Introduction
Despite the rapid decline of colorectal cancer (CRC)
incidence, due to introduction of CRC screening, in recent years,
CRC is still a major cause of incidence and mortality in many
countries, especially in more developed ones, though in very recent
years its incidence is increasing also in developing areas of the
world (1). CRC incidence and
mortality has been increasing rapidly in Korea during last few
decades (2). Shin et al
reported that the age-standardized incidence rate (ASR) of CRC was
27 (per 100,000) in 1999 and increased to 50.2 in 2009 among men
(annual percentage changes, 6.6%) in Korea (3). There are considerable advances in
neoadjuvant chemotherapy and improved surgical techniques have been
achieved in the past decades, the 5-year survival rate of colon
cancer of stage IV was only 8.1% after treatments (4). In addition, long-term use of
chemotherapy can make patient condition worse and develop
resistance to chemotherapy. Therefore, novel non-toxic therapeutic
agents which are safe, affordable and effective are urgently
needed.
Trans-3,4,5′-trihydroxystilbene (resveratrol)
(Fig. 1A) is a natural polyphenol
and has been shown to prevent tumor formation and development in
several cancer types (5–8). This polyphenol has also been shown to
kill multiple types of cancer cells (9–11), and
to suppress angiogenesis and metastasis in a variety of animal
tumor models (12,13). Besides anticancer properties,
resveratrol has multiple biological and pharmacologic activities:
it has been described as an antidiabetic agent, an anti-aging
agent, a platelet aggregation inhibitor, a cardioprotective agent
and an anti-inflammatory agent (14,15).
Although accumulating evidence on health benefits and anticancer
effect of resveratrol exist, the compound has limited its use as a
cancer chemoprevention agent, since resveratrol is not a potent
cytotoxic agent when compared with other chemotherapeutic agents.
Therefore, exposure to high doses of resveratrol is required to
induce apoptosis in cancer cells. In addition, the biological
activity of resveratrol is limited by its photosensitivity and
metabolic instability (16,17).
Multiple approaches are being sought to overcome
these limitations, including the design and synthesis of novel
structural analogues (18,19). One compound in particular,
4-(6-hydroxy-2-naphthyl)-1,3-benzenediol (HS-1793), has stronger
antitumor effects than resveratrol in most cancer cells tested
(20–23). Moreover, HS-1793 overcomes the
resistance conferred by Bcl-2 by inducing apoptosis (21) and inhibited hypoxia-induced
hypoxia-inducible factor-1 and vascular endothelial growth factor
expressions (24). However, the
direct molecular target and mode of HS-1793's anticancer mechanism
in human colon cancer cells have not been fully clarified.
Therefore, this study used human colon cancer HCT116 cells to
identify additional molecular mechanisms supporting the
antiproliferative and apoptotic effects of HS-1793.
Materials and methods
Chemicals
Trans-3,4,5′-trihydroxystilbene (resveratrol)
was purchased from Sigma-Aldrich Co. (St. Louis, MO, USA).
4-(6-Hydroxy-2-naphthyl)-1,3-benzendiol (HS-1793) was synthesized,
kindly supplied by Professor Hongsuk Suh (Pusan National
University, Busan, Korea). A 100 mM solution of resveratrol or
HS-1793 was prepared in ethanol, and stored in small aliquots at
−20°C. The stock solution was diluted as needed in cell culture
medium. The maximal concentration of ethanol did not exceed 0.1%
(v/v) in the treatment range, where there was no influence on the
cell growth. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide (MTT) was obtained from Amresco LLC (Solon, OH, USA).
Antibodies specific for pro-caspase-3, −8 and −9, poly(ADP-ribose)
polymerases (PARP), B-cell CLL/lymphoma-2 (Bcl-2), Bcl-2 associated
X protein (Bax), cyclin A, cyclin B1, cyclin D1, Cdc2, Cdc25C,
cyclin-dependent kinase (CDK) 2, CDK4, CDK6, cytochrome c,
p38 mitogen-activated protein kinase (MAPK), phospho-extracellular
signal-regulated kinase (ERK)1/2 (Thr202/Tyr204), c-Jun N-terminal
kinase (JNK), phospho-Akt (Ser473), Akt and glyceraldehyde
3-phosphate dehydrogenase (GAPDH) were obtained from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA). The anti-cleaved-caspase-3,
anti-cleaved-caspase-8, phospho-p38 MAPK (Thr180/Tyr182),
phospho-JNK (Thr183/Tyr185), and ERK1/2 were purchased from Cell
Signaling Technology (Danvers, MA, USA). Antibody against β-actin
and LY294002 were purchased from Sigma-Aldrich Co.
Cell culture and viability assay
HCT116 cells were obtained from American Type
Culture Collection (Manassas, VA, USA). Cells were maintained at
37°C in humidified 5% CO2 in RPMI-1640 supplemented with
10% fetal bovine serum, 100 U/ml penicillin, and 100 µg/ml
streptomycin (both from GE Healthcare Life Sciences, Logan, UT,
USA). Cell viability was determined by MTT assay. Cells were seeded
in each well of 24-well plate, allowed to adhere overnight, treated
with or without various reagents at the indicated concentrations,
and then incubated in the dark with 0.5 mg/ml MTT at 37°C for 2 h.
The formazan granules generated by the live cells were dissolved in
DMSO, and the absorbance at 540 nm was monitored using a multi-well
reader (Thermo Fisher Scientific, Vantaa, Finland).
Cell proliferation assay
To determine the effect of the test agents on cancer
cell proliferation, MTT assay was performed. Briefly, cells were
incubated in the presence or absence of the indicated concentration
of agents for 0, 1, 2 and 4 days. Thereafter, cell growth was
measured by MTT assay as described above.
Annexin V/PI staining assay
After treatment with various concentrations of
testing agents for 24 h, the cells were trypsinized, washed, and
collected. Apoptotic cells were detected using the BD Pharmingen
FITC Annexin V apoptosis detection kit (BD Biosciences, San Diego,
CA, USA) in accordance with the instruction provided by the
manufacturer. A total of 10,000 cells were subsequently collected
and analyzed using a flow cytometer (Accuri C6; BD Biosciences, Ann
Arbor, MI, USA).
Nuclear staining with Hoechst
33342
The control and treated cells were washed with
phosphate-buffered saline (PBS) and fixed with 3.7%
paraformaldehyde in PBS for 10 min at room temperature. Fixed cells
were washed with PBS and stained with 4 µg/ml Hoechst 33342 (Thermo
Fisher Scientific, Waltham, MA, USA) for 10 min at room
temperature. And then, the cells were washed with PBS and analyzed
by fluorescent microscope.
