Introduction
Gallic acid (GA; 3,4,5-triphydroxyl-benzoic acid)
and its derivatives are broadly distributed in a variety of plants,
fruits and foods. Especially, walnuts, green tea, grapes,
strawberries, bananas, lemons, pineapples, wines and apple peels
are recognized to be high in GA (1). GA is being used as food additives, and
preservatives. GA is very well absorbed in humans; free and
glucuronidated forms of GA and its main metabolite 4-O-methylgallic
have been observed to a large extent in human blood plasma after
intake of foods containing plenty of GA (2).
GA possesses various biological or pharmacological
activities including anti-bacterial (3), anti-viral (4) and anti-inflammatory effects (5). The major attention given to GA is due
to its antitumoral or anticancer effect. For example, GA inhibits
the growth of various cancer cells such as lung cancer (6,7),
leukemia (8), prostate cancer
(9), gastric, colon, breast,
cervical and esophageal cancers (10,11).
GA can trigger apoptosis via stimulating oxidative stress and/or
increasing intracellular Ca2+ levels (6–8,12).
However, GA shows somewhat lesser cytotoxicity against normal
endothelial and fibroblast cells (13). In addition, GA reveals an
anti-apoptotic potential in normal human lymphocytes (14). GA has been considered as a useful
phytochemical for cancer chemoprevention (15). Interestingly, GA can be a
pro-oxidant or an antioxidant depending on iron or
H2O2 in medium and plasma (16,17).
Therefore, further studies need to be performed to re-evaluate its
biological functions and roles under the different situations.
Reactive oxygen species (ROS) such as superoxide
anion (O2•−), hydroxyl radical
(•OH) and hydrogen peroxide (H2O2)
are involved in many important cellular functions of cell
proliferation, differentiation and apoptosis (18,19).
Alteration in the redox status of tissues and cells influences the
production or metabolism of ROS. They are generated as by-products
of mitochondrial respiration or certain oxidases such as
nicotinamide adenine dinucleotide phosphate oxidase and xanthine
oxidase (20). The primary
metabolic antioxidant enzyme is superoxide dismutase (SOD), which
metabolize O2•− to H2O2
(21). Further metabolism by other
antioxidant enzymes such as catalase and glutathione (GSH)
peroxidase, yields O2 and H2O (22). Cells possess various antioxidant
systems to control the redox state, which is important for their
survival. Oxidative stress may be the result of either
overproduction of ROS or accumulation of it due to the failure of
antioxidant systems, consequently inducing cell dysfunction or cell
death.
Cervical cancer is a foremost cause of
cancer-related death in women worldwide. The carcinogenesis is
considered to be connected with excessive inflammation mediated by
ROS. It was previously demonstrated that GA induces the growth
inhibition and death in GA-treated HeLa cervical cancer cells
(11). However, the underlying
mechanism remains unclear in view of redox state changes in
GA-treated HeLa cells. Thus, this study assessed the effects of GA
on ROS and GSH levels in HeLa cells and investigated the cellular
effects of N-acetyl cysteine (NAC; a well known antioxidant) and
buthionine sulfoximine (BSO; an inhibitor of GSH synthesis) on
GA-treated HeLa cells in relation to cell death.
Materials and methods
Cell culture
The human cervix adenocarcinoma HeLa cells obtained
from the American Type Culture Collection (ATCC, Manassas, VA, USA)
were cultured in RPMI-1640 supplemented with 10% fetal bovine serum
(Sigma-Aldrich Chemical Co., St. Louis, MO, USA) and 1%
penicillin-streptomycin (Gibco BRL, Grand Island, NY, USA). The
primary human umbilical vein endothelial cells (HUVEC) purchased
from PromoCell GmbH (Heidelberg, Germany) were cultured in complete
endothelial cell growth medium (ECGM, Promocell) with 2% FBS. HUVEC
were washed and detached with HEPES-BSS (30 mM HEPES), trypsin-EDTA
and trypsin neutralization solution (Promocell). The HUVEC between
passages four and eight were utilized for the experiments.
Reagents
GA was purchased from the Sigma-Aldrich Chemical Co.
and was dissolved in 100% ethanol at 200 mM. NAC and BSO were also
obtained from Sigma-Aldrich Chemical Co. NAC and BSO were obtained
from Sigma-Aldrich Chemical Co. NAC was dissolved in the buffer [20
mM HEPES (pH 7.0)]. BSO was dissolved in water. Based on a previous
study (23), exponentially growing
cells were treated with the indicated amounts of GA for 24 or 72 h
following one hour pre-incubation of 2 mM NAC or 10 µM BSO.
Growth inhibition assay
Changes in cell growth were assessed by measuring
the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
(MTT, Sigma-Aldrich Chemical Co.) dye absorbance as previously
described (24). Cells were exposed
to the indicated amounts of GA with or without NAC or BSO for 24 or
72 h.
Detection of intracellular ROS
levels
Intracellular ROS were assessed by a fluorescent
probe dye, 2′,7′-dichlorodihydrofluorescein diacetate
(H2DCFDA; Invitrogen Molecular Probes, Eugene, OR, USA).
