Introduction
Cervical cancer is the fourth most common malignancy
and cause of cancer-related death among women worldwide (1). Although the associated mortality rates
are generally decreasing because of early detection and effective
surgical excision as well as chemoradiotherapy, the 5-year survival
rate for women with metastatic cervical cancers is still only 16%
(2). Therefore, novel strategies
must be explored to improve the management of metastatic cervical
cancer.
Sodium iodide symporter (NIS) is a membrane
glycoprotein that mediates the transfer of iodide into thyroid
follicular cells (3). NIS
gene-mediated uptake of radioisotopes such as technetium-99m
(99mTc), iodide-125 (125I), and iodide-131
(131I) has been widely investigated as a potential
imaging and therapeutic method for thyroid and non-thyroid
carcinomas (4). However, to protect
normal cells from unnecessary radioisotope uptake, NIS expression
must be restricted to tumor cells only. Human telomerase reverse
transcriptase (hTERT) is an important component of telomerase,
which is highly active in most malignant tumors but inactive in
normal somatic cells (5).
Therefore, transfer of NIS genes under the control of the hTERT
promoter is an important breakthrough for ensuring tumor-specific
uptake of radioisotopes.
Rhenium-188 (188Re) is a chemical analog
of technetium and a useful radioisotope emitting powerful
β-particles that can be channeled for therapy and γ-rays for
imaging. Dosimetry calculations of β-particles have indicated that
188Re-perrhenate can generate a higher irradiation dose
with a wider irradiation range than 131I (6). The physical characteristics of
188Re support the notion that this β-emitter may be a
more suitable radioisotope for treatment and imaging of
NIS-expressing tumors.
In the present study, we constructed a lentiviral
vector to express a functional NIS gene under the control of the
hTERT promoter. The potential of NIS as an imaging and therapeutic
gene was investigated in vitro and in vivo using a
cervical cancer xenograft model.
Materials and methods
Generation of recombinant lentiviral
vector
A lentiviral vector harboring the puromycin
resistance gene (pLVX-puro) was purchased from Clontech (Takara,
Dalian, China). The pFastBac-hTERT-NIS vector was generated in our
laboratory (7). The
Lv-EF1α-OCT4-IRES-eGFP vector was kindly provided by the Institute
of Molecular Biology, Chinese Academy of Sciences. Polymerase chain
reaction (PCR) was used to introduce ClaI and XbaI
enzyme sites flanking the hTERT-NIS fragment in the
pFastBac-hTERT-NIS vector, and the fragment was then cloned into
pLVX-puro using the same enzymes, to generate a functional vector
with the NIS gene under the control of hTERT promoter
(pLVX-hTERT-NIS-puro). Similarly, the eGFP gene was amplified from
Lv-EF1α-OCT4-IRES-eGFP by PCR, digested with BamHI and
XbaI, and cloned into the BamHI and XbaI sites
of pLVX-hTERT-NIS-puro to generate a vector harboring the eGFP gene
under the control of the hTERT promoter (pLVX-hTERT-eGFP-puro).
The HEK293T cell line (Cell Bank of the Chinese
Academy of Science, Shanghai, China) was cultured in RPMI-1640
medium supplemented with 10% fetal bovine serum and 1%
penicillin/streptomycin.
Lentivirus particles were generated by
co-transfection of HEK293T cells with pLVX-hTERT-NIS-puro or
pLVX-hTERT-eGFP-puro and the three packaging plasmids pRsv-REV,
pMDIg-pRRE, and pMD2G (Biovector Science Lab, Shanghai, China). The
viral particles were harvested by collecting the cell culture
medium at 48 h post-transfection; the supernatants were filtered
through filters of pore size 0.45 µm and centrifuged at
10,000 × g for 15 min, and the resulting pellet was resuspended in
100 µl culture medium.
Cell lines and cell cultures
Human cervical cancer HeLa cells, human anaplastic
thyroid cancer C643 cells, human fibroblast MRC-5 cells (Cell Bank
of the Chinese Academy of Science, Shanghai, China) and human
glioma U87 cells (American Type Culture Collection, Manassas, VA,
USA) were maintained in Dulbecco's modified Eagle's medium. Human
proximal tubule epithelial HK-2 cells (Shanghai Cell Bank of the
Chinese Academy of Science) were cultured in keratinocyte
serum-free medium. Follicular thyroid carcinoma FTC-133 cells
(European Collection of Animal Cell Cultures, Salisbury, UK) were
cultured in Dulbecco's modified Eagle's medium:Ham's F12 (1:1)
medium. All media except keratinocyte serum-free medium were
supplemented with 10% fetal bovine serum and 1%
penicillin/streptomycin. All cell lines were incubated in a 5%
CO2 atmosphere at 37°C.
The cell lines were infected with
pLVX-hTERT-eGFP-puro at a multiplicity of infection of 100 for 24
h. To select cells stably transfected with pLVX-hTERT-eGFP-puro,
0.5–1.0 µg/ml puromycin (Sigma, Sydney, Australia) was added
to the medium for four days. HeLa cells stably transfected with
pLVX-hTERT-NIS-puro (HeLa-TERTNIS) were obtained using a similar
selection method.
Fluorescence intensity measurement
All tumor or normal cell lines stably transfected
with pLVX-hTERT-eGFP-puro were seeded into 6-well plates at a
density of 2×105 cells/well. eGFP expression under the
control of the hTERT promoter was quantitatively analyzed using a
flow cytometry (BD Bioscience, San Jose, CA, USA) in all cell
lines. The mean fluorescence intensity (MFI) of 3×104
cells of each cell line was determined by subtracting the basal MFI
of the same uninfected cell line.
Quantitative real-time polymerase chain
reaction
Total RNA samples from HeLa and HeLa-TERTNIS cells
were extracted using the RNeasy Mini kit and reverse-transcribed
into cDNA using the Superscript RT kit (Invitrogen, Carlsbad, CA,
USA). Quantitative real-time PCR was performed using the SYBR
Premix Ex Taq kit (Takara). The NIS gene was amplified using the
forward and reverse primers 5′-GTACATTGTAGCCACGATGCTGTA-3′ and
5′-CCGTGTAGAAGGTGCAGATAATTC-3′, respectively. Additionally, GAPDH
was co-amplified using the primers 5′-GTCAAGCTCATTTCCTGGTATGAC-3′
(forward) and 5′-CTCTCTCTTCCTCTTGTGCTCTTG-3′ (reverse). The cycling
conditions were 95°C for 10 sec, 40 cycles at 95°C for 5 sec and
60°C for 31 sec, and one cycle of 95°C for 15 sec, 60°C for 1 min,
and 95°C for 15 sec. According to the manufacturer's protocol, NIS
expression levels were normalized to those of the GAPDH endogenous
reference using the formula: F-value = 2−ΔΔCt (8). Quantitative real-time PCR was repeated
three times, and the mean values were obtained for each
specimen.
Western blotting
Lysates of HeLa and HeLa-TERTNIS cells were prepared
using standard methods. Western blot analysis was then performed
using mouse anti-human NIS (1:500; Thermo Scientific, Fremont, CA,
USA) or mouse anti-human GAPDH (1:10000; Abgent, Suzhou, China)
antibody in Tris-buffered saline/Tween-20 with overnight incubation
at 4°C, followed by incubation with peroxidase-conjugated goat
anti-mouse IgG (1:2500; Santa Cruz Biotechnology, Santa Cruz, CA,
USA) for 1 h at room temperature. Immunodetection was performed
using ECL Western Blot Detection kit (Pierce, Waltman, MA,
USA).
