Introduction
Head and neck squamous cell carcinoma (HNSCC) is a
heterogeneous disease composed of hypopharyngeal, oropharyngeal,
oral, and laryngeal squamous cell carcinoma. Laryngeal carcinoma
(LC) is one of the most common malignant tumors among HNSCCs. As an
etiologically multifactorial disease, carcinogenesis of laryngeal
carcinoma may result from genetic and environmental factors
(1). Previous study on the origin
of LC suggests that genetic alterations in tumor-suppressor genes
and proto-oncogenes in multiple cellular pathways may be important
in multistage LC carcinogenesis. However, the molecular mechanism
and genetic basis of the development of LC have not fully been
elucidated.
Overexpression of Bcl-2 in primary tumors is
associated with tumor cell differentiation, tumor metastasis,
recurrence, and poor prognosis in patients (2) and appears to suggest apoptosis
resistance in many types of cancer (3), including HNSCC (4–6).
Overexpression of Bcl-2 is also related to chemotherapy resistance
(4). However, molecular targeting
of Bcl-2 with small-molecule inhibitors or short peptides was found
to promote apoptosis and chemosensitivity in HNSCC cells (7,8). These
studies suggest that Bcl-2 plays a crucial role in the development
and progression of cancer.
In the present study, we report the mechanism by
which Bcl-2 functions in LC as an anti-apoptotic factor in relation
to its association with proteins by proteomes. Our data provide
novel evidence that Hsp90β is associated with Bcl-2 and that this
interaction facilitates optimal Bcl-2 anti-apoptotic function.
Further results showed that disruption of Bcl-2-Hsp90β interaction
inhibited the anti-apoptotic ability of Bcl-2 and decreased the
caspase activation in LC, which will have broad implications for
the better understanding of tumor formation, tumor cell survival,
development of metastasis due to Bcl-2 and potentially identify a
Bcl-2/Hsp90β axis as a novel target for LC therapy.
Materials and methods
Reagents
Mouse monoclonal anti-Bcl-2, anti-GAPDH, and
anti-Hsp90β antibodies were purchased from Santa Cruz Biotechnology
(Santa Cruz, CA, USA). Anti-mouse antibody was purchased from
Abcam, Inc. (Cambridge, MA, USA). Mercaptoethanol, iodoacetamide,
and HCl were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Bromophenol blue, bis, TEMED, Commassie Brilliant Blue G-250,
molecular weight marker, Tris-base, SDS, glycine, secondary
antibodies conjugated with horseradish peroxidase, and the enhanced
chemiluminescence (ECL) system were obtained from Amersham
Biosciences (Stockholm, Sweden). Sequencing-grade modified trypsin
was purchased from Promega Corp. (Madison, WI, USA). PVDF membranes
and ZipTip C18 columns were obtained from Millipore
Corp. (Boston, MA, USA).
Cell and cell culture
The HNSCC cell line SCC10A was derived from the
primary lesion of a larynx carcinoma, and has been extensively
characterized for its in vitro and in vivo phenotypes
(9). Cells were normally maintained
at low passage in Dulbeccos modified Eagles medium (DMEM)
supplemented with 10% fetal bovine serum (FBS) (both from
Invitrogen), 100 U/ml penicillin and 100 µg/ml streptomycin.
Co-immunoprecipitation
LC SCC10A cells were lysed at 4°C for 30 min in a
lysis buffer [50 mmol/l Tris (pH 7.5), 500 mmol/l NaCl, 1% Triton
X-100, 0.5% sodium deoxycholate, 0.1% SDS, 10 mmol/l
MgCl2, and complete protease inhibitor mixture (Roche
Molecular Biochemicals, Mannheim, Germany)]. The lysates were
centrifuged at 11,000 rpm for 15 min at 4°C. Protein concentrations
were measured with the bicinchoninic acid protein assay kit
(Pierce, Rockford, IL, USA). The clarified supernatants were
collected and used immediately for co-immunoprecipitation.
Approximately 500 mg of total protein was first precleared with
control (non-immune) serum, bound to 100 µl protein G-Sepharose
(Amersham Biosciences). The clarified supernatants were then
incubated with the anti-Bcl-2 antibody (10 µg) for 6 h. Protein
G-Sepharose (100 µl) was added and the mixture was incubated
overnight at 4°C. Samples were centrifuged for 30 sec and washed
three times with lysis buffer, and run on a SDS-PAGE. Subsequently,
SDS-PAGE electrophoresis was performed and proteins in the gels
were detected by Coomassie blue R-250 staining, followed by in-gel
trypsin digestion and MS analysis as previously described by us
(10). For western blot analysis,
proteins in the gels were transferred to nitrocellulose membranes
(Millipore Corp.). Then the membranes were incubated with an
anti-Bcl-2 or an anti-Hsp90β antibody. The Bcl-2 antibody was then
replaced by the non-immune IgY antibody (GenWay Biotech, Inc., San
Diego, CA, USA) which was used as a negative control.
MS and database analysis
ESI-Q-TOF-MS analysis of proteins was performed as
described by Cheng et al and Huang et al,
respectively (10,11).
Western blot analysis
The immunoprecipitated complexes or cell lysates
were separated by 10% SDS-PAGE, and transferred to nitrocellulose
membranes (Millipore Corp.). Blots were blocked with 5% non-fat dry
milk for 30 min at room temperature and washed three times with
phosphate-buffered saline (PBS) buffer. Then they were incubated
with primary anti-Bcl-2, anti-Hsp90β, or anti-GAPDH antibodies
overnight at 4°C, followed by incubation with a horseradish
peroxidase-conjugated secondary antibody for 1 h at room
temperature. The signal was visualized with ECL detection reagent.
GAPDH was detected simultaneously using mouse anti-GAPDH antibody
as a loading control.
Bioinformatic analysis
Molecule function classification and cluster
analysis were performed through the GO and Cluster program DAVID
(http://david.abcc.ncifcrf.gov/). The
default parameters of classification in terms of stringency in the
DAVID Cluster program were medium. Protein-protein interaction
(PPI) analysis was performed using VisANT software (version 3.15)
(http://visant.bu.edu/) (12).
