Introduction
Lung cancer is one of the most common malignant
tumors. According to the statistics data (GLOBOCAN) issued by the
World Health Organization, lung cancer is the most common cancer
with the highest fatality rate for male in 2008; while for female,
it is the fourth common one with the fatality rate ranked the
second (1). In 2008, lung cancer
accounted for 13% (1.6 million) of new onset cancer cases, and 18%
of deaths (1.4 million) (2). Lung
cancer is the malignant tumor with the highest morbidity in China
as well. Its morbidity in 2003–2007 was 48.90/100,000, accounting
for 18.39% of all new onset cancer cases; lung cancer was ranked in
the top position among regions with cancer registry in China in
2003–2007 (3). The mortality rate
was 43.48/100,000, accounting for 25.30% of all new onset cancer
cases (4).
Similar to other chemotherapeutics, drug resistance
to Taxanes has always been a great obstacle for further enhancing
therapeutic effect of chemotherapy, prolonging patient lifetime and
improving QOL. A variety of factors may lead to drug resistance to
Taxanes, including gene mutation of α-tubulin and p-tubulin,
diversified constitutions of p-tubulin hypotype, overexpression of
P-gp and increasing of microtubule activity related to the changed
microtubule-associated protein. In addition, among a variety of
molecular pathways associated with cell cycle and cell apoptosis,
some changes on protein expression may be associated with
chemotherapy resistance.
Eukaryotic initiation factor-4E (EIF4E) is a
protooncogene detected recently. It plays significant role at the
initial period of protein synthesis (5). EIF4E overexpression can change
expression quantity of malignant tumor related genes. It is the
determinant of tumor malignant phenotype (6). It may make cells break through the
limitation of normal growth thus leading to carcinogenesis by
adjusting expression translation amount of specific malignant
related molecules (such as VEGF, cylinD1 and C-myc) (7). Generally, a small amount of expression
of eIF4E can maintain mRNA translation required by normal life
activities of cells. In case of overexpression, it may lead to
increasing of mRNA expression translation of a variety of protein
coding carcinoma and tumor promoters thus to promote genesis and
development of tumor. Research has shown that overexpression of
eIF4E exists in many malignant tumors (such as: bladder cancer,
cervical cancer, leukemia, head and neck malignant tumor, laryngeal
cancer, breast cancer, esophagus cancer, colon cancer, prostatic
cancer and bile duct carcinoma) (7,8).
Research has verified that resistance mechanism
involves a variety of factors, including drug accumulation
reduction, drug detoxification increase, changes of
drug-resistance-related genes, inhibition of tumor cell apoptosis
and enhancement of DNA rehabilitation capacity, and involvement of
multiple signal pathways and key cytokines (9). In the last two years, it has been
detected that some miRNAs are related to sensitivity and drug
resistance of chemotherapy. In case of expression upregulation or
downregulation, it will directly lead to expression level abnormity
of target gene protein in related pathways of drug resistance
(10). Eventually, drug sensitivity
of tumor cells is changed through signal pathway of the cell. Such
target genes include those related to cell apoptosis, drug
transporter, drug target, cell repairing and cell cycle regulation
(11). At present, it has been
detected that some miRNAs is associated with sensitivity of
chemical drug treatment of tumors (12). The present study investigated the
role of miR-141 regulation of EIF4E expression in docetaxel
chemoresistance of NSCLCs.
Materials and methods
Patients
This study was conducted in accordance with the
ethical principles of The Affiliated Hospital of Qingdao
University. NSCLC patients had a histological diagnosis of NSCLC
from The Affiliated Hospital of Qingdao University. All patients
provided written informed consent before undergoing any study
procedure. In total, docetaxel chemoresistant patients with NSCLCs
was 16 and non-docetaxel chemoresistant patients with NSCLCs was
8.
Real-time quantitative reverse
transcription-PCR
Total RNA of tumor samples or cancer cells was
isolated using the TRIzol reagent (Invitrogen). Reverse
transcription (RT)-PCR was accomplished by the PrimeScript RT
Reagent kit (Takara Biotechnology Co., Ltd., Dalian, China) using
1–2 µg total RNA. Real-time quantitative reverse transcription-PCR
was performed using SYBR Premix Ex Taq™ II (Takara Biotechnology
Co., Ltd.). Expression mRNA levels of miR-141 and EIF4E were
defined as positive controls for the 2−∆∆Ct calculation.
The designed PCR primers of miR-141 and EIF4E are shown in Table I.
 | Table I.The designed PCR primers of miR-141
and EIF4E. |
Table I.
The designed PCR primers of miR-141
and EIF4E.
| Gene | PCR primers |
|---|
| miR-141 | F:
5′-CACATCCACCTCCTCCACATC-3′ |
|
| R:
5′-AATGCGGCCGCAACTCAATCAACATCACCAT-3′ |
| U6 | F:
5′-CTCGCTTCGGCAGCACATATACT-3′ |
|
| R:
5′-ACGCTTCACGAATTTGCGTGTC-3′ |
| EIF4E | F:
5′-ATGGCGACTGTCGAACCG-3′ |
|
| R:
5′-ATTAGATTCCGTTTTCTCCTCTTCTG-3′ |
| GAPDH | F:
5′-AAGGGAAGGTTGCTGGATAGG-3′ |
|
| R:
5′-CACATCCACCTCCTCCACATC-3′ |
Cell culture
Human NSCLC H1299 and H2009 cells were from the Cell
Bank of Chinese Academy of Medical Science. Cells were cultured and
maintained in RPMI-1640 medium (Gibco, Life Technologies, Waltham,
MA, USA) including L-glutamine, supplemented with 10% (v/v) fetal
calf serum (Gibco, Life Technologies) and 1% (v/v)
penicillin/streptomycin (100,000 U/l penicillin, 10 mg/l
streptomycin, Sigma-Aldrich, St. Louis, MO, USA) at 37°C in a
humidified atmosphere containing 5% carbon dioxide.
miRNA inhibitors and miRNA mimics
Negative controls, miR-141 inhibitors and miR-141
mimics were purchased from Shanghai GenePharma Co. Ltd. (Shanghai,
China). Plasmid was transfected into cells using Lipofectamine 2000
(Invitrogen) according to the manufacturer's protocol,
respectively. Transfected cells were cultured for 24 h before
analysis.
Cell viability assay
Transfected cells were seeded into 96-well plates
(2×103 cells/well) and cell viability was assessed using
the 50 µl of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide (MTT) assay. After 4-h culture, DMSO was dissolved for 10
min and the absorbance of each well at 490 nm (A490) was read on a
spectrophotometer.
Apoptosis analyses
Transfected cells were seeded into 6-well plates
(1–2×106 cells/well) and stained with Annexin V-FITC
Apoptosis Detection kit (BD Biosciences, La Jolla, CA, USA) for 30
min in the dark. Then, cells were stained with propidium iodide
(PI) solution (BD Biosciences) for 10 min in the dark. Cell
apoptosis was measured using a FACS sorter and the data were
analyzed using ModFit (both from BD Biosciences).
Caspase-3 activity
Transfected cells were seeded into 6-well plates
(1–2×106 cells/well), extracted by cell lysis (Beyotime
Institute of Biotechnology) and then centrifuged at 13,000 × g for
30 min. Protein content was measured using BCA assay (Beyotime
Institute of Biotechnology). Total cellular proteins were cultured
with Ac-DEVD-pNA for 2 h at room temperature. The absorbance of
each well at 405 nm was read on a spectrophotometer.
Western blotting
Transfected cells were seeded into 6-well plates
(1–2×106 cells/well), extracted by cell lysis (Beyotime
Institute of Biotechnology) and then centrifuged at 13,000 × g for
30 min. Protein content was measured using BCA assay (Beyotime
Institute of Biotechnology). Total cellular proteins were separated
on an 8–10% SDS-PAGE, transferred onto a PVDF membrane (BD
Biosciences). Membrane was incubated with primary antibodies
against EIF4E (1:500, Cell Signaling Technology), VEGF (1:500, Cell
Signaling Technology), c-Myc (1:500, Cell Signaling Technology),
Bax (1:500, Cell Signaling Technology) and GAPDH (1:500, Cell
Signaling Technology) at 1:500 overnight at 4°C. Membrane was
washed with TBSA containing 5% skim milk powder for 1 h at room
temperature and then with anti-rabbit IgG (1:5000, Cell Signaling
Technology) for 1 h at room temperature. Detection was carried out
using ECL Plus Western Blotting Detection System (Amersham,
UK).
