Introduction
The Epstein-Barr virus (EBV) is a common
γ-herpesvirus with a high prevalence in adults worldwide (1). EBV infection is considered a high risk
factor and is responsible for more than 1% of all human cancers
(2). EBV infection is associated
with various human proliferative diseases involving mostly
epithelial or lymphoid cells, including nasopharyngeal carcinoma
(NPC), gastric and lymphoepithelioma-like carcinomas (3–5). NPC
occurs in the epithelium of the throat and nose and can be
classified as two broad subtypes, i.e. keratinized (differentiated
epithelial) and non-keratinized (undifferentiated epithelial) cells
(6). NPC is prevalent in areas such
as southern China, Southeast Asia, Alaska and native populations of
Canada (7). EBV exhibits a type II
latency mechanism in NPC patients (8–10).
Type II latency infection is characterized by the expression of
viral Epstein-Barr nuclear antigen 1 (EBNA1), latent membrane
proteins (LMP1, LMP2A and LMP2B), and several EBV non-coding RNAs
(11,12). The expression of special viral
antigens in NPC associated with EBV make this disease an attractive
target for the therapy of NPC (9).
EBNA1 is the only viral protein expressed in all
three forms of latent EBV infections (13). As an important regulator of EBV
latency, EBNA1 has many functions influencing the EBV genome and
host cells. For example, EBNA1 plays an essential role in the
maintenance and replication of the EBV genome via sequence-specific
binding to the viral origin of replication (oriP) (14). EBNA1 has been shown to modulate
several cellular signaling pathways to regulate cell growth and
transformation, such as nuclear factor-κB (NF-κB), transforming
growth factor-β (TGF-β), and signal transducer and activator of
transcription (STAT) (15–17). EBNA1 also decreases the accumulation
of p53 in EBV-infected epithelial cells, leading to disruption of
the antitumor functions (18,19).
In NPC cell type II infection, EBNA1 transcription is mediated by
the Q promoter (Qp) (20,21). Previous studies have shown that
Janus kinase (JAK)-STAT activates Qp-driven EBNA1 expression
(22). Moreover, signal transducer
and activator of transcription 3 (STAT3) is likely to be the
biologically relevant STAT for EBNA1Qp and LMP1 L1-TR promoter
regulation (23). Aberrant
activation of STAT3 may be a necessary event for EBV-associated
malignancies (24). In addition,
increasing evidence has demonstrated that EBNA1 may be essential
for the proliferation of tumor cells (25,26).
Collectively, EBNA1 has become an attractive target for therapeutic
strategies.
Berberine is a natural compound belonging to the
alkaloids, which is present in the rhizome, roots and stem bark of
a number of medicinal plants such as Cortidis rhizome
(Huanglian), Coptis chinensis (Chinese goldthread),
Scutellaria caicalensis (Baikal Skullcap) and Berberine
vulgaris (barberry) (27,28).
Berberine is found to have multiple pharmacological functions such
as anti-diarrheal, anti-hypertensive, anti-microbial and
anti-inflammatory effects (29–32). A
previous study showed the potential cancer chemopreventive effect
of berberine (33). Moreover,
berberine has been reported to inhibit the growth of various types
of cancer, such as NPC, non-small lung cancer, primary effusion
lymphoma, and breast and liver cancer (34). Berberine was found to inhibit the
proliferation of human breast cancer cells (MCF-7) through the
downregulation of the expression of various tumor-related proteins
including HER2, Bcl-2, COX-2 and EGFR, and the upregulation of p21
and IFN-α expression (35–38). A previous study showed that
berberine induced mitochondrial apoptosis in EBV-transformed B
cells through the upregulation of XAF1 and GADD45α expression by
MAPK and functional p53 (39).
Various findings demonstrated that berberine induces the apoptosis
of NPC cells through the downregulation of the activity of STAT3
(40).
Our laboratory previously demonstrated that heat
shock protein 90 (Hsp90) inhibitors blocked outgrowth of
EBV-infected malignant cells through an EBNA1-dependent mechanism
in vitro and in vivo (41). Recently, we found that triptolide
inhibited the proliferation of EBV-positive B lymphocytes via the
inhibition of LMP1 (42). Moreover,
our laboratory demonstrated that triptolide, a diterpenoid
triepoxide purified from the roots of Chinese herb Tripterygium
wilfordii, showed antitumor activity against Kaposi's
sarcoma-associated herpesvirus (KSHV)-related primary effusion
lymphoma through a KSHV latency-associated nuclear antigen
1-dependent mechanism (43). KSHV
and EBV, members of the γ-herpesvirus subfamily, are very similar.
Furthermore, in addition to inhibiting the human telomerase reverse
transcriptase (hTERT) transcription and protein expression,
triptolide decreased the stability of hTERT in KSHV-positive BCBL-1
and BC-3 cells (44).
In the present study, we demonstrated that berberine
effectively inhibited cell proliferation and induced cycle arrest
and apoptosis of EBV-positive NPC cells. Berberine decreased EBNA1
transcription by inhibiting the activation of EBNA1 promoter Qp.
Berberine inhibited the expression of p-STAT3 and overexpression of
EBNA1 attenuated the inhibitory effect of berberine. In addition,
the production of virions were decreased by berberine. Moreover,
our in vivo results revealed that tumor growth of
EBV-positive NPC in non-obese diabetic/severe-combined
immunodeficient (NOD/SCID) mice was effectively inhibited by a
non-toxic dose (27,45) of berberine. These findings may
provide insights into the underlying mechanism involved in the
inhibition of NPC cell proliferation by berberine.
Materials and methods
Cell lines and reagents
EBV-positive NPC cell lines HONE1 and HK1-EBV were
kindly provided by Professor Sai Wah Tsao (The University of Hong
Kong, Hong Kong, China). HeLa cells were obtained from Professor
Hui Li (Wuhan University, Wuhan, China). The EBV-negative HK2 cells
were kindly provided by Professor Ling Zheng (Wuhan
University).
HONE1 and HK1-EBV cells were maintained in RPMI-1640
medium containing 10% fetal bovine serum (FBS; Gibco-BRL,
Gaithersburg, MD, USA) and G418 (400 ng/ml). HeLa cells were
cultured in Dulbeccos modified Eagles medium (DMEM) with 10% FBS.
HK2 cells were cultured in DME/F-12 containing 10% FBS and hEGF
(Sigma-Aldrich, Shanghai, China). All cell lines were cultured at
37̊C with a humidified atmosphere of 5% CO2. Berberine
chloride, cycloheximide (CHX) (both from Sigma-Aldrich, Shanghai,
China), acyclovir (ACV; Selleck Chemicals, Shanghai, China), and
12-O-tetradecanoylphorbol-13-acetate (TPA; Sigma-Aldrich)
were dissolved in dimethyl sulfoxide (DMSO). Sodium butyrate (SB;
Sigma-Aldrich) was dissolved in phosphate-buffered saline
(PBS).
Plasmids
Plasmid pSG5-EBNA1 was constructed by ligating the
EcoRI-XbaI fragment, which contains the EBNA1
sequence, into the pSG5 vector (YouBio, Shanghai, China). Plasmid
pGL3.0-basic was purchased from Promega (Madison, WI, USA). PRL-TK,
a plasmid expressing Renilla luciferase, was kindly provided
by Professor Deyin Guo (Wuhan University). The sequence of Qp was
amplified from the HONE1-strain EBV genome sequence. The primers
used in the present study were as follows: Qp forward,
5′-TCAGATCTTATAACGCAGGTCCTG-3′ and reverse,
5′-CGCAAGCTTTGTAAGGATAGCATG-3′ (YouBio). The amplified sequence was
digested with BglII and HindIII (NEB, Ipswich, MA,
USA). All plasmids were purified through columns (Axygen Scientific
Inc., Union City, CA, USA) as described by the manufacturer and
confirmed by DNA sequencing.
Cell viability assay
The viability of the cells was determined by the
Cell Counting Kit-8 (CCK-8) assay (Dojindo Laboratories, Kumamoto,
Japan). Briefly, HONE1 and HK1-EBV cells were placed into 96-well
plates with DMEM at a density of 1×104 cells/100 µl. All
cells were treated with controls (DMSO; 0.006%, vol/vol) and
increasing concentrations of berberine for 24 and 48 h. Tetrazolium
substrates were added into each well (10 µl/well) after different
treatments. The plates were incubated at 37̊C for 1 h. The optical
density (OD) was assessed at 450 nm with an ELx800
microimmunoanalyser (BioTek Instruments, Inc., Winooski, VT,
USA).
Flow cytometric analysis
Apoptosis was quantified using an Annexin
V-FITC/propidium iodide (PI) apoptosis detection kit
(MultiSciences, Shanghai, China). Briefly, HONE1 and HK1-EBV cells
were placed in 6-well plates and treated with the vehicle control
(DMSO; 0.006%, vol/vol) or berberine (50 or 100 µM) for 24 h. Cells
were harvested, washed and re-suspended in 500 µl of binding
buffer. Then, 5 µl of Annexin V-FITC and 10 µl of PI were added
before being analyzed by a Beckman Coulter system (EPICS Altra II;
Beckman Coulter, Fullerton, CA, USA).
Cell transfection
For transfection, all cells (HONE1, HK1-EBV and
HeLa) were transiently transfected using X-tremeGENE HP DNA
transfection reagent (Roche, Basel, Switzerland). At 4 h
post-transfection, cells were treated with the control (DMSO;
0.006%, vol/vol) or berberine for 44 h before harvesting. CHX (50
µg/ml) was used in experiments as described in the following
sections.
Dual luciferase reporter assay
In brief, HeLa cells were cultured in 48-well plates
(1×105 cells/well) before transfection. After 24 h,
pGL3.0-Qp (100 ng/well) and pRL-TK (5 ng/well) were co-transfected
into the HeLa cells. Then, the cells were treated with the vehicle
control (DMSO; 0.006%, vol/vol) or berberine (25 and 50 µM,
respectively) for 24 h, at 4 h post-transfection. Subsequently, the
medium was removed and cells were rinsed twice. Total proteins were
harvested in 1X lysis buffer (100 µl/well). Luciferase activity was
assessed by the GLO-MAX 20/20 system (both from Promega) following
the manufacturer's instructions. Renilla luciferase was used
to normalize the firefly luciferase activity.
