Introduction
Esophageal cancer is the eighth most common
malignant disease worldwide and also ranks as the sixth most common
cause of cancer mortality (1). As
one of the main histological types of esophageal cancer, the
incidence rate of esophageal adenocarcinoma (EAC) was reported to
be rapidly increasing among the past three decades (2). In comparison to esophageal squamous
cell carcinoma (ESCC), EAC often shows less response to
chemotherapy and radiotherapy, which leads to a relative poor
prognosis of EAC patients. In addition, EAC is often diagnosed at
advanced stages due to its early metastasis which is also
considered as the most frequent cause of death in EAC patients
(3). As a consequence, it has
always been meaningful to explore the molecular mechanism
underlying EAC metastasis and identify novel potent
chemotherapeutic targets which may be applied to suppress cancer
metastasis and in turn improve the prognosis.
The development of EAC is mostly associated with
Barrett's esophagus, a precancerous lesion of esophagus, which is
considered as a complication of gastroesophageal reflux disease
(4). Accordingly, there has been
increasing scientific evidence suggesting that chronic inflammation
induced by duodenogastroesophageal refluxate plays a vital role in
the pathogenesis and development of EAC (5,6). In
the course of the carcinogenic process, the inflammatory cells and
pro-inflammatory cytokines provide a proper microenvironment for
tumor growth, invasion, and metastasis.
Interleukin-17A (IL-17A), a pro-inflammatory
cytokine mainly secreted by Th17 cells, is proved to play an
important role in many inflammatory and autoimmune diseases
(7). Some evidence has revealed
that IL-17A could cause an increase in generation of ROS, leading
to a proinflammatory activation (8,9).
Recent studies have shown that IL-17A or IL-17-producing cells were
present in the microenvironment of certain inflammation-related
tumors such as ovarian, prostatic and gastric cancer (10–12).
However, the role of IL-17A in cancer development remains
controversial. Some studies revealed that IL-17A promoted tumor
growth through inducing IL-6 production, which in turn upregulated
pro-survival and pro-angiogenic genes (13,14).
Moreover, increased intratumoral IL-17-producing cells were found
to be correlated with poor survival in hepatocellular carcinoma
patients (15). On the contrary,
some studies revealed that IL-17A could induce ESCC tumor cells to
produce inflammatory chemokines, which are connected with the
migration of T cells, NK cells, DCs, and B cells (16,17).
Clinical evidence also proved that tumor infiltrating
IL-17A-producing cells correlated with better overall survival of
ESCC patients and might serve as a potential prognostic marker for
ESCC (18,19).
To date, little is known about the role of IL-17A in
EAC development. Therefore, the goal of the present study was to
investigate whether IL-17A is involved in the regulation of EAC
invasiveness, and if so, to explore the effect of ROS/NF-κB/MMP-2/9
signaling pathway underlying IL-17A-regulated EAC invasiveness.
Materials and methods
Cell line and cell culture
The human esophagus adenocarcinoma cell line OE19
was a gift from the Gastroenterology Department of Southwest
Hospital of Third Military Medical University. OE19 cells were
cultured in RPMI-1640 medium supplemented with 10% fetal bovine
serum (Gibco, Grand Island, NY, USA), L-glutamine, 100 U/ml
penicillin and 0.1 mg/ml streptomycin at 37°C, 5% CO2
air atmosphere.
Flow cytometry
Surface IL-17 receptor (IL-17R) expression on OE19
cells was examined using flow cytometry as described previously
(20). In brief, OE19 cells were
washed three times, harvested, and resuspended in cold PBS. Then
the cells were incubated with PE-labeled mouse anti-human IL-17RA
antibody (eBioscience, CA, USA) in the dark for 1 h at 4°C.
Isotype-matched antibody was used as a negative control. After
washing, at least 1×105 stained cells were analyzed
using flow cytometry (BD Biosciences). Fluorescence-positive cells
were quantified.
Cell proliferation assay
The effect of IL-17A on OE19 cell proliferation was
measured by MTT assay. The cells were seeded in a 96-well plate at
a density of 5,000 monolayer cells per well. After 24 h, the cells
were incubated with IL-17A (rhIL-17A, R&D System, Minneapolis,
MN, USA) in increasing concentrations (0, 1, 5, 10, 50 and 100
ng/ml) for 24, 48 and 72 h, respectively. Subsequently, the cells
were washed with PBS and incubated with 20 µl MTT solution (5 g/l)
for 4 h. After that, 150 µl DMSO was added to each well to dissolve
the crystals and then the plates were shaken for 10 min in the
dark. Finally, the optical density (OD) was measured at 490 nm
using multifunctional fluorescence microplate reader (Polarstar
Otima, BMG, Australia).
Cell migration assay
Cell migration ability in vitro was assessed
by wound-healing assay. OE19 cells were seeded in 12-well flat
bottomed plates and grown to ~80–90% monolayer confluence. A
sterile pipette tip was used to carefully scratch a straight strip
in the center of the well. After washing the cell debris, a wide
range of doses of IL-17A (0, 1, 10 and 100 ng/ml) was added to
cells. After 24 h incubation, the wound edges of different
treatment groups were observed and captured. The migration rate was
expressed as the percentage of average migration distance to
average starting (0 h) wound distance, respectively.
Cell invasion assay
The in vitro invasive assay was performed
with 24-well Transwell (8-µm pore size; Corning, NY, USA) precoated
with Matrigel (BD Biosciences, San Jose, CA, USA). Cells
(2×105), suspended in 200 µl serum-free medium in the
absence or presence of IL-17A (1, 10 and 100 ng/ml), were seeded
into the upper chamber, and 500 µl medium supplemented with 10% FBS
was placed into the lower chamber. After 24 h incubation, the cells
on the upper surface of the membrane were removed by cotton swabs.
