Introduction
Alternative splicing of precursor mRNAs (pre-mRNAs)
is critical for regulating transcriptome diversity and protein
multiplicity. During the process of alternative splicing, exons are
joined together to form different transcripts, leading to the
synthesis of many more proteins (1). It has been shown that alternative
splicing occurs widely in eukaryotes. For example, 95% of
intron-containing genes undergo alternative splicing in humans
(2). There are generally five
different mechanisms of alternative splicing: exon skipping,
mutually exclusive exons, alternative 3′ splice site, alternative
5′ splice site and intron retention. Among them, exon skipping is
the most common mode, that is, almost 35% of human alternative
splicing is caused by exon skipping. In addition to those basic
modes of alternative splicing, there are some other methods such as
multiple promoters and multiple polyadenylation sites in eukaryotes
that also occur (3–5).
Abnormally spliced mRNAs are found in many diseases,
particularly in cancers. The number and types of alternative
splicing differ in cancer cells compared with normal cells. For
instance, cancer cells exhibit higher levels of intron retention
but lower levels of exon skipping (6–9).
Colon cancer is the third leading cause of
cancer-related deaths worldwide. Several studies have investigated
the role of alternative splicing in colon cancer progression, and
discovered that the number of alternative splicing increases during
the transition from normal colon tissue to primary tumor, but
decreases during metastasis to the liver (10–13).
The differentially expressed alternative splicing genes in colon
cancer are involved in cell-cell and cell-matrix interactions
(14). Also, some alternative
splicing genes have been identified because of their close
association with cell growth and invasion in colon cancer,
including SLC39A14, VEGF, CyclinD1, VCL, CALD1, and B3GNT6
(14,15). Since alternative splicing is crucial
in colon cancer development, it is a promising target for novel
anti-colon cancer therapeutics.
Preoperative chemoradiation therapy is a common and
effective approach for cancer therapy, especially in colon cancer
(16). Many complicated cellular
responses are involved in chemoradiation therapy, which induce
cancer cell death (17).
Chemoradiation resistance that develops during treatment may be
caused by several genetic aberrations, such as a p53 mutation and
thymidylate synthase overexpression (18) However, the function of alternative
splicing events in chemoradiation resistance remains unclear. In
this study, we implemented a genome-wide transcriptome sequencing
in HCT116 and chemoradiation resistant HCT116 (RCR-HCT116) cells to
identify the alternative splicing events that affect tumor
sensitivity to chemoradiotherapy.
Materials and methods
Cell culture and the construction of the
RCR-HCT116 cells
HCT116 cells were cultured in McCoy's 5A
(Sigma-Aldrich, St. Louis, MO, USA) containing 10% fetal bovine
serum (FBS) (Gibco, Carlsbad, CA, USA) at 37°C under 5%
CO2 in a CO2 incubator (Thermo Labsystems,
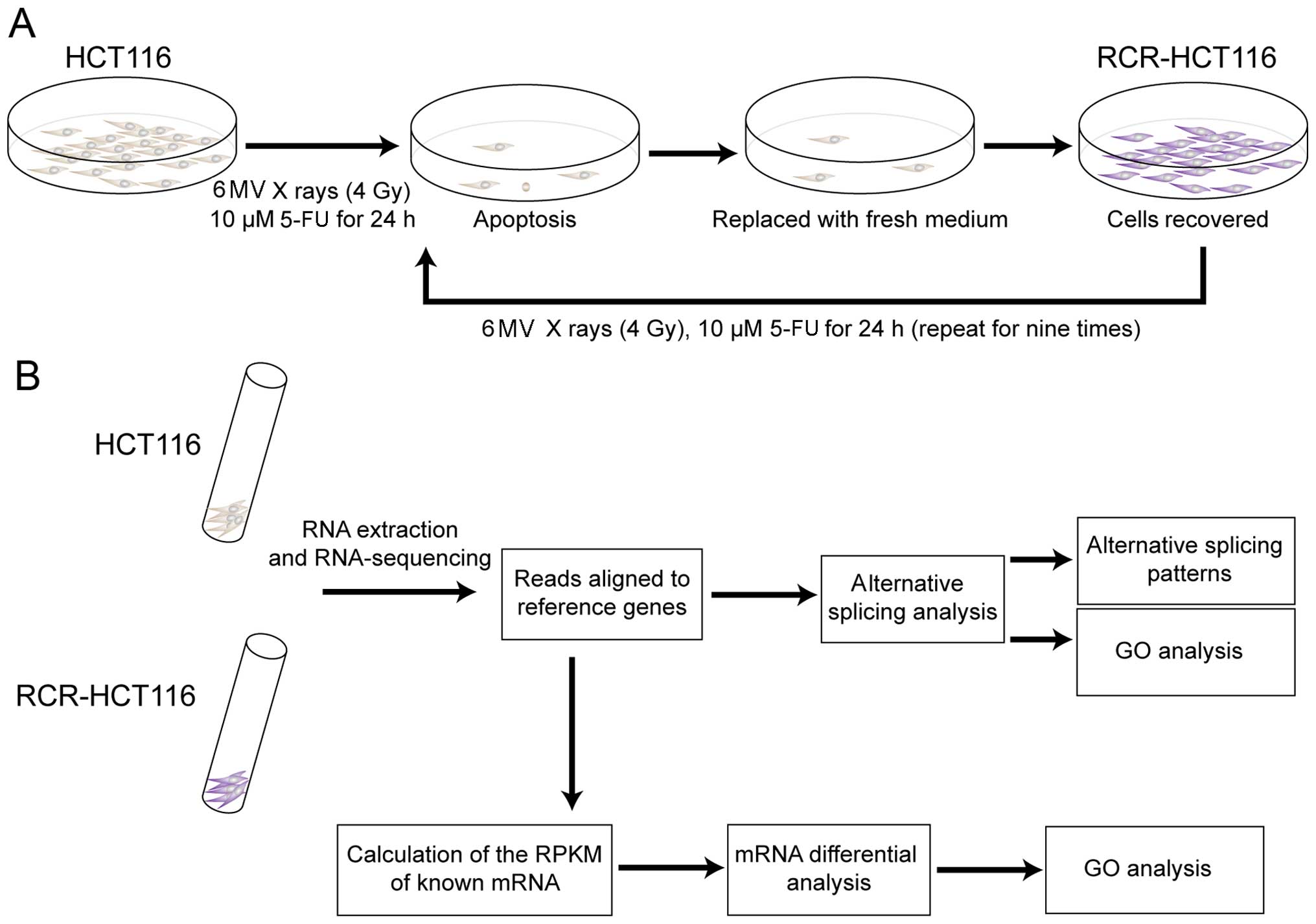
Vantaa, Finland). HCT116 cells were exposed to 6 MV X-rays (4 Gy)
at room temperature, followed by treatment with 10 µM
5-fluorouracil (5-FU) for 24 h to induce the apoptosis of tumor
cells. Then the medium was replaced with fresh medium and cultured
until the cells were recovered. The cells were treated with the
same aforementioned method, nine times. The chemoradiation-treated
cells were passaged and expanded to generate the RCR-HCT116
cells.
