Introduction
Since the National Comprehensive Cancer Network
Practice Guidelines for treatment of primary rectal cancer were
specified in 2009, neoadjuvant chemoradiation (NACR) has been
accepted as the standard therapy worldwide, except in Japan.
Numerous studies have shown that NACR increases local control of
the tumor, but no effect on overall patient survival has been
reported (1–6).
Conversely, recent studies have shown the benefits
of NACR, including good pathological responses to therapy and
increased rates of disease-specific and overall survival (7–15).
However, pathologic complete response (pCR) rates
are reported to be only 10–29.3%, and more than one-third of
patients do not respond to treatment. New strategies to achieve
complete responses using multimodality neoadjuvant therapy are
required for rectal cancer. Therefore, the present study aimed to
establish new strategies that may increase the pCR rate for
patients treated with NACR with concurrent thermal therapy.
Radiofrequency (RF) hyperthermia using the
Thermotron-RF8 has been performed mainly in Japan; however, it has
two major issues: i) this modality has not been approved as a
standardized treatment in oncology since each hyperthermic
treatment is not of the same quality; and ii) there are risks for
fatal hot spot or thermal runaway phenomena, which are induced by
the RF treatment itself (16,17).
As for the latter, we previously reported that a
good predictive equation for initial energy output at which
output-limiting symptoms occur was determined with two parameters:
initial time of output-limiting symptom onset and abdominal wall
fat thickness, using standardized power escalation principles
(18). This formula had an adjusted
R2 of 0.99, and all variance inflation factor (VIF)
values were <2. The initial energy output [initial RF output
(IRO)] at which an output-limiting symptom occurred (Watt) =
initial time at which an output-limiting symptom occurred (min) ×
6.162 - abdominal wall fat thickness (mm) × 17.155 + 967.995; i.e.,
larger number of IRO means thinner thickness of the fat of the
abdominal wall. In the present study, we considered 0 min as the
initial time at which an output-limiting symptom occurred and
compared it to the actual observed output.
The primary endpoint was toxicity and locoregional
tumor control and the main objective of the present study was to
identify pretreatment clinical parameters that predict pCR and
progressive disease (PD). We retrospectively evaluated whether or
not our predictive formula of output-limiting symptoms could
predict pCR and PD at the beginning of this modality.
Materials and methods
The present study included 81 consecutive patients
who were diagnosed with primary rectal adenocarcinoma localized in
the rectum [up to 12 cm from the anal verge: The National Cancer
Institute Rectal Cancer Focus Group has defined the rectum as
anything remaining within 12 cm from the anal verge (19)] between December 2011 and May 2015.
All patients received pre- and post-treatment diagnostic
examinations, including computed tomography (CT), positron emission
tomography/CT (PET/CT), and magnetic resonance imaging (MRI) at
Hidaka Hospital. The tumor extent and location were classified
according to the tumor-node-metastasis (TNM) staging (20).
All patients underwent NACR with concurrent thermal
therapy at Hidaka Hospital. Surgeries were performed at the
Department of General Surgical Science at Gunma University or the
Division of Surgery at Hidaka Hospital. Each resected specimen was
histologically evaluated in the Department of Pathology at Gunma
University. The present study was approved by the Ethics Committees
of Hidaka Hospital and Gunma University. Each patient provided
written informed consent.
Chemoradiotherapy
Intensity-modulated radiotherapy (IMRT) was
conventionally administered once daily 5 times/week using
TomoTherapy® (Hi-Art® treatment system;
Accuray®). For neoadjuvant radiotherapy, 50 Gy of
radiotherapy was administered to the posterior pelvis in 25 2-Gy
fractions. Concurrent neoadjuvant chemotherapy was administered in
5-day courses during the first through fifth week of NACR.
Capecitabine (Cap) was administered orally at a dose of 1,700
mg/m2/day for 5 days/week. Patients received Cap
throughout the radiation therapy course, beginning and ending on
the first and last days of radiation therapy administration,
respectively.
Thermal therapy
Thermal treatment was performed using the
Thermotron-RF8 (Yamamoto Vinita Co., Ltd., Osaka, Japan) and
administered once a week for 5 weeks during a 50-min irradiation.
Precise thermal therapy methods have been previously described
(18,21–23).
Objective response evaluation
The timing of objective response evaluations
according to the Response Evaluation Criteria in Solid Tumors
(RECIST) using MRI and PET/CT varied from weeks 2–18 after
completion of NACR with concurrent thermal therapy, with a median
time of 8 weeks (24). We
classified patient responses as follows: complete response (CR),
which describes total resolution of the lesions; partial response
(PR), which describes a 30% decrease in the sum of the diameters of
the lesions; stable disease (SD), which describes changes between a
30% decrease and 20% increase; and PD, which describes an increase
of at least 20% in the sum of the diameters of the lesions or new
distant metastasis. We evaluated CR as the disappearance of the
tumor on PET/CT and MRI and a positive to negative change by
PET/CT. The CRPD group included patients who experienced local CRs,
although new distant metastases appeared. Adverse effects of these
treatments were evaluated based on the criteria defined by the
Common Terminology Criteria for Adverse Events (Ver.4.0) (25).
Pathology
Resected specimens (n=54) were evaluated according
to the Japanese Classification of Colorectal Carcinoma. According
to this criteria, pathological grade 0 tumors have no fibrosis.
Grade 1 tumors exhibit denaturation and necrosis of cancer cells in
approximately <1/3 of the tumor (grade 1a) or denaturation and
necrosis in <2/3 of cancer cells plus fusion in >1/3 of the
tumor (grade 1b). Grade 2 tumors exhibit significant denaturation,
necrosis, fusion and loss in >2/3 of the tumor. In grade 3
tumors [pathological complete response (pCR)], no cancer cell is
observed in both primary and regional lymph nodes (19). The pathological grade 1-0PD group
included patients in whom local pathological grade 1 to 0 had
resolved even though new distant metastases appeared.
Statistical analysis
SPSS Statistics (IBM, Armonk, NY, USA) version 21
was used to analyze all data. Mean values were compared using
Student's t-tests. Categorical data were analyzed using the
χ2 test statistics. All reported P-values are two-tailed
and were considered significant when P<0.05.
From the thickness of the fat of the abdominal wall
(mm), IRO Watt and RO difference Watt at their first thermal
treatment were determined using the formulas below as follows:
IRO (Watt) = 967.995 - abdominal wall fat thickness
(mm) × 17.155
Average RO difference at the first thermal treatment
(Watt/min) = average actual observed RO (Watt/min) at the first
thermal treatment -IRO (Watt)
Average RO difference after treatment completion
(Watt/min/5) = average actual observed RO (Watt/min) at treatment
completion -IRO (Watt)
For predicted IRO, the quartiles were ≤635, 636 to
720, 721 to 792 and ≥793 Watt. For RO differences, the quartiles
were ≤-153, −152 to −77, −76 to 76, and ≥77 Watt at the first
thermal treatment and ≤-135, −134 to −32, −31 to 132 and ≥133 Watt
after treatment completion.
Results
Table I contains the
characteristics of the patients. Of the 81 consecutive patients, 56
received NACR with concurrent thermal therapy followed by surgery.
In total, 54 tumors were resected and evaluated for pathological
responses. Two patients did not have their tumors resected since
the tumors were widespread, and 25 patients did not undergo
surgery. Reasons for not undergoing surgery included the surgery
itself after CR (n=4), permanent ostomy (n=7), poor general
conditions (age and various complications; n=6) and PD (n=8), which
included 3 lung metastases, 2 liver metastases, and growth of
tumors or lymph nodes in 3 patients. The timing of surgical
resections varied from 8 to 42 weeks after NACR and thermal
therapy, with a median of 15 weeks after treatment completion. The
median distance to the anal verge was 3.0 cm (range, 0–12 cm). We
avoided permanent ostomy in 41/56 patients (73.2%).