RNA interference analysis
For RNA interference analysis, the cells were
transfected with Hsp90β siRNA or control siRNA (Dharmacon, Inc.)
using the Lipofectamine 2000 reagent (Invitrogen) according to the
siRNA transfection protocol provided by the manufacturer. Briefly,
the day before transfection, SCC10A were plated into 6-well plates
at the density of 105 cells/ml in DMEM containing 10%
FBS. When the cells reached 60–80% confluence, they were
transfected with 10 nmol/l of Hsp90β siRNA or control siRNA after a
preincubation for 20 min with siRNA transfection reagent in siRNA
transfection medium. Four hours after the beginning of the
transfection, the medium was replaced with DMEM containing 10% FBS
and the cells continued to culture for an additional 44 h. At the
end of the transfection, the Hsp90β expression level in the cells
was determined by western blot analysis.
Caspase activity assay
The caspase-3/7 fluorometric assay kit (Promega
Corp.) was used to measure caspase-3/7 activity following the
manufacturers instructions.
Immunohistochemistry
Immunohistochemical analysis of Bcl-2 and Hsp90β was
carried out with formalin-fixed and paraffin-embedded tissue
sections using the standard immunohistochemical technique according
to a report by Zuo et al (13).
Statistical analysis
A Student's t-test was used for the statistical
analysis, with P<0.05 considered as a significant
difference.
Results
Bcl-2-associated proteins are
identified by co-immunoprecipitation and MS
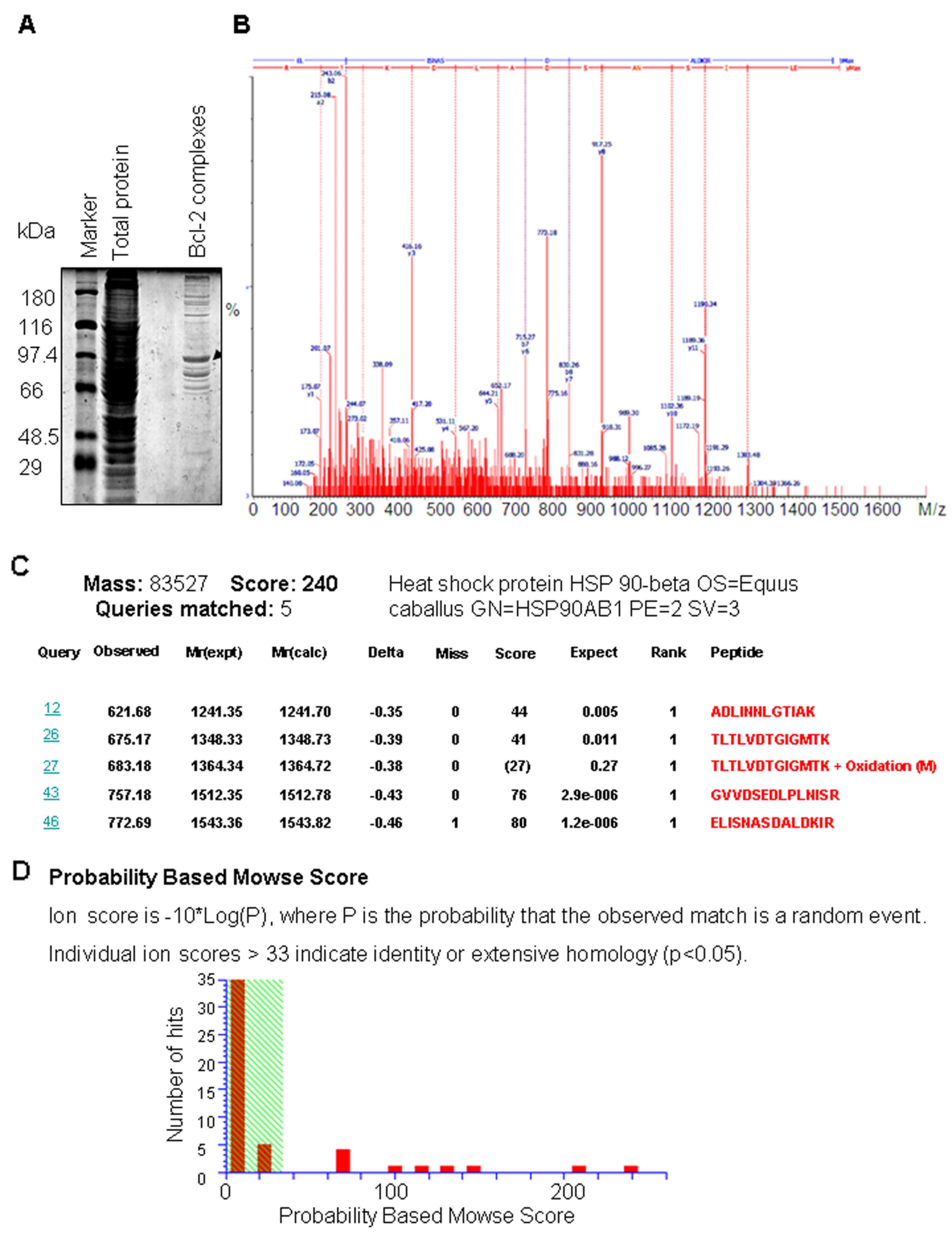
To isolate proteins that interact with Bcl-2, we
performed a proteomic analysis of the Bcl-2 complexes using
targeted proteomics (co-immunoprecipitation coupled with MS). The
complex was eluted, separated on an SDS-PAGE (Fig. 1A), and subjected to in-gel trypsin
digestion. The tryptic digests were analyzed through ESI-Q-TOF-MS.
To control for non-specific immune complexes, the Bcl-2 antibody
was replaced by a non-immune IgY antibody which was used as a
negative control. Thirty-five proteins were identified in the Bcl-2
complex after removing the proteins that were not found in the
replicate experiments, and subtracting the common proteins that
were found in the negative control (Table I).
 | Table I.Bcl-2 interacting proteins. |
Table I.