Subsequently, the cells on the lower surface were fixed with
paraformaldehyde, and stained with crystal violet. Finally,
invasive cells in five microscopic fields were counted and
captured.
Measurement of intracellular ROS
levels
Intracellular ROS levels were measured by
oxidation-sensitive fluorescent dye DCFH-DA. OE19 cells were
preconditioned with NAC (5 mM, Beyotime, Shanghai, China) or
vehicle for 2 h, then stimulated with IL-17A (100 ng/ml) for
another 6 h. After treatment, cells were washed twice and incubated
in 10 µM DCFH-DA (Invitrogen, Carlsbad, CA, USA) for 30 min. Then
the cells were washed three times and collected for further
analysis. Fluorescence was detected by flow cytometry and
fluorescence microscopy, respectively.
Western blot analysis
OE19 cells grown in 6-well plates were pretreated
with NAC or PDTC (100 µM, Sigma-Aldrich, St. Louis, MO, USA) for 2
h, then stimulated with IL-17A (100 ng/ml) for 6 h (for the
measurement of NF-κB p65, p50, p-IκB-α), or 24 h (for the
measurement of MMP-2/9). After incubation, whole cell proteins were
extracted using cell lysis buffer. Nuclear lysates were harvested
using NucBuster™ Protein Extraction kit (Novagen, Darmstadt,
Germany) according to the manufacturer's instructions. Proteins
were boiled for 5 min, separated by 8 or 10% SDS-PAGE, transferred
to PVDF membranes, and incubated overnight at 4°C with primary
antibodies against NF-κB p65 (1:1,000, Millipore), NF-κB p50
(1:1,000, Millipore), p-IκB-α (1:500, Cell Signaling Technology),
MMP-2 and MMP-9 (1:1,000, Abcam), then followed by a secondary
antibody for 2 h at room temperature. Histone H1 and GAPDH
(1:2,000, Santa Cruz Biotechnology) were used as loading
control.
Statistical analysis
All data are represented as mean ± SD from at least
three independent experiments performed in triplicate. One-way
ANOVA and Student's t-test were conducted to analyze the difference
between groups using SPSS 17.0 software. P<0.05 was considered
statistically significant.
Results
IL-17A has no direct effect on OE19
cell proliferation
In order to assess the biological effects of IL-17A
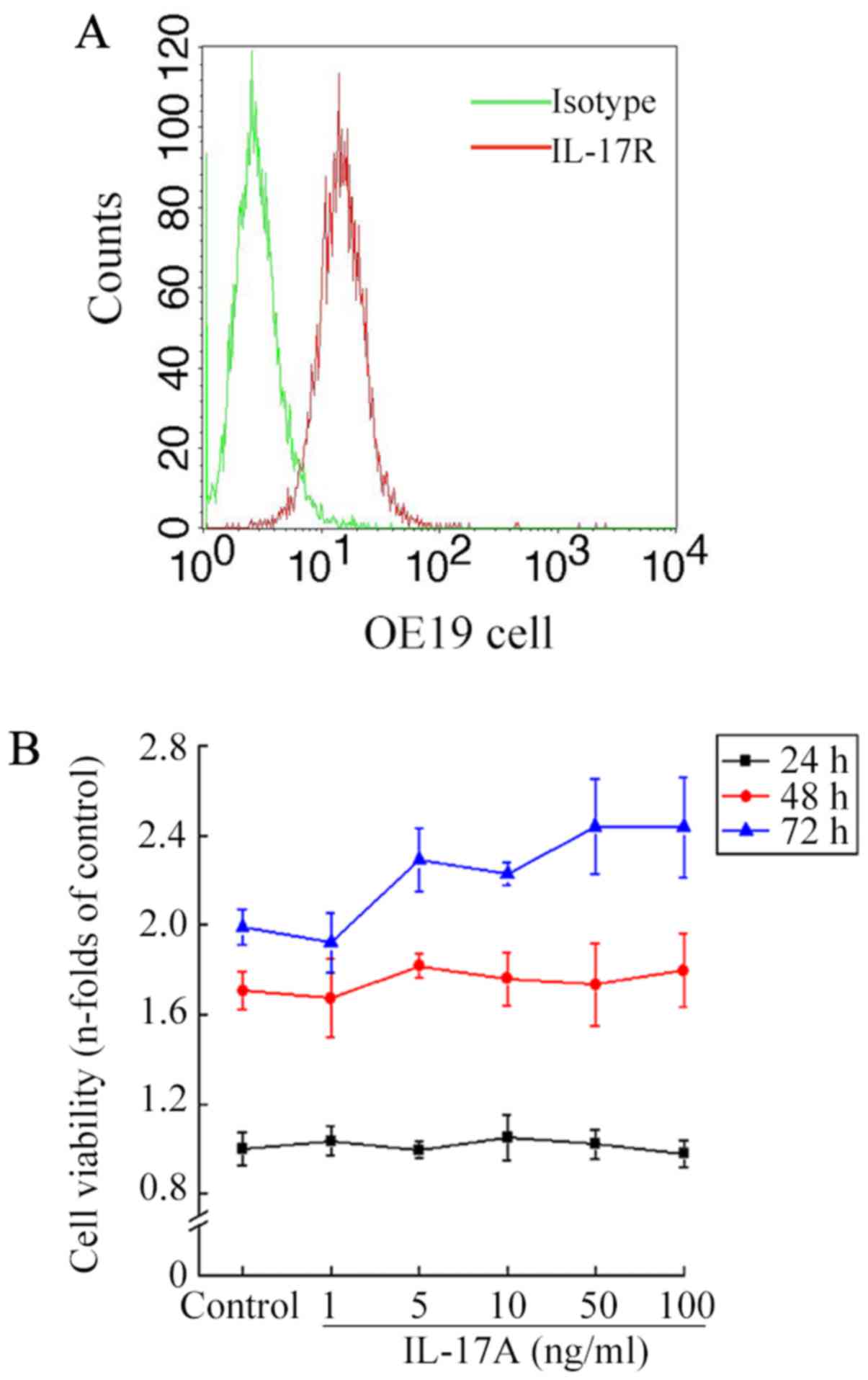
on OE19 cells, firstly we examined the expression of IL-17R on OE19
cells. Flow cytometry confirmed that the cells expressed IL-17RA on
the surface at the protein level (Fig.
