Introduction
HOXB9, a member of the class I homeobox (HOX) genes,
regulates several cellular processes, including angiogenesis and
maintenance of cell fate (1). It is
overexpressed in human breast cancer and its expression is
associated with increased tumorigenicity, lung metastasis and
radioresistance (2,3). HOXB9 transactivates several angiogenic
factors as well as erythroblastic leukemia viral oncogene homolog
(ErbB) and TGF-β ligands leading to epithelial-mesenchymal
transition (EMT), increased angiogenesis and distal metastasis. In
particular, HOXB9 induces tumor proliferation and metastasis by
activating angiogenesis; however, little is known concerning the
relationship between HOXB9 expression and angiogenesis in
hepatocellular carcinoma (HCC).
HCC is considered a serious public health concern
particularly in endemic areas of hepatitis B or C viral infection,
including Africa and Southeast Asia (4), despite recent progress in surgical and
non-surgical treatment including multikinase inhibitor therapy.
The prognosis for HCC patients is still dismal,
since the disease is discovered at an advanced stage in a
substantial number of patients when curative therapy is no longer
possible (5). Curative treatments
(e.g., resection, transplantation or local ablation) are available
only to patients with early-stage disease, however they are limited
by high recurrence rates, impairing patient outcomes. At advanced
stages, the multikinase inhibitor sorafenib is the only effective
treatment, although vigorous efforts are underway to better
characterize the molecular pathogenesis of liver cancer to refine
the efficacy of other molecular-targeted therapies (6).
Sorafenib (Nexavar®; Bayer HealthCare
Pharmaceuticals, Montville, NJ, USA) is an oral multikinase
inhibitor that inhibits the serine-threonine kinases Raf-1 and
B-Raf, the receptor tyrosine kinase activity of vascular
endothelial growth factor (VEGF) receptors 1-3, and
platelet-derived growth factor receptor β (7). It blocks tumor cell proliferation and
tumor angiogenesis, and increases the rate of apoptosis in a wide
range of tumor models by targeting the Raf/mitogen-activated
protein kinase/extracellular signal-regulated kinase (RAF/MEK/ERK)
and VEGF signaling pathways. Therefore, identification of accurate
prognostic biomarkers to distinguish patients at high risk of
recurrence or metastasis and surrogate markers for non-surgical
therapy is of the utmost importance for developing preventive
strategies to improve the outcome of HCC patients.
In the present study, we demonstrated that increased
expression of HOXB9 in HCC is associated with the induction of
angiogenic factors, increased vascular invasion and poor overall
survival of these patients. Sorafenib treatment was found to
suppress the expression of angiogenic factors and the Raf/MEF/ERK
pathway in HOXB9-expressing cells in vitro, and HCC patients
with increased HOXB9 expression exhibited increased overall
survival upon treatment with sorafenib. Collectively, these results
suggest that HOXB9 expression in HCC could be a surrogate marker
for a beneficial response to sorafenib treatment.
Materials and methods
Cell lines and cell culture
The human HCC cell lines, HepG2 and Hep3B, were
obtained from the American Type Culture Collection (ATCC; Manassas,
VA, USA). The identity of all cells was independently authenticated
by short tandem repeat genotyping in May 2014. The cells were
cultured in Dulbecco's modified Eagle's medium (DMEM), supplemented
with 10% bovine fetal serum, 100 U/ml penicillin, 100 µg/ml
streptomycin, 1X non-essential amino acids and 1 mM sodium
pyruvate, and incubated in humidified 37̊C incubators with 5%
CO2. Chemically synthesized Stealth RNAi™ siRNAs were
purchased from Invitrogen (Carlsbad, CA, USA) and were used to
knock down endogenous HOXB9 expression in HCC cells. Two different
siRNAs were individually transfected into the cells at a final
concentration of 100 nM using Lipofectamine RNAiMAX (Invitrogen).
In addition, siGFP was used as a control siRNA.
Ethical approval, consent to
participate and consent of publication
The present study was approved by the Ethics
Committee of Tokyo Medical University. The patients provided
consent for their participation and the publication of the present
study.