Flow cytometric analysis for
measurement of cell cycle population
The DNA content was measured following the staining
of the cells with propidium iodide (PI; Sigma-Aldrich Co.). After
treatment with various concentrations of testing agents, the cells
were harvested, washed with cold PBS, and further fixed in 70%
ethanol at −20°C overnight. The fixed cells were washed with cold
PBS and then stained with cold PI solution (50 µg/ml in PBS) at
37°C for 30 min in the dark. Flow cytometric analysis was performed
on an Accuri C6.
Western blot analysis
Cells were harvested and solubilized in whole cell
lysis buffer, and the supernatant was collected and protein
concentrations were then determined by protein assay reagents
(Bio-Rad, Hercules, CA, USA). Subcellular fractions of mitochondria
and cytosol were prepared using mitochondria isolation kit for
mammalian cells (Thermo Fisher Scientific). Equal amounts of
protein extracts were denatured by boiling at 100°C for 5 min in
sample buffer (Bio-Rad). Equal amount of protein was subjected to
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE) and transferred to polyvinylidene fluoride (PVDF)
membranes by immunoblotting. Blots were probed with the desired
primary antibodies overnight, incubated with horseradish peroxidase
(HRP)-conjugated secondary antibodies (Santa Cruz Biotechnology,
Inc.), and then visualized using the enhanced chemiluminescence
(ECL) detection system (GE Healthcare, Piscataway, NJ, USA).
Statistical analysis
Results are expressed as the mean ± SD of two or
three separate experiments and analyzed by Student's t-test. Means
were considered significantly different at *p<0.05 or
**p<0.01.
Results
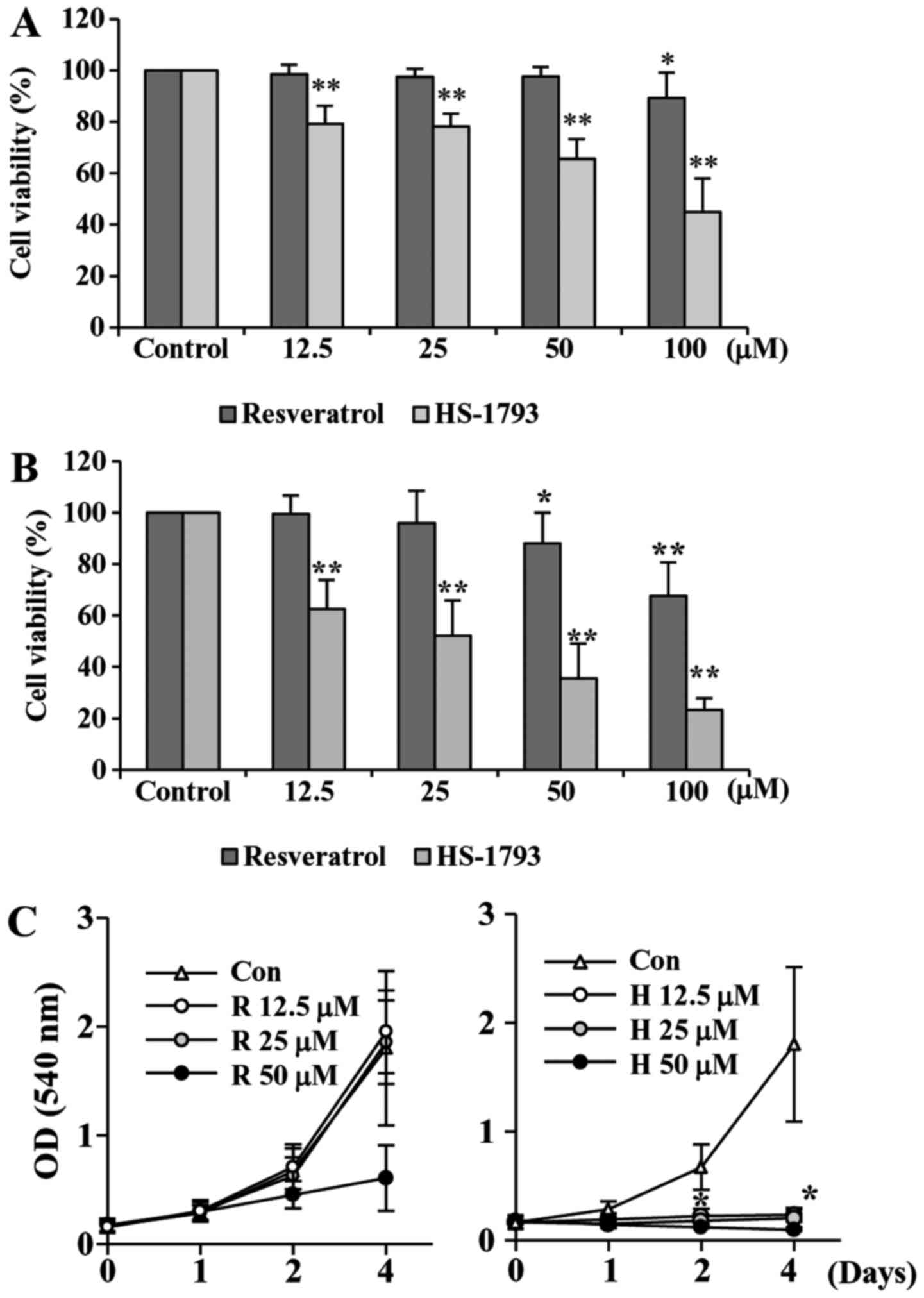
HS-1793 suppresses the proliferation
of HCT116 cells
To investigate the antiproliferative activities of
HS-1793, a synthetic resveratrol analogue, on HCT116 cells, we
first performed the cell viability assay. We also used resveratrol
for comparison. Treatment cells with resveratrol decreased the cell
viability slightly after 24 h, but after 48 h of treatment the
viability was reduced almost by 68% at 100 µM of resveratrol
(Fig. 2A). Our data showed that
HS-1793 significantly reduced the cell viability concentration- and
time-dependently (Fig. 2B). More
importantly, HS-1793 exhibited potent growth inhibitory effect when
compared to that of resveratrol under same experimental conditions
(Fig. 2A and B).
We also determined the effect of resveratrol and
HS-1793 on cell proliferation (Fig.
2C). Results indicated that resveratrol showed moderate
anti-proliferative effect in HCT116 cells (Fig. 2C, left). However, HS-1793
significantly suppresses proliferation of colon cancer cell line
HCT116 (Fig. 2C, right). More
importantly, the effects were observed at 12.5 µM, a concentration
at which resveratrol had no significant effect on HCT-116 cell
proliferation. The results demonstrate that HS-1793 is more potent
than resveratrol in the growth suppression of the human CRC cell
line HCT116.