Dihydroethidium (DHE, Invitrogen Molecular Probes) is a fluorogenic
probe which is highly selective for O2•−
among ROS. Cells were incubated with the indicated amounts of GA
with or without NAC or BSO for 0.5, 1, 2, 24 or 72 h. Cells were
then washed in PBS and incubated with 20 µM H2DCFDA or
DHE at 37°C for 30 min. DCF and DHE fluorescences were detected
using a FACStar flow cytometer (Becton-Dickinson, Franklin Lakes,
NJ, USA). ROS levels were expressed as mean fluorescence intensity
(MFI).
Detection of the intracellular
glutathione (GSH)
Cellular GSH levels were analyzed using a
5-chloromethylfluorescein diacetate dye (CMFDA, Invitrogen
Molecular Probes) as previously described (25). Cells were incubated with the
indicated amounts of GA with or without NAC or BSO for 0.5, 1, 2,
24 or 72 h. CMF fluorescence intensity was determined using a
FACStar flow cytometer (Becton-Dickinson). Negative CMF staining
(GSH depleted) cells were expressed as the percent of (−) CMF
cells. CMF levels in cells except (−) CMF cells were expressed as
MFI, which was calculated by CellQuest software
(Becton-Dickinson).
Annexin V staining for apoptosis
detection
Apoptosis was analyzed by staining cells with
Annexin V-fluorescein isothiocyanate (FITC; Invitrogen Molecular
Probes) as previously described (26). Cells were incubated with the
indicated amounts of GA with or without NAC or BSO for 24 h.
Annexin V-FITC staining cells were analyzed with a FACStar flow
cytometer (Becton-Dickinson).
Measurement of mitochondrial membrane
potential (MMP; ∆ψm)
MMP (∆ψm) levels were measured using a
Rhodamine 123 fluorescent dye (Sigma-Aldrich Chemical Co.; Ex/Em =
485/535 nm) as previously described (27). Cells were incubated with the
indicated amounts of GA with or without NAC or BSO for 24 h.
Rhodamine 123 staining intensity was determined by flow cytometry
(Becton-Dickinson). An absence of Rhodamine 123 from cells
indicated the loss of MMP (∆ψm) in HeLa cells.
Statistical analysis
The data were assessed using Instat software
(GraphPad Prism4, San Diego, CA, USA). The Student's t-test or
one-way analysis of variance (ANOVA) was used for parametric data.
Statistical significance was defined as p<0.05.
Results
Effects of GA on the growth of HeLa
cells and HUVEC
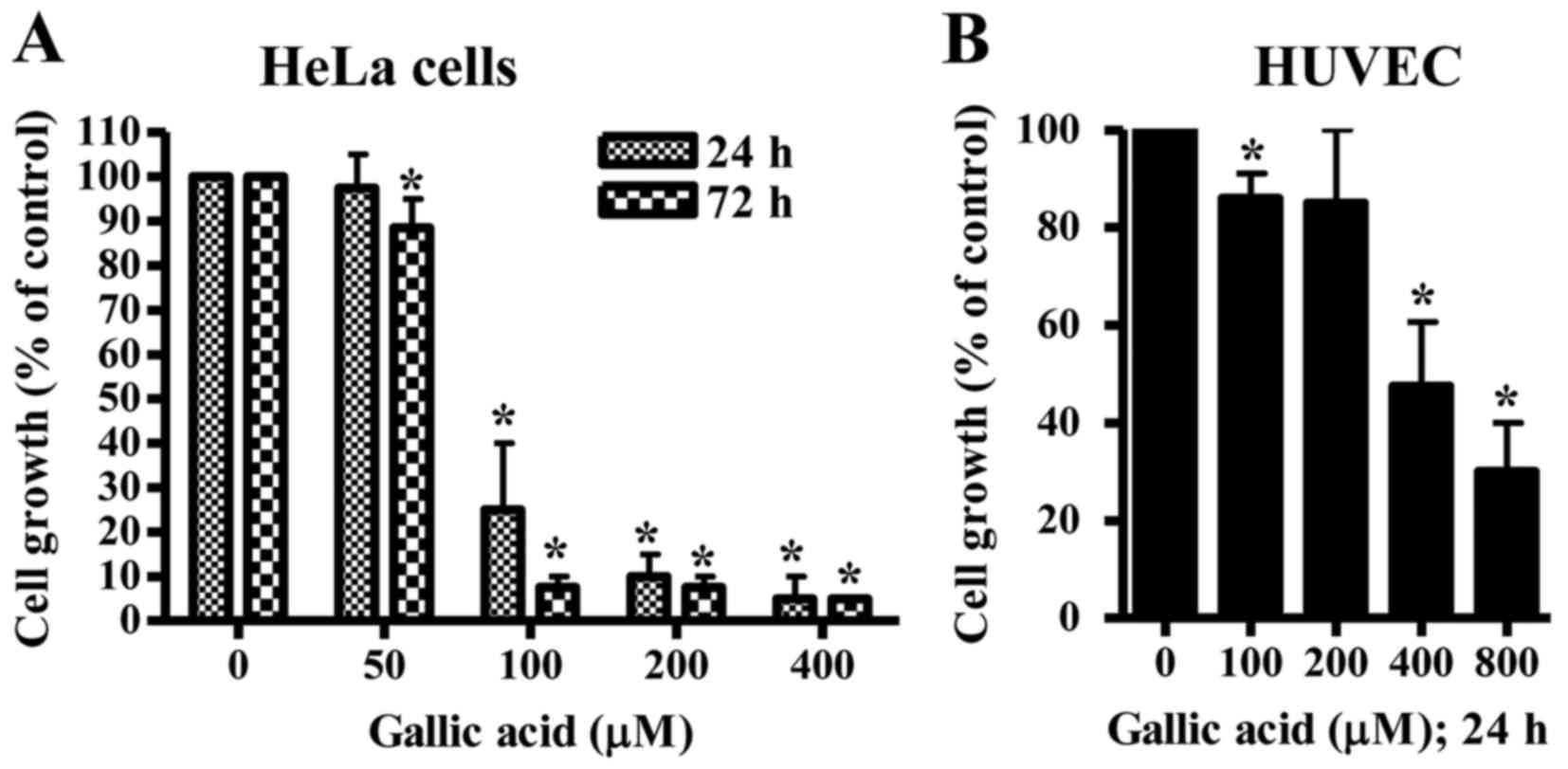
The anti-growth effect of GA was examined in HeLa
cells and HUVEC using MTT assays. In this study, HUVEC were used as
normal control cells since GA shows no cytotoxicity against normal
fibroblast and endothelial cells (13). After exposure to GA for 24 h, HeLa
cell growth was dose-dependently diminished with an IC50
of ~80 µM GA (Fig. 1A). At 72 h,
the growth of HeLa cells was completely inhibited at the
concentrations of >100 µM GA (Fig.