In vitro 188Re uptake
studies
HeLa and HeLa-TERTNIS cells (1×105) were
seeded in 24-well plates and cultured for 24 h. After subsequent
washing with buffered Hanks' balanced salt solution (bHBSS), the
cells were incubated for 10 min to 24 h at 37°C with 500 µl
bHBSS containing 10 µmol/l sodium iodide and 37 kBq
188Re in the form of Na188ReO4
(Xinke, Shanghai, China). HeLa-TERTNIS cells in the inhibition
group were treated with 300 µmol/l sodium perchlorate
(NaClO4). At various points of incubation, the cells
were washed twice with ice-cold bHBSS and lysed with 1 mol/l sodium
hydroxide. The radioactivity (counts per minute, CPM) was measured
using a γ-counter (Rihuan, Shanghai, China). All experiments were
performed in triplicate.
In vitro clonogenic assay
HeLa and HeLa-TERTNIS cells were plated in 10-cm
culture dishes (5×106 cells/dish), and 3.7 MBq
188Re in bHBSS was added. After 8 h, the cells were
washed three times with bHBSS and then trypsinized. Thousand cells
were plated into each well of 6-well plates. On day 7, the cells
were stained with 1 ml of crystal violet staining solution
(Beyotime Institute of Biotechnology, Shanghai, China) for 10 min,
and colonies containing more than 50 cells were counted by
observation under a microscope (Olympus, Tokyo, Japan). The
survival rate of each group was expressed as the percentage of
colonies to the number of colonies formed by HeLa cells not treated
with 188Re. Data are represented as means ± standard
deviation.
Establishment of xenograft tumors in nude
mice
Female BALB/c nude mice aged 4 weeks (Shanghai
Slaccas Experiment Animal Corp., Shanghai, China) were s.c.
injected with 5×106 HeLa cells in the left thigh and
5×106 HeLa-TERTNIS cells in the right thigh. This study
protocol was approved by the institutional review board and the
experimental animal center of Rui Jin Hospital affiliated to
Shanghai Jiao Tong University School of Medicine.
Micro-single photon-emission computed
tomography/computed tomography imaging
Three mice bearing both HeLa and HeLa-TERTNIS tumors
were i.v. injected with 37 MBq of 188Re for micro-single
photon-emission computed tomography/computed tomography
(micro-SPECT/CT) imaging. The mice was anesthetized by isoflurane
inhalation, placed in the spread-prone position, and scanned using
a small animal micro-SPECT/CT scanner (Bioscan, Washington, DC,
USA) at 0.5, 2, 4, 6, and 24 h after injection of the radioisotope.
CT images were acquired, after which whole-body SPECT images (10
sec/frame for systematic scans) were obtained. Regions of interest
(ROIs) were drawn in the visible tumors and organs including the
stomach, lung, liver, intestine, muscle, thyroid, bladder, and
heart at various time points. The radioactivity per volume unit
(Conc; µCi/mm3) in the ROIs was measured using
InVivoScope 1.44 software (Bioscan). To compare in vivo
distribution and kinetics of 188Re with those of
radioiodide, a micro-SPECT/CT imaging study with 37 MBq
125I was performed 1 week after 188Re imaging
in the same mice. 125I was used in this study instead of
131I, since both share pharmacokinetic properties but it
is easier to quantify energy emitted by 125I.
In vivo 188Re therapy
For 188Re therapy, 10 mice bearing both
HeLa and HeLa-TERTNIS tumors were divided into two groups: one
group received 37 MBq of 188Re while the other received
saline solution injected via the tail vein on day 0 and the same
volume on day 7. Tumor size was measured every 3 days after
injection and monitored for 21 days using calipers; tumor volume
was calculated using the formula: volume (mm3) = (length
× width2)/2.
Immunohistochemical analysis
At the end of the therapy experiments, the animals
were sacrificed by cervical vertebra dislocation, and the tumors
were removed and immunohistochemically analyzed using rabbit
anti-human NIS antibody (1:50; Proteintech Group, Chicago, IL,
USA), rabbit anti-human caspase-3 antibody (1:30; Epitomics,
Burlingame, CA, USA), and rabbit anti-human Ki67 antibody (1:200;
Thermo Scientific). The findings were semi-quantitatively analyzed
using Image Pro Plus software (Media Cybernetics, Rockville, MD,
USA). For every section, the integral optical density of each
visual field was calculated. Data are represented as means ±
SD.
Statistical analysis
Data were analyzed using SPSS 19.0 software (SPSS
Inc., Chicago, IL, USA). Each experiment was carried out in
triplicate, and results are presented as means ± SD. Experimental
groups were compared by analysis of variance. P<0.05 was
considered statistically significant.
Results
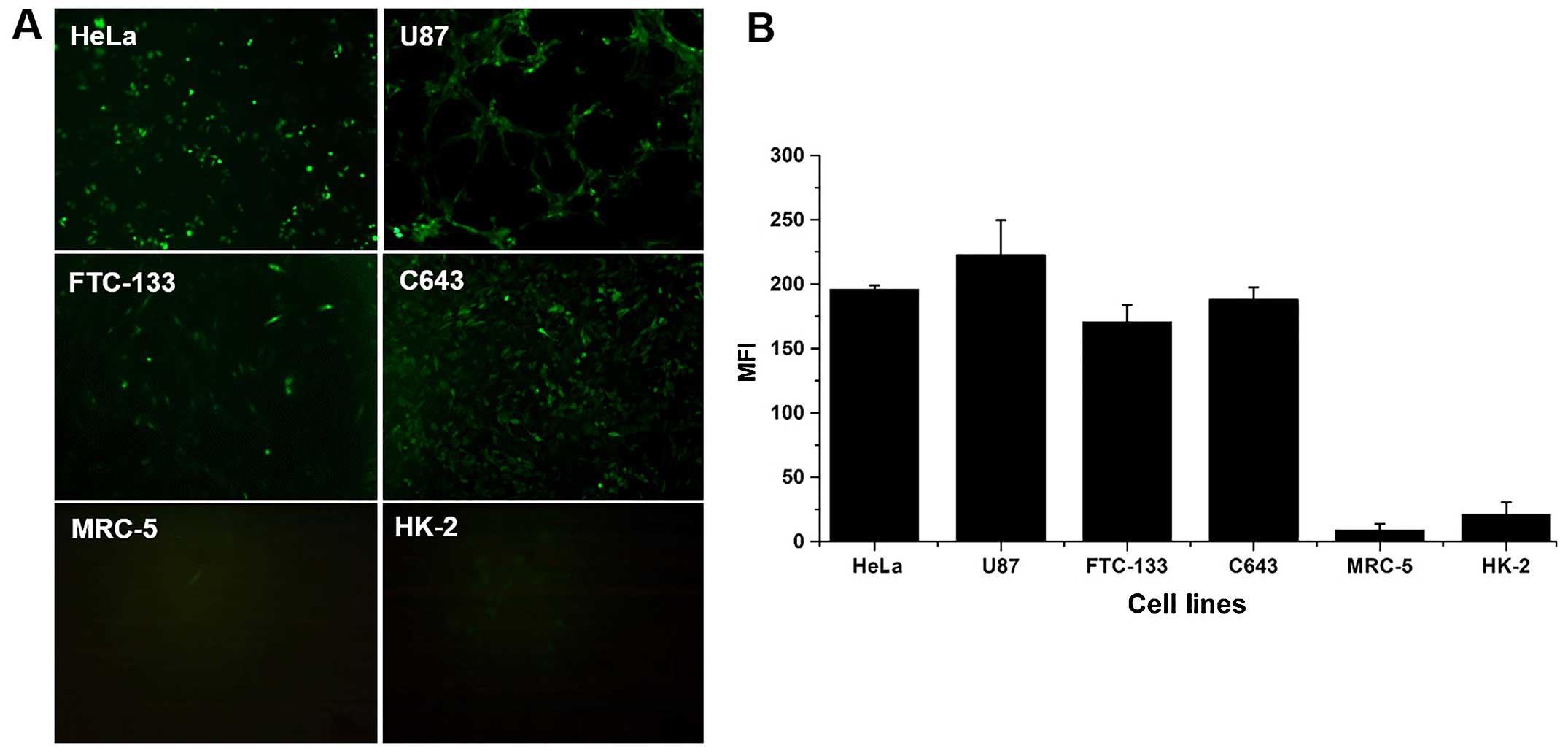
Fluorescence expression of eGFP under the
control of the hTERT promoter in tumor and normal cells
To confirm that the transcriptional activity of the
hTERT promoter is higher in telomerase-positive tumor cells than in
normal cells, eGFP was expressed under the control of the hTERT
promoter (Fig. 1A) and
quantitatively analyzed (Fig. 1B)
in four tumor cell lines (HeLa, U87, FTC-133, and C643) and two
normal cell lines (MRC-5 and HK-2) stably transfected with
pLVX-hTERT-eGFP-puro. The MFI of eGFP in all tumor cells was
approximately 10- to 20-fold higher than that in the two normal
cell lines (P<0.01).