mRNA expression
Our analysis included 79 HCC patients who underwent
consecutive resection between September 2008 and December 2014 at
Tokyo Medical University Hachioji Medical Center. All protocols
were performed in accordance with the Ethics Committee of Tokyo
Medical University. Consecutive frozen tissue specimens containing
cancerous and matched normal hepatocyte regions were available from
HCC patients (who received a pathological diagnosis) to assess gene
panels by quantitative reverse-transcription PCR (qRT-PCR). HOXB9
mRNA expression was examined in cancer and in normal hepatocytes
derived from consecutive samples of patients who had undergone
surgery at our institute during 2008–2014. The expression ratios
(cancer to normal hepatocytes surrounded by cancer) were examined
using qRT-PCR. RNA was extracted from cells using the RNeasy kit
(Qiagen, Valencia, CA, USA). Conditions for semi-quantitative
amplification of cDNA were 95̊C for 2 min, followed by 25 cycles of
95̊C for 30 sec, 56̊C for 30 sec, and 72̊C for 60 sec, with a final
extension cycle at 72̊C for 10 min. RT-PCR analysis was run in
triplicate for each sample on a Light Cycler 480 Real-Time PCR
System using SYBR-Green I Master Mix (Roche, Mannheim, Germany).
The following program was run: pre-incubation for 5 min at 95̊C,
amplification for 45 cycles (10 sec of denaturation at 95̊C, 10 sec
of annealing at 57̊C and a 10-sec extension at 72̊C), with melting
curve analysis. mRNA levels of the target genes were normalized
against mRNA levels of GAPDH, which was used as an internal
control. The sequences of all the primers used are listed as
follows: HoxB9 forward, 5′CACCATGTCCATTTCTGGGACGCTTAG3′ and
reverse, 5′AAACTCTTTGCCCTGCTCCTTATTC3′; GAPDH forward,
5′ATCATCCCTGCCTCTACTGG3′ and reverse, 5′TTTCTAGACCGGCAGGTCAGGT3′;
PDGF-b forward, 5′GATCCGCTCCTTTGATGATC3′ and reverse,
5′GTCTCACACTTGCATGCCAG3′; PDGFR-b forward, 5′AATGTCTCCAGCACCTTCGT3′
and reverse, 5′AGCGGA TGTGGTAAGGCATA3′; VEGF-A forward, 5′ACCATGCCA
AGTGGTCCCAG3′ and reverse, 5′CTTTCTTTGGTCTGCA TTCACA3′; VEGF-C
forward, 5′CTTCTTTAAACCTCCATG TGTGTC3′ and reverse,
5′GAATGAACTTGTCTGTAAACA TCCA3′; VEGFR-2 forward,
5′TCATCTGTTACAGCTTCC AAGT3′ and reverse, 5′GGTTTGATTCTTTCCAGGCTC3′;
VEGFR-3 forward, 5′CCAGCATCGTGTGGTACAAAGA3′ and reverse,
5′CTCCCCGGGGTCCATGATGAT3′.
Western blotting and
immunohistochemistry
Protein expression was detected by western blotting
using antibodies against HOXB9 and pERK (phospho-ERK) (Santa Cruz
Biotechnology, Santa Cruz, CA, USA); ERK2, pMEK (phospho-MEK) and
MEK1 (Cell Signaling Technology, Beverly, MA, USA); and pRAF1, Raf1
and actin (Abcam, Cambridge, MA, USA). The western blotting
protocols used have been previously described (8). Immunohistochemistry (IHC) was carried
out on 5-µm sections of formalin-fixed paraffin-embedded (FFPE)
tissue by heat-induced epitope retrieval in 10 mmol/l sodium
citrate (pH 6.0) before blocking with 5% BSA-PBS. For IHC, HOXB9
expression was detected using the anti-human HOXB9-specific
antibody at a dilution of 1:250 (Santa Cruz Biotechnology).
Sorafenib treatment
Cells were plated into 12- to 24-well tissue culture
plates or 10-cm2 dishes and cultured overnight.
Sorafenib (LC Laboratories, Woburn, MA, USA) was added to the cells
at two different concentrations (5 and 1 µM) for 48 h before mRNA
and protein extraction. The final dimethyl sulfoxide (DMSO)
concentration in all experiments was <0.05%. A total of 15
patients with tumor-node-metastasis (TNM) stage III HCC were
treated with sorafenib after curative resection.
Statistical analyses
Data represent the mean ± standard error of the
mean. Comparisons between groups were made using a two-tailed
t-test or U test for continuous variables, and Fishers exact test
for comparison of proportions. Correlations were assessed with the
non-parametric Spearman's coefficient. All calculations were
carried out with the SPSS software package (SPSS 18.0). Overall
survival (OS) curves were drawn according to Kaplan-Meier estimates
and compared by log-rank tests. P<0.05 was defined as
significant.
