Introduction
Colorectal cancer is the third most common cancer
and the leading cause of cancer-related mortality around the world
(1). The high mortality of
colorectal cancer is due to the high rate of synchronous liver
metastases which is found in newly diagnosed colorectal cancer
patients. Therefore, colorectal cancer is one of the diseases
seriously threating health (2,3). For
patients with advanced colorectal cancer, tumor resection is not
feasible and chemotherapy and biotherapy have become the only
strategies for treatment (4,5).
Unfortunately, however, these treatments are mostly ineffective
because of the development of chemoresistance in cancer patients
(6).
TNF-related apoptosis-inducing ligand (TRAIL) is a
member of the TNF superfamily, which can induce extrinsic apoptosis
depending on the activation of caspase-8 (7). TRAIL has been considered as a
potential therapeutic agent in cancer treatment due to its
capability to induce apoptosis selectively in tumor cells, but not
in normal cells (8). Therefore,
unlike TNF-α or chemotherapeutics, TRAIL shows little toxicity when
administered in vivo. However, a significant proportion of
cancer cells exhibit low sensitivity to TRAIL-induced apoptosis,
which precludes its use in many cases (9,10). It
is urgent to find a strategy to increase the sensitivity of tumor
cells to TRAIL.
MicroRNAs (miRNAs) are endogenous, small non-coding
RNAs consisting of approximately 21–25 nucleotides (nt) (11). They are involved in
post-transcriptional control of approximately 60% of the human
genes by binding to the 3-untranslated region (3-UTR) of target
mRNAs. Therefore, miRNAs participate in a wide array of biological
processes including cell proliferation, development,
differentiation, apoptosis and others (12,13).
Aberrant miRNA expression has been frequently observed in various
human tumors, which was indicated to be involved in cancer
development and progression (14).
In the present study, we demonstrated that miR-20a was dysregulated
in colorectal cancer and associated with the TRAIL sensitivity.
Therefore, this study presents significant insight into the role of
miR-20a, which shows potential for developing miRNA-related
colorectal cancer therapy.
Materials and methods
Clinical specimens and cell lines
Thirty pairs of colorectal cancer tissues and the
adjacent non-tumor tissues were obtained from the patients who
underwent tumor resection in The First Affiliated Hospital of
Wenzhou Medical University from June 2013 to September 2015. Both
tumor and non-cancerous samples were confirmed histologically. In
addition, 80 serum samples from 40 colorectal cancer patients and
40 healthy controls were collected from the same hospital before
the treatment. All samples were collected with patients informed
consent, and this project was approved by the Ethics Committee of
The First Affiliated Hospital of Wenzhou Medical University. FHC
which is a normal colorectal epithelial cell line (15) and the colorectal cancer cell lines
SW480, SW948, NCI-H508 and HT-29 were originated from the American
Type Culture Collection (ATCC; Manassas, VA, USA). SW480, SW948,
NCI-H508 and HT-29 cells were cultured in Dulbeccos modified Eagles
medium (DMEM) supplemented with 10% fetal bovine serum (FBS; Gibco-
Invitrogen), 100 U/ml penicillin and 100 µg/ml streptomycin. FHC
cells were cultured as previously described (15). All cells were cultured at 37°C with
5% CO2. TRAIL resistant SW480 (R-SW480) was established
by continuous exposure to TRAIL. Briefly, SW480 cells were treated
with TRAIL for 48 h and then the cells were moved to the TRAIL-free
medium for another 72 h after drug treatment. This process was
repeated three times before the cells were exposed to the next
higher dose of TRAIL. The initiating concentration of TRAIL was 1
ng/ml, and the concentration was increased by 0.5 ng/ml up to a
final concentration of 5 ng/ml. The R-SW480 cells were cultured in
medium with TRAIL, but before the experiments were performed,
R-SW480 cells were moved to the TRAIL-free medium for 2 weeks.
Quantitative real-time polymerase
chain reaction (qRT-PCR)
RNA was extracted from frozen tissues, serum and
colorectal cancer cell lines using the TRIzol reagent (Invitrogen,
Carlsbad, CA, USA) according to the manufacturers instructions. The
reverse transcription of miR-20a was performed by stem-loop RT
primer using the PrimeScript RT reagent kit (Takara, Dalian,
China), and quantified by qPCR using the SYBR Premix Ex Taq
(Takara) according to the manufacturers instructions. The primers
were synthesized as follows: miR-20a for RT primer:
5-CTCAACTGGTGTCGTGGAGT CGGCAATTCAGTTGAGATTTCACG-3. QPCR forward
primer: 5-ACACTCCAGCTGGGGATGGACGTGATATT CG-3 and reversed primer:
5-TGGTGTCGTGGAGTCG-3. U6 snRNA was used as the internal control.
The relative expression of miR-20a was determined using the
2−∆∆CT analysis method (16).
Transfection
BID siRNA, miR-20a mimics, miR-20a inhibitor
(anti-miR-20a) and the negative control (miR-NC) were synthesized
by Guangzhou RiboBio, Co., Ltd. (Guangzhou, China). The SW480 cells
were transfected with 50 pmol/ml BID siRNA
(5-GAAGACAUCAUCCGGAAUAUU-3), 50 pmol/ml miR-20a mimics
(5-GAUGGACGUGAUAUUC GUGAAAU-3), 50 pmol/ml 2-Omethyl modified
anti-miR-20a (5-AUUUCACGAAUAUCACGUCCAUC-3), 50 pmol/ml miR-NC
(5-GUCGAGAUUGGAUGAUAAUC AUG-3) using Lipofectamine 2000
(Invitrogen) according to the manufacturers guidance.
