Introduction
Glioma represents one of the most common brain
tumors causing severe mortality every year worldwide (1,2). Due
to the rapid growth, angiogenesis and metastasis of glioma cells,
it is extremely difficult to cure this disease (3). Despite multiple advanced treatments
over the past few decades, glioma remains an intractable disease
with a poor prognosis (2,4). Therefore, the urgent development of
new therapies for glioma is essential. However, the molecular
heterogeneity and the unclear pathogenesis of glioma still hamper
the development of new treatments for glioma.
In recent years, microRNAs (miRNAs) have drawn
considerable attention as potential and promising tools for cancer
diagnosis and treatments (5,6).
miRNAs can regulate target gene expression by interacting with the
3′-untranslated region (3-UTR) of mRNAs to inhibit translation or
induce mRNA degradation (7,8). Therefore, miRNAs are able to modulate
cancer cell proliferation, apoptosis, differentiation, migration
and invasion by targeting oncogenes or tumor suppressors (9). A growing body of evidence suggests
that various miRNAs which are aberrantly expressed in glioma
participate in glioma initiation and progression as well as
chemoresistance and radioresistance (10,11),
highlighting their potential as novel agents for glioma therapies.
However, their regulatory functions in glioma remain largely
unknown.
Twist1 is a basic-helix-loop-helix family
transcription factor and plays an important role in the development
of many pathological diseases (12). A prominent function of Twist1 is the
regulation of tumor metastasis by affecting epithelial-mesenchymal
transition (EMT) (13). EMT is an
essential process for cancer invasion and metastasis (14). Twist1 activation is sufficient to
promote EMT and the dissemination of tumor cells, and Twist1
overexpression is associated with more invasive cancer types and a
poor prognosis (15,16). The hypoxic condition can induce the
EMT process of tumor cells through activation of Twist1 (17). Overexpression of Twist1 is
frequently observed in a variety of types of cancers including
hepatocellular carcinoma, prostate, breast, gastric and lung
cancer, and plays an important role in regulating cancer
initiation, progression and metastasis involving multiple
regulatory pathways (12). High
expression of Twist1 has been detected in a significant proportion
of human glioma-derived cell lines and human glioma tissues
(18) and was found to predict poor
survival for glioma patients (19).
Twist1 overexpression was also found to promote cell invasion while
suppression of Twist1 was found to inhibit the cell invasion of
glioma cells (18,20). Therefore, targeting Twist1 may have
promising and potential therapeutic value as a glioma
treatment.
Emerging evidence indicates that microRNA-361-5p
(miR-361-5p) functions as a tumor suppressor in several types of
cancers, such as hepatocellular carcinoma (21), prostate (22), gastric and colorectal cancer
(23). However, the functional
significance of miR-361-5p in glioma has not been investigated. In
the present study, we aimed to investigate the biological functions
of miR-361-5p in regulating glioma progression and the underlying
molecular mechanism. We found that miR-361-5p expression was
significantly lower in glioma tissues and cell lines compared to
that obwerved in normal brain tissues. Overexpression of miR-361-5p
inhibited glioma cell migration, invasion and EMT. In addition,
bioinformatic analysis indicated that Twist1 was a direct target of
miR-361-5p in glioma cells. Our data suggest that miR-361-5p
regulates glioma cell EMT through targeting Twist1. Our findings
provide a better understanding of the mechanism of glioma
development and suggest that miR-361-5p may serve as an attractive
molecular target for the treatment of glioma.
Materials and methods
Tissue specimens
A total of 30 glioma tissue samples were obtained
from the Department of Neurosurgery, The Second Affiliated Hospital
of Xi'an Jiaotong University (Xi'an, China). The specimens were
collected during tumor resection surgery prior to any chemotherapy
or radiotherapies. The resected tumor samples were immediately
snap-frozen in liquid nitrogen and stored at −80°C. Glioma tissue
samples were classified according to the World Health Organization
(WHO) standards. Gliomas (12 of the 30) were classified as
low-grade (5 WHO I and 7 WHO II) and 18 were classified as
high-grade (8 WHO III and 10 WHO IV). Ten samples of normal brain
tissues were obtained from internal decompression patients
undergoing surgical operation at The Second Affiliated Hospital of
Xi'an Jiaotong University. Written informed consent was obtained
from all patients. The present study was approved by the
Institutional Human Experiment and Ethics Committee of The Second
Affiliated Hospital of Xi'an Jiaotong University and was performed
in accordance with the Helsinki Declaration.
Cell cultures
Human glioma cell lines U87, U251, SHG44 and A172,
and normal human astrocytes were purchased from the Type Culture
Collection of the Chinese Academy of Sciences (Shanghai, China).
All cells were routinely cultured in Dulbecco's modified Eagle's
medium (DMEM) plus 10% fetal bovine serum (FBS) (both from Gibco,
Rockville, MD, USA) and 1% penicillin/streptomycin mixture (Sigma,
St. Louis, MO, USA) at 37°C in a humidified incubator containing 5%
CO2.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted using TRIzol reagent
(Invitrogen, Carlsbad, CA, USA). For detection of miR-361-5p,
complementary DNA was synthesized by TaqMan MicroRNA Reverse
Transcription kit (Applied Biosystems, Carlsbad, CA, USA). To
detect Twist1, cDNA was synthesized by M-MLV reverse transcriptase
(Takara, Dalian, China) as per the manufacturer's instructions. PCR
amplification was performed by SYBR-Green Master Mix kit (Bio-Rad,
Hercules, CA, USA) on an Applied Biosystems AB7500 Real-Time PCR
system (Applied Biosystems) with specific primers: miR-361-5p
forward, 5′-ATAAAGTGCTGACAGTGCAGATAGTG-3′ and reverse,
5′-TCAAGTACCCACAGTGCGGT-3′; U6 forward,
5′-CGCTTCGGCAGCACATATACTAA-3′ and reverse,
5′-TATGGAACGCTTCACGAATTTGC-3′; Twist1 forward,
5′-CCAGGTACATCGACTTCCTCTA-3′ and reverse,
5′-CCATCCTCCAGACCGAGAA-3′; and GAPDH forward,
5′-CCATGTTCGTCATGGGTGTG-3′ and reverse,
5′-GGTGCTAAGCAGTTGGTGGTG-3′. U6 and GAPDH were used as internal
normalized references. Relative miRNA or mRNA expression was
calculated using the 2−ΔΔCt method, normalized against
U6 or GAPDH and then compared with the control group.
