Introduction
Hepatocellular carcinoma (HCC) is the fifth most
prevalent cancer among men and the ninth most common cancer among
women worldwide (1) and it is the
third leading cause of cancer-related deaths in China (2). Surgical resection is regarded as the
primary therapy for early-stage HCC with a 5-year survival rate of
~60–70% in patients who have undergone surgical treatment (3,4).
However, postoperative recurrence is very common (5) and intrahepatic metastases, vascular
invasion and satellite foci are the main reasons for this
recurrence (4). Therefore, it is
urgent to understand the mechanisms involved in HCC metastasis.
Activation of the epithelial-mesenchymal transition (EMT) process
is considered to be a critical event for the metastasis of tumors,
particularly in the early stage (6). After EMT, HCC cells lose their
epithelial phenotype with the downregulation of E-cadherin and
transform to a mesenchymal phenotype with the upregulation of
multiple mesenchymal genes (N-cadherin, vimentin and α-SMA)
(6).
Chinese herbal medicine has been demonstrated to
have a therapeutic effect on tumors (7,8). The
Chinese herbal medicine ‘Songyou Yin’ (SYY) contains 5 Chinese
medicinal herbal extracts with chromatographic fingerprints in the
following ratios (w/w): Astragalus membranaceus Bge, 14.3%;
Salvia miltiorrhiza Bge, 14.3%; Lycium barbarum L.,
23.8%; Crataegus pinnatifida Bge, 23.8% and Trionyx
sinensis Wiegmann, 23.8% (all from China) (9). In our previous studies, we found that
SYY suppressed the growth and invasion of HCC cells (9). Furthermore, SYY also enhanced the
sensitivity of HCC cells to chemotherapy through the inhibition of
the stemness of the hepatoma cells (10). Astragalus membranaceus is a
traditional Chinese herb. It is usually used to treat edemas of
acute nephritis, colds, vulnus and many other diseases (11). Various recent studies demonstrated
that Astragalus membranaceus has also anticancer bioactivity
(12–14). Due to its complex composition and
the lack of scientific basis to support its effects, it may be
useful to investigate which of its components has an antitumor
effect. Astragaloside-IV (AS-IV; chemical name,
3-O-β-D-xylopyranosyl-6-O-β-D-glucopyranosyl-cycloastragenol)
is a primary bioactive constituent of Astragalus
membranaceus (15), which has
an explicit chemical formula and an exact molecular weight
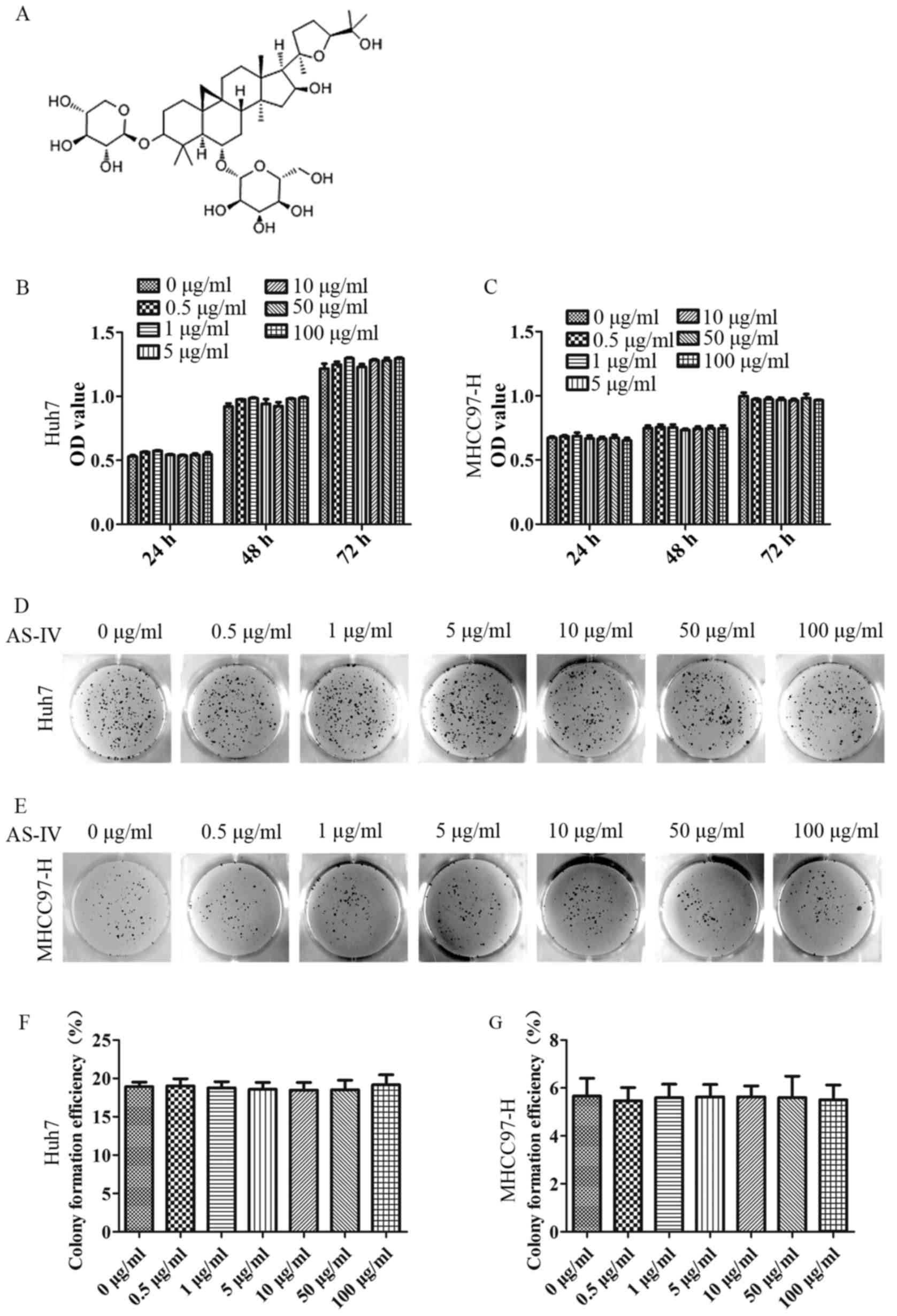
(16,17) (Fig.
1A). It was reported that AS-IV enhanced the expression of
smad7 to suppress the TGF-β1 induced EMT of peritoneal mesothelial
cells (18). Additionally, various
studies also proved that AS-IV was able to decrease the production
of reactive oxygen species (ROS), thus, glycated albumin induced
EMT which was inhibited in renal tubular cells (19). Our previous study also showed that
SYY functioned in changes in EMT-related genes in
oxaliplatin-treated HCC tissues and cell lines (20). In the present study, the aim was to
investigate the effects of AS-IV on the proliferation, invasion and
migration of HCC cells and the underlying mechanisms involved.
Materials and methods
Cell line culture
The human HCC cell line Huh7 with low metastatic
potential was obtained from the Cell Bank of the Chinese Academy of
Sciences (Shanghai, China), and the human HCC cell line MHCC97-H
with high metastatic potential was established at the authors'
institution (Liver Cancer Institute, Fudan University, Shanghai,
China) (21). All the HCC cell
lines were cultured in Dulbecco's modified Eagle's medium (DMEM;
Invitrogen, Carlsbad, CA, USA) containing 10% fetal bovine serum
(FBS) and 100 U/ml penicillin and 50 mg/ml streptomycin. All cells
were incubated at 37̊C with a humidified atmosphere of 5%
CO2.