Luciferase reporter assay
The 3′-UTR fragment of BID containing the seed
region was amplified and inserted downstream of the luciferase
reporter gene in the luciferase reporter pMIR-Report-Vector (Life
Technologies, Carlsbad, CA, USA). The mutant 3′-UTR of BID (GCACUUU
to GCAGAUU) was amplified by wild-type reporter containing BID
3′-UTR as the template, and the mutant plasmid was created by
Site-Directed Mutagenesis kit (Takara). For luciferase reporter
assays, the SW480 cells were co-transfected with RNAs and
luciferase reporters. Following transfection for 48 h, the cells
were collected and lysed. Luciferase activity was then measured by
using Dual-luciferase assay (Promega, Madison, WI, USA) according
to the manufacturers instructions. Firefly luciferase activity was
normalized to the Renilla luciferase activity, and the ratio
of firefly/Renilla was presented.
Cell viability assay
For cell viability assay, 24 h post-transfection of
RNAs, the cells were reseeded in 96-well plates at a density of
2×103 cells/well. Subsequently, after the cells were
treated with TRAIL for 48 h, they were stained with 20 µl
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
(5 mg/ml; Sigma-Aldrich, St. Louis, MO, USA) and incubated at 37°C
for 4 h. After removing the supernatant, 150 µl DMSO was added and
thoroughly mixed. The absorbance was read at 570 nm using a
microplate reader (Sunrise microplate reader; Tecan Group Ltd.,
Zürich, Switzerland).
Western blot analysis
For mitochondria isolation, the Mitochondria/Cytosol
fraction kit (BioVision, Inc., Milpitas, CA, USA) was used
according to the manufacturers instructions. Proteins from the
whole SW480 cells, cytosolic fractions and mitochondrial fractions
were separated by 12.5% sodium dodecyl sulfate polyacrylamide gel
electrophoresis (SDS-PAGE) and transferred to a PVDF membrane
(Millipore, Billerica, MA, USA). Membranes were blocked with 5%
skim milk for 1 h at room temperature and incubated overnight at
4°C with primary antibodies (caspase-8, caspase-9, caspase-3, BID,
cytochrome c, Smac/DIABLO and β-actin, all of them were
purchased from Cell Signaling Technology, Danvers, MA, USA) at a
1:1,000 dilution. The membranes were subsequently incubated with a
horseradish peroxidase (HRP)-conjugated secondary antibody (Cell
Signaling Technology) at a 1:2,000 dilution for 2 h at room
temperature. The blots were then detected with an enhanced
chemiluminescence detection kit (Pierce, Rockford, IL, USA).
Apoptosis assay
For apoptosis analysis, 24 h post-transfection of
RNAs, the cells were reseeded in 6-well plates at a density of
5×105 cells/well. Subsequently, after the cells were
treated with TRAIL for 48 h, they were stained with FITC-Annexin V
and propidium iodide (PI) according to the manufacturers
instructions (Sigma-Aldrich). The percentage of apoptotic cells was
quantified using a flow cytometry (Beckman Coulter, Inc., Brea, CA,
USA).
Measurement of mitochondrial membrane
potential (MMP, ∆Ψm)
For MMP measurement, 24 h post-transfection of RNAs,
the cells were reseeded in 6-well plates at a density of
5×105 cells/well. Subsequently, after the cells were
treated with TRAIL for 48 h, SW480 cells were incubated with 10
µg/ml JC-1 (Invitrogen) for 10 min at 37°C and then assessed for
red fluorescence using a flow cytometry.
Statistical analysis
All data are expressed as the mean ± standard
deviation and were carried out by three independent experiments.
The statistical significance of difference between the groups were
determined using the Students t-test performed by SPSS 13.0
software. P<0.05 was considered statistically significant.
Results
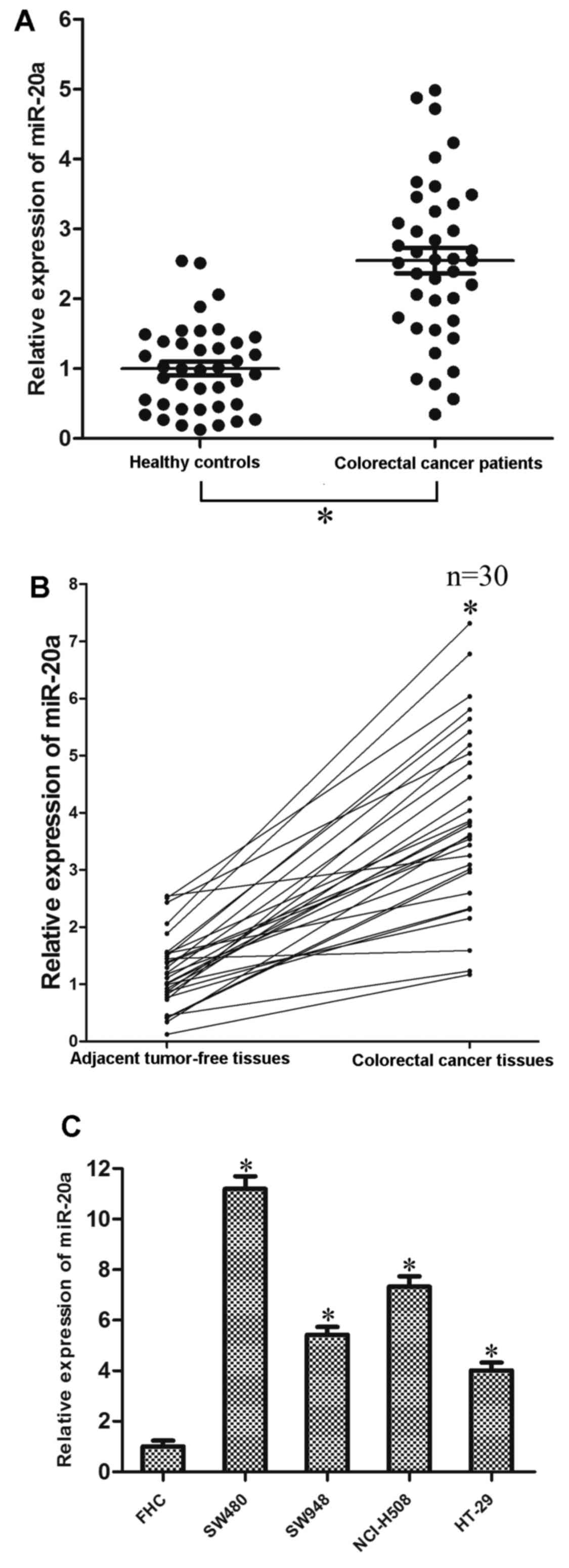
miR-20a is upregulated in colorectal
cancer
To investigate the potential role of miR-20a in
colorectal cancer, we analyzed the expression level of miR-20a in
colorectal cancer tissues and the adjacent tumor-free tissues, as
well as the serum samples of colorectal cancer patients and normal
controls. As shown in Fig. 1A, the
average expression level of miR-20a was significantly increased in
the serum of colorectal cancer patients compared with the healthy
controls. Furthermore, among the 30 patients with colorectal
cancer, miR-20a was upregulated in tumor tissues compared with the
adjacent tumor-free tissues (Fig.
