Introduction
Colorectal cancer (CRC) is the third most commonly
diagnosed cancer in humans around the world and accounts for
approximately 9% of all cancer-related deaths (1). Early diagnosis of CRC is critical to
perform surgical resection and contributes to improvement in the
CRC survival rate of patients (2).
However, to date, the long-term survival rate of patients with CRC
remains poor. Although molecular alterations including epigenetic
and genetic changes have been widely reported (3), knowledge of the molecular mechanisms
in the progression of CRC is still limited and further studies are
required to achieve a more complete understanding of CRC
pathogenesis.
Fibronectin is a glycoprotein of the extracellular
matrix which plays a crucial role in cell adhesion, growth,
migration and differentiation (4,5). New
findings suggest that fibronectin also participates in wound
healing and embryonic development (6). Fibronectin has been found highly
expressed in tumor vasculature and mediates angiogenesis during
tumorigenesis showing a potential role in cancer progression
(7,8). Actually, fibronectin has been
implicated in the development of several types of cancers (9,10).
Fibronectin participates in cell migration and invasion in
metastatic cancer cells (11).
Moreover, fibronectin has an anti-apoptotic function in standard
chemotherapy (12). In breast
cancer, high expression of fibronectin has been observed when
compared to that in normal breast parenchyma (9). The molecular mechanisms of fibronectin
in the progression of cancer cells have been identified as gene
expression changes induced by fibronectin. For instance,
fibronectin was found to enhance expression of MMPs which are also
key factors in the promotion of cancer invasion and metastasis
(13,14). Meanwhile, fibronectin was found to
stimulate expression of inflammatory factors including IL-8, CXCL-3
and Toll-like receptor (TLR)2 indicating the regulatory influence
of fibronectin on major inflammatory cells in the tumor
microenvironment (15,16). However, the function of fibronectin
in CRC demands to be resolved.
This study investigated the significance and
therapeutic potential of fibronectin as well as the effects of
fibronectin knockdown on the prognosis, cell proliferation and
malignancy of CRC. Our findings demonstrated that silencing of
fibronectin mediated by RNAi promoted G1 cell cycle arrest, leading
to apoptosis in CRC cells which could be a potential treatment
strategy.
Materials and methods
Patients
In total, 107 patients, who were diagnosed with CRC
at The Second Xiangya Hospital of Central South University, were
involved in the present study. The characteristics of these
patients are shown in Table I.
Normal control tissues were collected from 115 patients without
CRC. The tissues were embedded in formalin-fixed paraffin. The
follow-up study was performed for 60 months. All of the experiments
were approved by the Ethics Committee of the Second Xiangya
Hospital of Central South University and informed consent was
provided by the patients themselves or their relatives.
 | Table I.Correlation between characteristics
of the CRC patients and fibronectin expression. |
Table I.
Correlation between characteristics
of the CRC patients and fibronectin expression.
|
|
| Fibronectin
expression |
|
|---|
|
|
|
|
|
|---|
|
Characteristics | n | Low | High | P-value |
|---|
| Total | 107 | 49 | 58 |
|
| Age (years) |
|
|
|
|
|
<60 | 55 | 32 | 23 | 0.459 |
|
≥60 | 52 | 27 | 25 |
|
| Gender |
|
|
|
|
|
Male | 58 | 28 | 30 | 0.542 |
|
Female | 49 | 27 | 22 |
|
| TNM stage |
|
|
|
|
| I | 7 | 3 | 4 | 0.0025 |
| II | 47 | 17 | 30 |
|
|
III | 33 | 10 | 23 |
|
| VI | 20 | 3 | 17 |
|
| Distant
metastasis |
|
|
|
| No | 32 | 21 | 11 | 0.0013 |
|
Yes | 75 | 18 | 57 |
|
Immunohistochemistry and scoring
Protein expression was assayed by
immunohistochemistry using the paraffin-embedded sections from the
patients. The sections were first deparaffinized by xylene
(Shanghai Sangon, Shanghai, China). Then, the sections were
incubated with rabbit polyclonal anti-fibronectin antibody (1:500,
ab2413; Abcam, Cambridge, MA, USA) at 4°C for 24 h. After washing
with TBST buffer (Shanghai Sangon) for three times, the sections
were incubated with goat anti-rabbit IgG H&L (HRP, 1:500,
ab6721; Abcam) at room temperature for 1 h. Then, protein
immunostaining was performed using DAB Plus substrate (Thermo
Fisher Scientific, Waltham, MA, USA) following the manufacturer's
instructions. The scoring of the stained tumor cells were divided
into five grades: 0 (positive cells <5%), 1 (positive cells
between 5 and 25%), 2 (positive cells between 26 and 50%), 3
(positive cells between 51 and 75%), and 4 (positive cells between
75 and 100%). The cells were observed using a light microscope
(Axioskop; Zeiss, Germany). We designated grade 1 and 2 as low
expression of fibronectin and grade 3 and 4 as high expression of
fibronectin for the prognosis analysis.
Cell culture and transfection
CCD-18Co (normal colon cells), HT-29 (CRC cells),
CaCo2 (CRC cells) and SW480 (CRC cells) cell lines were obtained
from the American Type Culture Collection (ATCC; Rockville, MD,
USA). The cells were cultured in RPMI-1640 medium (Gibco-BRL,
Paisley, UK) supplemented with 10% fetal bovine serum (Hyclone,
Logan, UT, USA) at 37°C in 5% CO2. The fibronectin siRNA
(sc-29315) and control siRNA (sc-37007) were purchased from Santa
Cruz Biotechnology (Santa Cruz, CA, USA). Transfection was
performed using Lipofectamine® 2000 (Invitrogen Life
Technologies, Shanghai, China) following the manufacturer's
instructions. Negative small interfering RNAs (siRNAs) and
scrambled siRNA were synthesized by Shanghai Sangon.
Real-time quantitative PCR (qPCR)
RNA isolation and reverse transcription were
performed using RNAiso Plus kit and PrimeScript™ II First Strand
cDNA Synthesis kit (both from Takara, Dalian, China) according to
the manufacturer's instructions, respectively. The qPCR primers
were: fibronectin: forward, 5′-TTATG ACGAC GGGAA GACCT-3′ and
reverse, 5′-GCTGG ATGGA AAGAT TACTC-3′; GADPH forward, 5′-AATCC
CATCA CCATC TTCCA-3′ and reverse, 5′-TGGAC TCCAC GACGT ACTCA-3′;
caspase-3 forward, 5′-TTAAT AAAGG TATCC ATGGA GAACA CT-3′ and
reverse, 5′-TAGAG TTCTT TTGTG AG-3′; p53 forward, 5′-AACGG TACTC
CGCCA CC-3′ and reverse, 5′-CGTGT CACCG TCGTG GA-3′; PARP forward,
5′-AGGCT GCTTT GTCAA GAA-3′ and reverse, 5′-CTTGC TGCTT GTTGA
AGAT-3′; Bax forward, 5′-ACCAA GCTGA GCGAG TGTC-3′ and reverse,
5′-ACAAA GATGG TCACG GTCTG CC-3′; cytochrome c forward,
5′-CGTCG CATTC CAGAT TATCC A-3′ and reverse, 5′-CAACT ACGGA TATAT
AAGAG CCAAA ACTG-3′; and NF-κB forward, 5′-ACCTG AGTCT TCTGG ACCGC
TG-3′ and reverse 5′-CCAGC CTTCT CCCAA GAGTC GT-3′. The reaction
was performed on Applied Biosystems® ABI 7500 system
(Thermo Fisher Scientific). The reaction system SYBR®
Premix Ex Taq™ II (Takara) was used according to the manufacturers
instructions. The reaction condition was: 95°C for 15 min and 40
cycles of 95°C for 10 sec and 60°C for 10 sec. Melting curve
analysis was used to determine the specific amplification.
