Introduction
Prostate cancer is one of the most common
malignancies in men, representing the second leading cause of
cancer-related mortality (1). In
spite of current advances in cancer therapies, prostate cancer
remains an intractable disease because of its malignant
proliferation, high metastatic feature, and frequent relapses
(2). However, the mechanism of
prostate cancer tumorigenesis is still not fully understood.
Therefore, there is an imperative need to explore new and potential
therapeutic targets associated with prostate cancer malignant
proliferation and high metastasis.
MicroRNAs (miRNAs) are a family of endogenous, small
non-coding RNAs that participate in various cellular processes
(3). miRNAs function through
targeting the 3′-untranslated region (UTR) of target messenger RNA
(mRNA) that negatively regulates gene expression (4,5).
Increasing evidence reveals that a variety of miRNAs are
dysregulated in numerous types of cancers, functioning as either
oncogenes or tumor suppressors (6,7).
miRNAs have been suggested to exhibit potential value in cancer
diagnosis, prognosis, and treatment (3,8).
miRNAs perform important functions in the pathogenesis of prostate
cancer (9). However, the precise
role of miRNAs in prostate cancer remains largely unknown.
Chicken ovalbumin upstream promoter transcription
factor II (COUP-TFII), also known as nuclear receptor 2 family 2,
is an important transcriptional factor that belongs to the
steroid/thyroid hormone receptor superfamily (10). COUP-TFII performs a critical
function in regulating various biological processes, including
angiogenesis, organ genesis, neuronal development, cardiovascular
development, and metabolic homeostasis (11–14).
Thus, the dysregulated COUP-TFII contributes to numerous
pathological processes, especially cancer (15,16).
Increasing number of studies has demonstrated that COUP-TFII is
overexpressed in numerous types of cancers, including lung
(17), colorectal (18), pancreatic (19), and prostate cancers (20). COUP-TFII performs an important
function in regulating cancer microenvironment, cell growth and
proliferation, metastasis, and drug resistance (16,21).
These findings highlight that COUP-TFII is a potential molecular
target for anticancer interventions.
Recent studies have reported that miR-382 may
function as a tumor suppressor in colorectal cancer (22), ovarian cancer (23), esophageal squamous cell carcinoma
(24), and osteosarcoma (25). However, whether miR-382 functions in
prostate cancer remains unknown. In this study, we aimed at
investigating the potential function of miR-382 in prostate cancer.
We showed that the expression of miR-382 was significantly
downregulated in prostate cancer tissues and cell lines. The
overexpression of miR-382 markedly inhibited prostate cancer cell
proliferation, migration, and invasion. Furthermore, we predicted
the targets of miR-382 by bioinformatic algorithms and verified
that COUP-TFII represented a functional target gene in prostate
cancer. This study demonstrates that the interference of COUP-TFII
expression by miR-382 is a promising therapeutic strategy for
prostate cancer treatment.
Materials and methods
Clinical specimens
Fifteen paired prostate malignant cancer tissues and
adjacent normal prostate tissues were obtained from prostate cancer
patients who underwent resection operation in Xi'an Tangdu
Hospital. Written informed consent was obtained from the patients,
and the study was approved by Institutional Human Experiment and
Ethics Committee of Xi'an Tangdu Hospital.
Cell lines and cell culture
Human prostate cancer cell lines (LNCaP, PC-3,
22RV-1 and DU-145), normal prostate epithelial cells (RWPE-1), and
293T cells were purchased from American Type Culture Collection
(Manassas, VA, USA). Prostate cancer cell lines and 293T cells were
grown in RPMI-1640 and Dulbecco's modified Eagle's medium,
respectively, all supplemented with 10% fetal bovine serum (FBS),
100 mg/ml streptomycin and 100 U/ml penicillin (all reagents from
Gibco, Rockville, MD, USA). RWPE-1 cells were grown in keratinocyte
serum-free media supplemented with 5 ng/ml human recombinant
epidermal growth factor, 0.05 mg/ml bovine pituitary extract, 100
mg/ml streptomycin, and 100 U/ml penicillin (all reagents obtained
from Gibco). All cells were cultured in a humidified incubator
containing 5% CO2 at 37°C.
Cell transfection
The miR-382 mimics, antisense nucleotides of miR-382
(anti-miR-382), and control oligonucleotides (Ctrl.Oligo) were
purchased from Life Technologies (Carlsbad, CA, USA). Prostate
cancer cells were transiently transfected with 50 nM miRNA using
Lipofectamine 2000 (Life Technologies) according to the
manufacturer's instructions. After incubated for 48 h, transfection
efficiency was detected by real-time quantitative polymerase chain
reaction (RT-qPCR) analysis.
RT-qPCR
Total RNA was extracted using TRIzol reagent (Life
Technologies) according to the recommended protocols of the
manufacturer. A total of 500 ng total RNA was used for the reverse
transcription of cDNA and miRNA by M-MLV reverse transcriptase
(Clontech, Palo Alto, CA, USA) and miScript Reverse Transcription
kit (Qiagen, Dusseldorf, Germany), respectively. The mRNA and miRNA
transcripts were quantified by SYBR Premix Ex Taq GC kit (Takara,
Dalian, China) on an ABI7500 real-time PCR detection system
(Applied Biosystems, Carlsbad, CA, USA). Glyceraldehyde-3-phosphate
dehydrogenase (GAPDH) or U6 small nuclear RNA was used as the
internal reference. The relative gene expression was normalized
against internal reference gene expression by 2−∆∆Ct
method. Fold changes in gene expression were obtained by
normalization with the control group.
3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
Cells were plated in 96-well plates at a density of
1×104 cells/well and cultured for 24 h. Cells were
transfected with miR-382 mimics or anti-miR-382 for 24, 48 and 72
h. Then, 20 µl of MTT solution (5 mg/ml; Sigma, St. Louis, MO, USA)
was added to each well and incubated for 4 h. The medium was
discarded, and the formazan crystal was dissolved by adding 200 µl
dimethyl sulfoxide (Sigma) per well. The absorbance at a wavelength
of 490 nm was detected by a microplate spectrophotometer (Bio-Tek
Instruments, Winooski, VT, USA).
Colony formation assay
Cells were seeded into 6-well plates and transfected
with miR-382 mimics or anti-miR-382. After 48 h, the transfected
cells (200 cells/well) were cultured in growth medium containing
0.3% noble agar to allow formation of natural colonies. After 12
days, the plates were stained with crystal violet (Sigma) in 70%
ethanol. The number of stained colonies was counted under a
microscope (Olympus, Tokyo, Japan).
Cell migration assay
Cell migration assay was performed using a
24-Transwell plate (Corning Inc., Corning, Toledo, NY, USA). In
brief, cells were transfected with miR-382 mimics or anti-miR-382
for 48 h, and then starved overnight in serum-free medium. Then,
200 µl serum-free medium containing 1×104 transfected
cells were seeded into the upper chamber, and 500 µl growth medium
containing 10% FBS was added in the lower chamber. After incubation
for 20 h at 37°C, cells that had migrated to the lower filter side
were stained with 0.1% crystal violet (Sigma). The number of
migrated cells was counted under a microscope (Olympus).
Cell invasion assay
For the detection of cell invasion ability, the
undersurface of the Transwell filter was precoated with Matrigel
(BD Bioscience, San Jose, CA, USA). The transfected cells
(1×104 cells) in 200 µl of serum-free medium were added
to the upper chamber, and 500 µl of growth medium containing 10%
FBS was added in the lower chamber as a chemoattractant. After
incubation for 24 h at 37°C, the invaded cells on in the lower
filter side were stained with 0.1% crystal violet (Sigma) and
counted under the microscope (Olympus).