1B). Consistent with the above results, miR-20a expression
level was upregulated in all four examined colorectal cancer cell
lines (SW480, SW948, NCI-H508 and HT-29) compared with the FHC
which is the normal colorectal epithelial cell line (Fig. 1C).
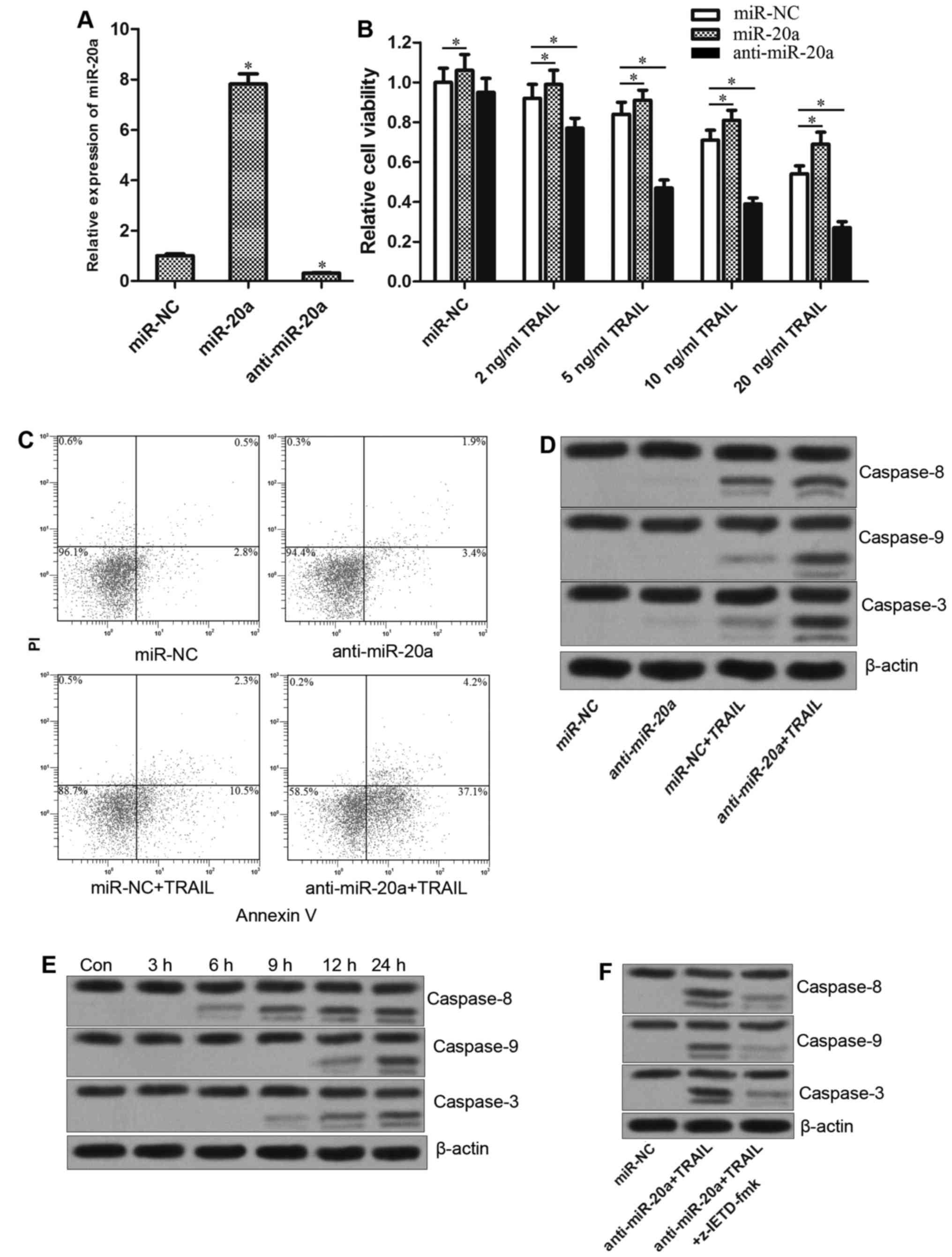
Anti-miR-20a increases the TRAIL
sensitivity via caspase-dependent apoptosis pathway
To explore the biological roles of miR-20a in TRAIL
treatment for colorectal cancer, SW480 cells were transfected with
miR-20a mimics or inhibitors. Since the miR-20a mimics or
inhibitors significantly changed the level of miR-20a in SW480
cells (Fig. 2A), the combination
treatment with these RNAs and TRAIL was performed. As shown in
Fig. 2B, knockdown of miR-20a
sensitized the SW480 cells to TRAIL-induced cell death, whereas the
miR-20a mimics decreased the antitumor effect of TRAIL. These
results suggested that miR-20a is associated with the TRAIL
sensitivity in colorectal cancer. To study the mechanism by which
anti-miR-20a enhanced the TRAIL-induced cell death, we treated the
SW480 cells with anti-miR-20a and TRAIL, either alone or in
combination, and then the cells were harvested and stained by
Annexin V and PI. As shown in Fig.
2C, co-treatment with anti-miR-20a and TRAIL induced apoptosis
more significantly compared with the either anti-miR-20a or TRAIL
alone treatment group, suggesting the apoptosis pathway was
involved in anti-miR-20a associated cell death induced by TRAIL.