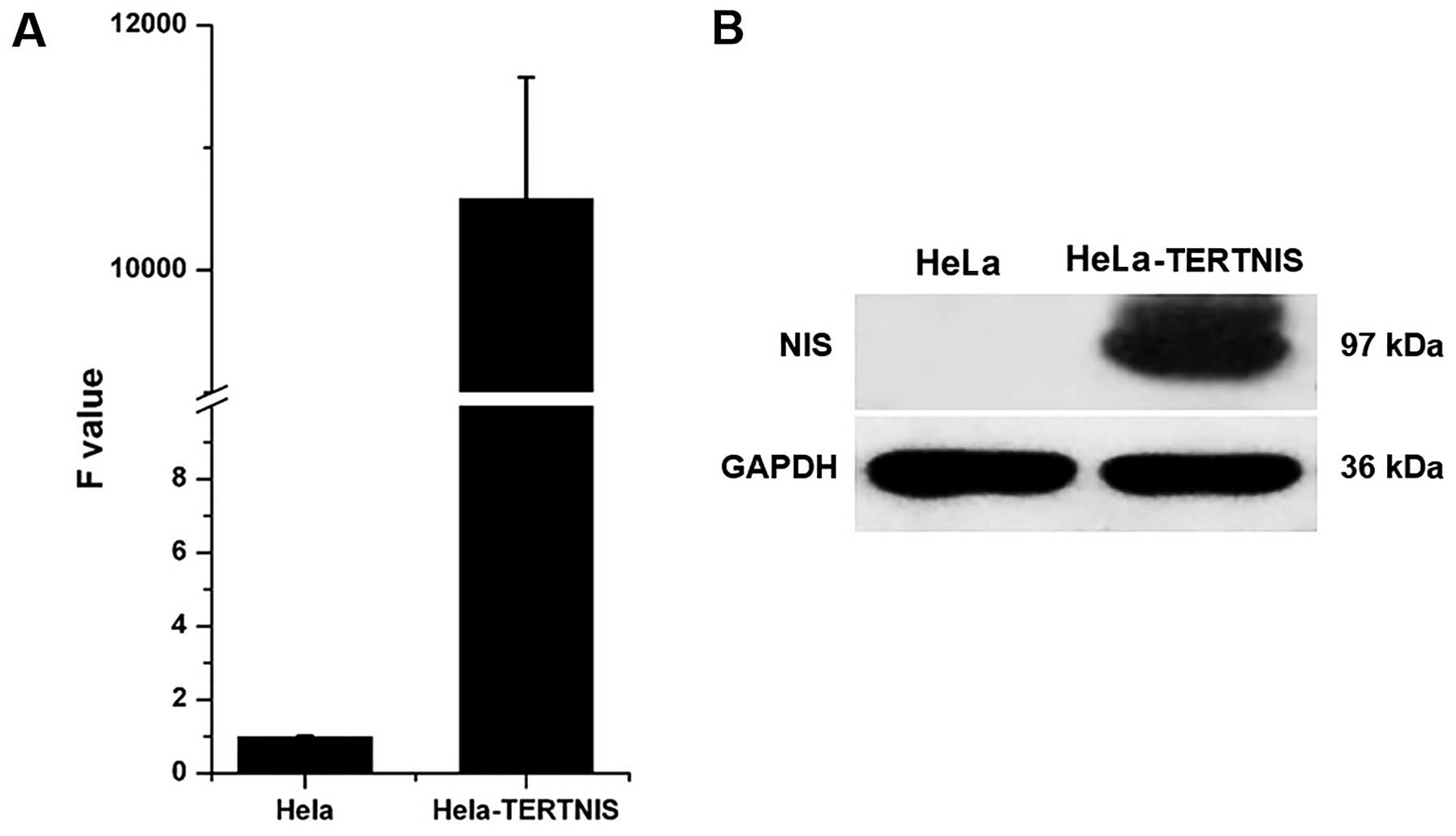
Stable expression of NIS in HeLa-TERTNIS
cells
Quantitative real-time PCR (Fig. 2A) and western blotting (Fig. 2B) confirmed that HeLa-TERTNIS cells
expressed high levels of NIS mRNA and protein (~97 kDa),
respectively.