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Patient
characteristics | Data |
|---|
| Total no. of
patients | 81 |
| Age (years) |
|
|
Median | 62 |
|
Range | 33–89 |
| Gender, n (%) |
|
|
Female | 20 (24.7) |
|
Male | 61 (75.3) |
| Distance to anal
verge (cm), n (%) |
|
|
0–3.0 | 55 (67.9) |
|
3.1–5.0 | 15 (18.5) |
|
5.1- | 11 (13.6) |
| Tumor location, n
(%) |
|
| Ra | 10 (12.3) |
| Rb | 46 (56.8) |
|
RbP | 25 (30.9) |
| Tumor stage, n
(%) |
|
| T2 | 20 (24.7) |
| T3 | 45 (55.6) |
| T4 | 16 (19.8) |
| Lymph node stage, n
(%) |
|
| N0 | 40 (49.4) |
| N1 | 38 (46.9) |
| N2 | 2 (2.5) |
| N3 | 1 (1.2) |
| Distant metastasis,
n (%) |
|
| M0 | 74 (91.4) |
| M1 | 7 (8.6) |
| Pretreatment TNM
stage, n (%) |
|
| Stage
1 | 12 (14.8) |
| Stage
2 | 24 (29.6) |
| Stage
3 | 38 (46.9) |
| Stage
4 | 7 (8.6) |
| Tumor
differentiation, n (%) |
|
|
Well-differentiated | 38 (46.9) |
|
Moderately-differentiated | 36 (44.4) |
|
Poorly-differentiated | 6 (7.4) |
|
Undifferentiated | 1 (1.2) |
| Type of surgery, n
(%) |
|
| APR
Miles | 12 (14.8) |
|
ISR | 7 (8.6) |
|
sLAR | 15 (18.5) |
|
LAR | 13 (16.0) |
| Local
incision | 6 (7.4) |
|
Pelvic | 1 (1.2) |
| No
resection | 2 (2.5) |
| No
surgery | 25 (30.9) |
| Clinical response
after completion of treatment, n (%) |
|
| CR | 26 (32.1) |
| PR | 32 (39.5) |
| SD | 10 (12.3) |
| PD | 13 (16.0) |
Reduced tumor burden was achieved in 37/54 patients
(68.5%), specifically in 10/15 patients (66.6%) with preoperative
T2 lesions, 20/31 patients (64.5%) with T3 lesions and 7/8 patients
(87.5%) with T4 lesions. Sixteen of the 54 patients (29.6%) had
postoperative yT0 lesions.
NACR with concurrent thermal therapy was
well-tolerated, with 96.3% of our patients receiving the full dose
of chemotherapy and 100% receiving the full dose of radiotherapy
and 5 cycles of thermal therapy. Sixty-three patients (77.8%) and
18 patients (22.2%) received thermal treatments with and without
standardized power escalation principles, which we called
neothermia, respectively (21).
In 81 tested patients, CR, PR, SD and PD with the
RECIST criteria were shown in 32.1, 39.5, 12.3 and 16.0% of the
patients, respectively (Table I).
Table II displays the treatment
response results for 81 patients with rectal cancer after
completion of NACR with concurrent thermal therapy according to
tumor stage, lymph node involvement, distant metastasis and
pretreatment tumor-node-metastasis (TNM) stage. In 54 resected
tumor samples, pCR grade 3, grade 2, grade 1-0, grade 1-0PD was
found in 20.4, 37.0, 35.2 and 7.4% of the samples, respectively.
The PD ratio included clinical PD and pathological grade 1-0PD.
Five patients (25.0%) and 3 patients (15.0%) who were diagnosed
with pretreatment T2 tumors achieved pCRs and CRs, respectively,
while 1 patient (6.2%) with T4 tumor achieved CR. Four patients
(33.3%), and 3 patients (25.0%) who were diagnosed with
pretreatment pTNM stage 1 tumors achieved pCRs and CRs,
respectively, while 5 patients (71.4%) diagnosed with TNM stage 4
disease experienced PD.
 | Table II.Treatment response results after
completion of NACR with concurrent thermal therapy, according to
tumor stages, lymph node involvement, distant metastasis and
pretreatment TNM stages in 81 patients with rectal cancer. |
Table II.
Treatment response results after
completion of NACR with concurrent thermal therapy, according to
tumor stages, lymph node involvement, distant metastasis and
pretreatment TNM stages in 81 patients with rectal cancer.
|
| Grade 3 | Grade 2 | Grade 1-0 | CR | PR-SD | PD |
|
|---|
|
|
|
|
|
|
|
|
|
|---|
|
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | Total |
|---|
| T2 | 5 (25.0) | 6 (30.0) | 3 (15.0) | 3 (15.0) | 1 (5.0) | 2 (10.0) | 20 |
| T3 | 6 (13.3) | 11 (24.4) | 12 (26.7) | 1 (2.2) | 7 (15.6) | 8 (17.8) | 45 |
| T4 | 0 (0.0) | 3 (18.8) | 4 (25.0) | 1 (6.2) | 4 (25.0) | 4 (25.0) | 16 |
| N- | 8 (20.0) | 12 (30.0) | 6 (15.0) | 4 (10.0) | 6 (15.0) | 4 (10.0) | 40 |
| N+ | 3 (7.3) | 8 (19.5) | 13 (31.7) | 1 (2.4) | 6 (14.6) | 10 (24.4) | 41 |
| M(−) | 11 (14.9) | 19 (25.7) | 18 (24.3) | 5 (6.8) | 12 (16.2) | 9 (12.2) | 74 |
| M(+) | 0 (0.0) | 1 (14.3) | 1 (14.3) | 0 (0.0) | 0 (0.0) | 5 (71.4) | 7 |
| Stage 1 | 4 (33.3) | 2 (16.7) | 2 (16.7) | 3 (25.0) | 0 (0.0) | 1 (8.3) | 12 |
| Stage 2 | 4 (16.7) | 9 (37.5) | 3 (12.5) | 1 (4.2) | 6 (25.0) | 1 (4.2) | 24 |
| Stage 3 | 3 (7.9) | 8 (21.1) | 13 (34.2) | 1 (2.6) | 6 (15.8) | 7 (18.4) | 38 |
| Stage 4 | 0 (0.0) | 1 (14.3) | 1 (14.3) | 0 (0.0) | 0 (0.0) | 5 (71.4) | 7 |
| Total | 11 (13.6) | 20 (24.7) | 19 (23.5) | 5 (6.2) | 12 (14.8) | 14 (17.3) | 81 |
Table III displays
the treatment response results according to the frequency of
RF-induced output-limiting symptoms during the 5 thermal
treatments. Patients who scored 0/5 experienced no output-limiting
symptoms during the thermal treatments, while those who scored 5/5
experienced output-limiting symptoms during each thermal treatment.
The highest pCR rate (25.0%) was seen in patients who had no
RF-induced output-limiting symptoms during the thermal
treatments.
 | Table III.Results according to the frequency of
RF-induced output-limiting symptoms. |
Table III.