Notably, anti-miR-20a plus TRAIL significantly activated caspase-8,
caspase-9 and caspase-3, whereas TRAIL alone treatment group
significantly triggered caspase-8 besides caspase-9 and caspase-3
(Fig. 2D). Furthermore, as shown in
Fig. 2E, anti-miR-20a plus TRAIL
rapidly triggered caspase-8 in SW480, whereas caspase-9 and
caspase-3 were activated after 12 h, suggesting caspase-8 acted as
the initiator caspase. To investigate this hypothesis, SW480 cells
were treated with the caspase-8-specific inhibitor z-IETD-fmk
(17) before the combined treatment
of anti-miR-20a with TRAIL. We found the z-IETD-fmk inhibited the
activation of caspase-8, as well as caspase-9 and caspase-3,
indicating that the caspase-9 and caspase-3 were downstream of
caspase-8 (Fig. 2F). Taken
together, these findings demonstrated that the activation of
caspase-9 and caspase-3, which was initiated by the caspase-8
activation, was involved in anti-miR-20a associated TRAIL-induced
apoptosis.
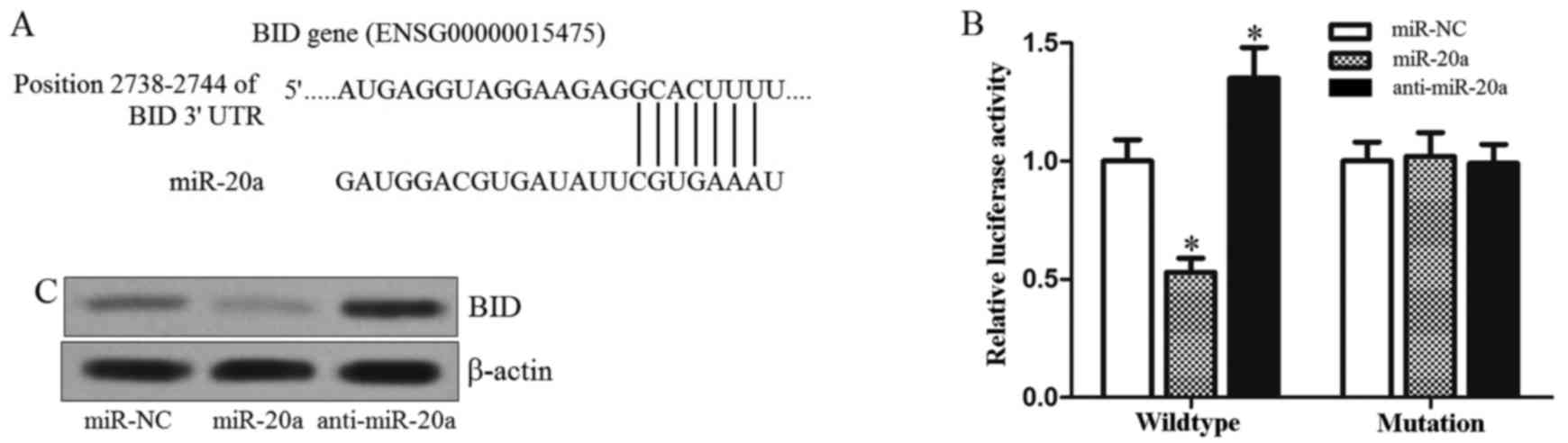
BID is the target of miR-20a in
colorectal cancer
To understand how miR-20a facilitates TRAIL-induced
apoptosis, we used TargetScan database to help identify miR-20a
targets in SW480. Of these target genes that were predicted by this
database, it was revealed that BID is one of the possible target
genes of miR-20a (Fig. 3A). BID is
a known BH3-only Bcl-2 family member which is the key regulator for
linking the extrinsic and mitochondrial (intrinsic) pathway of
apoptosis (18). Therefore, we
speculated that the gene of BID is a potential target of miR-20a.
To investigate this hypothesis, we cloned the BID 3-UTR sequences
containing the predicted target site of miR-20a into a luciferase
reporter vector. Luciferase assay revealed that co-transfection of
miR-20a mimics and luciferase reporter with wild-type of BID 3-UTR
significantly decreased the luciferase activities, whereas the
anti-miR-20a increased the luciferase activities. However, mutation
of the putative miR-20a sites in the 3-UTR of BID abrogated
luciferase responsiveness to miR-20a (Fig. 3B). To confirm that miR-20a regulates
the expression of BID, the BID protein was measured after the
miR-20a mimics or antagonist was transfected into SW480 cells. As
shown in Fig. 3C, miR-20a mimics
decreased the expression of BID, whereas the transfection of
anti-miR-20a increased the BID protein level in SW480 cells. Taken
together, these results suggested that the BID gene is a functional
target of miR-20a, which may be associated with the TRAIL
sensitivity in colorectal cancer.