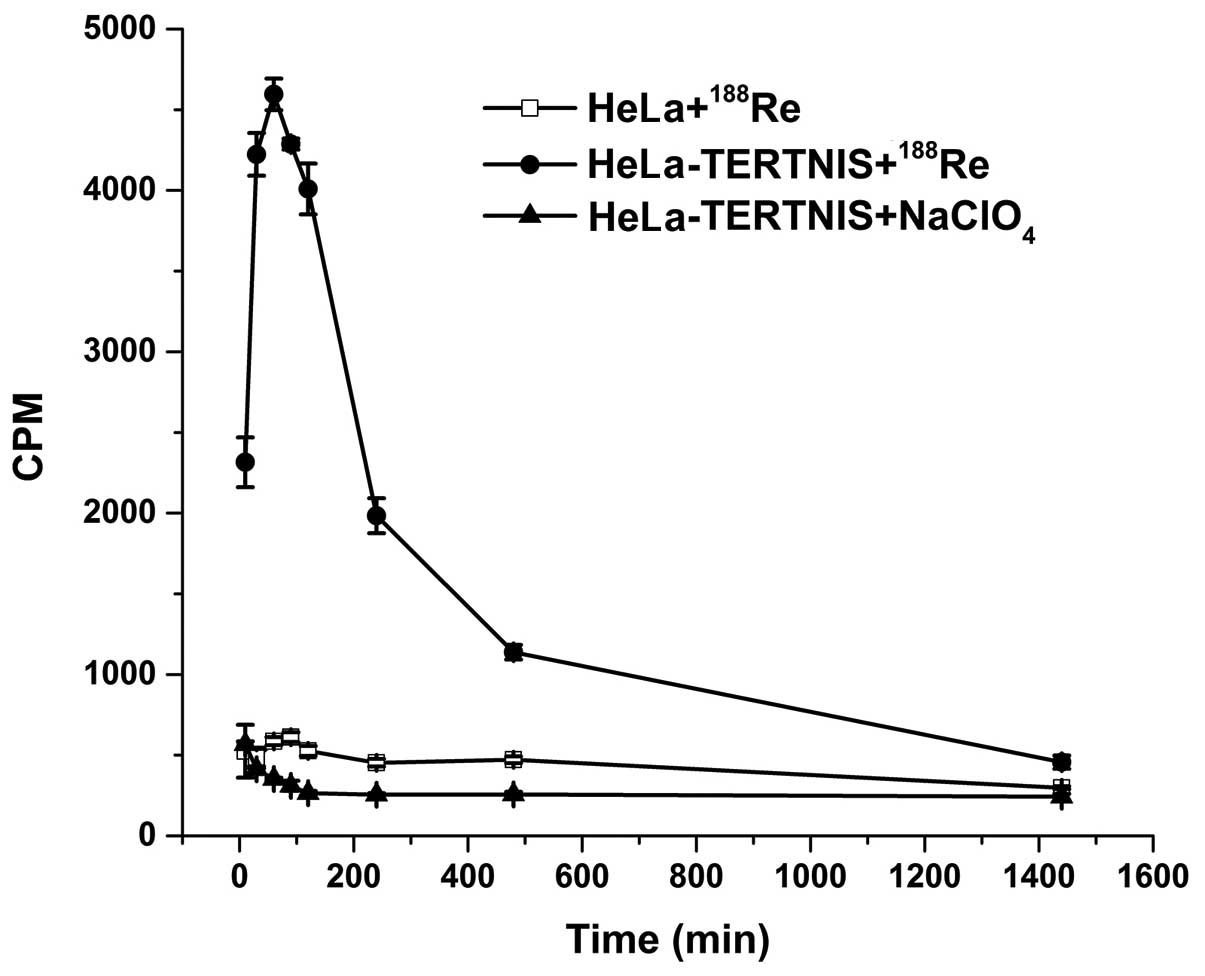
In vitro 188Re uptake
The functional activity of the NIS protein is shown
by its cellular uptake of radioisotopes such as 99mTc,
125I, 131I, and 188Re. As shown in
Fig. 3, the 188Re uptake
in HeLa-TERTNIS cells rapidly reached a peak (approximately 8-fold
higher than that in HeLa cells) after 60 min of incubation,
followed by a decline (half-life≈158.4 min). No functional
188Re uptake was observed in HeLa cells. Further, the
188Re uptake in HeLa-TERTNIS cells was completely
blocked by NaClO4.
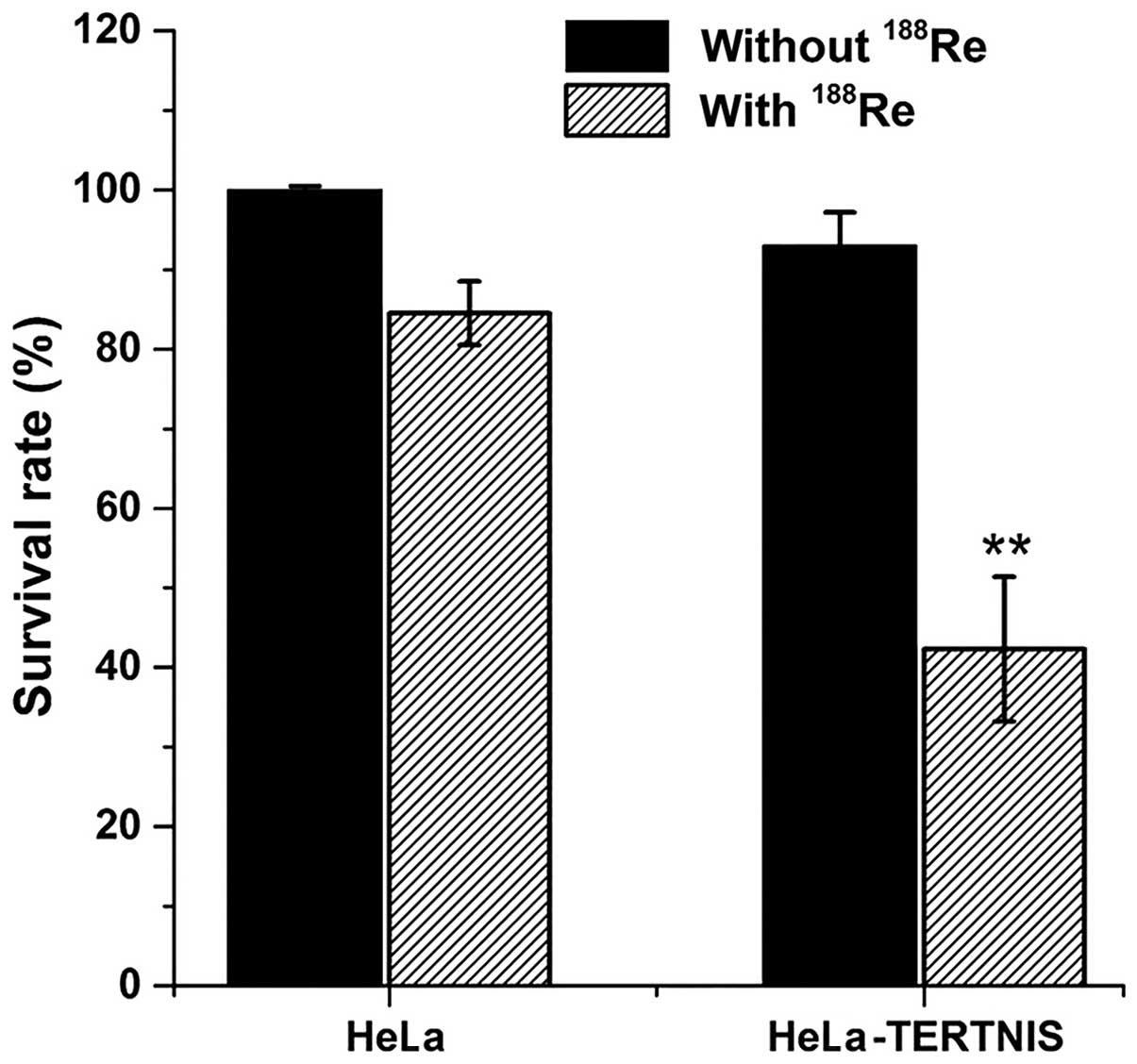
In vitro clonogenic assay
In vitro clonogenic assays were performed to
determine the effect of 188Re in HeLa-TERTNIS and HeLa
cells (Fig. 4). The survival rate
of HeLa-TERTNIS cells treated with 188Re markedly
decreased to 42.3% compared to the 84.5% survival rate of HeLa
cells treated with 188Re (P<0.01) and 93.0% survival
rate of HeLa-TERTNIS cells not treated with 188Re
(P<0.01). These findings indicating that 188Re
emitting β-particles had a significant cytotoxic effect in
HeLa-TERTNIS cells.