Bcl-2 interacting proteins.
| Protein accession
nos. | Protein molecular
weight (Da) | No. of unique
peptides | Score | Percentage sequence
coverage (%) |
|---|
| 4F2_HUMAN | 67,978.40 | 2 | 101 | 4.13 |
| ACTN1_HUMAN,
ACTN4_HUMAN | 103,043.00 | 2 | 120 | 2.69 |
| ALBU_HUMAN | 69,348.90 | 2 |
| 2.46 |
| COF1_HUMAN | 18,485.10 | 2 | 62 | 15.10 |
| DDX1_HUMAN | 82,415.10 | 2 | 127 | 3.51 |
| DDX3X_HUMAN | 73,227.70 | 2 | 83 | 3.93 |
| DDX5_HUMAN | 69,131.70 | 3 | 137 | 6.51 |
| EIF3L_HUMAN | 66,711.30 | 2 |
| 5.14 |
| GRP78_HUMAN | 72,316.70 | 3 | 115 | 6.57 |
| GTF2I_HUMAN | 112,399.90 | 2 | 41 | 2.71 |
| GTF2I_HUMAN | 112,399.90 | 3 | 149 | 4.01 |
| HS90β_HUMAN | 83,249.30 | 4 | 240 | 7.32 |
| HSP7C_HUMAN | 70,881.80 | 5 | 229 | 11.50 |
| K2C1_HUMAN | 66,022.30 | 3 | 133 | 6.37 |
| K2C5_HUMAN | 62,361.60 | 1 | 46 | 2.37 |
| K2C5_HUMAN | 62,361.60 | 3 | 126 | 6.44 |
| MYH9_HUMAN | 226,519.50 | 2 | 85 | 1.12 |
| PI51A_HUMAN | 62,617.40 | 3 | 137 | 6.94 |
| PKP3_HUMAN | 87,066.70 | 2 | 65 | 3.76 |
| PLEC1_HUMAN | 531,765.90 | 8 | 361 | 1.79 |
| PSPC1_HUMAN | 58,726.50 | 5 | 236 | 11.10 |
| RL11_HUMAN | 20,235.20 | 1 | 55 | 7.87 |
| RL22_HUMAN | 14,769.30 | 1 | 46 | 8.59 |
| RS16_HUMAN | 16,427.90 | 2 | 67 | 15.10 |
| RS23_HUMAN | 15,789.70 | 1 | 90 | 7.69 |
| SFPQ_HUMAN | 76,131.50 | 2 | 75 | 2.97 |
| TBB5_HUMAN | 49,652.60 | 4 | 134 | 11.70 |
All 35 protein bands were excised from the stained
gels, in situ digested with trypsin and analyzed by
MALDI-TOF MS. A total of 35 differential protein bands were
identified. The MALDI-TOF mass spectrometry map and database query
result of the representative bands of Hsp90β are presented in
Fig. 1B and C. The total of the
monoisotopic peaks was input into the Mascot search engine to
search the Swiss-Prot database, and the query result showed that
the protein bands belonged to Hsp90β (Fig. 1C and D). The annotation of all the
identified proteins is summarized in Table I.
Validation of the interactome of
Bcl-2
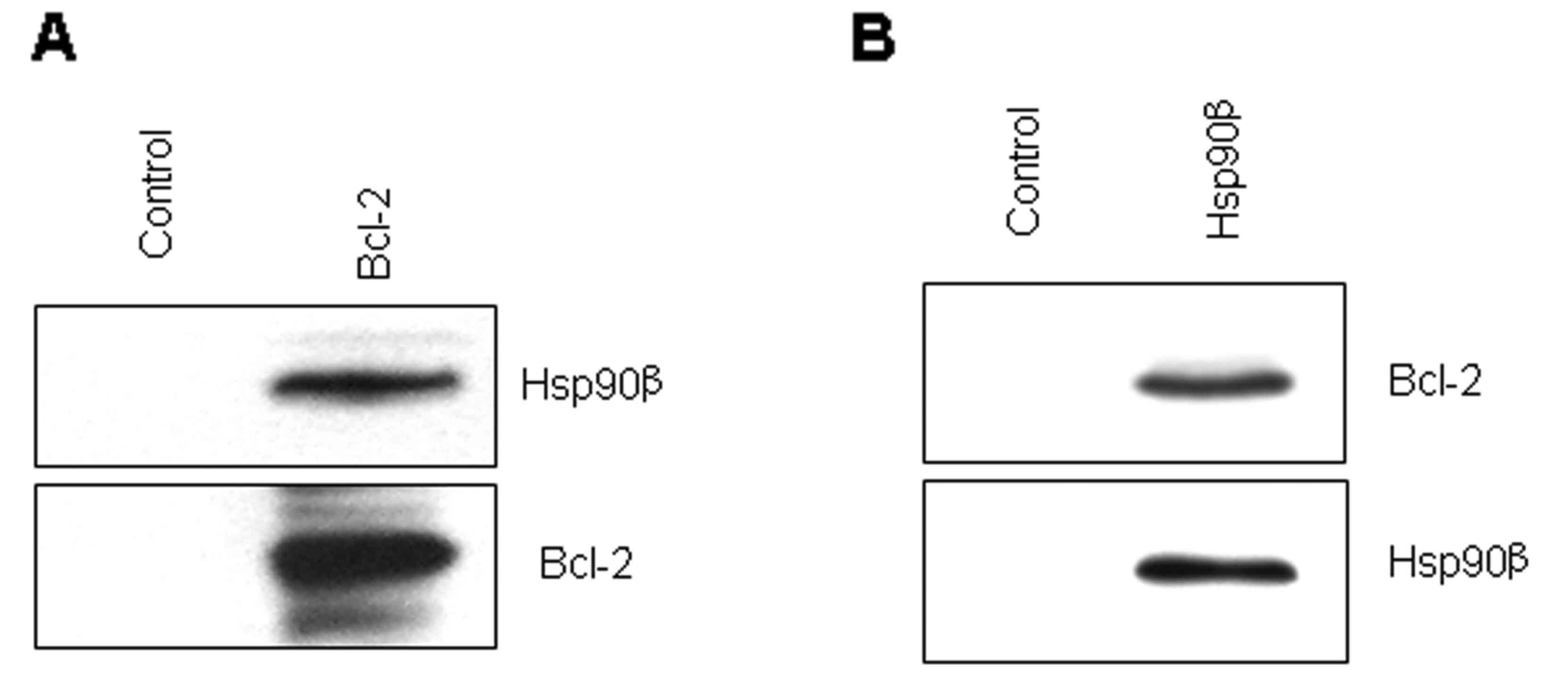
To confirm the MS analysis results, we selected the
protein of interest, Hsp90β, and detected its binding to Bcl-2 by
co-immunoprecipitation and western blot analysis based on the
availability and quality of the available antibodies. As shown in
Fig. 2A, Hsp90β was detected in the
Bcl-2 immune complex but not in the control. Concurrently, Bcl-2
was detected in the Hsp90β immune complex but not in the control
(Fig. 2B). These data showed that
the MS analysis results are reliable for bioinformatics and PPI
analysis.
