Introduction
Epithelial ovarian cancer (referred to as ovarian
cancer) is a common malignancy of the female reproductive system
and has a 5-year survival rate of less than 44% (1). Recurrence and drug resistance are the
main reasons for its poor prognosis (2). Cancer stem cells are a group of cells
capable of self-renewal and unlimited proliferation, and they can
also be drug-resistant and highly invasive (3,4).
According to the theory of cancer stem cells, although tumor cells
contain extremely few cancer stem cells, they are the root cause of
tumor initiation, metastasis, recurrence and therapeutic resistance
(5–9). Therefore, it is extremely important to
discover new approaches to regulate ovarian cancer stem cells
(OCSCs).
Ras homolog gene family member C (RhoC) is a small G
protein and is one of the three members of the Rho subfamily of
GTPases (10). Upregulation of RhoC
is closely linked to the growth, metastasis, invasion and
progression or adverse prognosis of various malignancies (11,12).
More importantly, RhoC plays a critical role in regulating cancer
stem cells, including head and neck squamous cell carcinoma and
breast cancer (13,14). Our previous studies showed that RhoC
overexpression in ovarian cancer may promote cancer invasion and
metastasis, and may be an independent prognostic factor for ovarian
cancer (15,16). A previous study reported that the
RhoC homolog RhoA is closely linked to the Wnt pathway, which has
been implicated in stem cell activity (17). Therefore, in the present study we
aimed to explore the role of RhoC in the proliferation, cisplatin
resistance and invasion ability of OCSCs.
Materials and methods
Cell culture
The OC cell lines A2780, A2780-PM and A2780-PTX were
purchased from the American Type Culture Collection (ATCC;
Manassas, VA, USA). The cells were maintained in Dulbeccos modified
Eagles medium (DMEM) supplemented with 10% fetal bovine serum
(FBS), 100 U/ml penicillin, and 100 µg/ml streptomycin in a
humidified atmosphere of 5% CO2 at 37°C. The cells were
harvested by centrifugation, rinsed with phosphate-buffered saline
(PBS), and were then used for extraction of total RNA.
Cell selection
The resistant ovarian cancer cell line A2780-PTX was
cultured in the top chambers of Matrigel™-coated
Transwell® inserts (Beckon-Dickinson Biosciences)
containing 1 ml of DMEM containing 10% FBS in the lower chamber.
A2780-PTX cells that were highly invasive were able to pass through
the membrane to the lower chamber. These cells have high resistance
and high invasiveness, and were termed A2780-PTX-PM. Flow cytometry
was used to determine the OCSCs from this population. Briefly, the
cells were trypsinized to prepare a single cell suspension,
adjusting the cell concentration to 5×105/tube cells.
Then, 2 ml of diluted CD117 antibody was added to each tube
containing the cell suspension, the mixture was incubated at room
temperature for 30 min, washed thrice with PBS, and centrifuged at
1,000 rpm for 5 min. The cells were then incubated with fluorescein
isothiocyanate (FITC)-labeled secondary antibodies at room
temperature for 30 min, washed thrice with PBS, centrifuged at
1,000 rpm for 5 min, and resuspended in 2 ml PBS, prior to
detection of fluorescence intensity and cell sorting. The sorted
cells were cultured after the completion of the relevant
experiments.
Proliferation assay
Cell proliferation was analyzed using the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay. Cells were directly seeded into 96-well plates
(5×103 cells/well) and allowed to adhere. At different
time points (0, 24, 48 and 72 h) after seeding, 20 µl of 5 mg/ml of
MTT (Sigma-Aldrich, St. Louis, MO, USA) was added to the cells, and
the cells were incubated at 37°C for 4 h. The supernatants were
removed, and 150 µl of dimethyl sulfoxide (DMSO; Sigma-Aldrich) was
added to each well. The absorbance of each well was measured at 490
nm.
Assessment of wound healing
Cells were seeded at 1×106 cells/well in
6-well culture plates and cultured to confluence. The cell
monolayer was then scratched with a pipette tip (200 µl). Cells
were washed thrice with PBS and cultured in an FBS-free medium. The
cells were photographed at 0, 24 and 48 h, and the scratched areas
were measured using ImageJ software. The wound healing rate was
calculated using the following formula: Wound healing rate = (area
of original wound - area of actual wound at different times)/area
of original wound × 100%.
Assessment of cell invasion
First, 5×104 cells were resuspended in
FBS-free DMEM and seeded into the top chambers of Matrigel™-coated
Transwell® inserts. The lower compartment of the chamber
contained 10% FBS as a chemoattractant. After incubation for 48 h
at 37°C in a 5% CO2 atmosphere, the cells on the upper
surface of the membrane were wiped away. Cells on the lower surface
of the membrane were washed with PBS, fixed in 4% methanol and
stained with crystal violet dye to quantify the extent of
invasion.