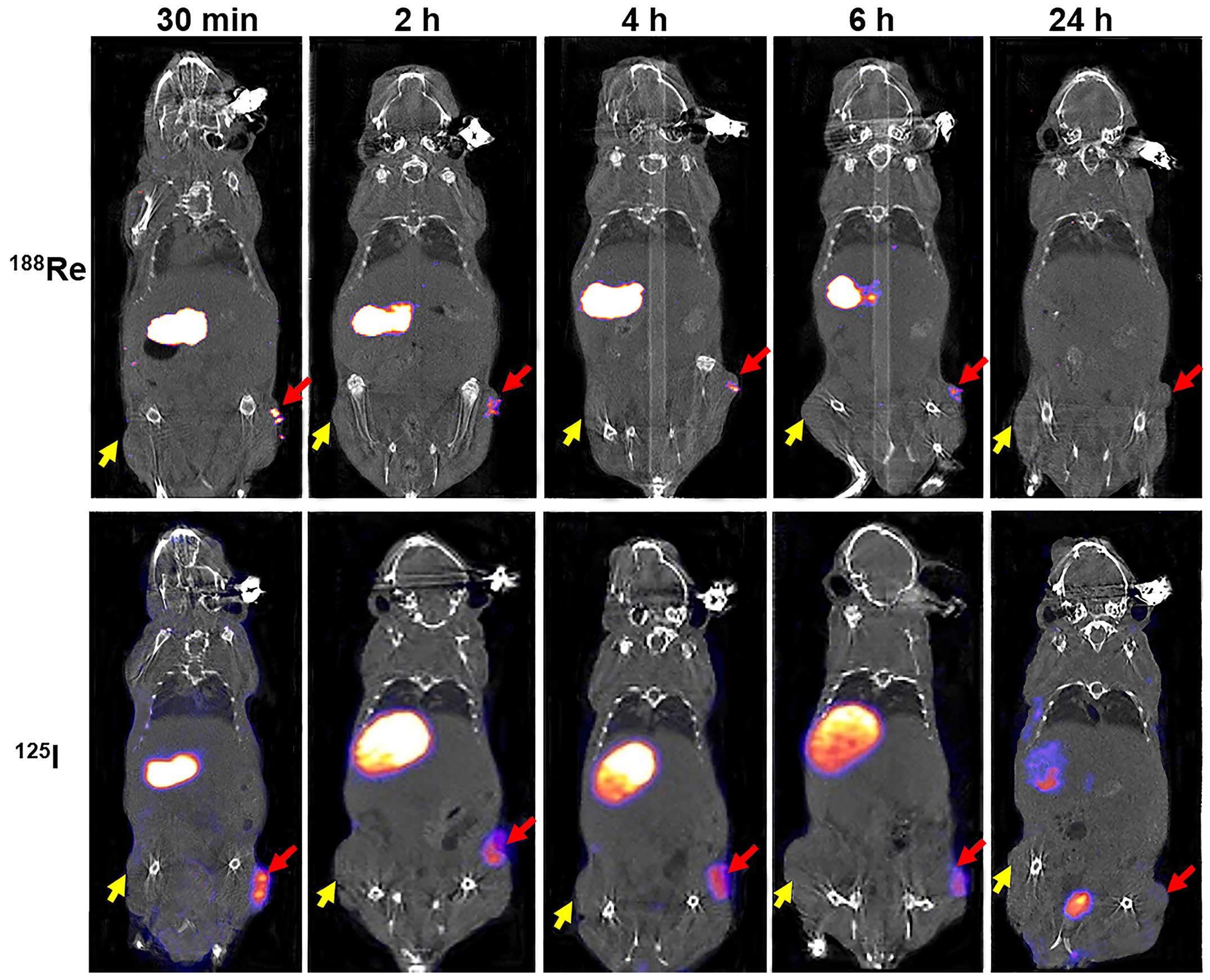
In vivo imaging and biodistribution of
188Re in mice bearing HeLa and HeLa-TERTNIS
xenografts
188Re uptake was clearly observed in
HeLa-TERTNIS tumors from 0.5 to 6 h after injection but was not
visible 24 h after injection. In contrast, 188Re
accumulation was not observed in HeLa tumors at any time point
after injection (Fig. 5).
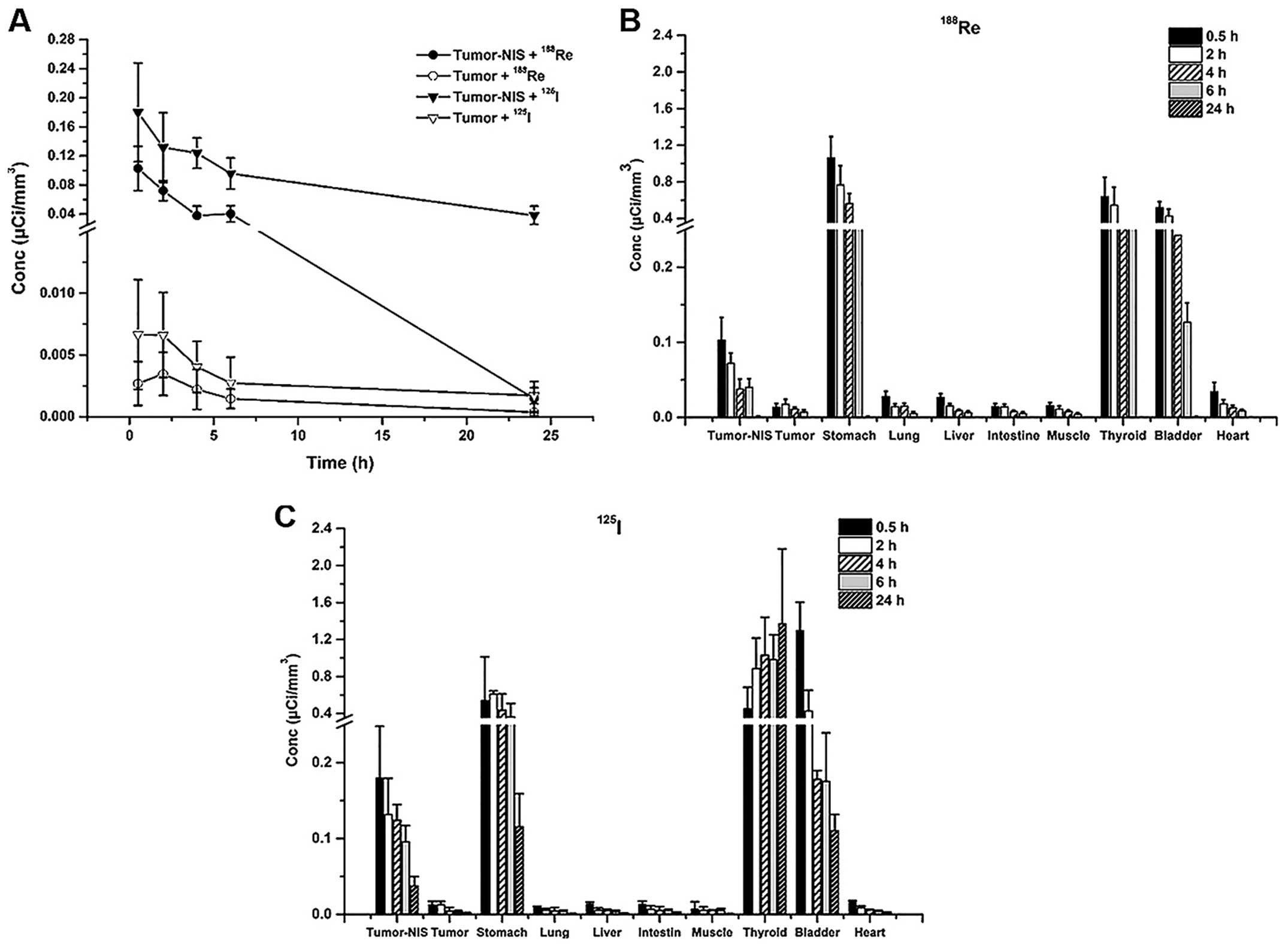
188Re accumulation in HeLa-TERTNIS tumors reached the
highest Conc value at 0.5 h after injection, which was
significantly (7.6-fold) higher than the corresponding value for
HeLa tumors. Additionally, compared to 125I uptake,
188Re uptake decreased more rapidly from 0.5 h onward
and reached a substantially lower level at 24 h (Fig. 6A). Both 188Re and
125I showed intense accumulation in the thyroid and
stomach, which express endogenous NIS, as well as in the bladder,
because of renal elimination. However, 188Re cleared at
a faster rate than 125I from the thyroid and stomach.