Western blot analysis
SW480 cells were collected at the treatment
time-points with reagents as suggested in each experiment. Cells
were lysed with RIPA buffer (Thermo Fisher Scientific) and 15%
electrophoresis (Thermo Fisher Scientific) was conducted under 90 V
for 2 h. After undergoing electrophoresis, the proteins were
transferred to polyvinylidene difluoride membranes. Primary
antibodies, rabbit polyclonal anti-fibronectin antibody (1:500),
rabbit polyclonal caspase-3 antibody (1:500, ab2302), rabbit
polyclonal NF-κB antibody (1:500, ab7971), rabbit polyclonal p53
antibody (1:500, ab1431), rabbit polyclonal PARP (1:500, ab6079),
rabbit polyclonal Bax antibody (1:500, ab53154), rabbit polyclonal
cytochrome c antibody (1:500, ab90529) and rabbit polyclonal
GAPDH antibody (1:500, ab9485) (all from Abcam) were incubated with
the membranes for 24 h at 4°C. After washing with TBST buffer three
times, the secondary antibody goat anti-rabbit IgG H&L (HRP)
was incubated at room temperature for 1 h. Immunostaining was
carried out using DAB Plus substrate and chemiluminescence system
(Amersham Biosciences, Freiburg, Germany). The results were
analyzed using chemiluminescence Molecular Imager®
ChemiDoc™ XRS+ system (Bio-Rad Laboratories, Inc., Hercules, CA,
USA).
MTT assay
In order to analyze the viability of the cells, the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay was performed. After undergoing transfection, the cells were
seeded in 96-well plates at a density of 1×104
cells/well for 24, 48 and 72 h. Consequently, 20 µl MTT (5 mg/ml;
Sigma-Aldrich, St. Louis, MO, USA) was added to the wells and
incubated at 37°C for 4 h. Then, the supernatant was removed, and
the cells were dissolved in 200 µl of dimethyl sulfoxide
(Sigma-Aldrich). The optical density was observed at 490 nm via a
spectrophotometer (SpectraMax Plus 384; Molecular Devices,
Sunnyvale, CA, USA). The experiments were performed in
triplicate.
Cell migration and invasion
assays
Transwell assays were performed using a 24-well
insert (Corning, Inc., Corning, NY, USA) to analyze the effect of
fibronectin on CRC cells. After transfection, the cells
(1×104 cells/well) were seeded in the top of the
chambers in triplicate for 48 h. The lower chambers with 10% fetal
bovine serum were co-cultured for another 72 h. For the invasion
assay, extracellular matrix gel (BD Biosciences, Bedford, MA, USA)
was used. The cells located on the top surface of the membrane were
discarded and the cells on the bottom surface were stained with
0.1% crystal violet (Shanghai Sangon). The number of cells on each
insert were calculated in five visual fields randomly and
calculated using a light microscope (Axioskop; Zeiss).
Flow cytometry
The cell cycle distribution was assessed by flow
cytometry. The control and transfected cells (48 h after
transfection) were collected and washed with PBS buffer (Shanghai
Sangon). After fixation in 70% ethanol, the cells were stained
using 20 µg/ml propidium iodide (PI) containing 20 µg/ml RNase
(DNase-free) (both from Shanghai Sangon) for 2 h. Then the cells
were assessed using flow cytometer Partec-PAS (Partec GmbH,
Muenster, Germany). The cells in the G0/G1,
S, G2/M, and sub-G1 phases were defined via
Multicycle Cell Cycle software (Phoenix Flow System, San Diego, CA,
USA).
Tumor formation assays
Female nude mice (6 to 8-weeks old) were provided by
The Second Xiangya Hospital of Central South University. To obtain
the tumor in vivo model, equal numbers of SW480 cells
(1×106) or SW480 cells transfected with control siRNAs
and fibronectin-siRNA were injected into the nude mice (four mice
per group). After injection, the mice were maintained under a 12-h
light/12-h dark cycle and fed with standard mouse diet for four
weeks. The tumor volume was measured and calculated as
(width2 × length)/2. The mice were then sacrificed using
sodium pentobarbital (Sigma-Aldrich) and the tumor tissues were
collected for further analysis. The study was performed following
the recommendations of the Guide for the Care and Use of Laboratory
Animals of The Second Xiangya Hospital of Central South
University.
Statistical analysis
All the results are presented as mean ± standard
deviation. Data analyses were processed via the two-tailed
Student's t-test and the log-rank test. P<0.05 was considered as
significant. SPSS v17.0 software was used to perform these analyses
(SPSS Inc., Chicago, IL, USA).
Results
Upregulation of fibronectin in CRC
tissues
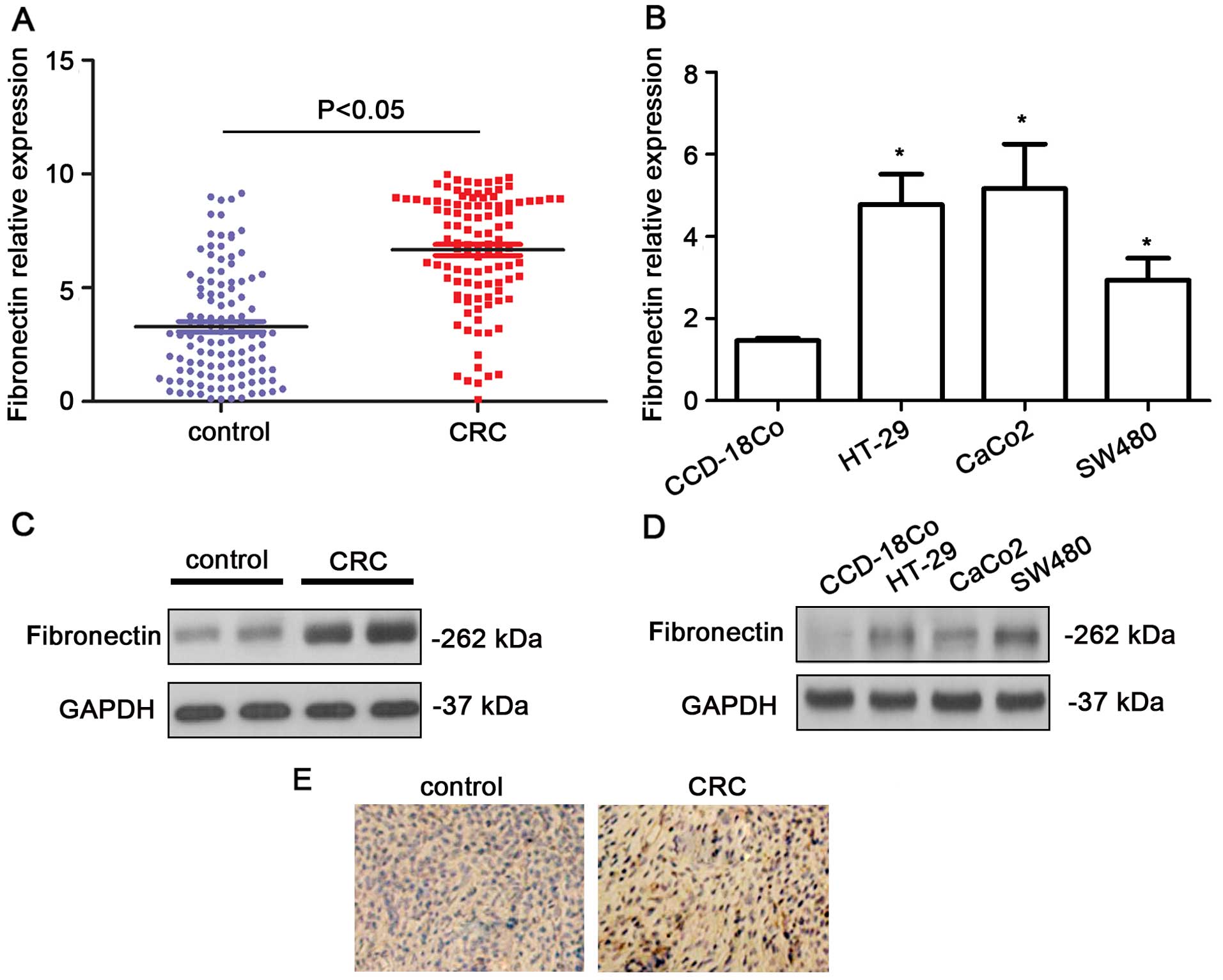
The expression levels of fibronectin were shown to
be upregulated in the CRC tissues in comparison with that in the
normal colon tissues using qPCR (P<0.05, Fig. 1A). Higher expression levels of
fibronectin were also noted in the CRC cell lines (P<0.05,
Fig. 1B). Western blot analysis
indicated that the fibronectin blot was remarkably denser in the
CRC samples (Fig. 1C). In the CRC
cell lines, the protein levels of fibronectin showed a significant
increase (Fig. 1D). In addition, as
shown in the immunohistochemistry results, the expression level of
fibronectin was obviously higher in the CRC tissues than that in
the non-cancerous, normal colorectal tissues (Fig. 1E). The fibronectin protein
expression was assessed in 107 CRC tissue samples by
immunohistochemistry. In the CRC tissues, 54.21% (58/107) of the
cases had high fibronectin expression (grade >2). Additionally,
we observed a marked difference in fibronectin expression in tumors
with different TNM stage (P=0.0025) and distant metastasis
(P=0.0013) (Table I).