Real-time reverse
transcription-polymerase chain reaction (RT-PCR)
Total RNA was extracted from OC cell lines using
TRIzol reagent (Takara Bio, Japan). Real-time RT-PCR was carried
out with 2 µg of total RNA using AMV reverse transcriptase and
random primers (Takara Bio). PCR primers were designed according to
the sequences in GenBank. cDNA amplification was carried out with a
SYBR™ Premix Ex Taq II kit (Takara), using glyceraldehyde
3-phosphate dehydrogenase (18s) as an internal control, according
to the manufacturer's instructions. Briefly, cDNA amplification for
each primer was carried out in a final volume of 20 µl containing
10 µl SYBR Premix Ex Taq (2X), 0.08 µl of each primer, 0.4
µl of ROX reference dye, and 1 µl of template cDNA (50 µg/µl). The
PCR protocol was as follows: initial incubation at 95°C for 30 sec
followed by 40 cycles of denaturation at 95°C for 5 sec and
annealing at 60°C for 34 sec. The relative gene expression levels
(amount of the target gene normalized to that of the endogenous
control gene) were calculated using the comparative Ct method
(2−ΔΔCt).
Immunofluorescence
For the immunofluorescence experiment, the cells
were cultured on glass coverslips, fixed with PBS containing 4%
formaldehyde for 10 min, and permeabilized with 0.2% Triton X-100