Transfection of miRNA
miR-361-5p mimics, a miR-361-5p inhibitor and a
scrambled miRNA control were purchased from GenePharma (Shanghai,
China) and transfected into cells using Lipofectamine 2000
(Invitrogen) at a final concentration of 50 nM according to the
manufacturer's instructions. After 48 h of transfection, the
transfection efficacy was determined by RT-qPCR analysis.
Migration and invasion assays
For detection of cell migration, 1×105
cells transfected with miR-361-5p mimics or miR-361 inhibitor were
resuspended in 500 µl of medium without FBS and placed in the upper
chambers of the Transwell (Costar, Cambridge, MA, USA). The lower
chambers were filled with medium containing 10% FBS as a
chemoattractant. For detection of cell invasion, the upper chambers
were coated with Matrigel (BD Biosciences, San Jose, CA, USA).
After 24 h at 37°C, the non-migrating or non-invading cells on the
top well were gently scraped. The cells on the lower surface of the
membrane were fixed with 70% ethanol and stained with 0.1% violet
(Sigma). The cells were observed and counted under a microscope
(Olympus, Tokyo, Japan).
Western blot analysis
Cells were lysed with a cell lysis buffer and the
protein concentration was detected using a BCA protein kit
(Beyotime, Haimen, China). A total of 50 µg of protein was
separated by SDS-PAGE and electro-transferred to a polyvinylidene
fluoride (PVDF) membrane (Bio-Rad). The membrane was then blocked
using 5% non-fat milk and incubated in the primary antibody
(anti-E-cadherin, anti-N-cadherin, anti-vimentin, anti-Twist1,
anti-Bmi-1 and anti-GAPDH; purchased from Santa Cruz Biotechnology,
Santa Cruz, CA, USA) at 4°C overnight. Thereafter, the membrane was
washed and incubated with horseradish peroxidase-conjugated
secondary antibodies (Bosis, Beijing, China) for 1 h at 37°C. The
protein bands were visualized using an ECL Western blotting kit
(Pierce, Rockford, IL, USA) according to the manufacturer's
instructions. Densitometric analysis of the protein bands was
performed using Image-Pro Plus 6.0 software (Media Cybernetics,
Inc., Rockville, MD, USA). The values were normalized against GAPDH
and were then compared to the control group.
Dual-luciferase reporter assay
A Twist1 cDNA fragment harboring seed-matched
sequences with or without a mutation was inserted into the pmirGLO
Dual-Luciferase vector (Promega, Madison, WI, USA). The pmirGLO
vector was then co-transfected with miR-361-5p mimics or miR-361
inhibitor into glioma cells by Lipofectamine 2000 and incubated for
48 h. Then, the relative luciferase activity was analyzed by a
Dual-Luciferase reporter assay system kit (Promega).
Rescue assay
Full length Twist1 cDNA without the 3′-UTR region
was cloned into the pcDNA3.0 vector (Invitrogen). The pcDNA/Twist1
vector was co-transfected with miR-361-5p mimics into glioma cells
using Lipofectamine 2000. After 48 h, TWIST1 expression was
detected by RT-qPCR and western blot analysis.
Statistical analysis
Data are expressed as means ± standard deviation.
Statistical analyses were performed using SPSS version 11.5 (SPSS,
Inc., Chicago, IL, USA). Differences were calculated by Student's
t-test or one-way analysis of variance followed by Bonferroni post
hoc test. At a p-value <0.05, the difference was considered to
be statistically significant.
Results
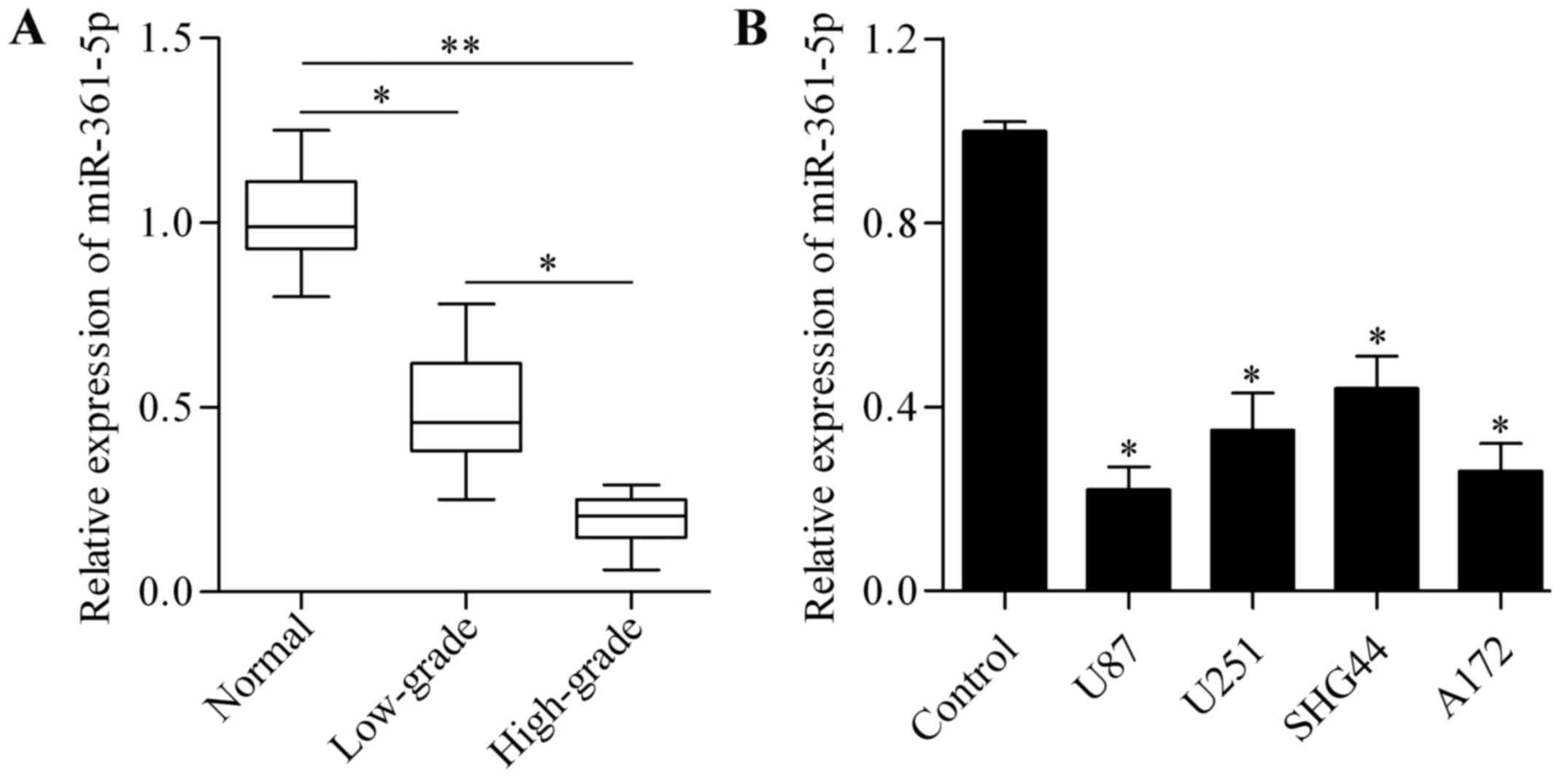
miR-361-5p is reduced in human glioma
tissues and cell lines
To investigate the potential biological role of
miR-361-5p in glioma, we first detected the expression of
miR-361-5p in glioma tissues and cell lines by RT-qPCR analysis.