Correlation between fibronectin
expression and prognosis in CRC patients
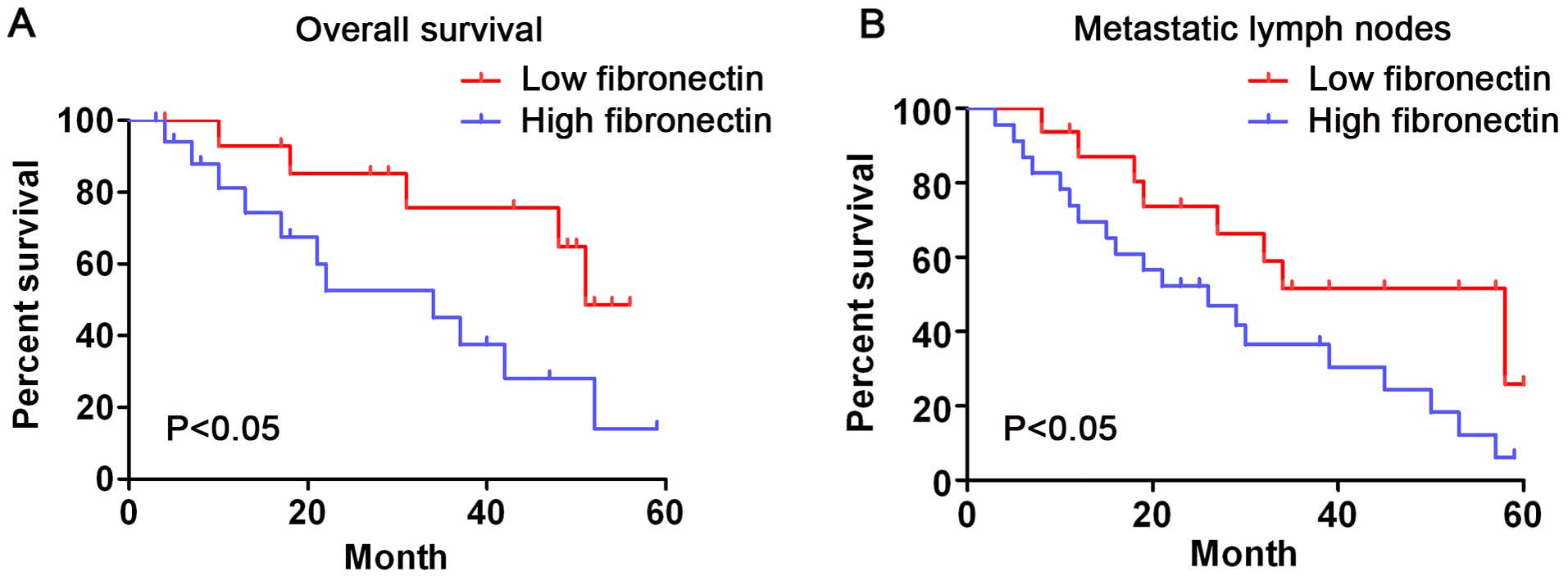
In short, the survival analysis showed that the
survival time of patients whose fibronectin expression was low
(grade 1–2) was significantly longer than that of those whose
fibronectin expression was high (grade 3–4) (log-rank test,
P<0.05, Fig. 2A). Patients with
metastatic lymph nodes whose fibronectin expression was low showed
a longer overall survival time than that in the patients whose
fibronectin expression was high (Fig.
2B, log-rank test, P<0.05). These outcomes indicate that
fibronectin expression is significantly related to the survival
rate of CRC patients.