Quantitative-PCR
The total RNA was extracted using TRIzol reagent
(Invitrogen, Grand Island, NY, USA) according to the manufacturer's
instructions. First-strand cDNA was synthesized from the RNA using
a reverse transcription kit (Takara, Tokyo, Japan) with random
primers. The expression of LMP1 and EBNA1 mRNAs was quantified by
CFX96 real-time PCR detection system using a SYBR Premix Ex
Taq™ kit (Takara). The relative amounts of LMP1 and EBNA1 mRNAs
were normalized to the housekeeping gene GAPDH. The primers were as
follows: LMP1 forward, 5′-CTATTCCTTTGCTCTCATGC-3′ and reverse,
5′-TGAGCAGGAGGGTGATCATC-3′; EBNA1 forward,
5′-GGTCGTGGACGTGGAGAAAA-3′ and reverse, 5′-GGTGGAGACCCGGATGATG-3′;
GAPDH forward, 5′-ACATCGCTCAGACACCATG-3′ and reverse,
5′-TGTAGTTGAGGTCAATGAAGGG-3′ (YouBio). The quantitative-PCR
conditions were as follows: a 30-sec denaturation at 95̊C, followed
by 40 cycles of 10 sec at 95̊C, 10 sec at 60̊C, and 20 sec at 72̊C.
The specificity of the reaction was controlled by melting curve
analysis (65–95̊C, 0.5̊C/sec).
Western blotting
Cells treated under different conditions were
harvested in RIPA buffer (Beyotime Institute of Biotechnology,
Shanghai, China) supplemented with 0.5 mM phenylmethylsufonyl
fluoride (PMSF) and 0.5% cocktail protease inhibitor (Roche) using
a micro-scraper. After being sonicated for 15 sec, whole cell
extracts were obtained by centrifugation at 12,000 × g for 15 min.
The supernatants were transferred to new tubes, and the protein
concentration was determined by the bicinchoninic acid method using
bovine serum albumin as a standard. Equal amounts of proteins were
mixed with 5X loading buffer [250 nM Tris-HCl (pH 6.8), 0.5% BPB,
10% SDS, 50% glycerol, 5% β-mercaptoethanol]. The proteins were
subjected to 10% SDS-polyacrylamide gel electrophoresis (SDS-PAGE).
The gels were run at 110 V, and then, the proteins were transferred
onto a polyvinylidene difluoride (PVDF) membrane (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). The membrane was blocked
with 5% skim milk in Tris-buffered saline and Tween-20 (TBST) for 1
h at room temperature, followed by incubation with the primary
antibody overnight at 4̊C. After 3×15 min washes with TBST, the
membrane was incubated with the appropriate secondary antibodies
for 1 h.
The primary antibodies used were as follows: GAPDH
(cat. no. 10494-1-AP, 1:10,000; Proteintech, Wuhan, China), EBNA1
(cat. no. sc81581, 1:200; Santa Cruz Biotechnology, Inc., Santa
Cruz, CA, USA), LMP1 (1:4,000; Abcam, Cambridge, UK), BZLF1 (1:500;
Dako, Glostrup, Denmark), caspase-3 (cat. no. 9668, 1:1,000),
cleaved caspase-3 (cat. no. 9664, 1:1,000), STAT3 (cat. no. 9139,
1:1,000), phospho-STAT3 (cat. no. 4113, 1:1,000, Tyr705), Mcl-1
(cat. no. 14765, 1:1,000) (all from Cell Signaling Technology Inc.,
Danvers, MA, USA). Secondary antibodies were horseradish
peroxidase-conjugated secondary anti-mouse IgG (cat. no. 7076,
1:10,000), anti-rabbit IgG (cat. no. 7074, 1:10,000) (both from
Cell Signaling Technology Inc.). Immunoreactivity was detected
using the ECL system (Bio-Rad Laboratories). Band gray values were
assessed by ImageJ software (National Institutes of Health,
Bethesda, MD, USA).
EBV copy number analysis
HONE1 and HK1-EBV cells were induced to lytic
replication phase by TPA (40 ng/ml) and SB (3 mM). After 3 h, cells
were cultured in the absence or presence of berberine for 48 h. EBV
virion-associated DNA was collected and amplified. The supernatants
of HONE1 and HK1-EBV cells were harvested and filtered with a 0.45
µl filter. Each supernatant was incubated with 2 µl DNase I and 10X
DNase I buffer (10 mM Tris-HCl, 0.5 mM CaCl2, 2.5 mM
MgCl2) at 37̊C. After 60 min, EDTA (2 mM, 20 µl, pH 8.0)
was added to inhibit the activity of DNase I. All the samples were
treated with proteinase K (0.1 mg/ml) [sample:proteinase K = 1:1
(vol/vol)] for 1 h at 50̊C. The reactions were stopped at 75̊C
after 20 min by inhibiting the activity of proteinase K (46). Subsequently, each sample and strand
was examined for the sequence of EBNA1 by quantitative-PCR assay
using a SYBR-Green PCR kit (cat no. RR420A; Takara Bio). Each
quantitative-PCR reaction mixture included 2 µl viral DNA from the
prepared sample, 2 µl specific primers (0.2 µM), 12.5 µl 2X SYBR
Premix Ex Taq, 0.4 µl ROX reference dye or Dyell and PCR-grade
water for a final volume of 25 µl. The primers were as follows:
EBNA1 forward, 5′-GGTCGTGGACGTGGAGAAAA-3′ and reverse,
5′-GGTGGAGACCCGGATGATG-3′ (YouBio). The quantitative-PCR conditions
were: a 5-sec denaturation at 95̊C, a 20-sec annealing at 60̊C, a
2-sec extension of primers at 72̊C for 45 cycles. Melting curve
analysis was performed from 65–95̊C (with 0.1̊C/sec).
In vivo tumor studies
All experimental procedures and protocols were
approved by the Medical Ethics Committee of Wuhan University. The
NOD/SCID mice were purchased and maintained in the Animal
Experiment Center of Wuhan University, Animal Biosafety Level-III
Laboratory.
Eight-week-old female NOD/SCID mice were
subcutaneously inoculated into flanks with 1×107 HONE1
cells suspended in 100 µl of PBS. Seven days later, the mice (five
mice/group) were treated with intraperitoneal injection of DMSO
(0.006%) or berberine (10 mg/kg, three times a week). Mice were
sacrificed after three weeks by cervical dislocation. Tumor size
was assessed, and then the tumors were fixed in 10% neutral
buffered formalin. Immunohistochemical analysis and hematoxylin and
eosin (H&E) staining were performed according to the method
described in a previous study (47).
Immunohistochemistry
Tumor samples were formalin-fixed and
paraffin-embedded and cut into sections of 4-µm thickness. The
sections were dewaxed in xylene, rehydrated in ethanol, washed in
PBS, and then stained with H&E. After staining, sections were
dehydrated through a series of increasing concentrations of ethanol
and xylene, and rehydrated in distilled water. After antigen
retrieval in a sodium citrate buffer in a microwave oven, the
endogenous peroxidase activity was blocked using 0.6%
H2O2 for 30 min. Sections were incubated
overnight at 4̊C with a primary antibody EBNA1 (cat. no. ab8329,
1:1,000; Abcam). After washing in PBS twice, the sample sections
underwent detection using the anti-mouse-specific HRP/AEC Detection
IHC kit (ab127055; Abcam) according to the manufacturer's
instructions.
Statistical analysis
All results were normalized to the control and are
presented as the mean ± standard deviation (mean ± SD) of at least
three independent experiments. The statistical significance of the
difference was evaluated by Student's t-test using GraphPad Prism
(GraphPad Software, La Jolla, CA, USA). Significance was assigned
at p<0.05.
Results
Berberine inhibits the viability of
EBV-positive NPC cells
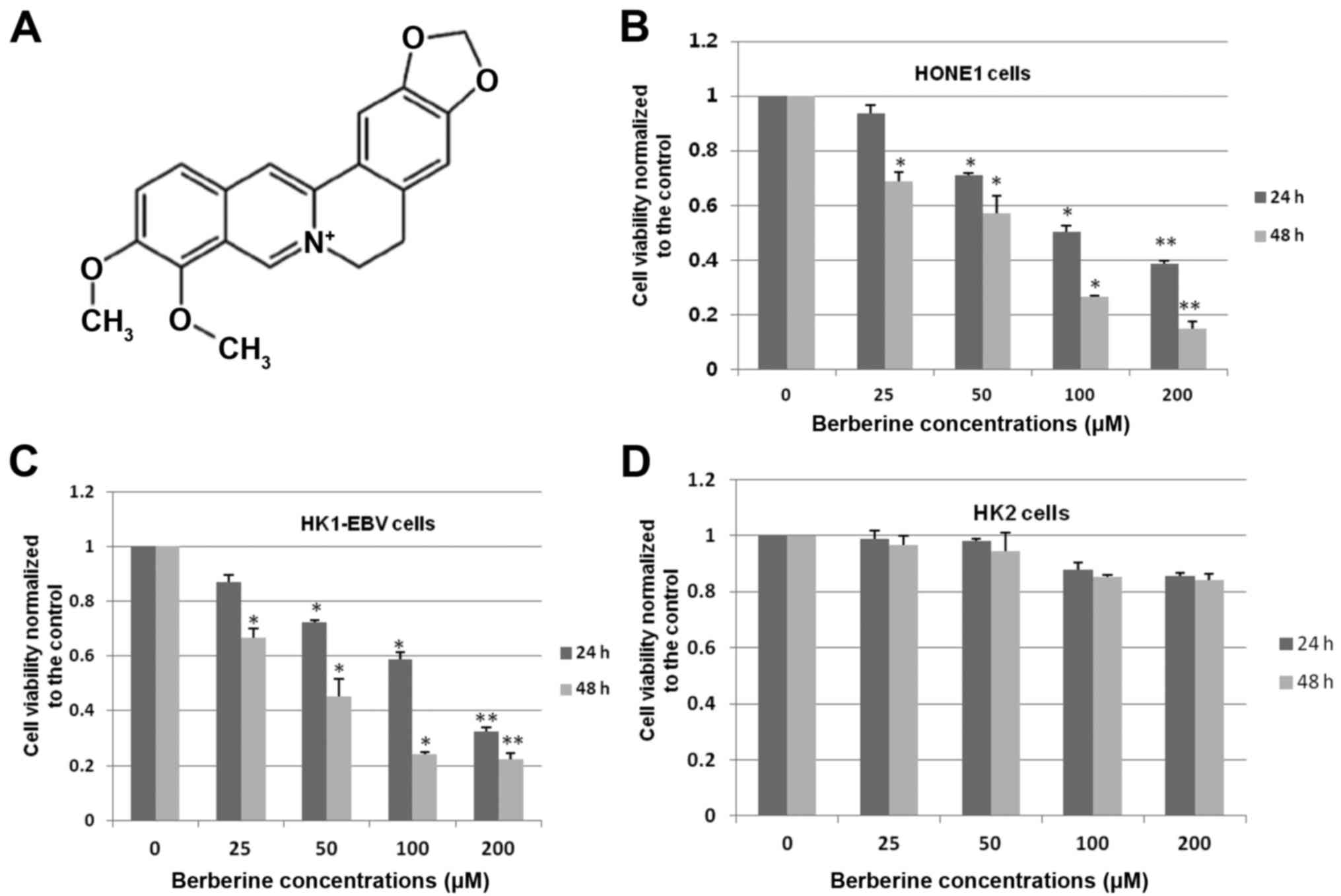
To determine whether berberine (Fig. 1A) affects the cell viability of
EBV-positive NPC cells, HONE1 and HK1-EBV cells were treated with a
vehicle control (0.006% DMSO) or a series of increasing
concentrations of berberine (25, 50, 100 and 200 µM) for 24 and 48
h, respectively. Cell viability was determined by CCK-8 assays. As
shown in Fig. 1B, berberine
inhibited the cell viability of HONE1 cells in a dose- and
time-dependent manner. The viability of HONE1 cells was decreased
from ~10 to 90% after berberine treatment for 24 and 48 h. The 50%
inhibitory concentration (IC50) for HONE1 cells was
101.3 and 56.7 µM with berberine treatment at 24 and 48 h,
respectively. Similar results were also found in the HK1-EBV cells
treated with a series of increasing concentrations of berberine.