Dual-luciferase reporter assay
The 3′-UTR of COUP-TFII matching miR-382 sequences
were cloned into pmirGLO dual-luciferase vector (Promega, Madison,
WI, USA) downstream of the luciferase open reading frame. The
mutant 3′-UTR of COUP-TFII containing the mutation of miR-382
recognition sites was synthesized using a Site-Directed Mutagenesis
kit (Agilent Technologies, Santa Clara, CA, USA) and cloned into
the pmirGLO dual-luciferase vector (Promega). The 293T cells were
cotransfected with miR-382 mimics and the pmirGLO vector.
Luciferase assay was performed using a dual-Glo luciferase assay
system (Promega).
Western blot analysis
Equal amounts of proteins from different samples
were subjected to sodium dodecyl sulfate polyacrylamide gel
electrophoresis. The separated proteins were then transferred to a
polyvinylidene difluoride membrane (Millipore, Boston, MA, USA).
The membrane was blocked by 3% non-fat milk in Tris-buffered saline
(TBS) for 1 h at 37°C. Then, the membrane was incubated with
primary antibodies in TBS at 4°C overnight. After three washes with
TBS containing 0.1% Tween-20, the membrane was incubated with
horseradish peroxidase-conjugated secondary antibodies (1:2,000;
Santa Cruz Biotechnology, Santa Cruz, CA, USA) at room temperature
for 4 h. Protein bands were detected by chemiluminescence (Pierce,
Woburn, MA, USA). Protein expression was quantified using Image-Pro
Plus 6.0 software (Media Cybernetics, Inc., Rockville, MD, USA)
normalized against internal control (GAPDH). Anti-COUP-TFII
(ab42672; 1:250) was purchased from Abcam (Cambridge, UK).
Anti-Snail (sc-28199; 1:200), anti-MMP2 (sc-10736; 1:200), and
anti-GAPDH (sc-25778; 1:500) were obtained from Santa Cruz
Biotechnology.
Statistical analysis
All numerical data were expressed as mean ± standard
deviation. Statistical analyses were performed using the commercial
statistical software, SPSS version 11.5 (SPSS Inc., Chicago, IL,
USA). Differences between two groups were analyzed by a paired
two-tailed Student's t-test. Data of more than two groups were
analyzed using one-way analysis of variance followed by a
Bonferroni post-analysis. p<0.05 was considered statistically
significant.
Results
Expression of miR-382 is decreased in
prostate cancer
To investigate the potential function of miR-382 in
prostate cancer, we examined the expression in prostate cancer
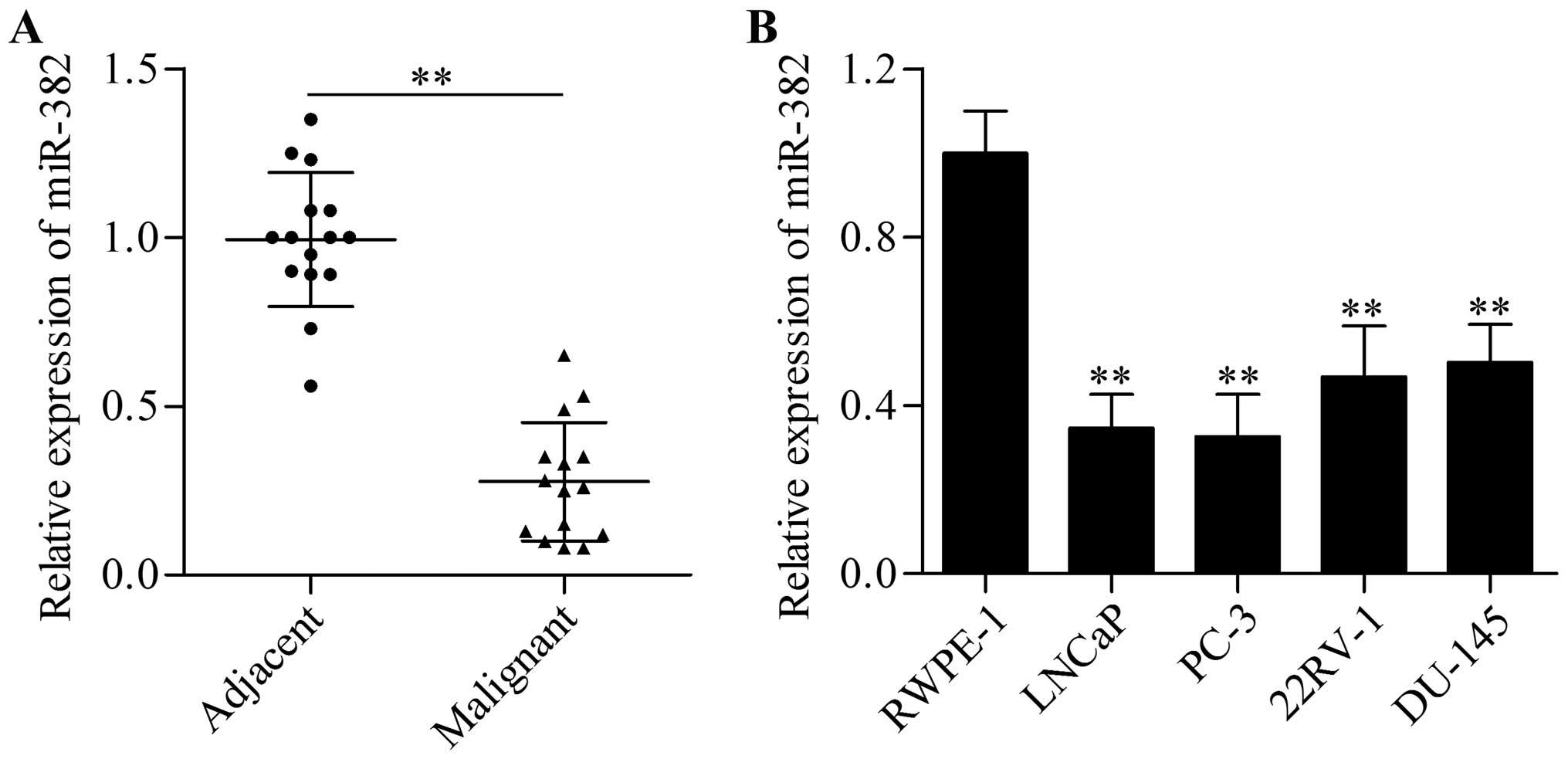
clinical specimens by RT-qPCR. Interestingly, our data showed that
miR-382 was significantly decreased in prostate cancer tissues
compared with adjacent normal prostate tissues (Fig. 1A). In addition, we found that the
expression of miR-282 was markedly decreased in human prostate
cancer cell lines compared with normal prostate RWPE-1 cells
(Fig. 1B). Taken together, our data
suggest that miR-382 may perform an important function in the
development and progression of prostate cancer.