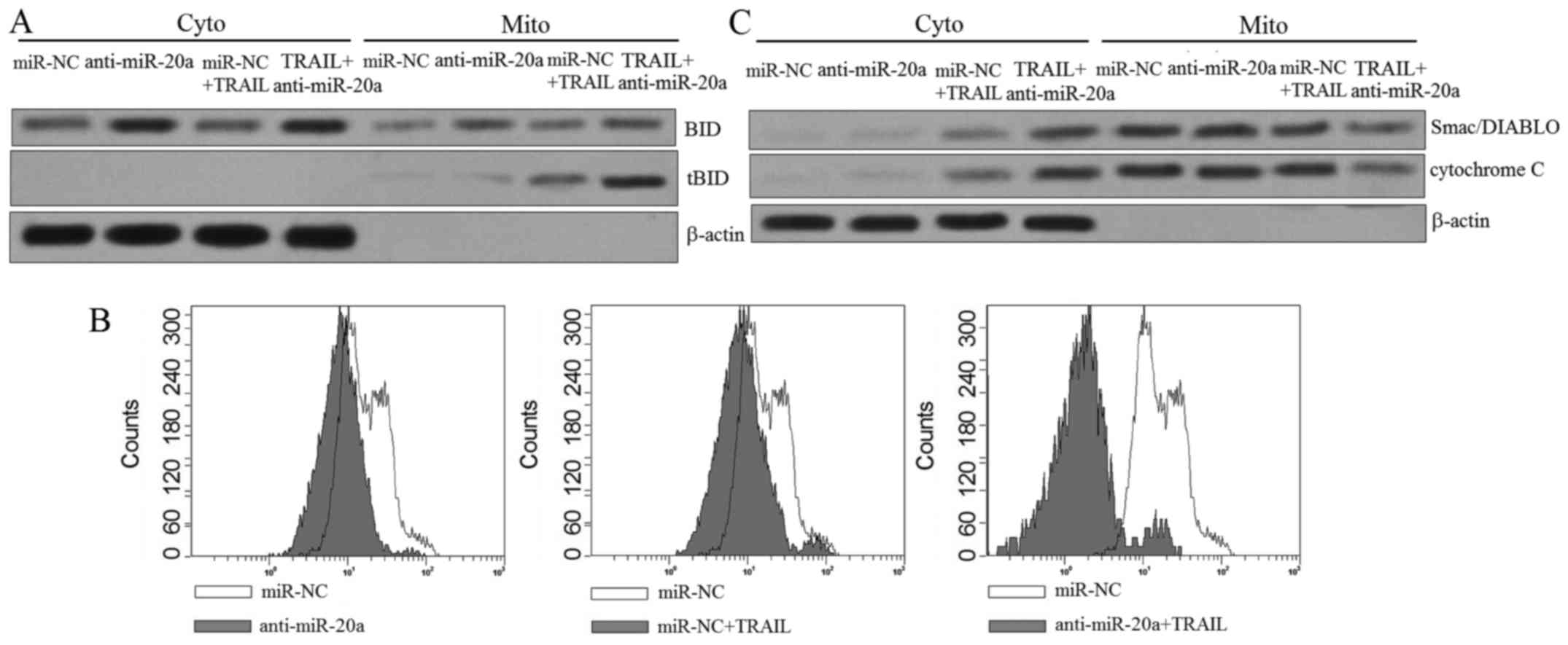
Knockdown of miR-20a increases the
translocation of tBid from the cytosolic fraction to the
mitochondria
It is acknowledged that BID is the substrate of
caspase-8 activated by TRAIL (19).
As we confirmed that BID is the target of miR-20a, we speculated
that transfection of anti-miR-20a may expand the TRAIL-induced
apoptosis via promoting the translocation of tBid from the
cytosolic fraction to the mitochondria. We therefore separated the
mitochondria from the cytoplasm in SW480 cells treated with
anti-miR-20a and TRAIL, and then the western blot assays were
performed. As shown in Fig. 4A,
transfection of miR-20a significantly increased the BID expression
level in the cytosolic fraction and promoted the translocation of
tBid to the mitochondria in SW480 cells. Since the translocation of
tBid induced mitochondria apoptosis, we measured the mitochondrial
membrane potential of SW480 cells by JC-1 staining. As expected,
although the anti-miR-20a alone hardly influenced ∆Ψm,
it significantly facilitated TRAIL to damage the mitochondria of
SW480 cells (Fig. 4B), resulting in
the release of mitochondria-derived pro-apoptotic inducers such as
the cytochrome c and Smac/DIABLO (Fig. 4C). Taken together, these results
suggested the dysfunction of mitochondria is the reason why
anti-miR-20a increased the sensitivity of SW480 to TRAIL-induced
cell death.
Mitochondrial apoptosis is induced by
the combination of TRAIL and anti-miR-20a dependent on the
upregulation of BID
Since the activation of caspase-9 and caspase-3 is
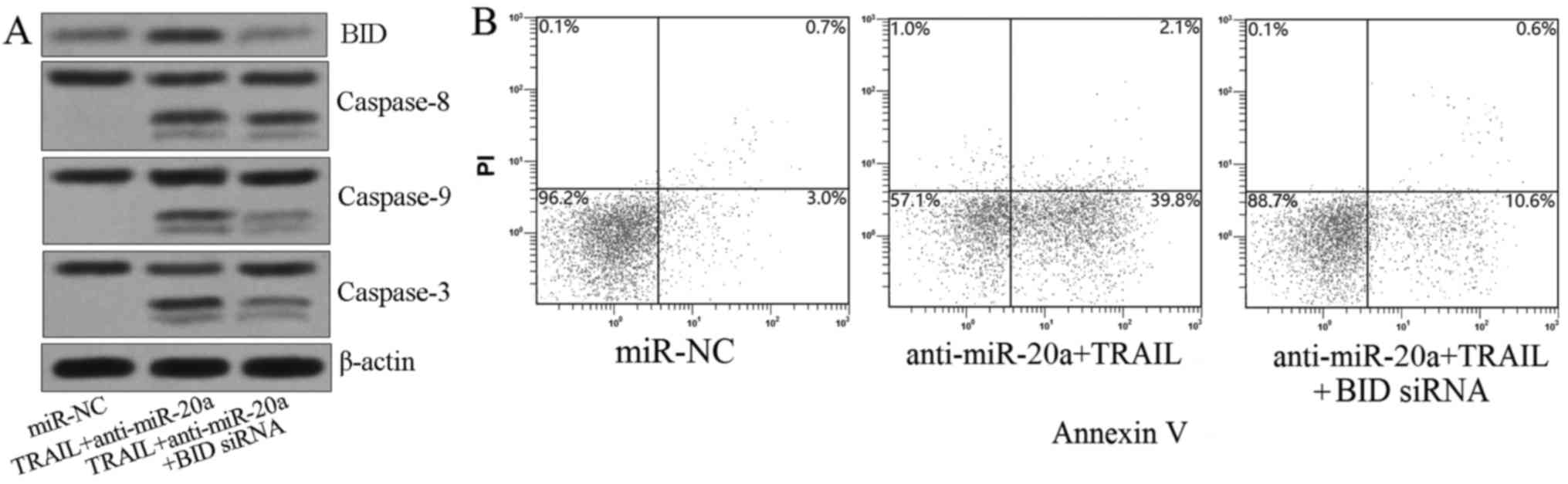
the downstream incidence of mitochondria damage (19), we knoced down BID by its specific
siRNA before the SW480 cells were treated with anti-miR-20a plus
TRAIL. As we observed, although BID siRNA did not influence the
activation of caspase-8, it significantly inhibited the cleavage of
caspase-9 and caspase-3 (Fig. 5A).