Reagents and antibodies
In the in vitro study, an AS-IV monomer
(purchased from the National Institutes for Food and Drug Control,
Beijing, China), a lyophilized powder with a purity of 99.99%,
first dissolved in dimethyl sulfoxide (DMSO) and then diluted with
phosphate-buffered saline (PBS) to the required concentration, was
used in the assays. GSK690693 (Selleck Chemicals, Houston, TX,
USA), an Akt-specific inhibitor was first dissolved in DMSO, and
then added to the cell culture medium for the required
concentration. Antibodies used for immunofluorescence and
immunoblotting were as follows: rabbit anti-human monoclonal
E-cadherin, rabbit anti-human monoclonal N-cadherin, rabbit
anti-human monoclonal vimentin, rabbit anti-human monoclonal Slug
(all from Cell Signaling Technology, Beverly, MA, USA), rabbit
anti-human monoclonal α-SMA (Abcam, Cambridge, UK), rabbit
anti-human monoclonal P-AKT and rabbit anti-human monoclonal AKT
(both from Cell Signaling Technology), rabbit anti-human monoclonal
β-catenin (Abcam), mouse anti-human monoclonal GAPDH and mouse
anti-human monoclonal β-tubulin (both from Beyotime, Haimen,
China).
Cell proliferation assays
Huh7 and MHCC97-H cells were incubated with
different concentrations of AS-IV for 72 h, and then plated in
96-well plates and exposed to increasing doses of AS-IV for 24, 48
and 72 h. Cell proliferation assays were performed by the Cell
Counting Kit-8 (CCK-8; Dojindo Laboratories, Kumamoto, Japan). The
optical density was read at a wavelength of 450 nm.
For colony formation assays, we counted one thousand
cells which were added to 6-well plates (Corning Inc., Corning, NY,
USA), and cultured with 1% FBS DMEM with various concentration of
AS-IV. The medium was changed every 4 days. After 14 days, ice-cold
4% paraformaldehyde was used to fix the colonies. The colonies were
stained with crystal violet and the images were captured using a
camera.
Cell migration and invasion
assays
Quantitative cell migration and invasion were
assessed using Transwell assays (Boyden chambers; Corning Inc.).
First, Huh7 and MHCC97-H cells were pretreated with different doses
of AS-IV for 72 h. Due to their different biological
characteristics, such as morphology and mobility, ~5×104
Huh7 cells/well and 3×104 MHCC97-H cells/well in DMEM,
respectively, were added into the upper chamber of each well of
24-well plates containing 8.0-µm pore size membranes, and the lower
chamber was filled with DMEM containing 5% FBS. Cells were allowed
to migrate for 24 h at 37̊C. The crystal violet was used on stained
cells that had reached the underside of the membrane. Finally, 5
random fields were photographed at a magnification of ×100, and the
number of stained cells was counted. Transwell assays to assess the
invasion were similarly performed except that 90 µl diluted
Matrigel (1:9 DMEM; BD Biosciences, San Jose, CA, USA) was added
into each well 3 h before cells were seeded in the upper
chamber.
The wound healing assay was also used to evaluate
cell migration. Briefly, ~5×105 HCC cells/well were
added into 6-well plates. Cells were incubated overnight to produce
a confluent monolayer. A 10-µl pipette tip was used to make a
straight scratch on the monolayer of the cells attached to the
bottom of a Petri dish. The suspended cells were washed 3 times
with PBS. Then, FBS-free DMEM with various concentrations of AS-IV
were added to each well for 24 h. The wound area was photographed
under an inverted microscope at 0 and 24 h.
Immunofluorescence assays
HCC cells were first pretreated with increasing
doses of AS-IV. Then, ~4×104 cells/well were added into
a glass bottom dish. After cells attached to the bottom, 4%
paraformaldehyde was used to fix the cells for 15 min at room
temperature. Then, cells were treated with 0.2% Triton X-100 for 15
min with the purpose of permeabilization and incubated in a
blocking buffer (5% BSA in PBS, pH 7.4) at room temperature for 30
min. Subsequently, the cells were incubated with the respective
primary antibody overnight at 4̊C. The next day, the cells were
incubated with the corresponding FITC-conjugated secondary antibody
(Beyotime) in the dark for 60 min at room temperature, and DAPI
solution was added in the last 10 min during this process. The
cells were observed and photographed under a fluorescence
microscope.
Western blotting
Cells were lysed using ice-cold RIPA buffer (150 mM
NaCl, 50 mM Tris-HCl, pH 8.0, 0.1% SDS, 1% Triton X-100) containing
protease and phosphatase inhibitors. Equal amounts of proteins from
each group were subjected to SDS-PAGE gel and transferred to
polyvinylidene difluoride (PVDF) membranes. The membranes were
blocked with 5% fat-free milk for 1 h, and then incubated with the
respective primary antibody overnight at 4̊C. Afterwards, the
membranes were washed in Tris-buffered saline with Tween-20 (TBST)
3 times and incubated with the corresponding HRP-conjugated
secondary antibody for 1 h at room temperature. Blots were
visualized with an enhanced chemiluminescence (ECL) detection kit
(Millipore, Bedford, MA, USA) and analyzed using Quantity One 1-D
Analysis Software (Bio-Rad, San Francisco, CA, USA).
Statistical analysis
All the experimental data were analyzed using Excel
2016 for Windows (Microsoft, Redmond, WA, USA) and SPSS 19.0 for
Windows (SPSS, Inc., Chicago, IL, USA). An unpaired Student's
t-test was used to compare quantitative variables and a level of
p<0.05 was considered to indicate a statistically significant
result.
Results
AS-IV suppresses HCC cell migration
and invasion, but not proliferation
A series of increasing doses (0–100 µg/ml) of AS-IV
was used to treat MHCC97-H and Huh7 cells in the CCK-8 and plate
clone formation assays, in order to evaluate the effects of AS-IV
on MHCC97-H and Huh7 cell proliferation. In the CCK-8 assay, there
were no significant differences in cell proliferation or apoptosis
between each of the groups at any of the time-points, as shown in
Fig. 1B and C at 72 h. In addition,
in the plate clone formation assay (2 weeks), the results
demonstrated that the average clone formation rate was not
significantly different between each group (Fig. 1D-F). Consequently, we chose 10, 50
and 100 µg/ml of AS-IV for further experiments.