Statistical analysis
All statistical analyses were performed using SPSS
19.0 software (SPSS, Inc., Chicago, IL, USA). The results are
presented as mean ± standard error. The difference between means
was analyzed with ANOVA and then Student's t-test. A p-value of
<0.05 was considered to indicate a statistically significant
difference.
Results
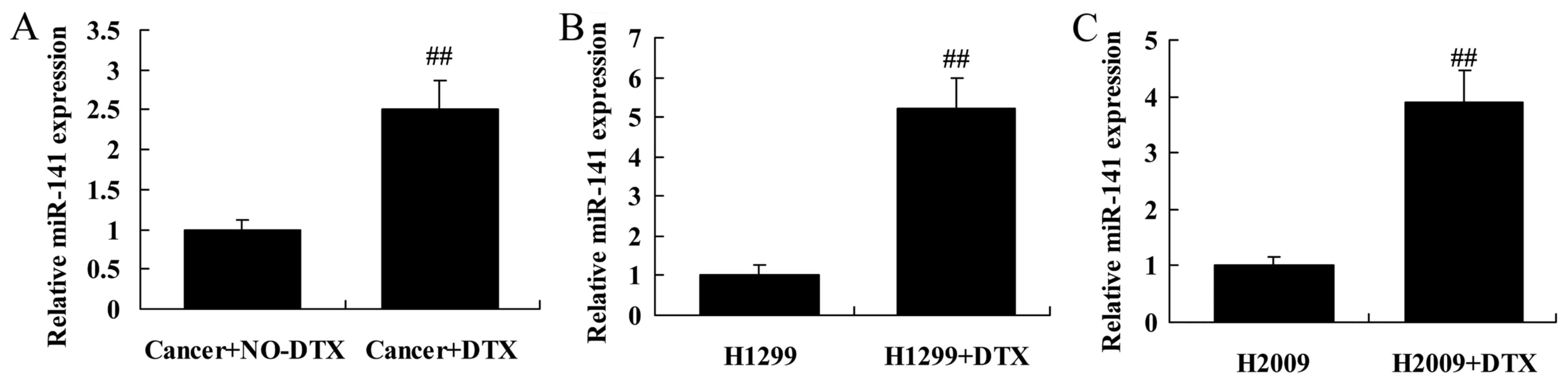
miR-141 expression in docetaxel
chemoresistance of human NSCLC patients
As shown in Fig. 1A,
the miRNA expression of miR-141 in docetaxel chemoresistant
patients with NSCLCs was markedly higher than those of
non-docetaxel chemoresistant patients with NSCLCs. The miRNA
expression of miR-141 in H1299 and H2009 cells treated by docetaxel
was also higher than that of H1299 and H2009 cells treated by
non-docetaxel group (Fig. 1B and
C).
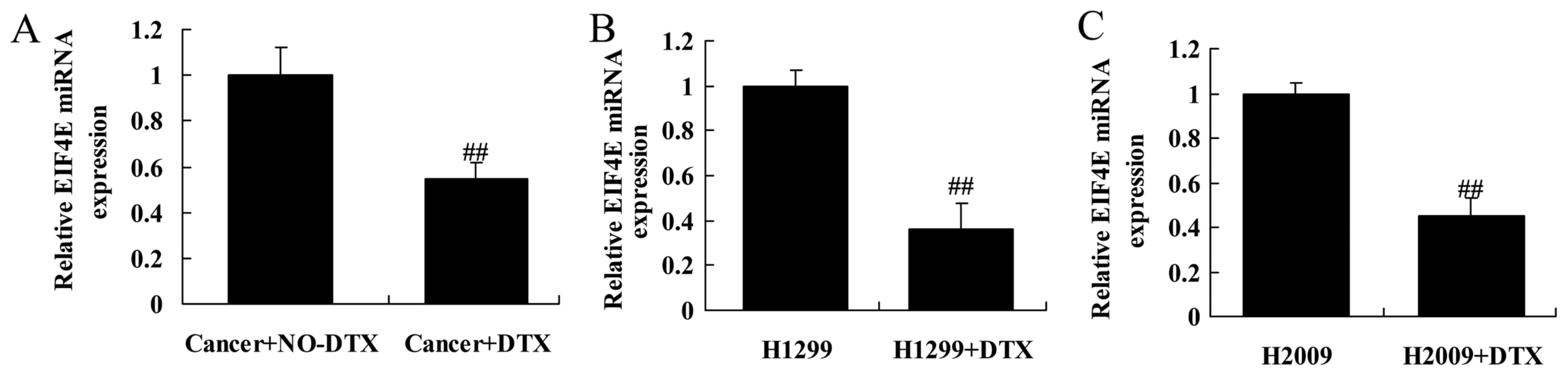
EIF4E expression in docetaxel
chemoresistance of human NSCLC patients
Then, we found that the miRNA expression of EIF4E in
docetaxel chemoresistant patients with NSCLCs was markedly lower
than those of non-docetaxel chemoresistant patients with NSCLCs
(Fig. 2A). Moreover, the miRNA
expression of EIF4E in H1299 and H2009 cells treated by docetaxel
was also lower than that of H1299 and H2009 cells treated by
non-docetaxel group (Fig. 2B and
C).
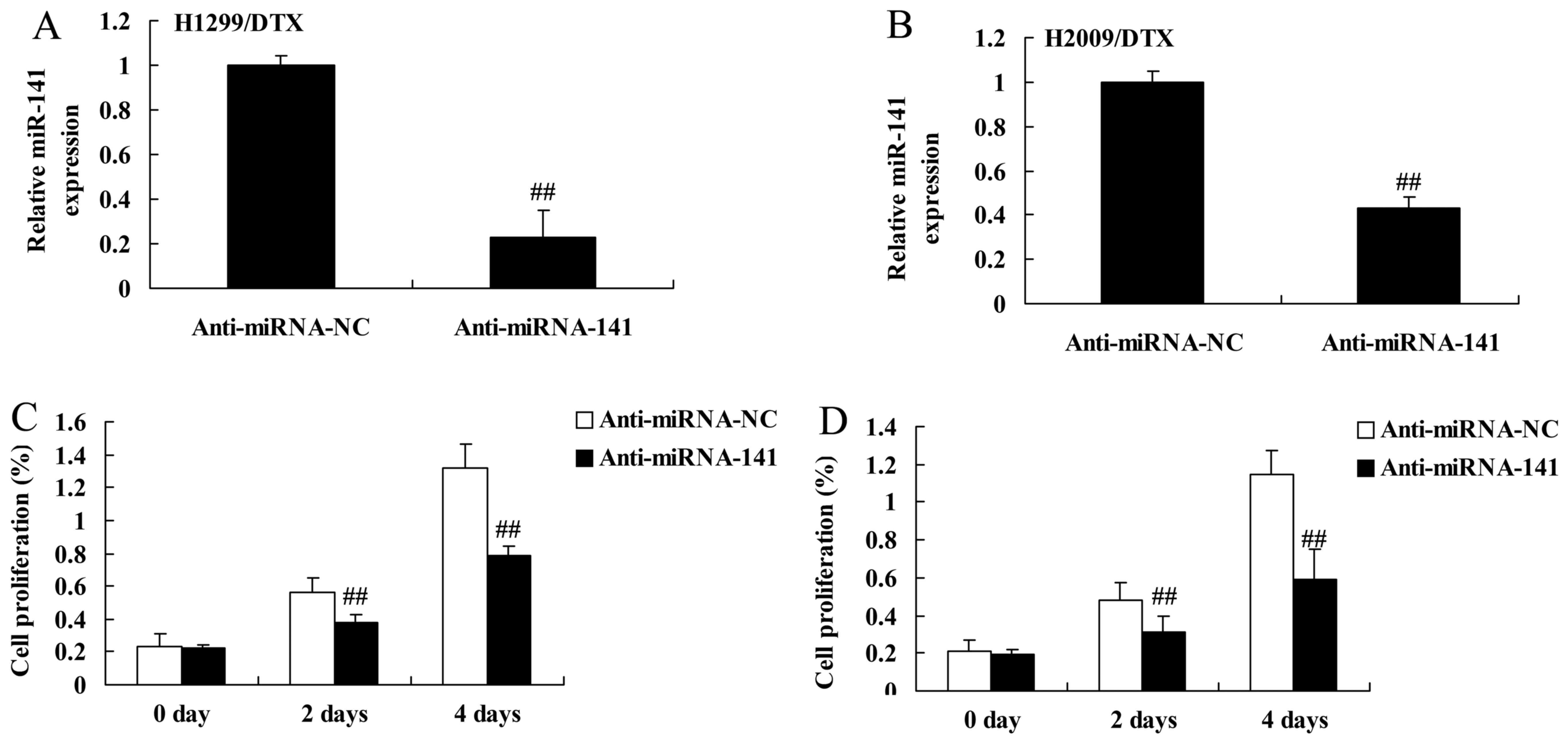
Downregulation of miR-141 reverses
docetaxel chemoresistance of human NSCLC growth
Therefore, negative control and miR-141 inhibitor
plasmids were transfected into H1299 and H2009 cells. MiR-141
inhibitor plasmids significantly inhibited the miR-141 expression
in H1299 and H2009 cells treated by docetaxel, compared with
negative control group (Fig. 3A and
B). Downregulation of miR-141 significantly inhibited cell
viability of H1299 and H2009 cells treated by docetaxel, compared
with negative control group (Fig. 3C
and D).