As a result, the apoptotic rate of treated SW480 cells was also
decreased by knockdown of BID (Fig.
5B). In summary, these results proved the conclusion that
knockdown of miR-20a expanded the TRAIL-induced apoptosis via
BID/mitochondrial pathway in colorectal cancer.
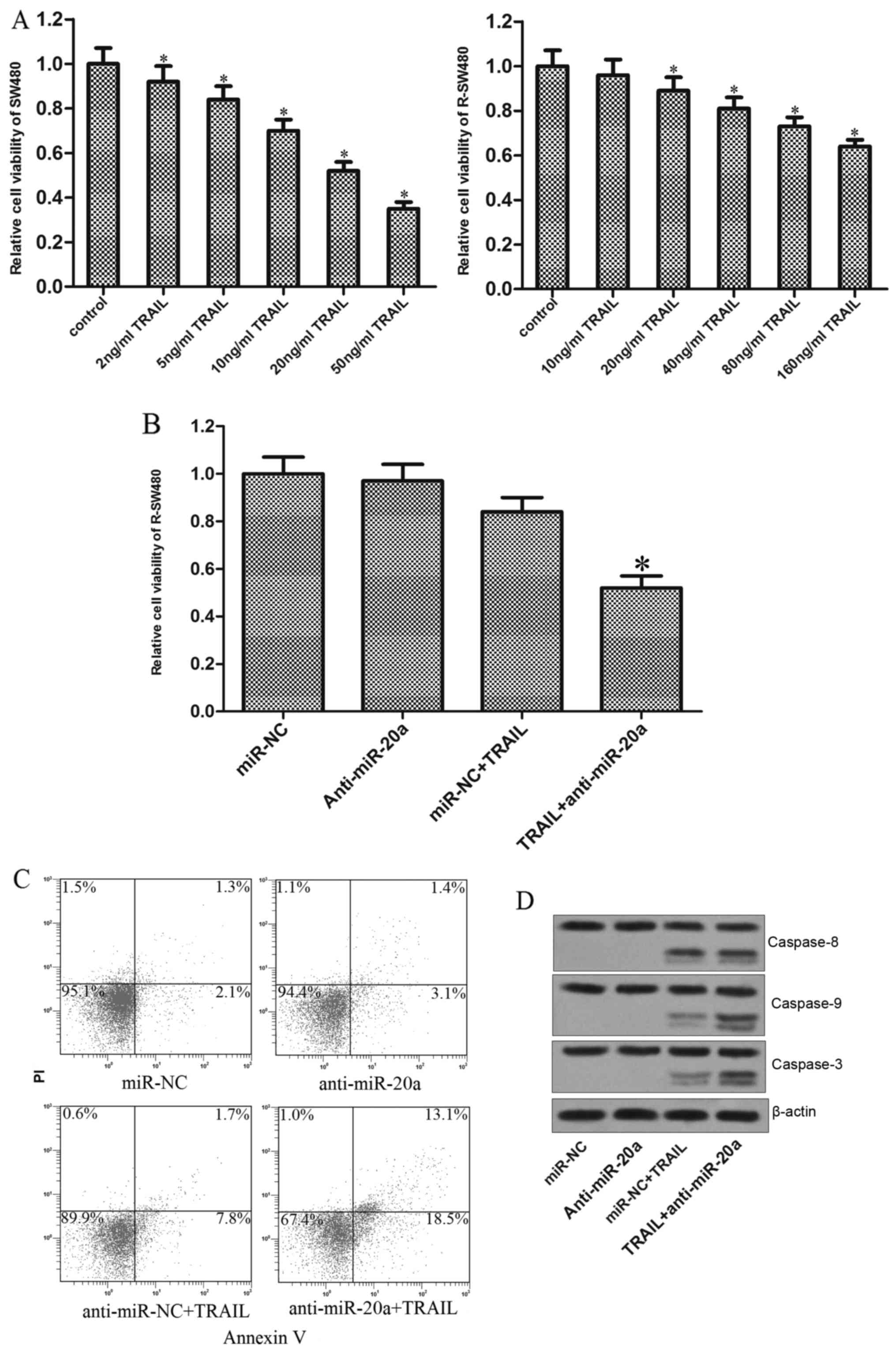
Knockdown of miR-20a reverses the
TRAIL resistance induced by continuous exposure in colorectal
cancer
Since the preceding results proved that knockdown of
miR-20a increased the sensitivity of SW480 cells to TRAIL, we next
investigate whether the anti-miR-20a reversed the TRAIL resistance
induced by repeated treatment in colorectal cancer cells. As shown
in Fig. 6A, the established TRAIL
resistant SW480 cells (R-SW480) showed obvious resistance to TRAIL.
Notably, knockdown of miR-20a in R-SW480 significantly resensitized
the cells to TRAIL-induced cell death (Fig. 6B). Similarly, although the R-SW480
cells were resistant to TRAIL-induced apoptosis, transfection of
anti-miR-20a impaired the tolerance, followed by strong apoptosis
(Fig. 6C). Furthermore, similarly
to the parental SW480, the TRAIL-treated R-SW480 cells also showed
obvious activation of caspase-9 and caspase-3 due to the knockdown
of miR-20a (Fig. 6D). Taken
together, our data strongly suggested that miR-20a is associated
with the TRAIL sensitivity in colorectal cancer, knockdown of which
can reverse the TRAIL resistance through BID/mitochondrial
pathway.