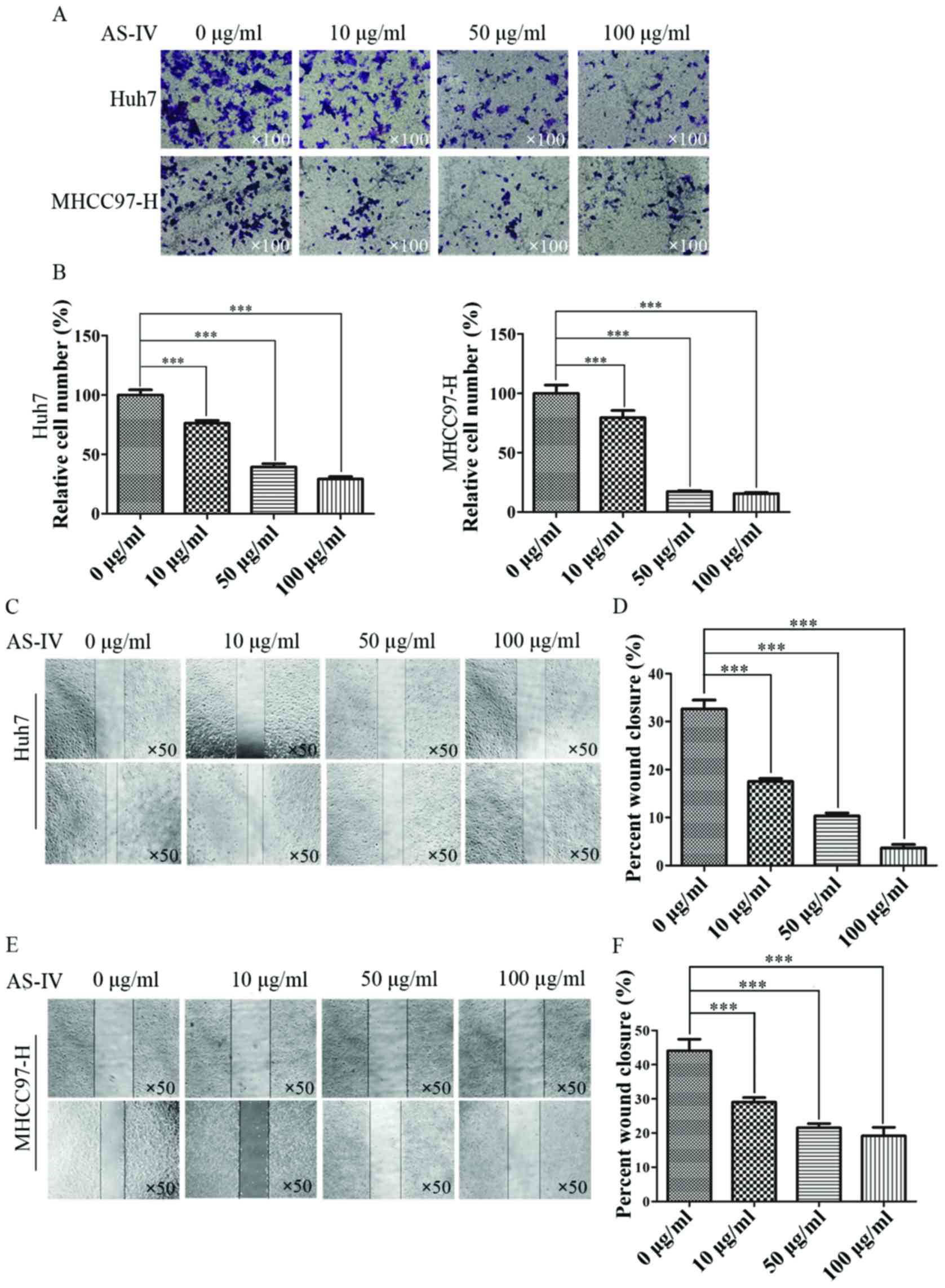
In order to evaluate the effects of AS-IV on HCC
cell migration and invasion, Transwell assays were performed. After
24 h, we stained the cells that had passed through the chamber
membrane and counted the number of cells from each group. Compared
with the control group, the number of migrating cells pretreated
with AS-IV decreased with increasing AS-IV concentration. The
relative percentage of migratory Huh7 cells treated with 10, 50 and
100 µg/ml AS-IV was 57.6±3.4, 33.5±2.2 and 20.7±1.8% compared to
the control group, respectively (Fig.
2A and B). The relative percentage of migratory MHCC97-H cells
treated with 10, 50 and 100 µg/ml AS-IV was 51.3±5.2, 29.7±1.1 and
27.7±0.5% of control group, respectively (Fig. 2A and B).
In the wound healing assay, we found that AS-IV
significantly suppressed the wound healing in the experiments with
both Huh7 and MHCC97-H cells. The closure rates of Huh7 cells
treated with 0, 10, 50 and 100 µg/ml AS-IV were 32.6±1.9, 17.5±0.6,
10.3±0.6 and 3.7±0.7%, respectively (Fig. 2C and D). The closure rates of
MHCC97-H cells treated with 0, 10, 50 and 100 µg/ml AS-IV were
44.1±3.4, 29.1±1.3, 21.5±1.2 and 19.2±2.5%, respectively (Fig. 2E and F).
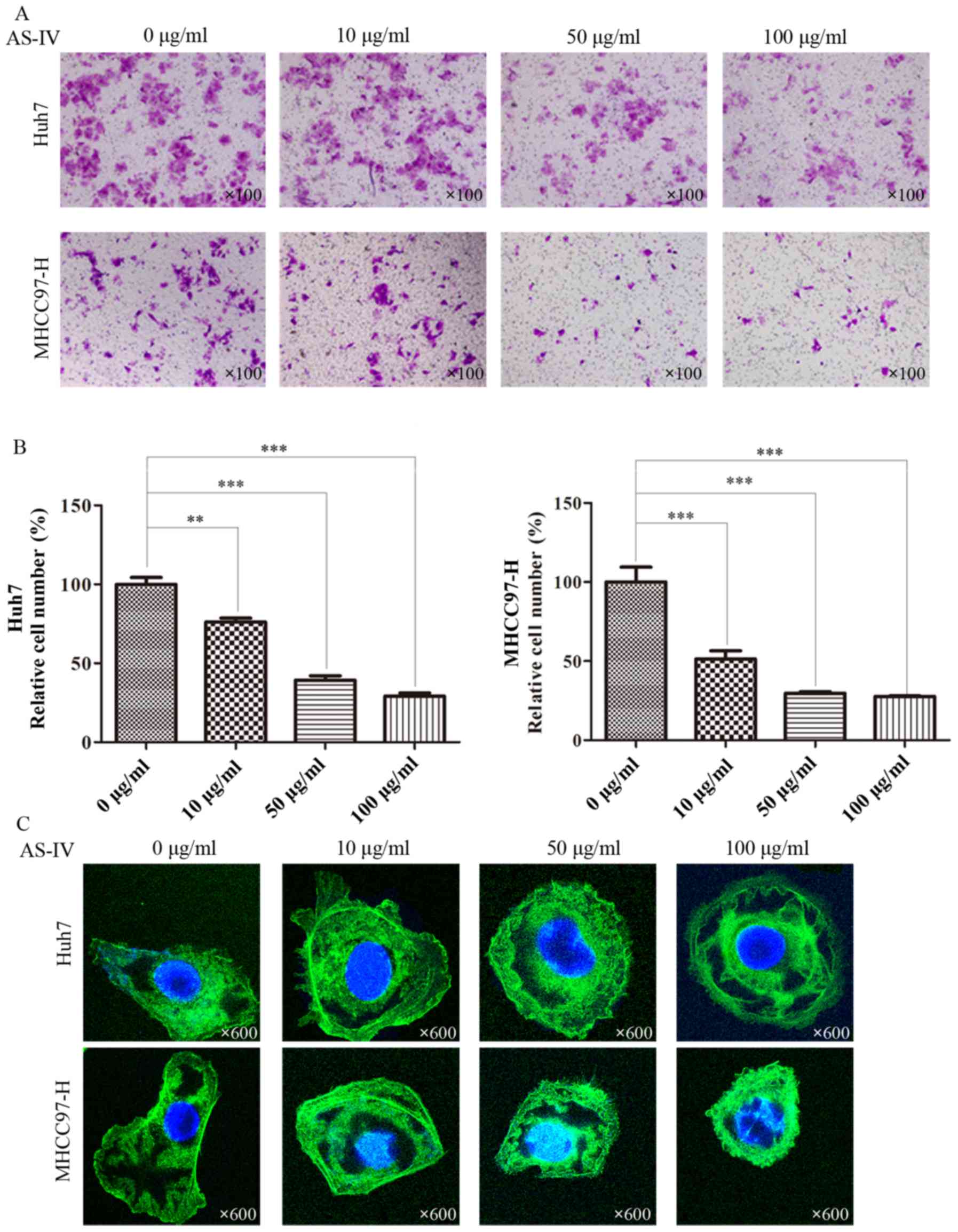
Transwell assays with Matrigel further revealed that
AS-IV clearly restrained the invasive capacity of Huh7 and MHCC97-H
cells as compared with the control group. In the group of Huh7
cells treated with 10, 50 and 100 µg/ml of AS-IV, the relative
percentage of migratory cells was 76.2±2.3, 39.3±2.8 and 29.1±2.0%
compared to the control group (Fig. 3A
and B). In the group of MHCC97-H cells treated with 10, 50 and
100 µg/ml of AS-IV, the relative percentage of migratory cells was
79.5±6.0, 17.8±0.8 and 16.2±1.0% compared to the control group
(Fig. 3A and B).
