Introduction
Osteosarcoma is a high-grade malignant tumor
frequently found in children and adolescents. The therapeutic
method has evolved obviously in recent years, from surgery only to
combined therapy of surgery, chemotherapy and radiation, and it
also improved the long-term survival rate from 20 to 70% (1,2).
However, most patients were first diagnosed as advanced and
metastatic osteosarcoma, and their 5-year survival rate was
<20%. New drugs to improve the survival rate are required.
Thalidomide has been reported to possess different
cytotoxic activity towards different tumor cell lines, such as
prostate, colorectal, non-small cell lung cancer, breast cancer,
and renal cell carcinoma (3–9). Tsai
et al (10) reported a
clinical case that a relapse osteosarcoma patient was treated with
a combination of thalidomide and celecoxib, then the tumors in the
lung became smaller 1 month later. Apoptosis plays an important
role in controlling tumorigenesis in many anticancer drugs
(11–13). Unfortunately, very few studies have
been carried on the inhibitory effect of thalidomide on
osteosarcoma and its mechanisms. Therefore, the aim of the present
study was to thoroughly investigate thalidomide-induced apoptosis,
and to explore its potential mechanisms.
Materials and methods
Reagents
Thalidomide was purchased from Sigma. Annexin
V-FITC/PI apoptosis detection kit, DNA content quantitation assay
(cell cycle), reactive oxygen species (ROS) detection kit,
apoptotic cell Hoechst 33258 detection kit were purchased from
KeyGEN (China). Cell Counting Kit-8 (CCK-8) was purchased from
Dojindo Laboratories in Japan. Mitochondrial membrane potential
assay kit with JC-1 was purchased from Beyotime Biotech (China).
DMEM, McCoy's 5A medium, trypsin and fetal bovine serum were
purchased from Gibco (USA). Rabbit anti-caspase-3, anti-Bcl-2,
anti-Bax, anti-NF-κB and anti-GAPDH antibodies were purchased from
Abcam. The secondary antibodies were purchased from Bioworld
Technology, Inc.
Cell culture and treatments
MG-63 and U2OS (osteosarcoma cells) were purchased
from the American Type Culture Collection. The cells were cultured
in DMEM or McCoy's 5A medium supplemented with 10% FBS at 37°C in
5% CO2 and 95% air, respectively. Thalidomide was
dissolved in DMSO, at concentration <0.1%.
Cytotoxicity assay in vitro
CCK-8 assay procedures were used to measure the cell
viability. Cells were seeded in 96-well plates overnight before
drug treatment. Thalidomide was added to the cells at various
concentrations (0, 12.5, 25, 50, 100, 200 and 400 µg/ml), with 5
wells used for each concentration. The plates were incubated at
37°C in a 5% CO2 incubator. After 24, 48 and 72 h, 10 µl
of CCK-8 solution were added to each well at 37°C for 3 h and
followed by a measurement of absorbance at 450 nm using a
microplate reader. The IC50 values were calculated. Each
experiment was repeated at least three times to obtain the mean
values. The two tumor cell lines used in the this study were MG-63
and U2OS.
Cell apoptosis assay
The morphological changes of apoptosis were measured
by Hoechst 33258 (Beyotime Biotech) after the cells were treated
with thalidomide. After 48 h, cells were washed with 1X PBS three
times and stained with 1 µg/ml of Hoechst 33258 nuclear dye for 10
min. Then the cells were observed and imaged by a fluorescence
microscope.
Flow cytometry detecting FITC-Annexin
V-positive apoptotic cells
Flow cytometry analysis was used to detect cell
apoptosis. After drug treatment, cells were collected by
trypsinization, and stained with FITC-Annexin V and propidium
iodide (PI). Both early (Annexin V+/PI−) and
late (Annexin V/PI+) apoptotic cells were sorted by FCM
(FACSCalibur; BD Biosciences).
Flow cytometry analysis on cell cycle
arrest studies
Flow cytometry analysis was used to detect the
distribution of cell cycle. After drug treatment, cells were
collected by trypsinization, and washed twice with ice-cold PBS,
suspended in 70% alcohol, and kept at 4°C overnight. Then cells
were stained with the Cycletest Plus. The cell cycle distribution
was detected with FCM (BD FACSCalibur; BD Biosciences).
Mitochondrial membrane potential (ΔΨm)
assay
The fluorescent dye JC-1 (Beyotime Biotech) was used
to assess ΔΨm. After drug treatment, MG-63 cells in 6-well plates
were collected and loaded with 1 µg/ml JC-1 at 37°C for 20 min in
the dark, and then rinsed twice with PBS. Then cell pellets were
suspended in PBS and ΔΨm was monitored by flow cytometry.
Assay of intracellular ROS
The non-fluorescent probe
2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA) was used to
measure ROS. The probe diffused into cells and reacted with ROS to
form the trapped fluorescent product DCF. After the treatment of
thalidomide for 24 h, MG-63 cells in 96-plate were washed three
times with PBS. DCFH-DA, diluted to a final concentration of 10 µM
with RPMI-1640 medium, was added to cover the cells and incubated
for 20 min at 37°C. The treated cells were then washed with cold
PBS twice, and involved in PBS. The fluorescence intensity was
measured at an excitation wavelength of 488 nm and emission at 525
nm with Thermo Scientific Varioskan Flash. The increase in value
compared to control was viewed as the increase of endocellular
ROS.
Western blot analysis
MG-63 cells were incubated with different
concentrations of thalidomide in the presence of 10% FBS for 48 h.