RNA extraction, library construction, and
sequencing
Total RNA from the HCT116 and RCR-HCT116 cells were
extracted using RNeasy Mini kit (Qiagen, Valencia, CA, USA)
according to the manufacturer's protocol, and treated with
RNase-free Dnase I (Invitrogen Life Technologies, Carlsbad, CA,
USA) to remove genomic DNA.
A total amount of 2 µg RNA per sample was
used for the construction of cDNA libraries. The cDNA libraries
were generated according to the TruSeq® RNA Sample Prep
kit v2 (Illumina, San Diego, CA, USA) following the manufacturer's
protocol and then sequenced by Illumina HiSeq 4000 platform
(Illumina) to generate 150-bp paired-end reads.
Read filtering and mapping
RNA-seq raw data of the HCT116 and RCR-HCT116 cells
were cleaned by removing the adaptor sequence and low quality reads
(mapping quality <20). Clean reads were aligned to the human
reference genome sequence hg19 using TopHat (19). The following parameters were set:
maximum number of mismatches permitted, 2; maximum alignments
allowed, 20; maximum number of mismatches permitted in each segment
alignment for reads mapped independently, 2; maximum insertion
length, 3; maximum deletion length, 3; maximum mismatches in the
anchor region, 0; min isoform-fraction, 0.15; minimum intron
length, 50; maximum intron length, 50,000.
Differential expression analysis
Genes that were differentially expressed between the
two groups were defined as differentially expressed genes (DEGs).
Cufflinks (20) software was used
to calculate the fragments per kilobase of transcript per million
fragments mapped (FPKM) value of the different genes. The DEGs from
the normal and RCR-HCT116 cells were identified by Cuffdiff
(20,21) at a q≤0.01 and a fold change ≥2.
Identification and quantification of
alternative splicing events
The alternative splicing events were classified into
five patterns by the mixture of isoforms (MISO) (22), including alternative 5′ splice site,
alternative 3′ splice site, mutually exclusive exon, intron
retention and exon skipping. The MISO Bayesian inference model was
used for the quantification of alternative splicing events. The
change of splicing isoforms was analyzed using the MISO Bayesian
inference model. The significant differentially spliced events were
determined by Bayes' factor (BF) and Psi values
(percent-spliced-in, Ψ) (BF≥10 and Ψ≥0.2).
Gene ontology (GO) analysis
The hypergeometric distribution test was used to
identify GO categories (biological process, cellular compartment
and molecular function) that were significantly enriched in a
specified gene set. GO analysis was implemented with Go.db package
(23).
Results
RNA-sequencing of normal and RCR-HCT116
cells
In order to generate chemoradiation-resistant colon
cancer cells, we first exposed the colon cancer-derived HCT116 cell
line to 6 MV X-rays (4 Gy), and then incubated the cells in 10
µM 5-fluorouracil (5-FU). As shown in Fig. 1A, after a 24-h incubation, the
medium was replaced with fresh medium to remove the apoptotic and
dead cells. The cells were cultured in fresh medium until they were
recovered and then treated with the same aforementioned method nine
times.