In order to further demonstrate these results, we
studied AS-IV-induced cytoskeletal and morphological changes in
Huh7 and MHCC97-H cells. The Huh7 and MHCC97-H cells with
spindle-shaped cellular morphology and stretched F-actin fibers
were altered to a cobblestone appearance and shrinkable F-actin
fibers after incubation with AS-IV (Fig. 3C).
AS-IV suppresses EMT in HCC cells
The aforementioned results demonstrated that after
AS-IV treatment, the morphology of HCC cells changed from a spindle
into an oval shaped. In addition, the mobility and invasion of HCC
cells were inhibited compared with the control group. We speculated
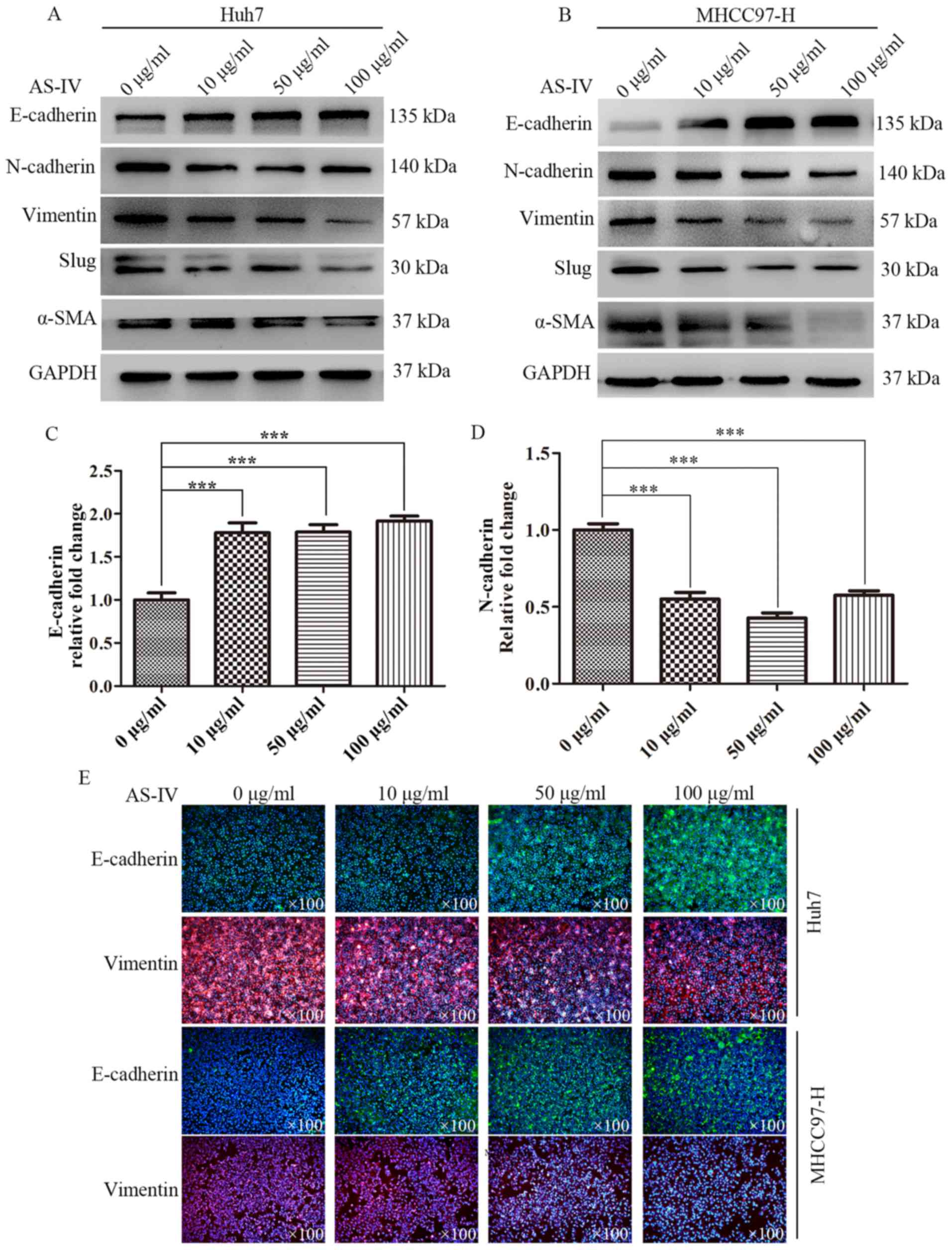
that AS-IV inhibits EMT in HCC cells. Western blotting was used to
further examine the effects of AS-IV on epithelial marker
(E-cadherin) and mesenchymal markers (N-cadherin, vimentin, Slug
and α-SMA). After incubating Huh7 cells with 10, 50 and 100 µg/ml
AS-IV for 48 h, the expression level of E-cadherin significantly
increased to 1.77±0.10-, 1.78±0.07- and 1.92±0.04-fold compared
with the control group (Fig. 4A and
C). Conversely, mesenchymal markers markedly decreased. The
expression level of N-cadherin decreased to 0.55±0.04-, 0.43±0.03-
and 0.57±0.02-fold compared to the control group (Fig. 4A and D). The same phenomena were
observed in the MHCC97-H cells treated with AS-IV; the increased
expression of E-cadherin was accompanied by decreased expression of
mesenchymal cell markers (Fig.
4B).
In order to further validate the aforementioned
results, we performed an immunofluorescence assay to test the
influence of AS-IV on E-cadherin and vimentin in both cell lines.
After incubating both cell lines with AS-IV for 48 h, we found that
AS-IV greatly increased E-cadherin expression and significantly
decreased vimentin expression in a concentration-dependent manner
(Fig. 4E).
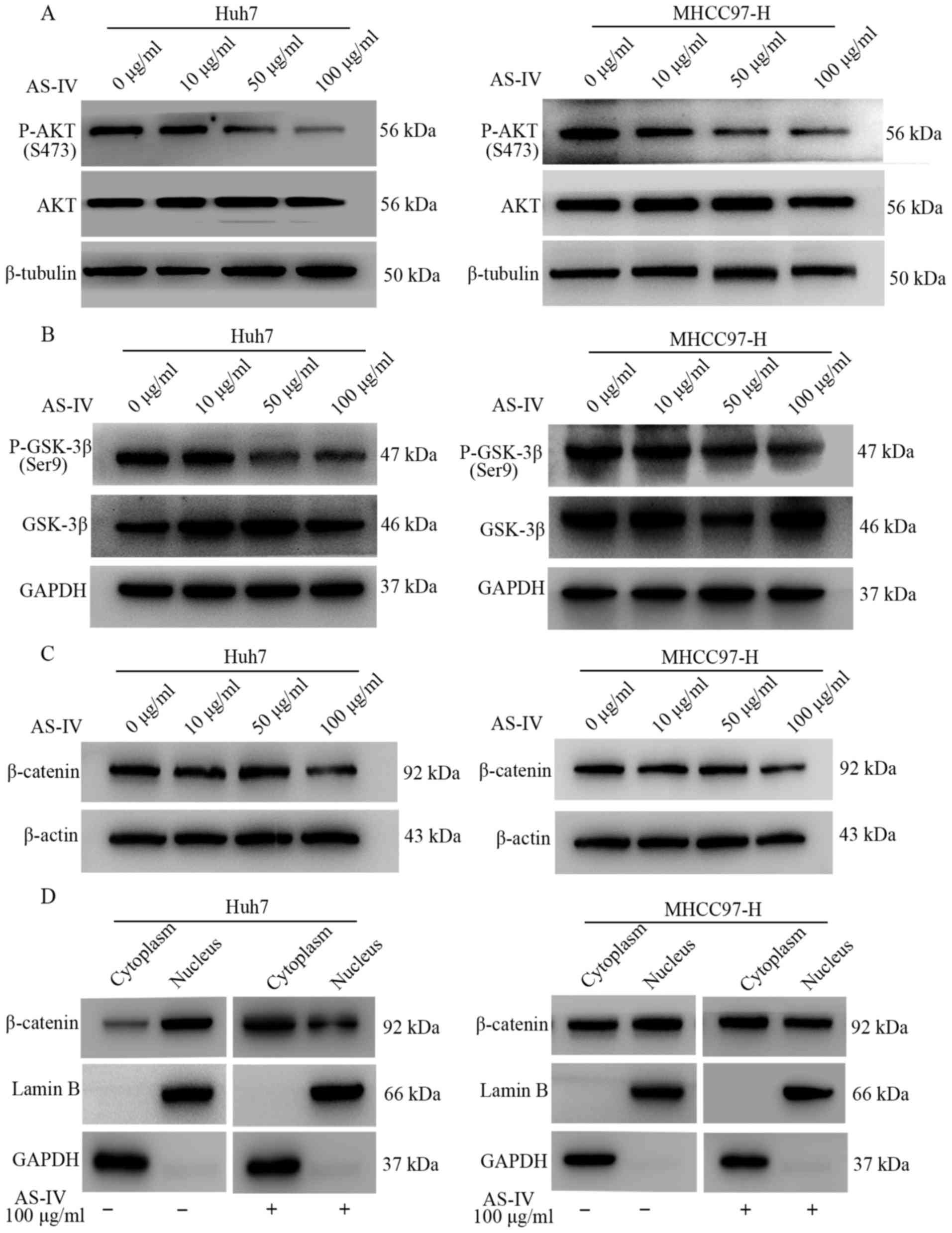
AS-IV inhibits EMT in HCC cells
through the regulation of the Akt/GSK-3β/β-catenin pathway
Various signaling pathways have been proven to play
important roles in the EMT process. It has been confirmed that the
oncogenic serine/threonine kinase AKT regulates EMT in many tumors
(22,23). AKT suppresses transcription of the
E-cadherin gene, resulting in the decrease of E-cadherin protein
expression and loose intercellular junctions (24). In order to verify whether AKT could
be modulated by AS-IV, western blotting was performed. The results
demonstrated that AS-IV attenuated the expression of phosphorylated
AKT (P-AKT), particularly at the dose of 100 µg/ml (Fig. 5A).
