Introduction
Colon cancer is a common malignant tumor in the
clinic, and its mortality is ranked third of all cancers. The
incidence of colon cancer has been increasing over the past few
decades (1). The main causes of
failure of the treatment are postoperative metastasis and drug
resistance to chemotherapy drugs (2).
Multidrug resistance (MDR), is the major cause for
chemotherapy failure and it happens when cancer cells resist
simultaneously to multiple chemotherapeutic agents, which are both
structurally and functionally unrelated (3). The mechanisms of MDR include increased
efflux of drugs, decreased uptake of drugs, impaired apoptotic
pathways, and altered cell cycle checkpoints. The overexpression of
ATP binding cassette (ABC) membrane transporter proteins that
actively pump anti-cancer drugs out of the cells is the most
important mechanism for MDR (4).
Three major ABC transporters, i.e., ABCB1 (P-glycoprotein/P-gp),
ABCC1 (multidrug resistance-associated proteins/MRP1), and ABCG2
(breast cancer resistance protein/BCRP), are commonly observed in
cancer cells and critical to mediate MDR (5).
P-gp, a 170-kDa transmembrane glycoprotein encoded
by the MDR1 gene, is the most studied member of ABC transporter
family. It is extensively distributed in intestinal epithelium,
hepatocytes, and kidneys, which is responsible for protecting
tissues from a variety of toxins and xenobiotics (6). P-gp is also overexpressed in cancer
cells, which can cause MDR and chemotherapy failure caused by
reduction of the concentration of anti-tumor drugs within the cells
(7). To date, three generations of
P-gp inhibitors have been developed, such as verapamil, quinine,
dexverapamil, emopamil, valspodar, and tariquidar (8). However, few of these inhibitors have
progressed beyond clinical trials due to them exhibiting
non-specific toxicity. Therefore, it is necessary to explore novel
P-gp inhibitors with improved specificity and higher potency. A
large number of traditional Chinese medicines have been observed to
present potent anti-cancer activities, and some have become
promising candidates as potential P-gp reversing agents (9,10).
ChanSu, a traditional Chinese medicine, derived from
the skin and postauricular glands of the Asiatic toad (Bufo
gargarizans) has been widely and successfully used for
centuries for analgesia, and in the treatment of inflammation and
cardiac arrhythmias (11).
Moreover, it is also used to treat various cancers, such as
colorectal, liver, and lung cancer in China (12). Cinobufagin (CBF) is one of the
principal bioactive components of ChanSu, which is a traditional
Chinese medicine. CBF is a major digoxin-like, bufadienolide
steroid isolated from ChanSu, which has been reported to exhibit
significant antitumor activity with the mechanism of inhibiting
cell proliferation, inducing cell differentiation and apoptosis
(13–15). Zhang et al reported that CBF
suppressed tumor growth through intrinsic mitochondria apoptosis
via AKT signaling pathway in human non-small cell lung cancer cells
(16). However, very little is
known concerning the role of CBF in circumvention MDR in colon
cancer. Our group previously investigated ChanSu and its active
ingredients (17–19) indicating that CBF can reverse
chemoresistance of cancer cells, but its mechanism was not clear.
In this study, we investigated the role and mechanism of CBF on
reversing P-gp mediated MDR both in vitro and in
vivo.
Materials and methods
Materials
CBF was purchased from Chengdu Herbpurify Co., Ltd.
(Sichuan, China). Doxorubicin (DOX), Rho123, verapamil and Lucifer
yellow were obtained from Sigma-Aldrich Chemical Co. (St. Louis,
MO, USA). Oxaliplatin (L-OHP) was obtained from Tokyo Chemical
Industry Co., Ltd. (Tokyo, Japan). Minimum Essential Media (MEM),
fetal bovine serum (FBS), non-essential amino acids (NEAA), Ham's
F-12K (Kaighn's) medium (F12K), and Hank's balanced salt solution
(HBSS), were obtained from Gibco BRL (Carlsbad, CA, USA). RPMI-1640
and phosphate-buffered saline (PBS) were from Hyclone (Thermo
Scientific, Logan, UT, USA). Annexin V-FITC Apoptosis Detection kit
was from BD Biosciences (Beijing, China). A P-gp ATPase assay
system was purchased from Promega (Madison, WI, USA). The primary
antibodies for caspase-3, caspase-9, P-gp and β-actin were from
Cell Signaling Technology (Boston, MA, USA). The secondary
antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz,
CA, USA). Cell Counting Kit-8 (CCK-8) was purchased from Dojindo
(Kumamoto, Japan).
Cell lines and culture conditions
LoVo, Caco-2, and HCT116 human colorectal carcinoma
cells were obtained from the Cell Bank of the Chinese Academy of
Sciences. LoVo cells were cultured in F12k medium. Caco-2 cells
were cultured in MEM medium, and HCT116 cells were maintained in
RPMI-1640 medium. P-gp-overexpressing HCT116/L-OHP (HCT116/L) cells
were established by our laboratory (20). L-OHP (5 µg/ml) was added to the
medium of HCT116/L cells to maintain resistance, and then incubated
in drug-free medium for minimum one week before use.
P-gp-overexpressing LoVo/ADR and Caco-2/ADR cells were obtained
from Shanghai Yan Sheng Industrial Co., Ltd. LoVo/ADR, HCT116/L and
Caco-2/ADR cells were cultured in RPMI-1640 medium. DOX (8 µg/ml)
was added to the medium of LoVo/ADR or Caco-2/ADR cells to maintain
resistance and incubated for minimum one week in drug-free medium
before use. All of the above cells lines were grown in culture
flasks or dishes with medium supplemented with 10% FBS, 100 U/ml
penicillin, and 100 µg/ml streptomycin in an atmosphere of 5%
CO2 and 90% relative humidity at 37°C.
Cell cytotoxicity by CCK-8 assay
Cell viability was determined by CCK-8 assay
(21). In brief, the cells were
seeded at 1×105 cells/ml in 96-well plates and were
incubated overnight. A range of different concentrations of
chemotherapeutic drugs, with or without verapamil (20 µM) or CBF,
was added to the plates and incubated at 37°C. After 48 h of
incubation, 10 µl CCK was added to the each well and the plates
were incubated for 1–4 h. The optical density was measured at 450
nm by Thermo Varioskan Flash (Thermo Scientific, Waltham, MA, USA).
The degree of resistance was estimated by dividing the
IC50 for the drug-resistant cells by that for the
sensitive parental cells; the fold-reversal factor (RF) of MDR was
calculated by dividing the IC50 values of the anticancer
drug obtained in the absence of CBF by those obtained in the
presence of CBF.
Apoptosis detection assay
The cell apoptosis rate of LoVo/ADR, HCT116/L and
Caco-2/ADR cells was measured by flow cytometry using the Annexin
V/PI Apoptosis Detection kit in accordance with the manufacturer's
protocols. Cells were seeded at 1×105 cells/ml onto
6-well plates and cultured with CBF, DOX and CBF+DOX for 48 h.
After cells were trypsinized and collected into 5 ml tubes in 500
µl of 1X binding buffer, 5 µl PI and 5 µl Annexin V-FITC were added
to each samples. Then samples were analyzed (FL-1:Ex=
488 nm, Em= 530 nm; FL-2:Ex= 488 nm,
Em=620 nm) by FACS (BD Biosciences, San Jose, CA,
USA).
Doxorubicin and Rho123 accumulation
assay by flow cyto-metry
The intracellular accumulation of Dox and Rho123 in
LoVo/ADR, HCT116/L and Caco-2/ADR cells was measured by flow
cytometry as previously described (22). First, the cells were plated onto
6-well plates at a density of 105/well and were then
incubated with CBF or verapamil for 48 h. Then cells were exposed
to Dox (5 µg/ml) and Rho123 (1 µg/ml) at 37°C for 90 min. After
treatment, cells were trypsinized and collected, washed three times
with cold PBS, and analyzed by FACS (BD Biosciences). Verapamil was
used as a positive control.
P-gp-mediated drug transport
assay
The transport experiments were performed using a
method described previously (23).
