Introduction
Esophageal cancer is one of the most common and
aggressive types of cancer worldwide, it is ranked as the 8th in
morbidity and 6th in cancer-related mortality (1,2).
Esophageal squamous cell carcinoma (ESCC) and esophageal
adenocarcinoma (EA) are the two major histological types of
esophageal cancer (3). However,
ESCC is the main subtype of esophageal cancer and comprises ~70% of
cases worldwide (4,5). The incidence of ESCC has been
increasing steadily in high-incidence areas such as northern China,
Iran and South Africa (4–6), and the overall survival still remains
extremely poor due to lack of effective biomarkers for early
diagnosis, and therapeutic target. Therefore, a better
understanding of the mechanism of this malignancy to identify and
characterize novel therapies is definitely necessary and of great
urgency.
Paclitaxel is a cytotoxic apoptosis inducer and
antineoplastic drug against a broad-spectrum of cancers such as
ovarian cancer, lung cancer, breast cancer and melanoma (7). As a single agent, paclitaxel has been
shown to have a response rate of 32% in esophageal cancer (8). In addition, several phase II studies
indicated that paclitaxel-based regimens play an important role in
patients with locally advanced and metastatic esophageal cancer
(9–11). Moreover, paclitaxel may increase the
sensitivity of tumor cells to radiation (12–14).
Signal transducers and activators of transcription 3 (STAT3)
involved in cell proliferation, differentiation, cell survival and
death in response to various signaling through the phosphorylation
and nuclear translocation (15,16).
STAT3 was constitutively activated in cancer tissues of ESCC
(17). Wegrzyn et al found
that STAT3 may translocate to mitochondria, and the mitochondrial
STAT3 (mtSTAT3) is important for the functions of mitochondrial
electron transport chain (ETC) since the activities of complex I
and II of mitochondrial ETC were significantly decreased in
STAT3−/− cells (18).
Moreover, mtSTAT3 supports Ras-dependent malignant transformation
by regulating the glycolysis and oxidative phosphorylation (OXPHOS)
of cancer cells (19). Previous
studies on paclitaxel-induced apoptosis mainly focused on the
mitochondrial apoptotic pathways (20–22).
However, the regulatory mechanism of how it induces apoptosis and
the role of STAT3 in ESCC cells remains to be further
investigated.
Materials and methods
Reagents and antibodies
Paclitaxel was obtained from Sangon Biotech Co.
(Shanghai, China). Horseradish peroxidase (HRP)-conjugated
anti-rabbit, anti-mouse immunoglobulin G, MTT Cell Proliferation
and Cytotoxicity Assay kit, ROS Assay kit (DCFH-DA) and
mitochondrial membrane potential (MMP) Assay kit (JC-1) were all
obtained from Beyotime Biotech (Jiangshu, China). Annexin
V-Fluorescein Isothiocyanate (FITC) Apoptosis Detection kit was
obtained from BD Biosciences (Franklin Lakes, NJ, USA). BCA Protein
Assay kit and Pierce ECL Western Blotting Substrate were both
obtained from Thermo Fisher Scientific, Inc. (Waltham, MA, USA).
Monoclonal antibody against β-actin was from Abmart (Shanghai,
China). Polyclonal antibody against PARP, caspase-3, cleaved
caspase-3, caspase-7, cleaved caspase-7, caspase-9, cleaved
caspase-9, STAT3, phospho-STAT3 (Ser727) were all obtained from
Cell Signaling Technology, Inc. (Beverly, MA, USA). Polyclonal
antibody against cytochrome c, COX IV, GAPDH, VDAC were all
purchased from Abcam (Cambridge, UK).
Cell lines and cell culture
The ESCC cell lines, EC-1 and Eca-109 were both
purchased from the Cell Bank of the Chinese Academy of Sciences
(Shanghai, China). Cells were grown in RPMI-1640 medium
supplemented with 10% fetal bovine serum (FBS) (both from
Invitrogen, Carlsbad, CA, USA) and antibiotics (100 U/ml penicillin
and 100 µg/ml streptomycin) at 37°C in a humidified atmosphere of
5% CO2.