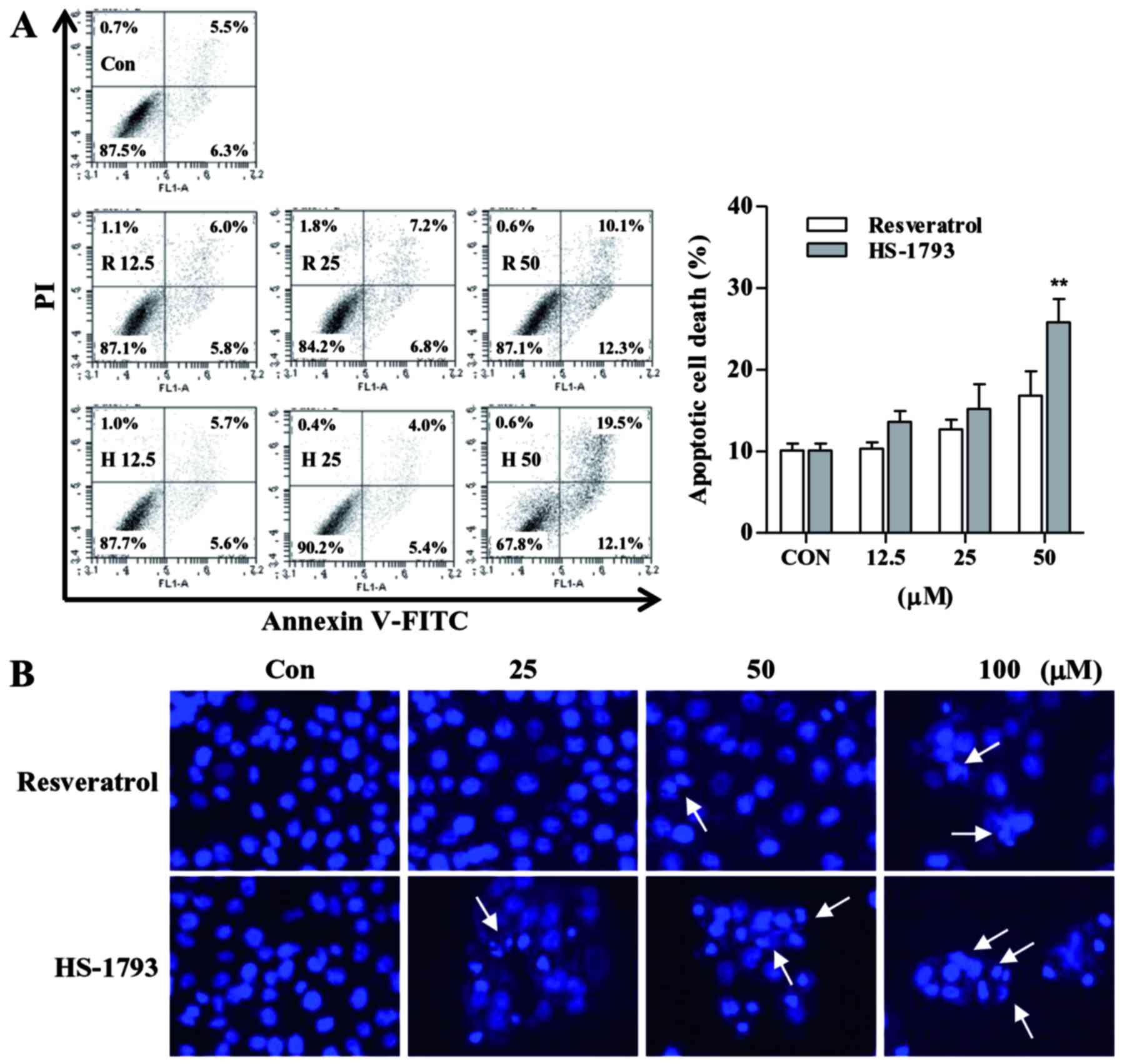
HS-1793 induces apoptosis in HCT116
cells
To investigate the underlying mechanism of growth
inhibition observed in the cell viability and cell proliferation
assay, we next examined apoptosis effect on HCT116 cells induced by
resveratrol and HS-1793 using Annexin V/PI staining as described in
Materials and methods section. Cells showed concentration-dependent
apoptosis after a 24 h treatment with resveratrol or HS-1793
(Fig. 3A). The analysis
demonstrated that treatment with 50 µM resveratrol induced
apoptosis in ~22.4%, whereas same concentration of HS-1793 induced
apoptosis in ~31.6% of HCT116 cells.
In order to determine whether HS-1793 induces
morphological changes, one characteristic of apoptosis, Hoechst
staining was performed. By Hoechst staining, it was shown that
resveratrol and HS-1793 caused chromatin condensation and
fragmentation which are typical apoptotic nuclear morphological
changes (Fig. 3B). The untreated
cells exerted oval nuclear structure, while the cells treated with
resveratrol and HS-1793 exhibited evident apoptotic
characteristics, including shrinkage and nuclear condensation
(Fig. 3B). Compared with
resveratrol, there was an evident increase in the number of
nuclear-condensed cells following treatment with HS-1793 at the
same concentration.
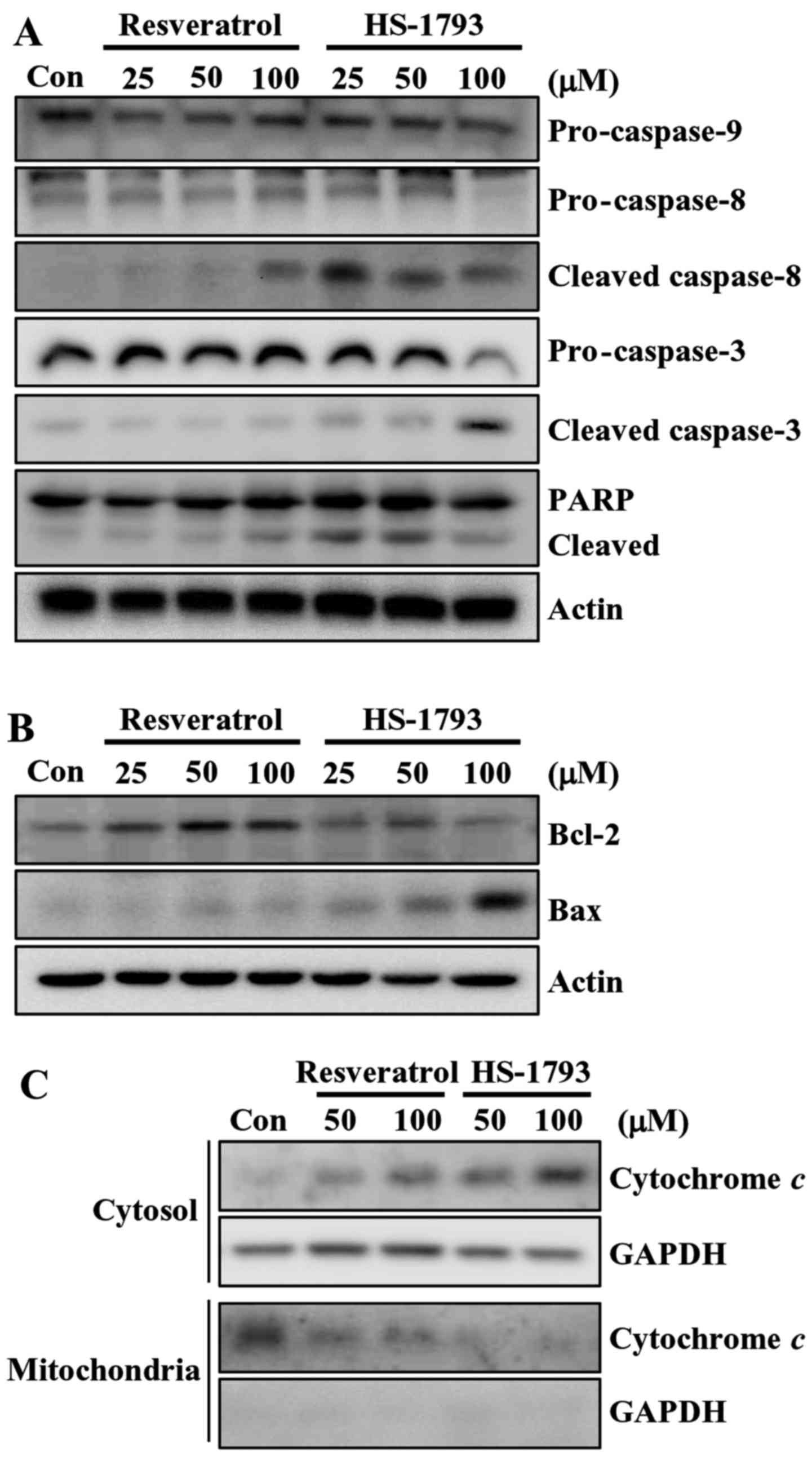
Then we determined the effect of resveratrol and
HS-1793 on the levels of apoptosis-related proteins in HCT116
cells. At 100 µM, HS-1793 effectively induced the reduction of
pro-caspase-8 and pro-caspase-3, whereas resveratrol did not
(Fig. 4A). HS-1793 also activated
caspase-8 and caspase-3 as indicated by the presence of cleaved
caspases. Similar result was observed in cleavage of PARP in HCT116
cells (Fig. 4A). It is noticeable
that HS-1793 (25 µM) caused the PARP cleavage, whereas resveratrol