miR-382 inhibits prostate cancer cell
proliferation
To investigate the function of miR-382 in prostate
cancer, we performed gain-of-function and loss-of-function
experiments by transfection of miR-382 mimics and anti-miR-382 in
prostate cancer cell lines, respectively, and detected their effect
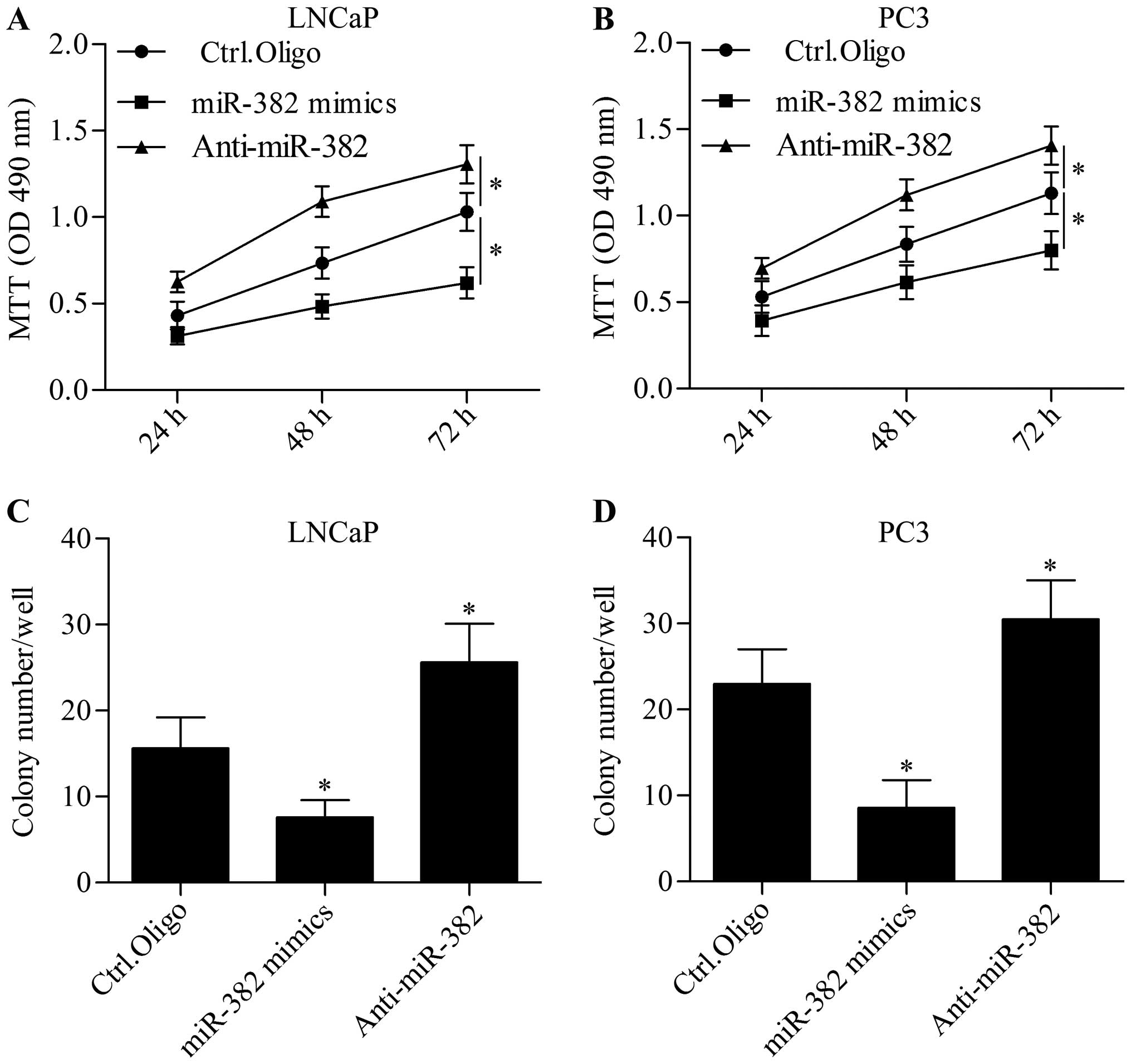
on cell proliferation. MTT assay showed that the overexpression of
miR-382 significantly inhibited prostate cancer cell proliferation,
but the inhibition of miR-382 significantly increased prostate
cancer cell proliferation (Fig. 2A and
B). Furthermore, the colony-forming capacity of prostate cancer
cells was markedly suppressed by miR-382 overexpression whereas
suppression of miR-382 showed an opposite effect (Fig. 2C and D). These results indicate that
miR-382 performs an antitumor function in prostate cancer.
miR-382 inhibits prostate cancer cell
metastasis in vitro
To further evaluate the biological function of
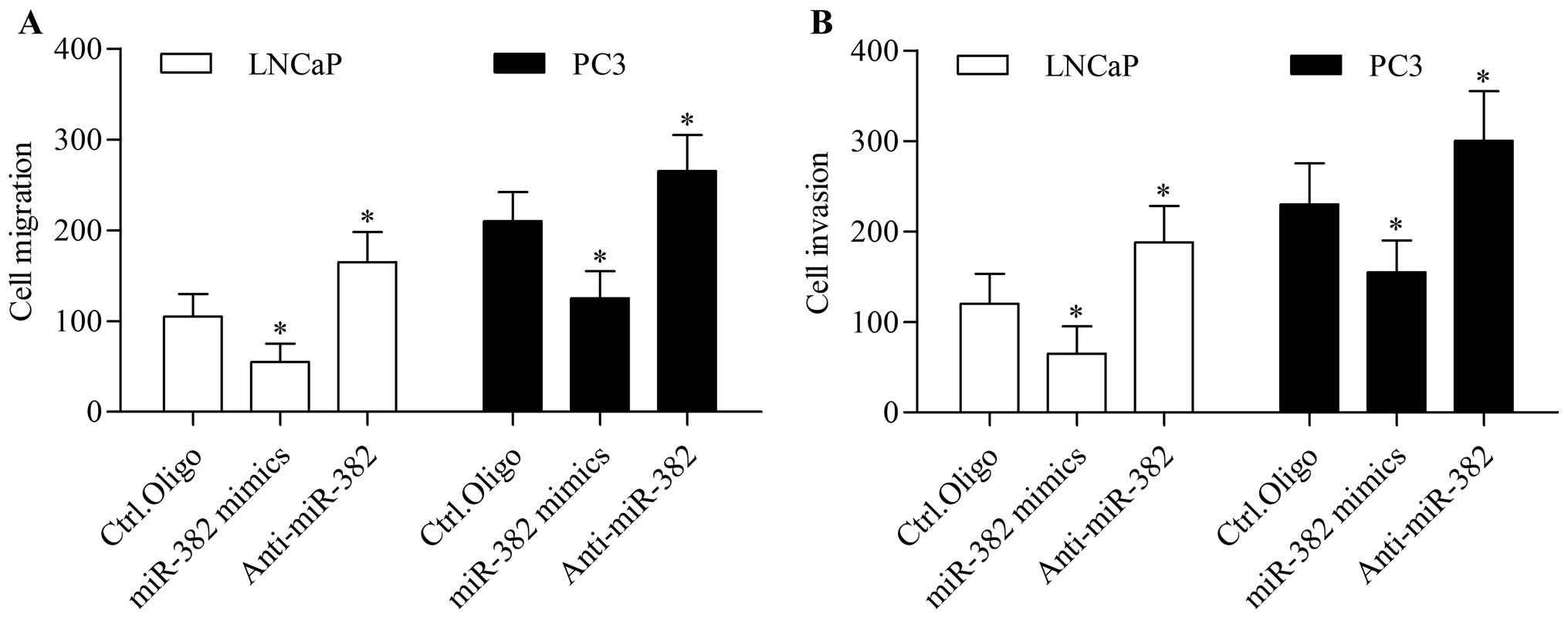
miR-382 in prostate cancer, we detected the function of miR-382 in
regulating prostate cancer cell migration and invasion. The results
showed that the overexpression of miR-382 significantly inhibited
LNCaP and PC3 cell migration (Fig.