Total protein was extracted with RIPA and PMSF buffer and
quantified using a bicinchoninic acid (BCA) Protein Assay kit
(Beyotime Biotech). A total of 40 µg of protein was subjected to
10% SDS-PAGE electrophoresis and transferred to a polyvinylidene
difluoride (PVDF) membrane later. The membrane was incubated with
Bax (1:1,000 dilution), Bcl-2 (1:250 dilution), caspase-3 (1:500
dilution), NF-κB (1:1,000 dilution), GAPDH (1:1,000 dilution) (all
from Abcam) at 4°C overnight and incubated with goat anti-rabbit
second antibody (1:5,000 dilution; Bioworld Technology, Inc.) at
room temperature for 1 h. The intensity of the specific
immunoreactive bands was detected by enhanced chemiluminescence
(ECL).
Statistical analysis
All data were analyzed with the statistical software
GraphPad Prism 5.0, and all values are expressed as means ± SD. The
differences between two groups were analyzed using Student's
unpaired t-test, and differences between three or more groups were
evaluated via one-way ANOVA with Bonferroni correction. A
probability value of <0.05 was considered significant.
Results
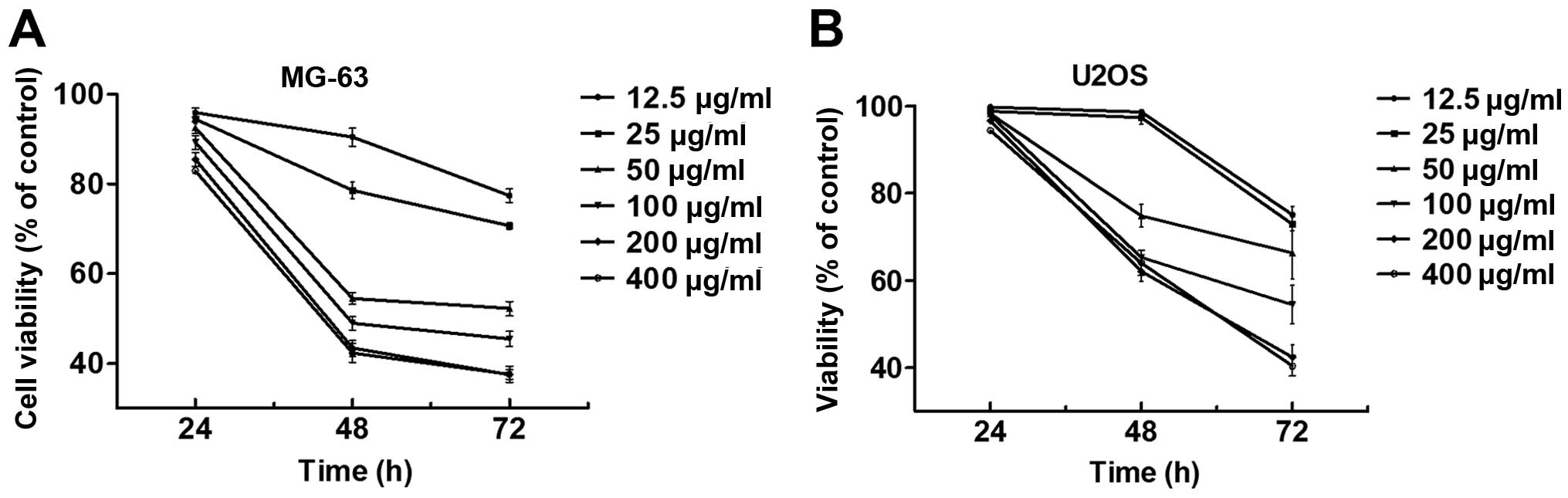
Cytotoxicity assay in vitro
After exposure to the desired concentration ranged
from 12.5 to 400 µg/ml for 24, 48 and 72 h, the cytotoxicity of
thalidomide against MG-63 and U2OS cells was evaluated by cell
viability using the CCK-8 assay. From the results (Fig. 1A and B), the inhibition of
thalidomide to MG-63 and U2OS cells was observed to be time- and
concentration-dependent, which indicates that thalidomide could
effectively inhibit the cell proliferation. The IC50
values are also shown in Table I,
which indicate 151.05±8.09 and 94.76±10.52 µg/ml for 48 and 72 h in
MG-63 cells.
 | Table I.The IC50 (µM) values of
thalidomide on osteosarcoma cells. |
Table I.
The IC50 (µM) values of
thalidomide on osteosarcoma cells.