Results according to the frequency of
RF-induced output-limiting symptoms.
| Patients | Grade 3 | Grade 2 | Grade 1-0 | CR | PR-SD | PD |
|---|
|
|
|
|
|
|
|
|---|
| Scores | N | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) |
| 0/5 | 8 | 2 (25.0) | 1 (12.5) | 2 (25.0) | 0 (0.0) | 2 (25.0) | 1 (12.5) |
| 1/5 | 38 | 6 (15.8) | 8 (21.1) | 10 (26.3) | 2 (5.3) | 6 (15.8) | 6 (15.8) |
| 2/5 | 15 | 2 (13.3) | 3 (20.0) | 2 (13.3) | 2 (13.3) | 3 (20.0) | 3 (20.0) |
| 3/5 | 6 | 0 (0.0) | 3 (50.0) | 1 (16.7) | 0 (0.0) | 1 (16.7) | 1 (16.7) |
| 4/5 | 11 | 1 (9.1) | 4 (36.4) | 2 (18.2) | 1 (9.1) | 1 (9.1) | 2 (18.2) |
| 5/5 | 3 | 0 (0.0) | 1 (33.3) | 1 (33.3) | 0 (0.0) | 0 (0.0) | 1 (33.3) |
| Total | 81 | 11 (13.6) | 20 (24.7) | 18 (22.2) | 5 (6.2) | 13 (16.0) | 14 (17.3) |
As for RF-induced output-limiting symptoms during
thermal therapy, 69 patients (85.2%) experienced RF-induced
output-limiting symptoms, with 54 of those (66.7%) experiencing
pain, including 4 patients (4.9%) who stopped RF due to severe
symptoms, 8 experiencing skin discomfort, 5 experiencing
subcutaneous induration, 1 experiencing micturition desire and 1
experiencing cold sensations.
Table IV summarizes
the toxicity incidence among the 81 patients. Grade 3 toxicities
were observed in 7/81 patients (8.6%), with 1 patient experiencing
palmar-plantar erythrodysesthesia syndrome, 3 experiencing anal
mucositis, 2 experiencing diarrhea and 1 experiencing anemia.
 | Table IV.Summary of the toxicity in response
to NACR with concurrent thermal therapy in 81 patients. |
Table IV.
Summary of the toxicity in response
to NACR with concurrent thermal therapy in 81 patients.
|
| Negative response
(−) | Grade 1 | Grade 2 | Grade 3 |
|---|
|
|
|
|
|
|
|---|
| Toxicities | n (%) | n (%) | n (%) | n (%) | Total |
|---|
| Palmar-plantar
erythrodysesthesia | 20 (24.7) | 46 (56.8) | 14 (17.3) | 1 (1.2) | 81 |
| syndrome |
|
|
|
|
|
| Peripheral sensory
neuropathy | 66 (81.5) | 15 (18.5) | 0 (0.0) | 0 (0.0) | 81 |
| Anal mucositis | 14 (17.3) | 33 (40.7) | 31 (38.3) | 3 (3.7) | 81 |
| Nausea | 68 (84.0) | 10 (12.3) | 3 (3.7) | 0 (0.0) | 81 |
| Anorexia | 75 (92.6) | 4 (4.9) | 2 (2.5) | 0 (0.0) | 81 |
| Diarrhea | 32 (39.5) | 40 (49.4) | 7 (8.6) | 2 (2.5) | 81 |
| Fatigue | 76 (93.8) | 5 (6.2) | 0 (0.0) | 0 (0.0) | 81 |
| White blood cell
count decrease | 58 (71.6) | 11 (13.6) | 12 (14.8) | 0 (0.0) | 81 |
| Anemia | 51 (63.0) | 22 (27.2) | 7 (8.6) | 1 (1.2) | 81 |
| Platelet count
decrease | 64 (79.0) | 16 (19.8) | 1 (1.2) | 0 (0.0) | 81 |
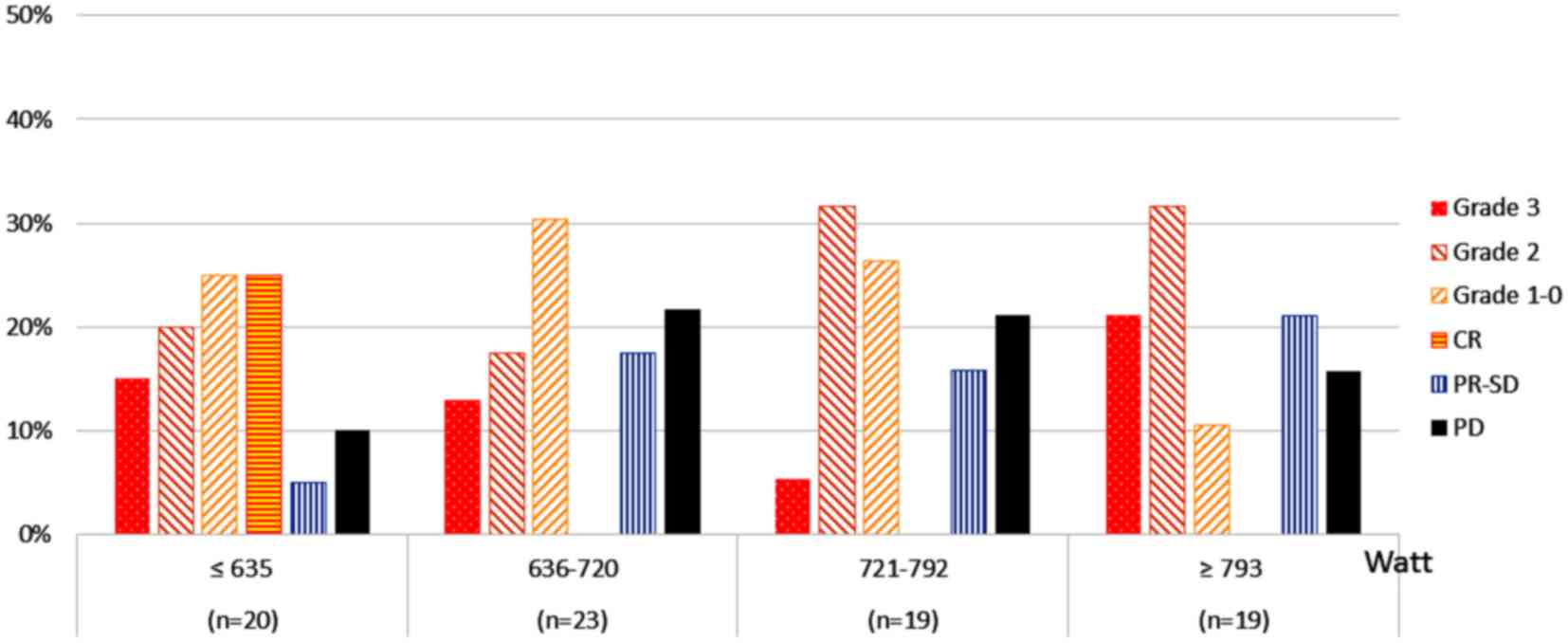
Fig. 1 shows the
treatment response results according to the predicted IRO (Watt) of
output-limiting symptoms. The highest proportion of patients with
grade 3 disease (21.2%) was in the upper quartile (≥793 Watt),
followed by 15.0% in the lowest quartile (≤635 Watt).