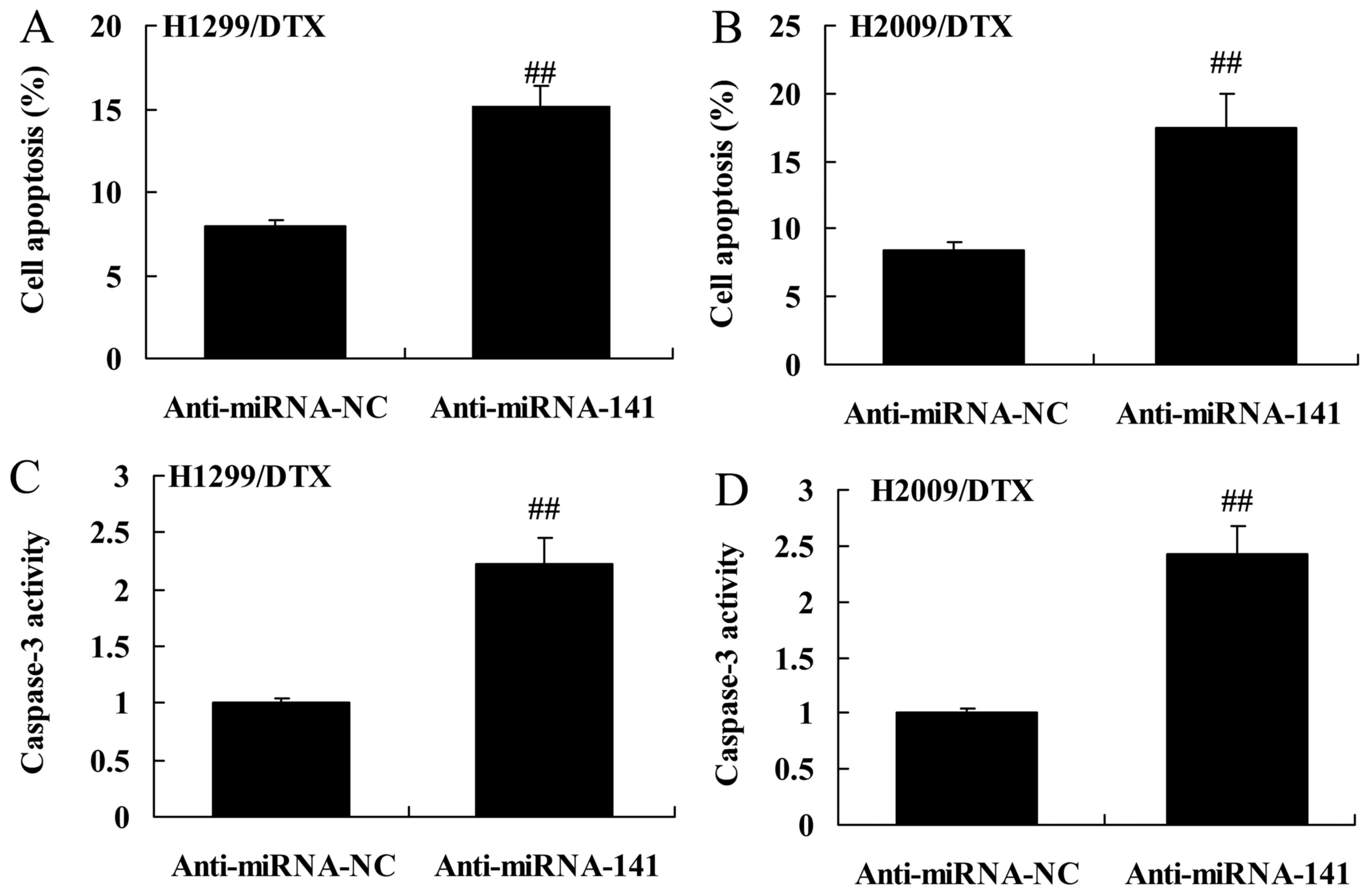
Downregulation of miR-141 reverses
docetaxel chemoresistance of human NSCLC death and caspase-3
activity
Then, we detected the downregulation of miR-141
affect on docetaxel chemoresistance of human NSCLC death and
caspase-3 activity. As shown in Fig.
4A-D, the downregulation of miR-141 significantly increased
cell apoptosis and caspase-3 activity of H1299 and H2009 cells
treated by docetaxel, compared with negative control group.
Downregulation of miR-141 reverses
EIF4E protein expression in docetaxel chemoresistance of human
NSCLC
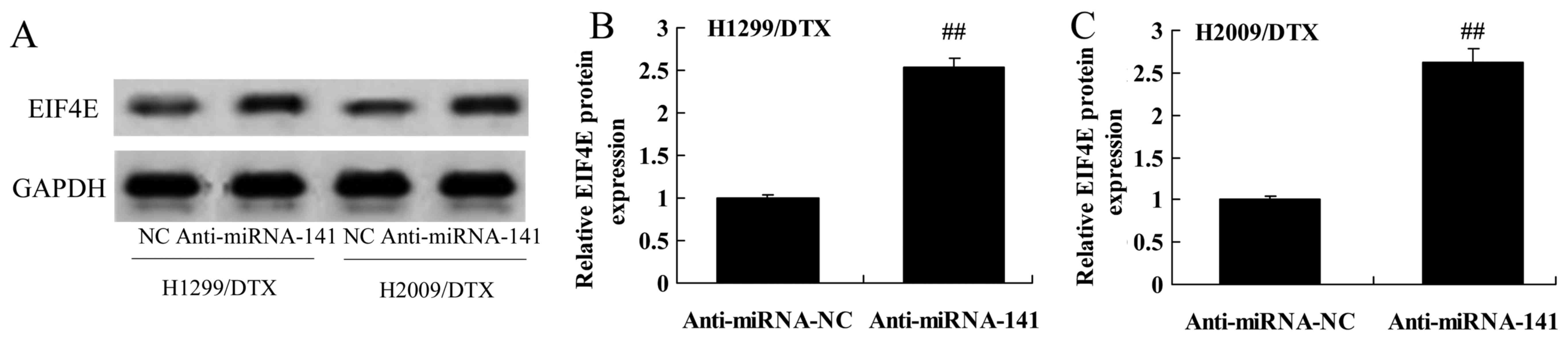
We evaluated whether the downregulation of miR-141
reverses EIF4E protein expression in docetaxel chemoresistance of
human NSCLC. Downregulation of miR-141 significantly induced the
protein expression of EIF4E in H1299 and H2009 cells treated by
docetaxel, compared with negative control group (Fig. 5A-C).