Results
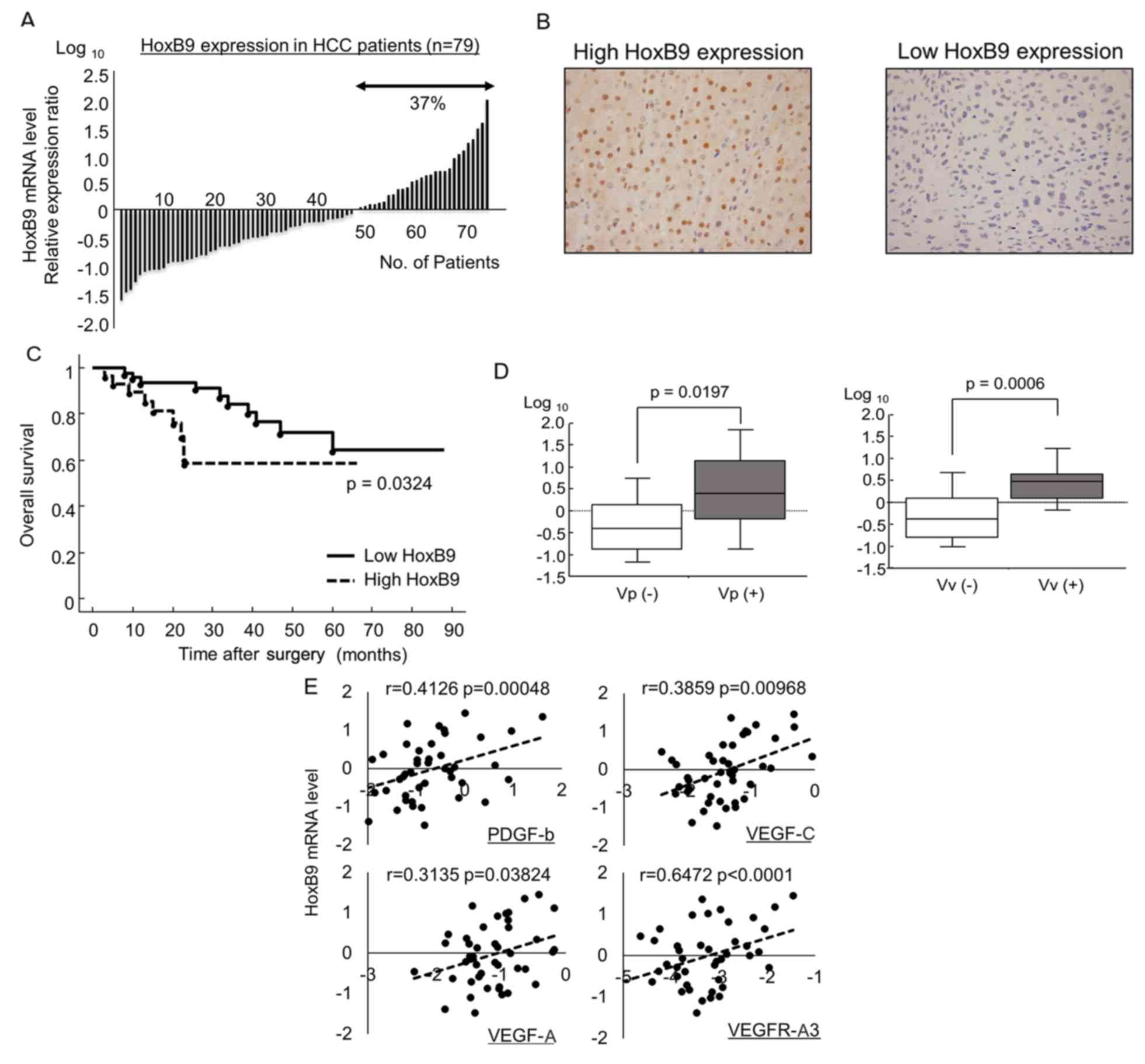
HOXB9 expression in HCC
To investigate HOXB9 expression in HCC, we examined
samples from 79 HCC patients who received curative resection at our
institute. HOXB9 mRNA was expressed in ~40% of the patients
(Fig. 1A) and was upregulated in
tumors relative to uninvolved adjacent normal hepatocytes as
determined by both qRT-PCR and immunohistochemical staining
(Fig. 1B). Although, HOXB9
expression in HCC did not affect disease-free survival after
surgery (data not shown), increased HOXB9 expression in HCC was
significantly associated with a poor prognosis in OS (5-year OS
rate; 63.5 vs. 58.5%; p=0.0324) (Fig.
1C), as previously reported for breast and colorectal cancer
(9,10). Moreover, HOXB9 mRNA expression was
significantly higher in patients exhibiting vascular invasion (both
portal and hepatic vein) compared to those who were negative for
vascular invasion (Fig. 1D). A
significant positive correlation was observed between HOXB9 mRNA
expression and several angiogenic factors targeted by sorafenib:
PDGF-b, VEGF-C, VEGF-C and VEGFR-A3 (p<0.05) (Fig. 1E) (11).