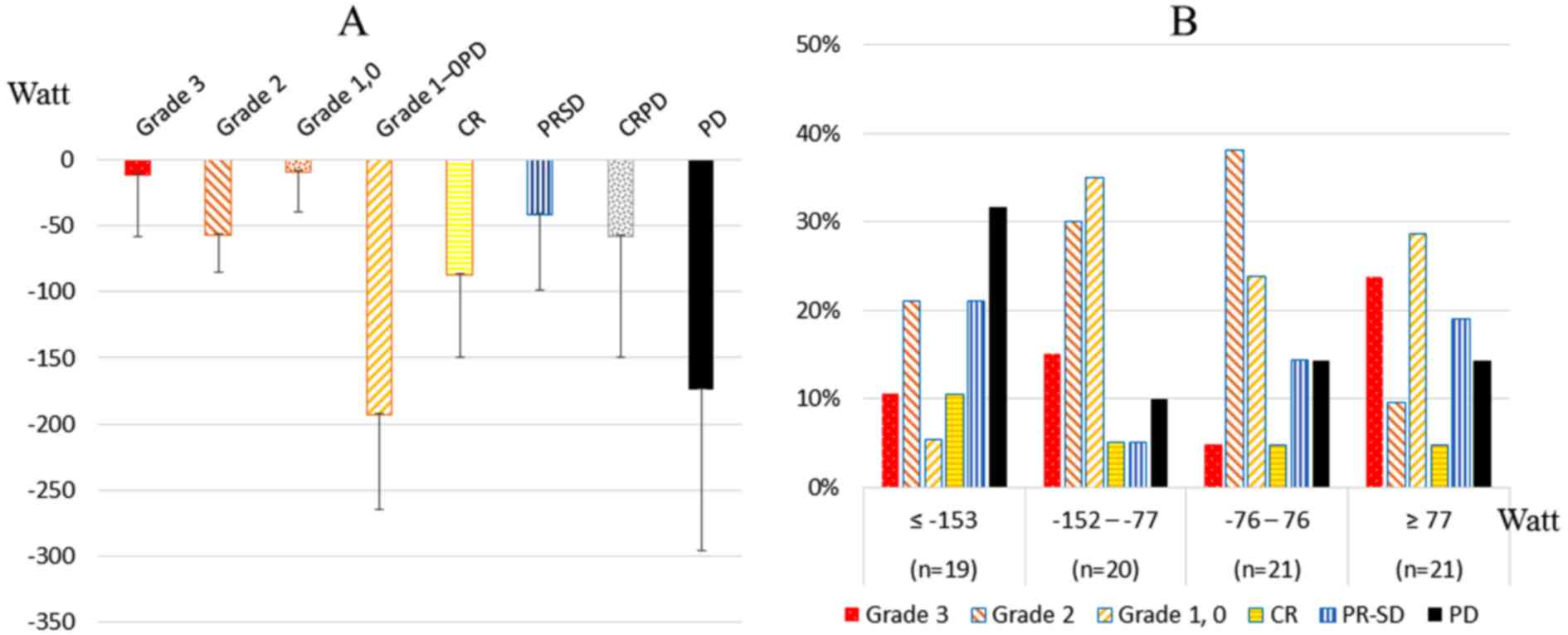
Fig. 2 shows the
average of the RO difference (Watt) (Fig. 2A) and the treatment responses at the
first RF thermal therapy (Fig. 2B).
There was a significant difference in the RO difference between
patients with pathological grade 1-0PD and grade 1–0 (p=0.021). All
patients with PD had negative scores of RF outputs, as PD patients
did not receive increased RF output over the predictive output. The
highest proportion of patients with grade 3 disease (23.8%) was in
the upper quartile (≥77 Watt) and the highest proportion of
patients with PD (31.6%) was in the lowest quartile (≤-153
Watt).
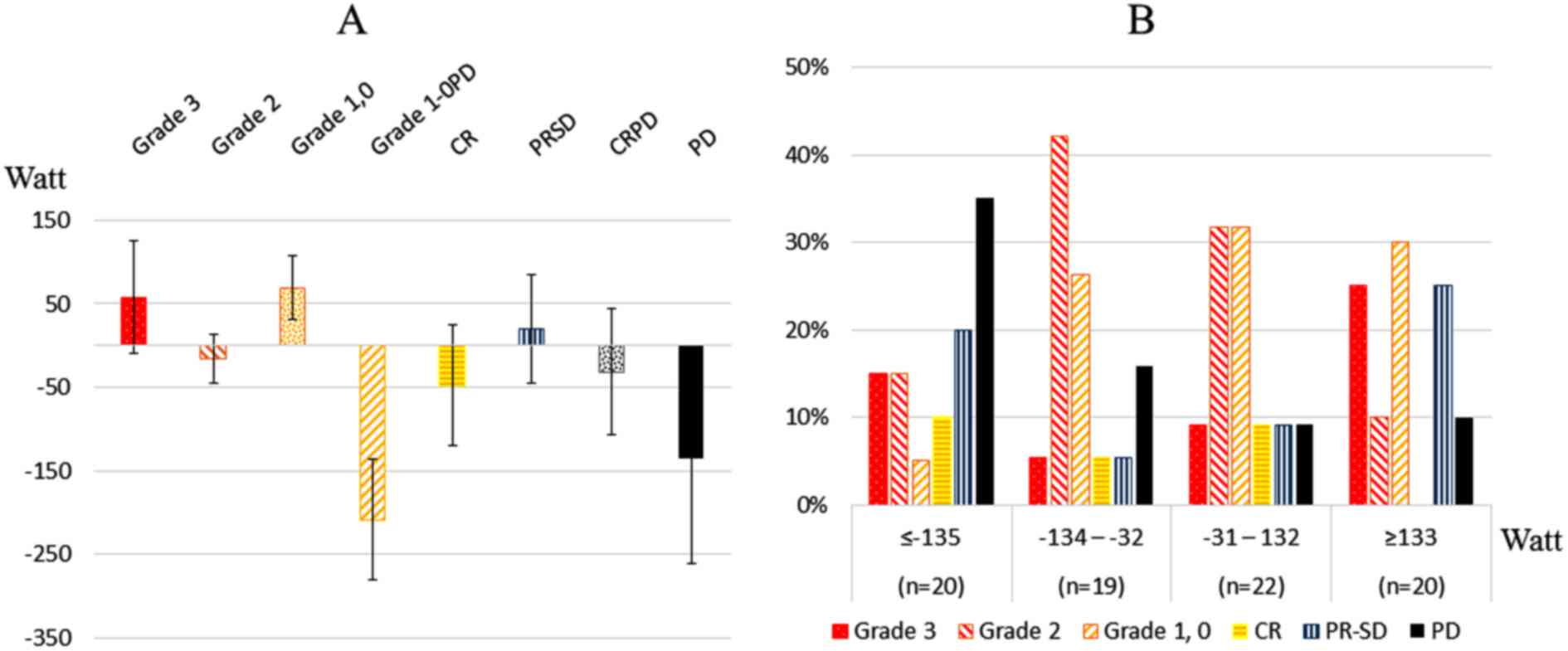
Fig. 3 shows the
average RO difference (Watt) (Fig.
3A) and treatment response after the fifth thermal treatment
(Fig. 3B). There were significant
differences between patients with grade 3, grade 2 and grade 1-0
disease and patients with pathological grade 1-0PD (p=0.046,
p=0.016 and p=0.005, respectively). There was a weak relationship
between patients with grade 3 disease and all patients with PD
(p=0.054). The highest proportion of patients with grade 3 disease
(25.0%) was in the upper quartile (≥133 Watt), and the highest
proportion of patients with PD (35.0%) was in the lowest quartile
(≤-135 Watt).