In brief, Caco-2 cells were seeded onto permeable polycarbonate
filter inserts in 12-well transwell plate (0.4 µm pore size, 1.13
cm2 of growth area, 12-mm diameter, Corning Costar Co.,
Corning, NY, USA) at a density of 1×105 cells/ml and
were allowed to grow for 21 days. The integrity of the monolayer
was monitored by detecting the transepithelial electrical
resistance (TEER) and Lucifer yellow permeability. Caco-2
monolayers with Lucifer yellow permeability <1% and TEER values
>250 Ω·cm2 were considered intact and used for the
transport studies. The experiment was carried out in HBSS solution
(pH 7.4). The monolayer cells were washed three times with HBSS and
equilibrated for approximately 15 min. DOX (20 µM) was then added
to the apical (A) or basolateral (B) side, and samples (0.1 ml)
were taken at different time points (0, 15, 30, 45, 60, 90, 120
min) from the other side. Equal volumes of blank buffer were
supplied after each sample withdrawal during the experiment. The
concentrations of DOX were analyzed by Thermo Varioskan Flash.
P-gp ATPase assay
The changes of ATPase activity were measured by
Pgp-Glo™ assay systems (24). In
brief, samples containing verapamil (0.20 mM) and CBF (5, 10, 20,
50, 100 nM) was cultured with recombinant human P-gp membranes in
untreated, white, opaque, 96-well plates (Inc., Lowell, MA, USA)
for about 5 min at 37°C. Reactions were initiated by adding 10 µl
of 25 mM MgATP to all wells, and then the wells were incubated at
37°C for 40 min on a heat block. Subsequently, the reaction was
terminated by mixing with 50 µl ATP detection reagent. Finally the
plate was incubated at room temperature for 20 min to allow
luminescent signals to develop. The relative light unit (RLU)
values were read on Thermo Varioskan Flash.
Western blot analysis
LoVo/ADR, Caco-2/ADR or HCT116/L cells were treated
with different concentrations of CBF, then incubated for 48 h.
Cells were washed three times and scraped in lysed RIPA containing
protease inhibitors. The concentration of protein was measured
using a BCA assay (Pierce Biotechnology, Rockford, IL, USA).
Protein (50 µg) was loaded onto SDS-PAGE gels and then transferred
to PVDF membranes. The membranes were blocked by incubating with 5%
BSA for 1 h, and then incubated with the following primary
monoclonal antibody: anti P-gp, anti-caspase-3, anti-caspase-9,
anti-Bcl 2, anti-Bax (1:1000) overnight at 4°C. The membrane was
washed 3×15 min with TBST buffer and subsequently incubated with
the HRP-conjugated secondary antibody (1:5000) for 2 h. Finally,
the membranes were visualized using enhanced chemiluminescence
detection (GE Healthcare Lifesciences, Pittsburgh, PA, USA), as
previously described (25). β-actin
was used as a loading control.
Nude mouse xenograft model
Six to eight weeks old athymic nude mice
(BALB/c-nu/nu), weighing 18–24 g, were purchased from Shanghai SLAC
Laboratory Animal Co., Ltd. All mice were fed with sterilized food
and water. LoVo/ADR (1×107) cells were resuspended in
200 µl PBS and inoculated subcutaneously into the nude mice
(26). When the tumor size reached
150–200 mm3, the mice were randomly divided into four
groups (n=6 per group): saline solution (control); DOX (0.1 mg/kg);
CBF (0.2 mg/kg); and DOX (0.1 mg/kg) plus CBF (0.2 mg/kg). All the
drugs were administered via i.p. injection every 3 days for a total
of 5 doses. The body weight of the animals was recorded every 3
days and tumor volumes (V) were calculated by the formula: V =
(tumor length × width2)/2.
The curve of tumor growth was drawn according to
tumor volume and time of implantation. At the end of experiments,
mice were sacrificed, and whole blood, tumor and other tissues
(heart, liver, lung, spleen, kidney and intestine) were harvested
and used for further analysis. Tumor tissues were analyzed by
immunohistochemical staining (IHC) for TUNEL, Ki67 and P-gp. All of
the experiments were carried out under the approval of the
Administrative Panel on Laboratory Animal Care of the Putuo
District Center Hospital.
Toxicity analysis
Normal tissues (heart, liver, lung, spleen, kidney
and intestine) were harvested for H&E histology studies. Venous
blood samples were collected in EDTA-coated tubes for hematology
studies. Samples were analyzed for white blood cells (WBC), red
blood cells (RBC), platelets (PLT), aspartate aminotransferase
(AST), alanine aminotransferase (ALT) and blood urea nitrogen (BUN)
in the clinical laboratory at our hospital.
Statistics
Values were expressed as the mean ± standard
deviation (SD). The differences between two groups were analyzed by
the unpaired Student's t-test. Statistical analysis was performed
using Prism 5.0. p<0.05 was considered statistically
significant.
Results
Characterization of colorectal
parental cells and drug-resistant cells
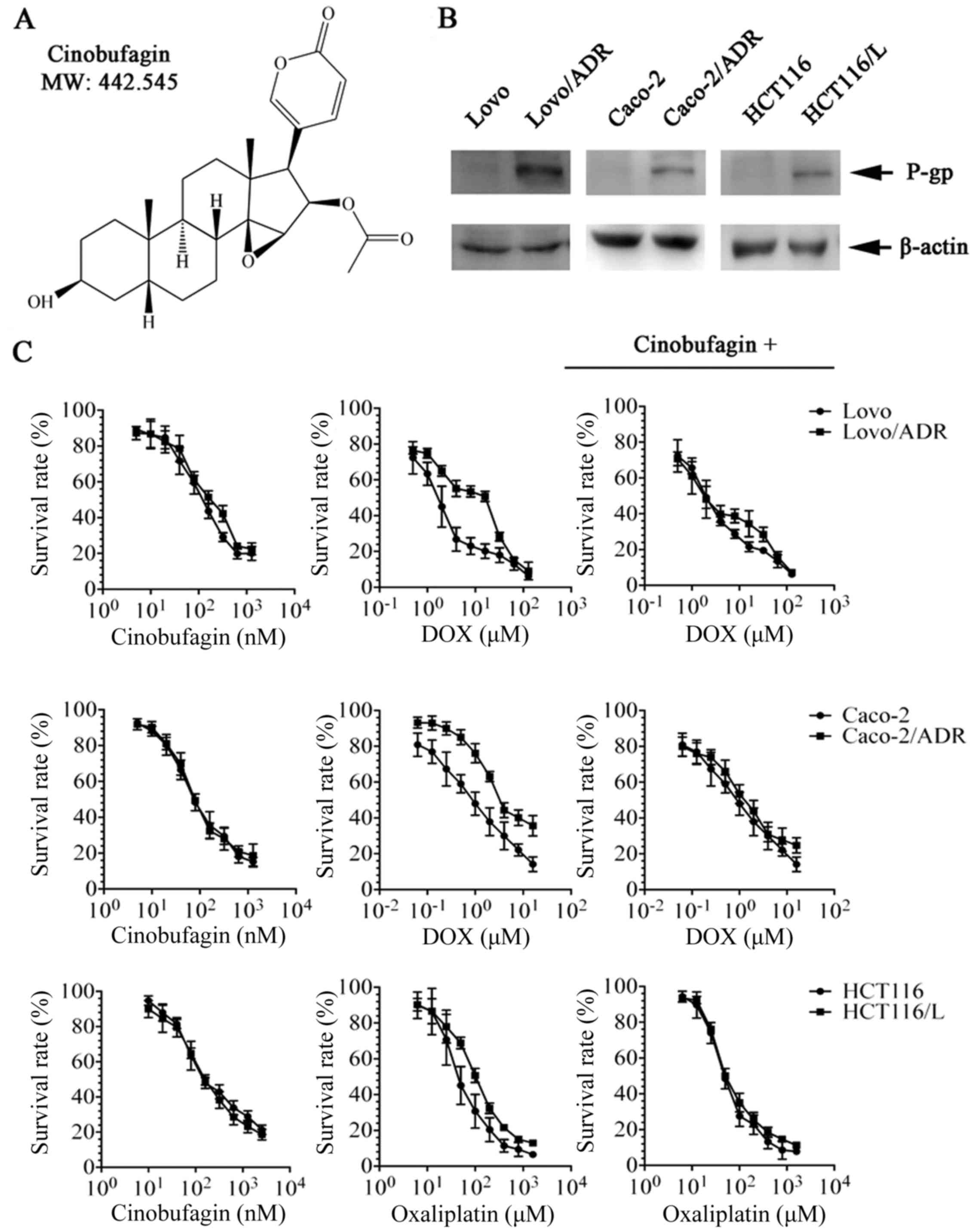
P-gp/MDR1 is a common biomarker of MDR. To confirm
this in our cell lines, we determined the protein expression of
P-gp on cell extracts with western blot analysis. As shown in
Fig. 1B, LoVo/ADR, Caco-2/ADR,
HCT116/L overexpressed P-gp transporter therefore showing a band at
170 kDa, whereas there parental cells have no band at the same
position.