A previous study showed that phosphorylated Akt
suppresses the activity of GSK-3β by phosphorylating its Ser9
residues, which is accompanied by the accumulation of β-catenin in
the nucleus and activation of the Wnt pathway. In the present
study, we found that 50 and 100 µg/ml of AS-IV markedly
downregulated the phosphorylation level of GSK-3β in both Huh7 and
MHCC97-H cells, as compared with the control group (Fig. 5B).
Past studies have suggested that once the expression
of E-cadherin is suppressed, the β-catenin/E-cadherin complex at
the cell membrane is also destroyed and a massive amount of
β-catenin enters the nucleus (25,26).
Therefore, the influence of AS-IV on the expression of β-catenin
was investigated. The results showed that 10 and 50 µg/ml of AS-IV
had little influence on β-catenin in both cell lines, but the
concentration of 100 µg/ml of AS-IV significantly suppressed the
expression of the β-catenin protein (Fig. 5C). Next, we investigated the effects
of AS-IV on the location of the β-catenin protein. The results from
western blotting demonstrated that 100 µg/ml of AS-IV inhibited the
accumulation of β-catenin in the nucleus as compared with the
control group in both HCC cell lines (Fig. 5D).
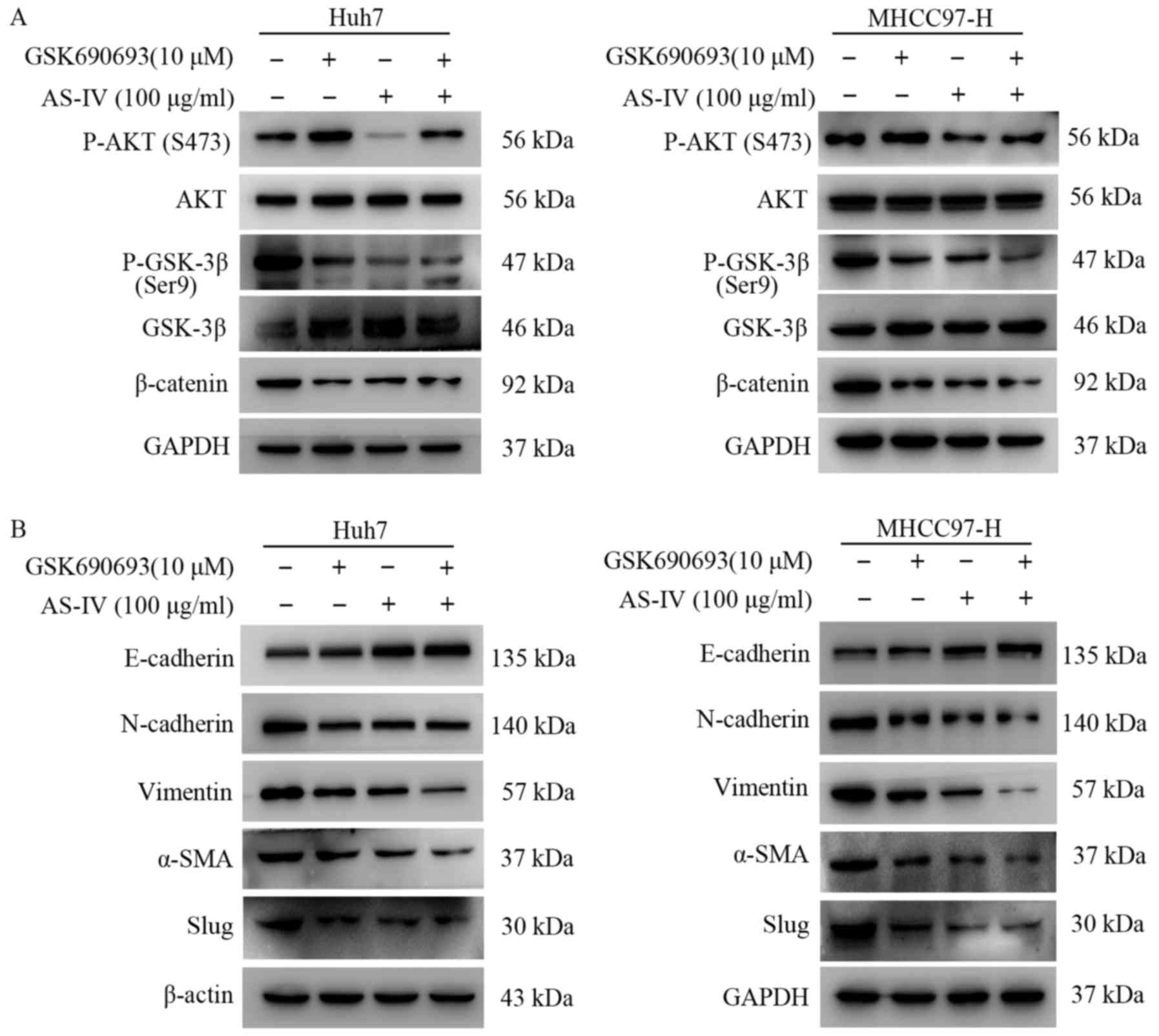
In order to further confirm the relationship between
AS-IV and the Akt/GSK-3β/β-catenin pathway, GSK690693, an AKT
inhibitor, was used to incubate Huh7 and MHCC97-H cells with or
without AS-IV for 24 h. The results demonstrated that GSK690693
increased the phosphorylation level of AKT at the Ser473 site in
both cell lines (Fig. 6A). This was
consistent with the feedback mechanism previously demonstrated
(27,28). In addition, treatment with GSK690693
decreased the phosphorylation level of GSK-3β and the expression
level of β-catenin in the Huh7 and MHCC97-H cells (Fig. 6A). GSK690693 also inhibited the
occurence of EMT in both cell lines (Fig. 6B). In the group using both the AS-IV
and GSK690693, the level of P-AKT at the Ser473 site was decreased,
while, the P-GSK-3β level was further decreased in both cell lines
(Fig. 6A). In addition, the
expression of β-catenin was also inhibited (Fig. 6A). The results of western blotting
also demonstrated that using AS-IV and GSK690693 together increased
the epithelial marker (E-cadherin) and decrease the mesenchymal
markers (N-cadherin, vimentin, Slug and α-SMA) more effectively in
the HCC cells (Fig. 6B).