3A) and invasion (Fig. 3B)
in vitro. Conversely, the inhibition of miR-382 markedly
promoted the migration and invasion of LNCaP and PC3 cells
(Fig. 3). These data imply that
miR-382 performs an anti-metastatic function in prostate
cancer.
miR-382 directly targets COUP-TFII in
prostate cancer cells
To understand the underlying mechanism of miR-382 in
exerting its function in regulating cell proliferation and
metastasis of prostate cancer, we used algorithms to predict mRNA
targets of miR-382 and identified COUP-TFII as a putative target
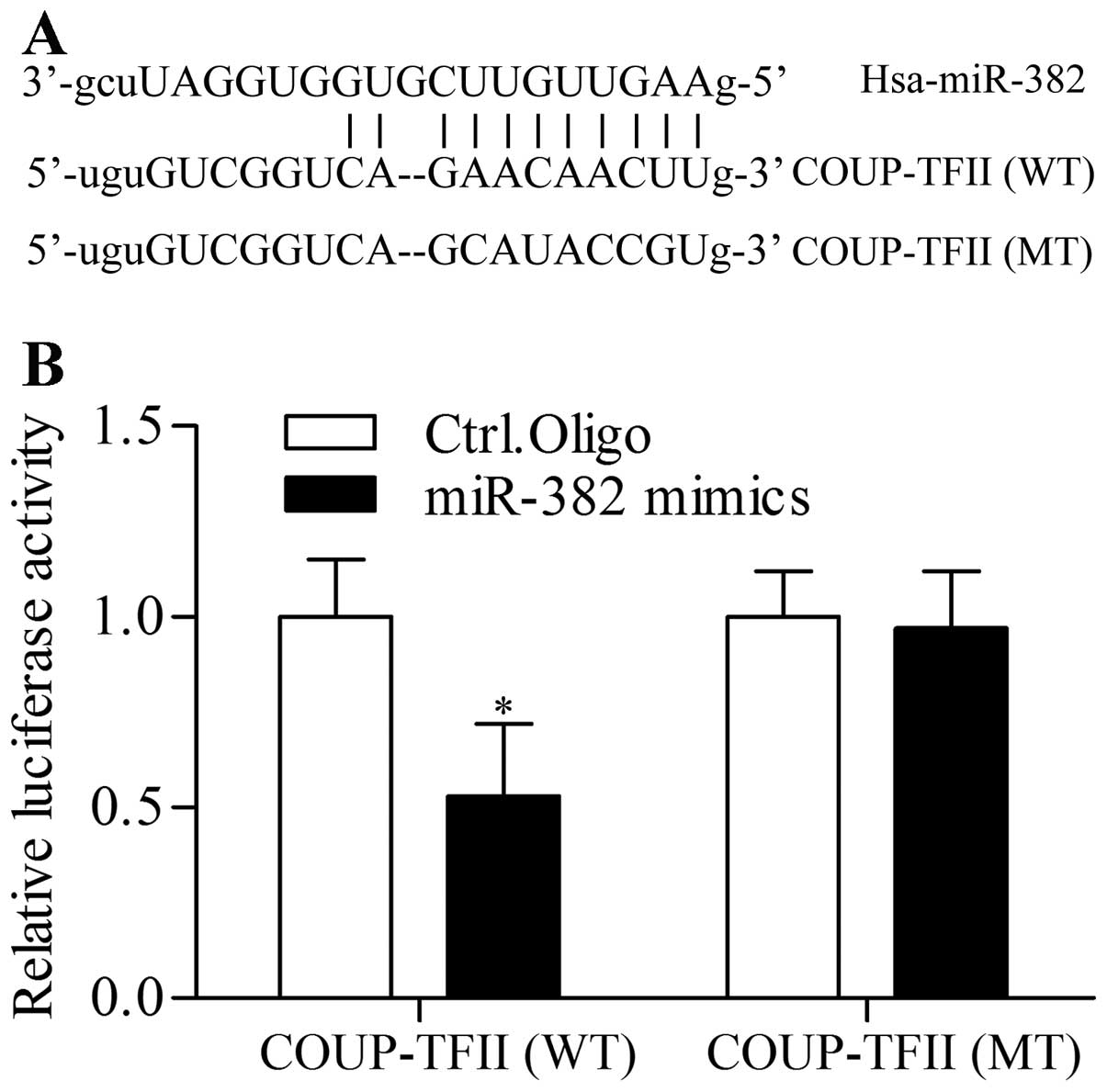
gene of miR-382 (Fig. 4A). To
confirm the direct binding between miR-382 and the 3′-UTR of
COUP-TFII, we performed dual-luciferase reporter assay. The 3′-UTR
of COUP-TFII containing complementary sequences to miR-382 seed
sequences was cloned into a reporter vector. Cotransfection of the
wild-type (WT) COUP-TFII 3′-UTR construct with miR-382 mimics into
293T cells resulted in a significant decrease in luciferase
activity compared with control (Fig.
4B). However, mutation of the predicated matching sites in the
3′-UTR of COUP-TFII (MT) significantly abrogated the suppressive
effect of miR-382 mimics on luciferase activity (Fig. 4B). This result indicates that
miR-382 directly targets the 3′-UTR of COUP-TFII. To assess whether
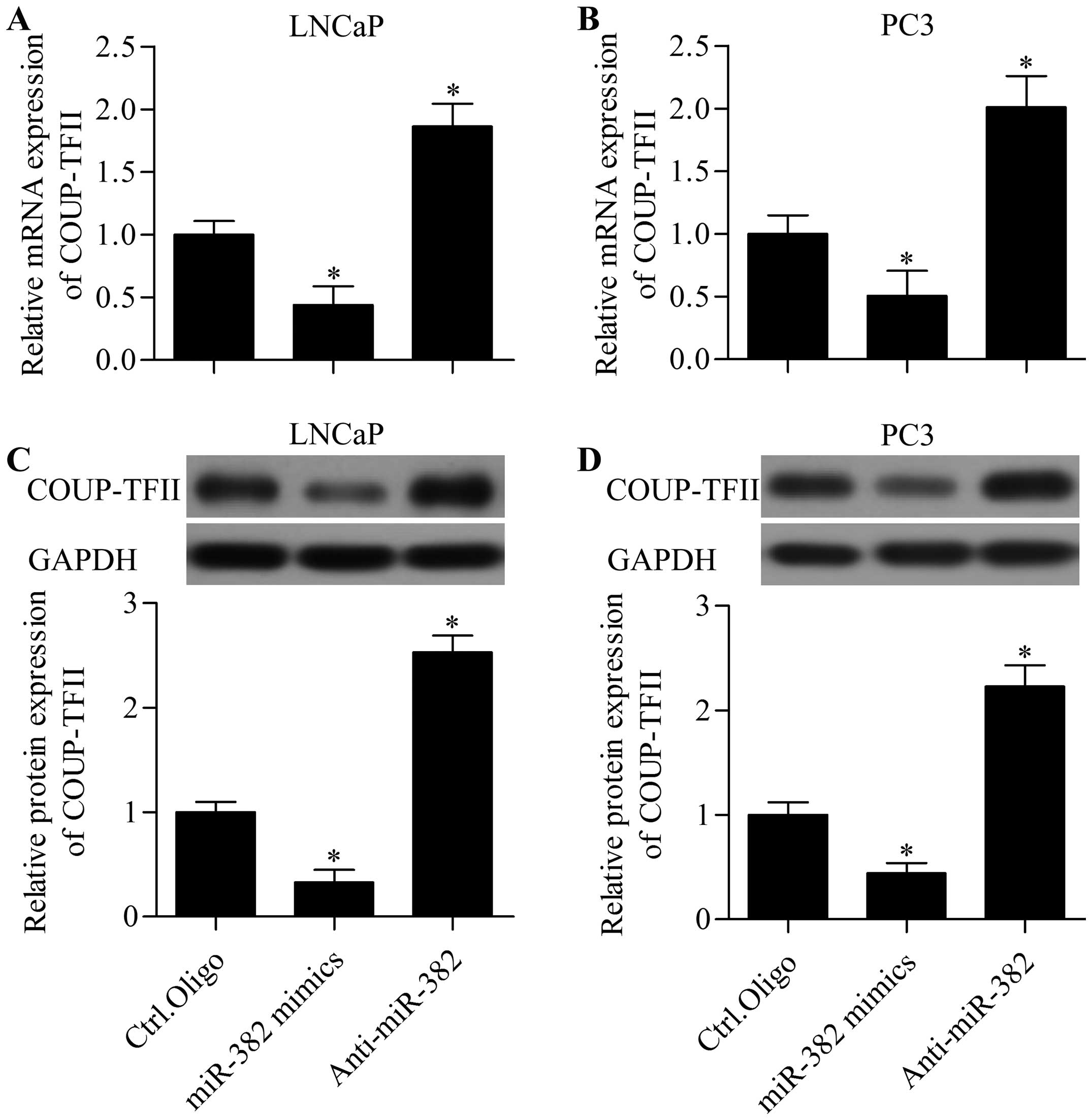
miR-382 regulates COUP-TFII expression, we detected the effect of
