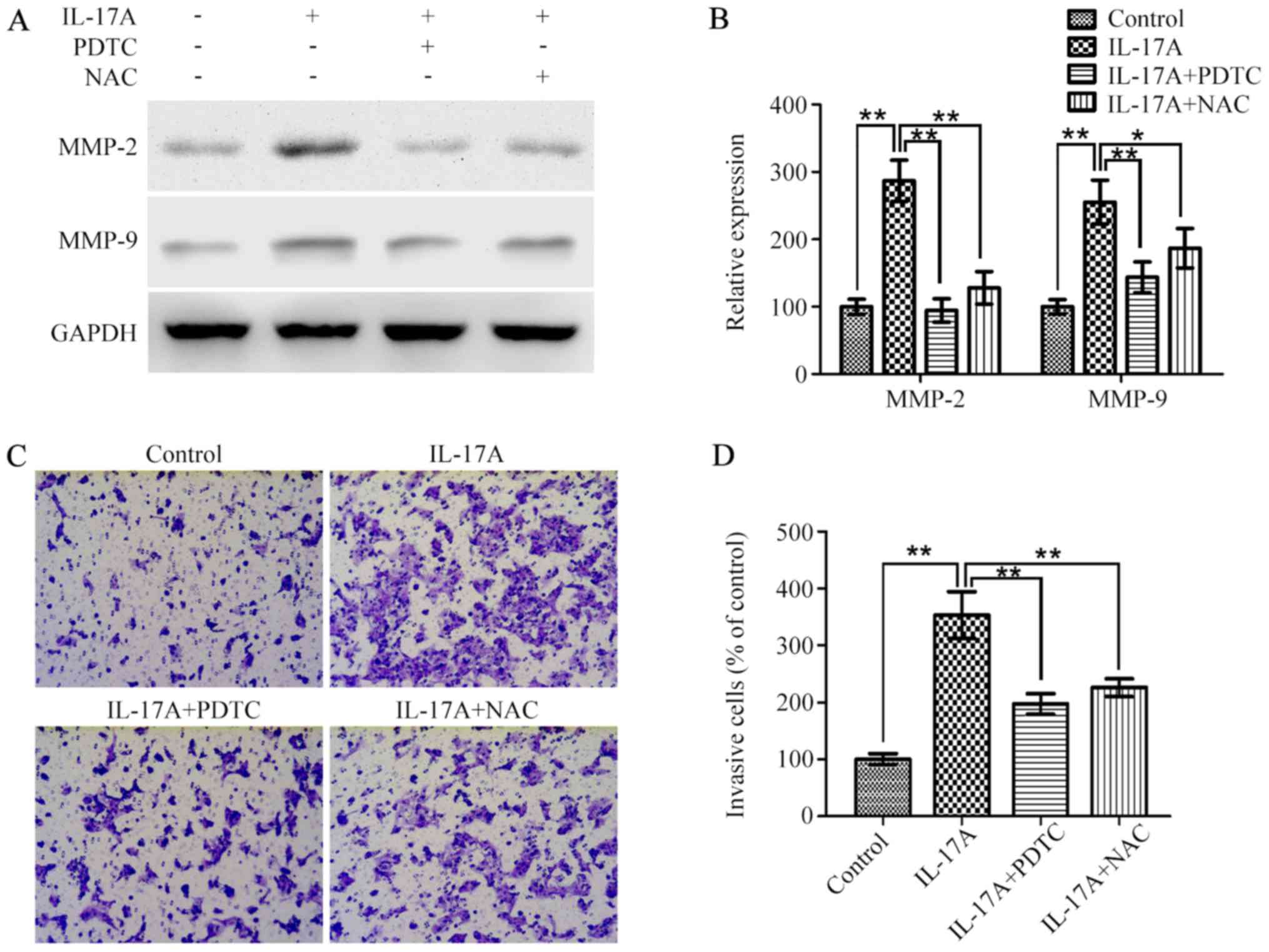
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pohl H, Sirovich B and Welch HG:
Esophageal adenocarcinoma incidence: Are we reaching the peak?
Cancer Epidemiol Biomarkers Prev. 19:1468–1470. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Holmes RS and Vaughan TL: Epidemiology and
pathogenesis of esophageal cancer. Semin Radiat Oncol. 17:2–9.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hvid-Jensen F, Pedersen L, Drewes AM,
Sørensen HT and Funch-Jensen P: Incidence of adenocarcinoma among
patients with Barrett's esophagus. N Engl J Med. 365:1375–1383.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
O'Sullivan KE, Phelan JJ, O'Hanlon C,
Lysaght J, O'Sullivan JN and Reynolds JV: The role of inflammation
in cancer of the esophagus. Expert Rev Gastroenterol Hepatol.