| Cells | 24 h (µg/ml) | 48 h (µg/ml) | 72 h (µg/ml) |
|---|
| MG-63 | >500 | 151.05±8.09 | 94.76±10.52 |
| U2OS | >500 | 476.13±93.3l | 156.61±40.65 |
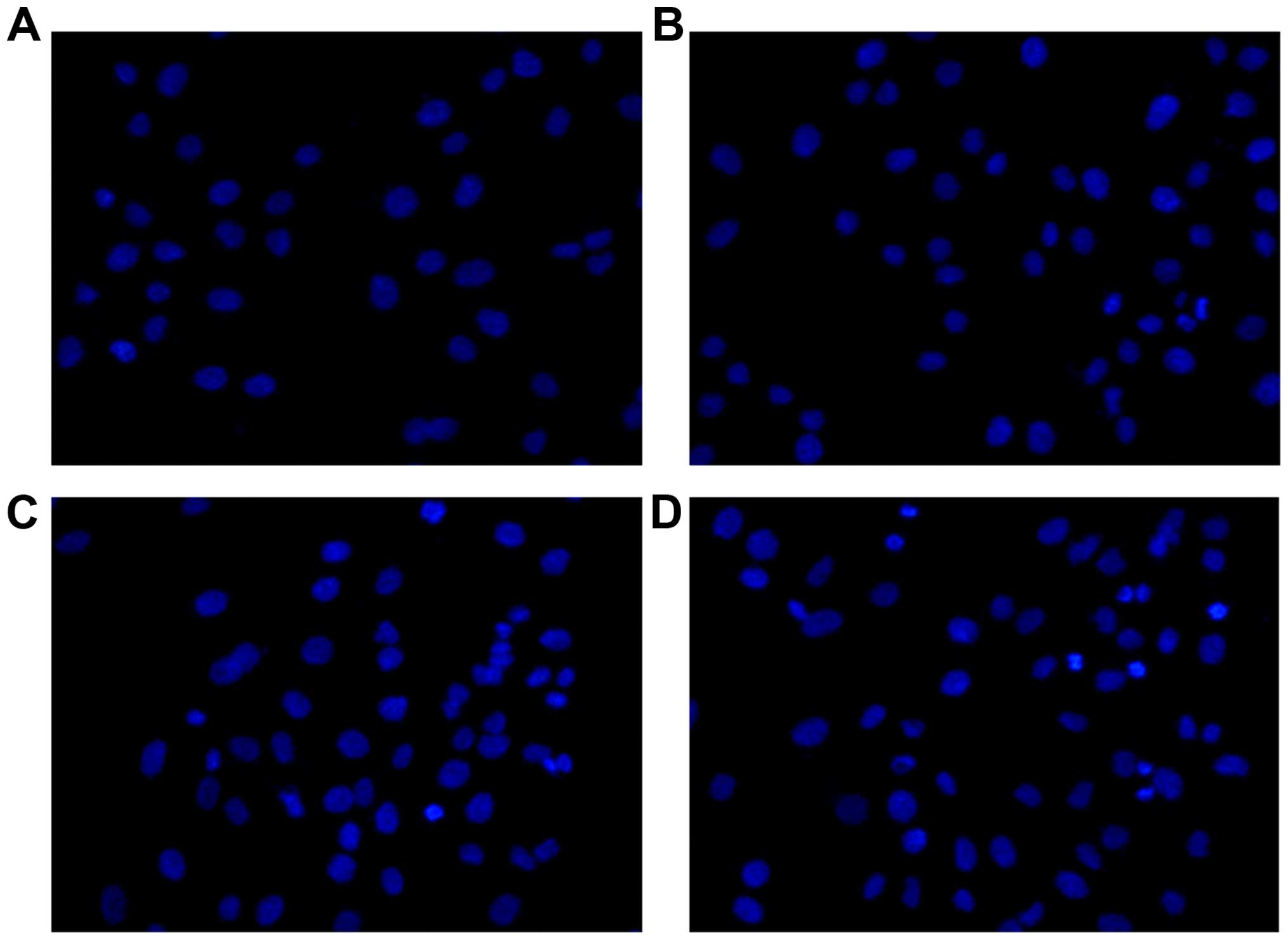
Apoptosis studies by Hoechst 33258
staining and by flow cytometry
After the treatment of thalidomide of different
concentration (50–200 µg/ml) for 48 h, MG-63 cells were stained
with Hoechst 33258 and imaged under a fluorescent microscope. As
shown in Fig. 2, significant
nuclear condensation and morphological changes, such as nuclear
shrinkage and chromatin condensation, were observed in MG-63
cells.
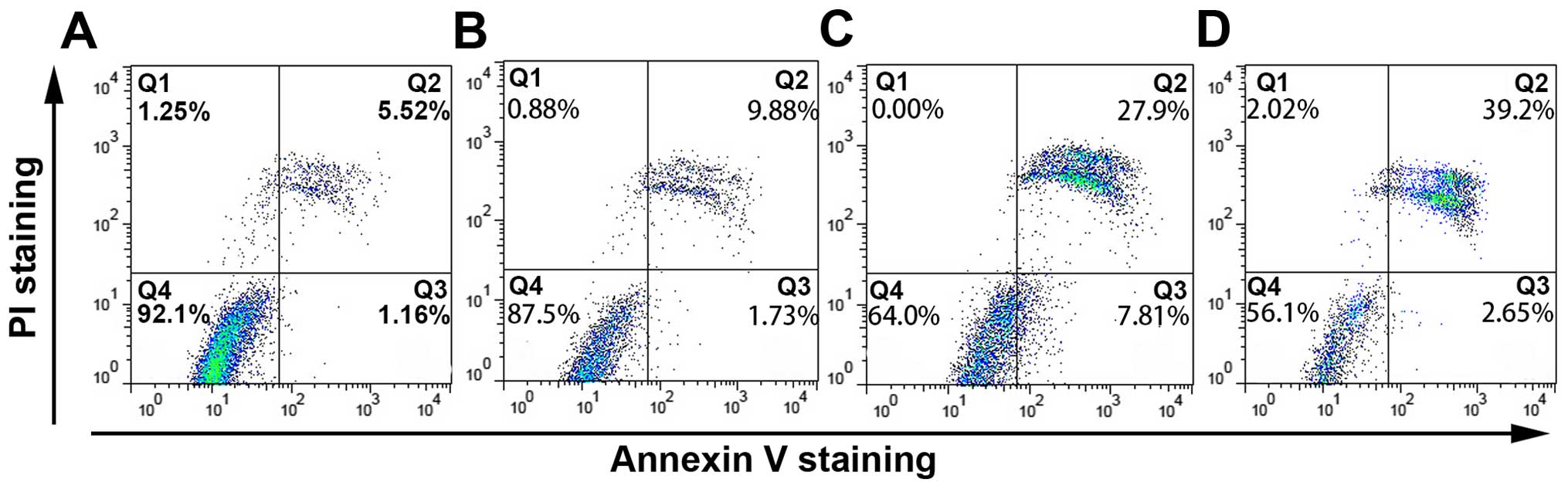
In Annexin V-FITC/PI double staining by FACS
analysis, Annexin V-FITC/PI double-positive cells significantly
increased after treatment with thalidomide for 48 h in a
concentration-dependent manner. As shown in Fig. 3, the percentage of apoptotic cells
was 7.98±1.26% in negative control. Exposure to 50, 100 and 200
µg/ml of thalidomide, the percentage of apoptotic cells was
10.58±1.18, 28.74±6.08 and 38.00±6.40%, respectively. The data
appeared to suggest that the apoptotic effect of thalidomide to
MG-63 cells was concentration-dependent.