miR-382 mimics or anti-miR-382 on COUP-TFII expression by RT-qPCR
and western blot analysis in LNCaP and PC3 cells. RT-qPCR analysis
showed that miR-382 overexpression significantly inhibited the mRNA
expression level of COUP-TFII in LNCaP (Fig. 5A) and PC3 (Fig. 5B) cells. Furthermore, western blot
analysis showed that miR-382 overexpression markedly suppressed the
protein expression of COUP-TFII in LNCaP (Fig. 5C) and PC3 (Fig. 5D) cells. In contrast, miR-382
suppression significantly upregulated the mRNA and protein
expression of COUP-TFII (Fig. 5).
Taken together, these results suggest that miR-382 directly targets
the 3′-UTR of COUP-TFII and regulates COUP-TFII expression in
prostate cancer cells.
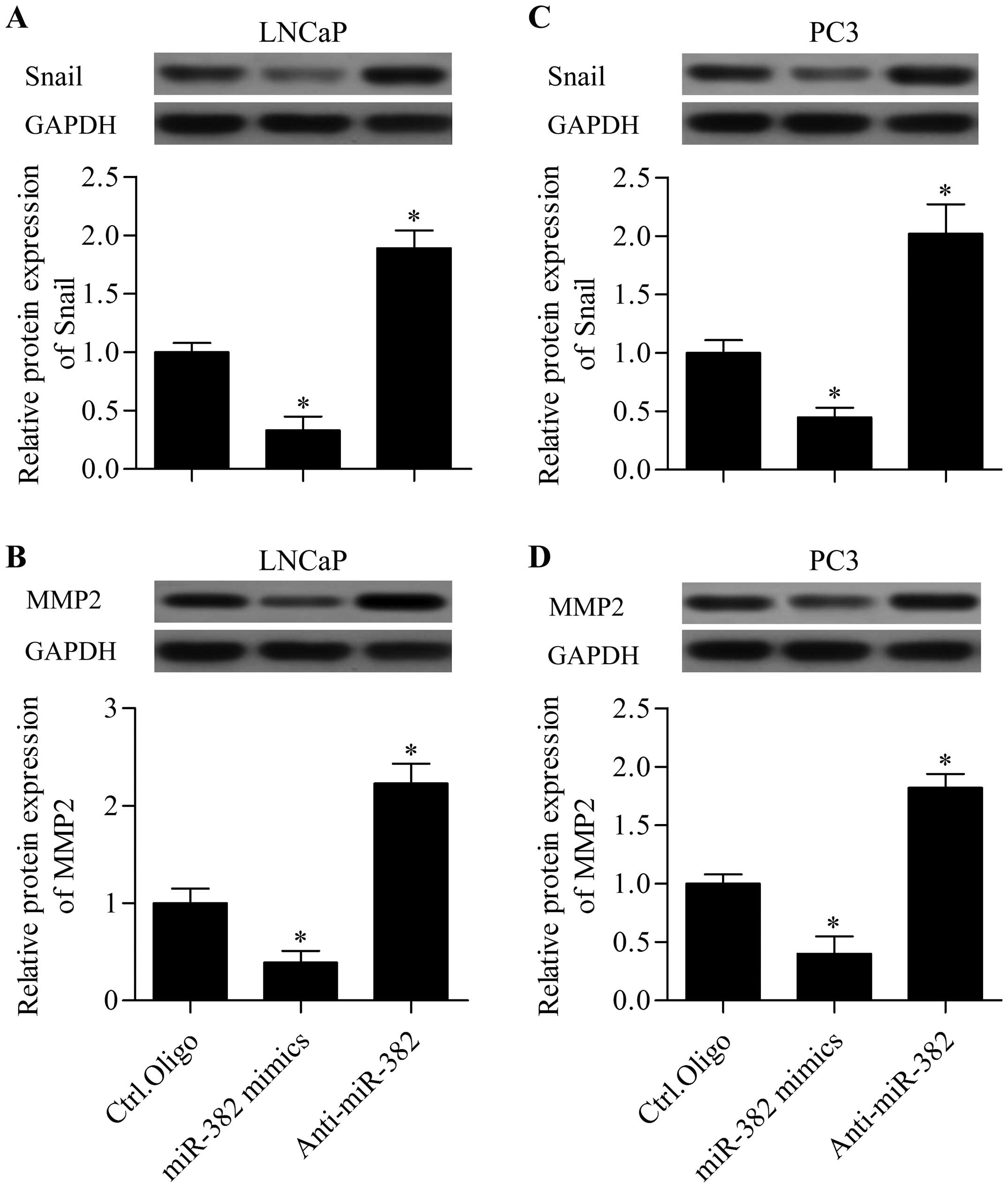
miR-382 inhibits Snail and MMP2
expression
To further understand the molecular basis of miR-382
in regulating prostate cancer, we detected the regulatory effect of
miR-382 on COUP-TFII downstream genes, including Snail and MMP2.
Our data showed that miR-382 overexpression significantly inhibited
the protein expression of Snail (Fig.
6A) and MMP2 (Fig. 6B) in LNCaP
cells transfected with miR-382 mimics. Similarly, miR-382
overexpression markedly suppressed the protein expression of Snail
(Fig. 6C) and MMP2 (Fig. 6D) in PC3 cells. However, miR-382
suppression exhibited opposite effect. These data indicate that
miR-382 regulates Snail and MMP2 expression.