1A). When the growth of HUVEC was assessed after treatment with
GA, the dose-dependent reduction of cell growth was observed with
an IC50 of ~400 µM GA at 24 h (Fig. 1B).
Effects of GA on intracellular ROS
levels in HeLa cells and HUVEC
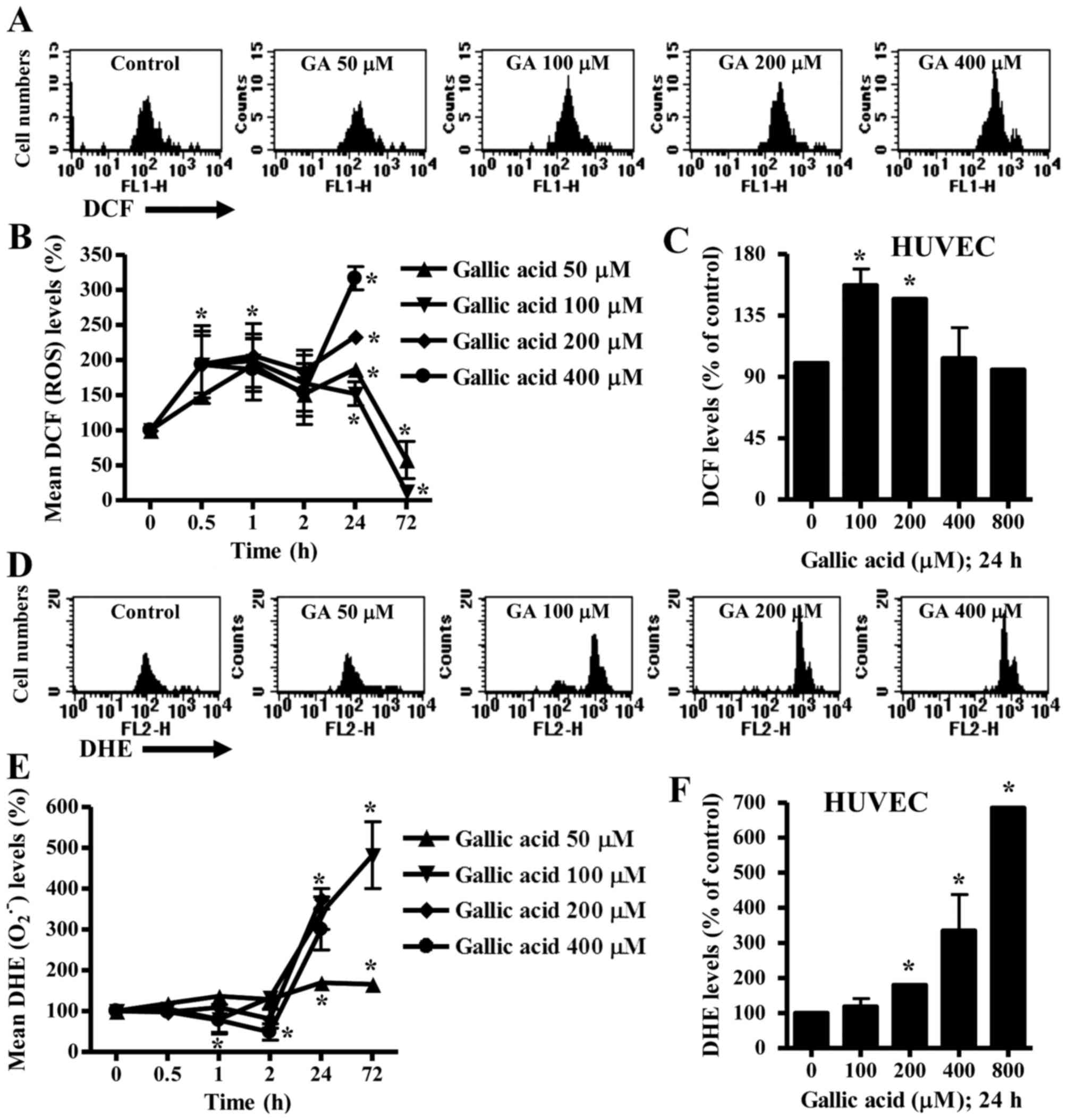
To assess ROS levels in GA-treated HeLa cells and
HUVEC, H2DCFDA and DHE dyes were used for the detection
of non-specific ROS and O2•− levels,
respectively. As shown in Fig. 2A and
B, intracellular ROS (DCF) levels increased in HeLa cells
treated with 50–400 µM GA from the early time phase of 30 min to 24
h. ROS levels dose-dependently increased at 24 h (Fig. 2A and B). However, 50 or 100 µM GA
decreased ROS levels in HeLa cells at 72 h (Fig. 2B). In HUVEC, 100 or 200 µM GA
increased ROS levels at 24 h whereas 400 and 800 µM GA did not
affect ROS levels at this time (Fig.