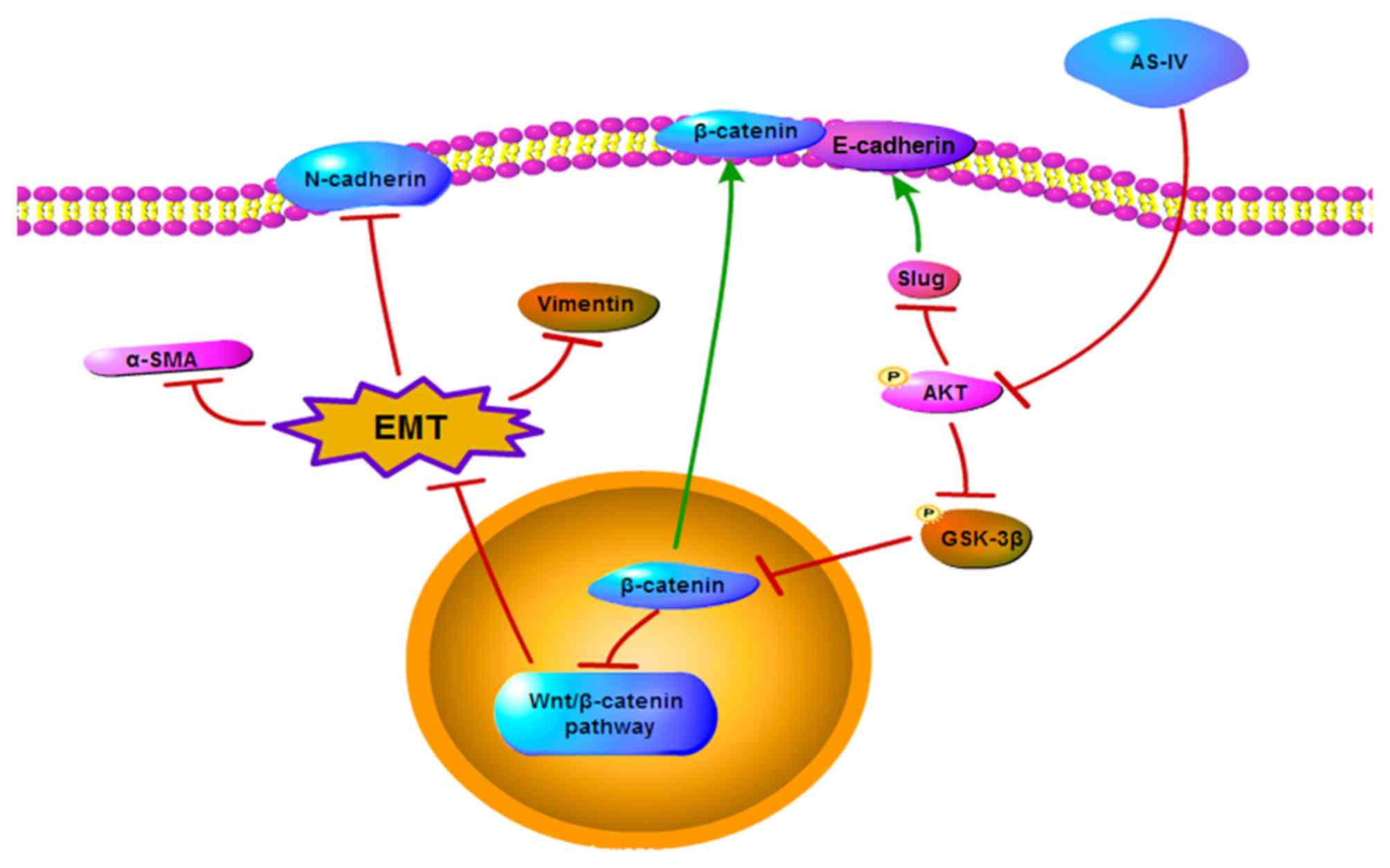
Collectively, these results suggest that AS-IV inhibits the EMT of
HCC through the modulation of the Akt/GSK-3β/β-catenin pathway
(Fig. 7).
Discussion
Invasion and metastasis are the main causes of tumor
relapse, and are regarded as important characteristics of HCC. EMT
plays an important role in the process of tumor invasion and
metastasis, particularly in the early stage (6). Traditional Chinese medicine contains a
variety of active ingredients. Many types of ingredients play a
role in the inhibition of tumor metastasis (29). In the present study, we investigated
the effects of AS-IV on HCC cell proliferation and metastasis in
vitro. Our results demonstrated that AS-IV had little effect on
the proliferation of HCC cells in vitro. However, we found
that AS-IV inhibited the invasion and metastasis of HCC cells in a
concentration-dependent manner in vitro. Moreover, AS-IV
significantly enhanced the expression of E-cadherin and suppressed
the expression of mesenchymal markers in HCC cells, along with
morphological changes in Huh7 and MHCC97-H cells after incubation
with AS-IV, indicating that AS-IV inhibits the EMT of HCC
cells.
EMT is a process involved in the conversion of early
tumors to aggressive malignancies. Tumor cells undergoing EMT lose
their epithelioid phenotype and gain more mesenchymal cell-like
features and then intercellular adhesion is reduced and cells
acquire invasive ability. Thereby, tumor cells can penetrate the
microvascular basic membrane more easily and be transported through
the circulation to distant sites (6,30,31).
Previous investigations have proven that some constituents of
Chinese medicine function as suppressors of EMT (32–34).
Our research team also confirmed that SYY inhibited the occurrence
of EMT in HCC (20). However, it
was not clear which ingredients of SYY played this role. In the
present study, we demonstrated that after incubation with AS-IV for
48 h, the expression level of the E-cadherin protein was
significantly increased and the mesenchymal markers (N-cadherin,
vimentin, α-SMA and Slug) were markedly decreased in both the Huh7
and MHCC97-H cell lines. E-cadherin acts as a tumor suppressor in
many types of tumors, since its gene is always inactive in a
variety of tumors and activation of the transcription of its gene
is sufficient to limit the invasion and metastasis of tumor cells
(35–37). The results revealed that AS-IV not
only increased the protein level of E-cadherin but also promoted
the accumulation of the E-cadherin protein on the cell membrane,
thus the cell-cell junctions became more compact and tumor cells
were less able to spread. Additionally, AS-IV also downregulated
the expression of Slug, which acts as a suppressor of E-cadherin
transcription (38,39), thus, AS-IV promoted E-cadherin
transcription to some extent.
In the present study, we also revealed the
underlying mechanism by which AS-IV suppresses EMT in HCC cells.
AKT is commonly regarded as a cancer gene and is overexpressed in
many types of solid tumors (40–42).
It participates in many basic cellular processes, and it has a
close relationship with EMT in cancer (23). The activation of AKT leads to the
loss of tumor cell-cell junctions, disruption of tumor cell
polarity and morphological changes of tumor cells, enhancing tumor
cell motility (43,44). Furthermore, AKT was confirmed to
suppress the transcription of the E-cadherin gene by binding to Ets
sites and palindromic E-boxes (45). Our results showed that the
phosphorylation level of the AKT protein gradually decreased with
the increase in the concentration of AS-IV. This was consistent
with the inhibition of tumor motility and the increase in the
E-cadherin protein. Moreover, the Akt inhibitor GSK690693 was used
to further investigate the relationship between AS-IV and AKT. The
results from western blotting showed that GSK690693 significantly
upregulated the phosphorylation of the AKT protein at the S473
site, as previously described (27,28).