(100 µM) had no significant effect on PARP cleavage in HCT116
cells. However, both resveratrol and HS-1793 had no effect on
pro-caspase-9 expression (Fig. 4A).
In addition, treatment of HS-1793 slightly downregulated the level
of antiapoptotic protein Bcl-2 at high concentration (100 µM) while
resveratrol further upregulated the level of Bcl-2 (Fig. 4B). In HCT116 cells, the level of
apoptosis-promoting protein Bax was induced by both HS-1793 and
resveratrol, and a more prominent effect was observed in
HS-1793-treated cells (Fig. 4B).
These data suggest that HS-1793 is a more potent inducer of
apoptosis than resveratrol.
HS-1793 induces cytochrome c release
in HCT116 cells
Mounting evidence suggests that mitochondria play an
essential role in apoptosis by releasing apoptogenic effectors such
as cytochrome c (25). In
order to determine the involvement of the mitochondrial pathway in
HS-1793-induced apoptosis in HCT116 cells, we analyzed the
cytosolic and mitochondrial levels of cytochrome c. The
results of western blot analyses demonstrated that both resveratrol
and HS-1793 promoted an increase in the release of cytochrome c
from the mitochondria into the cytosol (Fig. 4C). HS-1793 was stronger stimulatory
effects on inducing cytochrome c release when compared to that of
resveratrol.
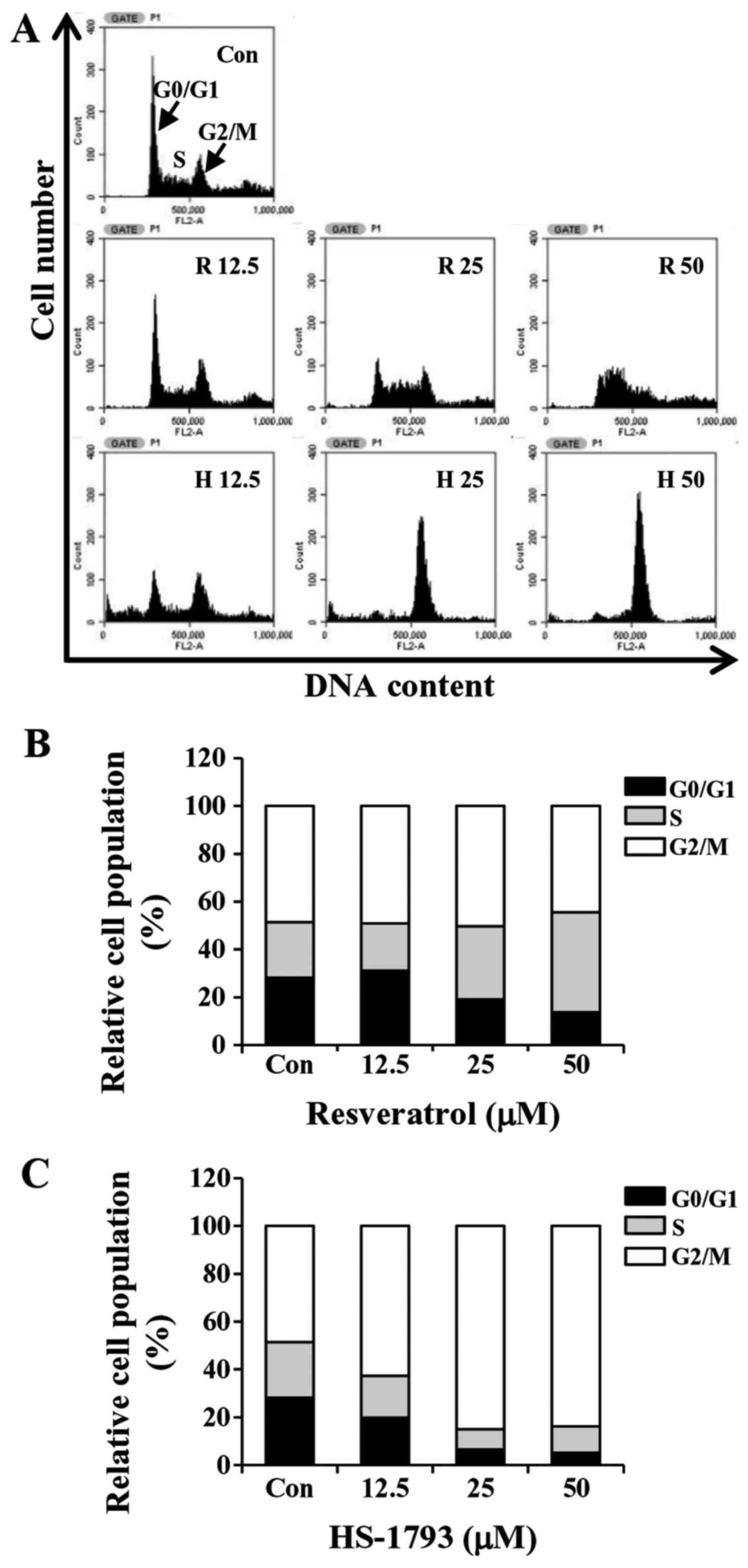
HS-1793 promotes G2/M cell cycle
arrest in HCT116 cells
To determine whether the growth inhibition by
HS-1793 or resveratrol was caused by cell cycle arrest, the cells
were incubated with various concentrations of HS-1793 for 24 h. The
cells were then fixed, stained and cell cycle populations were
determined by flow cytometry. The results showed that HS-1793
induced the accumulation of cells in the G2/M phase in a
concentration-dependent manner while S phase arrest was observed in
resveratrol-treated cells (Fig. 5).