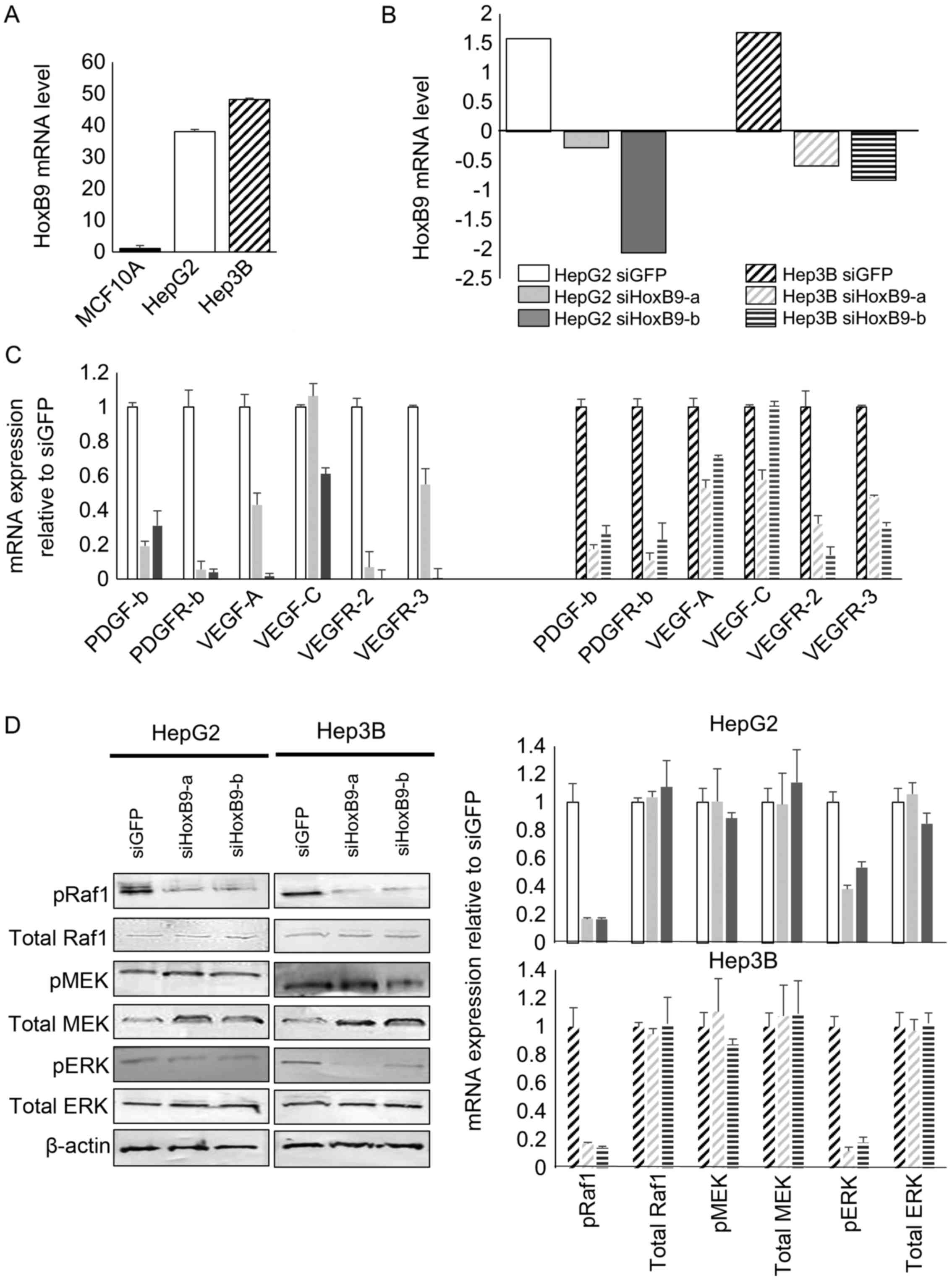
HOXB9 regulates the expression of
angiogenic factors and Raf/MEK/ERK signaling
HCC cell lines, HepG2 and Hep3B, showed high HOXB9
mRNA expression relative to the human mammary epithelial cell line,
MCF10A, which showed low HOXB9 mRNA (Fig. 2A) as previously reported (2). To determine the effect of the loss of
HOXB9 in HCC cells, we depleted HOXB9 mRNA in these cells using two
short interfering RNA (siRNA) oligonucleotides (Invitrogen)
targeting HOXB9. Both siRNAs decreased HOXB9 expression in the HCC
cells by −130 and −230% (Fig. 2B).