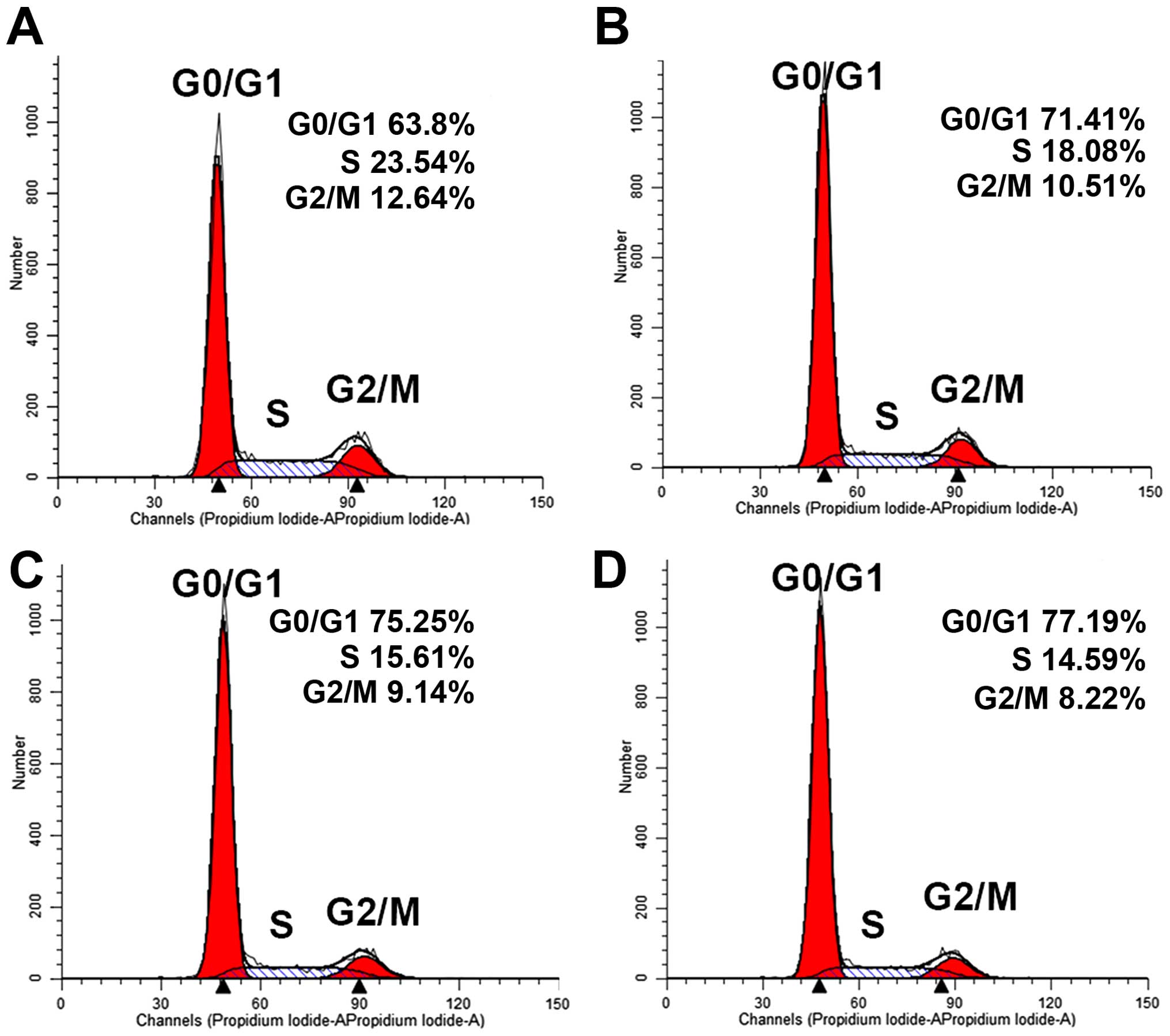
Cell cycle arrest
Flow cytometry was used to investigate the effect of
thalidomide on the cell cycle arrest. After the treatment with
different concentration (50–200 µg/ml) of thalidomide for 48 h, the
DNA distribution histogram is shown in Fig. 4. The differences between
thalidomide-treated culture and negative controls were significant.