The viability of HK1-EBV cells was decreased from 15 to 78% after
24 and 48 h of berberine treatment. The IC50 values were
calculated and were 124.5 and 43.1 µM at 24 and 48 h, respectively,
in the HK1-EBV cells treated with berberine (Fig. 1C). To further evaluate the effects
of berberine in the EBV-negative cell line, HK2 cells were treated
with the vehicle control (0.006% DMSO) or berberine for 24 and 48
h. As shown in Fig. 1D, treatment
with a series of increasing concentrations of berberine showed a
slight inhibition in the proliferation of HK2 cells at 24 and 48 h.
These results suggest that berberine inhibits the cell viability of
EBV-positive NPC cells efficiently, but not significantly in HK2
cells.
Berberine induces cell cycle arrest
and apoptosis in EBV-positive NPC cells
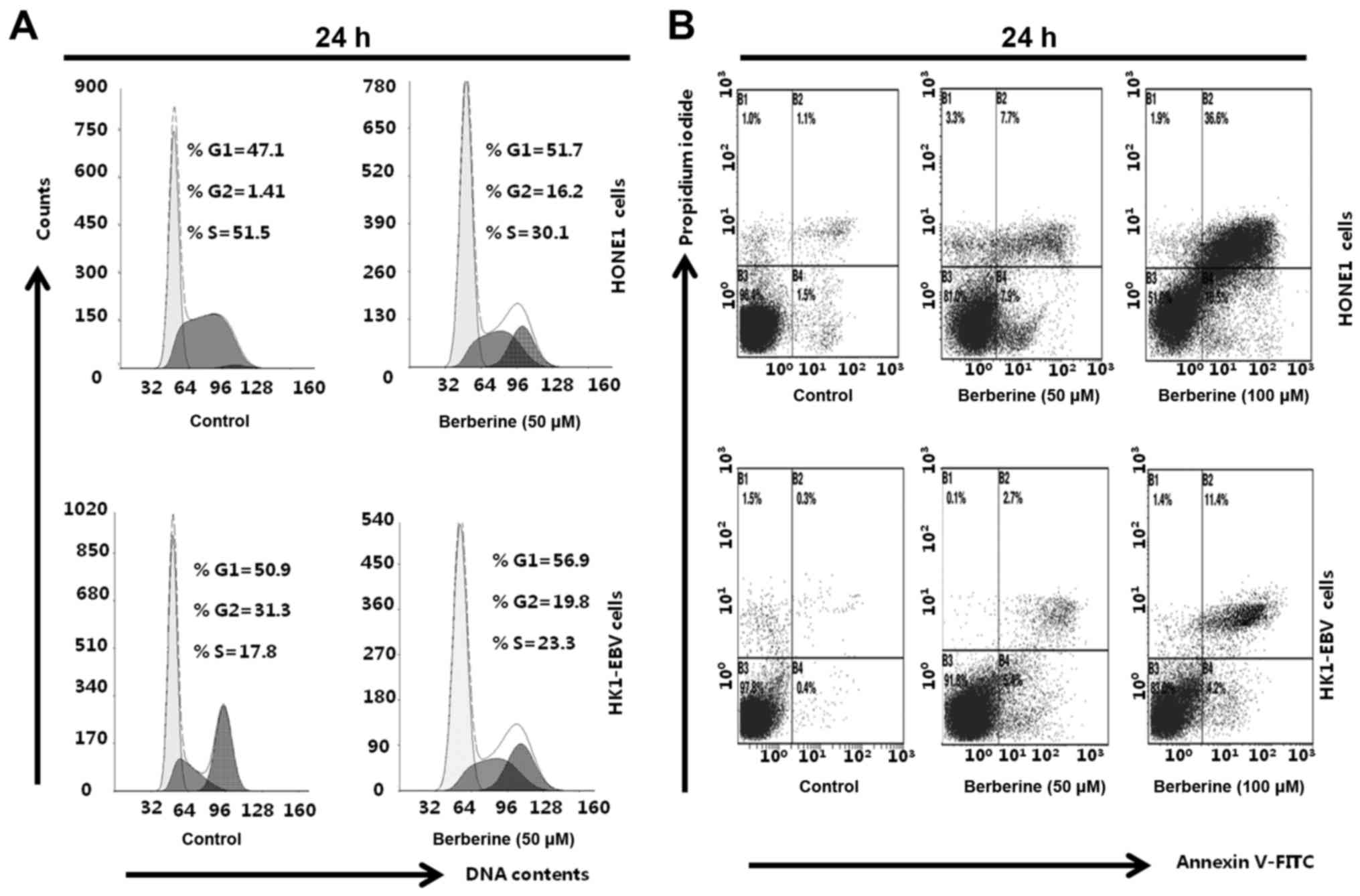
To examine whether berberine induces cell cycle
arrest and apoptosis, HONE1 and HK1-EBV cells were treated with the
vehicle control (0.006% DMSO) or berberine (50 and 100 µM) for 24
h. Cells were collected and stained with Annexin V-FITC/PI,
followed by analyses with flow cytometry. The cell cycle analysis
revealed that berberine-treated HONE1 cells underwent a G2 arrest;
the percentage of G2 phase cells was 1.41% in the control group,
and 16.2% in the berberine-treated group (Fig. 2A). Treatment of HK1-EBV cells with
berberine resulted in G1 phase arrest; the percentage of G1 phase
cells was 50.9% in the control (0.006% DMSO) and 56.9% in the
berberine-treated group (50 µM) (Fig.
2A). In addition, compared to the control group (0.006% DMSO),
berberine (50 or 100 µM) induced cell apoptosis in the HONE1 and
HK1-EBV cells (Fig. 2B). The
results suggest that berberine induced cell cycle arrest and
apoptosis in the EBV-positive HONE1 and HK1-EBV cells.
Berberine decreases EBNA1 expression
in EBV-positive NPC cells
To determine whether berberine alters the expression
of EBNA1 in EBV-positive NPC cell lines, HONE1 and HK1-EBV cells
were treated with the vehicle control (0.006% DMSO) or berberine
(25 or 50 µM) for 48 h. Whole-cell extracts were harvested and
subjected to western blotting. As shown in Fig. 3A, the expression levels of EBNA1 in
the HONE1 cells were decreased to 36.8 and 6.7%, with berberine
treatment at 25 and 50 µM, respectively, when compared to the
control group. HK1-EBV cells were also treated with the same
concentrations of berberine. When compared to the control group,
the expression levels of EBNA1 were not significantly affected in
the 25 µM berberine-treated group, but downregulated to 32.4% in
the 50 µM berberine-treated group. However, the expression level of
another EBV protein, LMP1, was not decreased by berberine in both
the HONE1 and HK1-EBV cells. Furthermore, compared to the control
group, the expression levels of cleaved-caspase-3 in the HONE1 and
HK1-EBV cells were increased while the levels of caspase-3
exhibited no obvious changes with berberine treatment. The
different response of the HONE1 and HK1-EBV cells to berberine is
possibly due to the different sensitivity of the cell lines to this
drug.