Sequencing was performed on the Illumina HiSeq 4000
platform to generate 150-bp paired-end reads. After the removal of
the adaptor sequence and the reads of low quality, a total of
162,398,220 and 134,409,494 reads of 101 bp were generated from the
HCT116 and RCR-HCT116 cells, respectively. There were 80.28 and
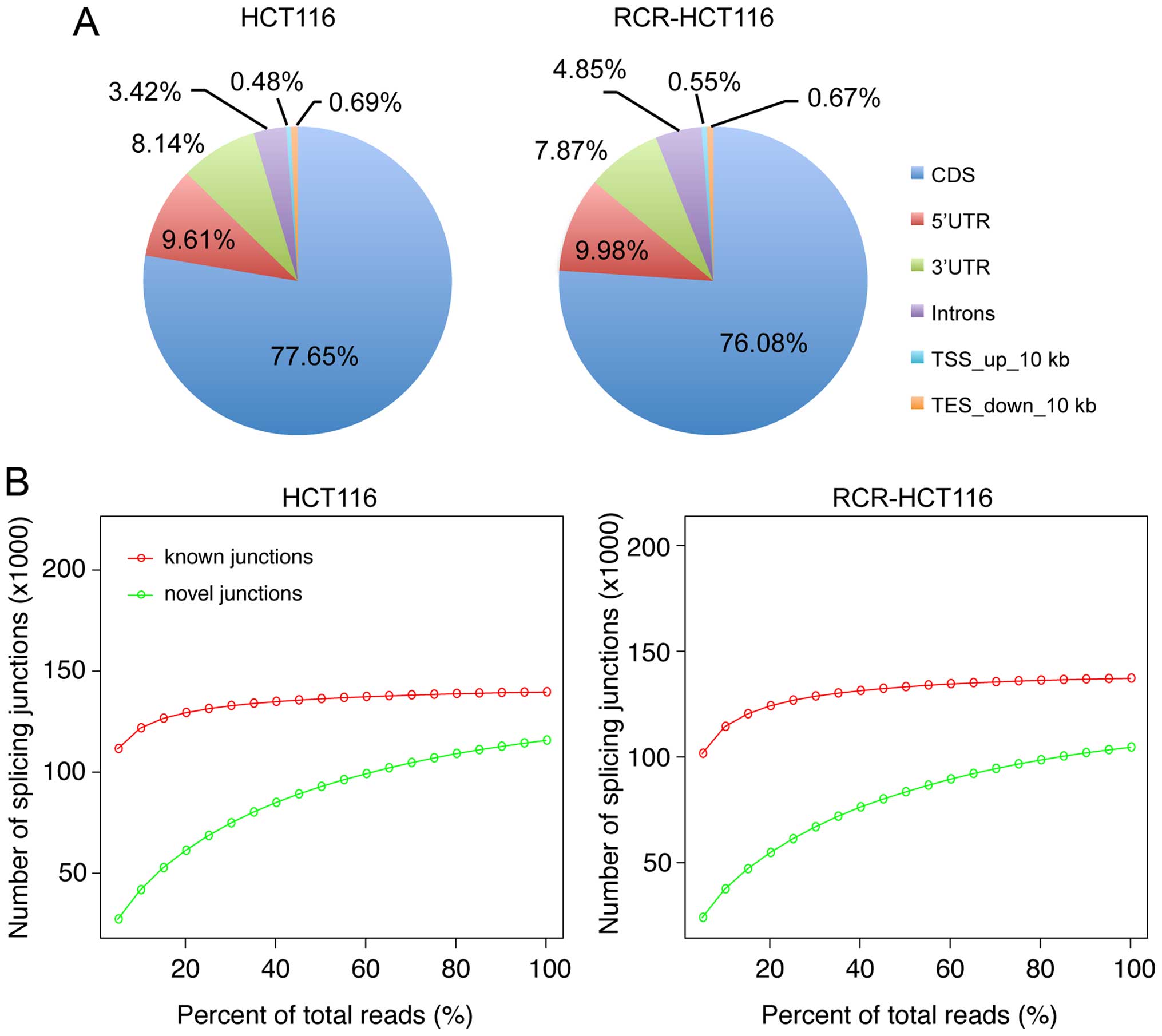
80.30% of the total reads from HCT116 and RCR-HT116 cells mapped to
the human reference genome (Fig.
1B, Table I). Comparison of the
RNA-seq data to the annotated human reference genome revealed that
~77% of the mapping reads were mapped to the CDS region. Meanwhile,
the two RNA-seq libraries showed similar genomic distribution
patterns from the mapping reads (Fig.
2A).
 | Table IStatistics of the RNA-seq reads and
mapped reads ratio against the human reference genome. |
Table I
Statistics of the RNA-seq reads and
mapped reads ratio against the human reference genome.
| RNA-seq | Cells | Total reads | Total mapped
reads | Mapped reads ratio
(%) |
|---|
| WGC053648R | HCT116 | 162,398,220 | 130,371,020 | 80.28 |
| WGC053649R | RCR-HCT116 | 134,409,494 | 107,929,116 | 80.30 |
DEG screening
To investigate the genes involved in chemoradiation
resistance in colon cancer, we compared the RNA-seq data of the
HCT116 and RCR-HCT116 cells to identify the DEGs. A total of 1,103
significant DEGs were identified (log2 FC>1,
FPKM1+FPKM2 >1, q<0.01), including 818 genes that were lowly
expressed and 285 genes that were highly expressed in the
RCR-HCT116 cells (Table II).
 | Table IITop 10 differentially expressed
genes. |
Table II
Top 10 differentially expressed
genes.
| Downregulated
genes | Log2
FC | Q-value | Upregulated
genes | Log2
FC | Q-value |
|---|
| TACSTD2 | −9.25 | 0 | RFPL4A | 7.43 | 6.27E-05 |
| RPS4Y1 | −7.49 | 0 | SFTA1P | 6.00 | 2.13E-03 |
| PDE4B | −6.66 | 0 | LARGE | 5.57 | 0 |
| SHF | −6.25 | 6.84E-08 | KRT34 | 5.50 | 1.92E-07 |
| HNF4A | −6.08 | 9.88E-06 | GADD45G | 5.46 | 1.05E-05 |
| EHF | −5.91 | 0 | KRTAP2-3 | 5.44 | 0 |
| MKX | −5.83 | 7.87E-13 | CREB5 | 5.35 | 3.72E-09 |
| NTSR1 | −5.79 | 0 | HEPH | 5.33 | 2.54E-09 |
| KLHL35 | −5.51 | 1.06E-07 | SNAI2 | 5.32 | 2.04E-05 |
| NEK3 | −5.42 | 4.50E-09 | TTYH1 | 5.17 | 1.77E-09 |
GO enrichment analysis of DEGs
We then carried out GO enrichment analysis on the
DEGs using Go.db package (23),
which calculates the p-values using hypergeometric distribution.
Genes that were expressed at a lower level in the RCR-HCT116 cells
than the level in the normal HCT116 cells were enriched for GO
categories related to cell cycle and cell division (e.g. cell
cycle, cell cycle phase, M phase, cell cycle process, mitosis and
nuclear division); whereas genes that were expressed at a higher
level in RCR-HCT116 cells than in the normal HCT116 cells were
enriched for GO categories related to the regulation of system
processes, response to wounding, negative regulation of
phosphorylation and regulation of phosphorylation (Table III).
 | Table IIIGene ontology analysis on
differentially expressed genes in HCT116 and RCR-HCT116 cells. |
Table III
Gene ontology analysis on
differentially expressed genes in HCT116 and RCR-HCT116 cells.