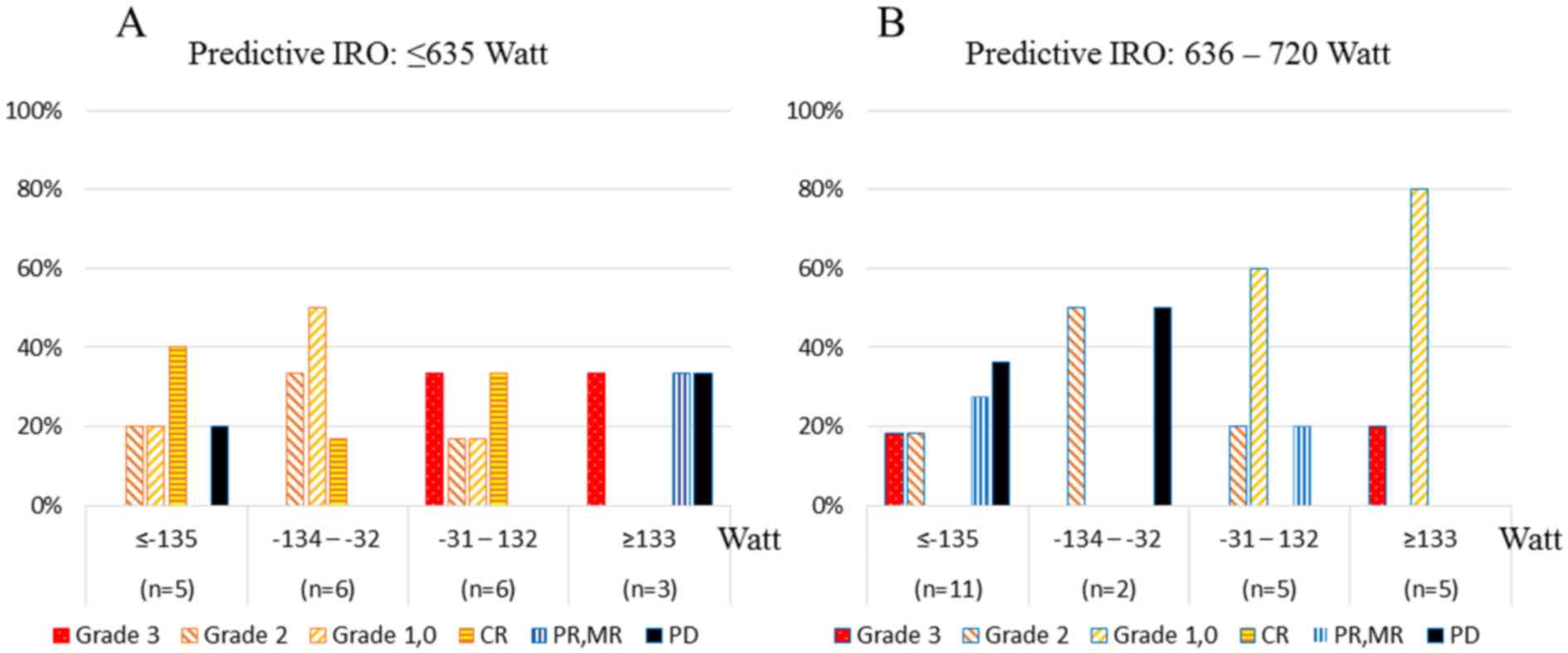
Fig. 4 shows the
correlations between both the predicted IRO and the RO difference
(Watt) and treatment response after the fifth thermal
treatment.
The highest proportion of patients with grade 3
disease (37.5%) were in the upper quartiles (predicted IRO ≥793
Watt and predicted IRO with ≥133 Watt) (Fig. 4D), and the highest proportion of
patients with PD were in the predicted IRO 721–792 Watt quartile
and the RO difference ≤-135 Watt quartile (Fig. 4C).
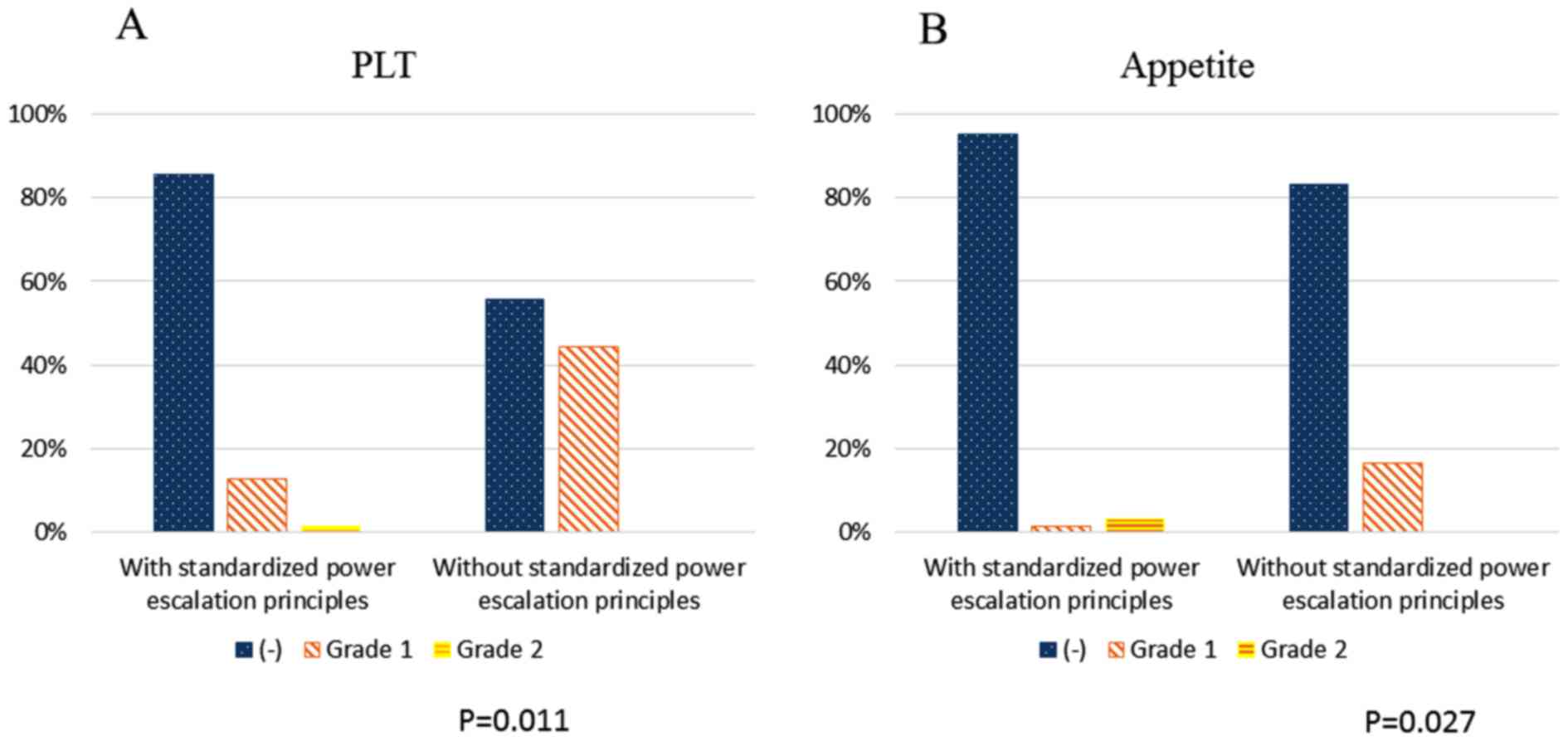
Fig. 5 shows the
toxicities in patients receiving thermal treatment with or without
standardized power escalation principles. There was no significant
difference between patients who received treatments with or without
standardized power escalation principles, with the exception of
platelet (PLT) and anorexia, which patients who received treatment
with standardized power escalation principles experienced with
significant less frequency (p=0.011 and p=0.027, respectively).
Discussion
Although numerous studies support the use of
capecitabine or infusional 5-FU, which are potentially
radiosensitizing agents (26–28).
as a standard of care in the neoadjuvant treatment of rectal
cancer, the optimal preoperative chemotherapy regimen and radiation
dose as a combined therapy for patients with rectal cancer are
unknown. Recently, the National Surgical Adjuvant Breast and Bowel
Project published its final study, in which the neoadjuvant Cap use
was comparable to continuous 5-FU infusion when combined with
radiation therapy (45 Gy/25 fractions) in 1,608 patients with stage
II or III rectal cancer, and the addition of oxaliplatin (OXA) did
not provide additional benefits with respect to toxicity (29,30).