Downregulation of miR-141 reverses
VEGF protein expression in docetaxel chemoresistance of human
NSCLC
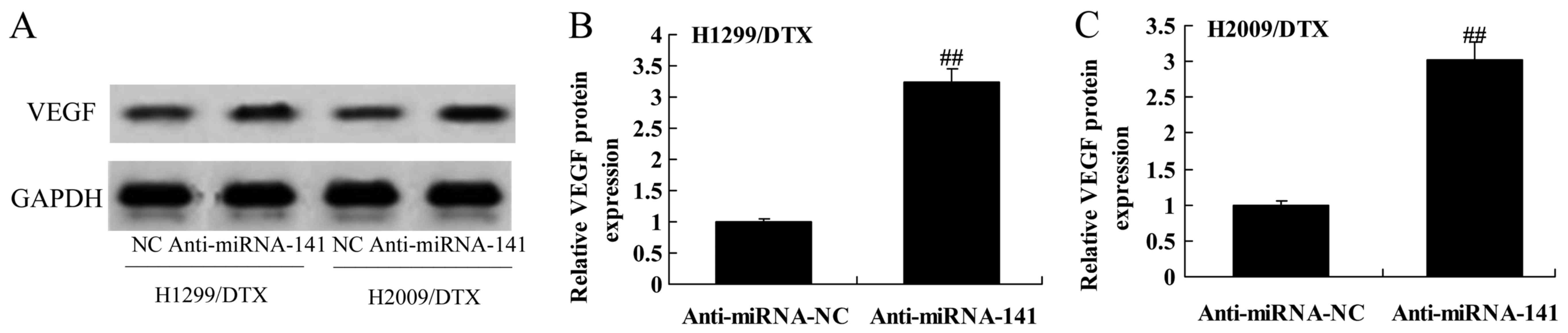
To assess the mechanism of anticancer effect of
miR-141 on docetaxel chemoresistance of human NSCLC, VEGF protein
expression was measured using western blotting. The results from
Western blot showed that the downregulation of miR-141
significantly activated the protein expression of VEGF in H1299 and
H2009 cells treated by docetaxel, compared with negative control
group (Fig. 6A-C).
Downregulation of miR-141 reverses
c-Myc protein expression in docetaxel chemoresistance of human
NSCLC
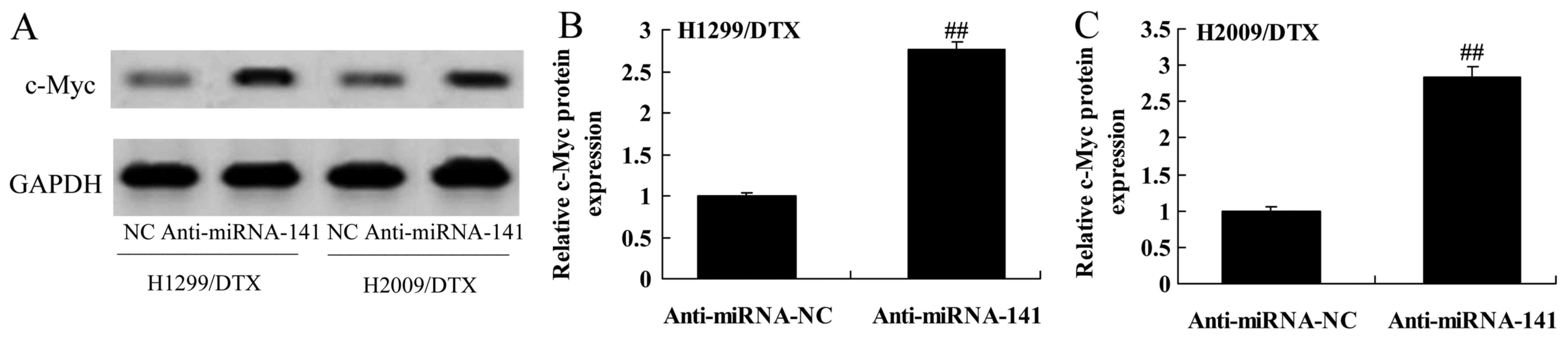
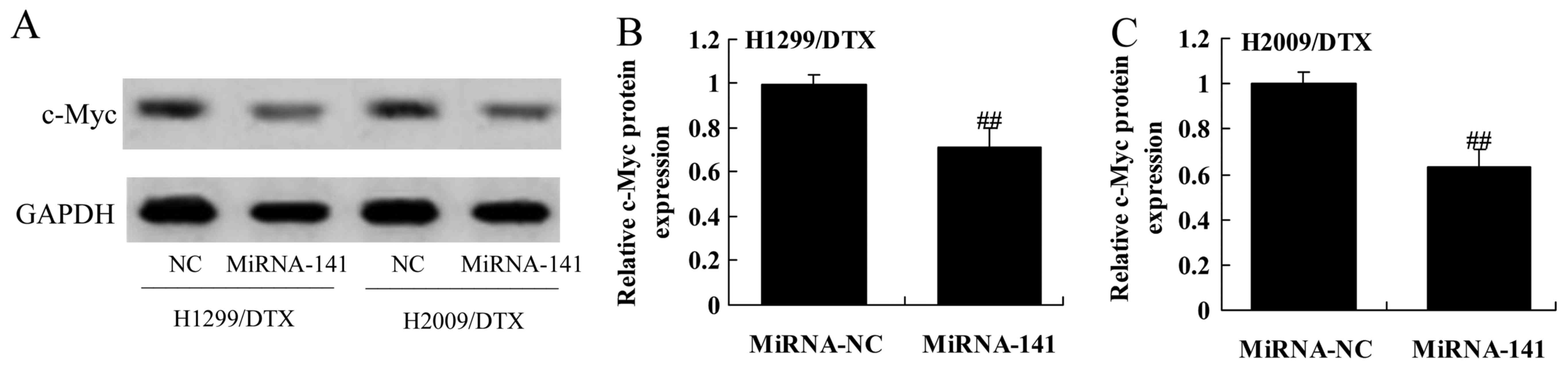
We further assessed the mechanism of anticancer
effect of miR-141 on docetaxel chemoresistance of human NSCLC,
c-Myc protein expression was also measured using western blotting.
Western blot showed that the downregulation of miR-141
significantly promoted the protein expression of c-Myc in H1299 and
H2009 cells treated by docetaxel, compared with negative control
group (Fig. 7A-C).