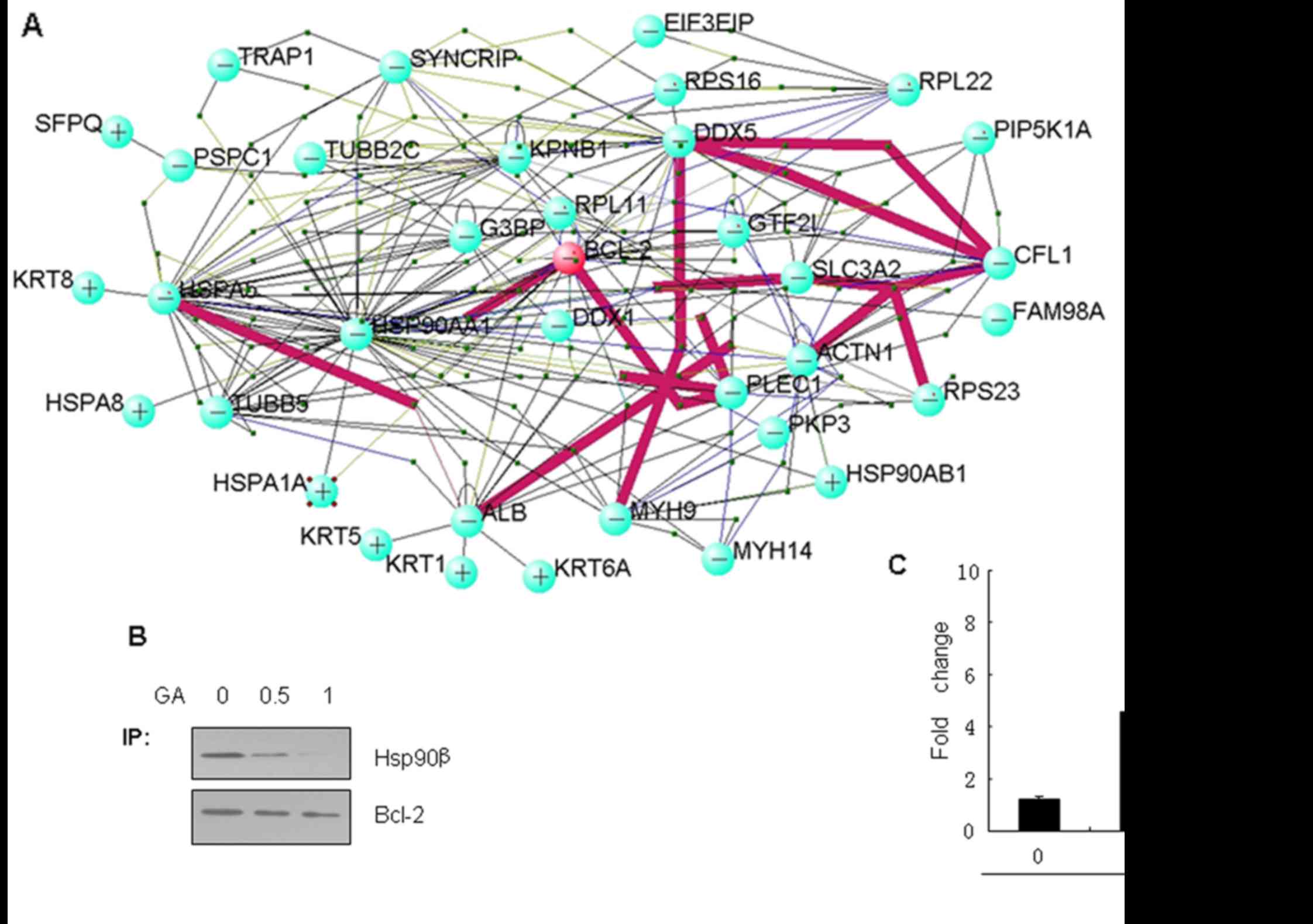
PPI analysis
In order to understand the interaction among the
Bcl-2-associated proteins, PPI analysis was performed using VisANT
software (http://visant.bu.edu/), a data
integrating visual framework for biological networks and modules.
Entrez-gene IDs of 35 Bcl-2-associated proteins were input into the
VisANT software and a complex net was obtained. The PPIs are
presented in Fig. 3A and it was
revealed that Bcl-2 could interact with Hsp90β. In order to detect
the relationship between Hsp90β and susceptibility to apoptosis in
LC cells, we chose the Bcl-2/Hsp90β interaction for further
study.
Hsp90β expression is necessary for
Bcl-2 anti-apoptosis
In order to detect whether Hsp90β is important to
the anti-apoptotic function of Bcl-2, we applied geldanamycin (GA),
as a specific inhibitor of Hsp90β, to study the chaperone function
of Hsp90β in its association with Bcl-2. LC cells were treated with
various concentrations of GA for 24 h. As expected, the data showed
that the association of Hsp90β with Bcl-2 was effectively blocked
in a dose-dependent manner by GA in the Bcl-2 immunoprecipitation
(Fig. 3B). Moreover, caspase-3/7
activities in the cells treated with GA showed a dose-dependent
increase (Fig. 3C), which suggested
that the inhibition of the chaperone function of Hsp90β in its
association with Bcl-2 decreased the ability of the anti-apoptotic
efffect of Bcl-2.