CBF sensitized P-gp-overexpressing
cells to chemotherapeutic drugs
We firstly tested the cytotoxicity of CBF in
different colon cancer cell lines by CCK-8 assay. The
IC50 values were 150.8±6.9, 166.6±10.5, 145.4±8.8,
160.2±12.0, 77.5±6.6, and 79.0±4.3 nM for LoVo, LoVo/ADR, HCT116,
HCT116/L, Caco-2, and Caco-2/ADR, respectively. More than 85% of
the cells survived at 20 nM CBF in LoVo, LoVo/ADR, HCT116, and
HCT116/L cells, and at 10 nM in Caco-2 and Caco-2/ADR cells
(Fig. 1C). Based on these data, CBF
concentrations of 20 nM (LoVo, LoVo/ADR, HCT116, and HCT116/L) or
10 nM (Caco-2 and Caco-2/ADR) were chosen as the maximal safe
concentrations for the reversal assays. The IC50 values
of the anticancer drugs (DOX or L-OHP) in sensitive and resistant
cells, with or without different concentrations of CBF, are shown
in Table I. CBF decreased the
IC50 values of L-OHP in HCT116/L cells, as well as the
IC50 values of DOX in LoVo/ADR and Caco-2/ADR cells, but
no effect was observed on their parental cells (Table I). The fold-reversal (RF) of CBF to
DOX was 8.0 and 2.1 at the given concentration of CBF in LoVo/ADR
and Caco-2/ADR, respectively. Similar to DOX, the RF value of CBF
to L-OHP was 4.6 at 20.0 µM CBF in HCT116/L, which was superior to
that of 20 µM verapamil. However, we found that CBF has no effect
on MIT (mitoxantrone, BCRP substance) or CDF [5(6)-carboxy-2′,7′-dichlorofluorescein, MRP2
substance] (Table II). These
findings suggested that CBF significantly improve the efficacy of
P-gp substrate drugs in resistance cells, indicating that CBF may
be a potent reversal agent of P-gp-mediated MDR in
vitro.
 | Table I.Effect of cinobufagin on the
sensitivity of Lovo, Lovo/ADR, Caco-2, Caco-2/ADR, HCT116 and
HCT116/L cells to anticancer drugs. |
Table I.
Effect of cinobufagin on the
sensitivity of Lovo, Lovo/ADR, Caco-2, Caco-2/ADR, HCT116 and
HCT116/L cells to anticancer drugs.
|
| IC50
(µM) |
|---|
|
|
|
|---|
| Compound | Lovo | Lovo/ADR |
|---|
| DOX | 1.8±0.2 | 16.0±0.5 |
| + CBF (5.0 nM) | 2.1±0.3 (0.9) | 4.0±0.3
(4.0)a |
| + CBF (10.0
nM) | 1.9±0.3 (0.9) | 2.5±0.2
(6.4)a |
| + CBF (20.0
nM) | 1.6±0.1 (1.1) | 2.0±0.2
(8.0)b |
| Verapamil (20
µM) | 1.7±0.2 (1.1) | 1.9±0.3
(8.0)b |
|
|
Caco-2 | Caco-2/ADR |
| DOX | 1.1±0.4 | 3.5±0.2 |
| + CBF (2.5 nM) | 1.3±0.2 (0.8) | 2.3±0.2 (1.5) |
| + CBF (5.0 nM) | 1.5±0.4 (0.7) | 2.6±0.3 (1.4) |
| + CBF (10.0
nM) | 1.2±0.3 (0.9) | 1.7±0.2
(2.1)a |
| Verapamil (20
µM) | 1.0±0.2 (1.1) | 0.9±0.1
(3.5)a |
|
| HCT116 | HCT116/L |
| L-OHP | 48.8±5.0 | 107.3±12.1 |
| +CBF (5.0 nM) | 43.3±4.9 (1.1) | 93.2±8.2 (1.1) |
| +CBF (10.0 nM) | 41.7±6.1 (1.2) | 43.5±7.4
(2.5)a |
| +CBF (20.0 nM) | 38.3±3.6 (1.3) | 23.3±5.0
(4.6)a |
| Verapamil (20
µM) | 31.2±3.2 (1.5) | 44.5±3.8
(2.4)a |
 | Table II.Effect of cinobufagin on the
sensitivity of Lovo, Lovo/ADR, Caco-2, Caco-2/ADR, HCT116 and
HCT116/L cells to MIT/CDF. |
Table II.
Effect of cinobufagin on the
sensitivity of Lovo, Lovo/ADR, Caco-2, Caco-2/ADR, HCT116 and
HCT116/L cells to MIT/CDF.
|
| IC50
(µM) |
|---|
|
|
|
|---|
| Compound | Lovo | Lovo/ADR |
|---|
| MIT (µM) | 1.05±0.09 | 1.23±0.08 |
| + CBF (20 nM) | 1.08±0.06 | 1.12±0.07 |
| CDF (mM) | 1.02±0.11 | 1.13±0.05 |
| + CBF (20 nM) | 0.95±0.10 | 1.01±0.08 |
|
| Caco-2 | Caco-2/ADR |
| MIT (µM) | 0.58±0.08 | 0.70±0.10 |
| + CBF (20 nM) | 0.52±0.09 | 0.59±0.05 |
| CDF (mM) | 0.61±0.08 | 0.78±0.04 |
| + CBF (20 nM) | 0.56±0.10 | 0.68±0.18 |
|
| HCT116 | HCT116/L |
| MIT (µM) | 0.85±0.15 | 1.02±0.28 |
| + CBF (20 nM) | 0.69±0.08 | 1.07±0.03 |
| CDF (mM) | 0.70±0.10 | 0.96±0.16 |
| + CBF (20 nM) | 0.68±0.07 | 0.98±0.15 |
CBF reverses P-gp mediated MDR in nude
mouse xenograft model
In order to substantiate our observation, we
established an in vivo LoVo/ADR cell xenograft model in
BALB/c-nu/nu mice to investigate the efficacy of CBF to reverse
resistance to DOX. There was no apparent difference in tumor size
between mice treated with saline, DOX, or CBF alone. However, the
combination of CBF and DOX produced a significant decrease in tumor
size compared with the other three groups, with an inhibition rate
of 40.9% (Fig. 2A and B). As shown
in Fig. 2C and D, the results
showed that the cell proliferation of the combined group was
decreased compare with the other three groups (Ki67 level), and the
apoptosis rate was increased (TUNEL assay). This result indicated
that CBF could increase the anticancer activity of DOX in
vivo.