The Conc values of both 188Re (Fig. 6B) and 125I (Fig. 6C) in the heart, lung, liver, muscle,
and intestine remained relatively low compared to thyroid, stomach
and bladder at all time points.
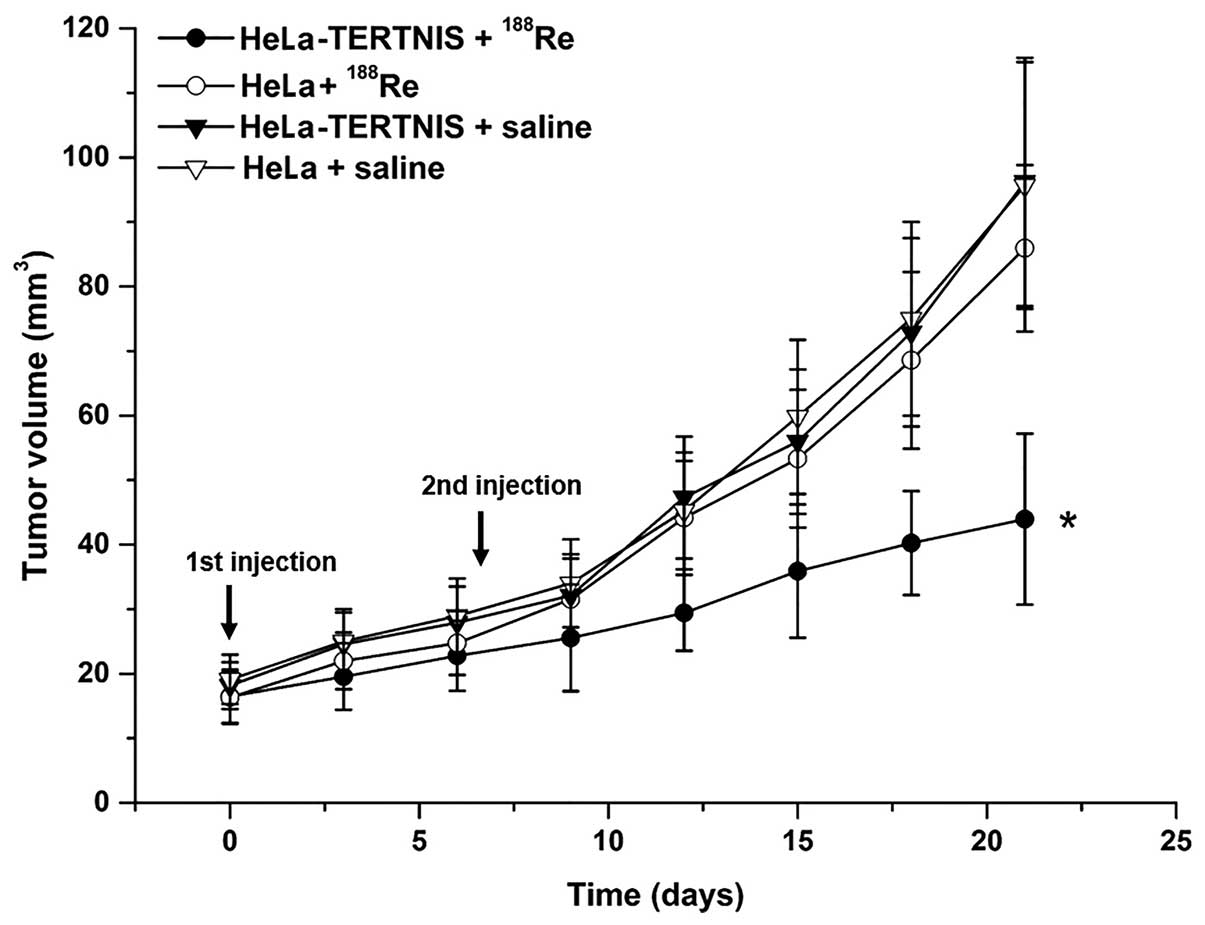
Therapeutic effects of 188Re
in HeLa xenograft tumors expressing NIS
Therapy with 37 MBq 188Re was initiated
when the tumors reached 3–5 mm in diameter. Seven days after the
first 188Re injection, the mice were reinjected with 37
MBq 188Re or saline. The growth of HeLa-TERTNIS tumors
was significantly inhibited from days 12 to 21 compared to other
tumors of the control groups, including HeLa tumors treated with
188Re and HeLa-TERTNIS and HeLa tumors treated with
saline (P<0.05). No significant differences in tumor volume were
noted among the three control groups (Fig. 7).