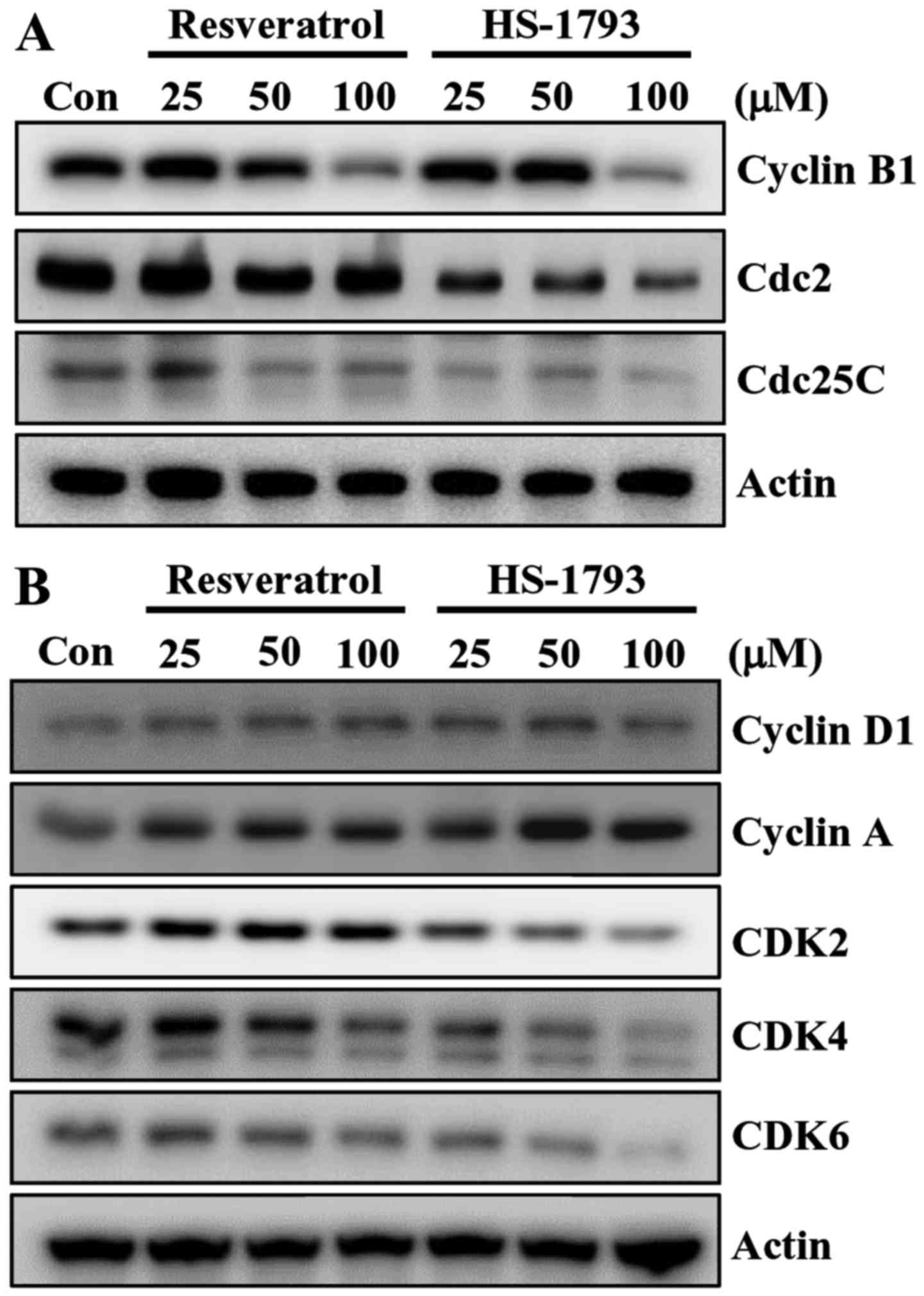
Next, we examined the effect of HS-1793 on the expression of G2/M
cell cycle regulators. Cell cycle checkpoints are mainly regulated
by several kinds of cyclin-dependent kinase (CDK) complexes. Above
all, G2/M transition is largely dependent on cyclin B1/Cdc2 (Cdk1)
activity (26). Thus the activity
of cyclin B1/Cdc2 complex is regulated by the positive regulator
Cdc25C, and two negative regulators, the protein kinases Weel and
Myt1 (27). The western blot
results indicated that the expression of G2/M cell cycle regulatory
protein cyclin B1, Cdc2 and Cdc25C decreased by increasing
concentration of HS-1793 (Fig. 6A).
Resveratrol also downregulated the protein expressions of cyclin B1
and Cdc25C in HCT116 cells but with different potency (Fig. 6A).
Recent report demonstrated that resveratrol induced
G1/S-phase arrest in human colon carcinoma cells (28), we investigated whether resveratrol
as well as HS-1793 affect the levels of proteins involved in G1/S
phase arrest. Resveratrol and its analogue HS-1793 showed
differential levels of downregulation of CDK2, CDK4 and CDK6 in
HCT116 cells (Fig. 5B). HS-1793 at
25 µM decreased levels of CDK4 while 50 and 100 µM of resveratrol
induced downregulation of CDK4. HS-1793 at 50 and 100 µM reduced
the level of CDK6 while a slight decrease of CDK6 was observed at
100 µM resveratrol (Fig. 6B).
Neither resveratrol nor HS-1793 altered the level of cyclin D1 and
cyclin A in HCT116 cells (Fig.
6B).