8:749–760. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Miyashita T, Tajima H, Shah FA, Oshima M,
Makino I, Nakagawara H, Kitagawa H, Fujimura T, Harmon JW and Ohta
T: Impact of inflammation-metaplasia-adenocarcinoma sequence and
inflammatory microenvironment in esophageal carcinogenesis using
surgical rat models. Ann Surg Oncol. 21:2012–2019. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pappu R, Ramirez-Carrozzi V and Sambandam
A: The interleukin-17 cytokine family: Critical players in host
defence and inflammatory diseases. Immunology. 134:8–16. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dhillion P, Wallace K, Herse F, Scott J,
Wallukat G, Heath J, Mosely J, Martin JN Jr, Dechend R and LaMarca
B: IL-17-mediated oxidative stress is an important stimulator of
AT1-AA and hypertension during pregnancy. Am J Physiol Regul Integr
Comp Physiol. 303:R353–R358. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pietrowski E, Bender B, Huppert J, White
R, Luhmann HJ and Kuhlmann CR: Pro-inflammatory effects of
interleukin-17A on vascular smooth muscle cells involve NAD(P)H-
oxidase derived reactive oxygen species. J Vasc Res. 48:52–58.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Miyahara Y, Odunsi K, Chen W, Peng G,
Matsuzaki J and Wang RF: Generation and regulation of human CD4+
IL-17-producing T cells in ovarian cancer. Proc Natl Acad Sci USA.
105:15505–15510. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Steiner GE, Newman ME, Paikl D, Stix U,
Memaran-Dagda N, Lee C and Marberger MJ: Expression and function of
pro-inflammatory interleukin IL-17 and IL-17 receptor in normal,
benign hyperplastic, and malignant prostate. Prostate. 56:171–182.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang B, Rong G, Wei H, Zhang M, Bi J, Ma
L, Xue X, Wei G, Liu X and Fang G: The prevalence of Th17 cells in
patients with gastric cancer. Biochem Biophys Res Commun.
374:533–537. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang L, Yi T, Kortylewski M, Pardoll DM,
Zeng D and Yu H: IL-17 can promote tumor growth through an
IL-6-Stat3 signaling pathway. J Exp Med. 206:1457–1464. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gu FM, Li QL, Gao Q, Jiang JH, Zhu K,
Huang XY, Pan JF, Yan J, Hu JH, Wang Z, et al: IL-17 induces
AKT-dependent IL-6/JAK2/STAT3 activation and tumor progression in
hepatocellular carcinoma. Mol Cancer. 10:1502011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang JP, Yan J, Xu J, Pang XH, Chen MS,
Li L, Wu C, Li SP and Zheng L: Increased intratumoral
IL-17-producing cells correlate with poor survival in
hepatocellular carcinoma patients. J Hepatol. 50:980–989. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lu L, Pan K, Zheng HX, Li JJ, Qiu HJ, Zhao
JJ, Weng DS, Pan QZ, Wang DD, Jiang SS, et al: IL-17A promotes
immune cell recruitment in human esophageal cancers and the
infiltrating dendritic cells represent a positive prognostic marker
for patient survival. J Immunother. 36:451–458. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lu L, Weng C, Mao H, Fang X, Liu X, Wu Y,
Cao X, Li B, Chen X, Gan Q, et al: IL-17A promotes migration and
tumor killing capability of B cells in esophageal squamous cell
carcinoma. Oncotarget. 7:21853–21864. 2016.PubMed/NCBI
|
|
18
|
Lv L, Pan K, Li XD, She KL, Zhao JJ, Wang
W, Chen JG, Chen YB, Yun JP and Xia JC: The accumulation and
prognosis value of tumor infiltrating IL-17 producing cells in
esophageal squamous cell carcinoma. PLoS One. 6:e182192011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang B, Li L, Liao Y, Li J, Yu X, Zhang Y,
Xu J, Rao H, Chen S, Zhang L, et al: Mast cells expressing
interleukin 17 in the muscularis propria predict a favorable
prognosis in esophageal squamous cell carcinoma. Cancer Immunol
Immunother. 62:1575–1585. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Numasaki M, Watanabe M, Suzuki T,
Takahashi H, Nakamura A, McAllister F, Hishinuma T, Goto J, Lotze
MT, Kolls JK, et al: IL-17 enhances the net angiogenic activity and
in vivo growth of human non-small cell lung cancer in SCID mice
through promoting CXCR-2-dependent angiogenesis. J Immunol.
175:6177–6189. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gaffen SL: Structure and signalling in the
IL-17 receptor family. Nat Rev Immunol. 9:556–567. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hadler-Olsen E, Winberg JO and
Uhlin-Hansen L: Matrix metalloproteinases in cancer: Their value as
diagnostic and prognostic markers and therapeutic targets. Tumour
Biol. 34:2041–2051. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tochhawng L, Deng S, Pervaiz S and Yap CT:
Redox regulation of cancer cell migration and invasion.