| GO ID | Term | Count | P-value | Pop hits | Fold
enrichment | Category |
|---|
| Go categories
enriched in genes that were expressed higher in the HCT116 than the
RCR-HCT116 cells |
| GO:0007049 | Cell cycle | 156 | 2.21E-62 | 776 | 4.56 | BP |
| GO:0022403 | Cell cycle
phase | 102 | 4.16E-48 | 414 | 5.59 | BP |
| GO:0000279 | M phase | 91 | 2.89E-47 | 329 | 6.28 | BP |
| GO:0022402 | Cell cycle
process | 117 | 3.91E-47 | 565 | 4.70 | BP |
| GO:0007067 | Mitosis | 70 | 1.74E-40 | 220 | 7.22 | BP |
| GO:0000280 | Nuclear
division | 70 | 1.74E-40 | 220 | 7.22 | BP |
| GO:0048285 | Organelle
fission | 71 | 3.07E-40 | 229 | 7.04 | BP |
| GO:0000087 | M phase of mitotic
cell cycle | 70 | 6.53E-40 | 224 | 7.09 | BP |
| GO:0000278 | Mitotic cell
cycle | 84 | 1.73E-36 | 370 | 5.15 | BP |
| GO:0051301 | Cell division | 73 | 3.25E-34 | 295 | 5.62 | BP |
| GO:0006259 | DNA metabolic
process | 93 | 9.49E-33 | 506 | 4.17 | BP |
| GO:0031981 | Nuclear lumen | 158 | 3.60E-32 | 1450 | 2.64 | CC |
| GO:0005694 | Chromosome | 81 | 3.70E-29 | 460 | 4.26 | CC |
| GO:0070013 | Intracellular
organelle lumen | 172 | 5.91E-29 | 1779 | 2.34 | CC |
| GO:0043233 | Organelle
lumen | 173 | 2.95E-28 | 1820 | 2.30 | CC |
| GO:0031974 | Membrane-enclosed
lumen | 175 | 3.27E-28 | 1856 | 2.28 | CC |
| GO:0043228 |
Non-membrane-bounded organelle | 214 | 2.08E-27 | 2596 | 2.00 | CC |
| GO:0043232 | Intracellular
non-membrane-bounded organelle | 214 | 2.08E-27 | 2596 | 2.00 | CC |
| GO:0000793 | Condensed
chromosome | 43 | 7.82E-27 | 129 | 8.07 | CC |
| GO:0044427 | Chromosomal
part | 69 | 3.73E-25 | 386 | 4.33 | CC |
| Go categories
enriched in genes that were expressed higher in the RCR-HCT116 than
the HCT116 cells |
| GO:0044057 | Regulation of
system process | 18 | 3.18E-06 | 309 | 3.94 | BP |
| GO:0009611 | Response to
wounding | 22 | 3.58E-05 | 530 | 2.81 | BP |
| GO:0006937 | Regulation of
muscle contraction | 8 | 8.80E-05 | 72 | 7.52 | BP |
| GO:0043005 | Neuron
projection | 15 | 4.55E-04 | 342 | 3.01 | CC |
| GO:0042326 | Negative regulation
of phosphorylation | 6 | 4.96E-04 | 45 | 9.02 | BP |
| GO:0042325 | Regulation of
phosphorylation | 18 | 5.30E-04 | 466 | 2.61 | BP |
| GO:0010563 | Negative regulation
of phosphorus metabolic process | 6 | 6.71E-04 | 48 | 8.46 | BP |
| GO:0045936 | Negative regulation
of phosphate metabolic process | 6 | 6.71E-04 | 48 | 8.46 | BP |
| GO:0051174 | Regulation of
phosphorus metabolic process | 18 | 8.28E-04 | 485 | 2.51 | BP |
| GO:0019220 | Regulation of
phosphate metabolic process | 18 | 8.28E-04 | 485 | 2.51 | BP |
| GO:0008285 | Negative regulation
of cell proliferation | 15 | 9.19E-04 | 361 | 2.81 | BP |
| GO:0042127 | Regulation of cell
proliferation | 24 | 1.28E-03 | 787 | 2.06 | BP |
| GO:0005201 | Extracellular
matrix structural constituent | 7 | 1.66E-03 | 86 | 5.50 | MF |
| GO:0042981 | Regulation of
apoptosis | 24 | 1.69E-03 | 804 | 2.02 | BP |
| GO:0043067 | Regulation of
programmed cell death | 24 | 1.92E-03 | 812 | 2.00 | BP |
| GO:0040013 | Negative regulation
of locomotion | 6 | 2.00E-03 | 61 | 6.65 | BP |
| GO:0010941 | Regulation of cell
death | 24 | 2.02E-03 | 815 | 1.99 | BP |
| GO:0040012 | Regulation of
locomotion | 10 | 2.15E-03 | 192 | 3.52 | BP |
| GO:0044459 | Plasma membrane
part | 48 | 3.05E-03 | 2203 | 1.50 | CC |
| GO:0006954 | Inflammatory
response | 13 | 3.14E-03 | 325 | 2.71 | BP |
Identification and annotation of
alternative splicing events
To determine the relationship between sequencing
depth and the detection power of alternative splicing, the
sequencing libraries (HCT116 and RCR-HCT116 cells) were randomly
selected to create sub-libraries (i.e. 5–95% of the whole library)
to determine the known and novel junctions. As shown in Fig. 2B, the sequencing depth was
correlated with the detection of unknown junctions, however, when
the sequencing depth was more than 20% of the whole library, the
increase of sequencing depth did not significantly increase the
number of known junctions. This implies that our sequencing data
was capable of supporting the identification of unknown
junctions.
We further examined the splicing patterns in the
normal and chemoradiation-resistant colon cancer cells using the
MISO (22) package. MISO quantifies
the level of inclusion of a given differentially expressed exon as
the 'percent spliced in' (Psi or Ψ), which reflects the fraction of
a gene's mRNA that includes the exon, intron or alternative splice
site. Ψ values vary between 0 (the exon, intron or alternative
splice site is excluded from every transcript) and 1 (the exon,
intron or alternative splice site is included in every transcript).
MISO also calculates a Bayes factor for each differential splicing
event, which is a measure of the odds that there is differential
inclusion of a particular exon in different samples. The five main
alternative splicing patterns, 3′ splice site (A3SS), alternative
5′ splice site (A5SS), mutually exclusive exon (MXE), intron
retention (IR) and exon skipping (ES), were analyzed in the RNA-seq
data of the HCT116 and the RCR-HCT116 cells (Fig. 3A). Fig.