1A). This result is in accordance with the ubiquitous
expression of IL-17R (21).
Subsequently, MTT assay was used to test the possibility that
IL-17A might modulate OE19 cell proliferation in vitro.
Cells were treated with IL-17A at concentrations ranging from 1 to
100 ng/ml. The result showed that the cell viability did not differ
significantly among groups with different IL-17A concentrations at
the exposure times of 24, 48 or 72 h, respectively (all P>0.05,
Fig. 1B).
IL-17A promotes OE19 cell migration
and invasion as well as upregulates MMP-2 and MMP-9 expression
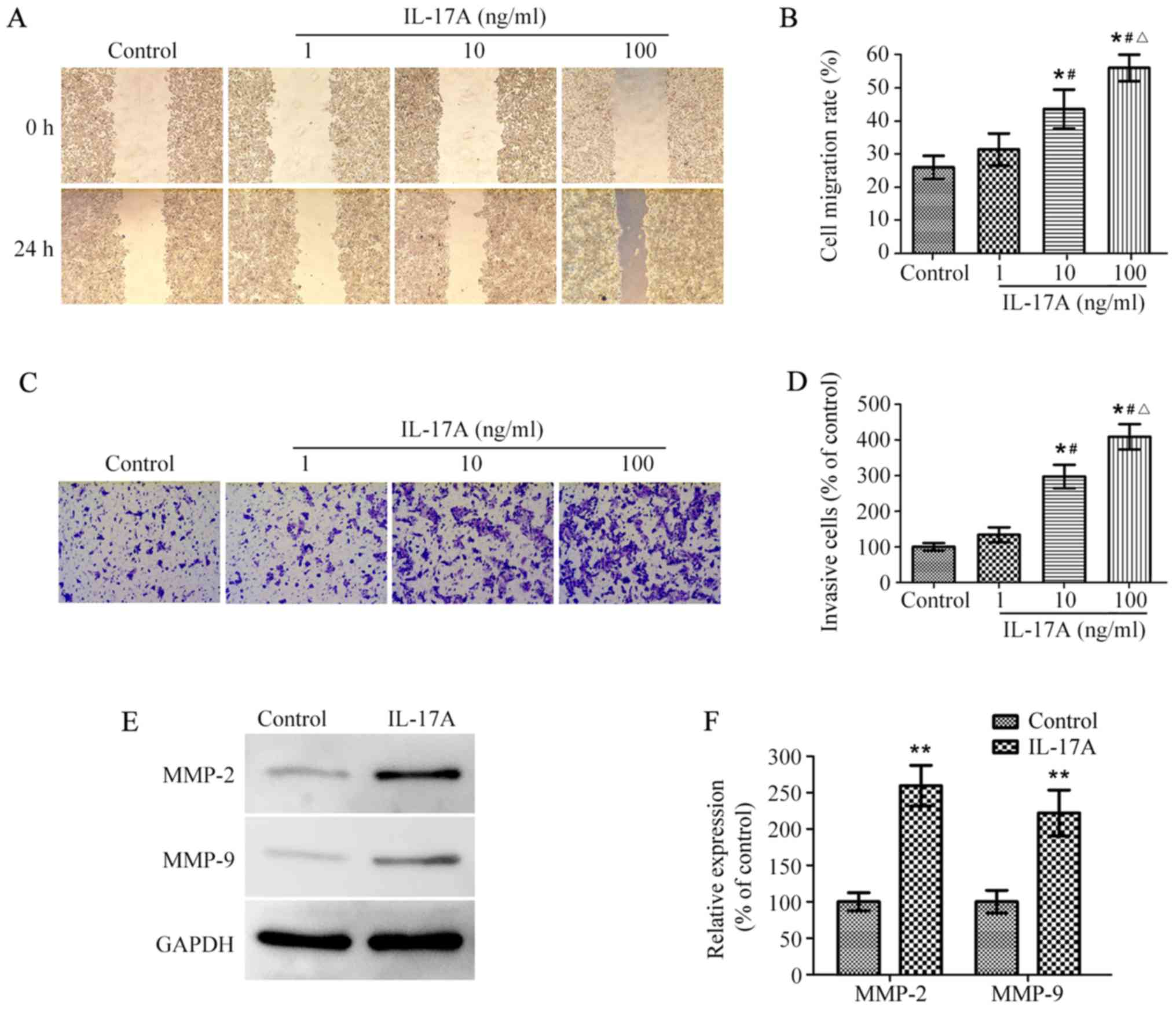
To determine whether IL-17A could promote OE19 cell
migration and invasion, wound healing and Matrigel-coated Transwell
invasion assay were performed. The wound healing assay showed that
the migration rate of OE19 cells reached ~26% in the absence of
IL-17A, however, IL-17A treatment remarkably promoted cell
migration rate in a dose-dependent manner (Fig. 2A and B). The following Transwell
invasion assay indicated that the number of invasive cells was
significantly higher in IL-17A-treated group than the control
group, and similarly the number markedly increased along with the
increasing concentrations of IL-17A (Fig. 2C and D).
MMPs are known to play crucial roles in cancer
metastasis by degrading the extra cellular matrix (22), we next investigated the effect of
IL-17A on MMP-2 and MMP-9 expression in OE19 cells. Western
blotting showed that the protein levels of MMP-2 and MMP-9 were
significantly upregulated in IL-17A-treated cells compared with the
untreated control, respectively (Fig.