in PBS for 30 min at room temperature. After washing with PBS, the
cells were incubated overnight at 4°C with antibodies to RhoC,
CD117 and CD133 (1:50; Proteintech, Shanghai, China). Then, the
cells were washed three times with PBS and treated with the
secondary antibody for 2 h at room temperature in a dark and
humidified chamber. After washing thrice with PBS, the
immunostained cells were mounted in mounting medium containing
4,6-diamidino-2-phenylindole (DAPI) for 5 min, and washed thrice
with PBS. The cells were then visualized under a fluorescence
microscope equipped with a camera.
Statistical analyses
Ranked data were analyzed using Spearman's rank
correlation coefficient. The Mann-Whitney U test was used to
differentiate between the mean values of different groups. A
P-value of <0.05 was considered to indicate a statistically
significant result. SPSS v.17.0 (IBM, Armonk, NY, USA) was employed
to analyze data.
Results
Expression of stem cell markers and
proliferation, migration and invasion ability of ovarian cancer
cells
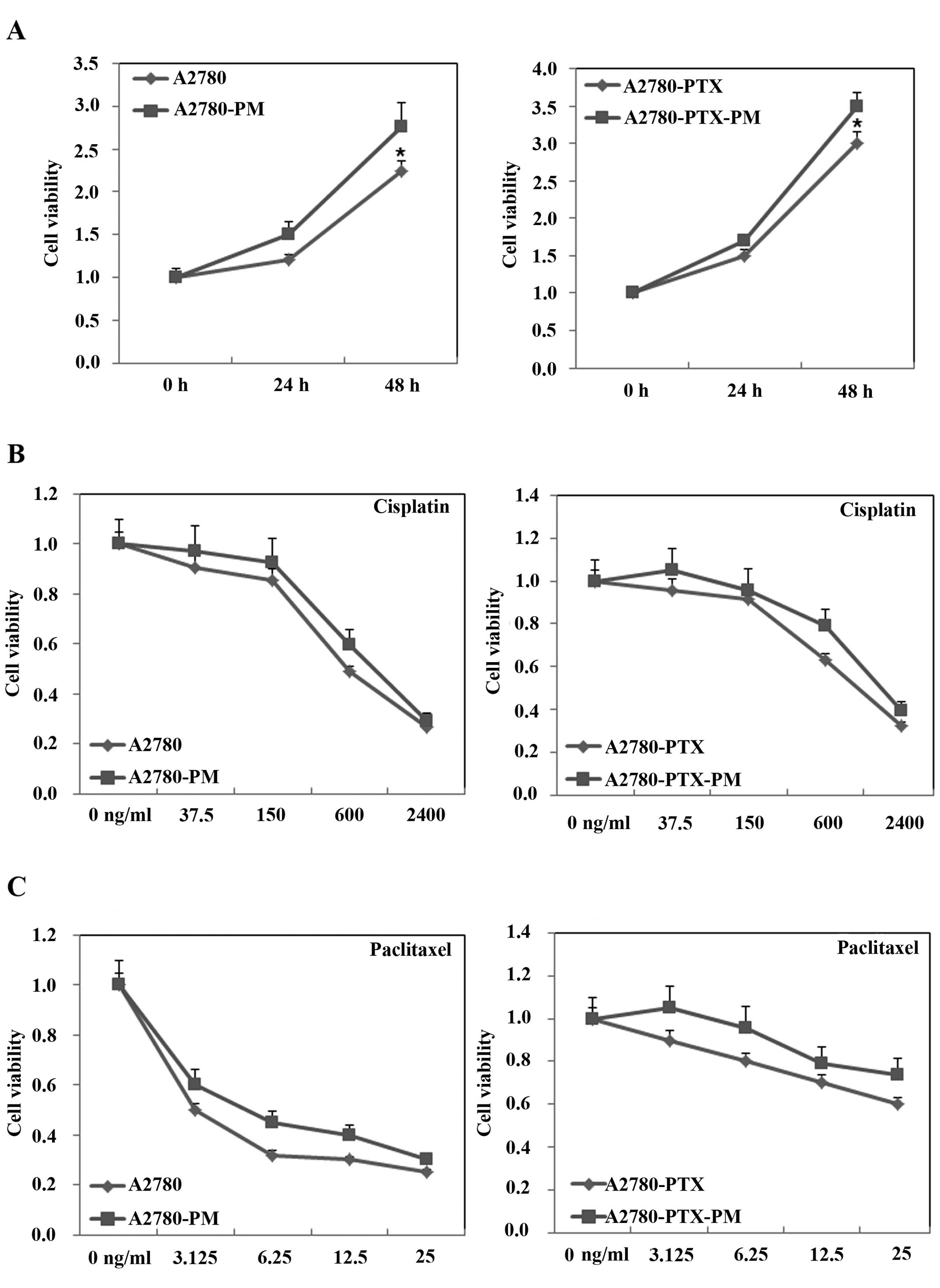
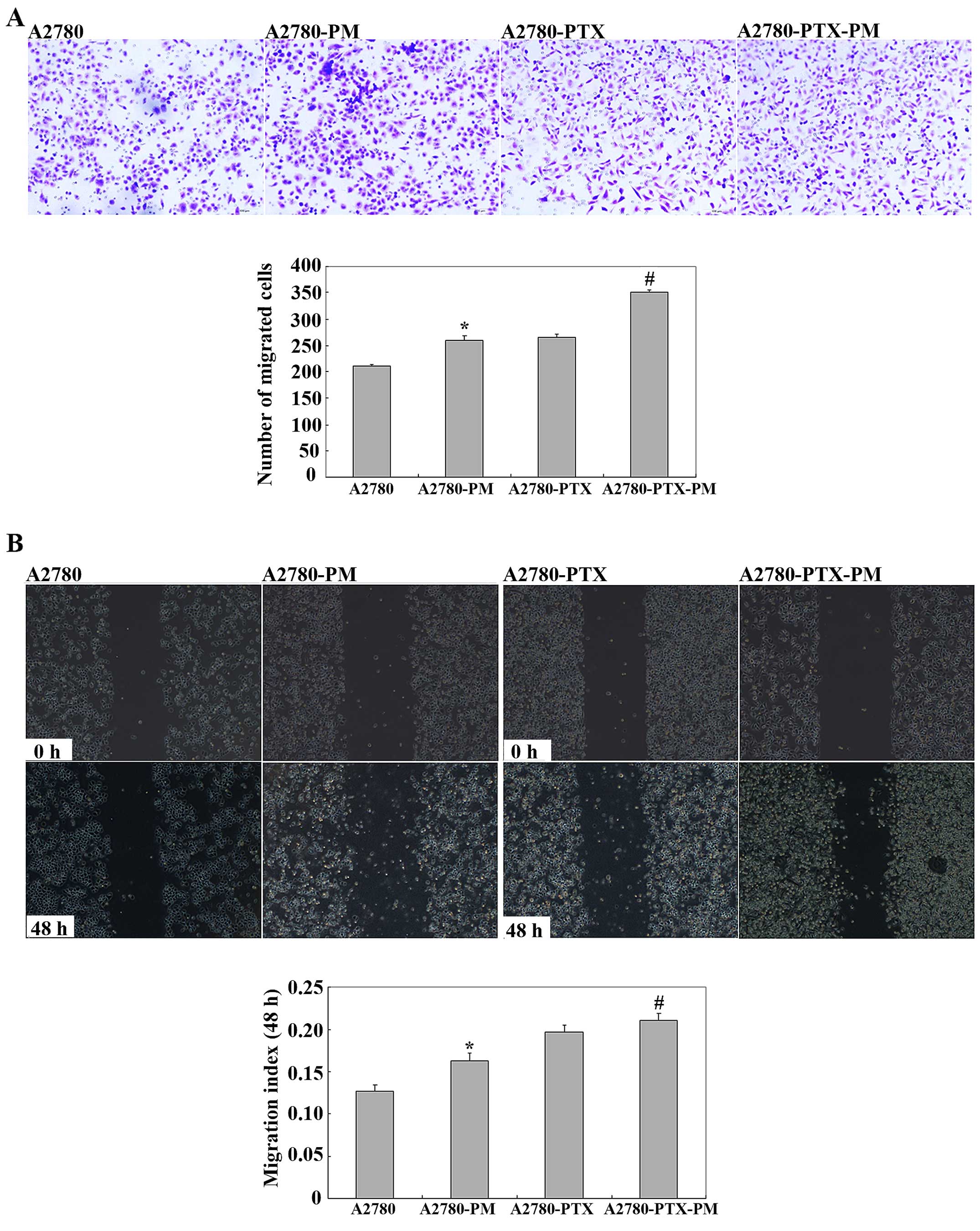
A2780-PM and A2780-PTX-PM cells showed higher cell
proliferation (Fig. 1A), drug
resistance (Fig. 1B and C) and
invasion and migration ability (Fig.
2) than these parameters in the A2780 and A2780-PTX cell lines.