However, after treating the cells with AS-IV as well, the
phosphorylation of AKT at the S473 site was downregulated. This
further illustrated that AS-IV restricted the activity of AKT.
Wnt/β-catenin is a critical pathway for EMT.
Accumulating evidence suggests that the Wnt/β-catenin pathway
induces EMT in different tumors (46,47).
Blocking Wnt/β-catenin-induced EMT suppresses cancer metastasis and
progression (47,48). Previous studies demonstrated that
β-catenin forms a complex with E-cadherin on the cell membrane and
that downregulation of E-cadherin leads to the release of β-catenin
and the translocation of β-catenin to the nucleus, fully activating
the Wnt/β-catenin pathway (25,26).
Furthermore, β-catenin is also modulated by GSK-3β. It has been
proven that GSK-3β induces the phosphorylation of β-catenin and
forces it to undergo ubiquitin/proteasome-mediated degradation
(45). GSK-3β is also a proven
target gene of AKT and phosphorylated Akt is known to induce an
inactive form of GSK-3β by phosphorylating its Ser9 residues
(49). This constitutes an
important event in the stabilization of β-catenin and its
subsequent translocation to the nucleus. Our results showed that
AS-IV and GSK690693 markedly decreased the phosphorylation of Ser9
residues of GSK-3β respectively, and it was more effective by
combining them together, which was consistent with previous studies
(49). This indicated that AS-IV
relieved the inhibition of GSK-3β by downregulating the
phosphorylation of Akt, and may have a regulatory role in the
expression and location of β-catenin. Western blotting was
performed to examine the effect of AS-IV on the expression and
location of the β-catenin protein. We found that both 100 µg/ml of
AS-IV and 10 µM of GSK690693 significantly inhibited β-catenin
expression. Additionally, the accumulation of β-catenin in the
nucleus was also decreased after treatment with 100 µg/ml of AS-IV.
In addition, the results from western blotting demonstrated that
the treatment of Huh7 and MHCC97-H cells with both AS-IV and
GSK690693 at the same time, could inhibit the process of EMT more
significantly. These results confirm that Akt/GSK-3β/β-catenin is
an objective pathway by which AS-IV suppresses EMT in HCC
cells.
However, this study still has some deficiencies. Our
results demonstrated that 10 and 50 µg/ml of AS-IV have little
effect on β-catenin and this may be because the dosage is not
enough, or there may exist other signaling pathways that modulate
EMT that could be mediated by AS-IV. In addition, our preliminary
investigation did not explore the effects of AS-IV on the tumor
microenvironment in order to achieve the inhibition of tumor
metastasis and invasion.
In conclusion, the present study demonstrated that
AS-IV suppressed the invasion and metastasis of HCC cells. In
addition, the in vitro assays confirmed that the inhibitory
effect of AS-IV on EMT was achieved by targeting the
Akt/GSK-3β/β-catenin signaling pathway. AS-IV, is an important
component of SYY and our results highlighted its potential as an
adjuvant therapy for the treatment of HCC.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (nos. 81272565 and
81172275).
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhou XD, Tang ZY, Yang BH, Lin ZY, Ma ZC,
Ye SL, Wu ZQ, Fan J, Qin LX and Zheng BH: Experience of 1000
patients who underwent hepatectomy for small hepatocellular
carcinoma. Cancer. 91:1479–1486. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Raphael S Waly, Yangde Z and Yuxiang C:
Hepatocellular carcinoma: Focus on different aspects of management.
ISRN Oncol. 2012:421673. 2012.PubMed/NCBI
|
|
5
|
Chaffer CL and Weinberg RA: A perspective
on cancer cell metastasis. Science. 331:1559–1564. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Puisieux A, Brabletz T and Caramel J:
Oncogenic roles of EMT-inducing transcription factors. Nat Cell
Biol. 16:488–494. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Guerram M, Jiang ZZ, Yousef BA, Hamdi AM,
Hassan HM, Yuan ZQ, Luo HW, Zhu X and Zhang LY: The potential
utility of acetyltanshinone IIA in the treatment of
HER2-overexpressed breast cancer: Induction of cancer cell death by
targeting apoptotic and metabolic signaling pathways. Oncotarget.
6:21865–21877. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lam W, Jiang Z, Guan F, Huang X, Hu R,
Wang J, Bussom S, Liu SH, Zhao H, Yen Y, et al: PHY906(KD018), an
adjuvant based on a 1800-year-old Chinese medicine, enhanced the
anti-tumor activity of Sorafenib by changing the tumor
microenvironment. Sci Rep. 5:93842015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Huang XY, Wang L, Huang ZL, Zheng Q, Li QS
and Tang ZY: Herbal extract ‘Songyou Yin’ inhibits tumor growth and
prolongs survival in nude mice bearing human hepatocellular
carcinoma xenograft with high metastatic potential. J Cancer Res
Clin Oncol. 135:1245–1255. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jia QA, Ren ZG, Bu Y, Wang ZM, Zhang QB,
Liang L, Jiang XM, Zhang QB and Tang ZY: Herbal compound ‘Songyou
Yin’ renders hepatocellular carcinoma sensitive to oxaliplatin
through inhibition of stemness. Evid Based Complement Alternat Med.
2012:9086012012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li X, Qu L, Dong Y, Han L, Liu E, Fang S,
Zhang Y and Wang T: A review of recent research progress on the
astragalus genus. Molecules. 19:18850–18880. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Boye A, Wu C, Jiang Y, Wang J, Wu J, Yang
X and Yang Y: Compound Astragalus and Salvia miltiorrhiza extracts
modulate MAPK-regulated TGF-β/Smad signaling in hepatocellular
carcinoma by multi-target mechanism. J Ethnopharmacol. 169:219–228.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang Y, Auyeung KK, Zhang X and Ko JK:
Astragalus saponins modulates colon cancer development by
regulating calpain-mediated glucose-regulated protein expression.
BMC Complement Altern Med. 14:401. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Auyeung KK, Han QB and Ko JK: Astragalus
membranaceus: A Review of its protection against inflammation and
gastrointestinal cancers. Am J Chin Med. 44:1–22. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li W and Fitzloff JF: Determination of
astragaloside IV in Radix astragali (Astragalus membranaceus var.
monghulicus) using high-performance liquid chromatography with
evaporative light-scattering detection. J Chromatogr Sci.
39:459–462. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lai PK, Chan JY, Cheng L, Lau CP, Han SQ,
Leung PC, Fung KP and Lau CB: Isolation of anti-inflammatory
fractions and compounds from the root of Astragalus membranaceus.
Phytother Res. 27:581–587. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhao M, Zhao J, He G, Sun X, Huang X and
Hao L: Effects of astragaloside IV on action potentials and ionic
currents in guinea-pig ventricular myocytes. Biol Pharm Bull.
36:515–521. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang L, Li Z, He W, Xu L, Wang J, Shi J
and Sheng M: Effects of astragaloside IV against the TGF-β1-induced
epithelial-to-mesenchymal transition in peritoneal mesothelial
cells by promoting Smad 7 expression. Cell Physiol Biochem.
37:43–54. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Qi W, Niu J, Qin Q, Qiao Z and Gu Y:
Astragaloside IV attenuates glycated albumin-induced
epithelial-to-mesenchymal transition by inhibiting oxidative stress
in renal proximal tubular cells. Cell Stress Chaperones.