2E and F). These results suggested that IL-17A promoted the
migration and invasion of OE19 cells possibly by upregulating
expression of MMP-2 and MMP-9.
IL-17A increases intracellular ROS
levels
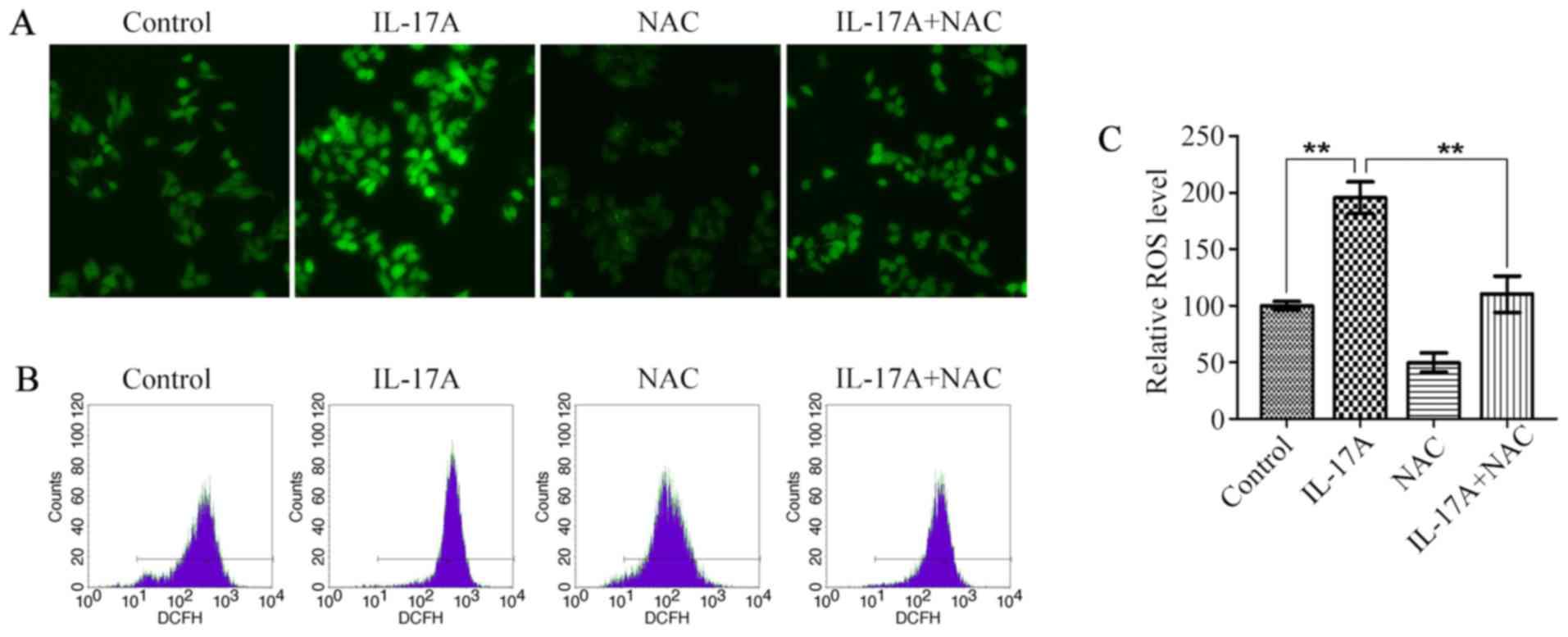
There is accumulating evidence that ROS perform an
important function in tumor development (23,24),
and IL-17A was also reported to cause an increase in intracellular
ROS levels (8,9), so we next examined the effect of
IL-17A on ROS production in OE19 cells. Fluorescence microscopy
showed that IL-17A at the concentration of 100 ng/ml caused ~2-fold
increase in intracellular ROS levels (Fig. 3A and C). The promoting effect of
IL-17A on ROS production was further confirmed by flow cytometry
(Fig. 3B), whereas it was
strikingly attenuated by the pretreatment with NAC (Fig. 3).