3B shows the Sashimi plots of five examples with different
patterns of alternative splicing events, with the number of reads
that span each part of the splice junction shown on the plots for
the two samples analyzed. Furthermore we calculated the number of
alternative splicing events for both types of cells and as shown in
Fig. 3C, there was no significant
difference in the number of detected A3SS, A5SS, MXE and IR in the
normal and RCR-HCT116 cells, indicating that these types of
alternative splicing may not function in chemoradiation-resistant
colon cancer cells. Nevertheless, the number of ES was
significantly increased in the chemoradiation-resistant colon
cancer cells (Fig. 3C), suggesting
that chemoradiation may functions via ES.
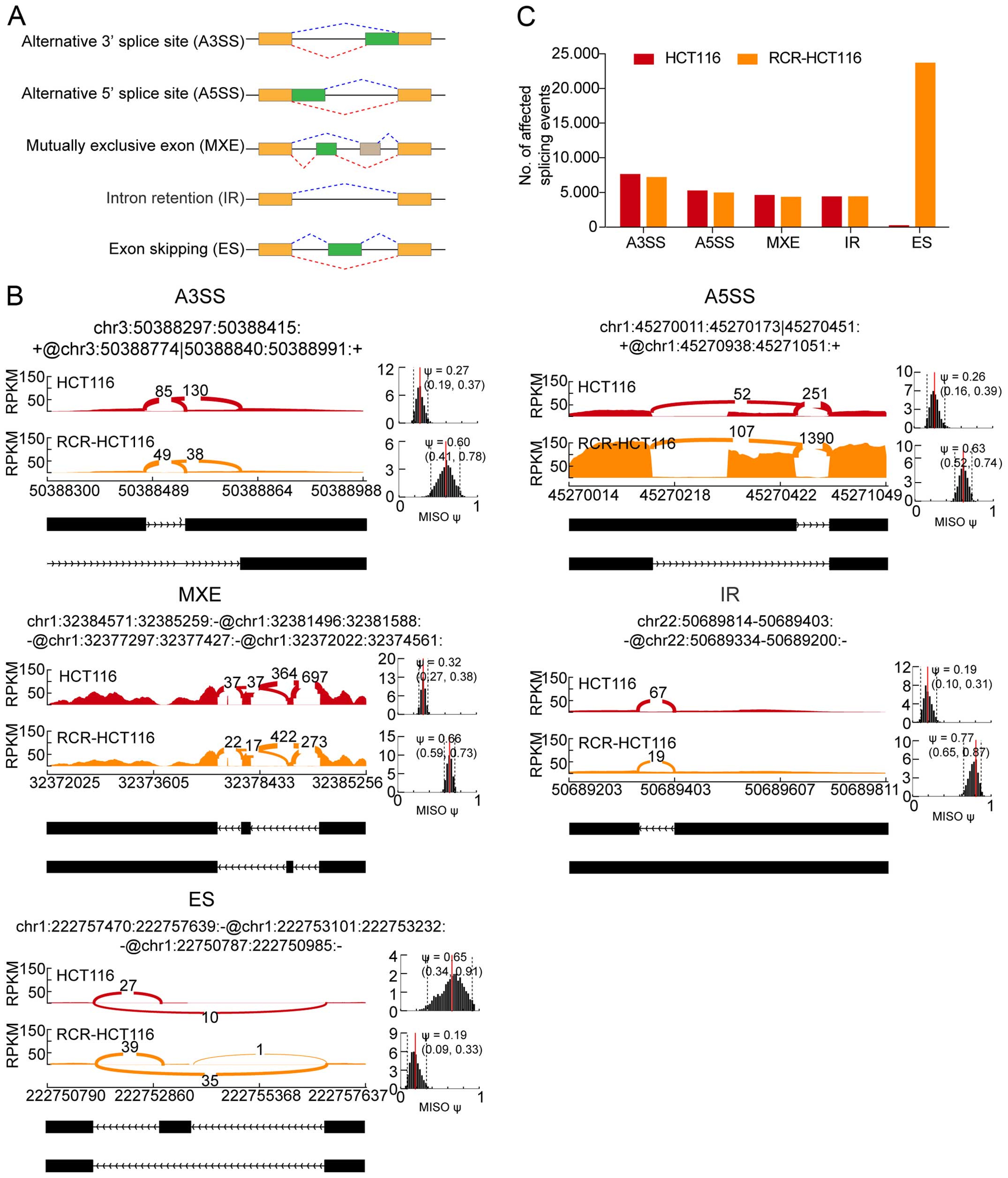 | Figure 3Statistical analysis of the different
alternative splicing events. (A) The schematic plots show the five
types of alternative splicing patterns. (B) The Sashimi plots of
the RNA-seq data for the five types of alternative splicing events
that are expressed differentially in the HCT116 and RCR-HCT116
cells. The main panel shows the counts of RNA-seq reads that span
the junctions in each region. The coordinates for each splicing
event are shown at the top, and the schematic of this splicing
event is shown at the bottom. Dark pink indicates the HCT116 cells,
orange indicates the RCR-HCT116 cells. The estimated MISO values
are shown in the right panels, the dashed lines indicate the 95%
confidence intervals of the MISO values. The Sashimi plot was
produced using the MISO package. (C) The number of different
splicing events in the normal and chemoradiation-resistant colon
cancer cells. A3SS, alternative 3′ splice site; A5SS, alternative
5′ splice site; MXE, mutually exclusive exon; IR, intron retention;
ES, exon skipping; RCR-HCT116, chemoradiation-resistant HCT116
cells; MISO, mixture of isoforms; RPKM, reads per kilobase
million. |
Go enrichment analysis of genes with
differentially alternative splicing levels
To gain further insight into the role of these
alternative splicing level altered genes, we performed GO analysis
on the genes that had different alternative splicing levels in the
normal and RCR-HCT116 cells. Our dataset identified that 323
alternative splicing events in 293 genes were significantly
different between the normal and RCR-HCT116 cells (data not shown).