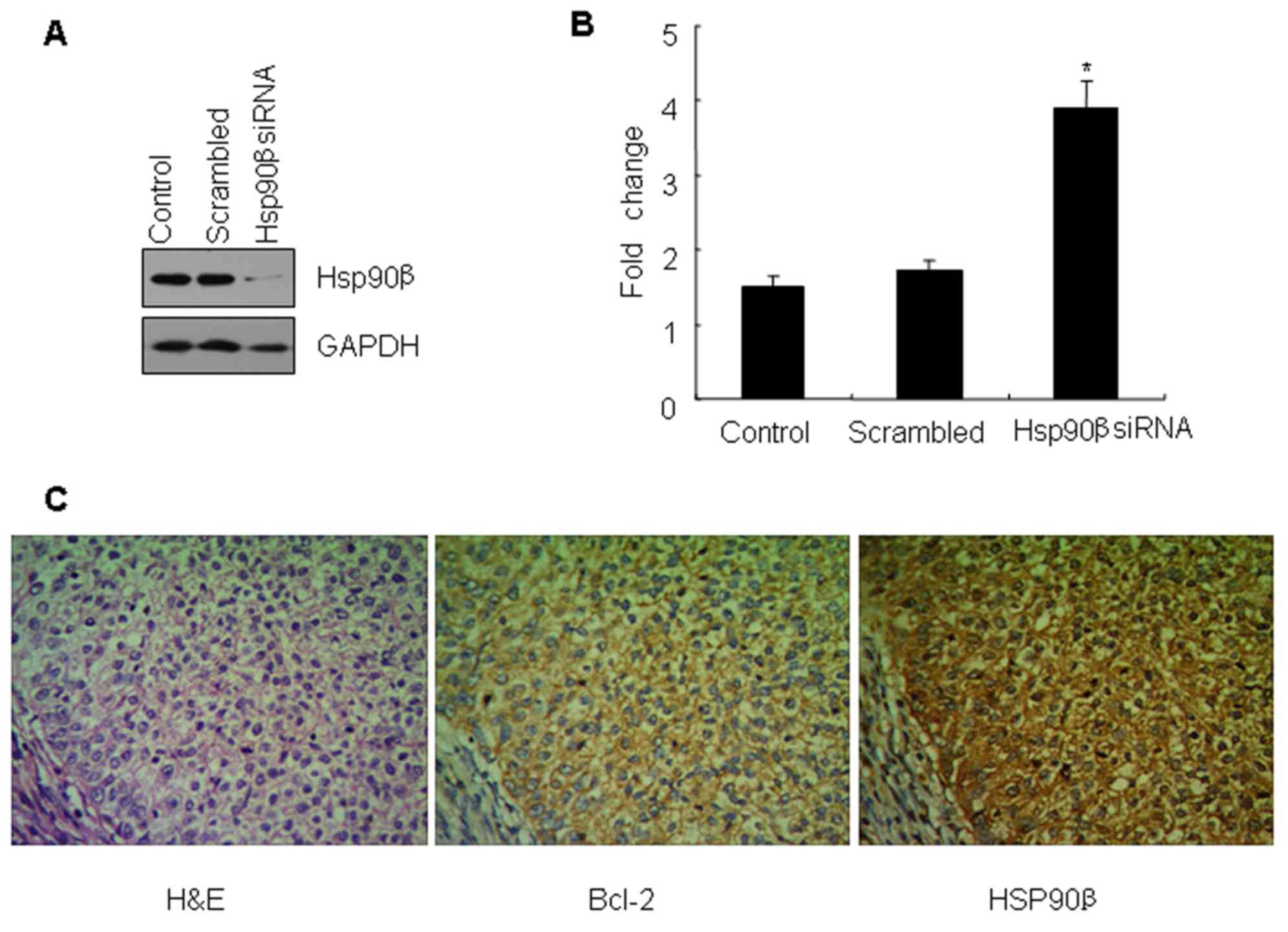
Knockdown of Hsp90β decreases the
ability of anti-apoptosis by Bcl-2
To further study the function of the Hsp90β-Bcl-2
association, specific siRNA of Hsp90β was carried out to knock down
the expression of Hsp90β. The results revealed that the protein
level of Hsp90β was significantly decreased 48 h after the
treatment with the specific Hsp90β RNAi. However, the change in
expression of Hsp90β was not detected in the untreated cells or the
cells treated with scrambled siRNA as determined by western blot
analysis (Fig. 4A). Concurrently,
it is of note that the knockdown of Hsp90β expression led to a
significant increase in the activity of caspase-3/7 in the SCC10A
cells compared to the control cells (Fig. 4B).
Correlation between Bcl-2 and Hsp90β
expression and clinicopathological factors in LC
In order to further verify whether the Bcl-2 target
we identified is also associated with Hsp90β in vivo, we
examined the expression levels of the Bcl-2 and Hsp90β proteins,
which are both actually highly expressed in LC (Fig. 4C). By Spearman correlation analysis
it was concluded that there was a positive correlation between the
expression of Bcl-2 and Hsp90β in laryngeal squamous cell carcinoma
(P<0.05) (Table II). The
expression of Bcl-2 and Hsp90β in LC tissues was not associated
with the age and gender of the patients (P>0.05) whereas it was
correlated with tumor differentiation, clinical stage and lymph
node metastasis (P<0.05) (Table
III).
 | Table II.Correlation between Bcl-2 and Hsp90β
expression in LC. |
Table II.
Correlation between Bcl-2 and Hsp90β
expression in LC.
|
| Hsp90β |
|---|
|
|
|
|---|
|
| + | − |
|---|
| Bcl-2 |
|
|
| + | 38 | 2 |
| − | 6 | 11 |
 | Table III.Correlation between Bcl-2 and Hsp90β
expression and clinicopathologic factors in LC. |
Table III.