Fibronectin-siRNA inhibits fibronectin
expression in the SW480 cells
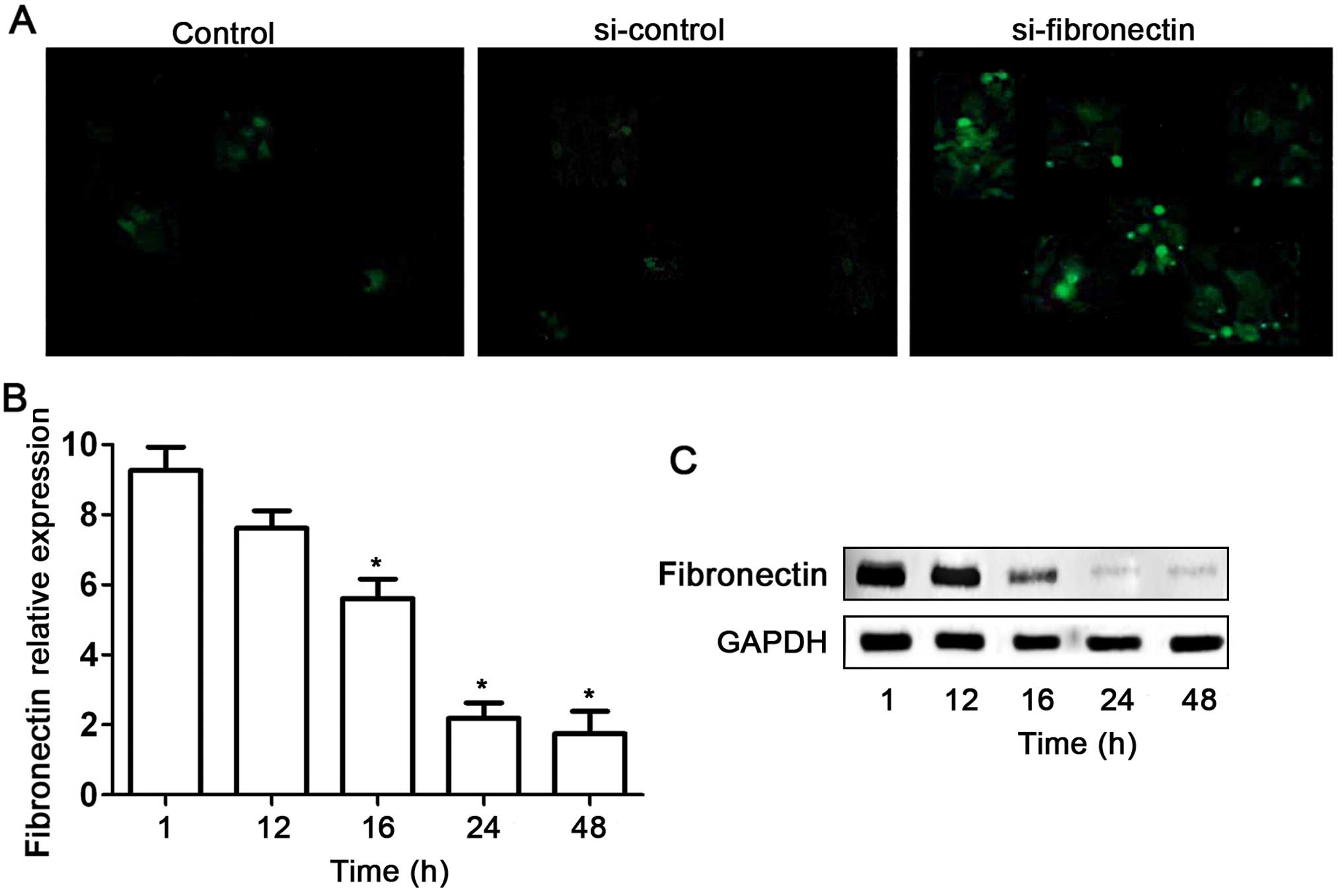
Based on our preliminary data of the high expression
level of fibronectin in SW480 cell lines, we used RNAi technique to
silence this gene in the SW480 cells. Fibronectin-siRNA
(si-fibronectin) effectively suppressed fibronectin expression in
the SW480 cells (Fig. 3). First,
si-fibronectin was successfully transfected into the SW480 cells as
indicated by fluorescent labels (Fig.
3A). Meanwhile the efficiency of the transfection had a
time-dependent effect (Fig. 3B).
Although no obvious decrease in expression of fibronectin was found
after si-fibronectin transfection of SW480 cells for 12 h,
fibronectin mRNA and protein levels were significantly suppressed
at 16, 24 and 48 h (Fig. 3B and
C).
Knockdown of fibronectin suppresses
CRC cell proliferation, migration and invasion
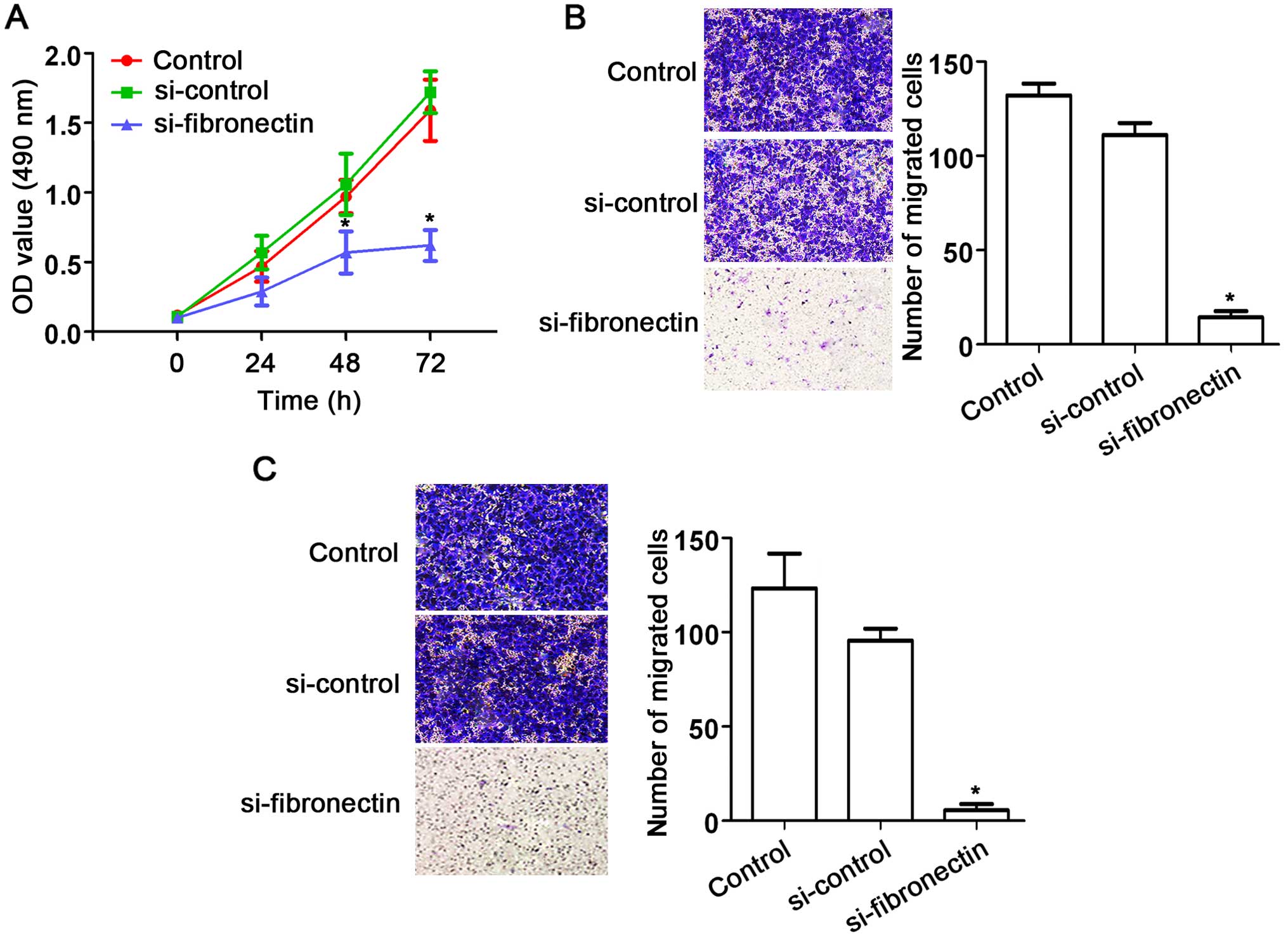
The effects of fibronectin on CRC SW480 cells were
assessed using siRNA approach. After transfection with
si-fibronectin, cell proliferation was significantly decreased
compared to that observed in the control and si-control groups
(Fig. 4A). In addition, fibronectin
knockdown also suppressed cell migration (Fig. 4B) and cell invasion (Fig. 4C) of the SW480 cells. These results
indicate the suppression of cell activities by fibronectin
knockdown.