IL-17A activates NF-κB in a
ROS-dependent manner
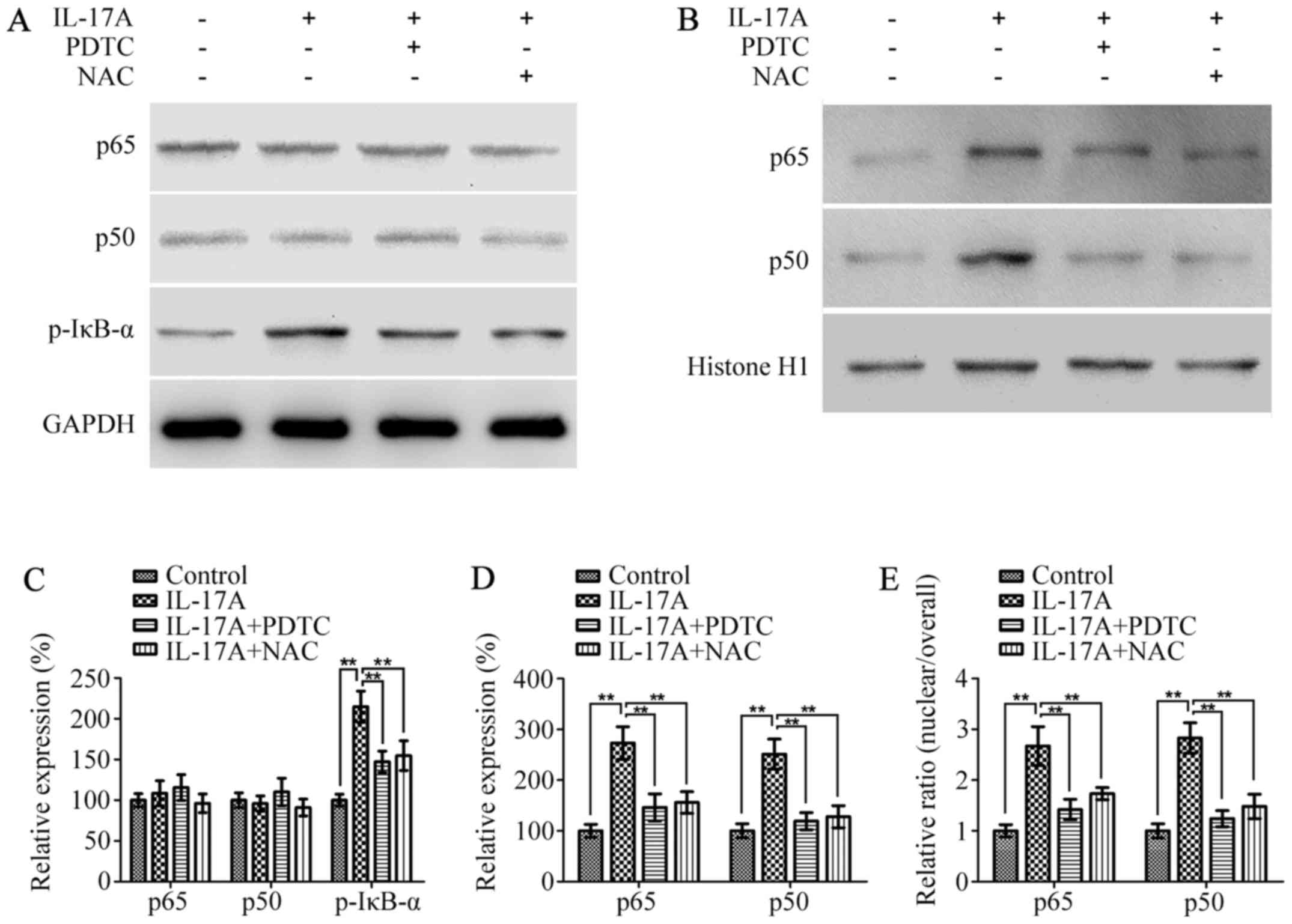
It has been well-documented that NF-κB is a major
transcription factor that mediates migration and invasion of cancer
cells, which is also downstream of oxidative stress (25). Therefore, we next detected whether
IL-17A could induce NF-κB activation in a ROS-dependent manner,
which in turn promotes cell invasion. As shown in Fig. 4A and C, the protein levels of both
p65 and p50 in the whole cell did not differ between groups with or
without IL-17A, however, they were remarkably elevated in the
nucleus in IL-17A-treated cells compared with the untreated cells
(Fig. 4B and D). The
nuclear/overall ratio of p65 and p50 were also increased by IL-17A
(Fig. 4E). Furthermore, the protein
level of p-IκB-α was markedly upregulated in IL-17A-treated cells
(Fig. 4A and C). Whereas,
pretreatment with NAC or PDTC significantly reversed these changes,
which indicated that IL-17A could induce NF-κB activation in a
ROS-dependent manner.
IL-17A promotes cell invasiveness
through ROS/NF-κB/MMP-2/9 signaling pathway
To address the effect of ROS/NF-κB signaling pathway
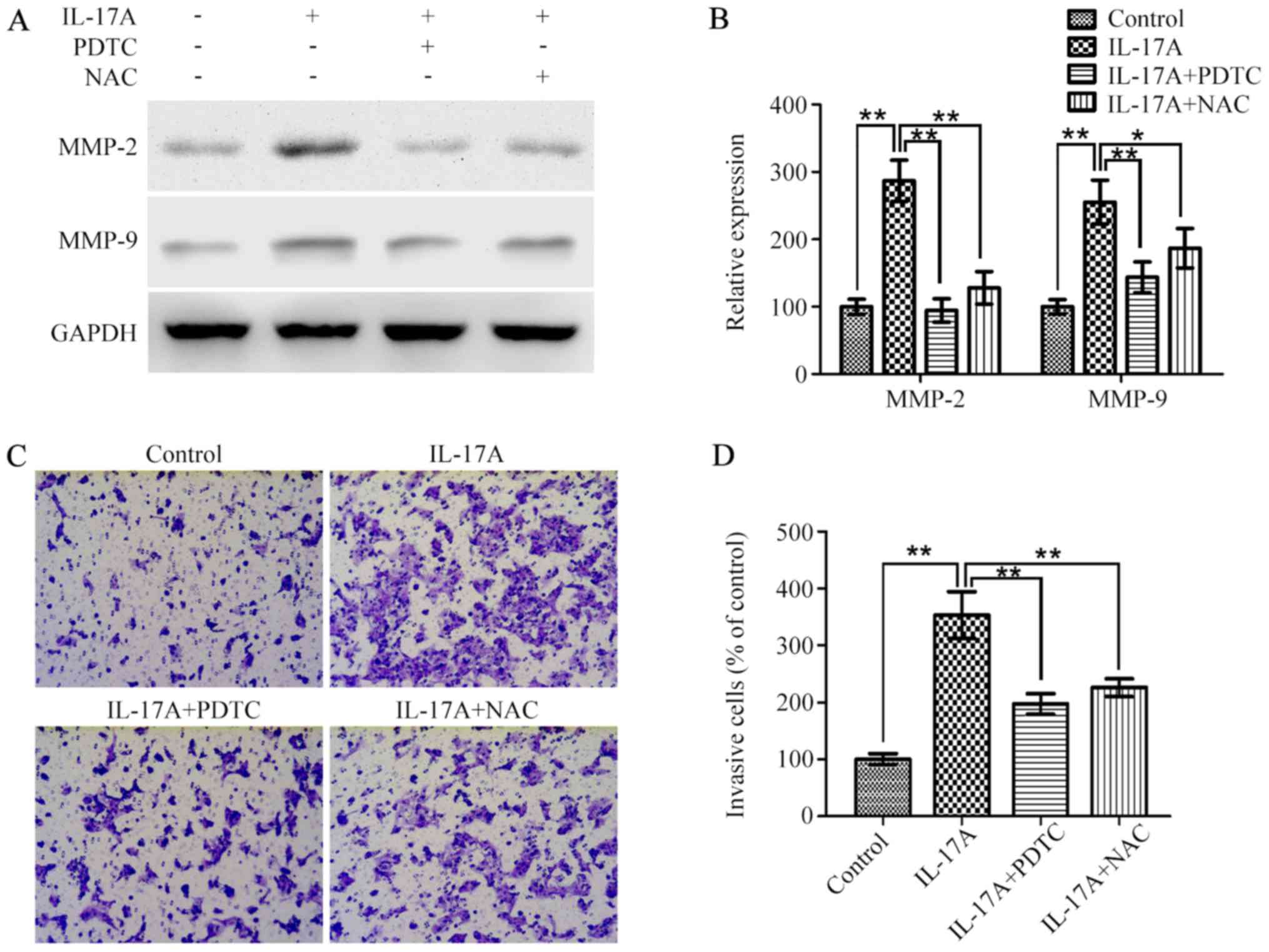
on IL-17A-induced MMP-2/9 expression and cell invasion, OE19 cells
were pretreated with NAC or PDTC for 2 h and then incubated with
IL-17A (100 ng/ml) for 24 h. Western blotting showed that the
effect of IL-17A on the upregulation of MMP-2/9 expression was both
significantly inhibited (Fig. 5A and
B). Furthermore, Transwell invasion assay indicated that
IL-17A-induced cell invasiveness was also markedly attenuated by
NAC or PDTC in vitro (Fig. 5C
and D).
Discussion
It has been well documented that chronic
inflammation plays a pivotal role in the pathogenesis and
development of malignant diseases, however, the role of IL-17A in
cancer is currently under debate. In this study, we aimed to
address whether IL-17A is involved in the regulation of EAC
metastasis, and the results suggested that IL-17A could promote EAC
cell invasiveness through ROS-dependent, NF-κB-mediated MMP-2 and
MMP-9 activation.
Since proinflammatory cytokine was reported to be
closely linked with the inflammation-intestinal
metaplasia-dysplasia-adenocarcinoma sequence in the lower
esophagus, our research group has been engaged in clarifying the
role of proinflammatory cytokine in the pathogenesis and
development of EAC (26,27). Growing scientific evidence showed
that IL-17A is a vital inflammatory cytokine in many inflammatory
diseases, and Th17 cells increased in a variety of human malignant
diseases (10,12). Previous study suggested that high
levels of intratumoral IL-17A-producing cells were correlated with
overall survival and disease-free survival in hepatocellular cancer
patients (15). Also, higher
proportion of circulating Th17 cells was detected in patients with