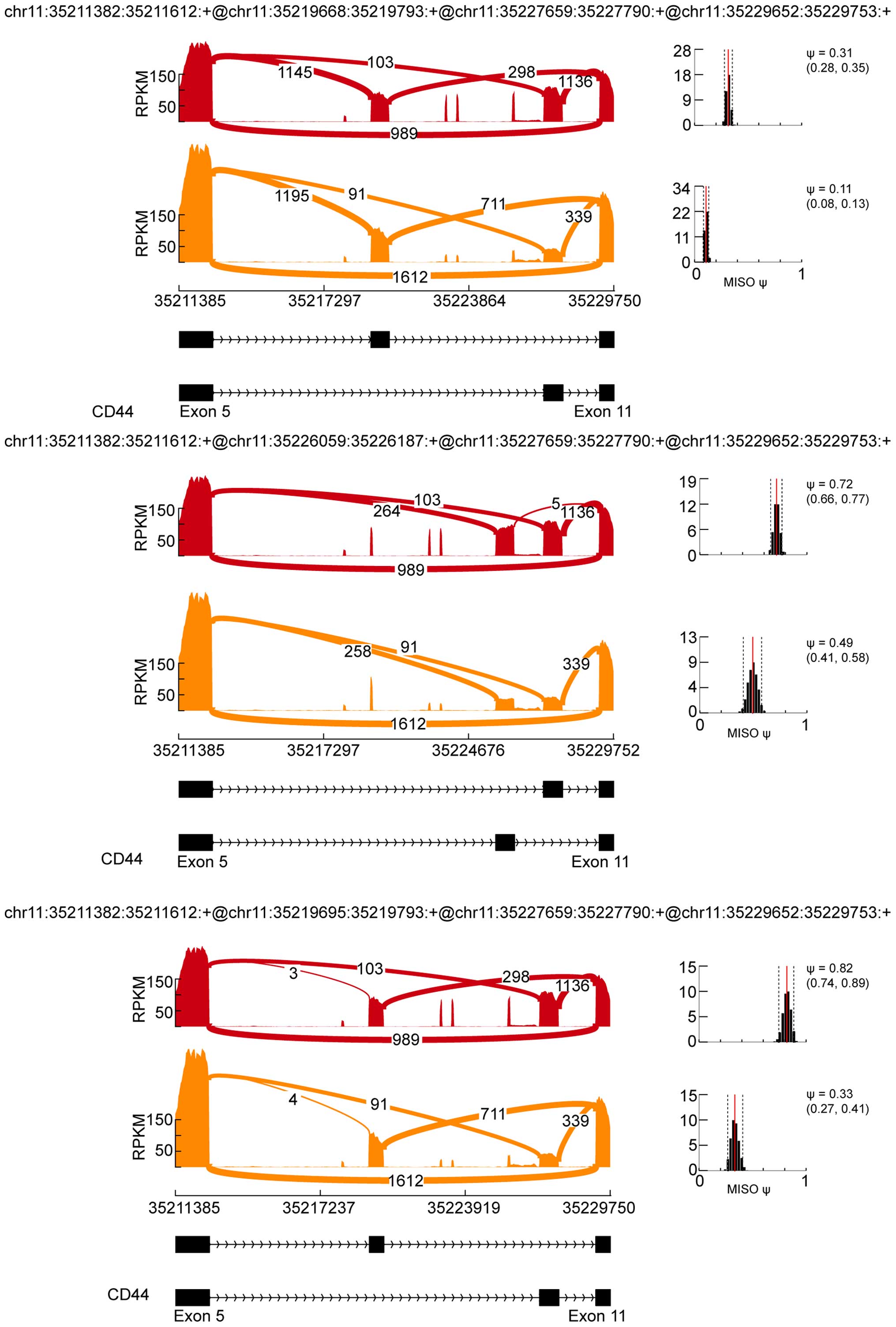
Some of the alternative splicing-containing genes were previously
reported in colon cancers, such as CD44. Tumors carrying the CD44
v6 epitope (exon v6) acquire selective advantages during tumor
progression. We further identified three new MXEs between exon 5
and exon 11 of CD44 (Fig. 4), which
may be associated with chemoradiation resistance of colon cancer.
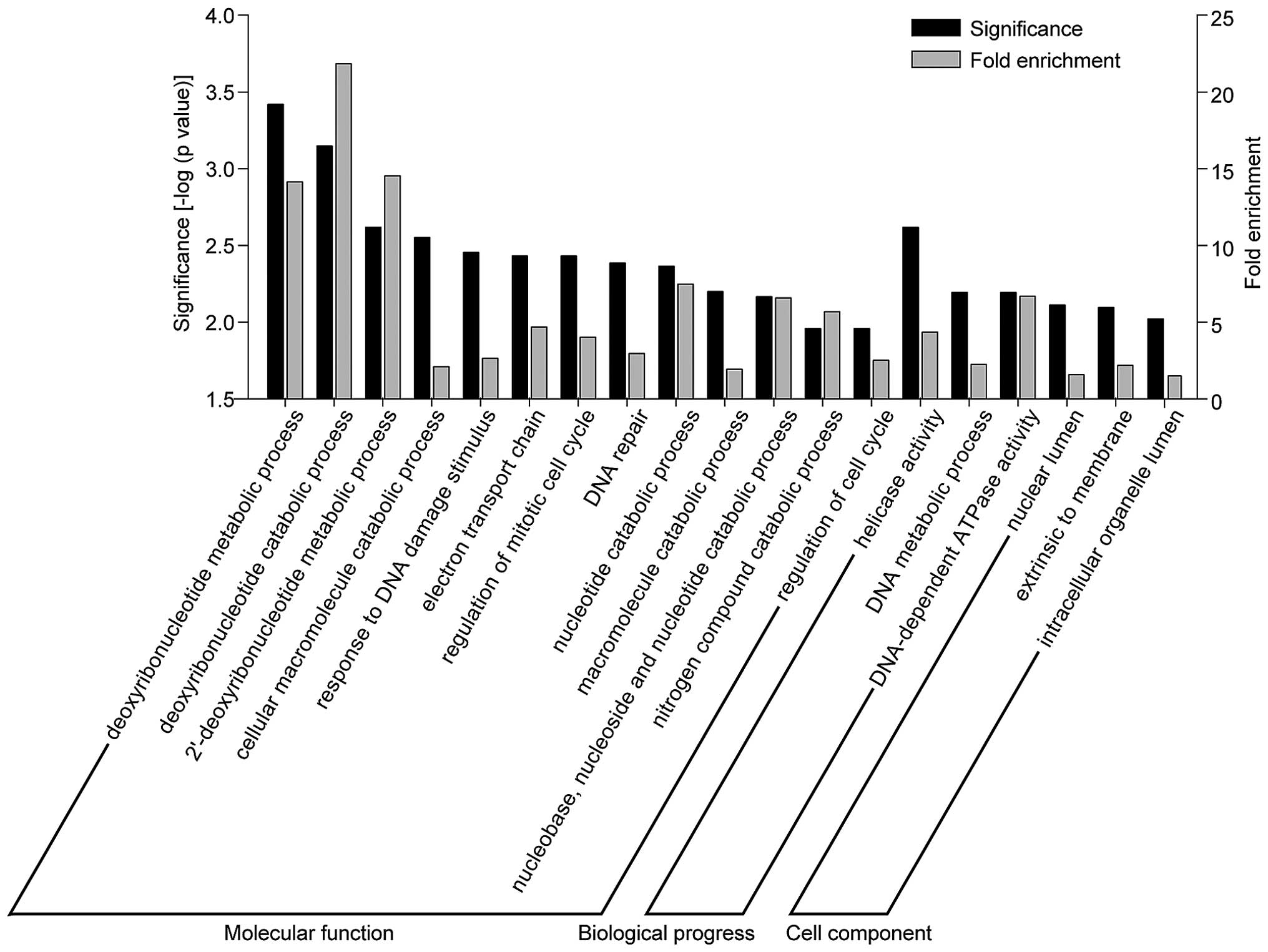
Go analysis showed that these genes were clustered functionally
into several groups related with DNA replication, such as
deoxyribonucleotide metabolic/catabolic processes (MPG, NT5M,
RRM2B, OGG1, NT5C), response to DNA damage-stimulus (POLL, RECQL4,
MPG, C17ORF70, PCBP4, POLG, ZMAT3, MUS81, GTF2H4, ZSWIM7, RRM2B,
OGG1, ERCC3) and helicase activity (RECQL4, MOV10, DDX11, SKIV2L,
EIF4A1, SNORA67, HLTF, ERCC3, SMARCA4) (Fig. 5).
Discussion
Alternative splicing, a key molecular event that
allows for protein diversity, is an important post-transcriptional
regulatory mechanism to control cell processes. Aberrant splicing
is related with various diseases, including colon cancer (24–26).
The importance of alternative splicing in colon cancer progression
has been emphasized in many studies (24). Until now, many alternative splicing
genes have been identified in colon cancer because of their close
association with cell growth and invasion, including SLC39A14,
VEGF, CyclinD1, VCL, CALD1, B3GNT6, ACTN1, TPM1, FN1, COL6A3,
SLC3A2 (13–15). Evidence suggests the role of CD44
alternative splicing in the progression of colon cancer. The
expression of CD44 splice variants (exon v6) is increased during
colon cancer progression and the expression level of CD44 v6 is
associated with tumor-related mortality (27–29).
In this study, we identified three novel MXEs between the exon 5
and exon 11 of the CD44 gene. The levels of these MXEs were
different between the normal and RCR-HCT116 cells, indicating that
these novel alternative-splicing events occurring in CD44 may be
related with the chemoradiation resistance of colon cancer. Thus,
different alternative splicing events, that even exist on the same
gene, may have diverse functions in tumor development and
therapy.
Preoperative chemoradiation therapy is increasingly
used in colon cancer therapy (30,31).
Some patients exhibit a marked pathologic response with standard
chemoradiation treatment, however others remain non-responsive.
Thus, the identification of markers that can predict sensitivity to
chemoradiation is exceedingly useful to avoid unnecessary
preoperative treatment. Previous studies have identified several
markers that predict the sensitivity or resistance to
chemoradiation therapy. A clinical study showed that thymidylate
synthase (TS) is overexpressed in chemoradiation-resistant rectal