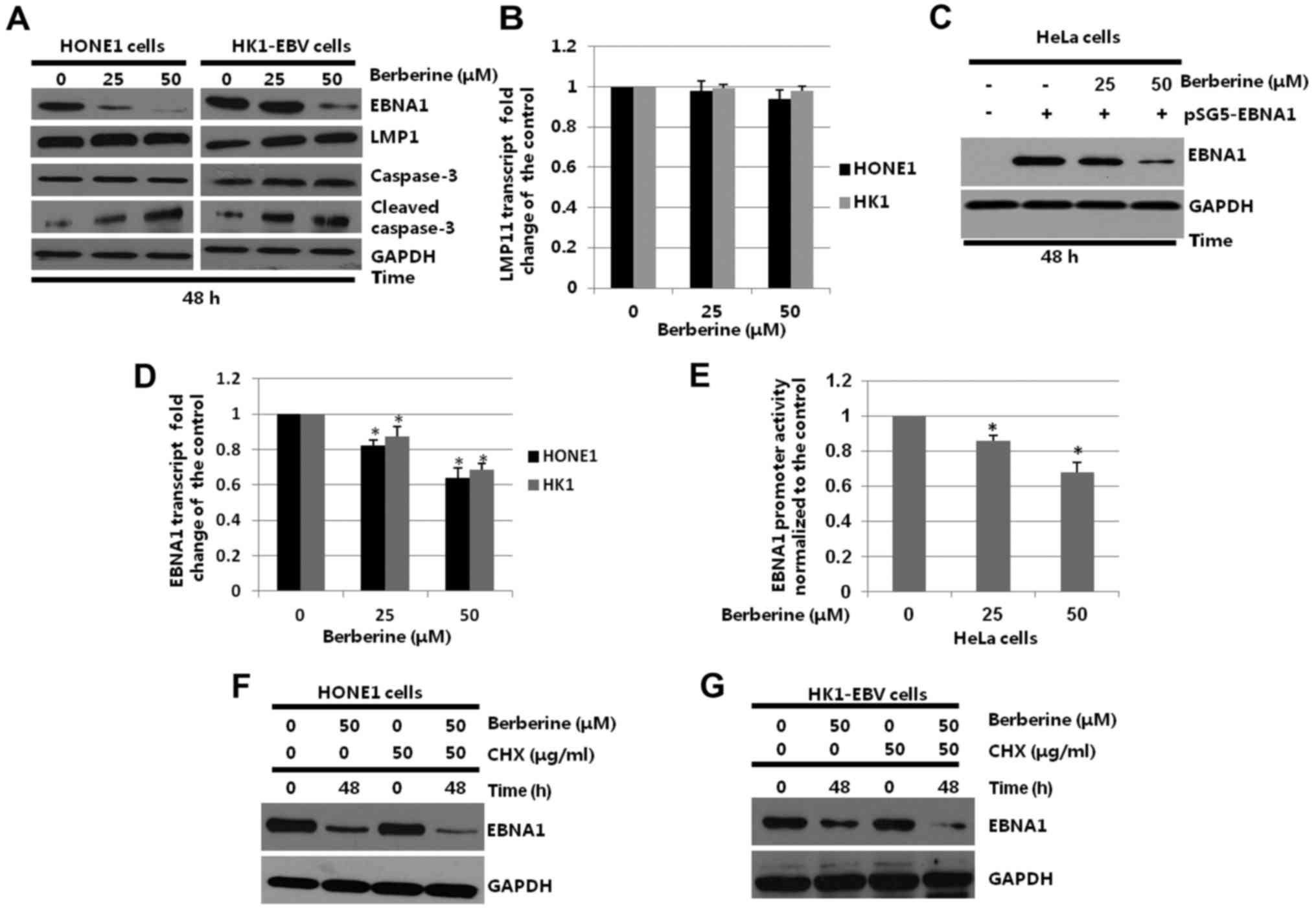 | Figure 3.Effect of berberine on the
transcription, expression and half-life of the EBNA1 protein in
HONE1 or HK1-EBV cells. (A) HONE1 or HK1-EBV cells were placed in
6-well plates and treated with the vehicle control (0.006% DMSO) or
berberine (25 or 50 µM) for 48 h. Whole-cell extracts were prepared
and western blotting was performed to analyze the expression of
EBNA1, caspase-3, cleaved caspase-3 and GAPDH. (B) HONE1 and
HK1-EBV cells were treated with the vehicle control (0.006% DMSO)
or berberine (25 or 50 µM) for 24 h. The mRNA levels of LMP1 were
detected by quantitative-PCR. The level of LMP1 transcript in cells
treated with the vehicle control was set as 1. (C) HeLa cells were
transfected with pSG5 or pSG5-EBNA1 as indicated, followed by a
44-h berberine (25 or 50 µM) treatment beginning at 4 h after
transfection. Whole-cell extracts were prepared and western
blotting was performed to analyze the expression of EBNA1 and
GAPDH. (D) HONE1 or HK1-EBV cells were treated with the vehicle
control (0.006% DMSO) or berberine (25 or 50 µM) for 48 h. The mRNA
levels of EBNA1 were determined by quantitative-PCR. The level of
EBNA1 transcript in cells treated with the vehicle control was set
as 1; *p<0.05, compared with vehicle control (0.006% DMSO). (E)
HeLa cells were co-transfected with pGL3.0-Qp and pRL-TK, followed
by a 24 h treatment with the vehicle control (0.006% DMSO) or
berberine (25 or 50 µM) beginning at 4 h post-transfection. Cell
lysates were harvested and the activity of the promoter was
determined by a dual-luciferase reporter assay; *p<0.05,
compared with the vehicle control (0.006% DMSO). (F) HONE1 and (G)
HK1-EBV cells were treated with the vehicle (0.006% DMSO) or
berberine (50 µM) for 48 h in the presence or absence of CHX (50
µg/ml) added to the medium 12 h before cell harvesting. Whole-cell
extracts were harvested and subjected to western blotting. EBNA1,
Epstein-Barr nuclear antigen 1; EBV, Epstein-Barr virus; DMSO,
dimethyl sulfoxide; LMP1, latent membrane protein 1; CHX,
cycloheximide. |
In addition, quantitative PCR analysis was used to
detect whether berberine affects LMP1 at the mRNA level. HONE1 and
HK1-EBV cells were treated with the vehicle control (0.006% DMSO)
or berberine (25 or 50 µM) for 24 h, respectively. The total RNAs
were collected and subjected to quantitative-PCR. The relative
expression of LMP1 was detected by comparison to the GAPDH gene. As
shown in Fig. 3B, the mRNA levels
of LMP1 in the HONE1 and HK1-EBV cells were not decreased by
berberine.
Berberine treatment in a previous study was not
observed to affect the viability of EBV-negative HeLa cells
(48). Therefore, HeLa cells were
used to determine whether berberine specifically decreases the
expression of EBNA1 in the present study. In order to determine
whether berberine specifically decreases the expression of EBNA1,
p-SG5 and pSG5-EBNA1 were transiently transfected into HeLa cells
for 4 h, followed by treatment with or without berberine. As shown
in Fig. 3C, when compared to the
control group, the expression levels of EBNA1 were not
significantly affected in the 25 µM berberine-treated group, but
downregulated to 32.5% in the 50 µM berberine-treated group.
To further determine whether the decreased
expression of EBNA1 was due to possible alterations at the
transcriptional levels, HONE1 and HK1-EBV cells were treated with
the vehicle control (0.006% DMSO) or berberine (25 or 50 µM). After
24 h, the total RNAs were harvested and subjected to
quantitative-PCR. As shown in Fig.
3D, berberine decreased EBNA1 mRNA levels to 81.23% in the 25
µM and 63.17% in the 50 µM berberine-treated groups, respectively,
when compared with the vehicle control (0.006% DMSO) group in the
HONE1 cells. In the HK1-EBV cells, the EBNA1 mRNA levels were
decreased to 85.39% (p<0.05) in the 25 µM and 65.73% (p<0.05)
in the 50 µM berberine-treated groups, respectively, when compared
with the vehicle control (0.006% DMSO) group. These results
demonstrated that berberine inhibited the mRNA levels of EBNA1 in
HONE1 and HK1-EBV cells.
Berberine inhibits the activity of
EBNA1 Qp
EBNA1 transcription is initiated from Qp in
EBV-associated NPC cells. According to our previous studies
(42,44), the promoters of latent membrane
protein 1 (LMP1) and human telomerase reverse transcriptase (hTERT)
were studied in HeLa and 293T cells. To determine whether berberine
inhibited the activity of EBNA1 promoter, pGL3.0 vector control or
pGL3.0-Qp was co-transfected with plasmid pRL-TK into HeLa cells.
The HeLa cells were treated with the control (0.006% DMSO) or
berberine (25 or 50 µM) for 24 h, at 4 h post-transfection. The
whole protein was harvested and the luciferase activity was
assessed using the GLO-MAX 20/20 system (Promega). Compared to the
control group, the activity of Qp was decreased to 83.4%
(p<0.05) and 62.7% (p<0.05) in the 25 and 50 µM
berberine-treated groups, respectively (Fig. 3E). The results suggest that the
transcriptional activity of EBNA1 was decreased by berberine in the
HeLa cell line.
Berberine decreases the half-life of
EBNA1
To determine whether berberine decreases the protein
half-life of EBNA1, HONE1 and HK1-EBV cells were treated with the
vehicle control or berberine, in the presence of CHX. As shown in
Fig. 3F and G, berberine decreased
the half-life of the EBNA1 protein in the CHX-treated HONE1 and
HK1-EBV cells when compared to the vehicle control. The results
suggest that berberine decreases the half-life of EBNA1.
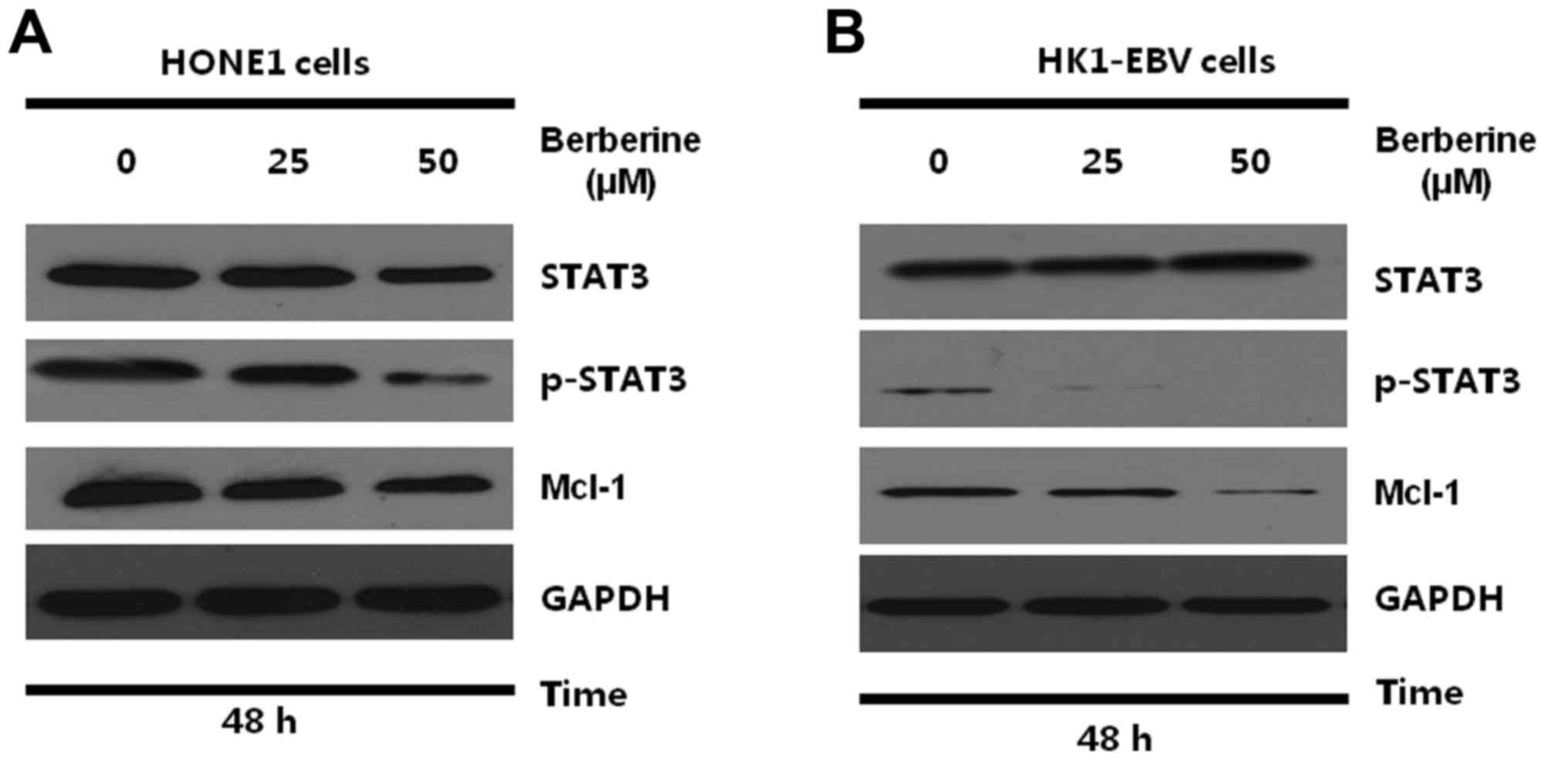
Berberine downregulates STAT3
signaling in the NPC cells
The Qp promoter that drives the expression of EBNA1
in EBV-associated tumors is regulated by the JAK-STAT signaling
pathway (22,23). STAT3 is essential for the activation
of Qp and raises the possibility that aberrant activation of STAT3
may be an essential factor in EBV-associated diseases (22). Since STAT3 represents a potential
target for treatment of EBV-associated tumors, it may be beneficial
to determine whether the activity of STAT3 is affected by
berberine. Therefore, HONE1 and HK1-EBV cells were treated with the
vehicle control (0.006% DMSO) or berberine (25 or 50 µM). After 48
h, the total proteins were collected and subjected to western
blotting. As shown in Fig. 4A and
B, the expression of STAT3 and p-STAT3 were detected in the
HONE1 and HK1-EBV cells. Berberine suppressed the expression of
p-STAT3 effectively in the HONE1 and HK1-EBV cells. Furthermore,
the present study demonstrated that the downregulation of p-STAT3
was associated with the suppressed expression of Mcl-1, which is a
downstream survival protein of STAT3. These results suggest that
downregulation of EBNA1 by berberine is possibly related to the
inhibition of the STAT3 signaling pathway in NPC cells.