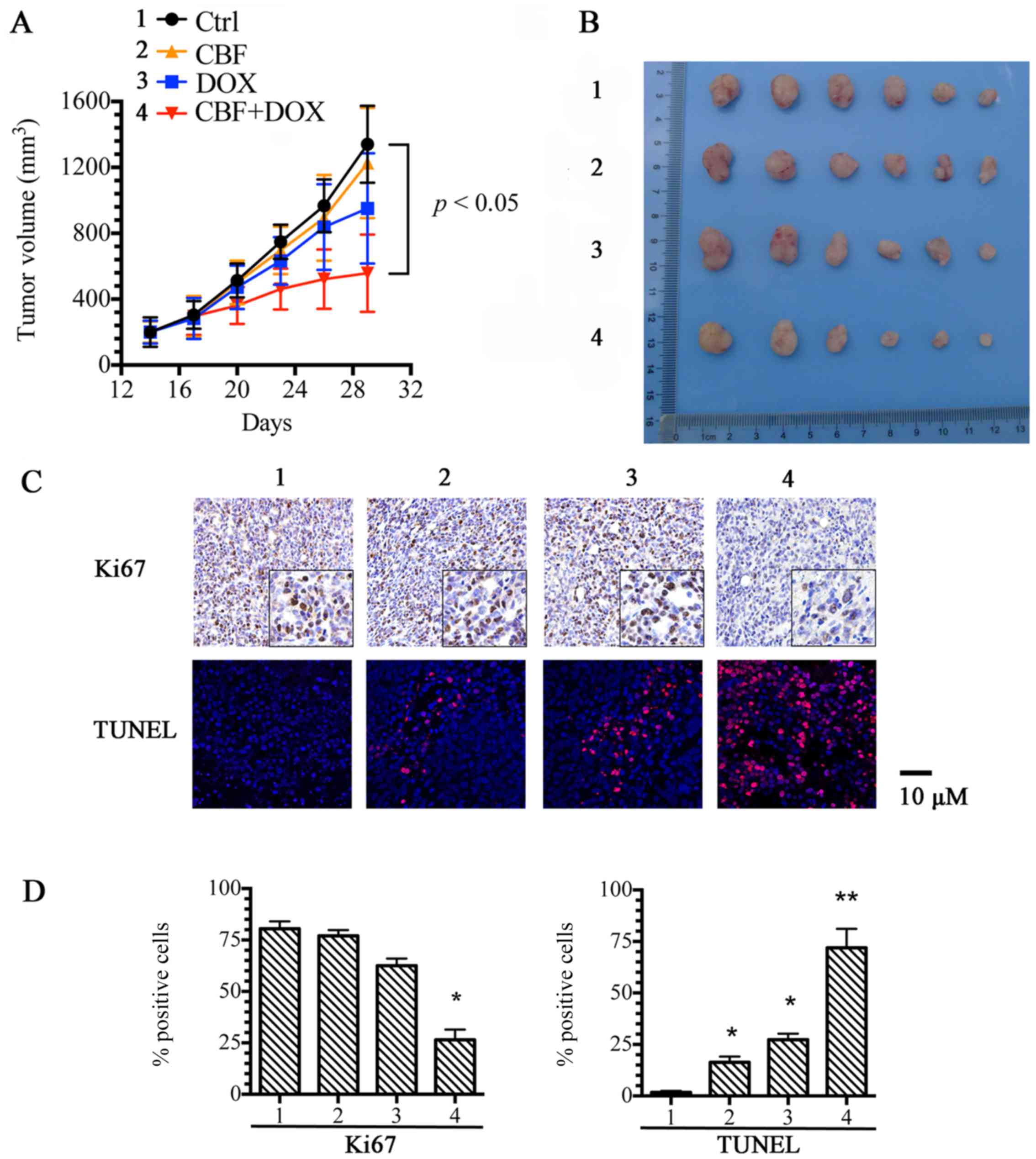 | Figure 2.Potentiation of the antitumor effects
of doxorubicin by cinobufagin (CBF) in a nude mouse xenograft
model. (A) Changes in tumor volume with time after tumor cell
inoculation. Points, mean tumor volume for each group of six mice
after implantation; bars, SD. (B) Tumor size. The image was taken
on the 29th day after implantation. The treatments were: control;
CBF (0.2 mg/kg, i.p., q3d ×5); DOX (0.1 mg/kg, i.p., q3d ×5) and
DOX (0.1 mg/kg, i.p., q3d ×5) plus CBF (0.2 mg/kg, i.p., q3d ×5,
given 1 h before DOX administration). (C) IHC for Ki67 and
immunofluorescence for TUNEL assay were performed in tumor tissues
at the end of experiments. (D) The positive rate of Ki67 and TUNEL
are based on IHC. Scale bar represents 10 µm. *p<0.05, comparing
with control group, **p<0.01, comparing with control group. |
Combination of CBF and DOX had no
significant toxicity in vivo
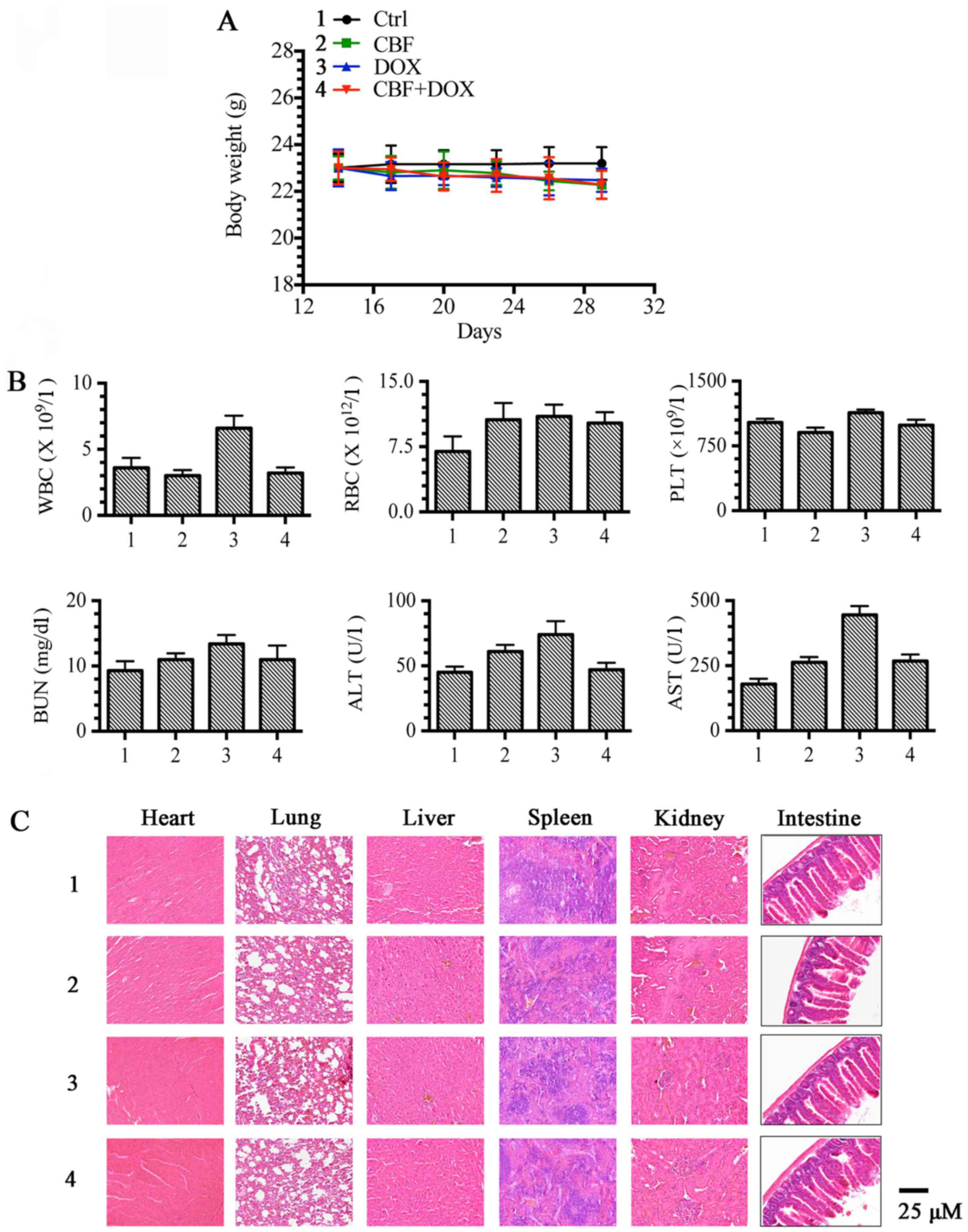
Furthermore, we investigated the toxicity effect of
CBF and DOX in vivo. As shown in Fig. 3A, none of the test subjects lost
body weight or died in any of the four groups at the doses tested.
Moreover, the routine blood parameters tested (WBCs, RBCs, PLTs),
liver (ALT and AST) and kidney (BUN) function were in the normal
range, without significant changes (Fig. 3B). Histopathology of harvested
tissues (heart, liver, lung, spleen, kidney and intestine)
displayed no abnormal changes as compared with normal tissues after
treatment with DOX, CBF, or the combination of DOX and CBF, at the
indicated doses (Fig. 3C). These
findings suggest that the combination of DOX and CBF did not
display any significant toxicity compared to the controls.