2C).
Intracellular O2•− levels
slightly decreased in 200 or 400 µM GA-treated HeLa cells from 1 h
to 2 h whereas O2•− levels were not
significantly changed in 50 or 100 µM GA-treated HeLa cells during
these times (Fig. 2E). At 24 h,
O2•− levels dose-dependently increased in
GA-treated HeLa cells (Fig. 2D and
E). The O2•− levels increased by 50 or
100 µM GA lasted for 72 h (Fig.
2E). In addition, 200–800 µM GA increased
O2•− levels in HUVEC at 24 h (Fig. 2F).
Effects of GA on intracellular GSH
levels in HeLa cells and HUVEC
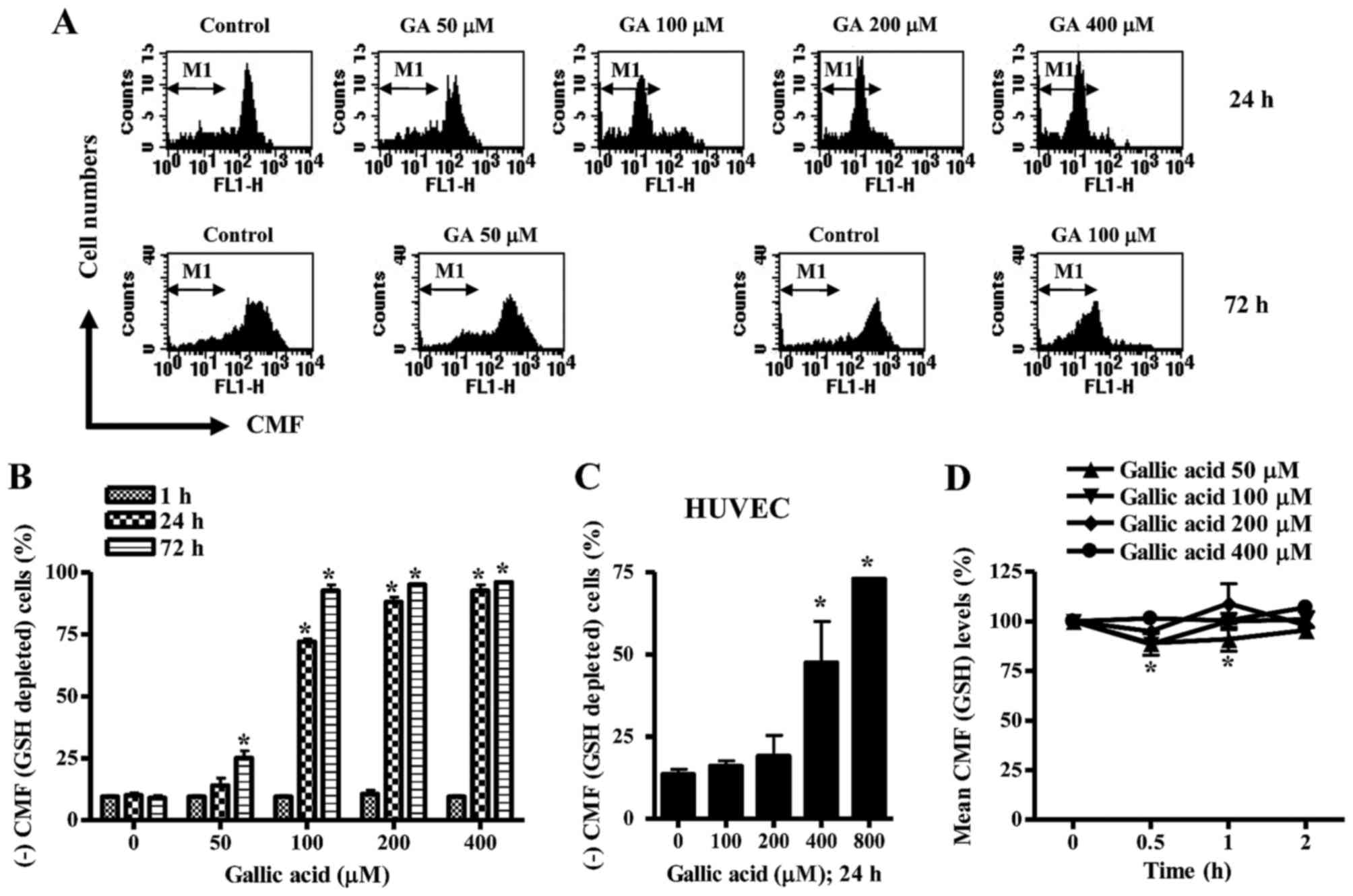
Next, changes in GSH levels were analyzed in
GA-treated HeLa cells and HUVEC using CMF fluorescence dye. As
shown in Fig. 3A and B, the numbers
of GSH-depleted cells in GA-treated HeLa cells were
dose-dependently increased at 24 or 72 h. A dramatic increase in
GSH-depleted cell number was observed in above 100 µM GA-treated
cells at 24 or 72 h (Fig. 3A and
B). However, the tested doses of GA did not induce GSH
depletion in HeLa cells at 1 h (Fig.