The results showed that the expression of miR-361-5p was
significantly downregulated in glioma tissues and miR-361-5p
expression was significantly lower in high-grade than low-grade
glioma tissues (Fig. 1A).
miR-361-5p was also markedly lower in the U87, U251, SHG44 and A172
glioma cell lines as compared with the level in the normal human
astrocytes (Fig. 1B). These results
indicate that miR-361-5p may play an important role in glioma.
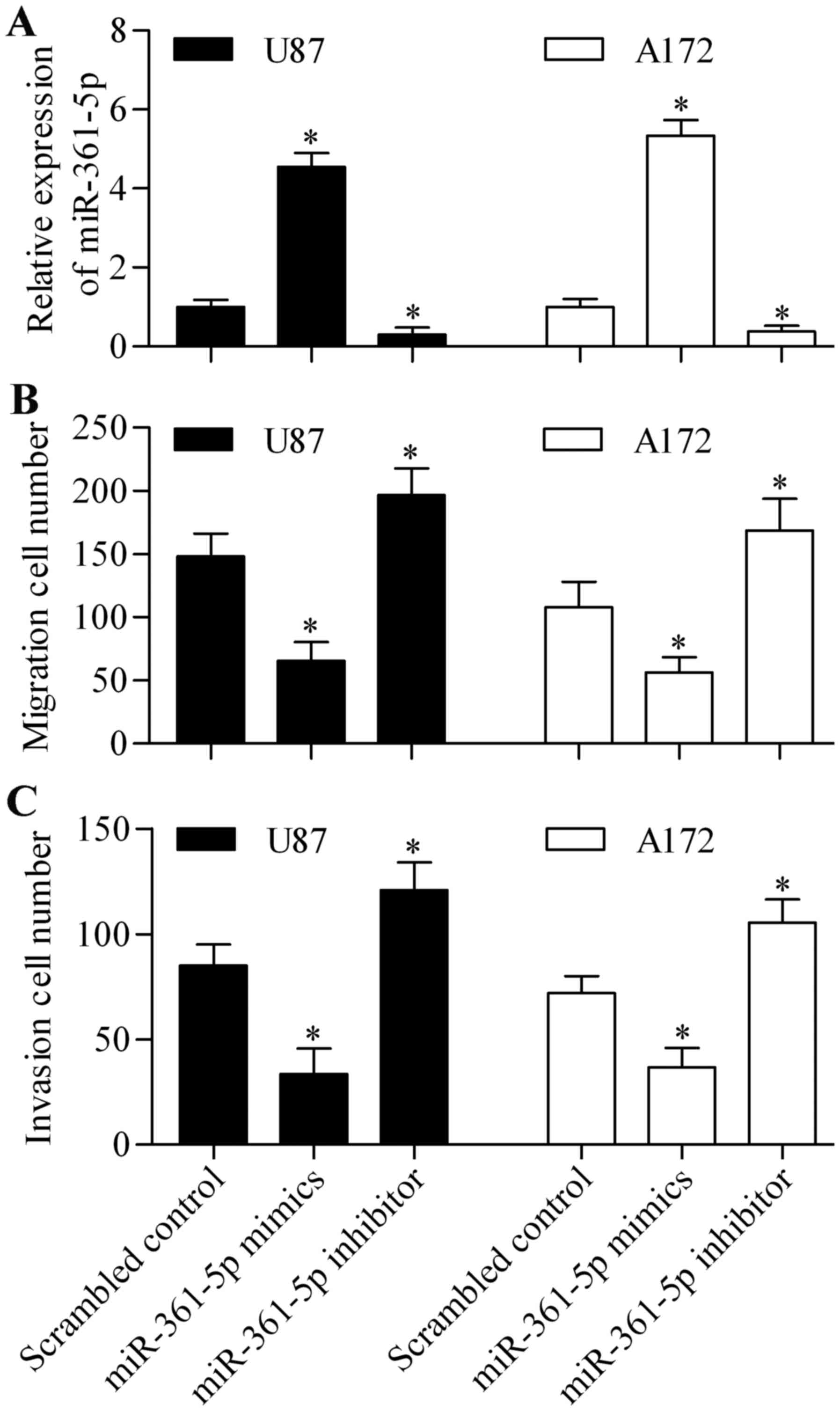
miR-361-5p inhibits cell migration and
invasion of glioma cells
To investigate the biological effect of miR-361-5p
in glioma cells, we performed gain-of-function and loss-of-function
experiments by transiently transfecting miR-361-5p mimics or
miR-361-5p inhibitors into U87 and A172 cells. Transfection of the
miR-361-5p mimics significantly increased miR-361-5p expression
whereas transfection of the miR-361-5p inhibitors significantly
suppressed miR-361-5p expression (Fig.
2A). We then examined the role of miR-361-5p in glioma cell
migration and invasion. We determined that overexpression of
miR-361-5p significantly decreased migration (Fig. 2B) and invasion (Fig. 2C). In contrast, miR-361-5p
suppression markedly promoted cell migration (Fig. 2B) and invasion (Fig. 2C). These data suggest that
miR-361-5p functions as a suppressor of cancer cell metastasis.