A decrease in HOXB9 expression by these siRNAs also decreased the
expression of several angiogenic factors. These angiogenic factors
which are targeted by sorafenib were highly expressed in the
siGFP-transfected control HepG2 and Hep3B lines (Fig. 2C). These results are consistent with
previously reported results in breast cancer (2,10).
Moreover, a decrease in HOXB9 expression in the two HCC cell lines
also mitigated the Raf/MEK/ERK pathway (Fig. 2D), which is targeted by sorafenib1
(2). Knockdown of HOXB9 expression
led to a decrease in phospho(p)Raf and pERK. The quantification of
these western blotting bands are shown (Fig. 2D, right panel).
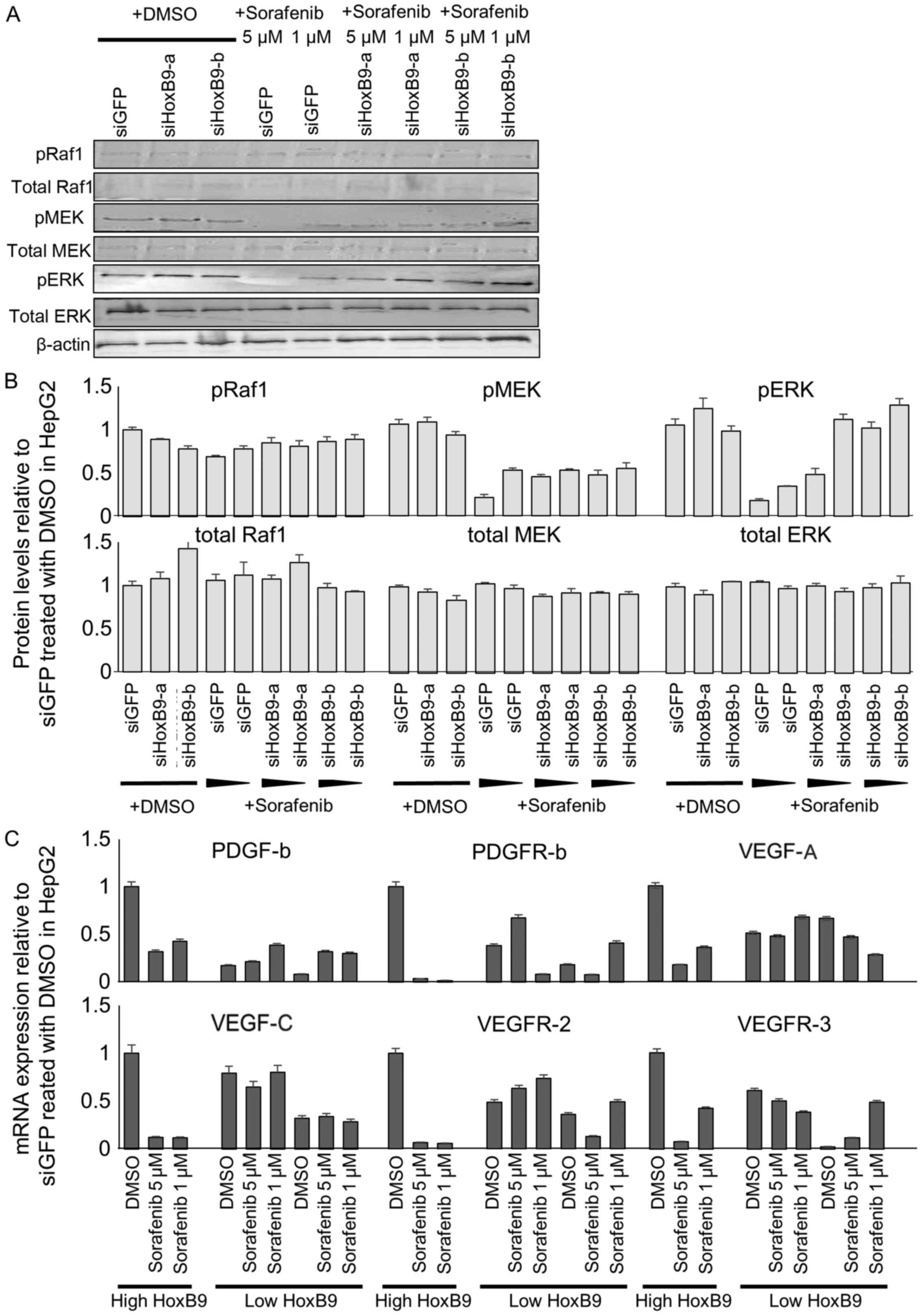
Significance of HOXB9 expression on
sorafenib response
To determine whether sorafenib, the multikinase
inhibitor suppresses the Raf/MEK/ERK pathway upregulated in
HOXB9-expressing cells, we treated the cells transfected with siGFP
and siHOXB9 (expressing high or low HOXB9, respectively) with 1 and
5 µM of sorafenib. The proteins of the Raf/MEK/ERK pathway were
more markedly suppressed in the cells expressing high levels of
HOXB9 compared to those expressing low HOXB9 mRNA (Fig. 3A). The quantification of these
western blotting bands is shown (Fig.