cancer patients, which indicates that the level of TS in tumors is
the best predictor of sensitivity to chemoradiation (31). In our study, we identifed 323
alternative splicing events in 293 genes that were significantly
different between the normal and chemoradiation-resistant HCT116
cells. Notably, there were no significant differences in the
expression of most of these alternative splicing affected genes (26
out of 293, data not shown). It is deducible that apart from the
expression level of some crucial genes, alternative-splicing events
of these genes may also affect tumor sensitivity to
chemoradiotherapy.
In this study, we defined a set of 293 genes showing
different alternative splicing events in a normal and
chemoradiation-resistant colon cancer cell line. This group of
genes were enriched in molecular functions and biological processes
relevant to DNA replication, such as deoxyribonucleotide
metabolic/catabolic processes and helicase activity. We identified
for the first time, to the best of our knowledge, the alternative
splicing events that are associated with the chemoradiation
resistance of colon cancer. Thus, from a clinical point of view,
our study is expected to provide insight into potential novel
therapeutic targets, such as alternative splicing, to improve
treatment response.
Acknowledgments
This study was supported by the Joint Funds of the
Department of Science and Technology of Yunnan Province and Kunming
Medical University (no. 2013FB167) and the Key Project of the
Department of Education of Yunnan Province (no. 2014Z061).
References
|
1
|
Leff SE, Rosenfeld MG and Evans RM:
Complex transcriptional units: Diversity in gene expression by
alternative RNA processing. Annu Rev Biochem. 55:1091–1117. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pan Q, Shai O, Lee LJ, Frey BJ and
Blencowe BJ: Deep surveying of alternative splicing complexity in
the human transcriptome by high-throughput sequencing. Nat Genet.
40:1413–1415. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Black DL: Mechanisms of alternative
pre-messenger RNA splicing. Annu Rev Biochem. 72:291–336. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Matlin AJ, Clark F and Smith CW:
Understanding alternative splicing: Towards a cellular code. Nat
Rev Mol Cell Biol. 6:386–398. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sammeth M, Foissac S and Guigó R: A
general definition and nomenclature for alternative splicing
events. PLOS Comput Biol. 4:e10001472008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
David CJ and Manley JL: Alternative
pre-mRNA splicing regulation in cancer: Pathways and programs
unhinged. Genes Dev. 24:2343–2364. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Oltean S and Bates DO: Hallmarks of
alternative splicing in cancer. Oncogene. 33:5311–5318. 2014.