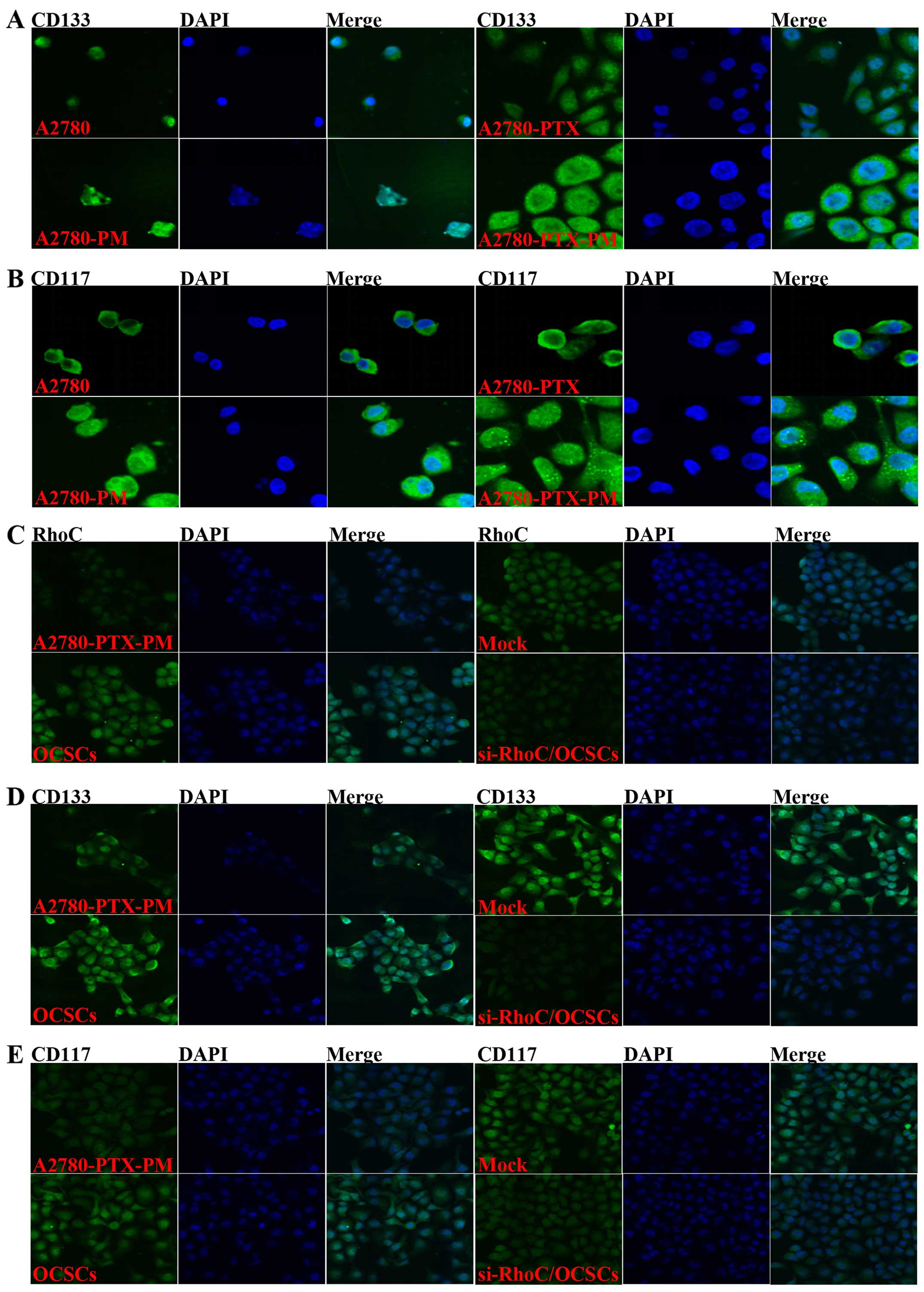
Additionally, CD133 and CD117 expression levels were higher in the
A2780-PM and A2780-PTX-PM cells than these levels in the A2780 and
A2780-PTX cells (Fig. 3A and
B).
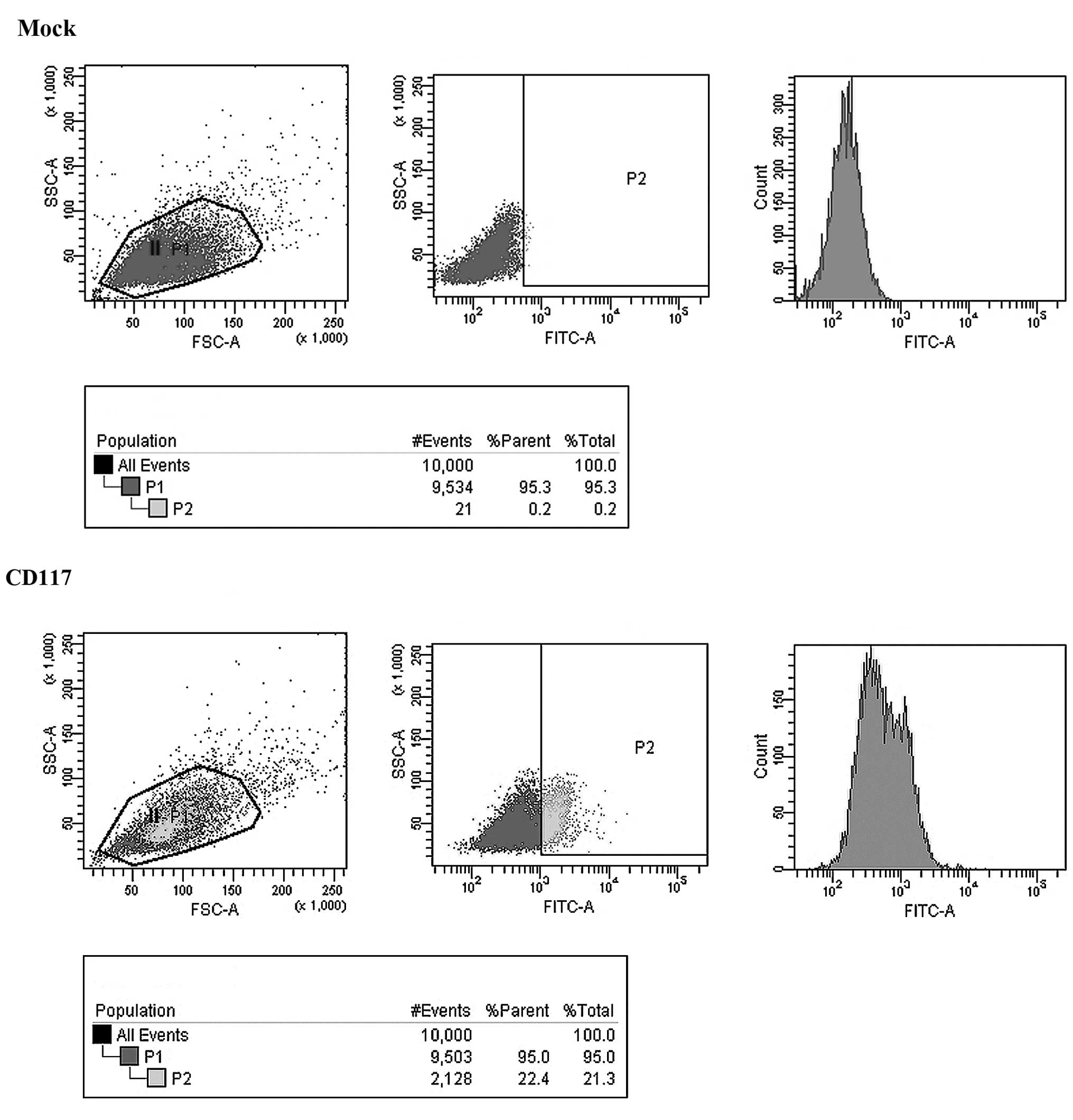
Selection of OCSCs
Cells were sorted through flow cytometric analysis
to select ovarian cancer cells with stem cell-like characteristics
(Fig. 4).
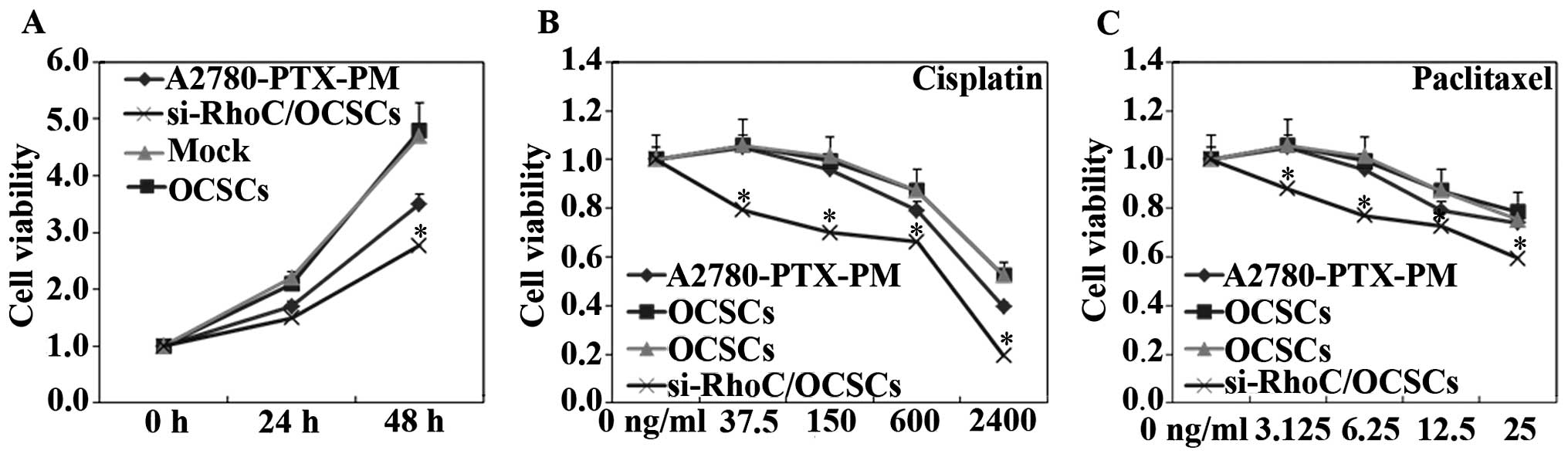
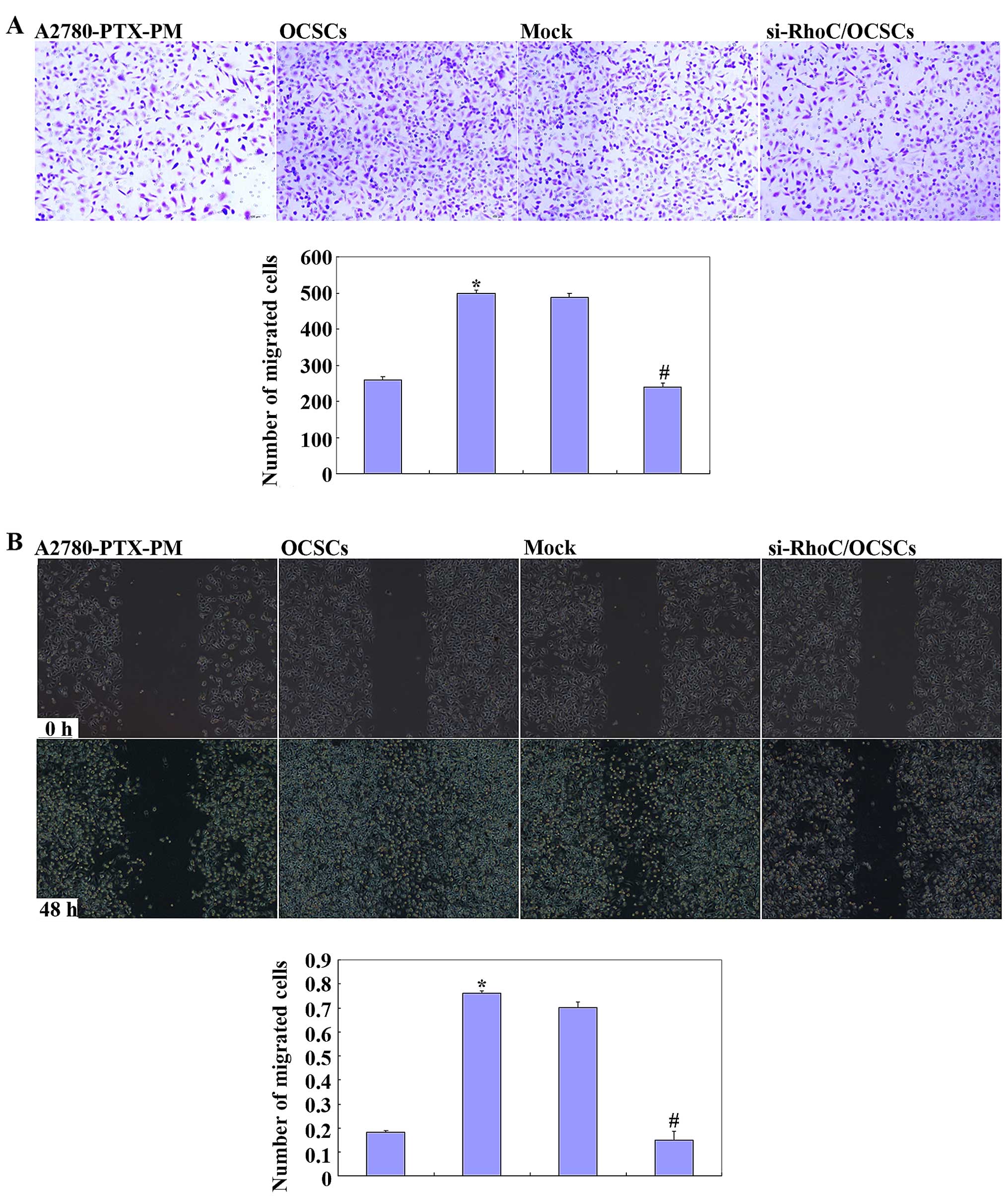
Effects of si-RhoC on OCSCs
OCSCs showed induced cell proliferation, drug
resistance and migration, and invasion ability. si-RhoC
transfection in OCSCs reduced cell proliferation (Fig. 5A), drug resistance (Fig. 5B and C), and migration and invasion
ability (Fig. 6). Results of the
immunofluorescence analysis revealed that the expression of RhoC,
CD133 and CD117 were also induced in the OCSCs, while transfection
with si-RhoC reduced the expression of RhoC, CD133 and CD117
(Fig. 3C-E).
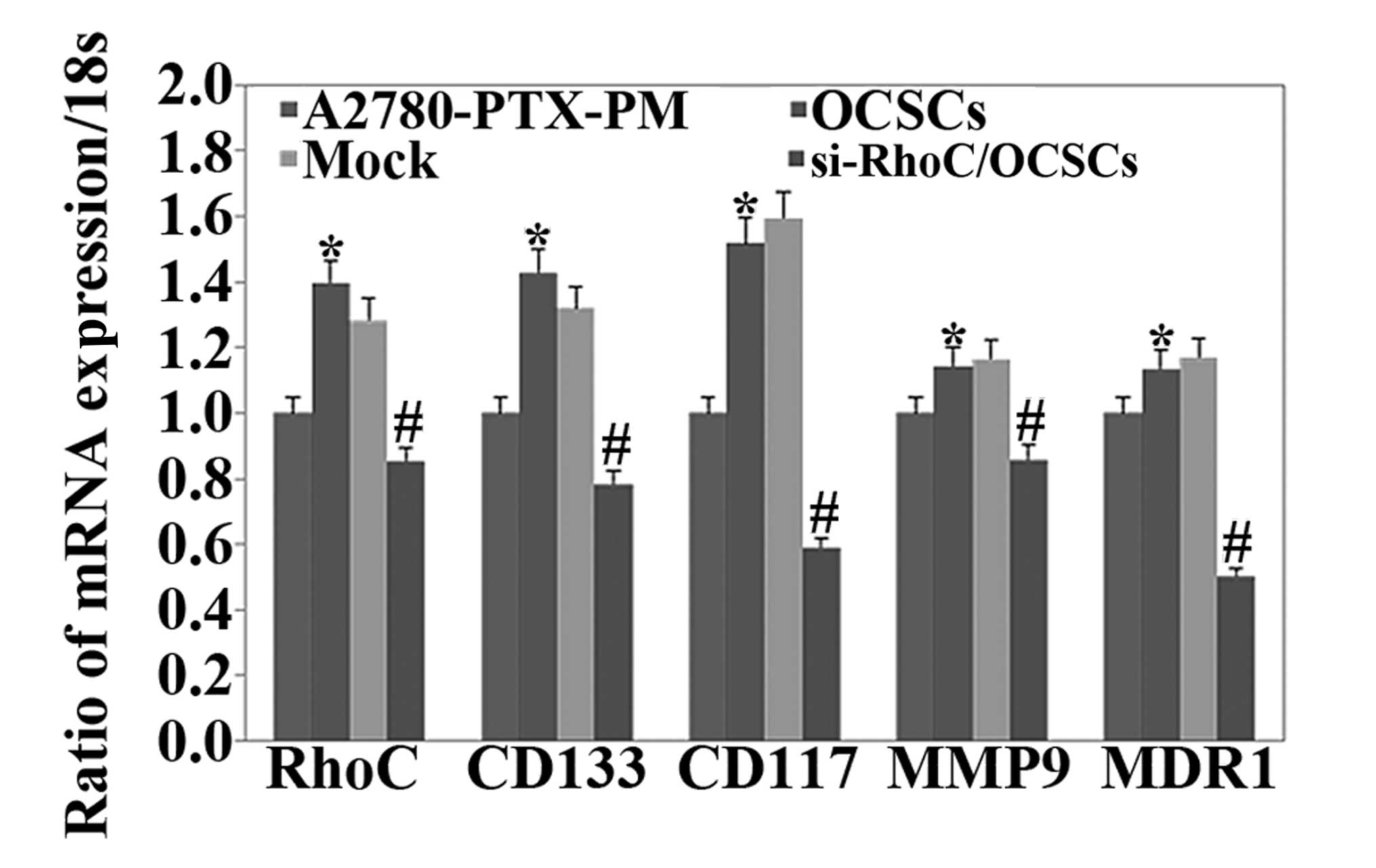
Expression of the mRNA of
phenotype-related molecules in ovarian cancer cells