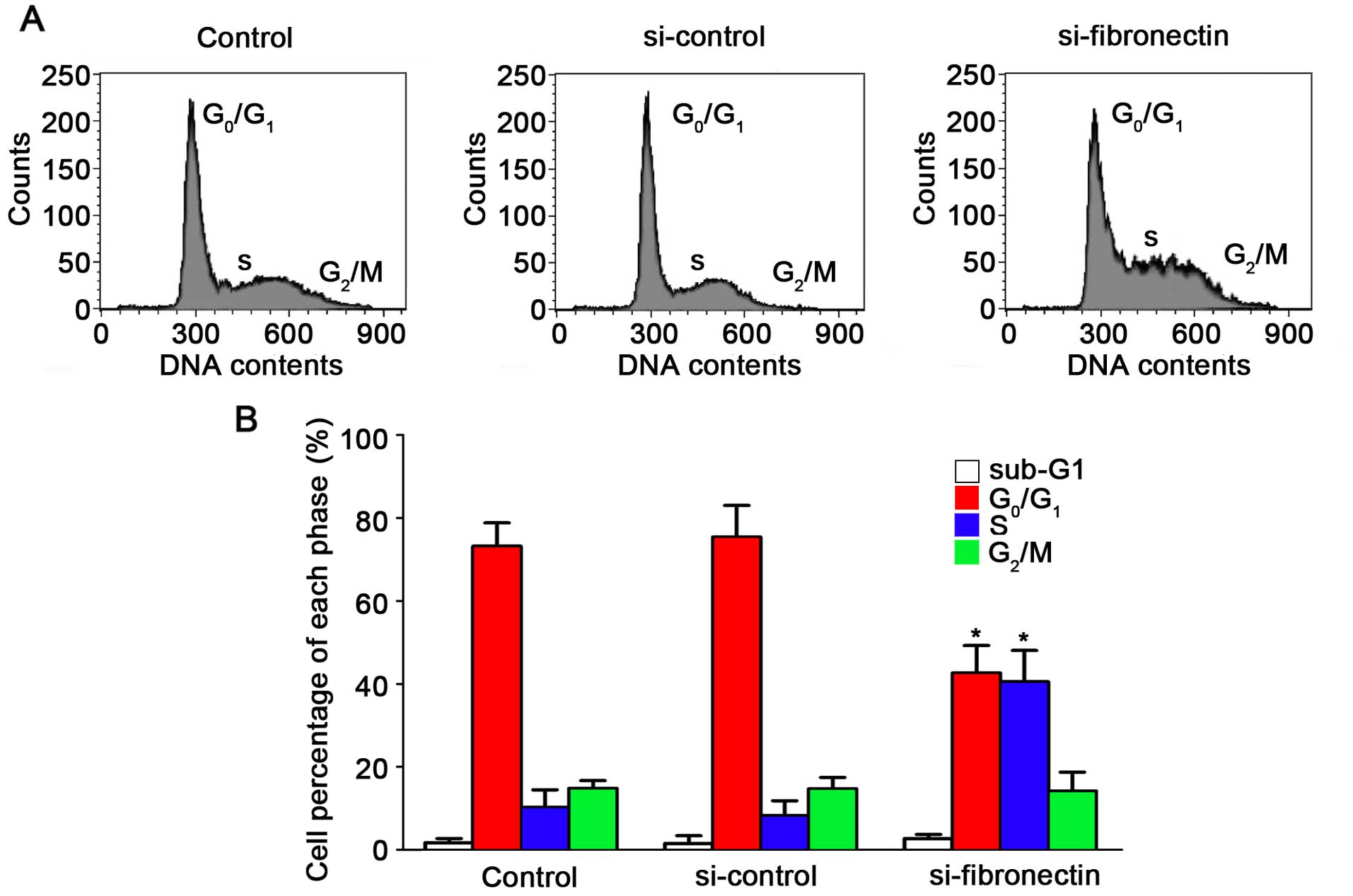
Knockdown of fibronectin causes
S-phase cell cycle arrest in CRC cells
Effects of the silencing of fibronectin on the
suppression of cell progression have been proven. To demonstrate
the effect of fibronectin knockdown on the cell cycle, flow
cytometry was performed. The results for the SW480 cells are shown
in Fig. 5A after transfection with
si-fibronectin, si-control and in the untreated control. After
transfection with si-fibronectin, the percentage of cells in the S
phase increased to 40.54% which was significantly higher compared
to the control and si-control groups (Fig. 5B). The percentage of cells in the
G0/G1 phases after si-fibronectin
transfection was decreased to 42.62% (Fig. 5B). Hence, these results showed that
the si-fibronectin transfection suppressed CRC cell viability. The
cells could not proliferate normally by arrest in the S phase of
the cell cycle and S-phase arrest may induce cell death and
morphological changes during progression of CRC.
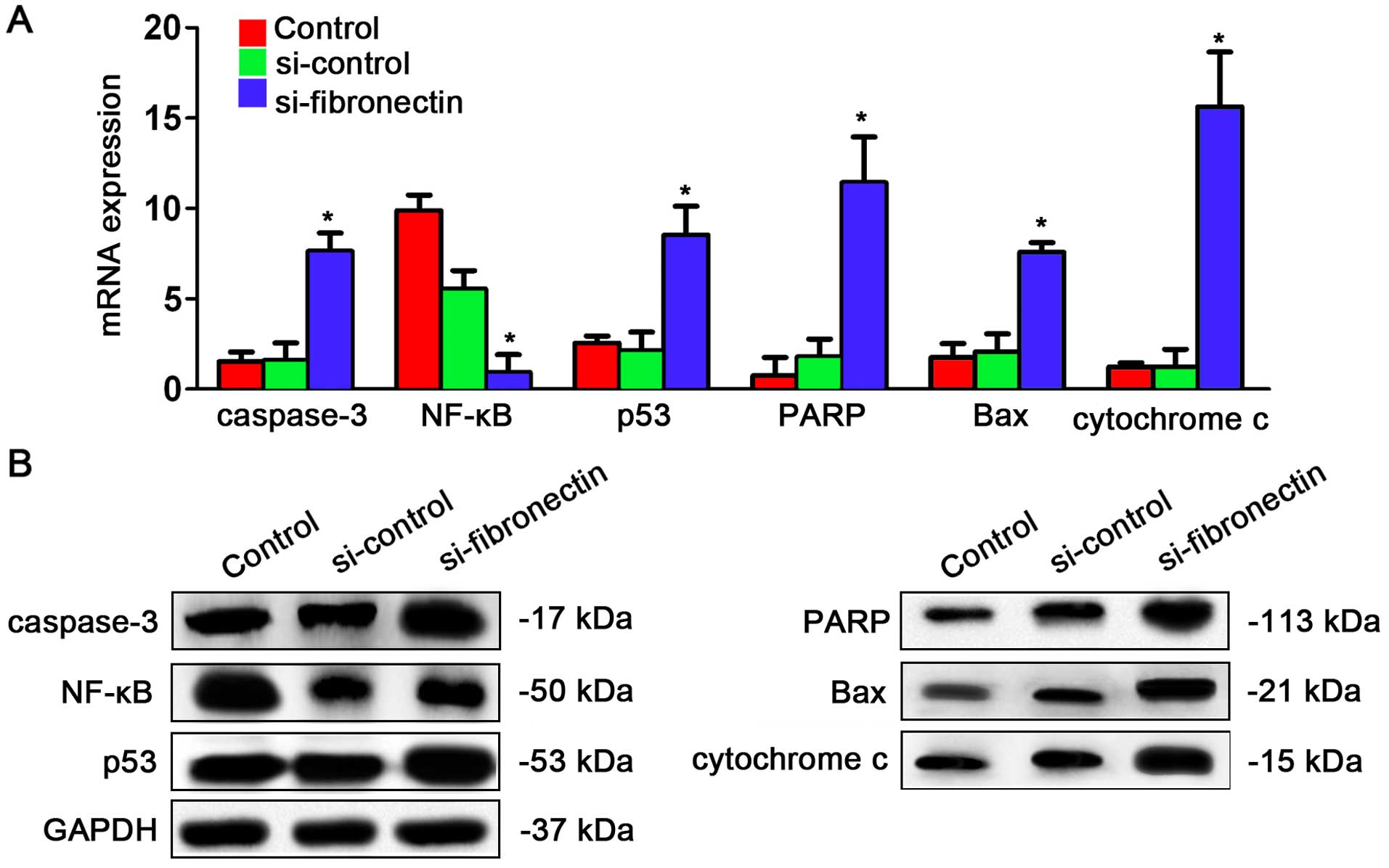
Fibronectin-siRNA transfection affects
expression of genes in the NF-κB/p53-apoptosis signaling pathway in
CRC cells
After understanding the effects of si-fibronectin
transfection on the cell cycle, we explored whether the
NF-κB/p53-apoptosis signaling pathway is regulated by fibronectin.