CBF enhances apoptosis rate of
chemotherapy agents in P-gp-overexpressing cells
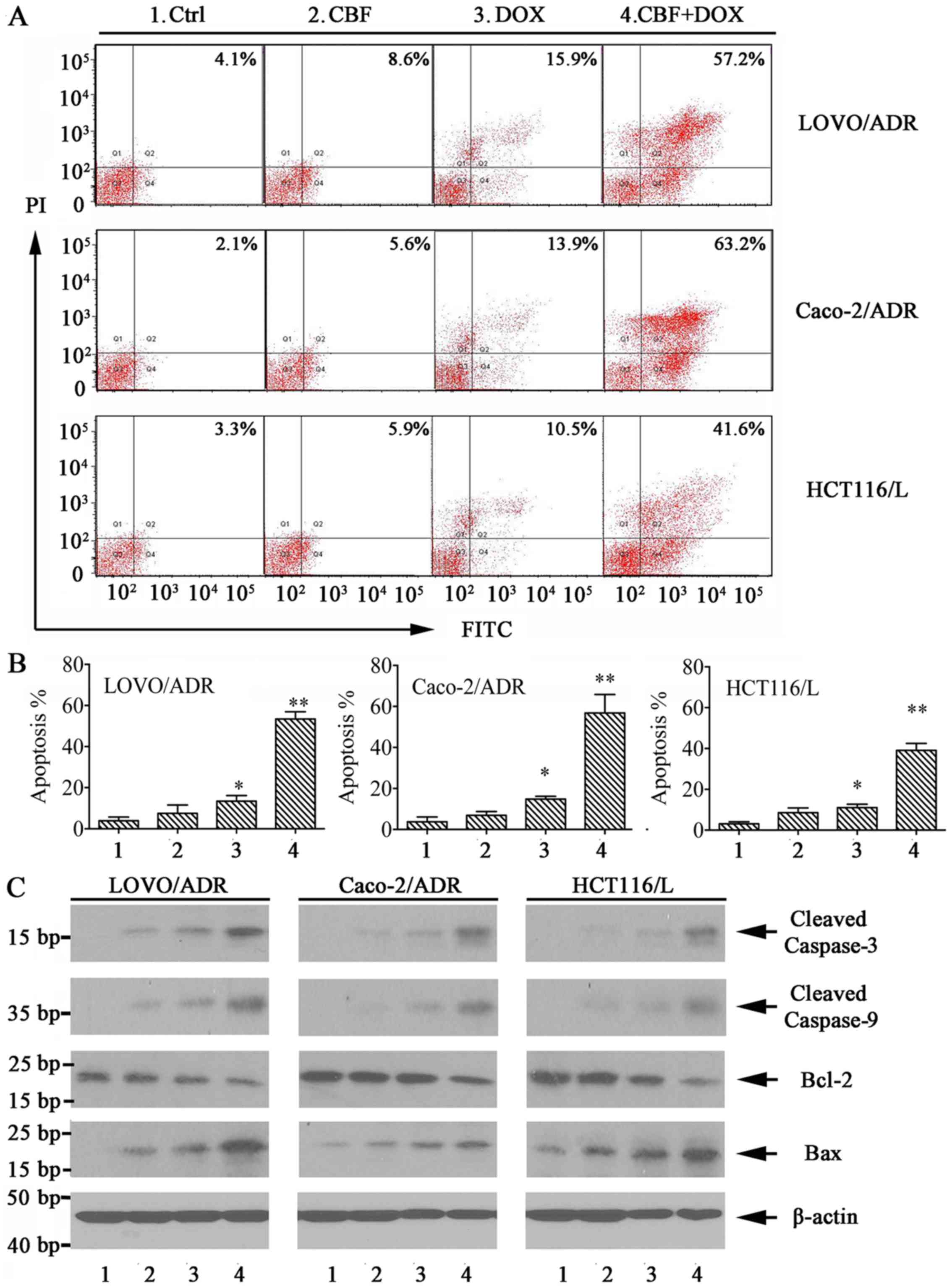
To determine whether cell apoptosis contributes to
CBF-induced cell growth inhibition, we investigated the effects of
CBF on cell apoptosis of DOX or LOHP in P-gp-overexpressing cells
by flow cytometry using the Annexin V/PI staining. The results
indicated that, the combination of CBF (20 nM) and DOX/LOHP greatly
enhanced apoptosis of LoVo/ADR, HCT116/L, and Caco-2/ADR cells
(Fig. 4A and B). Furthermore, we
examined the expression of apoptosis-related proteins, such as
cleaved caspase-3, caspase-9, Bcl-2 and Bax in P-gp-overexpressing
cells by western blot assays. As shown in Fig. 4C, expression levels of cleaved
caspase-3, caspase-9 and Bax were increased in treatment with CBF
plus DOX, while levels of Bcl-2 expression were reduced. These
findings suggested that CBF-induced apoptosis may be involved in
the enhanced cell growth inhibition of chemotherapy agents in P-gp
overexpressing cells.
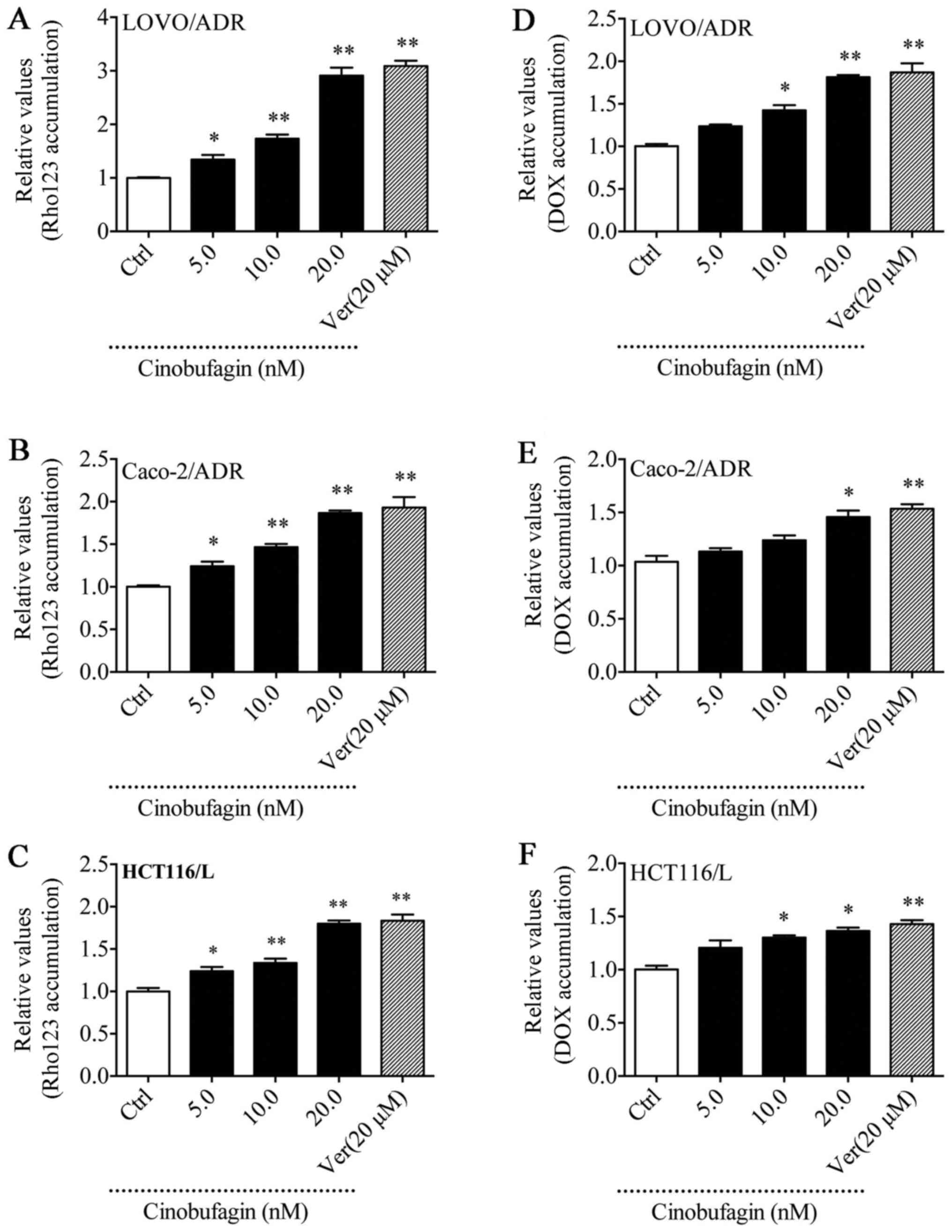
CBF increases the intracellular
accumulation of Dox and Rho123 in P-gp-overexpressing cells
Rho123 is a P-gp substrate routinely used to study
the MDR phenomenon (27). To
elucidate whether the reversal ability of CBF was associated with
increasing the intracellular concentration of drugs, we examined
the accumulation of DOX and Rho123 in LoVo/ADR, HCT116/L, and
Caco-2/ADR cells treated with CBF by flow cytometric analysis. As
shown in Fig. 5, intracellular
fluorescence intensity (MFI) of DOX and Rho123 was increased
compared with control in the presence of CBF in MDR cells. These
findings showed that CBF increased the intracellular concentration
of DOX and Rho123 in a dose-dependent manner, indicating that CBF
is able to inhibit P-gp-mediated drug efflux, thus enhancing the
intracellular accumulation of chemotherapeutic drugs.