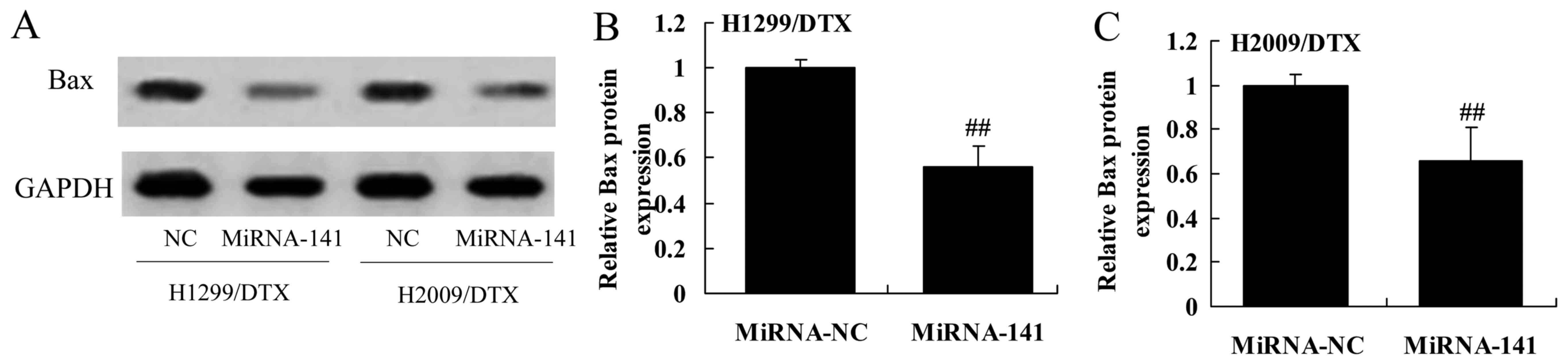
Downregulation of miR-141 reverses Bax
protein expression in docetaxel chemoresistance of human NSCLC
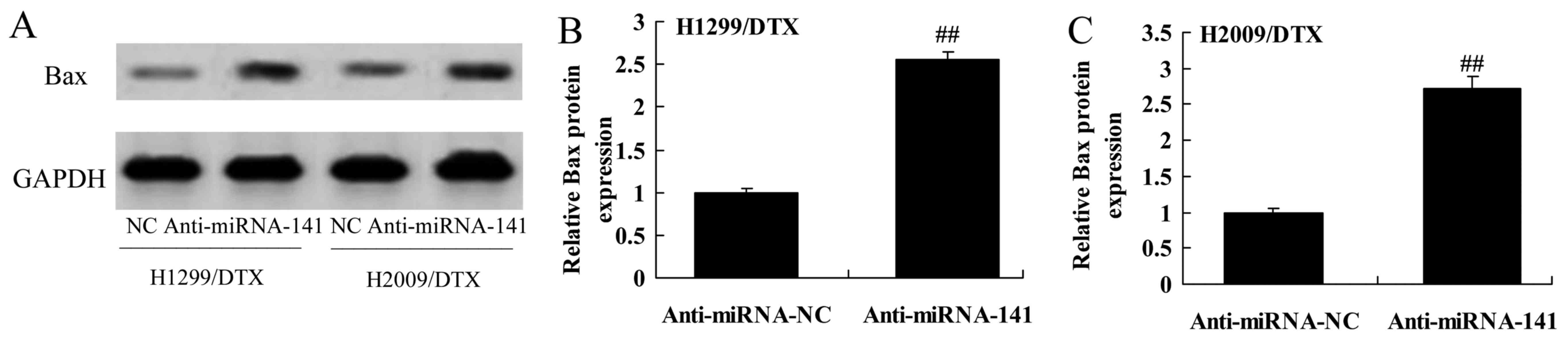
We explored the downregulation of miR-141 affect on
Bax protein expression in docetaxel chemoresistance of human NSCLC.
As shown in Fig. 8A-C,
downregulation of miR-141 significantly activated Bax protein
expression in H1299 and H2009 cells treated by docetaxel, compared
with negative control group.
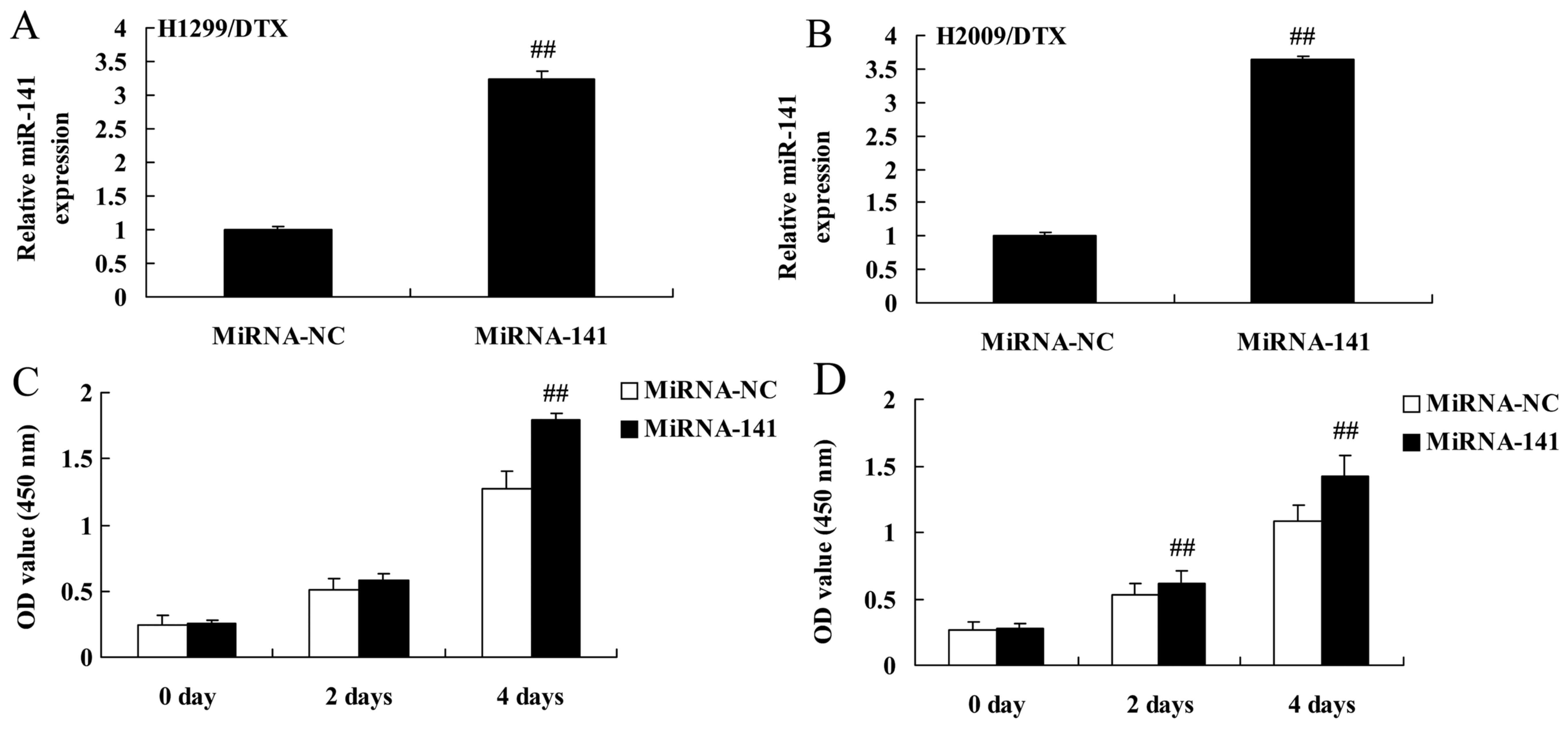
Upregulation of miR-141 reverses
docetaxel chemoresistance of human NSCLC growth
On the contrary, we used miR-141 mimics to increase
miR-141 expression in H1299 and H2009 cells treated by docetaxel,
which was used to analyze the upregulation of miR-141 affect on
docetaxel chemoresistance of human NSCLC growth. As shown in
Fig. 9A and B, miR-141 mimics
significantly increased the miR-141 expression in H1299 and H2009
cells treated by docetaxel. In addition, the upregulation of
miR-141 significantly increased cell viability of H1299 and H2009
cells treated by docetaxel, compared with negative control group
(Fig. 9C and D).