Correlation between Bcl-2 and Hsp90β
expression and clinicopathologic factors in LC.
|
| Bcl-2 |
| Hsp90β |
|
|---|
|
|
|
|
|
|
|---|
| Parameter | − | + | P-value | − | + | P-value |
|---|
| Overall | 17 | 40 |
| 13 | 44 |
|
| Age (years) |
|
| 0.340 |
|
| 0.381 |
|
>60 | 7 | 22 |
| 8 | 21 |
|
|
≤60 | 10 | 18 |
| 5 | 23 |
|
| Gender |
|
| 0.279 |
|
| 0.240 |
|
Male | 11 | 36 |
| 8 | 39 |
|
|
Female | 6 | 4 |
| 5 | 5 |
|
| Tumor
differentiation |
|
| 0.023 |
|
| 0.006 |
|
Well/moderate | 14 | 20 |
| 12 | 22 |
|
|
Poor | 3 | 20 |
| 1 | 22 |
|
| Lymph node
metastasis |
|
| 0.014 |
|
| 0.010 |
|
Yes | 5 | 26 |
| 3 | 28 |
|
| No | 12 | 14 |
| 10 | 16 |
|
| TNM stage |
|
| 0.025 |
|
| 0.002 |
|
I–II | 7 | 29 |
| 13 | 23 |
|
|
III–IV | 10 | 11 |
| 0 | 21 |
|
Discussion
Human Bcl-2 is located near the junction of
chromosomes 18 and 14 (t14;18) and has been discovered in the tumor
cells of follicular lymphoma patients. The chromosome translocation
results in misregulation of the normal Bcl-2 expression pattern,
which leads to abnormal cell growth and certainly contributes to
the development of certain types of tumors (14,15).
Bcl-2 overexpression occurs in a wide range of human cancers
(16) and causes resistance to
apoptosis, autophagic-associated cell death and treatment (16–19).
Elevated expression of Bcl-2 in some tumors is often associated
with enhanced invasion and metastasis (20–22),
shorter survival time and generally poorer clinical outcomes
(23). Thus, Bcl-2 plays an
important role in the orientation and development of tumors. Bcl-2
is one of the key regulators of apoptosis because it endows a
survival advantage on cells by protecting cells from apoptotic
death (17). However, this unknown
mechanism needs to be elucidated, especially in LC. In the present
study, we investigated in detail, the molecular pathways by which
Bcl-2 overexpression contributes to LC cell survival by forming a
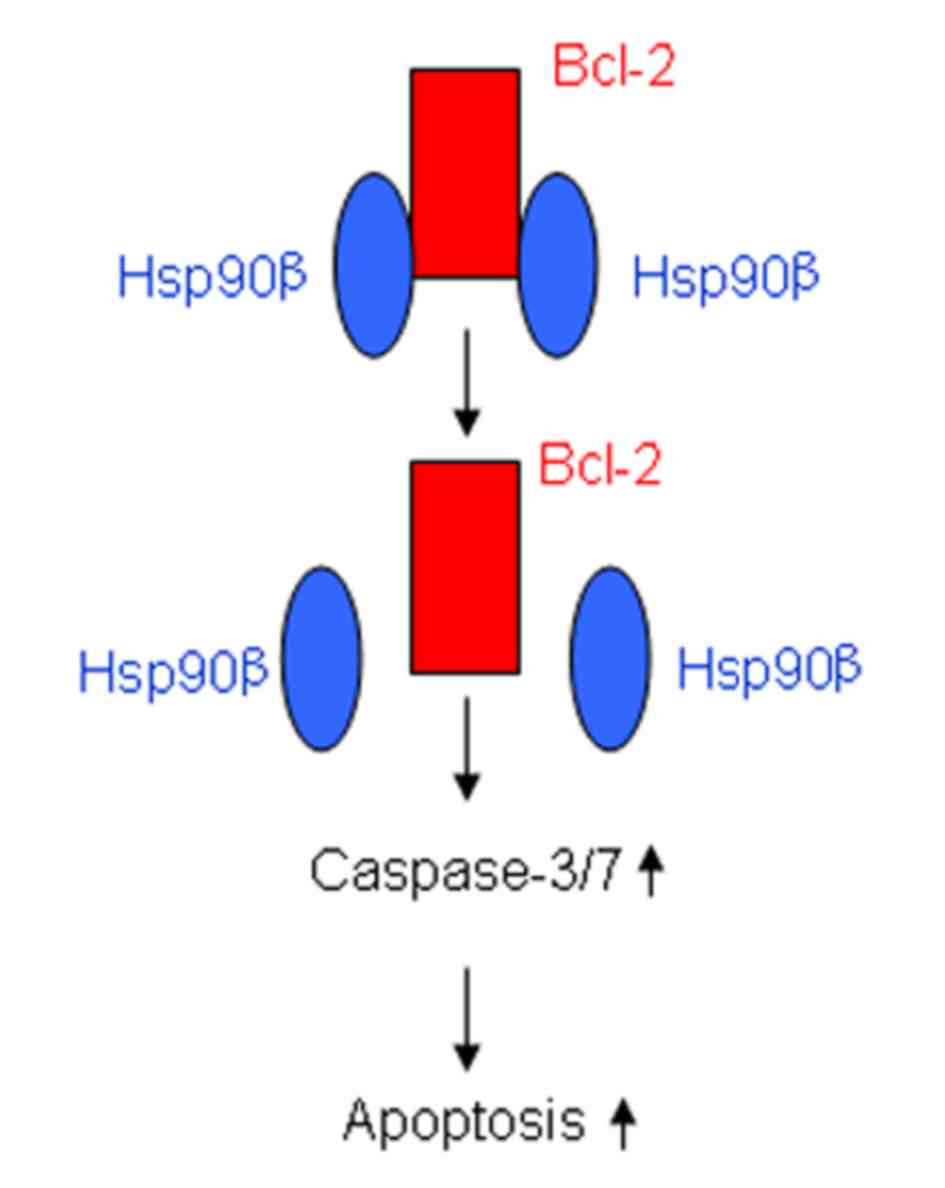
complex with Hsp90β (Fig. 5).