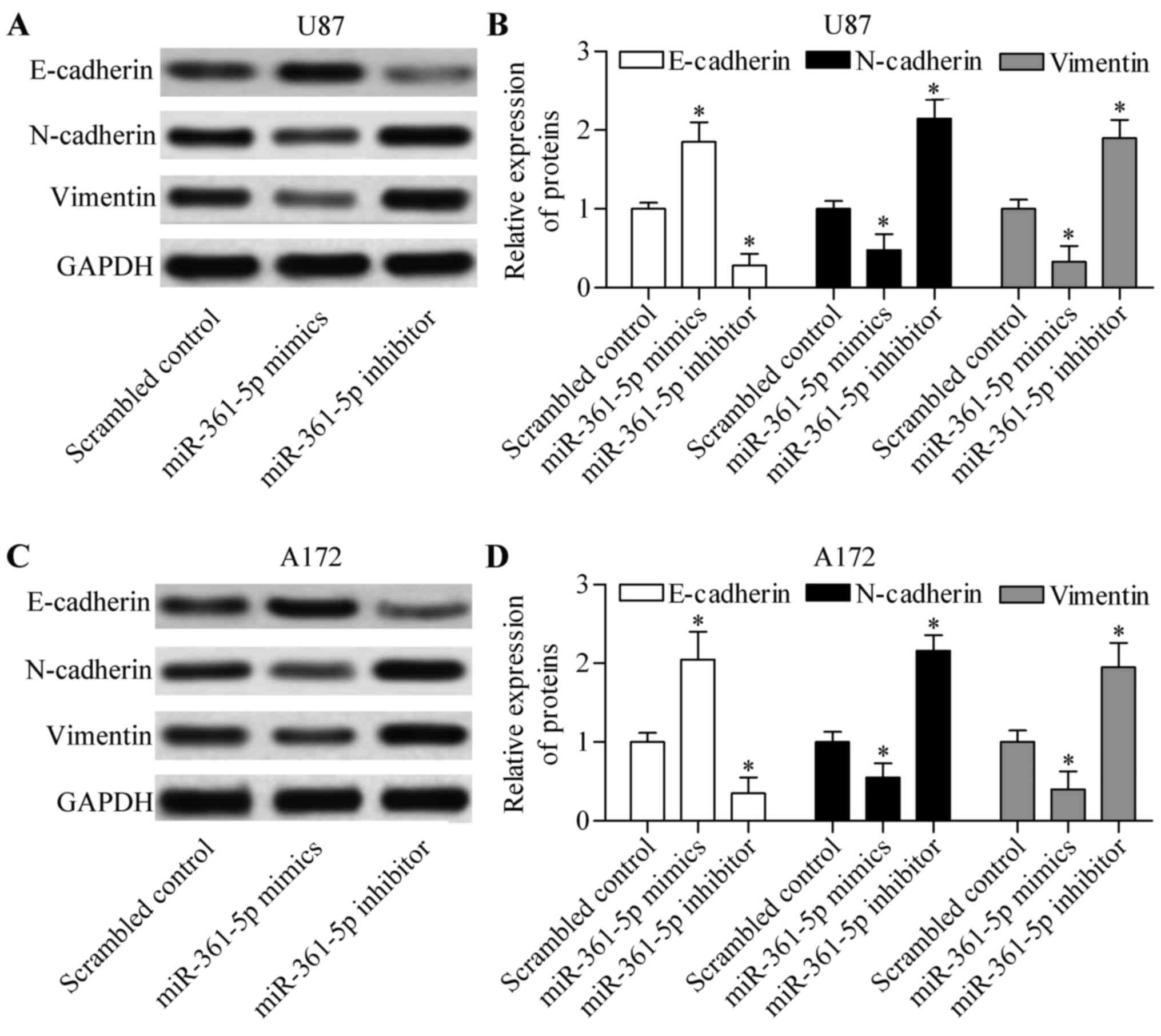
miR-361-5p inhibits EMT of glioma
cells
To further investigate the biological effect of
miR-361-5p on glioma, we investigated the role of miR-361-5p in
glioma cell EMT. We determined that miR-361-5p overexpression
significantly increased the expression of epithelial marker
E-cadherin and inhibited expression of mesenchymal markers
N-cadherin and vimentin in the U87 (Fig. 3A and B) and A172 cells (Fig. 3C and D). Conversely, miR-361-5p
suppression exhibited an opposite effect (Fig. 3A-D). These results suggest that
miR-361-5p suppresses EMT of glioma cells.