In the control, the percentage in G0/G1 phase was 63.68±1.76%. And
the percentage was 71.9±0.83, 73.87±1.72 and 76.37±1.12%,
respectively, after MG-63 cells were treated with 50, 100 and 200
µg/ml of thalidomide. The percentage in S phase was 15.08±3.35,
13.53±2.96 and 12.38±2%, compared with the control 19.95±3.11%,
respectively. These data showed that thalidomide induced cell cycle
arrest by increasing the number of cells in the G0/G1 phase and
decreasing the percentage of S phase in MG-63 cells. The effect of
thalidomide on the cell cycle arrest was also
concentration-dependent.
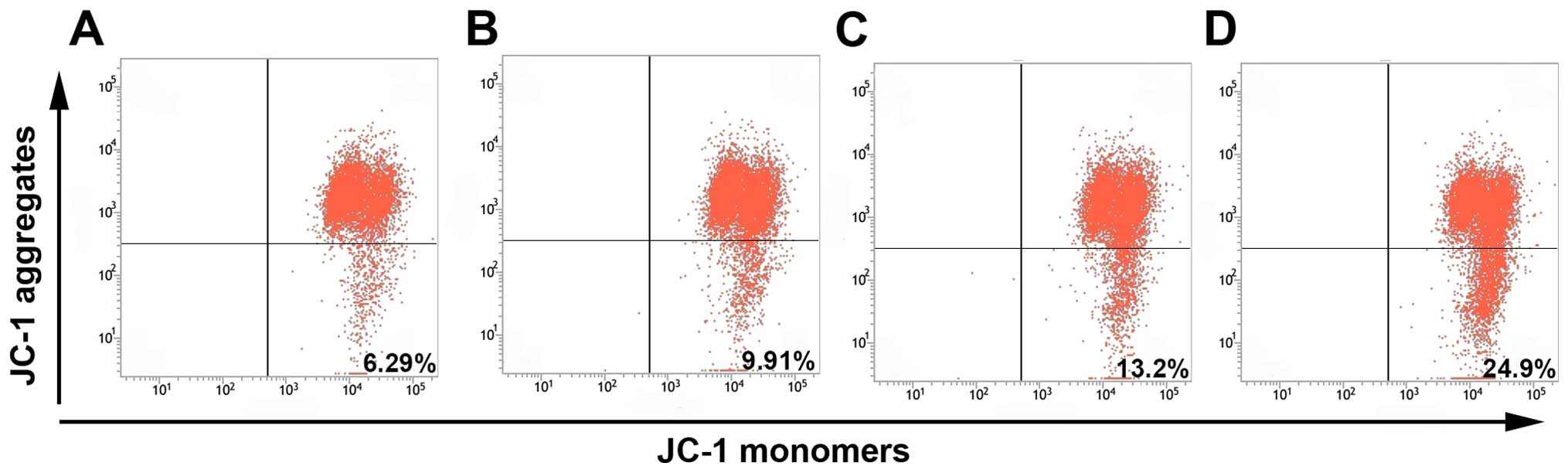
ΔΨm assay
To study the initiation of apoptosis, JC-1 was used
as a fluorescence probe in detecting the change of ΔΨm induced by
thalidomide. MG-63 cells were cultured with increasing
concentration (50–200 µg/ml) of thalidomide for 24 h, and then
analyzed by flow cytometry. As shown in Fig. 5, the percentage of cells showing an
intact mitochondrial membrane decreased 92.37±0.97, 90.4±2.62,
85.53±4.70% by concentration (50, 100 and 200 µg/ml), compared with
the negative control 95.17±0.31%, respectively. The number of cells
showing loss of mitochondrial membrane increased 7.63±0.94,
9.57±2.64, 13.62±5.92%, compared with negative control 4.84±0.31%,
respectively. This result suggested that thalidomide disrupted the
ΔΨm in a concentration-dependent manner. Taken together, these
results indicated that thalidomide induced apoptosis in MG-63 cells
through the mitochondrial pathway.