3B).
We similarly determined whether sorafenib treatment
suppresses the expression of HOXB9-regulating angiogenic factors in
cells expressing high and low HOXB9. Sorafenib potently suppressed
the expression of several angiogenic factors by 60–95% in cells
expressing high HOXB9 cells compared to the levels in cells
expressing low levels of HOXB9 (−50 and −10%) (Fig. 3C). Collectively these results show
that HOXB9-expressing cells, by expressing higher baseline levels
of angiogenic factors and activation of the Raf/MEK/ERK pathway,
are more likely to be responsive to sorafenib treatment.
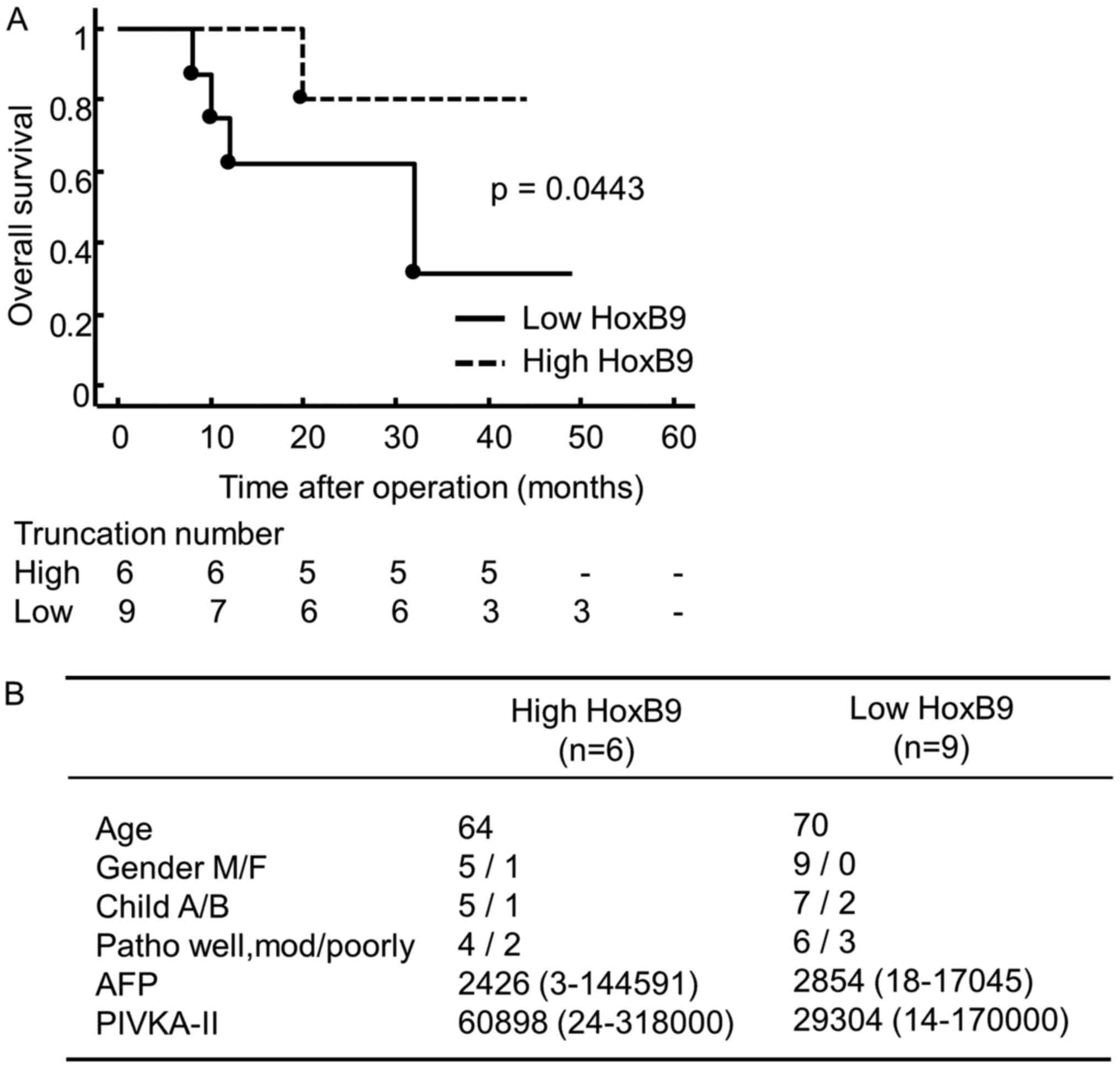
To clarify the clinical significance of these
findings, we conducted a retrospective study in HCC patients who
underwent resection before treatment with sorafenib at our
institute between 2008 and 2012 (n=15). Samples obtained from
patients before chemotherapy were analyzed by qRT-PCR. The
demographic and clinical data showed no significant differences
with regard to age, gender, pathological stage or chemotherapy
between groups with high and low expression of HOXB9. High
expression of HOXB9 mRNA was associated with longer OS after
treatment with sorafenib (3-years OS rate; 80 vs. 30%, p=0.0443)
compared with patients expressing low levels of HOXB9 (Fig. 4A). In addition, clinical findings
among the two groups had no significant difference (Fig. 4B).