We assessed the mRNA expression levels of RhoC,
CD133, CD117, MDR1 and MMP9 in A2780-PTX-PM cells, OCSCs and
si-RhoC-transfected OCSCs. The results revealed that the expression
levels of all of these markers were higher in OCSCs than levels in
the A2780-PTX-PM cells, but the expression levels were lower in the
si-RhoC-transfected OCSCs than levels in the OCSCs (Fig. 7).
Discussion
Given that stem cells have the ability of
self-renewal, unlimited proliferation, drug resistance, high
invasion and express high levels of CD117 and CD133 (18,19),
we identified highly invasive populations of A2780 and A2780-PTX
cells through Transwell assays. We screened the drug-resistant
ovarian cancer cell line A2780-PTX for a highly drug-resistant
ovarian cancer cell population with high invasiveness, and
designated this population A2780-PTX-PM. Flow cytometric analysis
was then applied to sort ovarian cancer cells bearing stem
cell-like characteristics, ovarian cancer stem cells (OCSCs)
expressing high levels of CD117 and CD133.
RhoC is known to be involved in the entire
process of tumor progression and resistance in a variety of tumors.
The RhoC protein regulates cell growth and proliferation mainly by
affecting the cell cycle. Xie et al studied hepatoma cells
in vitro and reported that RhoC expression regulated
growth and apoptosis, and si-RhoC-transfected cells exhibited
reduced cell proliferation and cell growth and a significantly
reduced S-G2/M phase cell population (20). RhoC can affect cells in a variety of
ways by altering the morphology and cell polarity, forming blood
vessels, and is involved in tumor invasion and metastasis. Studies
have shown that RhoGEF TEM4 (an activator of Rho family GTPases)
regulates the cell migration of endothelial cells. TEM4 regulates
cellular migration by signaling to RhoC as suppression of its
expression recapitulated the loss of TEM4 phenotypes, and RhoC
activation was impaired in TEM4-depleted cells (21). In addition, the destruction of the
extracellular cell transfer mechanism is an important condition
that is essential to the secretion of matrix metalloproteinases
(MMPs). Various researchers have found associations between MMPs
and the RhoC gene and reported that the RhoC gene
promotes the expression of the MMP2 and MMP9 genes
(20). Additionally, we found that
RhoC was expressed at a higher level in epithelial ovarian
carcinomas than this level in normal ovarian and benign ovarian
tumor tissues. The RhoC expression level was positively
correlated with ovarian cancer staging and differentiation. In
addition, functional studies have shown that overexpression of
RhoC promoted the invasion and metastasis of ovarian cancer
through expression of VEGF, MMP9 and Rho-associated kinase (ROCK).
After si-RhoC transfection in OCSCs, the proliferation, cisplatin
resistance, invasion and stemness were decreased; the expression of
associated proteins MDR1 and MMP9 were also decreased; and the
positive expression of CD117 and CD133 was decreased. Therefore,
based on our findings, we conclude that RhoC is an important
oncogene that is required for the maintenance and propagation of
OCSCs in ovarian cancer.