Twist1 is a target of miR-361-5p in
glioma cells
To understand the molecular basis of miR-361-5p in
regulating glioma cell EMT, we conducted bioinformatic analyses to
identify the target of miR-361-5p in glioma. Our analysis revealed
Twist1 as a potential target gene of miR-361-5p. The 3′-UTR of
Twist1 was found to contain a binding sequence for miR-361-5p
(Fig. 4A). To confirm that Twist1
is a target gene of miR-361-5p, a dual-luciferase reporter assay
was performed. The results showed that miR-361-5p overexpression
significantly inhibited the luciferase activity in the U87
(Fig. 4B) and A172 (Fig. 4C) cells transfected with the
luciferase reporter vector containing the wild-type (WT) Twist1
3′-UTR whereas miR-361-5p suppression promoted the luciferase
activity. However, neither miR-361-5p overexpression nor miR-361-5p
suppression showed an obvious effect on luciferase activity of the
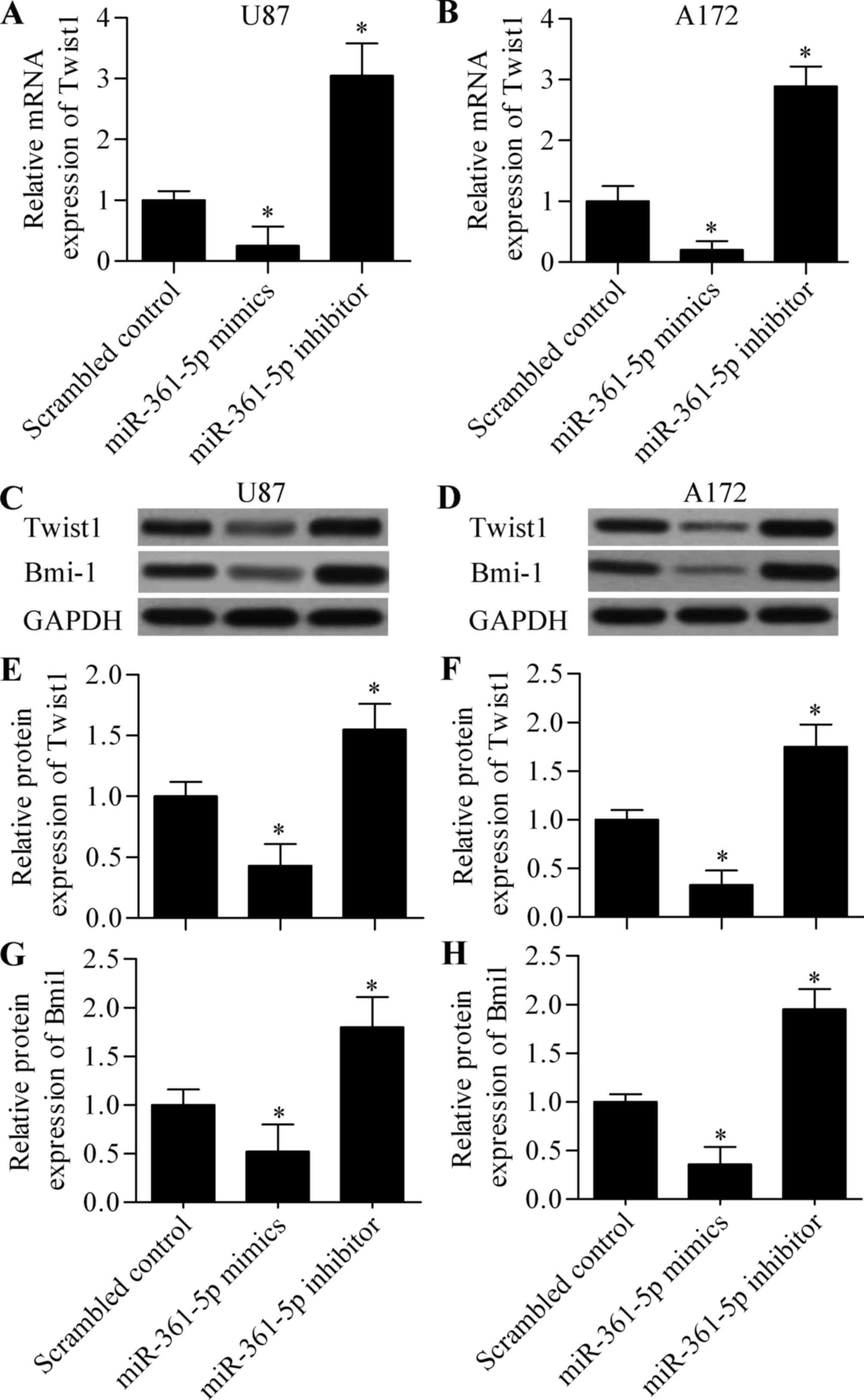
mutant-type (MT) Twist1 3′-UTR. Moreover, we next detected the
direct effect of miR-361-5p overexpression or miR-361-5p
suppression on Twist1 expression in glioma cells by RT-qPCR and
western blot analysis. The results showed that overexpression of
miR-361-5p significantly inhibited both the mRNA (Fig. 5A and B) and protein (Fig. 5C-F) expression levels of Twist1
which were markedly increased by miR-361-5p suppression. These
results suggest that miR-361-5p targets the 3′-UTR of Twist1 and
inhibits Twist1 expression.