CBF inhibited P-gp-mediated drug
transport
The permeability coefficients (Papp) of DOX (20 µM)
were tested in a Caco-2 cell monolayer model (Table III). The absorbable permeability
coefficient [Papp(A-to-B)] of DOX determined from the apical (A) to
the basolateral (B) side was 0.725±0.10×10−6 cm/sec and
the secretory permeability coefficient [Papp(B-to-A)] of DOX
obtained from the B to the A side was 3.65±0.52×10−6
cm/sec. The efflux ratio (ER), which is the ratio of
Papp(B-to-A)/Papp(A-to-B) was determined for assessing drug
transport across the cell membrane. The presence of CBF at 20 nM
reduced the ER values (2.63) compared with untreated control
monolayers (5.03), demonstrating that CBF was able to significantly
circumvent P-gp-mediated transport of DOX in the monolayers.
 | Table III.Papp and ER values of Dox in the
absence or presence of cinobufagin. |
Table III.
Papp and ER values of Dox in the
absence or presence of cinobufagin.
|
| Papp
(x10−6) |
|---|
|
|
|
|---|
| Compound | A to B | B to A | ER |
|---|
| DOX | 0.725±0.10 | 3.65±0.52 | 5.03 |
| DOX + CBF (20
nM) |
1.154±0.12b |
3.03±0.40a | 2.63c |
| DOX+Verapamil (20
µM) |
1.295±0.03b |
2.76±0.28b | 2.13c |
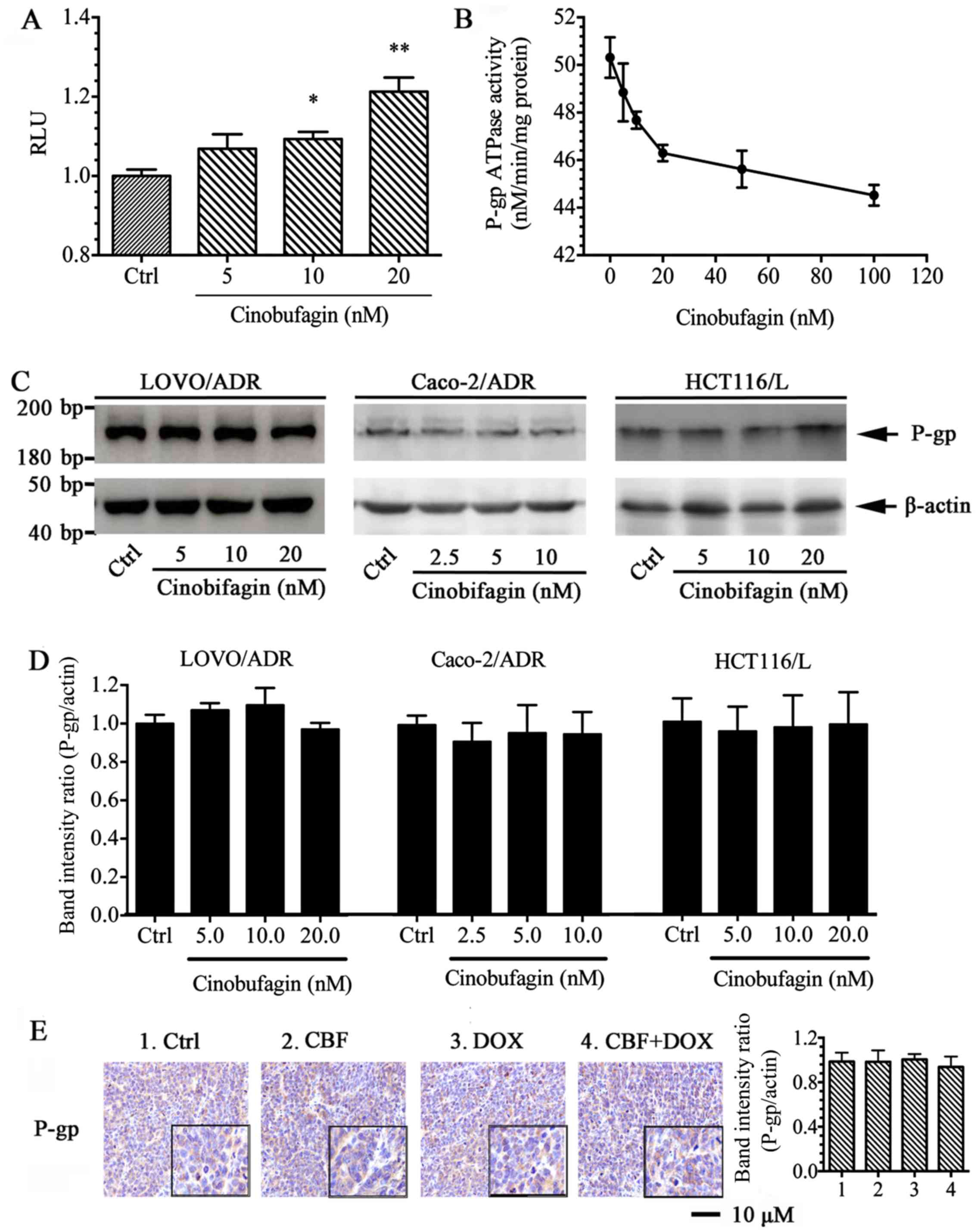
CBF inhibits P-gp ATPase activity but
had no effect on P-gp expression
In order to evaluate the effect of CBF on the P-gp
ATPase activity, we determined P-gp-mediated ATP hydrolysis at
different concentrations of CBF. In this assay the residual ATP
level, which is inversely correlated to the activity of P-gp
ATPase, was measured as luminescence in relative light units (RLU).
The results demonstrated that CBF inhibited basal- and
verapamil-stimulated ATPase activity in a dose-dependent manner
(Fig. 6A and B), suggesting that
the inhibition mechanism of P-gp ATPase by CBF was
non-competitive.
To further test whether the reversal ability of CBF
was mediated by affecting the expression of P-gp, the effects of
CBF on P-gp expression in MDR cells were analyzed by western
blotting (WB). Results indicated that, treatment with CBF at
different concentration did not alter the ABCB1 expression compared
to the negative control in LoVo/ADR, HCT116/L and Caco-2/ADR cells
(Fig. 6C and D). The
immunohistochemical analysis further supported the results that the
P-gp protein level remained unchanged in vivo (Fig. 6E). These results indicated that the
circumvention of P-gp-mediated MDR by CBF resulted from the
inhibition of P-gp transporter function but not the expression
level of P-gp.
Discussion
Multidrug resistance is the main reason for the
failure of cancer chemotherapy. Overexpression of ABC transporters
is the most important mechanism of multidrug resistance (28). P-gp is the most studied ABC
transporter, which can expel a wide range of anticancer agents from
cancer cells, resulting in drug resistance. Currently, very few
P-gp inhibitors are in clinical development, and the adverse drug
reactions seen during the clinical trials of all three generations
of P-gp inhibitors to date have prevented them continuing past the
clinical trial stage (29).
Therefore, developing novel reversal agents is a crucial and urgent
goal for overcoming multidrug resistance. Discovering effective
active ingredients or prodrugs that reverse MDR from traditional
Chinese medicines, and combining them with cytotoxic drugs has been
a promising strategy to overcome tumor multidrug resistance
(30–32). CBF is an effective active ingredient
of ChanSu, and has been proven to have antitumor activity. The
proposed mechanisms of action have been associated with killing
tumor cells, inhibiting multiplication, inducing differentiation,
inducing apoptosis, and anti-angiogenesis. However, the effect of
CBF on MDR remains unknown. Our investigation, for the first time,
assessed the potential activity of CBF in overcoming P-gp-mediated
multidrug resistance both in vivo and in vitro.