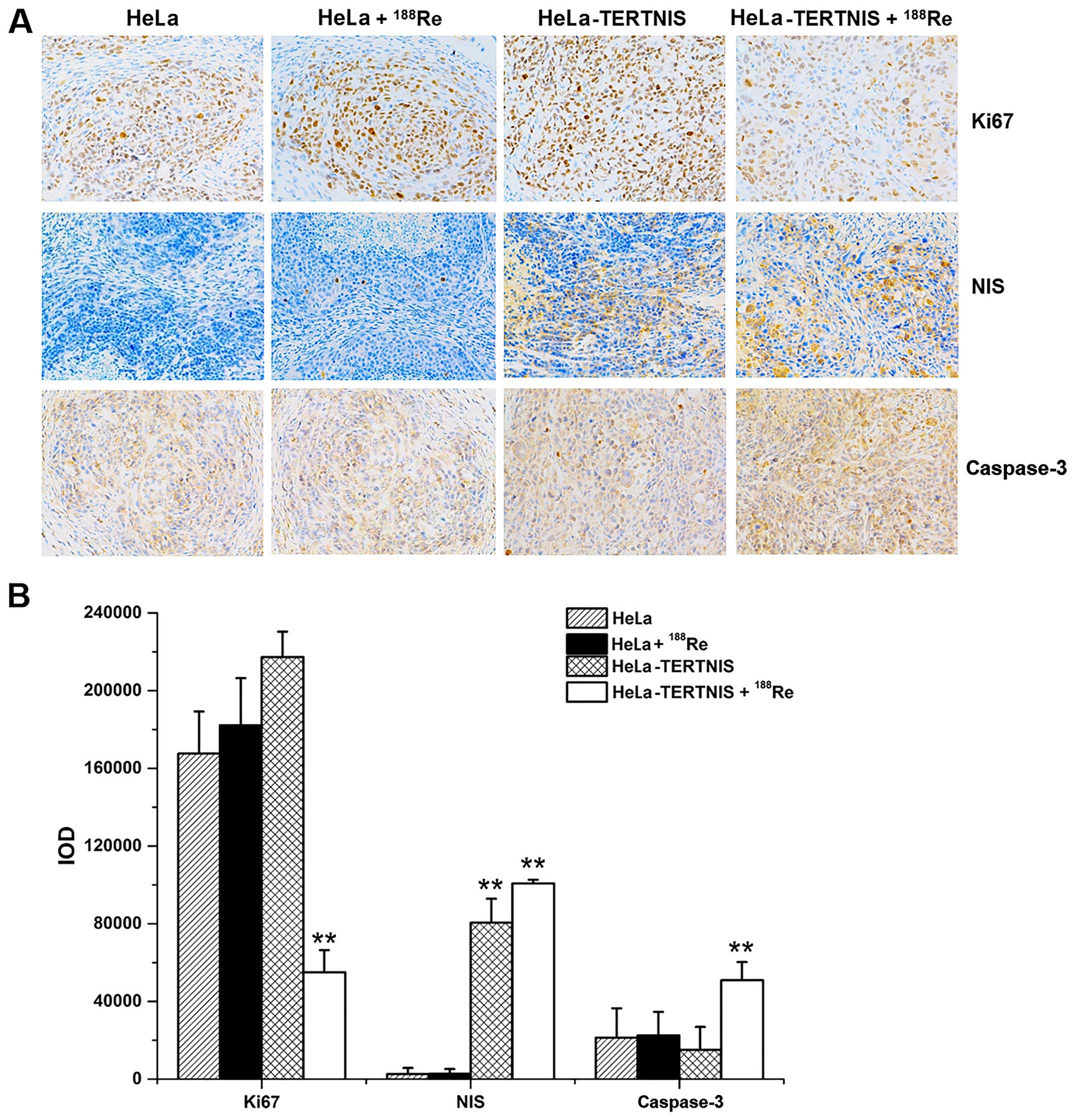
Fig. 8A shows the
results of immunohistochemical staining of the tumor xenografts,
while Fig. 8B shows the results of
quantitative analysis of staining intensity (Fig. 8B). The NIS protein was strongly
expressed in HeLa-TERTNIS xenografts treated with 188Re
or saline but not in HeLa xenografts with or without
188Re treatment (P<0.01). Caspase-3 protein
expression was significantly higher and Ki67 protein expression was
significantly lower in HeLa-TERTNIS xenografts treated with
188Re than HeLa xenografts treated with 188Re
HeLa and HeLa-TERTNIS xenografts treated with saline
(P<0.01).
Discussion
Novel strategies to improve the efficacy of cervical
cancer treatment are urgently needed since the survival rate of
patients with metastatic cervical cancer remains low even after
they receive conventional systematic therapy. NIS gene therapy is a
promising research area, whereby various radioisotopes can be
delivered in concentrated fashion into non-thyroid cancer cell
lines, and it has been successfully applied in gliomas, colon
tumors, nasopharyngeal carcinomas, and lung adenocarcinoma
(9–12) in our laboratory. However, a
tumor-specific expression system for the NIS gene needs to be
established in order to restrict the NIS-mediated uptake of
radioisotopes to tumor cells and protect normal cells from the
cytotoxic effects of radioisotopes.
Telomerase is almost undetectable in most normal
somatic cells but is strongly expressed in embryonic and stem cells
as well as in 85–90% cancer cells, in which it supports cell
proliferation by maintaining telomeres. hTERT is the rate-limiting
determinant of telomerase, and the hTERT promoter selectively
promotes hTERT gene expression in tumor cells (5). Therefore, the hTERT promoter has been
widely used to drive the expression of suicide and oncolytic genes
such as those encoding caspase-8, Bax, thymidine kinase, and E1,
resulting in cell apoptosis or lysis only in telomerase-positive
tumor cells (13–16). In the present study, eGFP expression
under the control of the hTERT promoter was significantly higher in
the four cancer cell lines than in the normal MRC-5 and HK-2 cell
lines, indicating that selective transcriptional activation by the
hTERT promoter in cancer cells is an effective strategy for
achieving the tumor-specific expression of transgenes. Of note, the
transcription efficiency of tumor-specific promoters is usually
weaker than that of commonly used promoters such as the
cytomegalovirus and the simian virus 40 promoter, but NIS gene
expression under the control of the hTERT promoter in the
HeLa-TERTNIS cells was high in the present study. This suggested
the unusually high transcription efficiency of the hTERT promoter,
which was almost equal to that of the cytomegalovirus promoter
described previously (7).
Reportedly, the transcription efficiency of the hTERT promoter is
even higher than that of the simian virus 40 promoter in human
prostate cancer cell DU145 and human malignant melanoma SK-MEL-5
cells (13), indicating that the
transcription efficiency of the hTERT promoter differed
considerably among various cancer cell lines.
Therapeutic effectiveness depends not only on the
level of NIS expression but also on the dose and retention time of
the radioisotope in the tumor expressing NIS. 131I (E
average=0.134 MeV; physical half-life = 8.1 days) is the most
frequently used radioisotope for NIS-based tumor therapy, but
because of the lack of organification of iodide in non-thyroid
cancers, it is not retained for an adequate time owing to its rapid
efflux despite sufficient iodide uptake (17). Several approaches have been proposed
to overcome this problem, one of which is the use of more powerful
β-emitting radioisotopes transported by NIS, such as
188Re (E average=0.764 MeV; physical half-life = 16.7
h). In our in vivo experiments, 188Re uptake in
HeLa-TERTNIS xenografts reached the maximal level at 0.5 h after
injection and then decreased more rapidly than 125I
levels did, especially at the 24 h point. The shorter retention
time of 188Re than 125I in the HeLa-TERTNIS
xenografts might be a result of its faster clearance from
circulation. Because of the thyroid gland reservoir and
entero-recirculation of iodide, 125I clearance from
circulation may be restricted. Despite the shorter retention time
of 188Re, a previous study found that the
188Re to 131I ratio of tumor-absorbed dose at
the same level of radioactivity was about 4.5:1 in a murine
xenograft model for breast cancer (6). Encouraging results regarding
188Re therapy have also been reported for
NIS-transfected hepatocarcinoma (18) and glioma (19).
In the present study, 188Re efficiently
and specifically inhibited the growth of HeLa-TERTNIS tumor cells
both in vitro and in vivo. Further,
immunohistochemical analysis for Ki67 and caspase-3 showed a
significant decrease in cell proliferation and increased levels of
apoptosis after 188Re treatment of HeLa-TERTNIS tumors.
188Re also has the advantage of emitting 155 keV γ-rays,
an energy comparable to that of 99mTc, whereby it
enables SPECT imaging for biokinetics evaluation. Collectively, the
results suggest that 188Re is a suitable alternative for
treatment of NIS-expressing non-thyroid tumors.
Gene therapy depends on transfer vectors that
facilitate the expression of a therapeutic gene. Viral vectors such
as adenovirus, adeno-associated virus, retrovirus, and baculovirus
have been widely employed for gene therapy in animal studies and
clinical trials (20). In addition
to possessing the useful features of previously developed
retroviral vectors, the lentiviral vector has the ability to
transfect dividing and non-dividing cells and can harbor large
target gene fragments, and recipients are unlikely to have
pre-existing immunity to this virus (21). In the present study, the lentiviral
vector facilitated strong and stable expression of NIS, which in
turn ensured high levels of 188Re or 125I
uptake in HeLa-TERTNIS xenografts. Our findings are superior to
those concerning baculoviral vector-mediated transfer of the NIS
gene obtained in a previous study (7) because of the higher transfection
efficiency of lentivirus than baculovirus for mammalian cells.