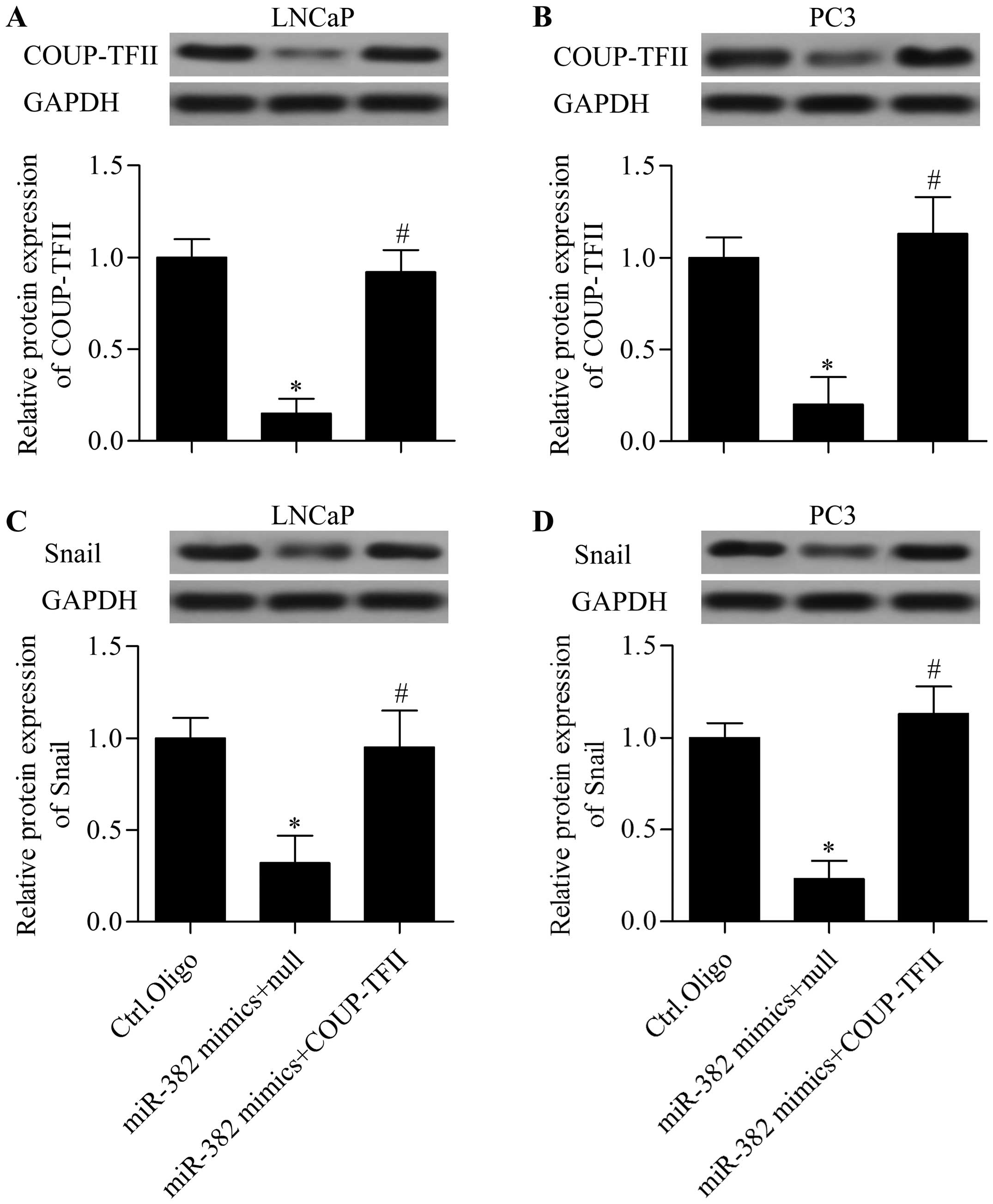
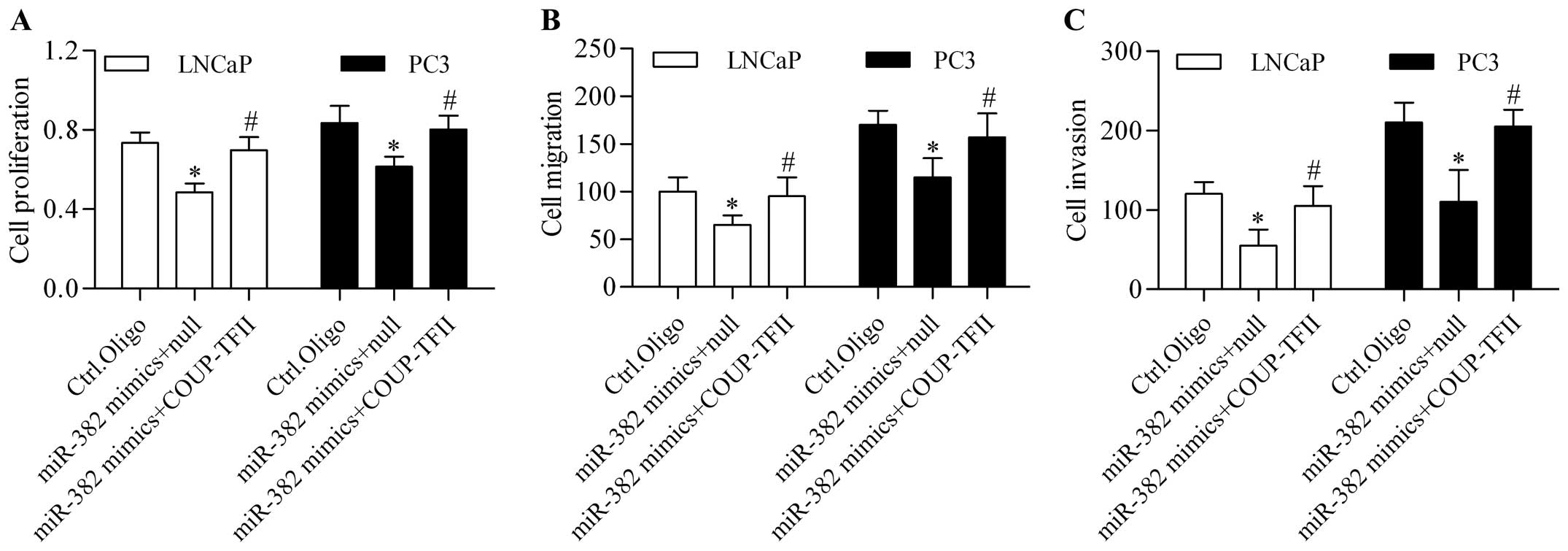
miR-382 functions via COUP-TFII
To verify whether miR-382 functions via COUP-TFII in
prostate cancer, we detected the restoration of COUP-TFII on the
suppressive effect of miR-382 by rescue experiments. We
cotransfected miR-382 mimics with COUP-TFII expressing vector
harboring no 3′-UTR into LNCaP and PC3. Results showed that
COUP-TFII expressing vector transfection significantly restored the
decreased COUP-TFII expression induced by miR-382 mimics (Fig. 7A and B). Furthermore, the decreased
Snail expression induced by miR-382 overexpression was also
significantly reversed by the restoration of COUP-TFII (Fig. 7C and D). In addition, the inhibitory
effect of miR-382 on prostate cancer proliferation (Fig. 8A), migration (Fig. 8B), and invasion (Fig. 8C) was significantly reversed by the
restoration of COUP-TFII. Taken together, these results imply that
miR-382 represses prostate cancer proliferation and metastasis via
COUP-TFII.
Discussion
We investigated the function of miR-382 in prostate
cancer, and showed that miR-382 expression was significantly
decreased in specimens from prostate cancer patients, as well as in
prostate cancer cell lines. Interestingly, the overexpression of
miR-382 inhibited prostate cancer cell proliferation, migration,
and invasion, implying a tumor suppressor function of miR-382.