advanced esophageal cancer, and the proportion of Th17 cells in
patients with lymph node metastasis was higher than those with
non-lymph node metastasis (28).
Whereas, further studies revealed that IL-17A probably enhanced the
tumor killing capability via stimulating expression of Granzyme B
and FasL (17), and also induced
ESCC tumor cells to produce more chemokines, which subsequently
promote the migration of T cells, NK cells, and dendritic cells
(16). However, the role of IL-17A
in EAC development is still unknown, and the related molecular
mechanisms remain to be elucidated. In this study, we found that
IL-17A had less effect on EAC cell proliferation, whereas
wound-healing and Transwell invasion assays showed that IL-17A
significantly promoted the migration and invasion of EAC cells.
These results indicated that IL-17A had no biological action on
promoting or suppressing EAC cell growth, however it could enhance
cell motility in vitro.
Cancer cell metastasis relies on the degradation of
the extra cellular matrix, which is mainly catalyzed by MMPs
(29). Thus, we subsequently
investigated whether MMPs were involved in IL-17A-induced EAC cell
invasion. Our study revealed that IL-17A treatment could markedly
increase the protein levels of both MMP-2 and MMP-9, indicating
that upregulation of MMP-2 and MMP-9 expression might be
responsible for this pro-invasive behavior. Similarly, previous
study also demonstrated that IL-17A increased cell motility in lung
cancer by activating MMPs (30).
However, it differs between the mechanisms underlying
IL-17A-induced expression of MMPs.
Compelling evidence has been found to show that ROS
and NF-κB are downstream of IL-17A (31,32)
and perform an important function in proinflammatory signaling and
EAC development (33,34). Therefore, we next investigated
whether the pro-invasion effect of IL-17A was through activating
ROS/NF-κB signaling pathway. In our study, the intracellular ROS
levels, IκB-α phosphorylation as well as NF-κB nuclear
translocation were all enhanced in IL-17A-treated OE19 cells. ROS
scavenger could remarkably inhibit IL-17A-induced NF-κB activation,
which further demonstrated that the activation of NF-κB was
ROS-dependent. These findings are supported by studies of others
describing oxidative stress as an important regulator of NF-κB
activation (35,36). Furthermore, the following
experiments showed that ROS-scavenging and NF-κB inhibition both
remarkably diminished the promoting effect of IL-17A on MMP-2/9
expression as well as cell invasiveness. Taken together, these
results suggested that IL-17A could activate ROS/NF-κB signaling
pathway, subsequently upregulate MMP-2/9 expression and thereby
promote OE19 cell invasiveness. As previously reported, IL-17A was
proved to promote gastric and colorectal cancer invasiveness via
NF-κB-mediated MMP expression (37,38).