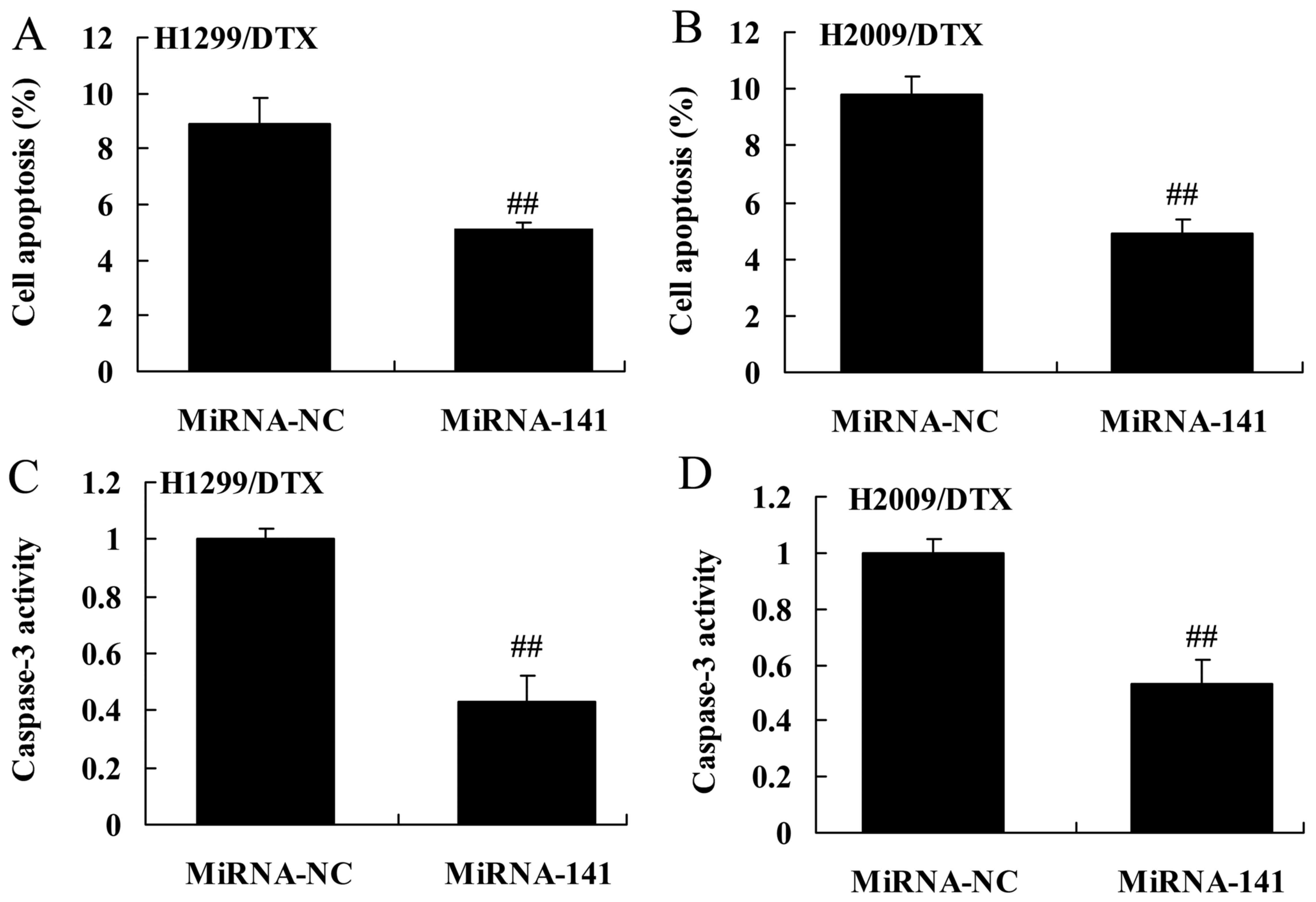
Upregulation of miR-141 reverses
docetaxel chemoresistance of human NSCLC death and caspase-3
activity
We confirmed whether the upregulation of miR-141
reverses docetaxel chemoresistance of human NSCLC death and
caspase-3 activity. Upregulation of miR-141 significantly inhibited
cell apoptosis and caspase-3 activity of H1299 and H2009 cells
treated by docetaxel, compared with negative control group
(Fig. 10A-D).
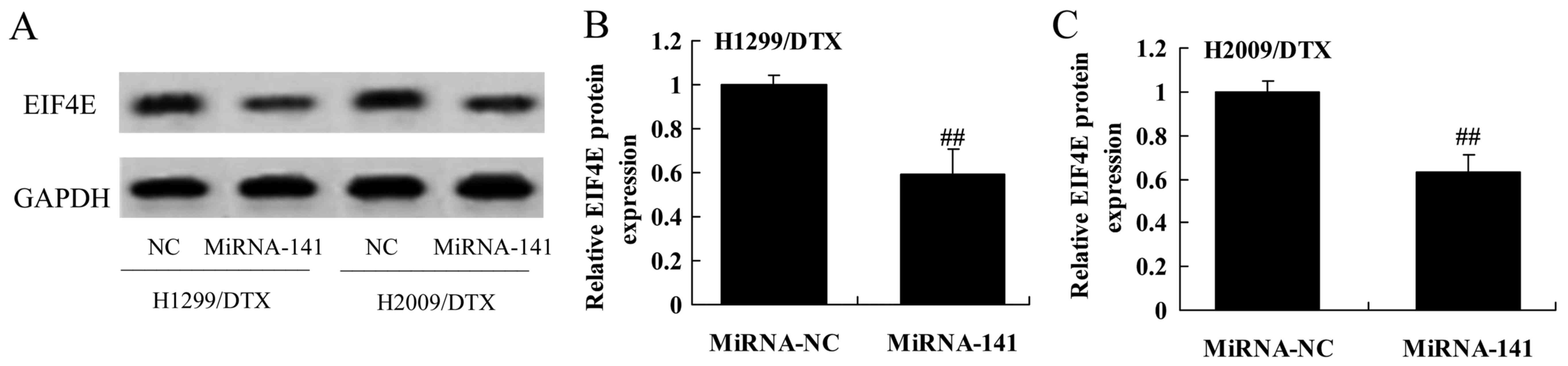
Upregulation of miR-141 reverses EIF4E
protein expression in docetaxel chemoresistance of human NSCLC
To further confirm that the upregulation of miR-141
reverses EIF4E protein expression in docetaxel chemoresistance of
human NSCLC. In contrast, the EIF4E protein expression of H1299 and
H2009 cells treated by docetaxel was significantly inhibited by
upregulation of miR-141, compared with negative control group
(Fig. 11A-C).
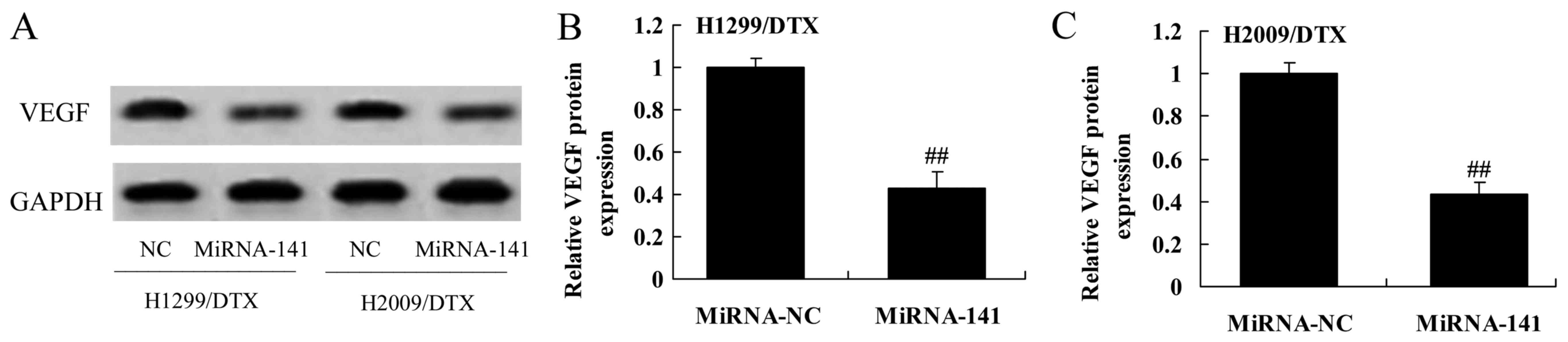
Upregulation of miR-141 reverses VEGF
protein expression in docetaxel chemoresistance of human NSCLC
To measure VEGF protein expression in docetaxel
chemoresistance of human NSCLC following upregulation of miR-141,
VEGF protein was measured using western blotting. Upregulation of
miR-141 significantly suppressed VEGF protein of H1299 and H2009
cells treated by docetaxel, compared with negative control group
(Fig. 12A-C).