This is the first study to investigate the role and
molecular mechanisms of RhoC in OCSCs, RhoC is an important
gene that can be used for the treatment of OCSCs. We believe that
the findings of the present study can shed insight into the root
cause of ovarian cancer recurrence.
Acknowledgements
We would like to thank the native English speaking
scientists of Elixigen Corporation (Huntington Beach, CA, USA) for
editing our manuscript. The present study was supported by grants
from the Liaoning Science and Technology Grant (no. 2013021077),
and the Natural Scientific Foundation of China (nos. 81202049,
81472440 and 81472502).
References
|
1
|
Baldwin LA, Huang B, Miller RW, Tucker T,
Goodrich ST, Podzielinski I, DeSimone CP, Ueland FR, van Nagell JR
and Seamon LG: Ten-year relative survival for epithelial ovarian
cancer. Obstet Gynecol. 120:612–618. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bae T, Weon KY, Lee JW, Eum KH, Kim S and
Choi JW: Restoration of paclitaxel resistance by CDK1 intervention
in drug-resistant ovarian cancer. Carcinogenesis. 36:1561–1571.
2015.PubMed/NCBI
|
|
3
|
Yoo YD and Kwon YT: Molecular mechanisms
controlling asymmetric and symmetric self-renewal of cancer stem
cells. J Anal Sci Technol. 6:282015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Beachy PA, Karhadkar SS and Berman DM:
Tissue repair and stem cell renewal in carcinogenesis. Nature.
432:324–331. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Levina V, Marrangoni AM, DeMarco R,
Gorelik E and Lokshin AE: Drug-selected human lung cancer stem
cells: Cytokine network, tumorigenic and metastatic properties.
PLoS One. 3:e30772008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Klonisch T, Wiechec E, Hombach-Klonisch S,
Ande SR, Wesselborg S, Schulze-Osthoff K and Los M: Cancer stem
cell markers in common cancers - therapeutic implications. Trends
Mol Med. 14:450–460. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li F, Tiede B, Massagué J and Kang Y:
Beyond tumorigenesis: Cancer stem cells in metastasis. Cell Res.
17:3–14. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Reya T, Morrison SJ, Clarke MF and
Weissman IL: Stem cells, cancer, and cancer stem cells. Nature.
414:105–111. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vezzoni L and Parmiani G: Limitations of
the cancer stem cell theory. Cytotechnology. 58:3–9. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sahai E and Marshall CJ: RHO-GTPases and
cancer. Nat Rev Cancer. 2:133–142. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hakem A, Sanchez-Sweatman O, You-Ten A,
Duncan G, Wakeham A, Khokha R and Mak TW: RhoC is dispensable for
embryogenesis and tumor initiation but essential for metastasis.
Genes Dev. 19:1974–1979. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kleer CG, Teknos TN, Islam M, Marcus B,
Lee JS, Pan Q, Merajver SD and Rho C: RhoC GTPase expression as a
potential marker of lymph node metastasis in squamous cell
carcinomas of the head and neck. Clin Cancer Res. 12:4485–4490.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Islam M, Sharma S and Teknos TN: RhoC
regulates cancer stem cells in head and neck squamous cell
carcinoma by overexpressing IL-6 and phosphorylation of STAT3. PLoS
One. 9:e885272014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rosenthal DT, Zhang J, Bao L, Zhu L, Wu Z,
Toy K, Kleer CG and Merajver SD: RhoC impacts the metastatic
potential and abundance of breast cancer stem cells. PLoS One.
7:e409792012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen S, Chen X, Xiu YL, Sun KX and Zhao Y
and Zhao Y: Inhibition of ovarian epithelial carcinoma
tumorigenesis and progression by microRNA 106b mediated through the
RhoC pathway. PLoS One. 10:e01257142015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen X, Chen S, Xiu YL, Sun KX, Zong ZH
and Zhao Y: RhoC is a major target of microRNA-93-5P in epithelial
ovarian carcinoma tumorigenesis and progression. Mol Cancer.
14:312015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen S, Wang J, Gou WF, Xiu YL, Zheng HC,
Zong ZH, Takano Y and Zhao Y: The involvement of RhoA and Wnt-5a in
the tumorigenesis and progression of ovarian epithelial carcinoma.
Int J Mol Sci. 14:24187–24199. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lukenda A, Dotlic S, Vukojevic N, Saric B,
Vranic S and Zarkovic K: Expression and prognostic value of
putative cancer stem cell markers CD117 and CD15 in choroidal and
ciliary body melanoma. J Clin Pathol. 69:234–239. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wright MH, Calcagno AM, Salcido CD,
Carlson MD, Ambudkar SV and Varticovski L: Brca1 breast tumors
contain distinct CD44+/CD24 and CD133+ cells
with cancer stem cell characteristics. Breast Cancer Res.
10:R102008. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xie SL, Zhu MG, Chen GF, Wang GY and Lv
GY: Effects of Ras homolog gene family, member C gene silencing
combined with rapamycin on hepatocellular carcinoma cell growth.
Mol Med Rep. 12:5077–5085. 2015.PubMed/NCBI
|
|
21
|
Mitin N, Rossman KL, Currin R, Anne S,
Marshall TW, Bear JE, Bautch VL and Der CJ: The RhoGEF TEM4
regulates endothelial cell migration by suppressing actomyosin
contractility. PLoS One. 8:e662602013. View Article : Google Scholar : PubMed/NCBI
|