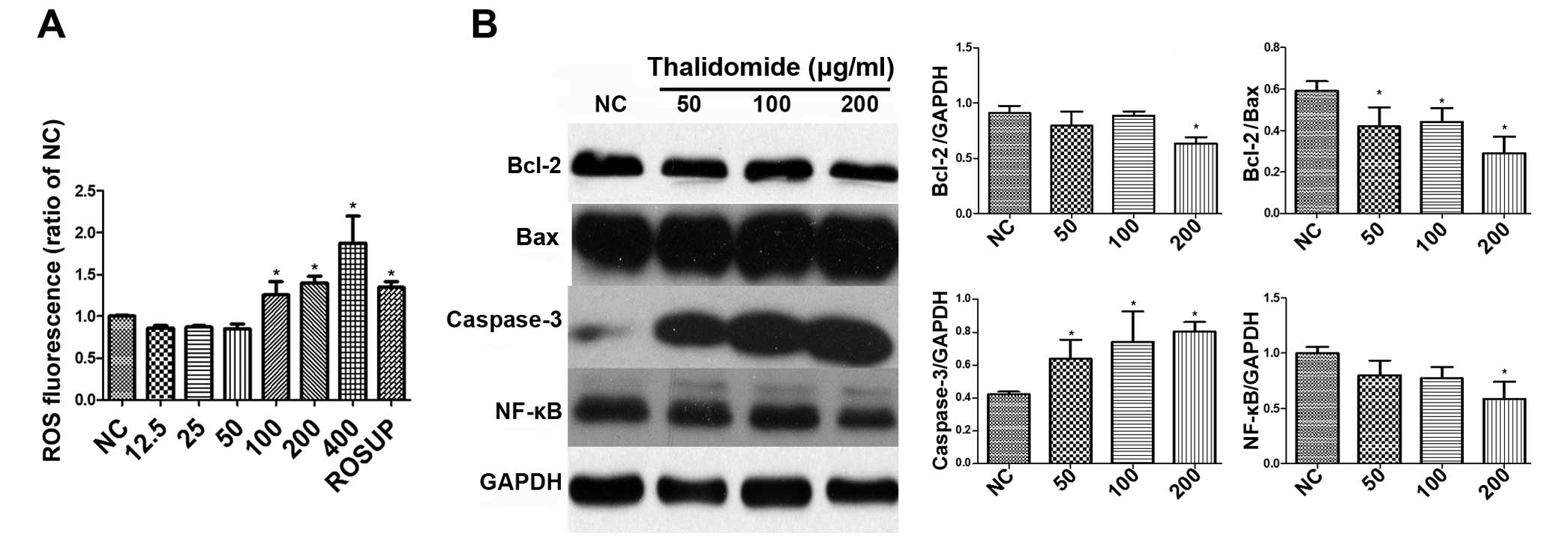
ROS level determination
Many potential anticancer agents induce apoptosis
through ROS generation. DCFH-DA was used as a fluorescent probe to
detect intracellular ROS production change. As shown in Fig. 6A, the result indicated that
thalidomide could increase the levels of ROS in MG-63 cells at
concentrations of 100–400 µg/ml. The fluorescent intensities of DCF
increased 1.26±0.16, 1.40±0.08 and 1.88±0.32 times of the negative
control in 100, 200 and 400 µg/ml of thalidomide group,
respectively, while the positive control Rosup was 1.34±0.07 times.
The differences of ROS between high concentration (100–400 µg/ml)
and low concentration (12.5–50 µg/ml) were also statistically
significant.
The expression of Bcl-2, Bax,
caspase-3 and NF-κB assay
Apoptosis was the major reason of cell death
produced by antitumor drugs. To clarify the underlying mechanism of
apoptosis, the effects of thalidomide to the expression of Bcl-2,
Bax, caspase-3 and NF-κB in MG-63 cells are shown in Fig. 6B. Bcl-2 family proteins play
important roles in the regulation of apoptosis via the control of
mitochondrial membrane permeability and the release of cytochrome
c and/or Smac/DIABLO (14).
The Bcl-2 is an oncogene and Bax is a cancer suppressor gene. An
imbalanced Bcl-2/Bax ratio has been recognized as a signature of
apoptosis acquisition in cancer cells (15,16).
Thalidomide treatment in MG-63 cells for 48 h
resulted in a decreasing expression of Bcl-2 (0.91±0.07, 0.79±0.13,
0.89±0.04 and 0.63±0.05 of GAPDH for 0, 50, 100 and 200 µg/ml of
thalidomide, respectively) and Bcl-2/Bax ratio (0.60±0.05,
0.42±0.09, 0.44±0.07 and 0.29±0.08 for 0, 50, 100 and 200 µg/ml of
thalidomide, respectively). Caspases are known to mediate the
apoptotic pathway (17,18), and processed effector caspase-3 can
create damage to the organelles. In this study, caspase-3 was
highly increased after the administration of thalidomide compared
with negative control (0.42±0.02, 0.64±0.12, 0.74±0.19 and
0.80±0.06 of GAPDH for 0, 50, 100 and 200 µg/ml, respectively).
Constitutive NF-κB activation has been noted in 95%
of all cancers (19–21). It plays an oncogenic role of in the
promotion of cell proliferation, control of apoptosis, promotion of
cell proliferation, control of apoptosis, stimulation of
angiogenesis and invasion/metastasis in cancer cells (22–26).
Significantly decreasing level of NF-κB is seen in Fig. 2C (1.00±0.05, 0.80±0.13, 0.77±0.11
and 0.59±0.16 of GAPDH for 0, 50, 100 and 200 µg/ml of thalidomide,
respectively).
Discussion
Osteosarcoma, occur predominantly in adolescents and
young adults, and is the most common malignant disease of primary
bone. The curative rate is low, due to terminal prognosis at the
first diagnosis and declining effects of cytotoxic drugs (27–29).
Finding new therapeutic agents to osteosarcoma is important.
Thalidomide, together with its anti-angiogenic, antiproliferative,
and pro-apoptotic activities, is thought to regulate antitumor
responses (30,31). Here we observed that thalidomide
induced apoptosis in cultured osteosarcoma cells. Treatment of
MG-63 cells with thalidomide, the cell viability decreased in time-
and concentration-dependent manner. Morphological changes of
apoptosis were observed as well. Thalidomide could effectively
induce apoptosis of MG-63 cells and inhibit the cell growth at the
G0/G1 phase. The high concentration of thalidomide could increase
the levels of ROS. Thalidomide could also induce the decrease of
ΔΨm, and thalidomide could downregulate the expression of Bcl-2,
Bcl-2/Bax ratio and NF-κB, and simultaneously increase the level of
caspase-3.