During in vitro experiments CBF demonstrated
a strong reversal effect of MDR in P-gp-overexpressing LoVo/ADR,
HCT116/L and Caco-2/ADR cells, and effectively restored the
sensitivity of DOX and L-OHP in drug resistant cells. However, we
found that CBF has no effect on MIT or CDF, which are not P-gp
substances (33,34). These results suggested that the
reversal ability of CBF was specific to inhibit P-gp. Further
evaluation included study of apoptosis rate of chemotherapeutic
drugs and Rho123 accumulation in drug-resistant cells using flow
cytometry. It was demonstrated that CBF can greatly enhance
apoptosis of DOX/LOHP and accumulation of DOX and Rho123 in a
concentration-dependent fashion in drug resistant cells. These data
were in accordance with that of the CCK-8 assays, together
demonstrating that CBF sensitized P-gp overexpressing cells to
chemotherapy drugs by enhancing cell apoptosis and increasing their
intracellular concentration.
The reversal effect of CBF can be due to inhibiting
expression of P-gp or affecting its transport function (35–37).
To fully explore the potential mechanisms, P-gp protein expression
and P-gp ATPase activity were further studied. We studied the
influence of CBF on P-gp expression in drug-resistant cells using
the western blot method. Our results show that CBF does not alter
P-gp protein expression at the concentrations used, which
demonstrated reversal of MDR. Therefore, we suspect that the
reversal effect of CBF is due to inhibiting the transport function
of P-gp. Since ABC transporters utilize the energy released from
ATP hydrolysis to transport different substrates across the cell
membrane, testing ATPase activity is another extensively used
method to assess the inhibition of ABC transporters (38). It was demonstrated that CBF inhibits
the activity of the P-gp ATPase in a concentration-dependent
manner. Based on their effects on ATPase activity, transporter
modulators could be catagorized into three distinct classes. The
1st class reduced its ATPase activity at high dose but enhanced its
activity at low dose. The 2nd class increased the ATPase activity
in a concentration-dependent fashion. The 3rd class inhibited both
basal- and verapamil-stimulated ATPase activity (39–42).
CBF should be classified under the third class of modulator and
belongs to non-competitive inhibitor group. As reported, ABC
transporters are large, membrane-bound proteins consisting of two
transmembrane domains (TMDs) and two nucleotide-binding domains
(NBDs) which mediate the active transport of substrate out of the
cell (43). An ATP-modulator could
either bind to the drug-binding pocket in TMD as a competitive
inhibitor, or block ATP-binding in NBDs as a non-competitive
inhibitor, which do not compete for active sites with the normal
substrate, but change the molecular structure of active sites,
making it unsuitable for the inhibitors (44). Furthermore, how CBF binds to NBDs
warrants further investigation.
In vivo, we observed the inhibitory effect of
CBF in the LoVo/ADR nude mouse xenograft model. We found that the
combination of DOX and CBF significantly increased the efficacy of
the antitumor activity of DOX, without inducing any significant
toxicity in vivo. Moreover, the P-gp protein level remained
unaltered as demonstrated by IHC and WB, both in vitro and
in vivo.
Most of the reported P-gp inhibitors, such as
verapamil, can circumvent MDR by inhibiting the protein expression
of P-gp. Nevertheless, normal expression of P-gp is an important
normal physiological defense mechanism, since P-gp inhibits
absorption of toxins through the small intestine, facilitates
excretion of drugs and other metabolites from the liver, prevents
xenobiotics from passing through blood-brain barrier (45,46).
Therefore, a better way to reverse P-gp mediated multidrug
resistance is to inhibit its transport function rather than affect
its expression. Therefore, CBF has been demonstrated to be a safe
and effective P-gp reversal agent worthy of further research.
In conclusion, this study demonstrated that CBF
reverses P-gp-mediated MDR by inhibiting the efflux function of
P-gp via non-competitive inhibition of P-gp ATPase activity. The
efficacy and relative safety of using CBF in combination with DOX
in vivo was demonstrated using the nude mouse xenograft
model. These results indicate that CBF may have the potential to be
used as an adjuvant therapy in combination with current
chemotherapies to augment cancer chemotherapy and prevent or
mitigate MDR.
Acknowledgements
This work was supported by the National Natural
Science Foundation of China (no. 81473482), the twelfth five-year
key subject (Integrated Chinese and Western Medicine and General
practice training of Traditional Chinese Medicine) of traditional
Chinese medicine of State Administration of Traditional Chinese
medicine and Xinglin Scholars of Shanghai University of Traditional
Chinese Medicine (no. B-X-72). This research was also supported by
the Academic leader candi- date of ‘315’ Health and family planning
commission System Project in Putuo District, Shanghai.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gustavsson B, Carlsson G, Machover D,
Petrelli N, Roth A, Schmoll HJ, Tveit KM and Gibson F: A review of
the evolution of systemic chemotherapy in the management of
colorectal cancer. Clin Colorectal Cancer. 14:1–10. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lage H: An overview of cancer multidrug
resistance: A still unsolved problem. Cell Mol Life Sci.
65:3145–3167. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kathawala RJ, Gupta P, Ashby CR Jr and
Chen ZS: The modulation of ABC transporter-mediated multidrug
resistance in cancer: A review of the past decade. Drug Resist
Updat. 18:1–17. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Binkhathlan Z and Lavasanifar A:
P-glycoprotein inhibition as a therapeutic approach for overcoming
multidrug resistance in cancer: Current status and future
perspectives. Curr Cancer Drug Targets. 13:326–346. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Callaghan R: Providing a molecular
mechanism for P-glycoprotein; why would I bother? Biochem Soc
Trans. 43:995–1002. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Breier A, Gibalova L, Seres M, Barancik M
and Sulova Z: New insight into P-glycoprotein as a drug target.
Anticancer Agents Med Chem. 3:159–170. 2013. View Article : Google Scholar
|
|
8
|
Yang K, Wu J and Li X: Recent advances in
the research of P-glycoprotein inhibitors. Biosci Trends.
2:137–146. 2008.PubMed/NCBI
|
|
9
|
Liu J, Wang S, Zhang Y, Fan HT and Lin HS:
Traditional Chinese medicine and cancer: History, present
situation, and development. Thorac Cancer. 6:561–569. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nie J, Zhao C, Deng LI, Chen J, Yu B, Wu
X, Pang P and Chen X: Efficacy of traditional Chinese medicine in
treating cancer. Biomed Rep. 4:3–14. 2016.PubMed/NCBI
|
|
11
|
Li C, Hashimi SM, Cao S, Qi J, Good D,
Duan W and Wei MQ: Chansu inhibits the expression of cortactin in
colon cancer cell lines in vitro and in vivo. BMC Complement Altern
Med. 15:2072015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Qi F, Li A, Inagaki Y, Kokudo N, Tamura S,
Nakata M and Tang W: Antitumor activity of extracts and compounds
from the skin of the toad Bufo bufo gargarizans Cantor. Int
Immunopharmacol. 11:342–349. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yu CH, Kan SF, Pu HF, Jea Chien E and Wang
PS: Apoptotic signaling in bufalin- and cinobufagin-treated
androgen-dependent and -independent human prostate cancer cells.
Cancer Sci. 99:2467–2476. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Qi F, Inagaki Y, Gao B, Cui X, Xu H,
Kokudo N, Li A and Tang W: Bufalin and cinobufagin induce apoptosis
of human hepatocellular carcinoma cells via Fas- and
mitochondria-mediated pathways. Cancer Sci. 102:951–958. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Baek SH, Kim C, Lee JH, Nam D, Lee J, Lee
SG, Chung WS, Jang HJ, Kim SH and Ahn KS: Cinobufagin exerts
anti-proliferative and pro-apoptotic effects through the modulation
ROS-mediated MAPKs signaling pathway. Immunopharmacol
Immunotoxicol. 37:265–273. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang G, Wang C, Sun M, Li J, Wang B, Jin
C, Hua P, Song G, Zhang Y, Nguyen LL, et al: Cinobufagin inhibits
tumor growth by inducing intrinsic apoptosis through AKT signaling
pathway in human nonsmall cell lung cancer cells. Oncotarget.