Upregulation of miR-141 reverses c-Myc
protein expression in docetaxel chemoresistance of human NSCLC
We measured c-Myc protein expression in docetaxel
chemoresistance of human NSCLC using western blotting after
upregulation of miR-141. The results showed a significant
inhibition in c-Myc protein expression of H1299 and H2009 cells
treated by docetaxel after upregulation of miR-141 than the control
cells (Fig. 13A-C).
Upregulation of miR-141 reverses Bax
protein expression in docetaxel chemoresistance of human NSCLC
Next, we measured whether the upregulation of
miR-141 reverses Bax protein expression in docetaxel
chemoresistance of human NSCLC. As expected, Bax protein expression
of H1299 and H2009 cells treated by docetaxel significantly
suppressed the protein expression of Bax, compared with negative
control group (Fig. 14A-C).
Discussion
Lung cancer is one of the most common malignant
tumors. It is a malignant tumor with the highest fatality rate as
well. According to histologic classification, 80% of lung cancer is
non-small cell lung cancer (NSCLC) (2). Due to the absence of effective early
diagnosis method, most of the patients at preliminary diagnosis
have lost the opportunity for surgery (13). In consequence, chemotherapy has
become the main treatment method of NSCLC (14). Docetaxel, as a third generation
cytotoxic drug, has been applied on first-line therapy for NSCLC at
advanced stage (15). Similar to
other chemotherapeutics, the increasing drug resistance also
affects its clinical application and curative effect of
chemotherapy (4). Therefore, to
illuminate its resistance mechanism has important realistic
significance on selection of therapeutic strategy, preventing or
reversing the drug resistance to the maximum extent and improving
curative effect of chemotherapy. Our work demonstrates that miR-141
expression in docetaxel chemoresistant patients with NSCLCs was
markedly higher than those of non-docetaxel chemoresistant patients
with NSCLCs. Downregulation of miR-141 significantly inhibited cell
viability, and increased cell apoptosis and caspase-3 activity of
H1299 and H2009 cells treated by docetaxel.
Experiments have shown that many malignant tumors in
clinic are accompanied by overexpression of eIF4E (5). In addition, its expression level is
closely related to differentiation degree of malignant tumors and
metastasis. In the past 10 years, the cap binding protein eIF4E of
mRNA has obtained increased attention during tumorigenesis and the
development process (16). High
expression of eIF4E exists on a variety of malignant tumors, such
as breast cancer, laryngeal carcinoma, esophagus cancer, thyroid
cancer, gastric cancer, lung cancer and leukemia. Furthermore, its
expression degree is related to tumor malignant degree (17). High expression of eIF4E can
obviously affect normal cell phenotype, induce cell proliferation
activity, promote cell conversion and lead to genesis and
metastasis of tumor (16). Transfer
of eIF4E antisense RNA to the cell or inhibition of its combination
with competitiveness of eIF4E binding proteins, will reduce protein
translation, inhibit cell transformation, reduce tumorigenesis,
alleviate infiltration and metastasis (18). In the present study, we showed that
the expression of EIF4E in docetaxel chemoresistant patients with
NSCLCs was markedly lower than those of non-docetaxel
chemoresistant patients with NSCLCs. Downregulation of miR-141
significantly induced the protein expression of EIF4E in H1299 and
H2009 cells treated by docetaxel. Yao et al reported that
miR-141 regulates EIF4E expression in docetaxel chemoresistance of
breast cancer cells (19). These
data suggest that miR-141 is a potential target for
chemosensitizing BCs.
It has been detected that overexpression of dF4E
exists in a variety of malignant tumors (8). In addition, the overexpression is
positively correlated to infiltration and metastasis ability of
tumor. Overexpression of eIF4E can selectively strengthen the
expression of a series of malignant proteins. Such malignant
proteins include angiogenesis protein (such as FGF and VEGF),
proliferation adherence and infiltrating related proteins. VEGF is
a upregulated protein after overexpression of eIF4E (18). It is a key effective factor during
angiogenesis process. Angiogenesis will increase after
overexpression of VEGF which can provide adequate oxygen and
nutrients for ectopic endometrium and promote adhesion and plant
growth of ectopic cells; new vessels can produce and secrete a
variety of cytokines, growth factors and degradation enzymes thus
accelerate adherence infiltration of ectopic endometrium;
amplification of new blood vessels in endothelial cells provides
opportunity for distant metastasis of ectopic endometrium via
circulatory system (20,21). Importantly, our present results
showed that downregulation of miR-141 significantly activated the
protein expression of VEGF in H1299 and H2009 cells treated by
docetaxel. Tejero et al showed that miR-141 regulates VEGF
in early stage non-small cell lung cancer adenocarcinoma (22).
It has been proven by overexpression of eIF4E in
breast cancer, that NSCLC and prostatic cancer instead of typical
benign lesions overexpression of eIF4E may be an important mark at
the malignant transformation process (23). A large number of proteins shall be
generated to maintain rapid proliferation of tumor cells. It has
been detected in the cells from eIF4E that synthesis of only few
proteins has been significantly improved (24). The synthesis of most proteins
slightly increased. In vitro experiment has verified that
the ‘minority proteins’ of cells with overexpression of eIF4E
include: CCND1, c-myc and ODC, which can regulate cell cycle
process and promote tumorigenesis, as well as promoters for
fibroblast growth factor and vascular endothelial cell growth
factor (25). Our work also
demonstrates that downregulation of miR-141 significantly promoted
the protein expression of c-Myc in H1299 and H2009 cells treated by
docetaxel. Zhang et al proposed that microRNA-141 is
involved in nasopharyngeal carcinoma-related gene through c-Myc
pathways (26).
Bax is the first member of bcl-2 being confirmed
with cell apoptosis promotion effect (27). In case of high expression of Bax
protein, it can form Bax homodimer and/or Bax-Bcl-2 heterodimer
with Bcl-2 thus inhibiting Bcl-2 functions, accelerating apoptosis
(28). The expression of Bcl-2 and
Bax, as well as the proportion of the two (Bax/Bcl-2) have key
effects on the survival of cells after accepting apoptosis signal
(29). In case of overexpression of
Bcl-2, the cell will survive; and in case of overexpression of Bax,
the reaction of cell on death signal will be enhanced and cell
death will increase (23). In this
study, we found that downregulation of miR-141 significantly
activated Bax protein expression in H1299 and H2009 cells treated
by docetaxel. Upregulation of miR-141 suppressed EIF4E, VEGF, c-Myc
protein expression and inhibited Bax in H1299 or H2009/docetaxel
cells.
In conclusion, the results of the present study
demonstrated that miR-141 suppressed cell proliferation, induced
cell death and increased caspase-3 activity in H1299 or
H2009/docetaxel cell via EIF4E, VEGF, c-Myc and Bax signaling
pathway, and suggests that miR-141 may serve as a biomarker for
response to chemotherapy in NSCLCs.
References
|
1
|
Louie AV, van Werkhoven E, Chen H, Smit
EF, Paul MA, Widder J, Groen HJ, van den Borne BE, De Jaeger K,
Slotman BJ, et al: Patient reported outcomes following stereotactic
ablative radiotherapy or surgery for stage IA non-small-cell lung
cancer: Results from the ROSEL multicenter randomized trial.
Radiother Oncol. 117:44–48. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Aisner J, Manola JB, Dakhil SR, Stella PJ,
Sovak MA and Schiller JH: Vandetanib plus chemotherapy for
induction followed by vandetanib or placebo as maintenance for
patients with advanced non-small-cell lung cancer: A randomized
phase 2 PrECOG study (PrE0501). J Thorac Oncol. 8:1075–1083. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lal R, Bourayou N, Hillerdal G, Nicolson
M, Vikstrom A, Lorenzo M, D'yachkova Y, Barriga S and Visseren-Grul
C: Home administration of maintenance pemetrexed for patients with
advanced non-squamous non-small cell lung cancer: Rationale,
practicalities and phase II feasibility study design. Health Qual
Life Outcomes. 11:1632013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhu Z, Liu W, Gillin M, Gomez DR, Komaki
R, Cox JD, Mohan R and Chang JY: Assessing the robustness of
passive scattering proton therapy with regard to local recurrence
in stage III non-small cell lung cancer: A secondary analysis of a
phase II trial. Radiat Oncol. 9:1082014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Graff JR, Boghaert ER, De Benedetti A,
Tudor DL, Zimmer CC, Chan SK and Zimmer SG: Reduction of
translation initiation factor 4E decreases the malignancy of
ras-transformed cloned rat embryo fibroblasts. Int J Cancer.