The reported pCR rate was 20.7% for patients receiving Cap (825
mg/m2) 7 days a week beginning and ending on the first
and last days of radiation therapy, respectively, which was similar
to the rates in other studies (~15%) (2,3,15).
Craven et al reported results using similar
regimens to the one described in the present study (radiation, 45
Gy/25 fractions; Cap, 900 mg/m2 for 5 days/week vs. 7
days/week) in 70 patients with rectal cancer. The pCR and
progressive disease (PD) rates were 9.2 and 6%, respectively.
Sixty-two patients (88.6%) received the full dose of Cap without
suffering significant grade 3 or 4 toxicities. Three patients
(4.3%) had to discontinue Cap due to acute toxicities. Sixty-seven
patients (96%) received the full 45-Gy dose of radiotherapy. Three
patients (4.3%) suffered grade 3 diarrhea, while 1 patient
developed severe abdominal pain and diarrhea. Of the 65 patients
who underwent resections, 33 were anterior resections, 26 were
abdominoperineal resections, 5 were pelvic exenterations and in
33/64 (51.5%) the anus was preserved (31).
Gérard et al compared the Cap 45 group, which
received 45 Gy/25 fractions with concurrent 800 mg/m2
Cap twice daily 5 days/week, and the combined Cap and OXA group,
which received 50 Gy/25 fractions with 800 mg/m2 Cap
twice daily 5 days/week and 50 mg/m2 OXA once weekly.
The present study of 597 patients with rectal cancer reported pCR
rates of 13.9% for the Cap 45 group and 19.2% for the Cap + OXA
group (32).
Positive outcomes due to IMRT plus Cap treatment
have been observed, with pCR rates ranging from 14.1% to 30.6% in
patients with grade 3 disease, who comprised 11.1% to 17.6% of the
study populations (33–35), but these studies did not mention
cases of PD. Lu et al (36)
only reported a pCR in 20% of the cases with a 22% rate of grade 3
toxicity, and PD in 17% of the cases. Studies with controlled
trials also did not mention PD. Thus, it is likely that PD cases
were not analyzed in these studies.
To the best of our knowledge, Hernando-Requejo et
al reported excellent pCR rates using the concomitant-boost
IMRT dose fractionation scheme (35). Seventy-four patients were treated
with concomitant-boost IMRT (57.5 Gy in 23 fractions and concurrent
825 mg/m2 Cap bid), and the pCR and grade 3 toxicity
rates were 30.6 and 17.6%, respectively.
Before the year 2000, there was no standardized
thermal dose or standard hyperthermia treatment. Although the
results were not comparable, the pCR rates were reported to range
from 10 to 14%, in populations with 14% PD by adding hyperthermia
to chemoradiotherapy in patients with rectal cancer (37,38).
Since 2000, trials by the International Agency of Atomic Energy
(IAAE) and Issels et al failed to show a synergistic effects
between hyperthermia and radiotherapy (39–41).
Schroeder et al reported a retrospective
study comparing neoadjuvant radiation with concurrent 5-FU-based
chemotherapy with (n=61) and without (n=45) hyperthermia (90 min,
4–6 times) in 106 patients with rectal cancer, and pCR was achieved
in 6.7% of the patients in the chemoradiation group and 16.4% of
the patients in the hyperthermia group. The rate of
sphincter-sparing surgery was 57% in the
hyperthermo-radiochemotherapy group in comparison to 35% in the
radiochemotherapy group, while, our rate of sphincter-sparing
surgery was 73.2%. However, a total of 21 out of 61 patients
(34.4%) discontinued hyperthermia within the first three treatments
(42).
Maluta et al recently reported that
hyperthermia plus chemoradiotherapy (200 mg/m2
continuous 5-FU infusion for 6 weeks plus weekly 45
mg/m2 OXA, and 50 Gy in 2-Gy fractions for 5 weeks plus
a 10-Gy boost) resulted in pCRs for 18 out of 76 patients (23.6%),
and 4/76 patients (5.2%) developed PD (43).
This series of patients receiving tumor resection
and a 5-day/week schedule of Cap and radiotherapy with concurrent
thermal therapy, shows higher compliance (96.3%), lower toxicity
(8.6%), a better rate for preserving the anus (73.2%), better tumor
burden (68.5%), and fairly good local control (20.4%) compared to
previous studies.
We noticed that the rate of PD (17.3%) was higher
than that of pCR in this series. Therefore, the specific patients
who should be indicated to receive this therapy modality should be
clarified. If we can discover or predict patients who received good
outcomes using this treatment modality, this may add value in a
clinical setting.
We reported that correlations between RF output and
skin temperature from that RF output could be divided into three
groups: low, median and high output, according to the occurrence of
the output-limiting symptoms. From combination analyses of RF
output and temperature output, patient temperature in the low RF
output group did not increase. No patients achieved pCRs, and there
was a high rate of PD. We think that these patients cannot respond
to NACR with concurrent thermal therapy, and they possibly cannot
respond to NACR alone. From these results and with the low pCR rate
observed in these patients, additional and/or synergistic effects
with NACR with concurrent thermal therapy were not observed.
Consequently, the thermal therapy may offset the NACR effects in
these patients. From our results, we found that patients with cT2,
T3 and N0 lesions who were pretreated obtained good responses using
this modality. However, those with T4, N(+) or M(+) lesions did
not. This was similar to the results observed in other studies.
We also demonstrated in the present study that
patients with PD actually could not receive higher RF outputs than
the predicted output. We indicated the patient groups predicted to
be able to receive more output that actually could receive more
output than the predicted output as well as those who were
predicted to receive lower to moderate output and could not receive
more than the predicted output. The former group had a 37.5% pCR
rate and the latter group had a 66.7% rate of PD. From previous and
present data, we speculate that RF thermal therapy may offset the
chemoradiation effects in patients who could receive more than the
predicted output. Adding thermal therapy as a multimodality therapy
to NACR has a potential filter effect on patients based on whether
they experience potency with lower predicted outputs or higher
predicted outputs as well as lower or higher RO differences.
Thermal therapy, as a multidisciplinary therapy, may be used to
evaluate patients that should be treated with NACR to determine
whether they may experience true pCR or fallacious PD.
At present, unfortunately, we do not understand
these mechanisms. One explanation for our results concerning the
filter effects of RF thermal therapy is that RF thermal therapy and
IMRT which provide electromagnetic treatment affect cancer cells
with low energy and low-frequency waves (8-MHz) or high energy and
high-frequency waves (>3×1019 Hz), respectively.
Further study is needed to confirm these preliminary data in the
future.
The need for suitable markers for the identification
of patients who respond to cancer treatment is not limited to NACR
with concurrent thermal therapy, and it is essential to reliably
assess CRs and PD before or after completion of NACR treatment.
Therefore, developing tools that predict responses has become
exceedingly important, and a large number of studies have evaluated
molecular parameters (44–46). To date, none have been validated in
multi-institutional prospective trials. We may complete further
studies to find suitable markers that may fit more with our
predictive formula and offer more precise response to treatment
assessments of this modality.
In conclusion, this treatment modality was
beneficial for patients with T2, T3 and N0 rectal cancer in order
to avoid permanent ostomy, and the predictive formula for
output-limiting symptoms induced by RF is a good tool to predict
CRs or PD. This formula may be able to identify patients in the
clinical setting using chemoradiation treatment markers. These data
suggest that RF with low/high output conditions, were correlated
with output-limiting symptoms, which had a good or bad synergistic
effect with chemoradiation regimens. Our predictive equation for
the initial energy output at which output-limiting symptoms occur
may also be able to use early assessment of treatment efficacy, and
as a result, it can be used to decide on the continuation or
cessation of this treatment modality.