Discussion
HOXB9 has been reported to be overexpressed in
neoplastic tissues (13). HOXB9
promotes the expression of angiogenic factors, as well as ErbB and
TGF-β ligands. From a biological perspective, multiple HOX-binding
sites are present in the promoters of angiopoietin-like 2, IL-8,
TGF-β2, VEGF and bFGF. AREG, ERG, VEGF and bFGF are direct targets
of HOXB9-induced transcriptional activation (14,15).
In the present study, we demonstrated that HOXB9 promoted the
expression of angiogenic factors and the Raf/MEK/ERK pathway in
HCC. Both angiogenesis and signaling through the RAF/MEK/ERK
cascade play critical roles in the development of HCC and may lead
to increased tumorigenesis and poor overall survival in HCC
patients.
Sorafenib is a multikinase inhibitor with activity
against the Ser/Thr kinase Raf, known to be important in tumor cell
signaling and tumor cell proliferation, and in the regulation of
several receptor tyrosine kinases involved in angiogenesis,
including VEGFR-2 and PDGFR-12. Although the HOXB9 gene has not
been described as a direct target of sorafenib, its ability to
induce angiogenesis may render HOXB9-expressing HCC cells sensitive
to sorafenib. This is consistent with our data showing increased
overall survival of HOXB9-overexpressing HCC patients treated with
sorafenib. There is increasing evidence of the potential crosstalk
between, for example, HOXB9 and wingless/T-cell factor (Wnt/TCF)
signaling pathways (16).
Expression profiling of HCC patients treated with sorafenib may
shed light on potential correlations between HOXB9-related
molecular classes and treatment responses.
Surrogate biomarkers that predict the biological and
clinical efficacy of sorafenib may help tailor treatment on an
individual patient basis. The predictive value of the Raf/MEK/ERK
signaling activity for the efficacy of sorafenib in HCC remains
uncertain (17,18). In the present study, we found a
strong correlation between increased HOXB9 activity in HCC and poor
prognosis, but better therapeutic responses to sorafenib.
Evaluation of HOXB9 activity in HCC can be useful to differentiate
between responders and non-responders before commencing sorafenib
treatment and to help select patients who are likely to benefit
from the treatment. However, the present study, was based on a
retrospective analysis of a small number of patients at a single
site, and selection of patients for sorafenib treatment was
subjective. The clinical significance of HOXB9 must therefore be
addressed in a prospectively planned multicenter trial. Multigene
assays involving a large number of specimens before and after
sorafenib exposure may provide more reliable insights into tumor
biology and the response to sorafenib treatment.
Acknowledgements
We thank Dr Shyamala Meheswaran (Cancer Center,
Massachusetts General Hospital and Harvard Medical School) for the
critical reading of the manuscript. This research was funded by a
JSPS KAKENHI Grant-in-Aid for Young Scientists (B) (no. 25861217)
and by the Tokyo Medical University Cancer Research Foundation.