VisANT extends the application of the GO database,
in network visualization, analysis and inference as an integrative
software platform used for the visualization, mining, analysis and
modeling of biological networks (12,24).
VisANT supports biological network mining and analysis, meaning
that it is able to find previously unidentified networks, annotate
them, and display their hierarchical organization (25). VisANT allows the different types of
networks to analyze the correlations between disease, therapy,
genes and drugs systematically (26). VisANTs ability to use nodes to model
more complex entities such as protein complexes or pathways allows
for more informative visualizations. VisANT also implements
algorithms for analyzing node degrees, clusters, path lengths,
network motifs and network randomizations (27). In this study, PPI was performed
using VisANT software to discover how Bcl-2 interacts with its
associated proteins. The results indicated that Bcl-2 interacts
with Hsp90β, which regulates cell apoptosis.
Heat shock proteins represent a diverse group of
chaperones that play a critical role in the protection of cells
against numerous environmental stresses (27). Heat shock proteins also carry out
the functions of the folding of synthesized proteins, and play a
role in the maturation and activity of many proteins. Many Hsp90
clients such as Raf, Bcr-Abl, and C-kit are oncoproteins that are
either mutated or overexpressed in cancer cells, which in turn lead
to the disregulation of cell growth and proliferation (28). Hsp90 can regulate cell motility,
inflammation, angiogenesis, matrix remodeling, tumor progression
and metastasis (27). Hsp90β is one
of the isoforms of Hsp90, which plays an important role in forming
a multiprotein complex with ATPase activity. It is also involved in
the folding, activation and assembly of several proteins, such as
the tumor-suppressor protein p53, the NOS family members,
Akt/protein kinase B and Raf-1 (29,30),
which result in signal transduction and transcriptional regulation.
Hsp90β is a calcium-binding protein and a novel regulatory factor
of MMP-13 expression in osteoarthritic chondrocytes (31). As many client proteins of Hsp90
control cell survival, proliferation, and apoptosis, Hsp90 is
closely associated with human health, especially tumors.
Concurrently, the expression of Hsp90 is 2- to 10-fold higher in
tumor cells than in normal cells (32). Therefore, Hsp90 has emerged as an
important target in cancers such as non-small cell lung cancer,
melanoma and breast cancer (33) in
recent years.
In the present study, GA, a benzoquinone ansamycin
antibiotic, has been used as an inhibitor of Hsp90β since it
specifically binds to the ATP/ADP pocket binding site in the
N-domain of Hsp90β, which leads to disruption of the Hsp90β
interaction with certain proteins. Thus, the GA inhibitor was
applied to test the chaperone function of Hsp90 in its association
with Bcl-2. Co-immunoprecipitation experiments showed that Hsp90β
binding to Bcl-2 was abrogated by GA, which suggests that Hsp90β
may fail to bind to Bcl-2. We also found that decreased Hsp90β
binding to Bcl-2 by GA led to these cells being less resistant to
apoptosis than the control cells. As predicted, GA induced cell
apoptosis to a greater degree when compared with the control.
Notably, the data suggest that GA has a potent antitumor effect and
may result in its use in clinical trials in LC in the future.
In this study, we immunohistochemically determined
Bcl-2 and Hsp90β expression in 57 patients with LC, and found a
70.18 and 77.20% positive frequency, respectively, similar to that
reported previously. Moreover, we showed that Bcl-2 and Hsp90β were
highly expressed in tumors, and positively associated with tumor
differentiation, clinical stage and lymph node metastasis. Finally
we demonstrated a positive correlation between Bcl-2 expression and
Hsp90β expression, when the percentage of positively stained cells
was determined in the LC tissues. All in all, these aforementioned
findings strongly support the relationship of Bcl-2 and Hsp90β and
their role in LC.
Collectively, in the present study we found a novel
molecular mechanism of anti-apoptosis via Bcl-2/Hsp90β interaction
in LC, which is strong evidence for new targets for LC therapy.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (no. 81272960), the Key Research
Program from the Science and Technology Department of Hunan
Province, China (no. 2013WK2010, 2014SK2015) and the Key Research
Program from the Ministry of Human Resources and Social Security of
the People's Republic of China (2016) (no. 176).
References
|
1
|
Jiang LY, Lian M, Wang H, Fang JG and Wang
Q: Inhibitory effects of 5-Aza-2-deoxycytidine and trichostatin A
in combination with p53-expressing adenovirus on human
laryngocarcinoma cells. Chin J Cancer Res. 24:232–237. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zuo J, Ishikawa T, Boutros S, Xiao Z,
Humtsoe JO and Kramer RH: Bcl-2 overexpression induces a partial
epithelial to mesenchymal transition and promotes squamous
carcinoma cell invasion and metastasis. Mol Cancer Res. 8:170–182.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kang MH and Reynolds CP: Bcl-2 inhibitors:
Targeting mitochondrial apoptotic pathways in cancer therapy. Clin
Cancer Res. 15:1126–1132. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lu S, Yu L, Mu Y, Ma J, Tian J, Xu W and
Wang H: Role and mechanism of Twist1 in modulating the
chemosensitivity of FaDu cells. Mol Med Rep. 10:53–60.