Discussion
miRNAs are considered as important regulators in
substantial cellular processes, including tumorigenesis (20). In addition, previous reports have
indicated that miRNAs are related to cancer sensitivity to
chemotherapy (21,22). In the present study, we demonstrated
that miR-20a was upregulated in colorectal cancer tissues and cell
lines. Moreover, knockdown of miR-20a by its specific antagonist
was unexpectedly efficient in killing SW480 cells when it was
combined with TRAIL. In previous studies, miR-20a was reported to
act as an oncomiR in multiple cancers. For instance, overexpression
of miR-20a was reported to be associated with the enhancement of
the tumor metastasis of colorectal cancer cells (23). In non-small cell lung cancer
(NSCLC), the iron exporter ferroportin (FPN), which acts as a tumor
suppressor by inhibiting the proliferation and colorectaly
formation of cancer cells, was downregulated by the high level of
miR-20a (24). Despite the
convincing evidence that miR-20a promotes the development of
tumors, this study first observed the potential use of miR-20a
antagonist as sensitization agent to enhance the antitumor effect
of TRAIL in colorectal cancer.
Apoptosis via the TRAIL pathway requires the
formation of the death-inducing signaling complex (DISC), which
recruits procaspase-8 and activate it. As the results, the
extrinsic apoptosis could be induced directly by the cleaved
caspase-8. Importantly, however, the activated caspase-8 also acts
as a bridging element to trigger the mitochondrial pathway of
apoptosis, which is also called endogenous apoptosis (25–27).
In the present study, we found the combination with miR-20a
antagonist and TRAIL induced significant cleavage of caspase-9 and
caspase-3, whereas their cleavage was slight in SW480 cells when
treated with TRAIL alone. Furthermore, we discovered that the
activation of caspase-9 and caspase-3 followed caspase-8
activation. Therefore, we declared that anti-miR-20a could promote
TRAIL-induced apoptosis via mitochondrial pathway in colorectal
cancer.
BID (BH3 interacting domain death agonist), which is
the BH3-only protein in Bcl-2 family, acts as a bridging element
between the death receptor signaling and the mitochondrial
apoptosis (26). Following the
interaction between death receptor and the ligands such as TRAIL,
BID is cleaved by caspase-8 to become the activated form of protein
termed truncated BID (tBID) (28,29).
After the tBID translocates to the mitochondria, the conformation
of Bax and Bak is changed to form the Bax/Bak pores, which lead to
the mitochondrial outer membrane permeabilization and mitochondrial
depolarization (decrease of ∆Ψm) (30,31).
As a result, the mitochondria-derived pro-apoptotic inducers (such
as the cytochrome c and Smac/DIABLO) are released into the
cytosol, followed by the activation of effector caspases (such as
caspase-9 and caspase-3) and apoptosis (7,32).
Previous studies have suggested that the activation
of BID is an important strategy in cancer therapy (33,34).
In the present study, we also demonstrated that the miR-20a
antagonist promoted the TRAIL-dependent mitochondrial apoptosis by
regulating the expression of BID directly. As a result of BID
upregulation, the miR-20a antagonist promoted the translocation of
tBID in TRAIL-treated SW480 cells, followed by the mitochondrial
depolarization and the release of cytochrome c and
Smac/DIABLO. Previous studies indicated that the cytochrome
c in cytosol triggers caspase-3 activation by inducing the
formation of Apaf-1/caspase-9 apoptosome, the Smac/DIABLO in
cytosol neutralizes the inhibitor of apoptosis proteins (IAPs)
(35), the mitochondrial apoptosis
occurred finally because of the combination of anti-miR-20a with
TRAIL.
Drug-resistance is a major challenge to effective
TRAIL treatment (36). Therefore,
we finally tested the role of miR-20a antagonist in reversing the
TRAIL resistance in colorectal cancer. We observed that the
anti-miR-20a can increase the sensitivity of experimental
TRAIL-resistant SW480 to TRAIL-induced cell death, suggesting the
attractive prospect of miR-20a antagonist on colorectal cancer
treatment with TRAIL.
In summary, we have provided several pieces of
evidence suggesting that miR-20a is associated with the TRAIL
sensitivity in colorectal cancer by regulating the pro-apoptotic
gene of BID. The identification of miR-20a antagonist as the
promoter of TRAIL might have significant therapeutic potential and
may provide a novel strategy for overcoming drug-resistance.
Acknowledgements
The present study was supported by the Wenzhou
Science and Technology Bureau (grant no. Y20150050).
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Manfredi S, Lepage C, Hatem C, Coatmeur O,
Faivre J and Bouvier AM: Epidemiology and management of liver
metastases from colorectal cancer. Ann Surg. 244:254–259. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bao S, Ouyang G, Bai X, Huang Z, Ma C, Liu
M, Shao R, Anderson RM, Rich JN and Wang XF: Periostin potently
promotes metastatic growth of colon cancer by augmenting cell
survival via the Akt/PKB pathway. Cancer Cell. 5:329–339. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yi HJ, Hong KS, Moon N, Chung SS, Lee RA
and Kim KH: Acute hyperammonemic encephalopathy after
5-fluorouracil based chemotherapy. Ann Surg Treat Res. 19:264–271.
2006.
|
|
5
|
Fuchs D, Metzig M, Bickeböller M, Brandel
C and Roth W: The Gβ5 protein regulates sensitivity to
TRAIL-induced cell death in colon carcinoma. Oncogene.