miR-361-5p regulates Bmi-1
expression
Bmi-1 is a well-known downstream gene of Twist1 that
participates in tumorigenesis and metastasis (24). We thus, investigated whether
miR-361-5p regulates Bim-1. We found that overexpression of
miR-361-5p significantly inhibited Bmi1 expression and miR-361-5p
suppression significantly increased Bmi-1 expression (Fig. 5C and D, and G and H). The results
suggest that miR-361-5p regulates the Twist1/Bmi-1 signaling
axis.
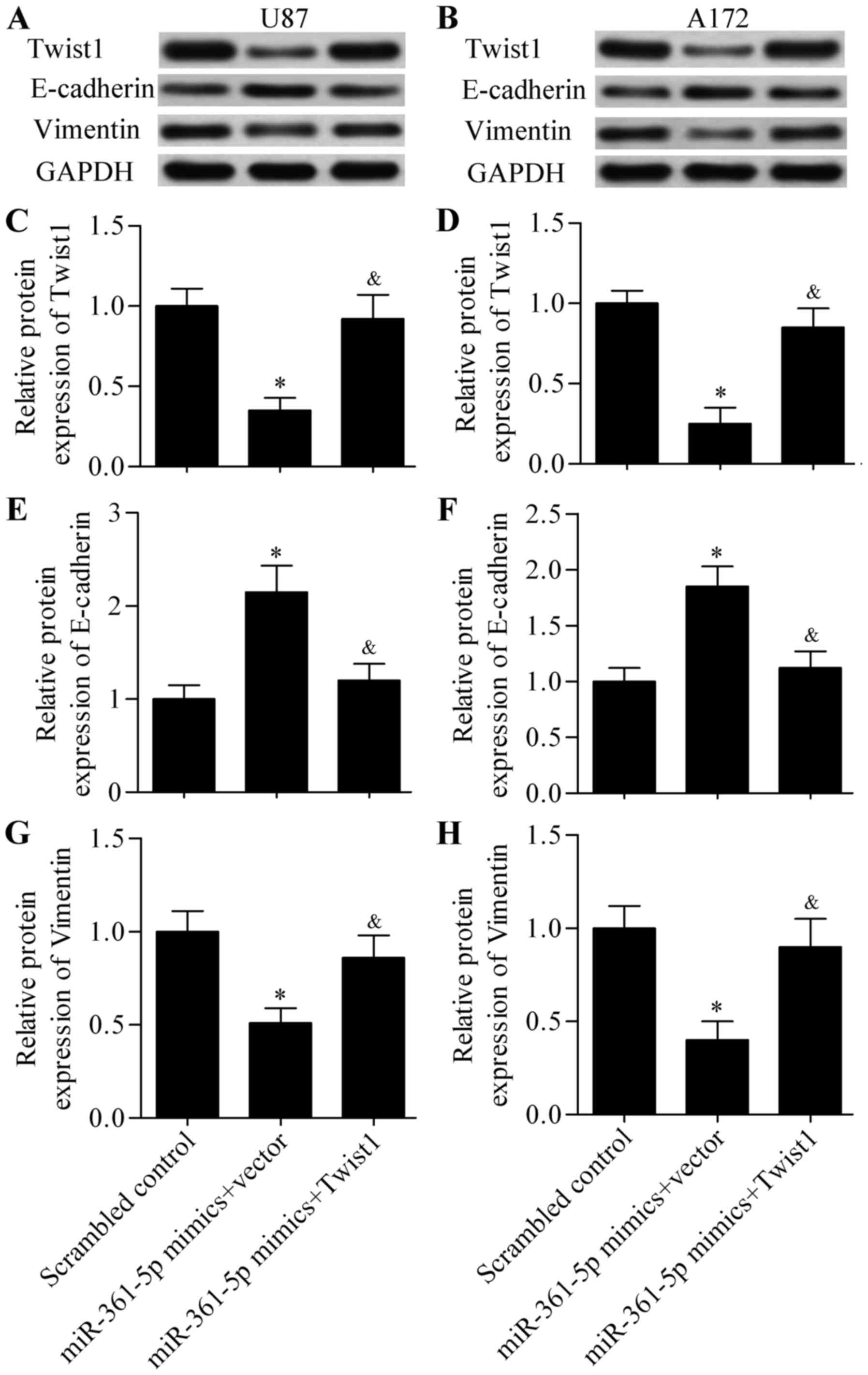
Overexpression of Twist1 rescues the
effects of miR-361-5p in glioma cells
To verify the functional relevance of Twist1
targeting by miR-361-5p, we performed a rescue assay to assess
whether Twist1 overexpression rescues the inhibitory effects of
miR-361-5p. Glioma cells were co-transfected with miR-361-5p mimics
and the Twist1-overexpressing plasmid. We determined that the
reduced protein expression induced by miR-361-5p overexpression was
significantly restored by transfection with the
Twist1-overexpressing plasmid in the U87 (Fig. 6A and C) and A172 (Fig. 6B and D) glioma cells. Overexpression
of Twist1 rescued the inhibitory effect of miR-361-5p on glioma
cell EMT (Fig. 6A, B and E-H).
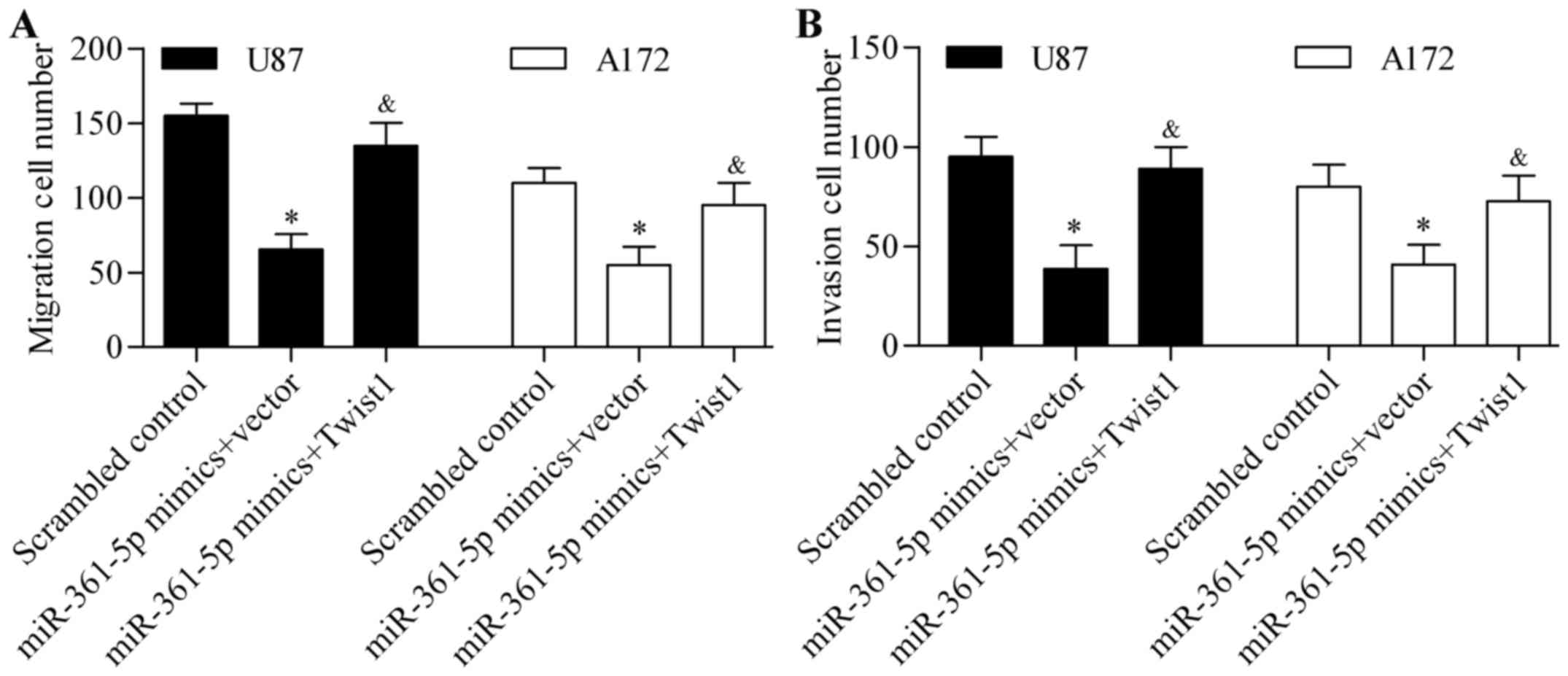
Furthermore, the inhibitory effects of miR-361-5p on glioma cell
migration (Fig. 7A) and invasion
(Fig. 7B) were significantly
reversed by Twist1 overexpression. These results suggest that
miR-361-5p regulates EMT through repressing Twist1.