The qPCR results showed that caspase-3, p53, PARP, Bax and
cytochrome c were significantly increased after
si-fibronectin transfection while only NF-κB was significantly
depressed by si-fibronectin transfection (Fig. 6A). Similar results were observed for
the protein expression levels. Caspase-3, p53, PARP, Bax and
cytochrome had higher expression after si-fibronectin transfection
while NF-κB had lower expression following si-fibronectin
transfection (Fig. 6B).
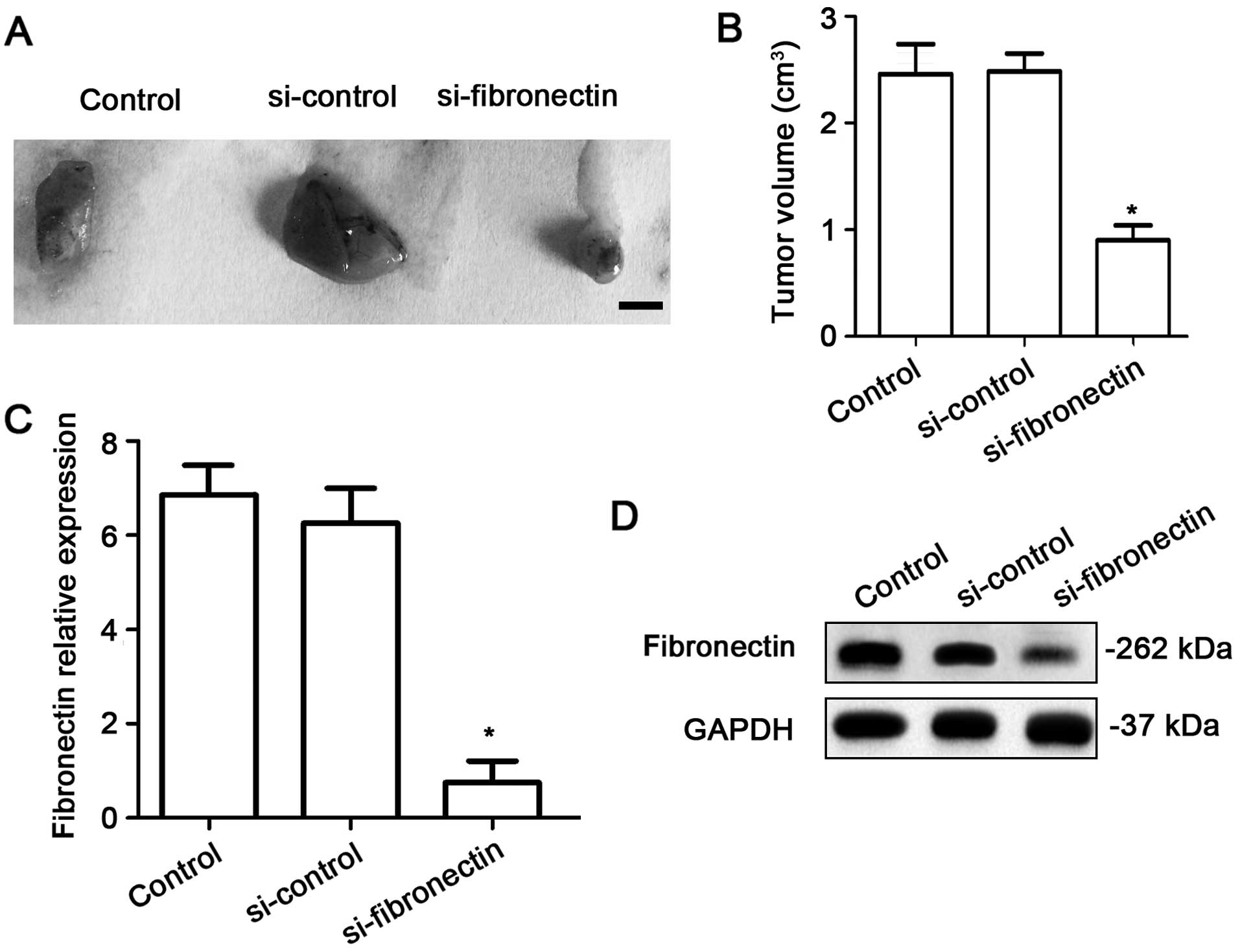
Fibronectin-siRNA transfection
decreases tumor growth in vivo
To investigate the effects of fibronectin on tumor
growth in vivo, the nude mice were injected using equal
numbers of SW480 cells (1×106) or SW480 cells
transfected with si-control or si-fibronectin. After 4 weeks,
tumors significantly appeared in the mice. The results showed that
si-fibronectin inhibited tumor growth in vivo (Fig. 7A and B). The expression of
fibronectin was measured using qPCR and western blotting. Both at
the RNA (Fig. 7C) and protein
levels (Fig. 7D), the fibronectin
expression was significantly decreased in the tumor tissue after
transfection with si-fibronectin.