Mitochondrion. 13:246–253. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang Y, Ma J, Shen H, Wang C, Sun Y,
Howell SB and Lin X: Reactive oxygen species promote ovarian cancer
progression via the HIF-1α/LOX/E-cadherin pathway. Oncol Rep.
32:2150–2158. 2014.PubMed/NCBI
|
|
25
|
Zhang H, Wang ZW, Wu HB, Li Z, Li LC, Hu
XP, Ren ZL, Li BJ and Hu ZP: Transforming growth factor-β1 induces
matrix metalloproteinase-9 expression in rat vascular smooth muscle
cells via ROS-dependent ERK-NF-κB pathways. Mol Cell Biochem.
375:11–21. 2013.PubMed/NCBI
|
|
26
|
Zhang R, Yin X, Shi H, Wu J, Shakya P, Liu
D and Zhang J: Adiponectin modulates DCA-induced inflammation via
the ROS/NF-κB signaling pathway in esophageal adenocarcinoma cells.
Dig Dis Sci. 59:89–97. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang R, Wu J, Liu D, Shan H and Zhang J:
Anti-inflammatory effect of full-length adiponectin and
proinflammatory effect of globular adiponectin in esophageal
adenocarcinoma cells. Oncol Res. 21:15–21. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen D, Hu Q, Mao C, Jiao Z, Wang S, Yu L,
Xu Y, Dai D, Yin L and Xu H: Increased IL-17-producing CD4(+) T
cells in patients with esophageal cancer. Cell Immunol.
272:166–174. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Deryugina EI and Quigley JP: Matrix
metalloproteinases and tumor metastasis. Cancer Metastasis Rev.
25:9–34. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xu B, Guenther JF, Pociask DA, Wang Y,
Kolls JK, You Z, Chandrasekar B, Shan B, Sullivan DE and Morris GF:
Promotion of lung tumor growth by interleukin-17. Am J Physiol Lung
Cell Mol Physiol. 307:L497–L508. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cho KA, Suh JW, Lee KH, Kang JL and Woo
SY: IL-17 and IL-22 enhance skin inflammation by stimulating the
secretion of IL-1β by keratinocytes via the ROS-NLRP3-caspase-1
pathway. Int Immunol. 24:147–158. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sønder SU, Saret S, Tang W, Sturdevant DE,
Porcella SF and Siebenlist U: IL-17-induced NF-kappaB activation
via CIKS/Act1: Physiologic significance and signaling mechanisms. J
Biol Chem. 286:12881–12890. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Song S, Guha S, Liu K, Buttar NS and
Bresalier RS: COX-2 induction by unconjugated bile acids involves
reactive oxygen species-mediated signalling pathways in Barrett's
oesophagus and oesophageal adenocarcinoma. Gut. 56:1512–1521. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Abdel-Latif MM, O'Riordan J, Windle HJ,
Carton E, Ravi N, Kelleher D and Reynolds JV: NF-kappaB activation
in esophageal adenocarcinoma: Relationship to Barrett's metaplasia,
survival, and response to neoadjuvant chemoradiotherapy. Ann Surg.
239:491–500. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yun HS, Baek JH, Yim JH, Lee SJ, Lee CW,
Song JY, Um HD, Park JK, Park IC and Hwang SG: Knockdown of
hepatoma-derived growth factor-related protein-3 induces apoptosis
of H1299 cells via ROS-dependent and p53-independent NF-κB
activation. Biochem Biophys Res Commun. 449:471–476. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sun L, Li W, Li W, Xiong L, Li G and Ma R:
Astragaloside IV prevents damage to human mesangial cells through
the inhibition of the NADPH oxidase/ROS/Akt/NF-κB pathway under
high glucose conditions. Int J Mol Med. 34:167–176. 2014.PubMed/NCBI
|
|
37
|
Wang Y, Wu H, Wu X, Bian Z and Gao Q:
Interleukin 17A promotes gastric cancer invasiveness via NF-κB
mediated matrix metalloproteinases 2 and 9 expression. PLoS One.
9:e966782014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ren H, Wang Z, Zhang S, Ma H, Wang Y, Jia
L and Li Y: IL-17A promotes the migration and invasiveness of
colorectal cancer cells through NF-κB mediated MMP expression.
Oncol Res. 23:249–256. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Feng M, Wang Y, Chen K, Bian Z, Jinfang Wu
and Gao Q: IL-17A promotes the migration and invasiveness of
cervical cancer cells by coordinately activating MMPs expression
via the p38/NF-κB signal pathway. PLoS One. 9:e1085022014.
View Article : Google Scholar : PubMed/NCBI
|