Discussion
A growing body of evidence suggests that miRNAs play
a pivotal role in regulating the tumorigenesis and metastasis of
glioma (25–27). Thus, identification of
glioma-associated miRNAs as biomarkers for glioma diagnosis,
prognosis and treatment is of great importance. In the present
study, we found that miR-361-5p expression was decreased in glioma
samples and cell lines. Functional experiments revealed that
miR-361-5p functioned as a tumor suppressor that inhibited glioma
cell migration, invasion and EMT. Further data indicated that
miR-361-5p functioned by targeting Twist1 in the glioma cells.
Taken together, our data suggest that miR-361-5p plays an important
role in glioma progression and represents a novel therapeutic agent
for glioma.
Increasing evidence indicates that miR-361-5p is a
tumor-associated miRNA that is frequently dysregulated in many
human cancer types. It has been reported that miR-361-5p exhibits a
lower expression pattern in castration-resistant prostate cancer
and overexpression of miR-361-5p inhibits the malignant progression
of prostate cancer by targeting signal transducer and activator of
transcription-6 (22). The
expression of miR-361-5p was found to be significantly decreased in
colorectal and gastric cancer tissues, and was negatively
correlated with lung metastasis and prognosis (23). Overexpression of miR-361-5p
significantly inhibited proliferation, migration and invasion of
colorectal and gastric cancer cells by inhibiting staphylococcal
nuclease domain containing-1 (23).
In hepatocellular carcinoma, the promoter of miR-361-5p was found
to be significantly hypermethylated leading to reduced expression
of miR-361-5p and overexpression of miR-361-5p inhibited the
proliferation and invasion of hepatocellular carcinoma cells
through targeting chemokine (C-X-C Motif) receptor 6 (21). Kanitz et al reported that
miR-361-5p targets and represses vascular endothelial growth factor
A in human cutaneous squamous cell carcinoma (28). These findings support a
tumor-suppressor role of miR-361-5p. In accordance with these
findings, our results support miR-361-5p as a tumor suppressor. We
demonstrated that miR-361-5p was significantly decreased in glioma
tissues, particularly in advanced tumor stages. We also
demonstrated that overexpression of miR-361-5p inhibited glioma
cell migration, invasion and EMT. However, an oncogenic role of
miR-361-5p was previously observed in cervical cancer (29). Thus, the precise role of miR-361-5p
requires further investigation.
To date, the targets of miR-361-5p remain unknown.
In the present study, we examined bioinformatic analysis predicted
targets to identify the potential target of miR-361-5p in glioma
cells. Notably, we identified Twist1, an important inducer of EMT,
as a direct target of miR-361-5p. Overexpression of Twist1 has
frequently been observed in a variety of human cancers playing an
important role in regulating cancer initiation, progression and
metastasis (12). Increasing
evidence suggests that Twist1 functions as a critical regulator of
EMT (13,15–17).
Twist1 promotes EMT by affecting downstream genes such as
E-cadherin, an epithelial marker (30). It has been reported that Twist1
activates the transcription of Bmi-1, and that Twist1 and Bmi-1
cooperate to inhibit E-cadherin expression by binding to its
promoter (24). High expression of
Twist1 has been detected in a majority of human glioma-derived cell
lines and human glioma tissues (18). In glioma patients, high expression
of Twist1 predicts poor survival for glioma patients (19). Twist1 overexpression was found to
promote cell invasion while suppression of Twist1 was found to
inhibit cell invasion in glioma cells (18,20).
Therefore, targeted therapy using Twist1 may have promising and
potential therapeutic value for glioma treatment.
miRNAs are important mechanisms of Twist1
post-transcriptional modification. A growing body of evidence has
revealed that a variety of miRNAs act as negative regulators of
Twist1 (31). miR-33b is reported
to inhibit breast cancer metastasis through targeting Twist1
(32). miR-548c regulates cell
migration and invasion of ovarian cancer cells by suppressing
Twist1 (33). Liu et al
reported that miR-1271 suppressed pancreatic cancer cell migration,
invasion and EMT through targeting Twist1 (34). A more recent study found that
miR-186 inhibits prostate cancer cell growth, vasculogenic mimicry
formation and EMT via targeting Twist1 (35). However, the miRNAs targeting Twist1
in glioma have not been well investigated. In the present study, we
found that miR-361-5p directly targeted Twist1 in glioma cells to
regulate migration, invasion and EMT. The decreased expression of
miR-361-5p partially contributed to the increased expression of
Twist1 in the glioma cells that promoted tumorigenesis and
metastasis. Our findings add new insight into the related molecular
mechanism of glioma pathogenesis.
In conclusion, the present study was the first to
show that miR-361-5p was decreased in glioma tissues and cell
lines. miR-361-5p exhibited a suppressive effect on glioma cell
migration, invasion and EMT indicating an important role in glioma
metastasis. More importantly, we elucidated that the underlying
mechanism involved direct regulation of its target gene Twist1.
Although detailed knowledge regarding the precise role and
mechanism of miR-361-5p in regulating tumorigenesis and metastasis
of glioma remains incomplete, we confirmed the role of miR-361-5p
as a tumor suppressor in glioma. These findings suggest that
miR-361-5p may serve as a novel potential therapeutic target for
glioma.
Acknowledgements
The present study was supported by the Social
Research and Development Program of Shaanxi Province (no.
2016SF-110).
Glossary
Abbreviations
Abbreviations:
|
miRNAs
|
microRNAs
|
|
EMT
|
epithelial-mesenchymal transition
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
|
3-UTR
|
3′-untranslated region
|
|
WHO
|
World Health Organization
|
|
FBS
|
fetal bovine serum
|
References
|
1
|
Westphal M and Lamszus K: The neurobiology
of gliomas: From cell biology to the development of therapeutic
approaches. Nat Rev Neurosci. 12:495–508. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Furnari FB, Fenton T, Bachoo RM, Mukasa A,
Stommel JM, Stegh A, Hahn WC, Ligon KL, Louis DN, Brennan C, et al:
Malignant astrocytic glioma: Genetics, biology, and paths to
treatment. Genes Dev. 21:2683–2710. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pollack IF: Neuro-oncology: Therapeutic
benefits of reirradiation for recurrent brain tumors. Nat Rev
Neurol. 6:533–535. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pang JC, Kwok WK, Chen Z and Ng HK:
Oncogenic role of microRNAs in brain tumors. Acta Neuropathol.
117:599–611. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Silber J, James CD and Hodgson JG:
microRNAs in gliomas: Small regulators of a big problem.