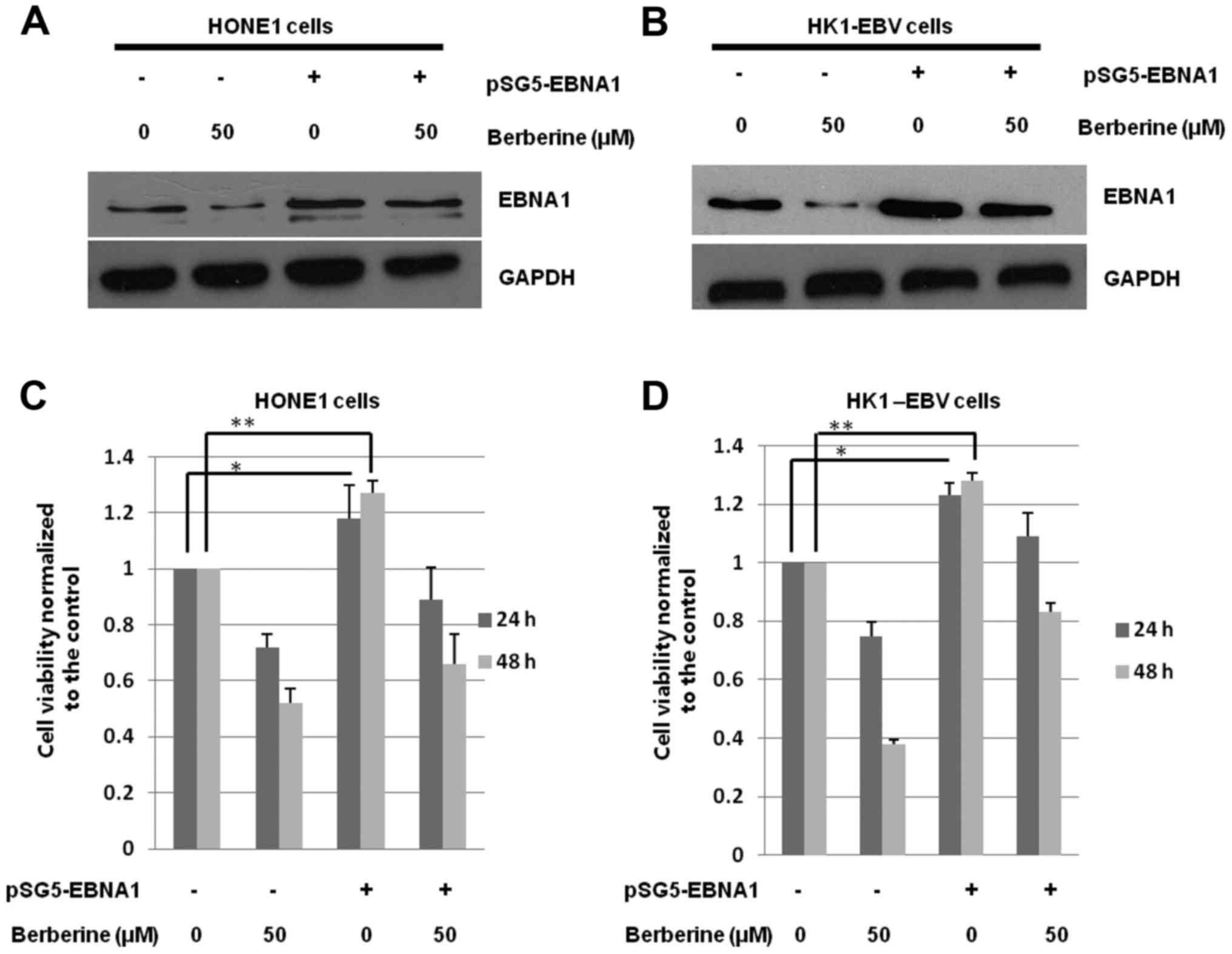
Overexpression of EBNA1 attenuates the
effect of berberine
To determine whether the berberine-induced decrease
in cell viability (Fig. 1B and C)
is mainly caused by the inhibited expression of EBNA1, pSG5-EBNA1
or the vehicle control (pSG5) were transfected into HONE1 and
HK1-EBV cells. The cells were treated with the vehicle control
(0.006% DMSO) or berberine (50 µM) at 4 h post-transfection. After
44 h, the total proteins were harvested and subjected to western
blotting. As shown in Fig. 5A and
B, the expression levels of EBNA1 were increased by 301.3% in
the HONE1 cells, and 368.1% in the HK1-EBV cells, respectively, by
transient transfection.
Cell viability was detected after 24 and 48 h. As
shown in Fig. 5C and D, the cell
viability of both HONE1 and HK1-EBV cells was significantly
decreased by berberine. Following the overexpression of EBNA1, the
cell viability was increased by 19.8% (p<0.05) in the HONE1
cells and 22.4% (p<0.05) in the HK1-EBV cells after 24 h,
respectively, when compared with the control group
(berberine-treated control group). After 48 h, compared to the
control group (berberine-treated control group), the cell viability
was increased by 24.5% (p<0.05) in the HONE1 cells and 27.6%
(p<0.05) in the HK1-EBV cells. Overexpression of EBNA1 increased
cell viability and decreased the inhibitory effect of berberine.
These results suggest that berberine inhibits the proliferation of
EBV-positive HONE1 and HK1-EBV cells possibly through a mechanism
related to the decreased expression of EBNA1.
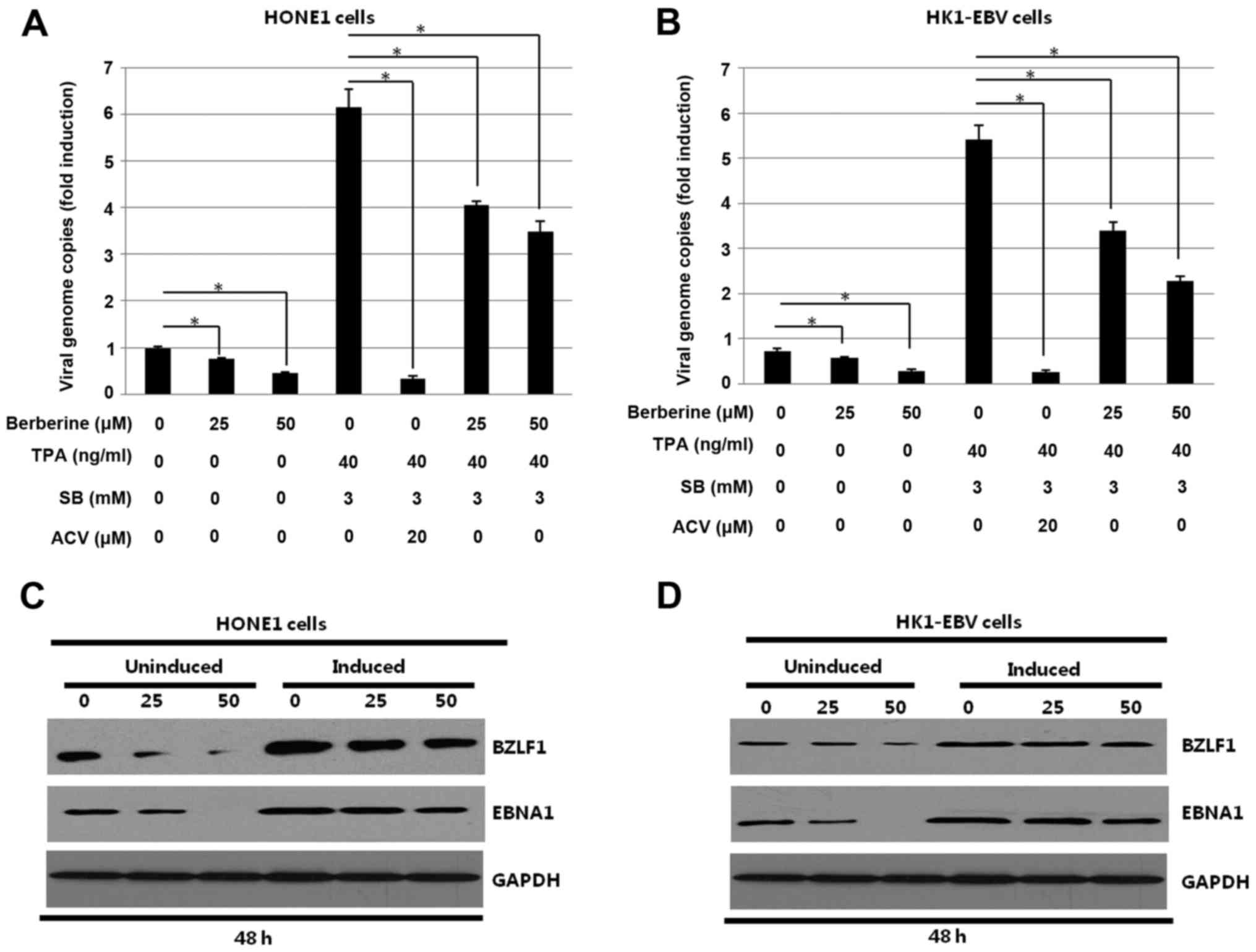
Berberine decreases latent and lytic
replication of EBV in EBV-positive NPC cells
To determine whether berberine inhibits lytic
replication of EBV, HONE1 and HK1-EBV cells uninduced or induced by
TPA and SB were treated with the indicated concentrations of
berberine for 48 h. Aciclovir (ACV) was used as a positive control,
efficiently decreasing virion production of EBV. After induction,
the culture media of HONE1 and HK1-EBV cells were prepared after 48
h incubation and processed for the detection of the EBV EBNA1
fragment. Quantitative-PCR and western blotting were used to detect
the effect of berberine on lytic replication of EBV. As shown in
Fig. 6A and B, berberine decreased
viral genome copies in both uninduced and induced HONE1 and HK1-EBV
cells. As expected, ACV, which was used as a positive control,
significantly decreased the viral genome copies in the induced
HONE1 and HK1-EBV cells. The results indicated that virions were
decreased by berberine in a dose-dependent manner. The
aforementioned cell lysates were harvested. Western blotting was
used to analyze the effect of berberine on the expression of EBV
transcriptional activator BZLF1 encoded by the EBV immediate-early
(IE) gene in the HONE1 and HK1-EBV cells. As shown in Fig. 6C and D, the expression levels of the
EBV transcriptional activator BZLF1 in the HONE1 and HK1-EBV cells
were decreased by berberine in both the uninduced and induced HONE1
and HK1-EBV cells. The results suggest that berberine decreased
latent and lytic replication of EBV in the HONE1 and HK1-EBV
cells.