3B). In HUVEC, 100 or 200 µM GA did not increase the numbers of
GSH-depleted cells at 24 h, but 400 or 800 µM GA strongly increased
the numbers (Fig. 3C). When GSH
levels were measured in GA-treated HeLa cells at the early time
phases of 0.5, 1 or 2 h, GSH levels seemed to be decreased by GA
during these times (Fig. 3D).
Effects of NAC and BSO on cell death
and MMP (∆ψm) in GA-treated HeLa cells
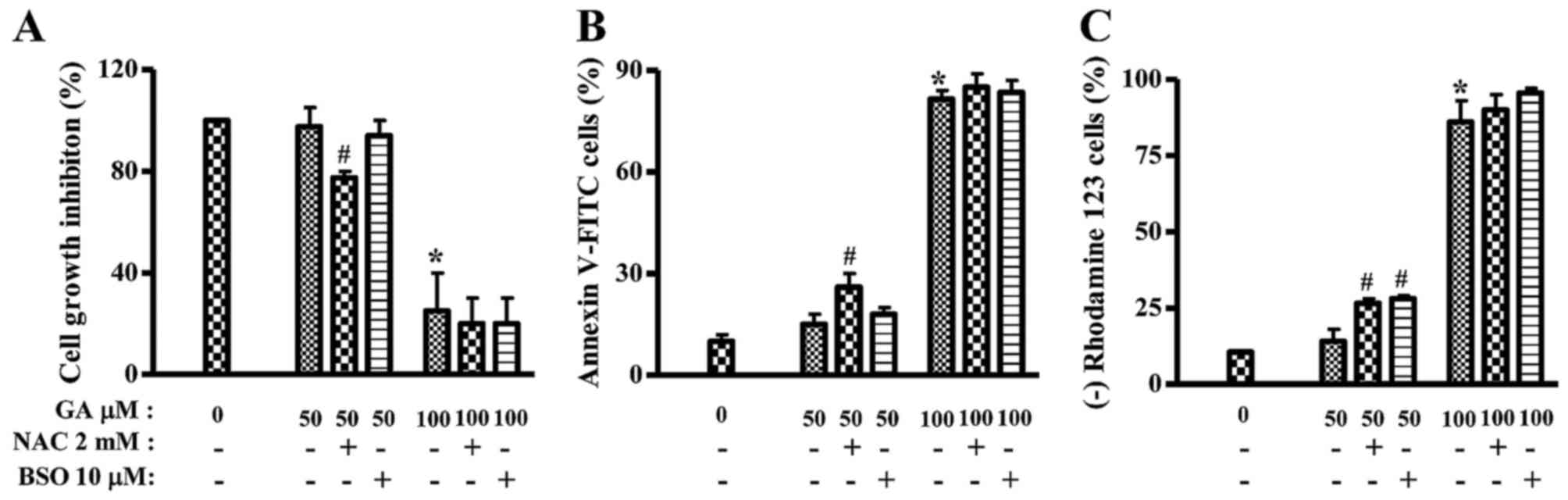
Because GA inhibited the growth of HeLa cells, this
study investigated the effects of NAC or BSO on the growth and
death of 50 or 100 µM GA-treated HeLa cells at 24 h. Treatment with
NAC significantly decreased the growth of 50 µM GA-treated HeLa
cells and BSO slightly decreased the growth (Fig. 4A). In addition, 50 µM GA slightly
increased the numbers of Annexin V-FITC-positive cells in HeLa
cells, but 100 µM GA strongly increased Annexin V-FITC-positive
cell numbers (Fig. 4B). NAC, but
not BSO, increased the numbers of Annexin V-FITC-positive cells in
50 µM GA-treated HeLa cells (Fig.
4B). Furthermore, mitochondrial membrane potential (MMP;
∆ψm) levels in GA-treated HeLa cells were analyzed in
the presence or absence of NAC and BSO at 24 h. Similar to the
Annexin V staining results, 100 µM, but not 50 GA µM, markedly
triggered the loss of MMP (∆ψm) in HeLa cells (Fig. 4C). Both NAC and BSO significantly
increased the loss of MMP (∆ψm) in 50 µM GA-treated HeLa
cells (Fig. 4C). NAC or BSO alone
did not trigger the loss of MMP (∆ψm) in HeLa control
cells (data not shown).