Neuromolecular Med. 11:208–222. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Winter J, Jung S, Keller S, Gregory RI and
Diederichs S: Many roads to maturity: microRNA biogenesis pathways
and their regulation. Nat Cell Biol. 11:228–234. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Manikandan J, Aarthi JJ, Kumar SD and
Pushparaj PN: Oncomirs: The potential role of non-coding microRNAs
in understanding cancer. Bioinformation. 2:330–334. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang Y, Dutta A and Abounader R: The role
of microRNAs in glioma initiation and progression. Front Biosci.
17:700–712. 2012. View
Article : Google Scholar
|
|
11
|
Besse A, Sana J, Fadrus P and Slaby O:
MicroRNAs involved in chemo- and radioresistance of high-grade
gliomas. Tumour Biol. 34:1969–1978. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhu QQ, Ma C, Wang Q, Song Y and Lv T: The
role of TWIST1 in epithelial-mesenchymal transition and cancers.
Tumour Biol. 37:185–197. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kang Y and Massagué J:
Epithelial-mesenchymal transitions: Twist in development and
metastasis. Cell. 118:277–279. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tam WL and Weinberg RA: The epigenetics of
epithelial-mesenchymal plasticity in cancer. Nat Med. 19:1438–1449.
2013. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Taube JH, Herschkowitz JI, Komurov K, Zhou
AY, Gupta S, Yang J, Hartwell K, Onder TT, Gupta PB, Evans KW, et
al: Core epithelial-to-mesenchymal transition interactome
gene-expression signature is associated with claudin-low and
metaplastic breast cancer subtypes. Proc Natl Acad Sci USA.
107:15449–15454. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang MH and Wu KJ: TWIST activation by
hypoxia inducible factor-1 (HIF-1): Implications in metastasis and
development. Cell Cycle. 7:2090–2096. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Elias MC, Tozer KR, Silber JR, Mikheeva S,
Deng M, Morrison RS, Manning TC, Silbergeld DL, Glackin CA, Reh TA,
et al: TWIST is expressed in human gliomas and promotes invasion.
Neoplasia. 7:824–837. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nordfors K, Haapasalo J, Mäkelä K,
Granberg KJ, Nykter M, Korja M, Paavonen T, Haapasalo H and Soini
Y: Twist predicts poor outcome of patients with astrocytic glioma.
J Clin Pathol. 68:905–912. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mikheeva SA, Mikheev AM, Petit A, Beyer R,
Oxford RG, Khorasani L, Maxwell JP, Glackin CA, Wakimoto H,
González-Herrero I, et al: TWIST1 promotes invasion through
mesenchymal change in human glioblastoma. Mol Cancer. 9:1942010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sun JJ, Chen GY and Xie ZT:
MicroRNA-361-5p inhibits cancer cell growth by targeting CXCR6 in
hepatocellular carcinoma. Cell Physiol Biochem. 38:777–785. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu D, Tao T, Xu B, Chen S, Liu C, Zhang
L, Lu K, Huang Y, Jiang L, Zhang X, et al: MiR-361-5p acts as a
tumor suppressor in prostate cancer by targeting signal transducer
and activator of transcription-6 (STAT6). Biochem Biophys Res
Commun. 445:151–156. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ma F, Song H, Guo B, Zhang Y, Zheng Y, Lin
C, Wu Y, Guan G, Sha R, Zhou Q, et al: MiR-361-5p inhibits
colorectal and gastric cancer growth and metastasis by targeting
staphylococcal nuclease domain containing-1. Oncotarget.
6:17404–17416. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang MH, Hsu DS, Wang HW, Wang HJ, Lan HY,
Yang WH, Huang CH, Kao SY, Tzeng CH, Tai SK, et al: Bmi1 is
essential in Twist1-induced epithelial-mesenchymal transition. Nat
Cell Biol. 12:982–992. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kwak PB, Iwasaki S and Tomari Y: The
microRNA pathway and cancer. Cancer Sci. 101:2309–2315. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Karsy M, Arslan E and Moy F: Current
progress on understanding microRNAs in glioblastoma multiforme.
Genes Cancer. 3:3–15. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tivnan A and McDonald KL: Current progress
for the use of miRNAs in glioblastoma treatment. Mol Neurobiol.
48:757–768. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kanitz A, Imig J, Dziunycz PJ, Primorac A,
Galgano A, Hofbauer GF, Gerber AP and Detmar M: The expression
levels of microRNA-361-5p and its target VEGFA are inversely
correlated in human cutaneous squamous cell carcinoma. PLoS One.
7:e495682012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wu X, Xi X, Yan Q, Zhang Z, Cai B, Lu W
and Wan X: MicroRNA-361-5p facilitates cervical cancer progression
through mediation of epithelial-to-mesenchymal transition. Med
Oncol. 30:7512013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sun T, Zhao N, Zhao XL, Gu Q, Zhang SW,
Che N, Wang XH, Du J, Liu YX and Sun BC: Expression and functional
significance of Twist1 in hepatocellular carcinoma: Its role in
vasculogenic mimicry. Hepatology. 51:545–556. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Haga CL and Phinney DG: MicroRNAs in the
imprinted DLK1-DIO3 region repress the epithelial-to-mesenchymal
transition by targeting the TWIST1 protein signaling network. J
Biol Chem. 287:42695–42707. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lin Y, Liu AY, Fan C, Zheng H, Li Y, Zhang
C, Wu S, Yu D, Huang Z, Liu F, et al: MicroRNA-33b inhibits breast
cancer metastasis by targeting HMGA2, SALL4 and Twist1. Sci Rep.
5:99952015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sun X, Cui M, Zhang A, Tong L, Wang K, Li
K, Wang X, Sun Z and Zhang H: MiR-548c impairs migration and
invasion of endometrial and ovarian cancer cells via downregulation
of Twist. J Exp Clin Cancer Res. 35:102016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu H, Wang H, Liu X and Yu T: miR-1271
inhibits migration, invasion and epithelial-mesenchymal transition
by targeting ZEB1 and TWIST1 in pancreatic cancer cells. Biochem
Biophys Res Commun. 472:346–352. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhao X, Wang Y, Deng R, Zhang H, Dou J,
Yuan H, Hou G, Du Y, Chen Q and Yu J: miR186 suppresses prostate
cancer progression by targeting Twist1. Oncotarget. 7:33136–33151.
2016.PubMed/NCBI
|