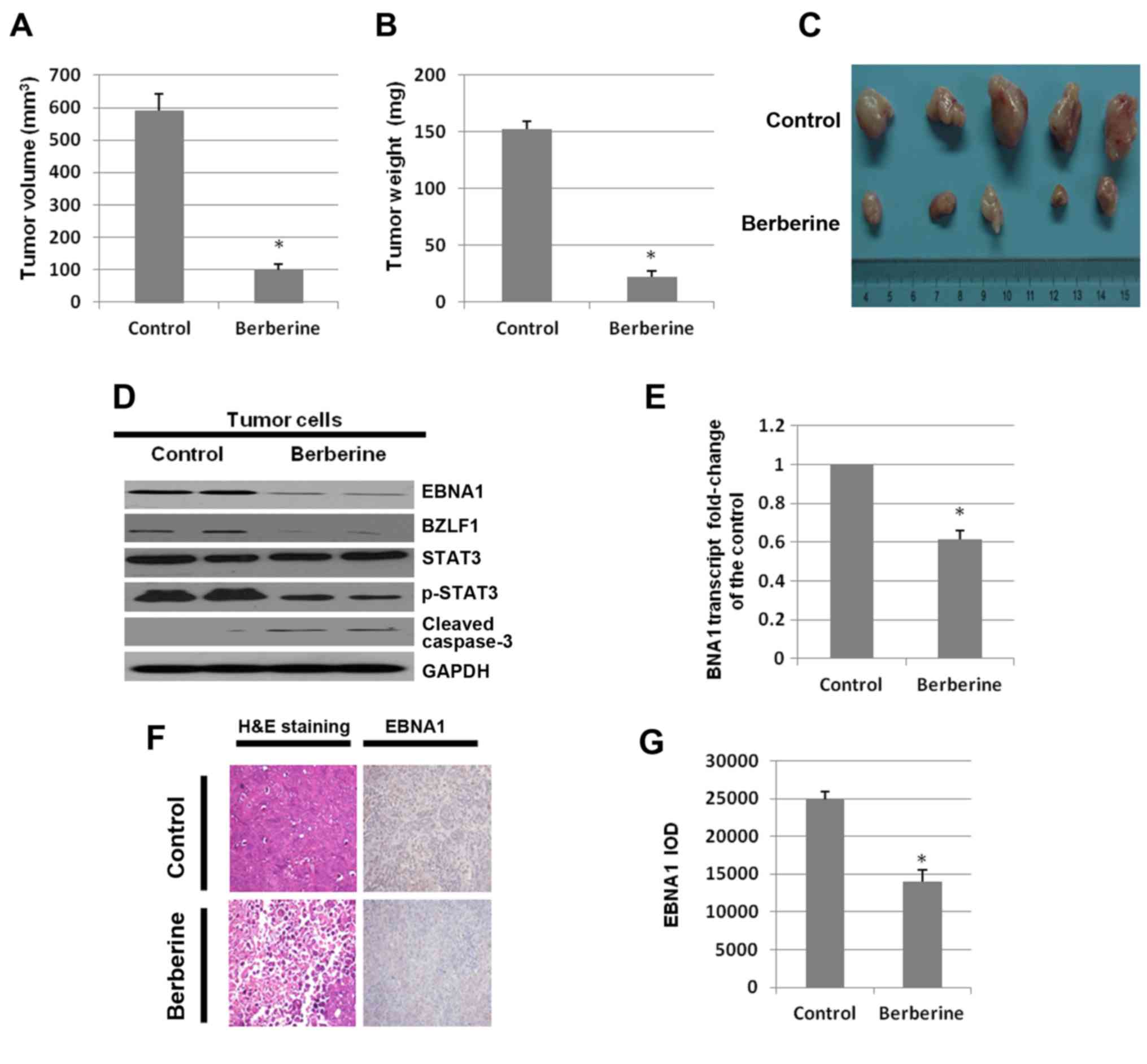
Berberine inhibits tumor growth in
severely immunodeficient mice
Since the aforementioned results demonstrate the
inhibition of NPC cell proliferation by berberine, the possible
effects of berberine in vivo were investigated. To determine
whether berberine inhibits the growth of the EBV-positive NPC in
NOD/SCID mice, 1×107 HONE1 cells were subcutaneously
inoculated in the flanks of the mice. After seven days, a dose of
10 mg/kg of berberine or DMSO was administered via subcutaneous
injection three times a week. After three weeks, the mice were
euthanized and the volumes and weights of the tumors were assessed.
The tumor volumes of the DMSO-treated group were larger than that
of the berberine-treated group (98.75±13 vs. 590.5±12.3
mm3, n=5, p<0.05; Fig.
7A). Mice treated with berberine had a significantly lower
tumor weight (22.25±7.5 vs. 152.5±10.2 mg, n=5, p<0.05; Fig. 7B). Images of tumors in mice treated
with DMSO or berberine are shown in Fig. 7C.
To determine the mechanism by which berberine
inhibits tumor growth, the expression levels of EBNA1, BZLF1,
p-STAT3 and cleaved caspase-3 in the tumor tissues were analyzed by
western blotting. Compared to the DMSO-treated group, berberine
downregulated the expression level of EBNA1 significantly and
induced tumor cell apoptosis via the caspase-3 pathway. In
addition, the expression of BZLF1 was decreased by berberine
(Fig. 7D). Then, total RNAs were
extracted from tumor tissues. The mRNA levels were analyzed by
quantitative-PCR. The mRNA level of EBNA1 in the berberine-treated
group was decreased to 61.3% of the control group (Fig. 7E).
Both the vehicle control (DMSO) and the
berberine-treated tumor tissue groups were analyzed by H&E
staining and EBNA1 immunostaining (Fig.
7F). The tumor tissues of the berberine-treated group were
found to have necrosis and a small amount of inflammatory cell
infiltration. The number of EBNA1-positive cells in the
berberine-treated mice was significantly decreased compared to the
control group. As shown in Fig. 7G,
the number of EBNA1-positive cells in the tumor tissues of the
berberine-treated group were decreased by 40.2% (p<0.05)
compared to those of the DMSO-treated group. The results suggest
that berberine significantly inhibits the growth of EBV-positive
NPC cells in vivo.
Discussion
Nasopharyngeal carcinoma (NPC) is distinguished from
other types of head and neck cancers due to its unique sensitivity
to radiotherapy and chemotherapy (49). Although a number of drugs have been
used as chemotherapy agents (such as cisplatin and cetuximab), a
relapse rate as high as 34% still confounds clinicians (50). Therefore, novel therapeutic
strategies such as molecular-targeted therapy are needed. EBNA1 has
been shown to alter the cellular environment and contribute to cell
survival, proliferation, immortalization and tumorigenesis,
providing an attractive target for Epstein-Barr virus
(EBV)-associated diseases (51).
Our previous study demonstrated that Hsp90 inhibitors block
outgrowth of EBV-infected malignant cells through an
EBNA1-dependent mechanism (41).
Thus, development of new drugs that can inhibit the activity of
EBNA1 may be therapeutically useful. In the present study, we
investigated the effect of berberine on EBV-positive NPC cells
in vitro and in vivo. Our results suggest that
berberine effectively inhibits EBV-positive NPC cell proliferation,
and induces cycle arrest and apoptosis.
Berberine, an active ingredient extracted from the
herb Coptidis rhizome, which has long been used as an
anti-diarrheal, a stomachic, an antibiotic, and an
anti-inflammatory agent in China has been reported to have
anticancer properties in multiple cancer cell lines, such as
breast, colon and liver cancer (30,31,52).
EBNA1 is reported to be an essential protein in all NPC tumors, and
is the only EBV protein needed for the persistence and replication
of EBV episomes (53). Our findings
revealed that berberine decreased EBNA1 expression in two different
EBV-positive NPC cell lines and EBV-negative HeLa cells transfected
with pSG5-EBNA1 transiently-expressing EBNA1. The activity of the
EBNA1 promoter in EBV latency II infection was inhibited by
berberine in HeLa cells. In addition, the stability or half-life of
EBNA1 was decreased by berberine. These results suggest that
berberine inhibited the expression EBNA1 at both the mRNA and
protein levels in HONE1 and HK1-EBV cells.