Apoptosis plays an important role in controlling
tumorigenesis in many anticancer drugs (18). It is well known that two major
pathways are involved in mammalian cells: the extrinsic and
intrinsic pathway. The latter leads to ΔΨm disruption, the early
event in mitochondrial-mediated apoptosis, and results in the
release of cytochrome c and the activation of caspase-9
(31). Then the apoptosomes cleave
pro-caspase-3 formed caspase-3, which plays a critical role in
implementing apoptosis (32). It
was also clear that Bcl-2 and Bax could regulate the release of
apoptogenic factors and the opening of the mitochondrial
permeability transition pore (33–35).
In the present study, early and late apoptotic cells quantitated by
Annexin V-FITC/PI double staining showed concentration-dependent
apoptosis. The sub-G1 population during cell cycle analysis
prompted the presence of apoptotic cells. The result of mechanistic
studies showed that thalidomide-induced apoptosis in MG-63 cells
was mediated by mitochondrial-mediated intrinsic pathway, followed
by the increase of caspase-3 and decrease of Bcl-2 protein and the
ratio of Bcl-2/Bax.
ROS at moderate levels represent significant
signaling molecules, which are widely involved in physiological
processes through oxidizing proteins, lipids and polynucleotides
(36). Oxidative stress is one of
the major causes for cell death and damage for oxidative damage to
DNA and biomolecules. Overexpression made internal defense
mechanism fail the fight against it. In the present research, ROS
was observed to be increasing in high concentration of thalidomide
in MG-63 cells. In addiction, ROS could reduce the level of Bcl-2
(37). ROS production might also
increase independently the metabolic state of mitochondria.
In many cancer cells NF-κB was persistently active
and located in the nucleus. The continuously expressing nuclear
Rel/NF-κB activity could protect cancer cells from apoptosis and
stimulate their growth. In this study, activation of NF-κB receded
in a concentration-dependent manner with treatment of thalidomide.
ROS stimulated the expression of NF-κB to activate MnSOD, which
could clear free radicals and reduced the activation of NF-κB in
return. In the present study, it was assumed that NF-κB pathway
work to decrease the level of ROS in low concentration of
thalidomide.
In conclusion, we found that thalidomide induced
apoptosis in osteosarcoma cells, which was accompanied by ROS,
disruption of ΔΨm and regulating the expression of Bcl-2, Bax,
caspases-3 and NF-κB. Therefore, thalidomide might play a role in
the therapy of osteosarcoma disease.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (no. 81272941) and the Guangdong
Provincial Department of Science and Technology (2014A020212009).
This study was also supported by the Guangdong Provincial Key
Laboratory of Malignant Tumor Epigenetics and Gene Regulation, Sun
Yat-Sen Memorial Hospital, Sun Yat-Sen University.
References
|
1
|
Raymond AK, Chawla SP, Carrasco CH, Ayala
AG, Fanning CV, Grice B, Armen T, Plager C, Papadopoulos NE,
Edeiken J, et al: Osteosarcoma chemotherapy effect: A prognostic
factor. Semin Diagn Pathol. 4:212–236. 1987.PubMed/NCBI
|
|
2
|
Rosen G, Caparros B, Huvos AG, Kosloff C,
Nirenberg A, Cacavio A, Marcove RC, Lane JM, Mehta B and Urban C:
Preoperative chemotherapy for osteogenic sarcoma: Selection of
postoperative adjuvant chemotherapy based on the response of the
primary tumor to preoperative chemotherapy. Cancer. 49:1221–1230.
1982. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Robak M, Treliński J and Chojnowski K:
Hemostatic changes after 1 month of thalidomide and dexamethasone
therapy in patients with multiple myeloma. Med Oncol. 29:3574–3580.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen Q, Lin RB, Ye YB, Fan NF, Guo ZQ,
Zhou ZF, Wang XJ, Chen MS, Chen SP and Li JY: The combined
administration of partially HLA-matched irradiated allogeneic
lymphocytes and thalidomide in advanced renal-cell carcinoma: A
case report. Med Oncol. 27:554–558. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rezvani H, Haghighi S, Ghadyani M and
Attarian H: Efficacy of taxotere, thalidomide, and prednisolone in
patients with hormone-resistant metastatic prostate cancer. Urol J.
9:673–677. 2012.PubMed/NCBI
|
|
6
|
Lv J, Liu N, Liu KW, Ding AP, Wang H and
Qiu WS: A randomised controlled phase II trial of the combination
of XELOX with thalidomide for the first-line treatment of
metastatic colorectal cancer. Cancer Biol Med. 9:111–114.
2012.PubMed/NCBI
|
|
7
|
Lee SM and Hackshaw A: A potential new
enriching trial design for selecting non-small-cell lung cancer
patients with no predictive biomarker for trials based on both
histology and early tumor response: Further analysis of a
thalidomide trial. Cancer Med. 2:360–366. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
de Souza CM, Araújoe Silva AC, de Jesus
Ferraciolli C, Moreira GV, Campos LC, dos Reis DC, Lopes MT,
Ferreira MA, Andrade SP and Cassali GD: Combination therapy with
carboplatin and thalidomide suppresses tumor growth and metastasis
in 4T1 murine breast cancer model. Biomed Pharmacother. 68:51–57.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tunio MA, Hashmi A, Qayyum A, Naimatullah
N and Masood R: Low-dose thalidomide in patients with metastatic
renal cell carcinoma. J Pak Med Assoc. 62:876–879. 2012.PubMed/NCBI
|
|
10
|
Tsai YC, Wu CT and Hong RL: Response of
refractory osteosarcoma to thalidomide and celecoxib. Lancet Oncol.