In addition, IL-17A also induced the migration and invasion of
cervical cancer cells by activating the p38/NF-κB signaling pathway
(39). Our results are consistent
with these studies, which imply that IL-17A might play a crucial
role in tumor migration and invasion.
In conclusion, this study provided evidence that
IL-17A could promote the migration and invasion of EAC cells.
Moreover, we revealed the molecular mechanism that IL-17A induced
EAC cell invasiveness through ROS-dependent, NF-κB-mediated MMP-2/9
activation. Considering that tumor metastasis is often associated
with poor prognosis and high mortality among EAC patients, our
findings may contribute a new molecular target for EAC therapy.
Acknowledgements
This study was supported by the Important Clinic
Project of the Chinese Ministry of Health (no. 2007353) and
National-Local Joint Engineering Research Center of Biodiagnostics
and Biotherapy (Zongfang Li, The Second Affiliated Hospital of
Xi'an Jiaotong University, China).
Glossary
Abbreviations
Abbreviations:
|
IL-17A
|
interleukin-17A
|
|
EAC
|
esophageal adenocarcinoma
|
|
ROS
|
reactive oxygen species
|
|
NAC
|
N-acetyl-L-cysteine
|
|
PDTC
|
pyrrolidine dithiocarbamate
|
References
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pohl H, Sirovich B and Welch HG:
Esophageal adenocarcinoma incidence: Are we reaching the peak?
Cancer Epidemiol Biomarkers Prev. 19:1468–1470. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Holmes RS and Vaughan TL: Epidemiology and
pathogenesis of esophageal cancer. Semin Radiat Oncol. 17:2–9.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hvid-Jensen F, Pedersen L, Drewes AM,
Sørensen HT and Funch-Jensen P: Incidence of adenocarcinoma among
patients with Barrett's esophagus. N Engl J Med. 365:1375–1383.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
O'Sullivan KE, Phelan JJ, O'Hanlon C,
Lysaght J, O'Sullivan JN and Reynolds JV: The role of inflammation
in cancer of the esophagus. Expert Rev Gastroenterol Hepatol.
8:749–760. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Miyashita T, Tajima H, Shah FA, Oshima M,
Makino I, Nakagawara H, Kitagawa H, Fujimura T, Harmon JW and Ohta
T: Impact of inflammation-metaplasia-adenocarcinoma sequence and
inflammatory microenvironment in esophageal carcinogenesis using
surgical rat models. Ann Surg Oncol. 21:2012–2019. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pappu R, Ramirez-Carrozzi V and Sambandam
A: The interleukin-17 cytokine family: Critical players in host
defence and inflammatory diseases. Immunology. 134:8–16. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dhillion P, Wallace K, Herse F, Scott J,
Wallukat G, Heath J, Mosely J, Martin JN Jr, Dechend R and LaMarca
B: IL-17-mediated oxidative stress is an important stimulator of
AT1-AA and hypertension during pregnancy. Am J Physiol Regul Integr
Comp Physiol. 303:R353–R358. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pietrowski E, Bender B, Huppert J, White
R, Luhmann HJ and Kuhlmann CR: Pro-inflammatory effects of
interleukin-17A on vascular smooth muscle cells involve NAD(P)H-
oxidase derived reactive oxygen species. J Vasc Res. 48:52–58.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Miyahara Y, Odunsi K, Chen W, Peng G,
Matsuzaki J and Wang RF: Generation and regulation of human CD4+
IL-17-producing T cells in ovarian cancer. Proc Natl Acad Sci USA.
105:15505–15510. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Steiner GE, Newman ME, Paikl D, Stix U,
Memaran-Dagda N, Lee C and Marberger MJ: Expression and function of
pro-inflammatory interleukin IL-17 and IL-17 receptor in normal,
benign hyperplastic, and malignant prostate. Prostate. 56:171–182.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang B, Rong G, Wei H, Zhang M, Bi J, Ma
L, Xue X, Wei G, Liu X and Fang G: The prevalence of Th17 cells in
patients with gastric cancer. Biochem Biophys Res Commun.
374:533–537. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang L, Yi T, Kortylewski M, Pardoll DM,
Zeng D and Yu H: IL-17 can promote tumor growth through an
IL-6-Stat3 signaling pathway. J Exp Med. 206:1457–1464. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gu FM, Li QL, Gao Q, Jiang JH, Zhu K,
Huang XY, Pan JF, Yan J, Hu JH, Wang Z, et al: IL-17 induces
AKT-dependent IL-6/JAK2/STAT3 activation and tumor progression in
hepatocellular carcinoma. Mol Cancer. 10:1502011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang JP, Yan J, Xu J, Pang XH, Chen MS,
Li L, Wu C, Li SP and Zheng L: Increased intratumoral
IL-17-producing cells correlate with poor survival in
hepatocellular carcinoma patients. J Hepatol. 50:980–989. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lu L, Pan K, Zheng HX, Li JJ, Qiu HJ, Zhao
JJ, Weng DS, Pan QZ, Wang DD, Jiang SS, et al: IL-17A promotes
immune cell recruitment in human esophageal cancers and the
infiltrating dendritic cells represent a positive prognostic marker
for patient survival. J Immunother. 36:451–458. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lu L, Weng C, Mao H, Fang X, Liu X, Wu Y,
Cao X, Li B, Chen X, Gan Q, et al: IL-17A promotes migration and
tumor killing capability of B cells in esophageal squamous cell
carcinoma. Oncotarget. 7:21853–21864. 2016.PubMed/NCBI
|
|
18
|
Lv L, Pan K, Li XD, She KL, Zhao JJ, Wang
W, Chen JG, Chen YB, Yun JP and Xia JC: The accumulation and
prognosis value of tumor infiltrating IL-17 producing cells in
esophageal squamous cell carcinoma. PLoS One. 6:e182192011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang B, Li L, Liao Y, Li J, Yu X, Zhang Y,
Xu J, Rao H, Chen S, Zhang L, et al: Mast cells expressing
interleukin 17 in the muscularis propria predict a favorable
prognosis in esophageal squamous cell carcinoma. Cancer Immunol
Immunother. 62:1575–1585. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Numasaki M, Watanabe M, Suzuki T,
Takahashi H, Nakamura A, McAllister F, Hishinuma T, Goto J, Lotze
MT, Kolls JK, et al: IL-17 enhances the net angiogenic activity and
in vivo growth of human non-small cell lung cancer in SCID mice
through promoting CXCR-2-dependent angiogenesis. J Immunol.