Our previous study demonstrated that triptolide
suppressed the activity of LMP1 promoter ED-L1 in latency III type
EBV-positive B lymphocytes (42).
In NPC, EBV exhibits a type II latency program. In the present
study, the mRNA level of EBNA1 was decreased by berberine.
Moreover, the activity of the EBNA1 promoter Qp was inhibited by
berberine, suggesting that inhibition of EBNA1 promoter activity
resulted in the inhibition of EBNA1 at the transcriptional
level.
Previous studies have shown that STAT3 is an
attractive target for anticancer therapy (24). STAT3 is an important transcriptional
factor that functions in numerous physiological processes including
cancer development, inflammation, immunity and wound healing. In
human breast (MDA-MB-231) and pancreatic (Panc-1) cancer cell
lines, the STAT3 inhibitor suppressed the viability, survival,
growth and malignant transformation, effectively (54). STAT3 also contributes to EBV and
KSHV-mediated cancers by promoting cell proliferation, angiogenesis
and invasion. In addition, recent evidence indicated that aberrant
activation of STAT3 is essential for the tumorigenesis and
progression of NPC (55). STAT3 was
activated almost immediately by EBV upon infection of primary
cells. EBV utilizes STAT3 to inhibit cellular self-protection
functions. A previous study implicated STAT3 as a biologically
relevant STAT for the activation of Qp and L1-TR promoters and
raises the possibility that aberrant activation of STAT3 may be a
contributing factor to EBV-associated tumorigenesis (23). Furthermore, evidence has
demonstrated that aberrant activation of the STAT3 pathway is
associated with tumor invasion and metastasis in various types of
cancer. STAT3 is constitutively activated without any stimulus in
HONE1 and HK1-EBV cells. In the present study, the expression
levels of p-STAT3 and Mcl-1 (a downstream survival protein of
STAT3) were significantly downregulated by berberine. Our results
indicate that downregulation of the expression levels of EBNA1 in
NPC may be at least partially a result from the inhibition of STAT3
activity by berberine. The mechanism in which STAT3 mediates
berberine-induced inhibition of EBNA1 may be studied in the
future.
In addition to LMP1, EBNA1 contributes to viral
transcription and multiple effects on cellular proteins and
pathways that promote cell survival and proliferation (56–58).
Our laboratory recently demonstrated that overexpression of LMP1
increases the cell viability of EBV-positive B lymphocytes, B95-8
and P3HR cells (42). In the
present study, we found that overexpression of EBNA1 increased the
cell viability of HONE1 and HK1-EBV cells. The results demonstrated
that berberine decreased the proliferation and activity of
EBV-positive NPC cells partly due to the decrease in EBNA1
expression.
In addition to the latent infection of EBV, the
lytic reactivation of EBV has been reported to be strongly
associated with several human diseases, including NPC. Inhibition
of the EBV lytic cycle has been shown to be of great benefit in the
treatment of EBV-associated diseases. Consistent with the effect of
berberine in decreasing the expression of EBNA1 in EBV-latent
infected cells, the present study demonstrated that berberine
decreased the expression of the lytic gene BZLF1 in uninduced and
induced EBV-positive NPC cells. At present, the mechanism involved
in the inhibition of gene expression and lytic replication of EBV
by berberine is not very clear and warrants further study.
Collectively, berberine induces cell cycle arrest and apoptosis,
downregulates the expression of EBNA1 and suppresses lytic
replication of EBV in EBV-positive NPC cells.
In addition to the inhibitory effect of berberine on
EBV-positive NPC cells in vitro, we also investigated the
therapeutic effect of berberine on NPC in a xenograft mouse model.
Consistent with the ability of Hsp90 inhibitors to suppress tumors
in NOD/SCID mice by decreasing the expression of EBNA1 (41), berberine displayed a significant
effect on the inhibition of tumor growth in NOD/SCID mice induced
by the injection of HONE1 cells. Moreover, berberine decreased the
expression of EBNA1 and BZLF1 in the xenograft tumor cells.
In the present study, our results demonstrated the
ability of berberine to decrease cell proliferation, induce cell
cycle arrest and promote apoptosis in the EBV-positive NPC cells.
The inhibitory activity of STAT3 provides evidence justifying the
decrease of EBNA1 levels induced by berberine. In addition,
berberine has the ability to inhibit the lytic replication of
EBV-positive NPC cells. Thus, berberine may be used as a novel
therapy in the treatment of EBV-associated tumors including
NPC.
Acknowledgements
The present study was supported by the Initiative
Research Program of Wuhan University (no. 410100020), the Advanced
Talent Independent Research Program of Wuhan University (no.
410500011), and the National Natural Science Foundation of China
(no. 31270205).
References
|
1
|
Martin KA, Lupey LN and Tempera I:
Epstein-Barr virus oncoprotein LMP1 mediates epigenetic changes in
host gene expression through PARP1. J Virol. 90:8520–8530. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cui Q, Feng FT, Xu M, Liu WS, Yao YY, Xie
SH, Li XZ, Ye ZL, Feng QS, Chen LZ, et al: Nasopharyngeal carcinoma
risk prediction via salivary detection of host and Epstein-Barr
virus genetic variants. Oncotarget. 2016.
|
|
3
|
Xu M, Cheung CC, Chow C, Lun SW, Cheung ST
and Lo KW: Overexpression of PIN1 enhances cancer growth and
aggressiveness with cyclin D1 induction in EBV-associated
nasopharyngeal carcinoma. PLoS One. 11:e01568332016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sivachandran N, Thawe NN and Frappier L:
Epstein-Barr virus nuclear antigen 1 replication and segregation
functions in nasopharyngeal carcinoma cell lines. J Virol.
85:10425–10430. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mansouri S, Pan Q, Blencowe BJ, Claycomb
JM and Frappier L: Epstein-Barr virus EBNA1 protein regulates viral
latency through effects on let-7 microRNA and dicer. J Virol.
88:11166–11177. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Houldcroft CJ and Kellam P: Host genetics
of Epstein-Barr virus infection, latency and disease. Rev Med
Virol. 25:71–84. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhu DD, Zhang J, Deng W, Yip YL, Lung HL,
Tsang CM, Law WT, Yang J, Lau VM, Shuen WH, et al: Significance of
NF-κB activation in immortalization of nasopharyngeal epithelial
cells. Int J Cancer. 138:1175–1185. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang FW, Wu XR, Liu WJ, Liang YJ, Huang
YF, Liao YJ, Shao CK, Zong YS, Mai SJ and Xie D: The nucleotide
polymorphisms within the Epstein-Barr virus C and Q promoters from
nasopharyngeal carcinoma affect transcriptional activity in vitro.
Eur Arch Otorhinolaryngol. 269:931–938. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shen Y, Zhang S, Sun R, Wu T and Qian J:
Understanding the interplay between host immunity and Epstein-Barr
virus in NPC patients. Emerg Microbes Infect. 4:e202015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sam CK, Brooks LA, Niedobitek G, Young LS,
Prasad U and Rickinson AB: Analysis of Epstein-Barr virus infection
in nasopharyngeal biopsies from a group at high risk of
nasopharyngeal carcinoma. Int J Cancer. 53:957–962. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kelly GL, Stylianou J, Rasaiyaah J, Wei W,
Thomas W, Croom-Carter D, Kohler C, Spang R, Woodman C, Kellam P,
et al: Different patterns of Epstein-Barr virus latency in endemic
Burkitt lymphoma (BL) lead to distinct variants within the
BL-associated gene expression signature. J Virol. 87:2882–2894.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kempkes B and Ling PD: EBNA2 and its
coactivator EBNA-LP. Curr Top Microbiol Immunol. 391:35–59.
2015.PubMed/NCBI
|
|
13
|
Frappier L: Contributions of Epstein-Barr
nuclear antigen 1 (EBNA1) to cell immortalization and survival.
Viruses. 4:1537–1547. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dheekollu J, Wiedmer A, Sentana-Lledo D,
Cassel J, Messick T and Lieberman PM: HCF1 and OCT2 cooperate with
EBNA1 to enhance OriP-dependent transcription and episome
maintenance of latent Epstein-Barr virus. J Virol. 90:5353–5367.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wood VH, O'Neil JD, Wei W, Stewart SE,
Dawson CW and Young LS: Epstein-Barr virus-encoded EBNA1 regulates
cellular gene transcription and modulates the STAT1 and TGFβ
signaling pathways. Oncogene. 26:4135–4147. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Valentine R, Dawson CW, Hu C, Shah KM,
Owen TJ, Date KL, Maia SP, Shao J, Arrand JR, Young LS, et al:
Epstein-Barr virus-encoded EBNA1 inhibits the canonical NF-kappaB
pathway in carcinoma cells by inhibiting IKK phosphorylation. Mol
Cancer. 9:12010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tempera I, De Leo A, Kossenkov AV,
Cesaroni M, Song H, Dawany N, Showe L, Lu F, Wikramasinghe P and
Lieberman PM: Identification of MEF2B, EBF1, and
IL6R as direct gene targets of Epstein-Barr virus (EBV)
nuclear antigen 1 critical for EBV-infected B-lymphocyte survival.
J Virol. 90:345–355. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Holowaty MN, Sheng Y, Nguyen T, Arrowsmith
C and Frappier L: Protein interaction domains of the
ubiquitin-specific protease, USP7/HAUSP. J Biol Chem.
278:47753–47761. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Saridakis V, Sheng Y, Sarkari F, Holowaty
MN, Shire K, Nguyen T, Zhang RG, Liao J, Lee W, Edwards AM, et al:
Structure of the p53 binding domain of HAUSP/USP7 bound to
Epstein-Barr nuclear antigen 1 implications for EBV-mediated
immortalization. Mol Cell. 18:25–36. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang FW, Wu XR, Liu WJ, Liao YJ, Lin S,
Zong YS, Zeng MS, Zeng YX, Mai SJ and Xie D: Heat shock factor 1
upregulates transcription of Epstein-Barr Virus nuclear antigen 1
by binding to a heat shock element within the BamHI-Q
promoter. Virology. 421:184–191. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Brooks L, Yao QY, Rickinson AB and Young
LS: Epstein-Barr virus latent gene transcription in nasopharyngeal
carcinoma cells: Coexpression of EBNA1, LMP1, and LMP2 transcripts.