Although a potentially dangerous situation may arise with the use
of the lentiviral vector, that is, the transgene may integrate into
the genomic DNA of host cells, the lentiviral vector remains a very
efficient tool for NIS gene transfer.
In conclusion, a lentiviral vector containing the
NIS gene driven by the hTERT promoter enabled efficient
188Re uptake into cervical cancer HeLa cells and showed
a significant therapeutic effect both in vitro and in
vivo. Further, it enabled in vivo imaging of tumors. Our
findings indicate the possibility of tumor-specific NIS gene
therapy and imaging using the powerful radioisotope
188Re.
Abbreviations:
|
NIS
|
sodium iodine symporter
|
|
188Re
|
rhemium-188
|
|
hTERT
|
human telomerase reverse
transcriptase
|
|
eGFP
|
enhanced green fluorescent protein
|
|
HeLa-TERTNIS
|
HeLa cell line stably expressing NIS
under the control of the hTERT promoter
|
|
micro-SPECT/CT
|
micro-single photon-emission computed
tomography/computed tomography
|
|
99mTc
|
technetium-99m
|
|
125I/131I
|
iodide-125/131
|
|
pLVX-puro
|
lentiviral vector harboring the
puromycin resistance gene
|
|
pLVX-hTERT-NIS-puro
|
lentiviral vector expressing NIS under
the control of the hTERT promoter
|
|
pLVX-hTERT-eGFP-puro
|
lentiviral vector expressing eGFP
under the control of the hTERT promoter
|
|
MFI
|
mean fluorescence intensity
|
|
bHBSS
|
buffered Hanks' balanced salt
solution
|
|
NaClO4
|
sodium perchlorate
|
|
SD
|
standard deviation
|
|
ROI
|
region of interest
|
|
Conc
|
radioactivity per volume unit
|
Acknowledgments
This work was supported by grants from the National
Natural Science Foundation of China (NSFC) (81071181), and Shanghai
Natural Science Foundation (13ZR1426000). We are indebted to the
staff of the Department of Nuclear Medicine, Fudan University
Shanghai Cancer Center for their technological support with
micro-SPECT/CT imaging.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Koh WJ, Greer BE, Abu-Rustum NR, Apte SM,
Campos SM, Cho KR, Chu C, Cohn D, Crispens MA, Dorigo O, et al:
Cervical Cancer, Version 2.2015. J Natl Compr Canc Netw.
13:395–404; quiz 404. 2015.PubMed/NCBI
|
|
3
|
Dai G, Levy O and Carrasco N: Cloning and
characterization of the thyroid iodide transporter. Nature.
379:458–460. 1996. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Riesco-Eizaguirre G and Santisteban P: A
perspective view of sodium iodide symporter research and its
clinical implications. Eur J Endocrinol. 155:495–512. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim NW, Piatyszek MA, Prowse KR, Harley
CB, West MD, Ho PL, Coviello GM, Wright WE, Weinrich SL and Shay
JW: Specific association of human telomerase activity with immortal
cells and cancer. Science. 266:2011–2015. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dadachova E, Bouzahzah B, Zuckier LS and
Pestell RG: Rhenium-188 as an alternative to Iodine-131 for
treatment of breast tumors expressing the sodium/iodide symporter
(NIS). Nucl Med Biol. 29:13–18. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang M, Guo R, Shi S, Miao Y, Zhang Y and
Li B: Baculovirus vector-mediated transfer of sodium iodide
symporter and plasminogen kringle 5 genes for tumor radioiodide
therapy. PLoS One. 9:e923262014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
9
|
Guo R, Zhang M, Xi Y, Ma Y, Liang S, Shi
S, Miao Y and Li B: Theranostic studies of human sodium iodide
symporter imaging and therapy using 188Re: A human
glioma study in mice. PLoS One. 9:e1020112014. View Article : Google Scholar
|
|
10
|
Shi S, Zhang M, Guo R, Miao Y, Hu J, Xi Y
and Li B: In vivo molecular imaging and radionuclide (131I) therapy
of human nasopharyngeal carcinoma cells transfected with a
lentivirus expressing sodium iodide symporter. PLoS One.
10:e01165312015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yin HY, Zhou X, Wu HF, Li B and Zhang YF:
Baculovirus vector-mediated transfer of NIS gene into colon tumor
cells for radionuclide therapy. World J Gastroenterol.
16:5367–5374. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Guo R, Zhang Y, Liang S, Xu H, Zhang M and
Li B: Sodium butyrate enhances the expression of
baculovirus-mediated sodium/iodide symporter gene in A549 lung
adenocarcinoma cells. Nucl Med Commun. 31:916–921. 2010.PubMed/NCBI
|
|
13
|
Koga S, Hirohata S, Kondo Y, Komata T,
Takakura M, Inoue M, Kyo S and Kondo S: A novel telomerase-specific
gene therapy: Gene transfer of caspase-8 utilizing the human
telomerase catalytic subunit gene promoter. Hum Gene Ther.
11:1397–1406. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gu J, Andreeff M, Roth JA and Fang B:
hTERT promoter induces tumor-specific Bax gene expression and cell
killing in syngenic mouse tumor model and prevents systemic
toxicity. Gene Ther. 9:30–37. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fujiwara T, Urata Y and Tanaka N:
Telomerase-specific oncolytic virotherapy for human cancer with the
hTERT promoter. Curr Cancer Drug Targets. 7:191–201. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yu B, Zhang Y, Zhan Y, Zha X, Wu Y, Zhang
X, Dong Q, Kong W and Yu X: Co-expression of herpes simplex virus
thymidine kinase and Escherichia coli nitroreductase by an
hTERT-driven adenovirus vector in breast cancer cells results in
additive antitumor effects. Oncol Rep. 26:255–264. 2011.PubMed/NCBI
|
|
17
|
Haberkorn U, Kinscherf R, Kissel M, Kübler
W, Mahmut M, Sieger S, Eisenhut M, Peschke P and Altmann A:
Enhanced iodide transport after transfer of the human sodium iodide
symporter gene is associated with lack of retention and low
absorbed dose. Gene Ther. 10:774–780. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kang JH, Chung JK, Lee YJ, Shin JH, Jeong
JM, Lee DS and Lee MC: Establishment of a human hepatocellular
carcinoma cell line highly expressing sodium iodide symporter for
radionuclide gene therapy. J Nucl Med. 45:1571–1576.
2004.PubMed/NCBI
|
|
19
|
Shen DH, Marsee DK, Schaap J, Yang W, Cho
JY, Hinkle G, Nagaraja HN, Kloos RT, Barth RF and Jhiang SM:
Effects of dose, intervention time, and radionuclide on sodium
iodide symporter (NIS)-targeted radionuclide therapy. Gene Ther.
11:161–169. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kohn DB and Candotti F: Gene therapy
fulfilling its promise. N Engl J Med. 360:518–521. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vigna E and Naldini L: Lentiviral vectors:
Excellent tools for experimental gene transfer and promising
candidates for gene therapy. J Gene Med. 2:308–316. 2000.
View Article : Google Scholar : PubMed/NCBI
|