhang P,
Zhao M and Li X: Perifosine downregulates MDR1 gene expression and
reverses multidrug-resistant phenotype by inhibiting PI3K/Akt/NF-κB
signaling pathway in a human breast cancer cell line. Neoplasma.
59:248–256. 2012. View Article : Google Scholar
|
|
17
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ma L, Teruya-Feldstein J and Weinberg RA:
Tumour invasion and metastasis initiated by microRNA-10b in breast
cancer. Nature. 449:682–688. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ma L, Reinhardt F, Pan E, Soutschek J,
Bhat B, Marcusson EG, Teruya-Feldstein J, Bell GW and Weinberg RA:
Therapeutic silencing of miR-10b inhibits metastasis in a mouse
mammary tumor model. Nat Biotechnol. 28:341–347. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cho WC: MicroRNAs: Potential biomarkers
for cancer diagnosis, prognosis and targets for therapy. Int J
Biochem Cell Biol. 42:1273–1281. 2010. View Article : Google Scholar
|
|
22
|
Cho WC: MicroRNAs in cancer - from
research to therapy. Biochim Biophys Acta. 1805:209–217. 2010.
|
|
23
|
Gabriely G, Teplyuk NM and Krichevsky AM:
Context effect: microRNA-10b in cancer cell proliferation, spread
and death. Autophagy. 7:1384–1386. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xiang Y, Ma N, Wang D, Zhang Y, Zhou J, Wu
G, Zhao R, Huang H, Wang X, Qiao Y, et al: MiR-152 and miR-185
co-contribute to ovarian cancer cells cisplatin sensitivity by
targeting DNMT1 directly: A novel epigenetic therapy independent of
decitabine. Oncogene. 33:378–386. 2014. View Article : Google Scholar
|
|
25
|
Shang Y, Zhang Z, Liu Z, Feng B, Ren G, Li
K, Zhou L, Sun Y, Li M, Zhou J, et al: miR-508-5p regulates
multidrug resistance of gastric cancer by targeting ABCB1 and
ZNRD1. Oncogene. 33:3267–3276. 2014. View Article : Google Scholar
|
|
26
|
Sui C, Meng F, Li Y and Jiang Y: miR-148b
reverses cisplatin-resistance in non-small cell cancer cells via
negatively regulating DNA (cytosine-5)-methyltransferase 1(DNMT1)
expression. J Transl Med. 13:1322015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fang L, Li H, Wang L, Hu J, Jin T, Wang J
and Yang BB: MicroRNA-17-5p promotes chemotherapeutic drug
resistance and tumour metastasis of colorectal cancer by repressing
PTEN expression. Oncotarget. 5:2974–2987. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yu ZW, Zhong LP, Ji T, Zhang P, Chen WT
and Zhang CP: MicroRNAs contribute to the chemoresistance of
cisplatin in tongue squamous cell carcinoma lines. Oral Oncol.
46:317–322. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sorrentino A, Liu CG, Addario A, Peschle
C, Scambia G and Ferlini C: Role of microRNAs in drug-resistant
ovarian cancer cells. Gynecol Oncol. 111:478–486. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wei B, Huang QY, Huang SR, Mai W and Zhong
XG: MicroRNA-34a attenuates the proliferation, invasion and
metastasis of gastric cancer cells via downregulation of MET. Mol
Med Rep. 12:5255–5261. 2015.PubMed/NCBI
|
|
31
|
Du Y, Zhu M, Zhou X, Huang Z, Zhu J, Xu J,
Cheng G, Shu Y, Liu P, Zhu W, et al: miR-20a enhances cisplatin
resistance of human gastric cancer cell line by targeting NFKBIB.
Tumour Biol. 37:1261–1269. 2016. View Article : Google Scholar
|
|
32
|
Yao Y, Suo AL, Li ZF, Liu LY, Tian T, Ni
L, Zhang WG, Nan KJ, Song TS and Huang C: MicroRNA profiling of
human gastric cancer. Mol Med Rep. 2:963–970. 2009.PubMed/NCBI
|
|
33
|
Wong MY, Yu Y, Walsh WR and Yang JL:
microRNA-34 family and treatment of cancers with mutant or
wild-type p53 (Review). Int J Oncol. 38:1189–1195. 2011.PubMed/NCBI
|
|
34
|
Kumar B, Yadav A, Lang J, Teknos TN and
Kumar P: Dysregulation of microRNA-34a expression in head and neck
squamous cell carcinoma promotes tumor growth and tumor
angiogenesis. PLoS One. 7:e376012012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shen Z, Zhan G, Ye D, Ren Y, Cheng L, Wu Z
and Guo J: MicroRNA-34a affects the occurrence of laryngeal
squamous cell carcinoma by targeting the antiapoptotic gene
survivin. Med Oncol. 29:2473–2480. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li Y, Guessous F, Zhang Y, Dipierro C,
Kefas B, Johnson E, Marcinkiewicz L, Jiang J, Yang Y, Schmittgen
TD, et al: MicroRNA-34a inhibits glioblastoma growth by targeting
multiple oncogenes. Cancer Res. 69:7569–7576. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cho WC: OncomiRs: The discovery and
progress of microRNAs in cancers. Mol Cancer. 6:602007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lutterbach B, Zeng Q, Davis LJ, Hatch H,
Hang G, Kohl NE, Gibbs JB and Pan BS: Lung cancer cell lines
harboring MET gene amplification are dependent on Met for growth
and survival. Cancer Res. 67:2081–2088. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Takeuchi H, Bilchik A, Saha S, Turner R,
Wiese D, Tanaka M, Kuo C, Wang HJ and Hoon DS: c-MET expression
level in primary colon cancer: A predictor of tumor invasion and
lymph node metastases. Clin Cancer Res. 9:1480–1488.
2003.PubMed/NCBI
|
|
40
|
Sawada K, Radjabi AR, Shinomiya N, Kistner
E, Kenny H, Becker AR, Turkyilmaz MA, Salgia R, Yamada SD, Vande
Woude GF, et al: c-Met overexpression is a prognostic factor in
ovarian cancer and an effective target for inhibition of peritoneal
dissemination and invasion. Cancer Res. 67:1670–1679. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Dang Y, Luo D, Rong M and Chen G:
Underexpression of miR-34a in hepatocellular carcinoma and its
contribution towards enhancement of proliferating inhibitory
effects of agents targeting c-MET. PLoS One. 8:e610542013.
View Article : Google Scholar : PubMed/NCBI
|