7:28935–28946. 2016.PubMed/NCBI
|
|
17
|
Hu Q, Liang B, Sun Y, Guo XL, Bao YJ, Xie
DH, Zhou M, Duan YR, Yin PH and Peng ZH: Preparation of
bufalin-loaded pluronic polyetherimide nanoparticles, cellular
uptake, distribution, and effect on colorectal cancer. Int J
Nanomedicine. 9:4035–4041. 2014.PubMed/NCBI
|
|
18
|
Yin P, Wang Y, Qiu Y, Hou L, Liu X, Qin J,
Duan Y, Liu P, Qiu M and Li Q: Bufalin-loaded mPEG-PLGA-PLL-cRGD
nanoparticles: Preparation, cellular uptake, tissue distribution,
and anticancer activity. Int J Nanomedicine. 7:3961–3969.
2012.PubMed/NCBI
|
|
19
|
Qiu YY, Hu Q, Tang QF, Feng W, Hu SJ,
Liang B, Peng W and Yin PH: MicroRNA-497 and bufalin act
synergistically to inhibit colorectal cancer metastasis. Tumour
Biol. 35:2599–2606. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang Z, Zhang L, Ni Z, Sun J, Gao H, Cheng
Z, Xu J and Yin P: Resveratrol induces AMPK-dependent MDR1
inhibition in colorectal cancer HCT116/L-OHP cells by preventing
activation of NF-κB signaling and suppressing cAMP-responsive
element transcriptional activity. Tumour Biol. 36:9499–9510. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhou RP, Chen G, Shen ZL and Pan LQ:
Cinobufacin suppresses cell proliferation via miR-494 in BGC-823
gastric cancer cells. Asian Pac J Cancer Prev. 15:1241–1245. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fu L, Liang Y, Deng L, Ding Y, Chen L, Ye
Y, Yang X and Pan Q: Characterization of tetrandrine, a potent
inhibitor of P-glycoprotein-mediated multidrug resistance. Cancer
Chemother Pharmacol. 53:349–356. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang L, Lin G, Kovács B, Jani M, Krajcsi
P and Zuo Z: Mechanistic study on the intestinal absorption and
disposition of baicalein. Eur J Pharm Sci. 31:221–231. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ambudkar SV: Drug-stimulatable ATPase
activity in crude membranes of human MDR1-transfected mammalian
cells. Methods Enzymol. 292:504–514. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kathawala RJ, Chen JJ, Zhang YK, Wang YJ,
Patel A, Wang DS, Talele TT, Ashby CR Jr and Chen ZS: Masitinib
antagonizes ATP-binding cassette subfamily G member 2-mediated
multidrug resistance. Int J Oncol. 44:1634–1642. 2014.PubMed/NCBI
|
|
26
|
Chen LM, Liang YJ, Ruan JW, Ding Y, Wang
XW, Shi Z, Gu LQ, Yang XP and Fu LW: Reversal of P-gp mediated
multidrug resistance in-vitro and in-vivo by FG020318. J Pharm
Pharmacol. 56:1061–1066. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim HJ, Lee KY, Kim YW, Choi YJ, Lee JE,
Choi CM, Baek IJ, Rho JK and Lee JC: P-glycoprotein confers
acquired resistance to 17-DMAG in lung cancers with an ALK
rearrangement. BMC Cancer. 15:5532015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wu Q, Yang Z, Nie Y, Shi Y and Fan D:
Multi-drug resistance in cancer chemotherapeutics: Mechanisms and
lab approaches. Cancer Lett. 347:159–166. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Coley HM: Overcoming multidrug resistance
in cancer: Clinical studies of P-glycoprotein inhibitors. Methods
Mol Biol. 596:341–358. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chai S, To KK and Lin G: Circumvention of
multi-drug resistance of cancer cells by Chinese herbal medicines.
Chin Med. 5:262010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hu T, To KK, Wang L, Zhang L, Lu L, Shen
J, Chan RL, Li M, Yeung JH and Cho CH: Reversal of P-glycoprotein
(P-gp) mediated multidrug resistance in colon cancer cells by
cryptotanshinone and dihydrotanshinone of Salvia miltiorrhiza.
Phytomedicine. 21:1264–1272. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen G, Wang K, Yang BY, Tang B, Chen JX
and Hua ZC: Synergistic antitumor activity of oridonin and arsenic
trioxide on hepatocellular carcinoma cells. Int J Oncol.
40:139–147. 2012.PubMed/NCBI
|
|
33
|
Aspenström-Fagerlund B, Tallkvist J,
Ilbäck NG and Glynn AW: Oleic acid increases intestinal absorption
of the BCRP/ABCG2 substrate, mitoxantrone, in mice. Toxicol Lett.
237:133–139. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zamek-Gliszczynski MJ, Xiong H, Patel NJ,
Turncliff RZ, Pollack GM and Brouwer KL: Pharmacokinetics of 5 (and
6)-carboxy-2′,7′-dichlorofluorescein and its diacetate promoiety in
the liver. J Pharmacol Exp Ther. 304:801–809. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu KJ, He JH, Su XD, Sim HM, Xie JD, Chen
XG, Wang F, Liang YJ, Singh S, Sodani K, et al: Saracatinib
(AZD0530) is a potent modulator of ABCB1-mediated multidrug
resistance in vitro and in vivo. Int J Cancer. 132:224–235. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhao XQ, Xie JD, Chen XG, Sim HM, Zhang X,
Liang YJ, Singh S, Talele TT, Sun Y, Ambudkar SV, et al: Neratinib
reverses ATP-binding cassette B1-mediated chemotherapeutic drug
resistance in vitro, in vivo, and ex vivo. Mol Pharmacol. 82:47–58.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ma B, Chai S, Li N, To KK, Kan WL, Yang D
and Lin G: Reversal of P-glycoprotein-mediated multidrug resistance
by a synthetic α-aminoxy peptidomimetic. Int J Pharm. 424:33–39.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Dwivedi GR, Tiwari N, Singh A, Kumar A,
Roy S, Negi AS, Pal A, Chanda D, Sharma A and Darokar MP: Gallic
acid-based indanone derivative interacts synergistically with
tetracycline by inhibiting efflux pump in multidrug resistant E.
coli. Appl Microbiol Biotechnol. 100:2311–2325. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mi Y and Lou L: ZD6474 reverses multidrug
resistance by directly inhibiting the function of P-glycoprotein.
Br J Cancer. 97:934–940. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Mi YJ, Liang YJ, Huang HB, Zhao HY, Wu CP,
Wang F, Tao LY, Zhang CZ, Dai CL, Tiwari AK, et al: Apatinib
(YN968D1) reverses multidrug resistance by inhibiting the efflux
function of multiple ATP-binding cassette transporters. Cancer Res.
70:7981–7991. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Dai CL, Tiwari AK, Wu CP, Su XD, Wang SR,
Liu DG, Ashby CR Jr, Huang Y, Robey RW, Liang YJ, et al: Lapatinib
(Tykerb, GW572016) reverses multidrug resistance in cancer cells by
inhibiting the activity of ATP-binding cassette subfamily B member
1 and G member 2. Cancer Res. 68:7905–7914. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Shi Z, Peng XX, Kim IW, Shukla S, Si QS,
Robey RW, Bates SE, Shen T, Ashby CR Jr, Fu LW, et al: Erlotinib
(Tarceva, OSI-774) antagonizes ATP-binding cassette subfamily B
member 1 and ATP-binding cassette subfamily G member 2-mediated
drug resistance. Cancer Res. 67:11012–11020. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chufan EE, Sim HM and Ambudkar SV:
Molecular basis of the polyspecificity of P-glycoprotein (ABCB1):
Recent biochemical and structural studies. Adv Cancer Res.
125:71–96. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang T, Sun Y, Ma W, Yang Z, Yang J, Liu
J, Fan H, Yang Y, Gu J, Fawcett JP, et al: Trantinterol, a novel
β2-adrenoceptor agonist, noncompetitively inhibits P-glycoprotein
function in vitro and in vivo. Mol Pharm. 12:1–9. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
McCormick JW, Vogel PD and Wise JG:
Multiple drug transport pathways through human P-glycoprotein.
Biochemistry. 54:4374–4390. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Aller SG, Yu J, Ward A, Weng Y,
Chittaboina S, Zhuo R, Harrell PM, Trinh YT, Zhang Q, Urbatsch IL,
et al: Structure of P-glycoprotein reveals a molecular basis for
poly-specific drug binding. Science. 323:1718–1722. 2009.
View Article : Google Scholar : PubMed/NCBI
|