Further results revealed that COUP-TFII was verified as a target
gene of miR-382, through which miR-382 regulated prostate cancer
cell proliferation and metastasis. These results suggest an
important function of miR-382 in prostate cancer.
A recent study demonstrates that the decreased
miR-382 is associated with poor outcome in esophageal squamous cell
cancer patients (24). In primary
ovarian tumors, miR-382 is significantly decreased and may function
through targeting the oncogene kinesin family member 14 (26). Tan and colleagues found that miR-382
was downregulated in human ovarian cancer tissues and cell lines;
moreover, they found that miR-382 could inhibit proliferation,
epithelial-mesenchymal transition, migration, and invasion of
ovarian cancer cells (23). Xu
et al reported that miR-382 is decreased in highly
metastatic osteosarcoma cell lines and that the overexpression of
miR-382 suppressed proliferation, epithelial-mesenchymal
transition, and metastasis and enhanced chemo-sensitivity in
osteosarcoma through targeting Y box-binding protein 1,
Krüppel-like factor 12, and homeodomain interacting protein kinase
3 (25,27). miR-382 is found to be decreased in
aggressive primary melanoma and miR-382 overexpression inhibits
tumor cell metastasis in vitro and in vivo through
targeting cortactin (28).
Consistent with these findings, we found that miR-382 was
significantly decreased in prostate cancer tissues and cell lines.
The overexpression of miR-382 inhibited prostate cancer cell
proliferation, migration, and invasion, indicating a tumor
suppressor function of miR-382. Further mechanistic study revealed
that miR-382 functioned through targeting COUP-TFII, which
regulated cancer cell proliferation and metastasis. Interestingly,
a more recent study reported that, in line with our findings,
miR-382 suppresses colorectal cancer growth and invasion through
targeting COUP-TFII (22). Although
these reports and our study support the notion that miR-382
functions as a tumor suppressor, the contrary function of miR-382
is also reported. Seok et al reported that miR-382 induced
by hypoxia promoted tumor growth and angiogenesis by inhibiting
tumor suppressor phosphatase and tensin homolog (PTEN) in gastric
cancer, supporting an oncogenic function of miR-382 (29). Similarly, Bei et al
demonstrated that miR-382 promotes hepatocyte proliferation and
cell growth by targeting PTEN signaling (30). These differences indicate that
miR-382 may exert its function in a context-dependent manner among
various cancer types.
COUP-TFII has been suggested as an important
regulator in cancers, especially in prostate cancer (16). Increased expression of COUP-TFII is
found in breast, ovarian, and colon cancers (31–33).
High expression of COUP-TFII, which is found in prostate cancer, is
associated with earlier tumor recurrences and decreased survival
after prostatectomy (20,34). COUP-TFII deficiency in PTEN-null
mice prevents prostate cancer progression and cancer cell
metastasis associated with TGF-β/SMAD4-induced growth barrier
(20). COUP-TFII regulates a series
of downstream genes that is associated with cell proliferation,
cell cycle, and metastasis (18,33).
Particularly, COUP-TFII can activate the transcription of the
Snail, a key regulator of tumor metastasis (35), through which COUP-TFII are related
to carcinogenesis and metastasis (18). Otherwise, the tumor suppressor
function of COUP-TFII is also proposed (36–38).
In this study, we have demonstrated that miR-382 could regulate
Snail through COUP-TFII, explaining the underlying mechanism of
miR-382 in regulating prostate cancer cell proliferation and
metastasis and supporting the tumor-promoter function of COUP-TFII.
A recent study reveals that miR-101 and miR-27a inhibit the
metastasis of prostate cancer by inhibiting COUP-TFII (21). In line with our study, the previous
study elucidated that specific miRNAs contribute to prostate cancer
progression and metastasis through the regulation of the
COUP-TFII.
miRNAs represent the novel therapeutics for cancer.
Besides miR-382 (22), miR-101, and
miR-27a (21), other miRNAs can
target COUP-TFII. miR-302 regulates stem cell differentiation
through directly targeting COUP-TFII (39,40).
Kang et al reported that miR-302a promotes osteoblastic
differentiation by repressing COUP-TFII (41). Similarly, miR-194 is reported to
promote osteogenesis and inhibit adipogenesis by regulating
COUP-TFII (42). Taken together,
these reports offer a possibility of targeting COUP-TFII by
specific miRNAs to treat diseases.
In conclusion, we show that miR-382 expression is
decreased in prostate cancer. The overexpression of miR-382
significantly suppresses prostate cancer cell proliferation,
migration, and invasion directly through targeting COUP-TFII. Thus,
miR-382 may function as a tumor suppressor in prostate cancer. Our
study suggests that miR-382 and COUP-TFII are potential and
promising molecular targets for prostate cancer therapy.
Glossary
Abbreviations
Abbreviations:
|
miRNAs
|
microRNAs
|
|
COUP-TFII
|
chicken ovalbumin upstream promoter
transcription factor II
|
|
UTR
|
untranslated region
|
|
MMP2
|
matrix metalloproteinase 2
|
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Coleman RE: Clinical features of
metastatic bone disease and risk of skeletal morbidity. Clin Cancer
Res. 12:S6243–S6249. 2006. View Article : Google Scholar
|
|
3
|
Mendell JT and Olson EN: MicroRNAs in
stress signaling and human disease. Cell. 148:1172–1187. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Winter J, Jung S, Keller S, Gregory RI and
Diederichs S: Many roads to maturity: microRNA biogenesis pathways
and their regulation. Nat Cell Biol. 11:228–234. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Esquela-Kerscher A and Slack FJ: Oncomirs
- microRNAs with a role in cancer. Nat Rev Cancer. 6:259–269. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cho WC: OncomiRs: The discovery and
progress of microRNAs in cancers. Mol Cancer. 6:602007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ranganathan K and Sivasankar V: MicroRNAs
- Biology and clinical applications. J Oral Maxillofac Pathol.
18:229–234. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang YL, Wu S, Jiang B, Yin FF, Zheng SS
and Hou SC: Role of microRNAs in prostate cancer pathogenesis. Clin
Genitourin Cancer. 13:261–270. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang LH, Tsai SY, Cook RG, Beattie WG,
Tsai MJ and O'Malley BW: COUP transcription factor is a member of
the steroid receptor superfamily. Nature. 340:163–166. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tang K, Rubenstein JL, Tsai SY and Tsai
MJ: COUP-TFII controls amygdala patterning by regulating neuropilin
expression. Development. 139:1630–1639. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li L, Xie X, Qin J, Jeha GS, Saha PK, Yan
J, Haueter CM, Chan L, Tsai SY and Tsai MJ: The nuclear orphan
receptor COUP-TFII plays an essential role in adipogenesis, glucose
homeostasis, and energy metabolism. Cell Metab. 9:77–87. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
You LR, Lin FJ, Lee CT, DeMayo FJ, Tsai MJ
and Tsai SY: Suppression of Notch signalling by the COUP-TFII
transcription factor regulates vein identity. Nature. 435:98–104.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yu CT, Tang K, Suh JM, Jiang R, Tsai SY
and Tsai MJ: COUP-TFII is essential for metanephric mesenchyme
formation and kidney precursor cell survival. Development.