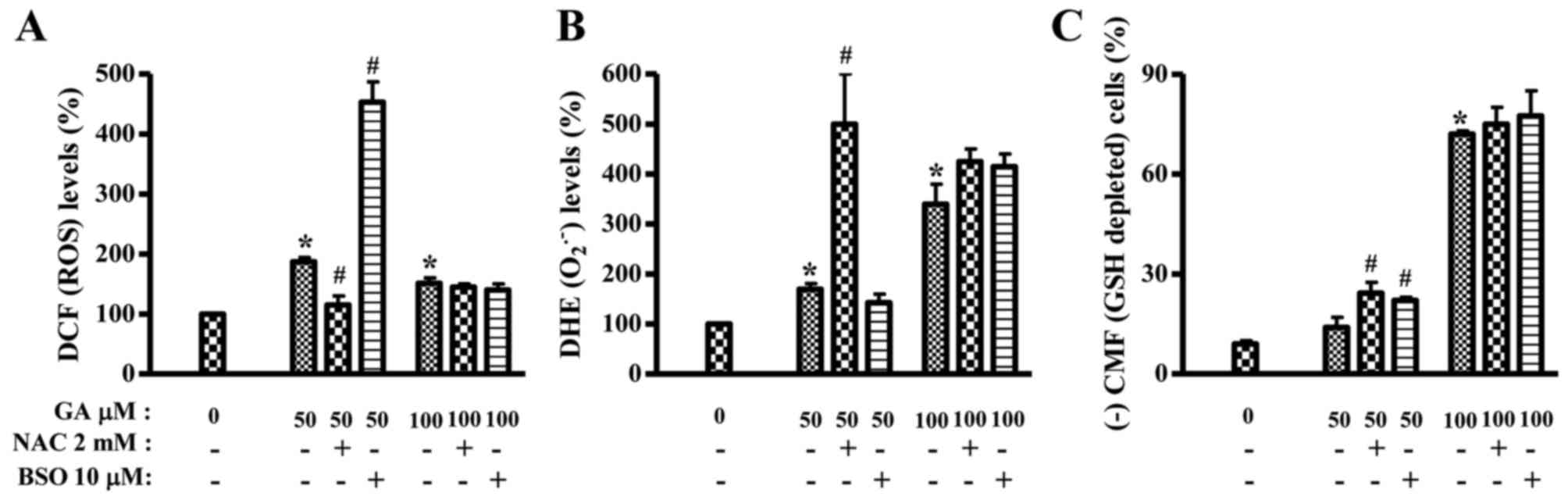
Effects of NAC and BSO on ROS and GSH
levels in GA-treated HeLa cells
It was assessed whether ROS and GSH levels in
GA-treated HeLa cells were changed or not by NAC or BSO at 24 h. As
shown in Fig. 5A, ROS (DCF) level
in 50 µM GA-treated HeLa cells was significantly decreased by NAC
whereas BSO strongly increased ROS (DCF) level in these cells. NAC
and BSO did not alter ROS (DCF) level in 100 µM GA-treated HeLa
cells (Fig. 5A). In contrast, NAC
intensified O2•− level in 50 µM GA-treated
HeLa cells and slightly increased O2•− level
in 100 µM GA-treated HeLa cells (Fig.
5B). BSO slightly decreased O2•− level in
50 µM GA-treated HeLa cells but it increased
O2•− level in 100 µM GA-treated HeLa cells
(Fig. 5B). In relation to GSH
level, NAC and BSO significantly increased the numbers of
GSH-depleted cells in 50 µM GA-treated HeLa cells at 24 h (Fig. 5C). Both agents also mildly increased
GSH-depleted cell numbers in 100 µM GA-treated HeLa cells (Fig. 5C). NAC or BSO alone did not
significantly change GSH and ROS levels including
O2•−.
Discussion
This study focused on evaluating the effects of GA
on the growth and death of HeLa cells in relation to ROS and GSH
levels. This study also demonstrated that the susceptibility of
HeLa cells to GA was higher than that of HUVEC. This result was
similar to the report that GA shows the lower cytotoxicity against
normal fibroblast and endothelial cells (13). GA is reported to induce apoptosis in
prostate cancer cells via mitochondrial dysfunction (28). Likewise, GA seemed to induce
apoptosis in HeLa cells and it triggered the loss of MMP
(∆ψm).
GA has been reported to have both pro-oxidant and/or
antioxidant properties (16,17).
Increasing evidence suggests that apoptosis induced by GA is
associated with oxidative stresses derived from ROS (8,12,28).
According to our results, the intracellular ROS (DCF) levels
increased in GA-treated HeLa cells from the early time phases. ROS
levels also dose-dependently increased at 24 h. Interestingly, 50
or 100 µM GA showing an apoptotic effect at 72 h decreased ROS
levels. In contrast, GA slightly decreased
O2•− levels at the early time phases and it
increased O2•− levels at 24 or 72 h. In
relation to HUVEC, 100 or 200 µM GA increased ROS levels at 24 h
whereas 400 and 800 µM GA showed a significant growth inhibition
and cell death did not increase ROS levels. Treatment with the
>200 µM GA increased O2•− levels in HUVEC
at 24 h. In addition, GA increases ROS levels including
O2•− at 24 h in lung cancer and normal cells
(7,29). These results suggest that GA can
individually affect different ROS levels depending on the
incubation times and doses, and cell types.
NAC showing the reduction of ROS (DCF) level in 50
µM GA-treated HeLa cells significantly enhanced growth inhibition
and cell death in these cells. In addition, NAC strongly increased
O2•− level in GA-treated HeLa cells.