19:105–114. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xiong W, Ren ZG, Qiu SJ, Sun HC, Wang L,
Liu BB, Li QS, Zhang W, Zhu XD, Liu L, et al: Residual
hepatocellular carcinoma after oxaliplatin treatment has increased
metastatic potential in a nude mouse model and is attenuated by
Songyou Yin. BMC Cancer. 10:219. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tian J, Tang ZY, Ye SL, Liu YK, Lin ZY,
Chen J and Xue Q: New human hepatocellular carcinoma (HCC) cell
line with highly metastatic potential (MHCC97) and its expressions
of the factors associated with metastasis. Br J Cancer. 81:814–821.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xue G, Restuccia DF, Lan Q, Hynx D,
Dirnhofer S, Hess D, Rüegg C and Hemmings BA: Akt/PKB-mediated
phosphorylation of Twist1 promotes tumor metastasis via mediating
cross-talk between PI3K/Akt and TGF-β signaling axes. Cancer
Discov. 2:248–259. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hsu CY, Lin CH, Jan YH, Su CY, Yao YC,
Cheng HC, Hsu TI, Wang PS, Su WP, Yang CJ, et al:
Huntingtin-interacting protein-1 is an early-stage prognostic
biomarker of lung adenocarcinoma and suppresses metastasis via
Akt-mediated epithelial-mesenchymal transition. Am J Respir Crit
Care Med. 193:869–880. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lamouille S, Xu J and Derynck R: Molecular
mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell
Biol. 15:178–196. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen HN, Yuan K, Xie N, Wang K, Huang Z,
Chen Y, Dou Q, Wu M, Nice EC, Zhou ZG, et al: PDLIM1 stabilizes the
E-cadherin/β-catenin complex to prevent epithelial-mesenchymal
transition and metastatic potential of colorectal cancer cells.
Cancer Res. 76:1122–1134. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Murata-Kamiya N, Kurashima Y, Teishikata
Y, Yamahashi Y, Saito Y, Higashi H, Aburatani H, Akiyama T, Peek RM
Jr, Azuma T, et al: Helicobacter pylori CagA interacts with
E-cadherin and deregulates the β-catenin signal that promotes
intestinal transdifferentiation in gastric epithelial cells.
Oncogene. 26:4617–4626. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rhodes N, Heerding DA, Duckett DR,
Eberwein DJ, Knick VB, Lansing TJ, McConnell RT, Gilmer TM, Zhang
SY, Robell K, et al: Characterization of an Akt kinase inhibitor
with potent pharmacodynamic and antitumor activity. Cancer Res.
68:2366–2374. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Levy DS, Kahana JA and Kumar R: AKT
inhibitor, GSK690693, induces growth inhibition and apoptosis in
acute lymphoblastic leukemia cell lines. Blood. 113:1723–1729.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang J, Wang P, Ouyang H, Yin J, Liu A,
Ma C and Liu L: Targeting cancer-related inflammation: Chinese
herbal medicine inhibits epithelial-to-mesenchymal transition in
pancreatic cancer. PLoS One. 8:e703342013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Qi X, Zhang L and Lu X: New insights into
the epithelial-to-mesenchymal transition in cancer. Trends
Pharmacol Sci. 37:246–248. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lai YJ and Tai CJ, Wang CW, Choong CY, Lee
BH, Shi YC and Tai CJ: Anti-cancer activity of Solanum nigrum
(AESN) through suppression of mitochondrial function and
epithelial-mesenchymal transition (EMT) in breast cancer cells.
Molecules. 21:pii: E5532016. View Article : Google Scholar
|
|
33
|
Lin W, Zhuang Q, Zheng L, Cao Z, Shen A,
Li Q, Fu C, Feng J and Peng J: Pien Tze Huang inhibits liver
metastasis by targeting TGF-β signaling in an orthotopic model of
colorectal cancer. Oncol Rep. 33:1922–1928. 2015.PubMed/NCBI
|
|
34
|
Lin X, Yi Z, Diao J, Shao M, Zhao L, Cai
H, Fan Q, Yao X and Sun X: ShaoYao decoction ameliorates
colitis-associated colorectal cancer by downregulating
proinflammatory cytokines and promoting epithelial-mesenchymal
transition. J Transl Med. 12:105. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li Y, Chen CQ, He YL, Cai SR, Yang DJ, He
WL, Xu JB and Zan WH: Abnormal expression of E-cadherin in tumor
cells is associated with poor prognosis of gastric carcinoma. J
Surg Oncol. 106:304–310. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Siu MK, Wong ES, Kong DS, Chan HY, Jiang
L, Wong OG, Lam EW, Chan KK, Ngan HY, Le XF, et al: Stem cell
transcription factor NANOG controls cell migration and invasion via
dysregulation of E-cadherin and FoxJ1 and contributes to adverse
clinical outcome in ovarian cancers. Oncogene. 32:3500–3509. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Qualtrough D, Rees P, Speight B, Williams
AC and Paraskeva C: The Hedgehog inhibitor cyclopamine reduces
β-catenin-Tcf transcriptional activity, induces E-cadherin
expression, and reduces invasion in colorectal cancer cells.
Cancers. 7:1885–1899. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang SP, Wang WL, Chang YL, Wu CT, Chao
YC, Kao SH, Yuan A, Lin CW, Yang SC, Chan WK, et al: p53 controls
cancer cell invasion by inducing the MDM2-mediated degradation of
Slug. Nat Cell Biol. 11:694–704. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Fernando RI, Litzinger M, Trono P,
Hamilton DH, Schlom J and Palena C: The T-box transcription factor
Brachyury promotes epithelial-mesenchymal transition in human tumor
cells. J Clin Invest. 120:533–544. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lee JK, Phillips JW, Smith BA, Park JW,
Stoyanova T, McCaffrey EF, Baertsch R, Sokolov A, Meyerowitz JG,
Mathis C, et al: N-Myc drives neuroendocrine prostate cancer
initiated from human prostate epithelial cells. Cancer Cell.
29:536–547. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Toker A and Rameh L: PIPPing on AKT1: How
many phosphatases does it take to turn off PI3K? Cancer Cell.
28:143–145. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Mayer IA and Arteaga CL: The PI3K/AKT
pathway as a target for cancer treatment. Annu Rev Med. 67:11–28.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Attoub S, Arafat K, Hammadi NK, Mester J
and Gaben AM: Akt2 knock-down reveals its contribution to human
lung cancer cell proliferation, growth, motility, invasion and
endothelial cell tube formation. Sci Rep. 5:127592015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhang Y, Liu S, Wang L, Wu Y, Hao J, Wang
Z, Lu W, Wang XA, Zhang F, Cao Y, et al: A novel PI3K/AKT signaling
axis mediates Nectin-4-induced gallbladder cancer cell
proliferation, metastasis and tumor growth. Cancer Lett.
375:179–189. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Larue L and Bellacosa A:
Epithelial-mesenchymal transition in development and cancer: Role
of phosphatidylinositol 3 kinase/AKT pathways. Oncogene.
24:7443–7454. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Gu Y, Wang Q, Guo K, Qin W, Liao W, Wang
S, Ding Y and Lin J: TUSC3 promotes colorectal cancer progression
and epithelial-mesenchymal transition (EMT) through WNT/β-catenin
and MAPK signalling. J Pathol. 239:60–71. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lee SC, Kim OH, Lee SK and Kim SJ: IWR-1
inhibits epithelial-mesenchymal transition of colorectal cancer
cells through suppressing Wnt/β-catenin signaling as well as
survivin expression. Oncotarget. 6:27146–27159. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Bernaudo S, Salem M, Qi X, Zhou W, Zhang
C, Yang W, Rosman D, Deng Z, Ye G, Yang B, et al: Cyclin G2
inhibits epithelial-to-mesenchymal transition by disrupting
Wnt/β-catenin signaling. Oncogene. 35:4816–4827. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Soutto M, Peng D, Katsha A, Chen Z,
Piazuelo MB, Washington MK, Belkhiri A, Correa P and El-Rifai W:
Activation of β-catenin signalling by TFF1 loss promotes cell
proliferation and gastric tumorigenesis. Gut. 64:1028–1039. 2015.
View Article : Google Scholar : PubMed/NCBI
|