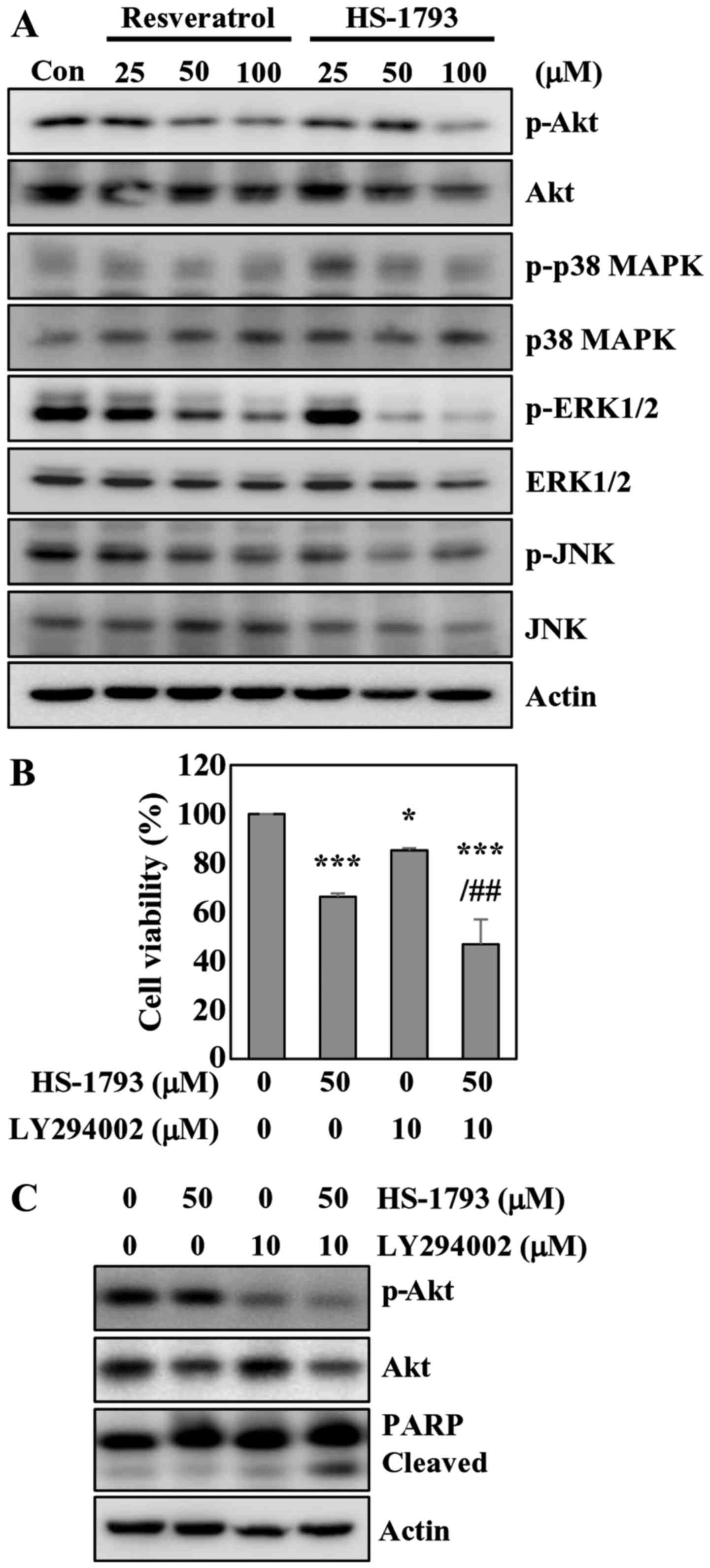
HS-1793 inhibits Akt and ERK
phosphorylation in HCT116 cells
The AKT/protein kinase B (PKB) kinases have been
shown to play critical roles in controlling the cellular processes
including cell growth, proliferation, survival and apoptosis
(29). Western blot analyses showed
a significant decrease in Akt phosphorylation in cells treated with
resveratrol or HS-1793 (Fig. 7A).
The results also indicated that resveratrol did not affect total
Akt level, while HS-1793 slightly reduced total Akt at
high-concentration (Fig. 7A). We
also investigated whether HS-1793 modulates the MAPK cascades
including JNK, ERK1/2 and p38 MAPK. HS-1793 decreased the
phosphorylation of ERK1/2 without affecting the protein level
(Fig. 7A). HS-1793 was more potent
than resveratrol in diminishing ERK1/2 phosphorylation. However,
HS-1793 and resveratrol did not affect the activation of JNK and
p38 MAPK.
To confirm the involvement of Akt signaling in
apoptosis induced by HS-1793, we employed LY294002 to inactivate
Akt, treating HCT116 cells with HS-1793. The results indicated that
HS-1793 and LY294002 treatment alone induced 33 and 15% cell death,
respectively. The MTT assay results also showed that enhanced
apoptotic effects of HS-1793 on HCT116 cells were observed when
co-treated with LY294002 in comparison to treatment with HS-1793
alone (Fig. 7B). Pharmacological
inhibition of Akt with HS-1793 treatment increased levels of
cleaved PARP compared with either treatment alone (Fig. 7C). Of note, treatment of HCT116
cells with LY294002 and HS-1793 was found to significantly suppress
Akt activation. These results suggest that the Akt pathway is
likely involved in HS-1793-induced growth inhibition and apoptosis
of HCT116 cells.
Discussion
In the present study we evaluated and compared the
anticancer activity of HS-1793 with resveratrol in human colon
cancer cell line HCT116. The MTT assay revealed that HS-1793 is
more potent than resveratrol in the inhibition of cell growth and
proliferation in HCT116 cells. At equimolar concentrations, HS-1793
is also more potent than resveratrol in the induction of apoptotic
cell death, evidenced by Annexin V staining, pro-caspase-3
reduction, cytochrome c release and cleaved PARP, in colon
cancer cells. Flow cytometric analysis indicated that resveratrol
caused S phase arrest, whereas HS-1793 induced G2/M arrest in
tested colon cancer cells. HS-1793 induced cell cycle progression
mainly by downregulating cyclins and CDKs. In addition, we found
that HS-1793 was substantially more potent than resveratrol at
inhibition of Akt.
We found that HS-1793 was more effective in
inhibiting cell growth and proliferation, and induced apoptosis in
CRC cells. The observed growth inhibitory efficacy and apoptogenic
cell death-inducing properties of HS-1793 are in agreement with
those observed by ours and others in prostate cancer (30), breast cancer (23,31,32),
colon cancer (22) and leukemia
(21). We reported previously that
HS-1793 triggered apoptosis in two breast cancer cell lines by
mediating p53-dependent and -independent pathways (23). The same study also described that
HS-1793 was respectively, 2-fold more potent in inducing apoptotic
cell death (23). In the present
study, we confirmed that HS-1793 exhibited more potent anticancer
property than resveratrol in HCT116 CRC cells, while HCT116 cells
seem to be less sensitive to resveratrol and its analogue HS-1793
in response to that in breast cancer cells (23). The ability of HS-1793 to decrease
the protein level of pro-caspases and the subsequent cleavage of
PARP further supports the apoptogenic property of HS-1793 against
CRC. We also found that HS-1793 upregulates the expression of
pro-apoptotic Bax at the protein level. The Bcl-2 family proteins
play critical roles in the induction of apoptosis. Indeed, the
ratio between anti-apoptotic Bcl-2 and apoptosis prompting Bax
helps determine, in part, the susceptibility of cells to death
signal (33). Although HS-1793 did
not affect the protein level of Bcl-2, the change in Bcl-2/Bax
ratio by HS-1793 is sufficient to induce apoptosis in HCT116 cells.