View Article : Google Scholar
|
|
8
|
Kim E, Goren A and Ast G: Insights into
the connection between cancer and alternative splicing. Trends
Genet. 24:7–10. 2008. View Article : Google Scholar
|
|
9
|
Fackenthal JD and Godley LA: Aberrant RNA
splicing and its functional consequences in cancer cells. Dis Model
Mech. 1:37–42. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Miura K, Fujibuchi W and Unno M: Splice
isoforms as therapeutic targets for colorectal cancer.
Carcinogenesis. 33:2311–2319. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Thorsen K, Sørensen KD, Brems-Eskildsen
AS, Modin C, Gaustadnes M, Hein AM, Kruhøffer M, Laurberg S, Borre
M, Wang K, et al: Alternative splicing in colon, bladder, and
prostate cancer identified by exon array analysis. Mol Cell
Proteomics. 7:1214–1224. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mojica W and Hawthorn L: Normal colon
epithelium: A dataset for the analysis of gene expression and
alternative splicing events in colon disease. BMC Genomics.
11:52010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gardina PJ, Clark TA, Shimada B, Staples
MK, Yang Q, Veitch J, Schweitzer A, Awad T, Sugnet C, Dee S, et al:
Alternative splicing and differential gene expression in colon
cancer detected by a whole genome exon array. BMC Genomics.
7:3252006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bisognin A, Pizzini S, Perilli L, Esposito
G, Mocellin S, Nitti D, Zanovello P, Bortoluzzi S and Mandruzzato
S: An integrative framework identifies alternative splicing events
in colorectal cancer development. Mol Oncol. 8:129–141. 2014.
View Article : Google Scholar
|
|
15
|
Thorsen K, Mansilla F, Schepeler T, Øster
B, Rasmussen MH, Dyrskjøt L, Karni R, Akerman M, Krainer AR,
Laurberg S, et al: Alternative splicing of SLC39A14 in colorectal
cancer is regulated by the Wnt pathway. Mol Cell Proteomics.
10:M110.0029982011. View Article : Google Scholar :
|
|
16
|
Gantt GA, Chen Y, Dejulius K, Mace AG,
Barnholtz-Sloan J and Kalady MF: Gene expression profile is
associated with chemo-radiation resistance in rectal cancer.
Colorectal Dis. 16:57–66. 2014. View Article : Google Scholar
|
|
17
|
Kim Y, Joo KM, Jin J and Nam DH: Cancer
stem cells and their mechanism of chemo-radiation resistance. Int J
Stem Cells. 2:109–114. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hiro J, Inoue Y, Toiyama Y, Miki C and
Kusunoki M: Mechanism of resistance to chemoradiation in p53 mutant
human colon cancer. Int J Oncol. 32:1305–1310. 2008.PubMed/NCBI
|
|
19
|
Trapnell C, Pachter L and Salzberg SL:
TopHat: Discovering splice junctions with RNA-Seq. Bioinformatics.
25:1105–1111. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Trapnell C, Williams BA, Pertea G,
Mortazavi A, Kwan G, van Baren MJ, Salzberg SL, Wold BJ and Pachter
L: Transcript assembly and quantification by RNA-Seq reveals
unannotated transcripts and isoform switching during cell
differentiation. Nat Biotechnol. 28:511–515. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Trapnell C, Roberts A, Goff L, Pertea G,
Kim D, Kelley DR, Pimentel H, Salzberg SL, Rinn JL and Pachter L:
Differential gene and transcript expression analysis of RNA-seq
experiments with TopHat and Cufflinks. Nat Protoc. 7:562–578. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Katz Y, Wang ET, Airoldi EM and Burge CB:
Analysis and design of RNA sequencing experiments for identifying
isoform regulation. Nat Methods. 7:1009–1015. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yu G, Li F, Qin Y, Bo X, Wu Y and Wang S:
GOSemSim: An R package for measuring semantic similarity among GO
terms and gene products. Bioinformatics. 26:976–978. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Grosso AR, Martins S and Carmo-Fonseca M:
The emerging role of splicing factors in cancer. EMBO Rep.
9:1087–1093. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Narla G, DiFeo A, Yao S, Banno A, Hod E,
Reeves HL, Qiao RF, Camacho-Vanegas O, Levine A, Kirschenbaum A, et
al: Targeted inhibition of the KLF6 splice variant, KLF6 SV1,
suppresses prostate cancer cell growth and spread. Cancer Res.
65:5761–5768. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Venables JP, Klinck R, Koh C, Gervais-Bird
J, Bramard A, Inkel L, Durand M, Couture S, Froehlich U, Lapointe
E, et al: Cancer-associated regulation of alternative splicing. Nat
Struct Mol Biol. 16:670–676. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wielenga VJ, Heider KH, Offerhaus GJ,
Adolf GR, van den Berg FM, Ponta H, Herrlich P and Pals ST:
Expression of CD44 variant proteins in human colorectal cancer is
related to tumor progression. Cancer Res. 53:4754–4756.
1993.PubMed/NCBI
|
|
28
|
Mulder JW, Kruyt PM, Sewnath M, Oosting J,
Seldenrijk CA, Weidema WF, Offerhaus GJ and Pals ST: Colorectal
cancer prognosis and expression of exon-v6-containing CD44
proteins. Lancet. 344:1470–1472. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Herrlich P, Pals S and Ponta H: CD44 in
colon cancer. Eur J Cancer. 31A:1110–1112. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bilchik AJ, Poston G, Curley SA, Strasberg
S, Saltz L, Adam R, Nordlinger B, Rougier P and Rosen LS:
Neoadjuvant chemotherapy for metastatic colon cancer: A cautionary
note. J Clin Oncol. 23:9073–9078. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Okonkwo A, Musunuri S, Talamonti M, Benson
A III, Small W Jr, Stryker SJ and Rao MS: Molecular markers and
prediction of response to chemoradiation in rectal cancer. Oncol.
8:497–500. 2001.
|