60:255–263. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rosenwald IB, Hutzler MJ, Wang S, Savas L
and Fraire AE: Expression of eukaryotic translation initiation
factors 4E and 2alpha is increased frequently in bronchioloalveolar
but not in squamous cell carcinomas of the lung. Cancer.
92:2164–2171. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kaufman JL, Glockner F, Chang BB, Koslow
AR, Shah DM and Leather RP: Impact of the presence of orthopedic
hardware on technical performance of major amputations. Ann Vasc
Surg. 4:356–358. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chang SH, Kim JE, Lee JH, Minai-Tehrani A,
Han K, Chae C, Cho YH, Yun JH, Park K, Kim YS, et al: Aerosol
delivery of eukaryotic translation initiation factor 4E-binding
protein 1 effectively suppresses lung tumorigenesis in K-rasLA1
mice. Cancer Gene Ther. 20:331–335. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zagryazhskaya A and Zhivotovsky B: miRNAs
in lung cancer: A link to aging. Ageing Res Rev. 17:54–67. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yamashita R, Sato M, Kakumu T, Hase T,
Yogo N, Maruyama E, Sekido Y, Kondo M and Hasegawa Y: Growth
inhibitory effects of miR-221 and miR-222 in non-small cell lung
cancer cells. Cancer Med. 4:551–564. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ye XW, Yu H, Jin YK, Jing XT, Xu M, Wan ZF
and Zhang XY: miR-138 inhibits proliferation by targeting
3-phosphoinositide-dependent protein kinase-1 in non-small cell
lung cancer cells. Clin Respir J. 9:27–33. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhu X, Li D, Yu F, Jia C, Xie J, Ma Y, Fan
S, Cai H, Luo Q, Lv Z, et al: miR-194 inhibits the proliferation,
invasion, migration, and enhances the chemosensitivity of non-small
cell lung cancer cells by targeting forkhead box A1 protein.
Oncotarget. 7:13139–13152. 2016.PubMed/NCBI
|
|
13
|
Komiyama K, Kobayashi K, Minezaki S,
Kotajima F, Sutani A, Kasai T, Mori K, Hoshi E, Takayanagi N,
Koyama S, et al: Kanto Respiratory Disease Study Group: Phase I/II
trial of a biweekly combination of S-1 plus docetaxel in patients
with previously treated non-small cell lung cancer (KRSG-0601). Br
J Cancer. 107:1474–1480. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang L, Wu S, Ou G, Bi N, Li W, Ren H, Cao
J, Liang J, Li J, Zhou Z, et al: Randomized phase II study of
concurrent cisplatin/etoposide or paclitaxel/carboplatin and
thoracic radiotherapy in patients with stage III non-small cell
lung cancer. Lung Cancer. 77:89–96. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shukuya T, Ko R, Mori K, Kato M, Yagishita
S, Kanemaru R, Honma Y, Shibayama R, Koyama R, Shimada N, et al:
Prognostic factors in non-small cell lung cancer patients who are
recommended to receive single-agent chemotherapy (docetaxel or
pemetrexed) as a second- or third-line chemotherapy: In the era of
oncogenic drivers and molecular-targeted agents. Cancer Chemother
Pharmacol. 76:771–776. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Satheesha S, Cookson VJ, Coleman LJ,
Ingram N, Madhok B, Hanby AM, Suleman CA, Sabine VS, Macaskill EJ,
Bartlett JM, et al: Response to mTOR inhibition: Activity of eIF4E
predicts sensitivity in cell lines and acquired changes in eIF4E
regulation in breast cancer. Mol Cancer. 10:192011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mizutani R, Imamachi N, Suzuki Y, Yoshida
H, Tochigi N, Oonishi T, Suzuki Y and Akimitsu N: Oncofetal protein
IGF2BP3 facilitates the activity of proto-oncogene protein eIF4E
through the destabilization of EIF4E-BP2 mRNA. Oncogene.
35:3495–3502. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Robichaud N, del Rincon SV, Huor B, Alain
T, Petruccelli LA, Hearnden J, Goncalves C, Grotegut S, Spruck CH,
Furic L, et al: Phosphorylation of eIF4E promotes EMT and
metastasis via translational control of SNAIL and MMP-3. Oncogene.
34:2032–2042. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yao YS, Qiu WS, Yao RY, Zhang Q, Zhuang
LK, Zhou F, Sun LB and Yue L: miR-141 confers docetaxel
chemoresistance of breast cancer cells via regulation of EIF4E
expression. Oncol Rep. 33:2504–2512. 2015.PubMed/NCBI
|
|
20
|
Smith KA, Zhou B, Avdulov S, Benyumov A,
Peterson M, Liu Y, Okon A, Hergert P, Braziunas J, Wagner CR, et
al: Transforming growth factor-β1 induced epithelial mesenchymal
transition is blocked by a chemical antagonist of translation
factor eIF4E. Sci Rep. 5:182332015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Inglis DJ, Lavranos TC, Beaumont DM, Leske
AF, Brown CK, Hall AJ and Kremmidiotis G: The vascular disrupting
agent BNC105 potentiates the efficacy of VEGF and mTOR inhibitors
in renal and breast cancer. Cancer Biol Ther. 15:1552–1560. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tejero R, Navarro A, Campayo M, Viñolas N,
Marrades RM, Cordeiro A, Ruíz-Martínez M, Santasusagna S, Molins L,
Ramirez J, et al: miR-141 and miR-200c as markers of overall
survival in early stage non-small cell lung cancer adenocarcinoma.
PLoS One. 9:e1018992014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li J, Huang Y, Gao Y, Wu H, Dong W and Liu
L: Antibiotic drug rifabutin is effective against lung cancer cells
by targeting the eIF4E-β-catenin axis. Biochem Biophys Res Commun.
472:299–305. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li L, Zhang L, Liu D, Cheng Y, Jing YT, Yu
H, Zhou P, Song J and Li WM: Overexpression of eukaryotic
translation initiation factor 4E-binding protein 1 induces the
alteration of immune status in H1299 lung cancer cells. Thorac
Cancer. 6:427–432. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li S, Jia Y, Jacobson B, McCauley J,
Kratzke R, Bitterman PB and Wagner CR: Treatment of breast and lung
cancer cells with a N-7 benzyl guanosine monophosphate tryptamine
phosphoramidate pronucleotide (4Ei-1) results in chemosensitization
to gemcitabine and induced eIF4E proteasomal degradation. Mol
Pharm. 10:523–531. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang L, Deng T, Li X, Liu H, Zhou H, Ma
J, Wu M, Zhou M, Shen S, Li X, et al: microRNA-141 is involved in a
nasopharyngeal carcinoma-related genes network. Carcinogenesis.
31:559–566. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jiang H, Zhao PJ, Su D, Feng J and Ma SL:
Paris saponin I induces apoptosis via increasing the Bax/Bcl-2
ratio and caspase-3 expression in gefitinib-resistant non-small
cell lung cancer in vitro and in vivo. Mol Med Rep. 9:2265–2272.
2014.PubMed/NCBI
|
|
28
|
Lee SH, Kim DY, Jing F, Kim H, Yun CO, Han
DJ and Choi EY: Del-1 overexpression potentiates lung cancer cell
proliferation and invasion. Biochem Biophys Res Commun. 468:92–98.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
You BR, Shin HR and Park WH: PX-12
inhibits the growth of A549 lung cancer cells via G2/M phase arrest
and ROS-dependent apoptosis. Int J Oncol. 44:301–308.
2014.PubMed/NCBI
|