The data here were consistent with the results of previous studies
(21,23,30),
which suggested that HS-1793 had stronger antitumor effects than
resveratrol and could induce cell death in part through the
modulation of Bcl-2 family proteins.
Kim et al (23) showed that HS-1793 caused G2/M phase
cell cycle arrest in the human breast cancer MCF-7 and MDA-MB-231
cells, and reduced the level of cell cycle regulatory proteins
(cyclin B1, Cdc2 and Cdc25C) involve in G2/M. The present study
observed that HS-1793 could induce G2/M phase cell cycle arrest in
HCT116 cells. While resveratrol caused the accumulation of cells in
the S phase in HCT116 cells. In addition, Liu et al
(28) reported that resveratrol
could inhibit proliferation of HCT116 cells by inducing G1/S phase
cell cycle arrest, while we observed increased number of cells in S
phase by resveratrol. The precise reason for this difference is not
clear but conditions for the experiments may count for this
discrepancy. Here, we found that resveratrol decreased the
expression of CDK4 and CDK6 but not cyclin D1. The G1/S transition
is regulated by complexes formed by cyclin D and its binding
partners CDK4 or CDK6 (34). We
also observed that HS-1793 markedly suppressed the expression of
CDK4 and CDK6 although this resveratrol analogue caused G2/M arrest
in colon cancer cells. Thus, HS-1793 was found to be more effective
than resveratrol in inhibiting these two cyclin-dependent kinases.
Therefore, it is likely that HS-1793 exerts its inhibitory effects
on cancer cell cycle progression by modulating cell cycle regulator
proteins, however, further mechanistic study is needed to elucidate
the define mode of action of HS-1793.
Our result showed that HS-1793 inhibited the
phosphorylation of Akt, which are involved in cancer cell growth
and proliferation. In addition, we found that HS-1793 was
substantially more potent than resveratrol at reduction of
phosphorylated Akt. It has been shown that activation of Akt
signaling pathway was frequently observed in patients with colon
cancer (35,36), and thus it has considered as
therapeutic targets for cancer prevention (37). Our results are also consistent with
another recent report that HS-1793 inhibits Akt activation in colon
cancer cells (22).
Overall, our results suggest that HS-1793 exhibits
anti-proliferative and apoptosis-inducing effect in human colon
cancer cells. A previous study demonstrated the role of endoplasmic
reticulum stress and Akt on HS-1793-induced cell death in HT-29
colon cancer cells, however, the study did not provide the cell
death mechanism on colon cancer cells in detail (22). Moreover, the anticancer ability from
single cell line may give only limited information on an agent's
biological response. Therefore, testing the activities in several
cell lines are required to characterize and understand the
mechanism of drug action, resistance and modulation. Thus, this
study provides strong evidence to constitute significant
advancement over the existing knowledge and this is needed before
consequent in vivo preclinical study with HS-1793. Moreover,
present study showed that HS-1793 is superior to its parental
chemical resveratrol as a good candidate for novel anticancer
agent. On the basis of these results, further studies are needed to
confirm and extend the present study and
pharmacokinetic/pharmacodynamics studies are required to use this
novel resveratrol analogue HS-1793 as an anticancer agent.
Acknowledgements
This study was supported by the Basic Science
Research Program through the National Research Foundation of Korea
(NRF) funded by the Ministry of Education, Science and Technology
(nos. 2012R1A1A2006753 and 2014R1A1A2055336). This study was also
supported by the National Research Foundation of Korea (NRF) grant
funded by the Korea government (MSIP) (no. 2009-0083538).
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jung KW, Won YJ, Kong HJ, Oh CM, Cho H,
Lee DH and Lee KH: Cancer statistics in Korea: Incidence,
mortality, survival, and prevalence in 2012. Cancer Res Treat.
47:127–141. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shin A, Kim KZ, Jung KW, Park S, Won YJ,
Kim J, Kim DY and Oh JH: Increasing trend of colorectal cancer
incidence in Korea, 1999–2009. Cancer Res Treat. 44:219–226. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li YH, Niu YB, Sun Y, Zhang F, Liu CX, Fan
L and Mei QB: Role of phytochemicals in colorectal cancer
prevention. World J Gastroenterol. 21:9262–9272. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bhat KP, Lantvit D, Christov K, Mehta RG,
Moon RC and Pezzuto JM: Estrogenic and antiestrogenic properties of
resveratrol in mammary tumor models. Cancer Res. 61:7456–7463.
2001.PubMed/NCBI
|
|
6
|
Schneider Y, Duranton B, Gossé F,
Schleiffer R, Seiler N and Raul F: Resveratrol inhibits intestinal
tumorigenesis and modulates host-defense-related gene expression in
an animal model of human familial adenomatous polyposis. Nutr
Cancer. 39:102–107. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li ZG, Hong T, Shimada Y, Komoto I, Kawabe
A, Ding Y, Kaganoi J, Hashimoto Y and Imamura M: Suppression of
N-nitrosomethylbenzylamine (NMBA)-induced esophageal tumorigenesis
in F344 rats by resveratrol. Carcinogenesis. 23:1531–1536. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sale S, Tunstall RG, Ruparelia KC, Potter
GA, Steward WP and Gescher AJ: Comparison of the effects of the
chemopreventive agent resveratrol and its synthetic analog
trans-3,4,5,4-tetramethoxystilbene (DMU-212) on adenoma development
in the Apc(Min+) mouse and cyclooxygenase-2 in
human-derived colon cancer cells. Int J Cancer. 115:194–201. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu HS, Pan CE, Yang W and Liu XM:
Antitumor and immunomodulatory activity of resveratrol on
experimentally implanted tumor of H22 in Balb/c mice. World J
Gastroenterol. 9:1474–1476. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen Y, Tseng SH, Lai HS and Chen WJ:
Resveratrol-induced cellular apoptosis and cell cycle arrest in
neuroblastoma cells and antitumor effects on neuroblastoma in mice.
Surgery. 136:57–66. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pan MH, Gao JH, Lai CS, Wang YJ, Chen WM,
Lo CY, Wang M, Dushenkov S and Ho CT: Antitumor activity of
3,5,4-trimethoxystilbene in COLO 205 cells and xenografts in SCID
mice. Mol Carcinog. 47:184–196. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen JC, Chen Y, Lin JH, Wu JM and Tseng
SH: Resveratrol suppresses angiogenesis in gliomas: Evaluation by
color Doppler ultrasound. Anticancer Res. 26:1237–1245.