139:2330–2339. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Qin J, Chen X, Xie X, Tsai MJ and Tsai SY:
COUP-TFII regulates tumor growth and metastasis by modulating tumor
angiogenesis. Proc Natl Acad Sci USA. 107:3687–3692. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Qin J, Tsai SY and Tsai MJ: The critical
roles of COUP-TFII in tumor progression and metastasis. Cell
Biosci. 4:582014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Navab R, Gonzalez-Santos JM, Johnston MR,
Liu J, Brodt P, Tsao MS and Hu J: Expression of chicken ovalbumin
upstream promoter-transcription factor II enhances invasiveness of
human lung carcinoma cells. Cancer Res. 64:5097–5105. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bao Y, Gu D, Feng W, Sun X, Wang X, Zhang
X, Shi Q, Cui G, Yu H, Tang C, et al: COUP-TFII regulates
metastasis of colorectal adenocarcinoma cells by modulating Snail1.
Br J Cancer. 111:933–943. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Polvani S, Tarocchi M, Tempesti S, Mello
T, Ceni E, Buccoliero F, D'Amico M, Boddi V, Farsi M, Nesi S, et
al: COUP-TFII in pancreatic adenocarcinoma: Clinical implication
for patient survival and tumor progression. Int J Cancer.
134:1648–1658. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Qin J, Wu SP, Creighton CJ, Dai F, Xie X,
Cheng CM, Frolov A, Ayala G, Lin X, Feng XH, et al: COUP-TFII
inhibits TGF-β-induced growth barrier to promote prostate
tumorigenesis. Nature. 493:236–240. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lin SC, Kao CY, Lee HJ, Creighton CJ,
Ittmann MM, Tsai SJ, Tsai SY and Tsai MJ: Dysregulation of
miRNAs-COUP-TFII -FOXM1-CENPF axis contributes to the metastasis of
prostate cancer. Nat Commun. 7:114182016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhou B, Song J, Han T, Huang M, Jiang H,
Qiao H, Shi J and Wang Y: MiR-382 inhibits cell growth and invasion
by targeting NR2F2 in colorectal cancer. Mol Carcinog. Jan
22–2016.(Epub ahead of print). doi: 10.1002/mc.22466. View Article : Google Scholar
|
|
23
|
Tan H, He Q, Gong G, Wang Y, Li J, Wang J,
Zhu D and Wu X: miR-382 inhibits migration and invasion by
targeting ROR1 through regulating EMT in ovarian cancer. Int J
Oncol. 48:181–190. 2016.PubMed/NCBI
|
|
24
|
Qi B, Lu JG, Yao WJ, Chang TM, Qin XG, Ji
YH, Wang TY, Liu SG, Li HC, Liu YZ, et al: Downregulation of
microRNA-382 is associated with poor outcome of esophageal squamous
cell carcinoma. World J Gastroenterol. 21:6884–6891.
2015.PubMed/NCBI
|
|
25
|
Xu M, Jin H, Xu CX, Sun B, Mao Z, Bi WZ
and Wang Y: miR-382 inhibits tumor growth and enhance
chemosensitivity in osteosarcoma. Oncotarget. 5:9472–9483. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Thériault BL, Basavarajappa HD, Lim H,
Pajovic S, Gallie BL and Corson TW: Transcriptional and epigenetic
regulation of KIF14 overexpression in ovarian cancer. PLoS One.
9:e915402014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xu M, Jin H, Xu CX, Sun B, Song ZG, Bi WZ
and Wang Y: miR-382 inhibits osteosarcoma metastasis and relapse by
targeting Y box-binding protein 1. Mol Ther. 23:89–98. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hanniford D, Segura MF, Zhong J, Philips
E, Jirau-Serrano X, Darvishian F, Berman RS, Shapiro RL, Pavlick
AC, Brown B, et al: Identification of metastasis-suppressive
microRNAs in primary melanoma. J Natl Cancer Inst. 107:1072015.
View Article : Google Scholar
|
|
29
|
Seok JK, Lee SH, Kim MJ and Lee YM:
MicroRNA-382 induced by HIF-1α is an angiogenic miR targeting the
tumor suppressor phosphatase and tensin homolog. Nucleic Acids Res.
42:8062–8072. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bei Y, Song Y, Wang F,
Dimitrova-Shumkovska J, Xiang Y, Zhao Y, Liu J, Xiao J and Yang C:
miR-382 targeting PTEN-Akt axis promotes liver regeneration.
Oncotarget. 7:1584–1597. 2016.PubMed/NCBI
|
|
31
|
Nagasaki S, Suzuki T, Miki Y, Akahira J,
Shibata H, Ishida T, Ohuchi N and Sasano H: Chicken ovalbumin
upstream promoter transcription factor II in human breast
carcinoma: Possible regulator of lymphangiogenesis via vascular
endothelial growth factor-C expression. Cancer Sci. 100:639–645.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shin SW, Kwon HC, Rho MS, Choi HJ, Kwak JY
and Park JI: Clinical significance of chicken ovalbumin upstream
promoter-transcription factor II expression in human colorectal
cancer. Oncol Rep. 21:101–106. 2009.PubMed/NCBI
|
|
33
|
Hawkins SM, Loomans HA, Wan YW,
Ghosh-Choudhury T, Coffey D, Xiao W, Liu Z, Sangi-Haghpeykar H and
Anderson ML: Expression and functional pathway analysis of nuclear
receptor NR2F2 in ovarian cancer. J Clin Endocrinol Metab.
98:E1152–E1162. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Qin J, Tsai S and Tsai MJ: COUP-TFII, a
prognostic marker and therapeutic target for prostate cancer. Asian
J Androl. 15:360–361. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kudo-Saito C, Shirako H, Takeuchi T and
Kawakami Y: Cancer metastasis is accelerated through
immunosuppression during Snail-induced EMT of cancer cells. Cancer
Cell. 15:195–206. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Song CH, Lee HJ, Park E and Lee K: The
chicken ovalbumin upstream promoter-transcription factor II
negatively regulates the transactivation of androgen receptor in
prostate cancer cells. PLoS One. 7:e490262012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Litchfield LM, Appana SN, Datta S and
Klinge CM: COUP-TFII inhibits NFkappaB activation in
endocrine-resistant breast cancer cells. Mol Cell Endocrinol.
382:358–367. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nakshatri H, Mendonca MS, Bhat-Nakshatri
P, Patel NM, Goulet RJ Jr and Cornetta K: The orphan receptor
COUP-TFII regulates G2/M progression of breast cancer cells by
modulating the expression/activity of p21(WAF1/CIP1), cyclin D1,
and cdk2. Biochem Biophys Res Commun. 270:1144–1153. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Rosa A and Brivanlou AH: A regulatory
circuitry comprised of miR-302 and the transcription factors OCT4
and NR2F2 regulates human embryonic stem cell differentiation. EMBO
J. 30:237–248. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hu S, Wilson KD, Ghosh Z, Han L, Wang Y,
Lan F, Ransohoff KJ, Burridge P and Wu JC: MicroRNA-302 increases
reprogramming efficiency via repression of NR2F2. Stem Cells.
31:259–268. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kang IH, Jeong BC, Hur SW, Choi H, Choi
SH, Ryu JH, Hwang YC and Koh JT: MicroRNA-302a stimulates
osteoblastic differentiation by repressing COUP-TFII expression. J
Cell Physiol. 230:911–921. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Jeong BC, Kang IH, Hwang YC, Kim SH and
Koh JT: MicroRNA-194 reciprocally stimulates osteogenesis and
inhibits adipogenesis via regulating COUP-TFII expression. Cell
Death Dis. 5:e15322014. View Article : Google Scholar : PubMed/NCBI
|