Glossary
Abbreviations
Abbreviations:
|
HOXB9
|
homeobox B9
|
|
HOX
|
homeobox
|
|
VEGF
|
vascular endothelial growth factor
|
|
OS
|
overall survival
|
|
siRNA
|
short interference RNA
|
|
qRT-PCR
|
quantitative reverse
transcription-polymerase chain reaction
|
References
|
1
|
Cantile M, Schiavo G, Terracciano L and
Cillo C: Homeobox genes in normal and abnormal vasculogenesis. Nutr
Metab Cardiovasc Dis. 18:651–658. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hayashida T, Takahashi F, Chiba N,
Brachtel E, Takahashi M, Godin-Heymann N, Gross KW, Vivanco M,
Wijendran V, Shioda T, et al: HOXB9, a gene overexpressed in
breast cancer, promotes tumorigenicity and lung metastasis. Proc
Natl Acad Sci USA. 107:1100–1105. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chiba N, Comaills V, Shiotani B, Takahashi
F, Shimada T, Tajima K, Winokur D, Hayashida T, Willers H, Brachtel
E, et al: Homeobox B9 induces epithelial-to-mesenchymal
transition-associated radioresistance by accelerating DNA damage
responses. Proc Natl Acad Sci USA. 109:2760–2765. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sherman M: Hepatocellular carcinoma:
Epidemiology, surveillance, and diagnosis. Semin Liver Dis.
30:3–16. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rampone B, Schiavone B, Martino A, Viviano
C and Confuorto G: Current management strategy of hepatocellular
carcinoma. World J Gastroenterol. 15:3210–3216. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Llovet JM, Ricci S, Mazzaferro V, Hilgard
P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A,
et al: SHARP Investigators Study Group: Sorafenib in advanced
hepatocellular carcinoma. N Engl J Med. 359:378–390. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wilhelm SM, Carter C, Tang L, Wilkie D,
McNabola A, Rong H, Chen C, Zhang X, Vincent P, McHugh M, et al:
BAY 43–9006 exhibits broad spectrum oral antitumor activity and
targets the RAF/MEK/ERK pathway and receptor tyrosine kinases
involved in tumor progression and angiogenesis. Cancer Res.
64:7099–7109. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ha TU, Segev DL, Barbie D, Masiakos PT,
Tran TT, Dombkowski D, Glander M, Clarke TR, Lorenzo HK, Donahoe
PK, et al: Mullerian inhibiting substance inhibits ovarian cell
growth through an Rb-independent mechanism. J Biol Chem.
275:37101–37109. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Seki H, Hayashida T, Jinno H, Hirose S,
Sakata M, Takahashi M, Maheswaran S, Mukai M and Kitagawa Y: HOXB9
expression promoting tumor cell proliferation and angiogenesis is
associated with clinical outcomes in breast cancer patients. Ann
Surg Oncol. 19:1831–1840. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hoshino Y, Hayashida T, Hirata A,
Takahashi H, Chiba N, Ohmura M, Wakui M, Jinno H, Hasegawa H,
Maheswaran S, et al: Bevacizumab terminates homeobox B9-induced
tumor proliferation by silencing microenvironmental communication.
Mol Cancer. 13:102–108. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wilhelm SM, Adnane L, Newell P, Villanueva
A, Llovet JM and Lynch M: Preclinical overview of sorafenib, a
multikinase inhibitor that targets both Raf and VEGF and PDGF
receptor tyrosine kinase signaling. Mol Cancer Ther. 7:3129–3140.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu L, Cao Y, Chen C, Zhang X, McNabola A,
Wilkie D, Wilhelm S, Lynch M and Carter C: Sorafenib blocks the
RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor
cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer
Res. 66:11851–11858. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Abate-Shen C: Deregulated homeobox gene
expression in cancer: Cause or consequence? Nat Rev Cancer.
2:777–785. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jain RK: Molecular regulation of vessel
maturation. Nat Med. 9:685–693. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shrestha B, Ansari KI, Bhan A, Kasiri S,
Hussain I and Mandal SS: Homeodomain-containing protein HOXB9
regulates expression of growth and angiogenic factors, facilitates
tumor growth in vitro and is overexpressed in breast cancer tissue.
FEBS J. 279:3715–3726. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nguyen DX, Chiang AC, Zhang XH, Kim JY,
Kris MG, Ladanyi M, Gerald WL and Massagué J: WNT/TCF signaling
through LEF1 and HOXB9 mediates lung adenocarcinoma metastasis.
Cell. 138:51–62. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Abou-Alfa GK, Schwartz L, Ricci S, Amadori
D, Santoro A, Figer A, De Greve J, Douillard JY, Lathia C, Schwartz
B, et al: Phase II study of sorafenib in patients with advanced
hepatocellular carcinoma. J Clin Oncol. 24:4293–4300. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Newell P, Toffanin S, Villanueva A, Chiang
DY, Minguez B, Cabellos L, Savic R, Hoshida Y, Lim KH,
Melgar-Lesmes P, et al: Ras pathway activation in hepatocellular
carcinoma and anti-tumoral effect of combined sorafenib and
rapamycin in vivo. J Hepatol. 51:725–733. 2009. View Article : Google Scholar : PubMed/NCBI
|