6:997–999. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zu C, Zhang M, Xue H, Cai X, Zhao L, He A,
Qin G, Yang C and Zheng X: Emodin induces apoptosis of human breast
cancer cells by modulating the expression of apoptosis-related
genes. Oncol Lett. 10:2919–2924. 2015.PubMed/NCBI
|
|
12
|
Huang W, Liu J, Feng X, Chen H, Zeng L,
Huang G, Liu W, Wang L, Jia W, Chen J, et al: DLC-1 induces
mitochondrial apoptosis and epithelial mesenchymal transition
arrest in nasopharyngeal carcinoma by targeting EGFR/Akt/NF-κB
pathway. Med Oncol. 32:1152015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu X, Yue P, Zhou Z, Khuri FR and Sun SY:
Death receptor regulation and celecoxib-induced apoptosis in human
lung cancer cells. J Natl Cancer Inst. 96:1769–1780. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cory S, Huang DC and Adams JM: The Bcl-2
family: Roles in cell survival and oncogenesis. Oncogene.
22:8590–8607. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang M, Zhang P, Zhang C, Sun J, Wang L,
Li J, Tian Z and Chen W: Prognostic significance of Bcl-2 and Bax
protein expression in the patients with oral squamous cell
carcinoma. J Oral Pathol Med. 38:307–313. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Faggiano A, Sabourin JC, Ducreux M,
Lumbroso J, Duvillard P, Leboulleux S, Dromain C, Colao A,
Schlumberger M and Baudin E: Pulmonary and extrapulmonary poorly
differentiated large cell neuroendocrine carcinomas: Diagnostic and
prognostic features. Cancer. 110:265–274. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Salvesen GS and Dixit VM: Caspases:
Intracellular signaling by proteolysis. Cell. 91:443–446. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Thornberry NA and Lazebnik Y: Caspases:
Enemies within. Science. 281:1312–1316. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Aggarwal BB and Shishodia S: Molecular
targets of dietary agents for prevention and therapy of cancer.
Biochem Pharmacol. 71:1397–1421. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lu T, Sathe SS, Swiatkowski SM, Hampole CV
and Stark GR: Secretion of cytokines and growth factors as a
general cause of constitutive NFkappaB activation in cancer.
Oncogene. 23:2138–2145. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lu T and Stark GR: Cytokine overexpression
and constitutive NFkappaB in cancer. Cell Cycle. 3:1114–1117. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Okayasu I, Ohkusa T, Kajiura K, Kanno J
and Sakamoto S: Promotion of colorectal neoplasia in experimental
murine ulcerative colitis. Gut. 39:87–92. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Greten FR, Eckmann L, Greten TF, Park JM,
Li ZW, Egan LJ, Kagnoff MF and Karin M: IKKbeta links inflammation
and tumorigenesis in a mouse model of colitis-associated cancer.
Cell. 118:285–296. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
DiDonato JA, Mercurio F and Karin M: NF-κB
and the link between inflammation and cancer. Immunol Rev.
246:379–400. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kashani-Sabet M, Shaikh L, Miller JR III,
Nosrati M, Ferreira CM, Debs RJ and Sagebiel RW: NF-κB in the
vascular progression of melanoma. J Clin Oncol. 22:617–623. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Karin M: Nuclear factor-kappaB in cancer
development and progression. Nature. 441:431–436. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
He H, Ni J and Huang J: Molecular
mechanisms of chemoresistance in osteosarcoma (Review). Oncol Lett.
7:1352–1362. 2014.PubMed/NCBI
|
|
28
|
Ek ET and Choong PF: The role of high-dose
therapy and autologous stem cell transplantation for pediatric bone
and soft tissue sarcomas. Expert Rev Anticancer Ther. 6:225–237.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang C, Hornicek FJ, Wood KB, Schwab JH,
Mankin H and Duan Z: RAIDD expression is impaired in multidrug
resistant osteosarcoma cell lines. Cancer Chemother Pharmacol.
64:607–614. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Scheele C, Nielsen S and Pedersen BK: ROS
and myokines promote muscle adaptation to exercise. Trends
Endocrinol Metab. 20:95–99. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Franks ME, Macpherson GR and Figg WD:
Thalidomide. Lancet. 363:1802–1811. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li M, Kondo T, Zhao QL, Li FJ, Tanabe K,
Arai Y, Zhou ZC and Kasuya M: Apoptosis induced by cadmium in human
lymphoma U937 cells through Ca2+-calpain and
caspase-mitochondria- dependent pathways. J Biol Chem.
275:39702–39709. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mohamad N, Gutiérrez A, Núñez M, Cocca C,
Martín G, Cricco G, Medina V, Rivera E and Bergoc R: Mitochondrial
apoptotic pathways. Biocell. 29:149–161. 2005.PubMed/NCBI
|
|
34
|
Orrenius S, Gogvadze V and Zhivotovsky B:
Mitochondrial oxidative stress: Implications for cell death. Annu
Rev Pharmacol Toxicol. 47:143–183. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yin XM: Signal transduction mediated by
Bid, a pro-death Bcl-2 family proteins, connects the death receptor
and mitochondria apoptosis pathways. Cell Res. 10:161–167. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ermak G and Davies KJ: Calcium and
oxidative stress: From cell signaling to cell death. Mol Immunol.
38:713–721. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pan JS, Hong MZ and Ren JL: Reactive
oxygen species: A double-edged sword in oncogenesis. World J
Gastroenterol. 15:1702–1707. 2009. View Article : Google Scholar : PubMed/NCBI
|