2006.PubMed/NCBI
|
|
13
|
Busquets S, Ametller E, Fuster G, Olivan
M, Raab V, Argilés JM and López-Soriano FJ: Resveratrol, a natural
diphenol, reduces metastatic growth in an experimental cancer
model. Cancer Lett. 245:144–148. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kosmeder JW II, Pezzuto JM, Pezzuto JM and
Bhat KP: Biological effects of resveratrol. Antioxid Redox Signal.
3:1041–1064. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Harikumar KB and Aggarwal BB: Resveratrol:
A multitargeted agent for age-associated chronic diseases. Cell
Cycle. 7:1020–1035. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Baur JA and Sinclair DA: Therapeutic
potential of resveratrol: The in vivo evidence. Nat Rev Drug
Discov. 5:493–506. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cai YJ, Wei QY, Fang JG, Yang L, Liu ZL,
Wyche JH and Han Z: The 3,4-dihydroxyl groups are important for
trans-resveratrol analogs to exhibit enhanced antioxidant and
apoptotic activities. Anticancer Res. 24:999–1002. 2004.PubMed/NCBI
|
|
18
|
Szekeres T, Fritzer-Szekeres M, Saiko P
and Jäger W: Resveratrol and resveratrol analogues -
structure-activity relationship. Pharm Res. 27:1042–1048. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Song S, Lee H, Jin Y, Ha YM, Bae S, Chung
HY and Suh H: Syntheses of hydroxy substituted
2-phenyl-naphthalenes as inhibitors of tyrosinase. Bioorg Med Chem
Lett. 17:461–464. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jeong SH, Lee JS, Jeong NY, Kim TH, Yoo
KS, Song S, Suh H, Kwon TK, Park BS and Yoo YH: A novel resveratrol
analogue HS-1793 treatment overcomes the resistance conferred by
Bcl-2 and is associated with the formation of mature PML nuclear
bodies in renal clear cell carcinoma Caki-1 cells. Int J Oncol.
35:1353–1360. 2009.PubMed/NCBI
|
|
21
|
Jeong SH, Jo WS, Song S, Suh H, Seol SY,
Leem SH, Kwon TK and Yoo YH: A novel resveratrol derivative,
HS1793, overcomes the resistance conferred by Bcl-2 in human
leukemic U937 cells. Biochem Pharmacol. 77:1337–1347. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Um HJ, Bae JH, Park JW, Suh H, Jeong NY,
Yoo YH and Kwon TK: Differential effects of resveratrol and novel
resveratrol derivative, HS-1793, on endoplasmic reticulum
stress-mediated apoptosis and Akt inactivation. Int J Oncol.
36:1007–1013. 2010.PubMed/NCBI
|
|
23
|
Kim JA, Kim DH, Hossain MA, Kim MY, Sung
B, Yoon JH, Suh H, Jeong TC, Chung HY and Kim ND: HS-1793, a
resveratrol analogue, induces cell cycle arrest and apoptotic cell
death in human breast cancer cells. Int J Oncol. 44:473–480.
2014.PubMed/NCBI
|
|
24
|
Kim DH, Hossain MA, Kim MY, Kim JA, Yoon
JH, Suh HS, Kim GY, Choi YH, Chung HY and Kim ND: A novel
resveratrol analogue, HS-1793, inhibits hypoxia-induced HIF-1α and
VEGF expression, and migration in human prostate cancer cells. Int
J Oncol. 43:1915–1924. 2013.PubMed/NCBI
|
|
25
|
Wang C and Youle RJ: The role of
mitochondria in apoptosis. Annu Rev Genet. 43:95–118. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sancar A, Lindsey-Boltz LA, Unsal-Kaçmaz K
and Linn S: Molecular mechanisms of mammalian DNA repair and the
DNA damage checkpoints. Annu Rev Biochem. 73:39–85. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Perry JA and Kornbluth S: Cdc25 and Wee1:
Analogous opposites? Cell Div. 2:122007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu B, Zhou Z, Zhou W, Liu J, Zhang Q, Xia
J, Liu J, Chen N, Li M and Zhu R: Resveratrol inhibits
proliferation in human colorectal carcinoma cells by inducing G1/S
phase cell cycle arrest and apoptosis through caspase/cyclin CDK
pathways. Mol Med Rep. 10:1697–1702. 2014.PubMed/NCBI
|
|
29
|
Bellacosa A, Kumar CC, Di Cristofano A and
Testa JR: Activation of AKT kinases in cancer: Implications for
therapeutic targeting. Adv Cancer Res. 94:29–86. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jeong NY, Yoon YG, Rho JH, Lee JS, Lee SY,
Yoo KS, Song S, Suh H, Choi YH and Yoo YH: The novel resveratrol
analog HS-1793-induced polyploid LNCaP prostate cancer cells are
vulnerable to downregulation of Bcl-xL. Int J Oncol. 38:1597–1604.
2011.PubMed/NCBI
|
|
31
|
Jeong SH, Song IS, Kim HK, Lee SR, Song S,
Suh H, Yoon YG, Yoo YH, Kim N, Rhee BD, et al: An analogue of
resveratrol HS-1793 exhibits anticancer activity against MCF-7
cells via inhibition of mitochondrial biogenesis gene expression.
Mol Cells. 34:357–365. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kim HJ, Yang KM, Park YS, Choi YJ, Yun JH,
Son CH, Suh HS, Jeong MH and Jo WS: The novel resveratrol analogue
HS-1793 induces apoptosis via the mitochondrial pathway in murine
breast cancer cells. Int J Oncol. 41:1628–1634. 2012.PubMed/NCBI
|
|
33
|
Gross A, McDonnell JM and Korsmeyer SJ:
BCL-2 family members and the mitochondria in apoptosis. Genes Dev.
13:1899–1911. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bates S, Bonetta L, MacAllan D, Parry D,
Holder A, Dickson C and Peters G: CDK6 (PLSTIRE) and CDK4 (PSK-J3)
are a distinct subset of the cyclin-dependent kinases that
associate with cyclin D1. Oncogene. 9:71–79. 1994.PubMed/NCBI
|
|
35
|
Malinowsky K, Nitsche U, Janssen KP, Bader
FG, Späth C, Drecoll E, Keller G, Höfler H, Slotta-Huspenina J and
Becker KF: Activation of the PI3K/AKT pathway correlates with
prognosis in stage II colon cancer. Br J Cancer. 110:2081–2089.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Rychahou PG, Kang J, Gulhati P, Doan HQ,
Chen LA, Xiao SY, Chung DH and Evers BM: Akt2 overexpression plays
a critical role in the establishment of colorectal cancer
metastasis. Proc Natl Acad Sci USA. 105:20315–20320. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Crowell JA, Steele VE and Fay JR:
Targeting the AKT protein kinase for cancer chemoprevention. Mol
Cancer Ther. 6:2139–2148. 2007. View Article : Google Scholar : PubMed/NCBI
|