2014.PubMed/NCBI
|
|
5
|
Wu M, Zhang H, Hu J, Weng Z, Li C, Li H,
Zhao Y, Mei X, Ren F and Li L: Isoalantolactone inhibits UM-SCC-10A
cell growth via cell cycle arrest and apoptosis induction. PLoS
One. 8:e760002013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rahman MA, Amin AR, Wang D, Koenig L,
Nannapaneni S, Chen Z, Wang Z, Sica G, Deng X, Chen ZG, et al: RRM2
regulates Bcl-2 in head and neck and lung cancers: A potential
target for cancer therapy. Clin Cancer Res. 19:3416–3428. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kawano K, Kantak SS, Murai M, Yao CC and
Kramer RH: Integrin α3β1 engagement disrupts intercellular
adhesion. Exp Cell Res. 262:180–196. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nurmenniemi S, Sinikumpu T, Alahuhta I,
Salo S, Sutinen M, Santala M, Risteli J, Nyberg P and Salo T: A
novel organotypic model mimics the tumor microenvironment. Am J
Pathol. 175:1281–1291. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zuo JH, Zhu W, Li MY, Li XH, Yi H, Zeng
GQ, Wan XX, He QY, Li JH, Qu JQ, et al: Activation of EGFR promotes
squamous carcinoma SCC10A cell migration and invasion via inducing
EMT-like phenotype change and MMP-9-mediated degradation of
E-cadherin. J Cell Biochem. 112:2508–2517. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cheng AL, Huang WG, Chen ZC, Peng F, Zhang
PF, Li MY, Li F, Li JL, Li C, Yi H, et al: Identification of novel
nasopharyngeal carcinoma biomarkers by laser capture
microdissection and proteomic analysis. Clin Cancer Res.
14:435–445. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huang WG, Cheng AL, Chen ZC, Peng F, Zhang
PF, Li MY, Li F, Li JL, Li C, Yi H, et al: Targeted proteomic
analysis of 14-3-3σ in nasopharyngeal carcinoma. Int J Biochem Cell
Biol. 42:137–147. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hu Z, Hung JH, Wang Y, Chang YC, Huang CL,
Huyck M and DeLisi C: VisANT 3.5: Multi-scale network
visualization, analysis and inference based on the gene ontology.
Nucleic Acids Res. 37:(Web Server). W115–W121. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zuo J, Wen M, Lei M, Peng X, Yang X and
Liu Z: MiR-210 links hypoxia with cell proliferation regulation in
human Laryngocarcinoma cancer. J Cell Biochem. 116:1039–1049. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hardwick JM and Soane L: Multiple
functions of BCL-2 family proteins. Cold Spring Harb Perspect Biol.
5:a0087222013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yip KW and Reed JC: Bcl-2 family proteins
and cancer. Oncogene. 27:6398–6406. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yu L and Liu S: Autophagy contributes to
modulating the cytotoxicities of Bcl-2 homology domain-3 mimetics.
Semin Cancer Biol. 23:553–560. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Marquez RT and Xu L: Bcl-2:Beclin 1
complex: multiple, mechanisms regulating autophagy/apoptosis toggle
switch. Am J Cancer Res. 2:214–221. 2012.PubMed/NCBI
|
|
18
|
Karnak D and Xu L: Chemosensitization of
prostate cancer by modulating Bcl-2 family proteins. Curr Drug
Targets. 11:699–707. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Buggins AG and Pepper CJ: The role of
Bcl-2 family proteins in chronic lymphocytic leukaemia. Leuk Res.
34:837–842. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mena S, Benlloch M, Ortega A, Carretero J,
Obrador E, Asensi M, Petschen I, Brown BD and Estrela JM: Bcl-2 and
glutathione depletion sensitizes B16 melanoma to combination
therapy and eliminates metastatic disease. Clin Cancer Res.
13:2658–2666. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xie L, Qin W, Li J, He X, Zhang H, Yao G,
Shu H, Yao M, Wan D and Gu J: BNIPL-2 promotes the invasion and
metastasis of human hepatocellular carcinoma cells. Oncol Rep.
17:605–610. 2007.PubMed/NCBI
|
|
22
|
Planas-Silva MD, Bruggeman RD, Grenko RT
and Smith JS: Overexpression of c-Myc and Bcl-2 during progression
and distant metastasis of hormone-treated breast cancer. Exp Mol
Pathol. 82:85–90. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Olsson Åkefeldt S, Ismail MB, Valentin H,
Aricò M, Henter JI and Delprat C: Targeting BCL2 family in human
myeloid dendritic cells: A challenge to cure diseases with chronic
inflammations associated with bone loss. Clin Dev Immunol.
2013:7013052013.PubMed/NCBI
|
|
24
|
Hu Z, Snitkin ES and DeLisi C: VisANT: An
integrative framework for networks in systems biology. Brief
Bioinform. 9:317–325. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hu Z, Chang YC, Wang Y, Huang CL, Liu Y,
Tian F, Granger B and Delisi C: VisANT 4.0: Integrative network
platform to connect genes, drugs, diseases and therapies. Nucleic
Acids Res. 41:(Web Server). W225–W231. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Suderman M and Hallett M: Tools for
visually exploring biological networks. Bioinformatics.
23:2651–2659. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hance MW, Nolan KD and Isaacs JS: The
double-edged sword: Conserved functions of extracellular hsp90 in
wound healing and cancer. Cancers (Basel). 6:1065–1097. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tsaytler PA, Krijgsveld J, Goerdayal SS,
Rüdiger S and Egmond MR: Novel Hsp90 partners discovered using
complementary proteomic approaches. Cell Stress Chaperones.
14:629–638. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Calamia V, de Andrés MC, Oreiro N,
Ruiz-Romero C and Blanco FJ: Hsp90b inhibition modulates nitric
oxide production and nitric oxide-induced apoptosis in human
chondrocytes. BMC Musculoskelet Disord. 12:2372011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jia W, Yu C, Rahmani M, Krystal G,
Sausville EA, Dent P and Grant S: Synergistic antileukemic
interactions between 17-AAG and UCN-01 involve interruption of
RAF/MEK- and AKT-related pathways. Blood. 102:1824–1832. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fan Z, Tardif G, Hum D, Duval N, Pelletier
JP and Martel-Pelletier J: Hsp90β and p130cas: Novel regulatory
factors of MMP-13 expression in human osteoarthritic chondrocytes.
Ann Rheum Dis. 68:976–982. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li J and Buchner J: Structure, function
and regulation of the hsp90 machinery. Biomed J. 36:106–117. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Whitesell L and Lin NU: HSP90 as a
platform for the assembly of more effective cancer chemotherapy.
Biochim Biophys Acta. 1823:756–766. 2012. View Article : Google Scholar : PubMed/NCBI
|