J Virol. 66:2689–2697. 1992.PubMed/NCBI
|
|
22
|
Chen H, Lee JM, Zong Y, Borowitz M, Ng MH,
Ambinder RF and Hayward SD: Linkage between STAT regulation and
Epstein-Barr virus gene expression in tumors. J Virol.
75:2929–2937. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen H, Lee JM, Wang Y, Huang DP, Ambinder
RF and Hayward SD: The Epstein-Barr virus latency BamHI-Q
promoter is positively regulated by STATs and Zta interference with
JAK/STAT activation leads to loss of BamHI-Q promoter
activity. Proc Natl Acad Sci USA. 96:9339–9344. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yu H and Jove R: The STATs of cancer - new
molecular targets come of age. Nat Rev Cancer. 4:97–105. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Imai S, Kuroda M, Yamashita R and Ishiura
Y: Therapeutic inhibition of Epstein-Barr virus-associated tumor
cell growth by dominant-negative EBNA1. Uirusu. 55:239–249.
2005.(In Chinese). View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Coppotelli G, Mughal N, Callegari S,
Sompallae R, Caja L, Luijsterburg MS, Dantuma NP, Moustakas A and
Masucci MG: The Epstein-Barr virus nuclear antigen-1 reprograms
transcription by mimicry of high mobility group A proteins. Nucleic
Acids Res. 41:2950–2962. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Goto H, Kariya R, Shimamoto M, Kudo E,
Taura M, Katano H and Okada S: Antitumor effect of berberine
against primary effusion lymphoma via inhibition of NF-κB pathway.
Cancer Sci. 103:775–781. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lin JP, Yang JS, Lee JH, Hsieh WT and
Chung JG: Berberine induces cell cycle arrest and apoptosis in
human gastric carcinoma SNU-5 cell line. World J Gastroenterol.
12:21–28. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Peng PL, Hsieh YS, Wang CJ, Hsu JL and
Chou FP: Inhibitory effect of berberine on the invasion of human
lung cancer cells via decreased productions of
urokinase-plasminogen activator and matrix metalloproteinase-2.
Toxicol Appl Pharmacol. 214:8–15. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim JB, Yu JH, Ko E, Lee KW, Song AK, Park
SY, Shin I, Han W and Noh DY: The alkaloid Berberine inhibits the
growth of Anoikis-resistant MCF-7 and MDA-MB-231 breast cancer cell
lines by inducing cell cycle arrest. Phytomedicine. 17:436–440.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kishimoto A, Dong SF, Negishi H, Yasui N,
Sun JN and Ikeda K: Effects of berberine on adipose tissues and
kidney function in 3T3-L1 cells and spontaneously hypertensive
rats. Nat Prod Commun. 10:1543–1546. 2015.PubMed/NCBI
|
|
32
|
Bae YA and Cheon HG: Activating
transcription factor-3 induction is involved in the
anti-inflammatory action of berberine in RAW264.7 murine
macrophages. Korean J Physiol Pharmacol. 20:415–424. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang J, Qi Q, Feng Z, Zhang X, Huang B,
Chen A, Prestegarden L, Li X and Wang J: Berberine induces
autophagy in glioblastoma by targeting the AMPK/mTOR/ULK1-pathway.
Oncotarget. 7:66944–66958. 2016.PubMed/NCBI
|
|
34
|
Barzegar E, Fouladdel S, Movahhed TK,
Atashpour S, Ghahremani MH, Ostad SN and Azizi E: Effects of
berberine on proliferation, cell cycle distribution and apoptosis
of human breast cancer T47D and MCF7 cell lines. Iran J Basic Med
Sci. 18:334–342. 2015.PubMed/NCBI
|
|
35
|
Liu J, He C, Zhou K, Wang J and Kang JX:
Coptis extracts enhance the anticancer effect of estrogen receptor
antagonists on human breast cancer cells. Biochem Biophys Res
Commun. 378:174–178. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Abukhdeir AM, Vitolo MI, Argani P, De
Marzo AM, Karakas B, Konishi H, Gustin JP, Lauring J, Garay JP,
Pendleton C, et al: Tamoxifen-stimulated growth of breast cancer
due to p21 loss. Proc Natl Acad Sci USA. 105:288–293. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kuo CL, Chi CW and Liu TY: Modulation of
apoptosis by berberine through inhibition of cyclooxygenase-2 and
Mcl-1 expression in oral cancer cells. In Vivo. 19:247–252.
2005.PubMed/NCBI
|
|
38
|
Hwang JM, Kuo HC, Tseng TH, Liu JY and Chu
CY: Berberine induces apoptosis through a mitochondria/caspases
pathway in human hepatoma cells. Arch Toxicol. 80:62–73. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Park GB, Park SH, Kim D, Kim YS, Yoon SH
and Hur DY: Berberine induces mitochondrial apoptosis of
EBV-transformed B cells through p53-mediated regulation of XAF1 and
GADD45α. Int J Oncol. 49:411–421. 2016.PubMed/NCBI
|
|
40
|
Tsang CM, Cheung YC, Lui VW, Yip YL, Zhang
G, Lin VW, Cheung KC, Feng Y and Tsao SW: Berberine suppresses
tumorigenicity and growth of nasopharyngeal carcinoma cells by
inhibiting STAT3 activation induced by tumor associated
fibroblasts. BMC Cancer. 13:6192013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sun X, Barlow EA, Ma S, Hagemeier SR,
Duellman SJ, Burgess RR, Tellam J, Khanna R and Kenney SC: Hsp90
inhibitors block outgrowth of EBV-infected malignant cells in vitro
and in vivo through an EBNA1-dependent mechanism. Proc Natl Acad
Sci USA. 107:3146–3151. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhou H, Guo W, Long C, Wang H, Wang J and
Sun X: Triptolide inhibits proliferation of Epstein-Barr
virus-positive B lymphocytes by down-regulating expression of a
viral protein LMP1. Biochem Biophys Res Commun. 456:815–820. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Long C, Guo W, Zhou H, Wang J, Wang H and
Sun X: Triptolide decreases expression of latency-associated
nuclear antigen 1 and reduces viral titers in Kaposi's
sarcoma-associated and herpesvirus-related primary effusion
lymphoma cells. Int J Oncol. 48:1519–1530. 2016.PubMed/NCBI
|
|
44
|
Long C, Wang J, Guo W, Wang H, Wang C, Liu
Y and Sun X: Triptolide inhibits transcription of hTERT through
down-regulation of transcription factor specificity protein 1 in
primary effusion lymphoma cells. Biochem Biophys Res Commun.
469:87–93. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Xu M, Xiao Y, Yin J, Hou W, Yu X, Shen L,
Liu F, Wei L and Jia W: Berberine promotes glucose consumption
independently of AMP-activated protein kinase activation. PLoS One.
9:e1037022014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wu CC, Fang CY, Hsu HY, Chen YJ, Chou SP,
Huang SY, Cheng YJ, Lin SF, Chang Y, Tsai CH, et al: Luteolin
inhibits Epstein-Barr virus lytic reactivation by repressing the
promoter activities of immediate-early genes. Antiviral Res.
132:99–110. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Choi ES, Nam JS, Jung JY, Cho NP and Cho
SD: Modulation of specificity protein 1 by mithramycin A as a novel
therapeutic strategy for cervical cancer. Sci Rep. 4:71622014.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Saha SK and Khuda-Bukhsh AR: Berberine
alters epigenetic modifications, disrupts microtubule network, and
modulates HPV-18 E6-E7 oncoproteins by targeting p53 in cervical
cancer cell HeLa: A mechanistic study including molecular docking.
Eur J Pharmacol. 744:132–146. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Paiar F, Di Cataldo V, Zei G, Pasquetti
EM, Cecchini S, Meattini I, Mangoni M, Agresti B, Iermano C, Bonomo
P, et al: Role of chemotherapy in nasopharyngeal carcinoma. Oncol
Rev. 6:e12012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Gu J, Yin L, Wu J, Zhang N, Huang T, Ding
K, Cao H, Xu L and He X: Cetuximab and cisplatin show different
combination effect in nasopharyngeal carcinoma cells lines via
inactivation of EGFR/AKT signaling pathway. Biochem Res Int.
2016:70169072016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Frappier L: Role of EBNA1 in NPC
tumourigenesis. Semin Cancer Biol. 22:154–161. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Li CH, Wu DF, Ding H, Zhao Y, Zhou KY and
Xu DF: Berberine hydrochloride impact on physiological processes
and modulation of twist levels in nasopharyngeal carcinoma CNE-1
cells. Asian Pac J Cancer Prev. 15:1851–1857. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
O'Neil JD, Owen TJ, Wood VH, Date KL,
Valentine R, Chukwuma MB, Arrand JR, Dawson CW and Young LS:
Epstein-Barr virus-encoded EBNA1 modulates the AP-1 transcription
factor pathway in nasopharyngeal carcinoma cells and enhances
angiogenesis in vitro. J Gen Virol. 89:2833–2842. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Aziz MH, Hafeez BB, Sand JM, Pierce DB,
Aziz SW, Dreckschmidt NE and Verma AK: Protein kinase Cε mediates
Stat3Ser727 phosphorylation, Stat3-regulated gene expression, and
cell invasion in various human cancer cell lines through
integration with MAPK cascade (RAF-1, MEK1/2, and ERK1/2).
Oncogene. 29:3100–3109. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Liu YP, Tan YN, Wang ZL, Zeng L, Lu ZX, Li
LL, Luo W, Tang M and Cao Y: Phosphorylation and nuclear
translocation of STAT3 regulated by the Epstein-Barr virus latent
membrane protein 1 in nasopharyngeal carcinoma. Int J Mol Med.
21:153–162. 2008.PubMed/NCBI
|
|
56
|
Kennedy G, Komano J and Sugden B:
Epstein-Barr virus provides a survival factor to Burkitt's
lymphomas. Proc Natl Acad Sci USA. 100:14269–14274. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Hong M, Murai Y, Kutsuna T, Takahashi H,
Nomoto K, Cheng CM, Ishizawa S, Zhao QL, Ogawa R, Harmon BV, et al:
Suppression of Epstein-Barr nuclear antigen 1 (EBNA1) by RNA
interference inhibits proliferation of EBV-positive Burkitt's
lymphoma cells. J Cancer Res Clin Oncol. 132:1–8. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Yin Q and Flemington EK: siRNAs against
the Epstein Barr virus latency replication factor, EBNA1, inhibit
its function and growth of EBV-dependent tumor cells. Virology.
346:385–393. 2006. View Article : Google Scholar : PubMed/NCBI
|