Therefore, although NAC decreased ROS (DCF) level in GA-treated
HeLa cells, NAC seemed to act as a pro-oxidant because of
increasing O2•− level and cell death in these
cells. Similarly, NAC intensified the growth inhibition and death
in GA-treated lung cancer, which were accompanied by a decrease in
ROS (DCF) level and an increase in O2•− level
(7). BSO showing a strong increased
effect of ROS (DCF) on GA-treated HeLa cells did not affect cell
growth and death but it increased the loss of MMP (∆ψm)
in these cells. Taken together, these results suggest that changes
in ROS levels by GA are not tightly but at least partially related
to HeLa cell death. The exact role of ROS, especially
O2•− needs to be defined further in
GA-induced HeLa cell death.
It has been reported that the intracellular GSH
content has a decisive effect on anticancer drug-induced apoptosis,
indicating that apoptotic effects are inversely comparative to GSH
content (30,31). The intracellular GSH content also is
involved in GA-induced cell death (7,29).
Likewise, GA dose-dependently increased the numbers of GSH-depleted
cells in HeLa cells and HUVEC. The decreased GSH levels in
GA-treated HeLa cells at the early times probably resulted from ROS
(DCF) level increased by GA. In addition, NAC showing the
enhancement of cell death in GA-treated HeLa cells significantly
increased the numbers of GSH-depleted cells. Similarly, NAC
enhances GSH depletion in GA-treated lung cancer and normal cells
(7,29). Although it is known that NAC as a
GSH precursor contains a thiol group, NAC used in this study did
not seem to be a GSH precursor in GA-treated HeLa cells. However,
NAC significantly prevented GSH depletion in propyl gallate-treated
HeLa cells (32). Therefore, it is
considered that NAC can be a GSH precursor or not depending on
co-incubated agents. BSO significantly increased the numbers of
GSH-depleted cells in GA-treated HeLa cells. BSO also augmented GSH
depletion and cell death in GA-treated lung cancer and normal cells
(7,29). However, BSO enhanced the loss of MMP
(∆ψm) in GA-treated HeLa cells without a significant
increase in cell death, implying that GSH depletion in these cells
was correlated with MMP (∆ψm) loss rather than cell
death. Taken together, these results suggest that the intracellular
GSH content seem to have a vital role on GA-induced cell death but
changes in GSH levels are not sufficient enough to precisely
predict cell death.
In conclusion, GA significantly inhibited the growth
of HeLa cells. Changes in ROS levels, especially
O2•− affected GA-induced HeLa cell death.
GA-induced HeLa cell death correlated with GSH depletion.
Acknowledgements
This study was supported by a grant from the
National Research Foundation of Korea (NRF) funded by the Korean
government (MSIP; nos. 2008-0062279 and 2016R1A2B4007773).
Glossary
Abbreviations
Abbreviations:
|
GA
|
gallic acid
|
|
HUVEC
|
human umbilical vein endothelial
cells
|
|
ROS
|
reactive oxygen species
|
|
MMP (∆ψm)
|
mitochondrial membrane potential
|
|
MTT
|
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
|
|
H2DCFDA
|
2′,7′-dichlorodihydrofluorescein
diacetate
|
|
DHE
|
dihydroethidium
|
|
GSH
|
glutathione
|
|
CMFDA
|
5-chloromethylfluorescein
diacetate
|
|
NAC
|
N-acetyl cysteine
|
|
BSO
|
L-buthionine sulfoximine
|
References
|
1
|
Chu YF, Sun J, Wu X and Liu RH:
Antioxidant and antiproliferative activities of common vegetables.
J Agric Food Chem. 50:6910–6916. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shahrzad S, Aoyagi K, Winter A, Koyama A
and Bitsch I: Pharmacokinetics of gallic acid and its relative
bioavailability from tea in healthy humans. J Nutr. 131:1207–1210.
2001.PubMed/NCBI
|
|
3
|
Kang MS, Oh JS, Kang IC, Hong SJ and Choi
CH: Inhibitory effect of methyl gallate and gallic acid on oral
bacteria. J Microbiol. 46:744–750. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kratz JM, Andrighetti-Fröhner CR, Leal PC,
Nunes RJ, Yunes RA, Trybala E, Bergström T, Barardi CR and Simões
CM: Evaluation of anti-HSV-2 activity of gallic acid and pentyl
gallate. Biol Pharm Bull. 31:903–907. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim SH, Jun CD, Suk K, Choi BJ, Lim H,
Park S, Lee SH, Shin HY, Kim DK and Shin TY: Gallic acid inhibits
histamine release and pro-inflammatory cytokine production in mast
cells. Toxicol Sci. 91:123–131. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
You BR, Kim SZ, Kim SH and Park WH: Gallic
acid-induced lung cancer cell death is accompanied by ROS increase
and glutathione depletion. Mol Cell Biochem. 357:295–303. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
You BR and Park WH: Gallic acid-induced
lung cancer cell death is related to glutathione depletion as well
as reactive oxygen species increase. Toxicol In Vitro.
24:1356–1362. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Inoue M, Sakaguchi N, Isuzugawa K, Tani H
and Ogihara Y: Role of reactive oxygen species in gallic
acid-induced apoptosis. Biol Pharm Bull. 23:1153–1157. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Agarwal C, Tyagi A and Agarwal R: Gallic
acid causes inactivating phosphorylation of cdc25A/cdc25C-cdc2 via
ATM-Chk2 activation, leading to cell cycle arrest, and induces
apoptosis in human prostate carcinoma DU145 cells. Mol Cancer Ther.
5:3294–3302. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Faried A, Kurnia D, Faried LS, Usman N,
Miyazaki T, Kato H and Kuwano H: Anticancer effects of gallic acid
isolated from Indonesian herbal medicine, Phaleria macrocarpa
(Scheff.) Boerl, on human cancer cell lines. Int J Oncol.