The present study does have some limitations. We
used a small sample size. Due to this, we did not have a group that
received radiation therapy with concurrent Cap. We also did not
perform long-term follow-up of the patients after therapy.
Therefore, further studies should be performed to confirm these
results.
References
|
1
|
Bosset JF, Calais G, Mineur L, Maingon P,
Stojanovic-Rundic S, Bensadoun RJ, Bardet E, Beny A, Ollier JC,
Bolla M, et al: EORTC Radiation Oncology Group: Fluorouracil-based
adjuvant chemotherapy after preoperative chemoradiotherapy in
rectal cancer: Long-term results of the EORTC 22921 randomised
study. Lancet Oncol. 15:184–190. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sauer R, Liersch T, Merkel S, Fietkau R,
Hohenberger W, Hess C, Becker H, Raab HR, Villanueva MT, Witzigmann
H, et al: Preoperative versus postoperative chemoradiotherapy for
locally advanced rectal cancer: Results of the German
CAO/ARO/AIO-94 randomized phase III trial after a median follow-up
of 11 years. J Clin Oncol. 30:1926–1933. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gérard JP, Conroy T, Bonnetain F, Bouché
O, Chapet O, Closon-Dejardin MT, Untereiner M, Leduc B, Francois E,
Maurel J, et al: Preoperative radiotherapy with or without
concurrent fluorouracil and leucovorin in T3-4 rectal cancers:
Results of FFCD 9203. J Clin Oncol. 24:4620–4625. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Park JH, Yoon SM, Yu CS, Kim JH, Kim TW
and Kim JC: Randomized phase 3 trial comparing preoperative and
postoperative chemoradiotherapy with capecitabine for locally
advanced rectal cancer. Cancer. 117:3703–3712. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Peeters KC, Marijnen CA, Nagtegaal ID,
Kranenbarg EK, Putter H, Wiggers T, Rutten H, Pahlman L, Glimelius
B, Leer JW, et al: Dutch Colorectal Cancer Group: The TME trial
after a median follow-up of 6 years: Increased local control but no
survival benefit in irradiated patients with resectable rectal
carcinoma. Ann Surg. 246:693–701. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Das P, Lin EH, Bhatia S, Skibber JM,
Rodriguez-Bigas MA, Feig BW, Chang GJ, Hoff PM, Eng C, Wolff RA, et
al: Preoperative chemoradiotherapy with capecitabine versus
protracted infusion 5-fluorouracil for rectal cancer: A
matched-pair analysis. Int J Radiat Oncol Biol Phys. 66:1378–1383.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yeo SG, Kim DY, Kim TH, Chang HJ, Oh JH,
Park W, Choi DH, Nam H, Kim JS, Cho MJ, et al: Pathologic complete
response of primary tumor following preoperative chemoradiotherapy
for locally advanced rectal cancer: Long-term outcomes and
prognostic significance of pathologic nodal status (KROG 09-01).
Ann Surg. 252:998–1004. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Park IJ, You YN, Agarwal A, Skibber JM,
Rodriguez-Bigas MA, Eng C, Feig BW, Das P, Krishnan S, Crane CH, et
al: Neoadjuvant treatment response as an early response indicator
for patients with rectal cancer. J Clin Oncol. 30:1770–1776. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fokas E, Liersch T, Fietkau R, Hohenberger
W, Beissbarth T, Hess C, Becker H, Ghadimi M, Mrak K, Merkel S, et
al: Tumor regression grading after preoperative chemoradiotherapy
for locally advanced rectal carcinoma revisited: Updated results of
the CAO/ARO/AIO-94 trial. J Clin Oncol. 32:1554–1562. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huebner M, Wolff BG, Smyrk TC, Aakre J and
Larson DW: Partial pathologic response and nodal status as most
significant prognostic factors for advanced rectal cancer treated
with preoperative chemoradiotherapy. World J Surg. 36:675–683.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhu J, Gu W, Lian P, Sheng W, Cai G, Shi
D, Cai S and Zhang Z: A phase II trial of neoadjuvant IMRT-based
chemoradiotherapy followed by one cycle of capecitabine for stage
II/III rectal adenocarcinoma. Radiat Oncol. 8:1302013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Martin ST, Heneghan HM and Winter DC:
Systematic review and meta-analysis of outcomes following
pathological complete response to neoadjuvant chemoradiotherapy for
rectal cancer. Br J Surg. 99:918–928. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Maas M, Nelemans PJ, Valentini V, Das P,
Rödel C, Kuo LJ, Calvo FA, García-Aguilar J, Glynne-Jones R,
Haustermans K, et al: Long-term outcome in patients with a
pathological complete response after chemoradiation for rectal
cancer: A pooled analysis of individual patient data. Lancet Oncol.
11:835–844. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Maas M, Beets-Tan RG, Lambregts DM,
Lammering G, Nelemans PJ, Engelen SM, van Dam RM, Jansen RL, Sosef
M, Leijtens JW, et al: Wait-and-see policy for clinical complete
responders after chemoradiation for rectal cancer. J Clin Oncol.
29:4633–4640. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Roh MS, Colangelo LH, O'Connell MJ,
Yothers G, Deutsch M, Allegra CJ, Kahlenberg MS, Baez-Diaz L,
Ursiny CS, Petrelli NJ, et al: Preoperative multimodality therapy
improves disease-free survival in patients with carcinoma of the
rectum: NSABP R-03. J Clin Oncol. 27:5124–5130. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee CK, Song CW, Rhee JG, Foy JA and
Levitt SH: Clinical experience using 8 MHz radiofrequency
capacitive hyperthermia in combination with radiotherapy: Results
of a phase I/II study. Int J Radiat Oncol Biol Phys. 32:733–745.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wiersma J, van Wieringen N, Crezee H and
van Dijk JD: Delineation of potential hot spots for hyperthermia
treatment planning optimisation. Int J Hyperthermia. 23:287–301.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shoji H, Motegi M, Osawa K, Okonogi N,
Okazaki A, Andou Y, Asao T, Kuwano H, Takahashi T and Ogoshi K:
Output-limiting symptoms induced by radiofrequency hyperthermia.
Are they predictable? Int J Hyperthermia. 32:199–203. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Engstrom PF, Arnoletti JP, Benson AB III,
Chen YJ, Choti MA, Cooper HS, Covey A, Dilawari RA, Early DS,
Enzinger PC, et al: National Comprehensive Cancer Network: NCCN
Clinical Practice Guidelines in Oncology: Rectal cancer. J Natl
Compr Canc Netw. 7:838–881. 2009.PubMed/NCBI
|
|
20
|
Japanese Society for Cancer of the Colon
and Rectum, . Japanese Classification of Colon Carcinoma. 8th.
Kanehara; Tokyo: 2013
|
|
21
|
Shoji H, Motegi M, Osawa K, Okonogi N,
Okazaki A, Andou Y, Asao T, Kuwano H, Takahashi T and Ogoshi K: A
novel strategy of radiofrequency hyperthermia (neothermia) in
combination with preoperative chemoradiotherapy for the treatment
of advanced rectal cancer: A pilot study. Cancer Med. 4:834–843.
2015. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shoji H, Motegi M, Osawa K, Okonogi N,
Okazaki A, Andou Y, Asao T, Kuwano H, Takahashi T and Ogoshi K:
Does standardization of radiofrequency hyperthermia benefit
patients with malignancies? Ann Cancer Res Ther. 22:28–35. 2014.