175:6177–6189. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gaffen SL: Structure and signalling in the
IL-17 receptor family. Nat Rev Immunol. 9:556–567. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hadler-Olsen E, Winberg JO and
Uhlin-Hansen L: Matrix metalloproteinases in cancer: Their value as
diagnostic and prognostic markers and therapeutic targets. Tumour
Biol. 34:2041–2051. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tochhawng L, Deng S, Pervaiz S and Yap CT:
Redox regulation of cancer cell migration and invasion.
Mitochondrion. 13:246–253. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang Y, Ma J, Shen H, Wang C, Sun Y,
Howell SB and Lin X: Reactive oxygen species promote ovarian cancer
progression via the HIF-1α/LOX/E-cadherin pathway. Oncol Rep.
32:2150–2158. 2014.PubMed/NCBI
|
|
25
|
Zhang H, Wang ZW, Wu HB, Li Z, Li LC, Hu
XP, Ren ZL, Li BJ and Hu ZP: Transforming growth factor-β1 induces
matrix metalloproteinase-9 expression in rat vascular smooth muscle
cells via ROS-dependent ERK-NF-κB pathways. Mol Cell Biochem.
375:11–21. 2013.PubMed/NCBI
|
|
26
|
Zhang R, Yin X, Shi H, Wu J, Shakya P, Liu
D and Zhang J: Adiponectin modulates DCA-induced inflammation via
the ROS/NF-κB signaling pathway in esophageal adenocarcinoma cells.
Dig Dis Sci. 59:89–97. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang R, Wu J, Liu D, Shan H and Zhang J:
Anti-inflammatory effect of full-length adiponectin and
proinflammatory effect of globular adiponectin in esophageal
adenocarcinoma cells. Oncol Res. 21:15–21. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen D, Hu Q, Mao C, Jiao Z, Wang S, Yu L,
Xu Y, Dai D, Yin L and Xu H: Increased IL-17-producing CD4(+) T
cells in patients with esophageal cancer. Cell Immunol.
272:166–174. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Deryugina EI and Quigley JP: Matrix
metalloproteinases and tumor metastasis. Cancer Metastasis Rev.
25:9–34. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xu B, Guenther JF, Pociask DA, Wang Y,
Kolls JK, You Z, Chandrasekar B, Shan B, Sullivan DE and Morris GF:
Promotion of lung tumor growth by interleukin-17. Am J Physiol Lung
Cell Mol Physiol. 307:L497–L508. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cho KA, Suh JW, Lee KH, Kang JL and Woo
SY: IL-17 and IL-22 enhance skin inflammation by stimulating the
secretion of IL-1β by keratinocytes via the ROS-NLRP3-caspase-1
pathway. Int Immunol. 24:147–158. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sønder SU, Saret S, Tang W, Sturdevant DE,
Porcella SF and Siebenlist U: IL-17-induced NF-kappaB activation
via CIKS/Act1: Physiologic significance and signaling mechanisms. J
Biol Chem. 286:12881–12890. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Song S, Guha S, Liu K, Buttar NS and
Bresalier RS: COX-2 induction by unconjugated bile acids involves
reactive oxygen species-mediated signalling pathways in Barrett's
oesophagus and oesophageal adenocarcinoma. Gut. 56:1512–1521. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Abdel-Latif MM, O'Riordan J, Windle HJ,
Carton E, Ravi N, Kelleher D and Reynolds JV: NF-kappaB activation
in esophageal adenocarcinoma: Relationship to Barrett's metaplasia,
survival, and response to neoadjuvant chemoradiotherapy. Ann Surg.
239:491–500. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yun HS, Baek JH, Yim JH, Lee SJ, Lee CW,
Song JY, Um HD, Park JK, Park IC and Hwang SG: Knockdown of
hepatoma-derived growth factor-related protein-3 induces apoptosis
of H1299 cells via ROS-dependent and p53-independent NF-κB
activation. Biochem Biophys Res Commun. 449:471–476. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sun L, Li W, Li W, Xiong L, Li G and Ma R:
Astragaloside IV prevents damage to human mesangial cells through
the inhibition of the NADPH oxidase/ROS/Akt/NF-κB pathway under
high glucose conditions. Int J Mol Med. 34:167–176. 2014.PubMed/NCBI
|
|
37
|
Wang Y, Wu H, Wu X, Bian Z and Gao Q:
Interleukin 17A promotes gastric cancer invasiveness via NF-κB
mediated matrix metalloproteinases 2 and 9 expression. PLoS One.
9:e966782014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ren H, Wang Z, Zhang S, Ma H, Wang Y, Jia
L and Li Y: IL-17A promotes the migration and invasiveness of
colorectal cancer cells through NF-κB mediated MMP expression.
Oncol Res. 23:249–256. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Feng M, Wang Y, Chen K, Bian Z, Jinfang Wu
and Gao Q: IL-17A promotes the migration and invasiveness of
cervical cancer cells by coordinately activating MMPs expression
via the p38/NF-κB signal pathway. PLoS One. 9:e1085022014.
View Article : Google Scholar : PubMed/NCBI
|