30:605–613. 2007.PubMed/NCBI
|
|
11
|
You BR, Moon HJ, Han YH and Park WH:
Gallic acid inhibits the growth of HeLa cervical cancer cells via
apoptosis and/or necrosis. Food Chem Toxicol. 48:1334–1340. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Serrano A, Palacios C, Roy G, Cespón C,
Villar ML, Nocito M and González-Porqué P: Derivatives of gallic
acid induce apoptosis in tumoral cell lines and inhibit lymphocyte
proliferation. Arch Biochem Biophys. 350:49–54. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Inoue M, Suzuki R, Sakaguchi N, Li Z,
Takeda T, Ogihara Y, Jiang BY and Chen Y: Selective induction of
cell death in cancer cells by gallic acid. Biol Pharm Bull.
18:1526–1530. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sohi KK, Mittal N, Hundal MK and Khanduja
KL: Gallic acid, an antioxidant, exhibits antiapoptotic potential
in normal human lymphocytes: A Bcl-2 independent mechanism. J Nutr
Sci Vitaminol (Tokyo). 49:221–227. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Giftson JS, Jayanthi S and Nalini N:
Chemopreventive efficacy of gallic acid, an antioxidant and
anticarcinogenic polyphenol, against 1,2-dimethyl hydrazine induced
rat colon carcinogenesis. Invest New Drugs. 28:251–259. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Strlic M, Radovic T, Kolar J and Pihlar B:
Anti- and prooxidative properties of gallic acid in fenton-type
systems. J Agric Food Chem. 50:6313–6317. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sakagami H and Satoh K: Prooxidant action
of two antioxidants: Ascorbic acid and gallic acid. Anticancer Res
17A. 221–224. 1997.
|
|
18
|
Gonzalez C, Sanz-Alfayate G, Agapito MT,
Gomez-Niño A, Rocher A and Obeso A: Significance of ROS in oxygen
sensing in cell systems with sensitivity to physiological hypoxia.
Respir Physiol Neurobiol. 132:17–41. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Baran CP, Zeigler MM, Tridandapani S and
Marsh CB: The role of ROS and RNS in regulating life and death of
blood monocytes. Curr Pharm Des. 10:855–866. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zorov DB, Juhaszova M and Sollott SJ:
Mitochondrial ROS-induced ROS release: An update and review.
Biochim Biophys Acta. 1757:509–517. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zelko IN, Mariani TJ and Folz RJ:
Superoxide dismutase multigene family: A comparison of the CuZn-SOD
(SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures,
evolution, and expression. Free Radic Biol Med. 33:337–349. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wilcox CS: Reactive oxygen species: Roles
in blood pressure and kidney function. Curr Hypertens Rep.
4:160–166. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Han YH and Park WH: The effects of
N-acetyl cysteine, buthionine sulfoximine, diethyldithiocarbamate
or 3-amino-1,2,4-triazole on antimycin A-treated Calu-6 lung cells
in relation to cell growth, reactive oxygen species and
glutathione. Oncol Rep. 22:385–391. 2009.PubMed/NCBI
|
|
24
|
Han YH, Moon HJ, You BR, Kim SZ, Kim SH
and Park WH: Effects of carbonyl cyanide p-(trifluoromethoxy)
phenylhydrazone on the growth inhibition in human pulmonary
adenocarcinoma Calu-6 cells. Toxicology. 265:101–107. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Han YH, Kim SH, Kim SZ and Park WH:
Caspase inhibitor decreases apoptosis in pyrogallol-treated lung
cancer Calu-6 cells via the prevention of GSH depletion. Int J
Oncol. 33:1099–1105. 2008.PubMed/NCBI
|
|
26
|
Han YH, Kim SZ, Kim SH and Park WH:
Apoptosis in pyrogallol-treated Calu-6 cells is correlated with the
changes of intracellular GSH levels rather than ROS levels. Lung
Cancer. 59:301–314. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
You BR, Kim SH and Park WH: Reactive
oxygen species, glutathione, and thioredoxin influence suberoyl
bishydroxamic acid-induced apoptosis in A549 lung cancer cells.
Tumour Biol. 36:3429–3439. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen HM, Wu YC, Chia YC, Chang FR, Hsu HK,
Hsieh YC, Chen CC and Yuan SS: Gallic acid, a major component of
Toona sinensis leaf extracts, contains a ROS-mediated anti-cancer
activity in human prostate cancer cells. Cancer Lett. 286:161–171.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
You BR and Park WH: Enhancement of gallic
acid-induced human pulmonary fibroblast cell death by N-acetyl
cysteine and L-buthionine sulfoximine. Hum Exp Toxicol. 30:992–999.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Estrela JM, Ortega A and Obrador E:
Glutathione in cancer biology and therapy. Crit Rev Clin Lab Sci.
43:143–181. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Han YH, Kim SZ, Kim SH and Park WH:
Intracellular GSH level is a factor in As4.1 juxtaglomerular cell
death by arsenic trioxide. J Cell Biochem. 104:995–1009. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Han YH and Park WH: Propyl gallate
inhibits the growth of HeLa cells via regulating intracellular GSH
level. Food Chem Toxicol. 47:2531–2538. 2009. View Article : Google Scholar : PubMed/NCBI
|