View Article : Google Scholar
|
|
23
|
Shoji H, Motegi M, Osawa K, Okonogi N,
Okazaki A, Andou Y, Asao T, Kuwano H, Takahashi T and Ogoshi K:
Radiofrequency thermal treatment with chemoradiotherapy for
advanced rectal cancer. Oncol Rep. 35:2569–2575. 2016.PubMed/NCBI
|
|
24
|
Therasse P, Arbuck SG, Eisenhauer EA,
Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van
Oosterom AT, Christian MC, et al: New guidelines to evaluate the
response to treatment in solid tumors. European Organization for
Research and Treatment of Cancer, National Cancer Institute of the
United States, National Cancer Institute of Canada. J Natl Cancer
Inst. 92:205–216. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
US National Cancer Institute, Cancer
Therapy Evaluation Program, . Common Terminology Criteria for
Adverse Events (CTCAE). http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_40Accessed
Feb 16, 2016.
|
|
26
|
Lawrence TS, Davis MA, Tang HY and Maybaum
J: Fluorodeoxyuridine-mediated cytotoxicity and radiosensitization
require S phase progression. Int J Radiat Biol. 70:273–280. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen AY, Chou R, Shih SJ, Lau D and
Gandara D: Enhancement of radiotherapy with DNA topoisomerase
I-targeted drugs. Crit Rev Oncol Hematol. 50:111–119. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gmeiner WH, Willingham MC, Bourland JD,
Hatcher HC, Smith TL, D'Agostino RB Jr and Blackstock W: F10
inhibits growth of PC3 xenografts and enhances the effects of
radiation therapy. J Clin Oncol Res. 2:10282014.PubMed/NCBI
|
|
29
|
O'Connell MJ, Colangelo LH, Beart RW,
Petrelli NJ, Allegra CJ, Sharif S, Pitot HC, Shields AF, Landry JC,
Ryan DP, et al: Capecitabine and oxaliplatin in the preoperative
multimodality treatment of rectal cancer: Surgical end points from
National Surgical Adjuvant Breast and Bowel Project trial R-04. J
Clin Oncol. 32:1927–1934. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Allegra CJ, Yothers G, O'Connell MJ, Beart
RW, Wozniak TF, Pitot HC, Shields AF, Landry JC, Ryan DP, Arora A,
et al: Neoadjuvant 5-FU or capecitabine plus radiation with or
without oxaliplatin in rectal cancer patients: A Phase III
randomized clinical trial. J Natl Cancer Inst. 107:djv2482015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Craven I, Crellin A, Cooper R, Melcher A,
Byrne P and Sebag-Montefiore D: Preoperative radiotherapy combined
with 5 days per week capecitabine chemotherapy in locally advanced
rectal cancer. Br J Cancer. 97:1333–1337. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gérard JP, Azria D, Gourgou-Bourgade S,
Martel-Lafay I, Hennequin C, Etienne PL, Vendrely V, François E, de
La Roche G, Bouché O, et al: Clinical outcome of the ACCORD 12/0405
PRODIGE 2 randomized trial in rectal cancer. J Clin Oncol.
30:4558–4565. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
De Ridder M, Tournel K, Van Nieuwenhove Y,
Engels B, Hoorens A, Everaert H, Op de Beeck B, Vinh-Hung V, De
Grève J, Delvaux G, et al: Phase II study of preoperative helical
tomotherapy for rectal cancer. Int J Radiat Oncol Biol Phys.
70:728–734. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Huang MY, Chen CF, Huang CM, Tsai HL, Yeh
YS, Ma CJ, Wu CH, Lu CY, Chai CY, Huang CJ, et al: Helical
tomotherapy combined with capecitabine in the preoperative
treatment of locally advanced rectal cancer. Biomed Res Int.
2014:3520832014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hernando-Requejo O, López M, Cubillo A,
Rodriguez A, Ciervide R, Valero J, Sánchez E, Garcia-Aranda M,
Rodriguez J, Potdevin G, et al: Complete pathological responses in
locally advanced rectal cancer after preoperative IMRT and
integrated-boost chemoradiation. Strahlenther Onkol. 190:515–520.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lu JY, Xiao Y, Qiu HZ, Wu B, Lin GL, Xu L,
Zhang GN and Hu K: Clinical outcome of neoadjuvant chemoradiation
therapy with oxaliplatin and capecitabine or 5-fluorouracil for
locally advanced rectal cancer. J Surg Oncol. 108:213–219. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Rau B, Wust P, Hohenberger P, Löffel J,
Hünerbein M, Below C, Gellermann J, Speidel A, Vogl T, Riess H, et
al: Preoperative hyperthermia combined with radiochemotherapy in
locally advanced rectal cancer: A phase II clinical trial. Ann
Surg. 227:380–389. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Petrovich Z, Langholz B, Gibbs FA,
Sapozink MD, Kapp DS, Stewart RJ, Emami B, Oleson J, Senzer N,
Slater J, et al: Regional hyperthermia for advanced tumors: A
clinical study of 353 patients. Int J Radiat Oncol Biol Phys.
16:601–607. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Vasanthan A, Mitsumori M, Park JH, Zhi-Fan
Z, Yu-Bin Z, Oliynychenko P, Tatsuzaki H, Tanaka Y and Hiraoka M:
Regional hyperthermia combined with radiotherapy for uterine
cervical cancers: A multi-institutional prospective randomized
trial of the international atomic energy agency. Int J Radiat Oncol
Biol Phys. 61:145–153. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Mitsumori M, Zeng ZF, Oliynychenko P, Park
JH, Choi IB, Tatsuzaki H, Tanaka Y and Hiraoka M: Regional
hyperthermia combined with radiotherapy for locally advanced
non-small cell lung cancers: A multi-institutional prospective
randomized trial of the International Atomic Energy Agency. Int J
Clin Oncol. 12:192–198. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Issels RD, Lindner LH, Verweij J, Wust P,
Reichardt P, Schem BC, Abdel-Rahman S, Daugaard S, Salat C,
Wendtner CM, et al: European Organisation for Research and
Treatment of Cancer Soft Tissue and Bone Sarcoma Group
(EORTC-STBSG); European Society for Hyperthermic Oncology (ESHO):
Neo-adjuvant chemotherapy alone or with regional hyperthermia for
localised high-risk soft-tissue sarcoma: A randomised phase 3
multicentre study. Lancet Oncol. 11:561–570. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Schroeder C, Gani C, Lamprecht U, von
Weyhern CH, Weinmann M, Bamberg M and Berger B: Pathological
complete response and sphincter-sparing surgery after neoadjuvant
radiochemotherapy with regional hyperthermia for locally advanced
rectal cancer compared with radiochemotherapy alone. Int J
Hyperthermia. 28:707–714. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Maluta S, Romano M, Dall'oglio S, Genna M,
Oliani C, Pioli F, Gabbani M, Marciai N and Palazzi M: Regional
hyperthermia added to intensified preoperative chemo-radiation in
locally advanced adenocarcinoma of middle and lower rectum. Int J
Hyperthermia. 26:108–117. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Powathil GG, Adamson DJ and Chaplain MA:
Towards predicting the response of a solid tumour to chemotherapy
and radiotherapy treatments: Clinical insights from a computational
model. PLOS Comput Biol. 9:e10031202013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Holck S, Nielsen HJ, Pedersen N and
Larsson LI: Phospho-ERK1/2 levels in cancer cell nuclei predict
responsiveness to radiochemotherapy of rectal adenocarcinoma.
Oncotarget. 6:34321–34328. 2015.PubMed/NCBI
|
|
46
|
Ebara T, Kaira K, Saito J, Shioya M, Asao
T, Takahashi T, Sakurai H, Kanai Y, Kuwano H and Nakano T: L-type
amino-acid transporter 1 expression predicts the response to
preoperative hyperthermo-chemoradiotherapy for advanced rectal
cancer. Anticancer Res. 30:4223–4227. 2010.PubMed/NCBI
|