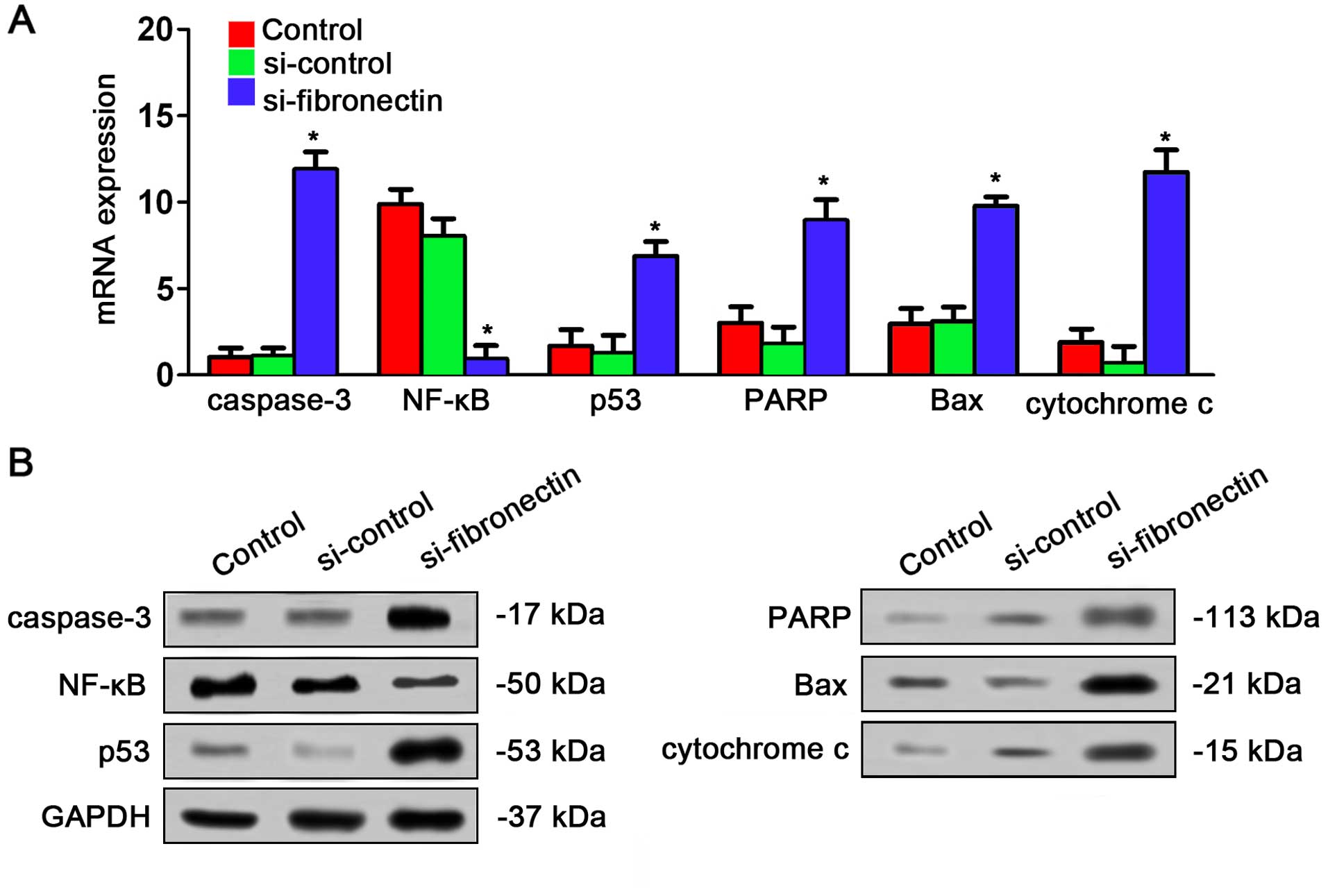
Fibronectin-siRNA affects expression
of the NF-κB/p53-apoptosis signaling pathway in vivo
The effects of si-fibronectin on expression of
NF-κB/p53-apoptosis signaling pathway in nude mice were assayed by
qPCR and western blot analysis. The result was very similar to the
results in vitro. qPCR indicated that caspase-3, p53, PARP,
Bax and cytochrome c were significantly increased by
si-fibronectin transfection in the tumor tissues from the nude mice
while NF-κB was decreased after transfection (Fig. 8A). The result of western blotting
also showed that caspase-3, p53, PARP, Bax and cytochrome c
had higher expression after si-fibronectin transfection while NF-κB
had lower expression after si-fibronectin transfection (Fig. 8B).
Discussion
CRC is one of the most dangerous cancers; thus, the
studies of CRC hold great value for human health. To date, the CRC
incidence and mortality worldwide have increased dramatically over
the past few decades (17). Early
detection and new treatment strategies to decrease death rates are
needed. Thus, the survival will be increased. However, this
approach is limited due to the lack of screening tools with high
specificity and sensitivity, appropriate for early-stage tumors
(18,19). In this way, understanding the
pathogenic mechanisms of CRC is urgently needed at present. It has
been indicated that fibronectin is a multifunctional, extracellular
matrix glycoprotein and takes part in regulating self-renewal, cell
cycle progression and proliferation in cancer cells (20,21).
To investigate the role of fibronectin in CRC, we assayed the
expression of fibronectin in CRC patient tissues and cell lines. As
far as we known, studies on the significant role of fibronectin in
CRC are limited. We found that fibronectin was highly expressed in
HT-29, CaCo2 and SW480 cells. This result is in line with previous
studies on the basis of fibronectin overexpression in other types
of human cancer cell lines (20,22).
In the present study, we found that expression of
fibronectin was associated with cancer cell metastasis, TNM stage
and survival. Moreover, no correlations were significant for age or
gender of the patients. These results suggest that high expression
of fibronectin could be regarded as a prognostic marker for CRC
diagnosis. Remarkably high fibronectin expression has been observed
in various human cancers, such as lung (21), bladder (23), ovarian (12) and breast cancers (12). Nevertheless, the mechanism involved
in the modulation of CRC by fibronectin remains unexplained. In our
study, fibronectin had higher expression in CRC tissues in
comparison with its expression level in non-cancerous tissues,
which indicates a similar expression pattern of fibronectin in
different cancers.
In human breast cells, fibronectin overexpression
has been found to promote chemotaxis of human malignant plasma cell
lines and to stimulate cell invasion as well as migration (24). Additionally, in the present study,
fibronectin was found to be a suitable potential prognostic marker
for CRC. Meanwhile, high expression of fibronectin was negatively
related with the prognosis of patients with breast cancer (9). Based on these results, a worse outcome
was hypothesized in CRC patients who had higher fibronectin
expression. The multivariate analysis demonstrated that high
expression of fibronectin could be an independent prognostic
parameter for CRC patients. In the cell lines, fibronectin-siRNA
transfection remarkably depressed proliferation and invasion of CRC
cells. Taken together, apart from acting as a prognostic marker,
fibronectin could be a candidate therapeutic target.
In the present study, the functions of fibronectin
in the progression of the cell cycle and apoptosis were defined by
fibronectin-siRNA. These oligos resulted in a remarkable reduction
in fibronectin mRNA expression. Knockdown of fibronectin suppressed
the increase in CRC cells after transfection. Apoptosis was also
induced by transfection. Silencing of fibronectin in SW480 cells
led to suppression of growth. All of these findings were coincident
with the cell cycle results, in which we detected an aggregation in
the S phase after fibronectin-siRNA transfection. To understand
apoptosis-related pathways, we detected the effects of
fibronectin-siRNA transfection on the NF-κB/p53 apoptosis signaling
pathway. The results showed that except for NF-κB, all the genes
were significantly increased by fibronectin-siRNA transfection.
NF-κB is a key factor which has transcriptional activation to
regulate multiple gene expression and participates in cell
proliferation, vasculogenesis and tumor metastasis (25,26).
Suppression of NF-κB activity could induce the sensitivity of
cancer cells to chemotherapy and radiotherapy (27). In addition, inhibition of NF-κB
increased p53 and promoted apoptosis in cancer cells by increasing
DNA damage (27). On the contrary,
caspase-3, p53, PARP, Bax and cytochrome c were upregulated
by fibronectin-siRNA transfection. These five genes are markers for
apopotsis. Caspase-3 has a typical role in apoptosis by affecting
chromatin condensation and DNA fragmentation (28). p53, an anticancer protein, plays a
role in apoptosis in cancers cells and inhibition of angiogenesis
(29). PARP maintains the
structural stability of chromosomes and helps DNA replication and
transcription (30). Bax
participates in the p53 pathway which also induces apoptosis in
cancer cells (31). Cytochrome
c is an intermediary of apoptosis which controls cell death
in response to DNA damage (32).
These genes control the cell cycle and apoptosis of cancer cells.
The present findings suggest that fibronectin-siRNA transfection
suppressed CRC cell growth via the NF-κB/p53-apoptosis signaling
pathway and arrested the cells in the S phase. Knockdown of
fibronectin could be a candidate treatment strategy.