34:2753–2763. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hwang JS, Lee HC, Oh SC, Lee DH and Kwon
KH: Shogaol overcomes TRAIL resistance in colon cancer cells via
inhibiting of survivin. Tumour Biol. 36:8819–8829. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Laussmann MA, Passante E, Hellwig CT,
Tomiczek B, Flanagan L, Prehn JH, Huber HJ and Rehm M: Proteasome
inhibition can impair caspase-8 activation upon submaximal
stimulation of apoptotic tumor necrosis factor-related apoptosis
inducing ligand (TRAIL) signaling. J Biol Chem. 287:14402–14411.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Walczak H, Miller RE, Ariail K, Gliniak B,
Griffith TS, Kubin M, Chin W, Jones J, Woodward A, Le T, et al:
Tumoricidal activity of tumor necrosis factor-related
apoptosis-inducing ligand in vivo. Nat Med. 5:157–163. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Thorburn A, Behbakht K and Ford H: TRAIL
receptor-targeted therapeutics: Resistance mechanisms and
strategies to avoid them. Drug Resist Updat. 11:17–24. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Finlay D, Vamos M, González-López M,
Ardecky RJ, Ganji SR, Yuan H, Su Y, Cooley TR, Hauser CT, Welsh K,
et al: Small-molecule IAP antagonists sensitize cancer cells to
TRAIL-induced apoptosis: Roles of XIAP and cIAPs. Mol Cancer Ther.
13:5–15. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee RC, Feinbaum RL and Ambros V: The C.
elegans heterochronic gene lin-4 encodes small RNAs with antisense
complementarity to lin-14. Cell. 75:843–854. 1993.
|
|
12
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
He H, Tian W, Chen H and Jiang K: MiR-944
functions as a novel oncogene and regulates the chemoresistance in
breast cancer. Tumour Biol. 37:1599–1607. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zheng Y, Lv X, Wang X, Wang B, Shao X,
Huang Y, Shi L, Chen Z, Huang J and Huang P: MiR-181b promotes
chemoresistance in breast cancer by regulating Bim expression.
Oncol Rep. 35:683–690. 2016.PubMed/NCBI
|
|
15
|
Kim AD, Zhang R, Han X, Kang KA, Piao MJ,
Maeng YH, Chang WY and Hyun JW: Involvement of glutathione and
glutathione metabolizing enzymes in human colorectal cancer cell
lines and tissues. Mol Med Rep. 12:4314–4319. 2015.PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yeh CC, Deng YT, Sha DY, Hsiao M and Kuo
MY: Suberoylanilide hydroxamic acid sensitizes human oral cancer
cells to TRAIL-induced apoptosis through increase DR5 expression.
Mol Cancer Ther. 8:2718–2725. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yin XM: Bid, a BH3-only multi-functional
molecule, is at the cross road of life and death. Gene. 369:7–19.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kantari C and Walczak H: Caspase-8 and
bid: Caught in the act between death receptors and mitochondria.
Biochim Biophys Acta. 1813:558–563. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Frixa T, Donzelli S and Blandino G:
Oncogenic MicroRNAs: Key players in malignant transformation.
Cancers (Basel). 7:2466–2485. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ye Z, Hao R, Cai Y, Wang X and Huang G:
Knockdown of miR-221 promotes the cisplatin-inducing apoptosis by
targeting the BIM-Bax/Bak axis in breast cancer. Tumour Biol.
37:4509–4515. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xie X, Hu Y, Xu L, Fu Y, Tu J, Zhao H,
Zhang S, Hong R and Gu X: The role of
miR-125b-mitochondria-caspase-3 pathway in doxorubicin resistance
and therapy in human breast cancer. Tumour Biol. 36:7185–7194.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xu T, Jing C, Shi Y, Miao R, Peng L, Kong
S, Ma Y and Li L: microRNA-20a enhances the
epithelial-to-mesenchymal transition of colorectal cancer cells by
modulating matrix metalloproteinases. Exp Ther Med. 10:683–688.
2015.PubMed/NCBI
|
|
24
|
Babu KR and Muckenthaler MU: miR-20a
regulates expression of the iron exporter ferroportin in lung
cancer. J Mol Med (Berl). 94:347–359. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Salvesen GS and Walsh CM: Functions of
caspase 8: The identified and the mysterious. Semin Immunol.
26:246–252. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li H, Zhu H, Xu CJ and Yuan J: Cleavage of
BID by caspase 8 mediates the mitochondrial damage in the Fas
pathway of apoptosis. Cell. 94:491–501. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fulda S and Debatin KM: Extrinsic versus
intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene.
25:4798–4811. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Billen LP, Shamas-Din A and Andrews DW:
Bid: A Bax-like BH3 protein. Oncogene. 27:(Suppl 1). S93–S104.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Orzechowska EJ, Girstun A, Staron K and
Trzcinska-Danielewicz J: Synergy of BID with doxorubicin in the
killing of cancer cells. Oncol Rep. 33:2143–2150. 2015.PubMed/NCBI
|
|
30
|
Eskes R, Desagher S, Antonsson B and
Martinou JC: Bid induces the oligomerization and insertion of Bax
into the outer mitochondrial membrane. Mol Cell Biol. 20:929–935.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wei MC, Lindsten T, Mootha VK, Weiler S,
Gross A, Ashiya M, Thompson CB and Korsmeyer SJ: tBID, a
membrane-targeted death ligand, oligomerizes BAK to release
cytochrome c. Genes Dev. 14:2060–2071. 2000.PubMed/NCBI
|
|
32
|
Billard C: Design of novel BH3 mimetics
for the treatment of chronic lymphocytic leukemia. Leukemia.
26:2032–2038. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Boonyarat C, Yenjai C, Vajragupta O and
Waiwut P: Heptaphylline induces apoptosis in human colon
adenocarcinoma cells through bid and Akt/NF-κB (p65) pathways.
Asian Pac J Cancer Prev. 15:10483–10487. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wu CS, Chen GS, Lin PY, Pan IH, Wang ST,
Lin SH, Yu HS and Lin CC: Tazarotene induces apoptosis in human
basal cell carcinoma via activation of caspase-8/t-Bid and the
reactive oxygen species-dependent mitochondrial pathway. DNA Cell
Biol. 33:652–666. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Saelens X, Festjens N, Walle L Vande, van
Gurp M, van Loo G and Vandenabeele P: Toxic proteins released from
mitochondria in cell death. Oncogene. 23:2861–2874. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Trivedi R and Mishra DP: Trailing TRAIL
resistance: Novel targets for TRAIL sensitization in cancer cells.
Front Oncol. 5:692015. View Article : Google Scholar : PubMed/NCBI
|