Taken together, fibronectin has the potential to be
a diagnostic biomarker of CRC and high fibronectin is related to
the poor prognosis of CRC patients. Knockdown of fibronectin may be
helpful for the treatment of CRC. Further investigations are needed
to identify the gene therapy strategies and efficacy of these
approaches. The mechanisms of the modulation of CRC development by
fibronectin need to be elucidated in more detail.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang S, Xiang J, Li Z, Lu S, Hu J, Gao X,
Yu L, Wang L, Wang J, Wu Y, et al: A plasma microRNA panel for
early detection of colorectal cancer. Int J Cancer. 136:152–161.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nishihara R, Morikawa T, Kuchiba A,
Lochhead P, Yamauchi M, Liao X, Imamura Y, Nosho K, Shima K,
Kawachi I, et al: A prospective study of duration of smoking
cessation and colorectal cancer risk by epigenetics-related tumor
classification. Am J Epidemiol. 178:84–100. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pierschbacher MD and Ruoslahti E: Cell
attachment activity of fibronectin can be duplicated by small
synthetic fragments of the molecule. Nature. 309:30–33. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pankov R and Yamada KM: Fibronectin at a
glance. J Cell Sci. 115:3861–3863. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Francis SE, Goh KL, Hodivala-Dilke K,
Bader BL, Stark M, Davidson D and Hynes RO: Central roles of α5β1
integrin and fibronectin in vascular development in mouse embryos
and embryoid bodies. Arterioscler Thromb Vasc Biol. 22:927–933.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rybak J-N, Roesli C, Kaspar M, Villa A and
Neri D: The extra-domain A of fibronectin is a vascular marker of
solid tumors and metastases. Cancer Res. 67:10948–10957. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Santimaria M, Moscatelli G, Viale GL,
Giovannoni L, Neri G, Viti F, Leprini A, Borsi L, Castellani P,
Zardi L, et al: Immunoscintigraphic detection of the ED-B domain of
fibronectin, a marker of angiogenesis, in patients with cancer.
Clin Cancer Res. 9:571–579. 2003.PubMed/NCBI
|
|
9
|
Ioachim E, Charchanti A, Briasoulis E,
Karavasilis V, Tsanou H, Arvanitis DL, Agnantis NJ and Pavlidis N:
Immunohistochemical expression of extracellular matrix components
tenascin, fibronectin, collagen type IV and laminin in breast
cancer: Their prognostic value and role in tumour invasion and
progression. Eur J Cancer. 38:2362–2370. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Saad S, Gottlieb DJ, Bradstock KF, Overall
CM and Bendall LJ: Cancer cell-associated fibronectin induces
release of matrix metalloproteinase-2 from normal fibroblasts.
Cancer Res. 62:283–289. 2002.PubMed/NCBI
|
|
11
|
Meng XN, Jin Y, Yu Y, Bai J, Liu GY, Zhu
J, Zhao YZ, Wang Z, Chen F, Lee KY, et al: Characterisation of
fibronectin-mediated FAK signalling pathways in lung cancer cell
migration and invasion. Br J Cancer. 101:327–334. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xing H, Weng D, Chen G, Tao W, Zhu T, Yang
X, Meng L, Wang S, Lu Y and Ma D: Activation of
fibronectin/PI-3K/Akt2 leads to chemoresistance to docetaxel by
regulating survivin protein expression in ovarian and breast cancer
cells. Cancer Lett. 261:108–119. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Stanton H, Gavrilovic J, Atkinson SJ,
d'Ortho MP, Yamada KM, Zardi L and Murphy G: The activation of
ProMMP-2 (gelatinase A) by HT1080 fibrosarcoma cells is promoted by
culture on a fibronectin substrate and is concomitant with an
increase in processing of MT1-MMP (MMP-14) to a 45 kDa form. J Cell
Sci. 111:2789–2798. 1998.PubMed/NCBI
|
|
14
|
Esparza J, Vilardell C, Calvo J, Juan M,
Vives J, Urbano-Márquez A, Yagüe J and Cid MC: Fibronectin
upregulates gelatinase B (MMP-9) and induces coordinated expression
of gelatinase A (MMP-2) and its activator MT1-MMP (MMP-14) by human
T lymphocyte cell lines. A process repressed through RAS/MAP kinase
signaling pathways. Blood. 94:2754–2766. 1999.PubMed/NCBI
|
|
15
|
La Fleur M, Beaulieu AD, Kreis C and
Poubelle P: Fibronectin gene expression in polymorphonuclear
leukocytes. Accumulation of mRNA in inflammatory cells. J Biol
Chem. 262:2111–2115. 1987.PubMed/NCBI
|
|
16
|
Hines KL, Kulkarni AB, McCarthy JB, Tian
H, Ward JM, Christ M, McCartney-Francis NL, Furcht LT, Karlsson S
and Wahl SM: Synthetic fibronectin peptides interrupt inflammatory
cell infiltration in transforming growth factor beta 1 knockout
mice. Proc Natl Acad Sci USA. 91:5187–5191. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Center MM, Jemal A and Ward E:
International trends in colorectal cancer incidence rates. Cancer
Epidemiol Biomarkers Prev. 18:1688–1694. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huang Z, Huang D, Ni S, Peng Z, Sheng W
and Du X: Plasma microRNAs are promising novel biomarkers for early
detection of colorectal cancer. Int J Cancer. 127:118–126. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Srivastava S, Verma M and Henson DE:
Biomarkers for early detection of colon cancer. Clin Cancer Res.
7:1118–1126. 2001.PubMed/NCBI
|
|
20
|
Fornaro M, Plescia J, Chheang S, Tallini
G, Zhu YM, King M, Altieri DC and Languino LR: Fibronectin protects
prostate cancer cells from tumor necrosis factor-α-induced
apoptosis via the AKT/survivin pathway. J Biol Chem.
278:50402–50411. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sethi T, Rintoul RC, Moore SM, MacKinnon
AC, Salter D, Choo C, Chilvers ER, Dransfield I, Donnelly SC,
Strieter R, et al: Extracellular matrix proteins protect small cell
lung cancer cells against apoptosis: A mechanism for small cell
lung cancer growth and drug resistance in vivo. Nat Med. 5:662–668.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shibata K, Kikkawa F, Nawa A, Thant AA,
Naruse K, Mizutani S and Hamaguchi M: Both focal adhesion kinase
and c-Ras are required for the enhanced matrix metalloproteinase 9
secretion by fibronectin in ovarian cancer cells. Cancer Res.
58:900–903. 1998.PubMed/NCBI
|
|
23
|
Eissa S, Swellam M, Sadek M, Mourad MS, El
Ahmady O and Khalifa A: Comparative evaluation of the nuclear
matrix protein, fibronectin, urinary bladder cancer antigen and
voided urine cytology in the detection of bladder tumors. J Urol.
168:465–469. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shibayama H, Tagawa S, Hattori H, Inoue R,
Katagiri S and Kitani T: Laminin and fibronectin promote the
chemotaxis of human malignant plasma cell lines. Blood. 86:719–725.
1995.PubMed/NCBI
|
|
25
|
Baldwin AS Jr: The NF-κB and IκB proteins:
New discoveries and insights. Annu Rev Immunol. 14:649–683. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Beg AA and Baltimore D: An essential role
for NF-kappaB in preventing TNF-α-induced cell death. Science.
274:782–784. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chariot A: The NF-kappaB-independent
functions of IKK subunits in immunity and cancer. Trends Cell Biol.
19:404–413. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Porter AG and Jänicke RU: Emerging roles
of caspase-3 in apoptosis. Cell Death Differ. 6:99–104. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Feng Z, Hu W, Rajagopal G and Levine AJ:
The tumor suppressor p53: Cancer and aging. Cell Cycle. 7:842–847.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Curtin NJ: PARP inhibitors for cancer
therapy. Expert Rev Mol Med. 7:1–20. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mackey TJ, Borkowski A, Amin P, Jacobs SC
and Kyprianou N: bcl-2/bax ratio as a predictive marker for
therapeutic response to radiotherapy in patients with prostate
cancer. Urology. 52:1085–1090. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kaufmann SH and Earnshaw WC: Induction of
apoptosis by cancer chemotherapy. Exp Cell Res. 256:42–49. 2000.
View Article : Google Scholar : PubMed/NCBI
|