Isolation of cytoplasmic and
mitochondrial fractions
EC-1 and Eca-109 cells were treated with 12 and 20
nM paclitaxel for 24 h, respectively. The cells harvested were
lysed in 2 ml 0.1X isolation buffer (IB, 3.5 mM Tris-HCl, 2.5 mM
NaCl, 0.5 mM MgCl2 and protease inhibitors, pH 7.8) and
incubated on ice for 2 min. The mixture was then transferred into
the Dounce glass homogenizer. After more than 90% of cells were
confirmed to be broken by using phase contrast microscope, 200 µl
10X IB and 0.1X IB were added into the mixture for further lysing
the cells. The lysate were then centrifuged at 1,000 × g for 3 min,
and the supernatant were transferred to a new Eppendorf tube,
centrifuged at 15,000 × g for 2 min, and the supernatant was
cytoplasmic fraction and the pellet obtained was mitochondrial
fraction which was resuspended in 200 µl buffer A (10 mM Tris-HCl,
pH 7.9, 1 mM EDTA, 0.32 M sucrose) and 2 µl 100 mM PMSF.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
For MTT assay, EC-1 and Eca-109 cells were seeded
into 96-well plates at a density of 2×103 cells/well and
incubated at 37°C in a humidified atmosphere of 5% CO2
overnight. Next day, EC-1 and Eca-109 cells were treated with 12
and 20 nM paclitaxel for 0, 24, 48 and 72 h, respectively. Cells
were stained with MTT reagent (5 mg/ml) for 4 h, and the crystals
produced were dissolved with DMSO. Once the crystals were dissolved
completely, the plate was measured at 570 nm by the plate reader
(Thermo Fisher Scientific, Inc.).
Apoptosis analysis
EC-1 and Eca-109 cells were seeded in a 6-well plate
at a density of 2×105 cells/well and treated with 12 and
20 nM paclitaxel, respectively. Following treatment for 24 h, the
cells were collected, washed twice with ice-cold phosphate-buffered
saline PBS and subsequently stained with Annexin V-FITC/PI for 20
min by incubation in the dark at room temperature. The samples were
then analyzed immediately using a BD Accuri C6 flow cytometer (BD
Biosciences).
Reactive oxygen species (ROS)
determination
DCFH-DA detection kit was used to measure the
intracellular ROS levels according to the manufacturer's
instructions. Briefly, EC-1 and Eca-109 cells were treated with
paclitaxel at concentrations of 12 and 20 nM, respectively.
Following the treatment for 24 h, cells were collected, washed with
RPMI-1640 and incubated with 10 mM DCFH-DA at 37°C for 15 min in
the dark. The cells were then washed with RPMI-1640 and resuspended
in 200 µl RPMI-1640. The generation of intracellular ROS was
analyzed by flow cytometry within 30 min.
JC-1 fluorescence analysis
The MMP was measured by means of JC-1 staining as
previously described (24).
Briefly, EC-1 and Eca-109 cells treated with paclitaxel were
incubated with 2.0 µM JC-1 probe for 20 min at 37°C in the dark.
The excess JC-1 probe was removed by washing cells with warm PBS
and pelleted by centrifugation. Cell pellets were resuspended in
500 µl PBS and the MMP was assayed by flow cytometry.
RNA interference
EC-1 and Eca-109 cells were transfected with control
or STAT3 siRNA (Genepharma, Shanghai, China) using Lipofectamine
2000 (Life Technologies, Grand Island, NY, USA) in serum-free
medium following manufacturers instructions. Thirty-six hours
post-transfection, cells were seeded on two 6-cm dishes,
respectively. Then, cells were treated with or without indicated
concentration of PTX (20 nM for EC-1 cells and 12 nM for Eca-109
cells, respectively) for another 24 h. Cells were collected and
further subjected to western blot analysis with indicated
antibodies. The following sequences for STAT3 siRNA are:
STAT3-homo-398 sense, 5-CCACUUUGGUGUUUCAUAATT-3 and
antisense, 5-UUAUGAAACACCAAAGUGGTT-3; STAT3-homo-978 sense,
5-GCAACAGAUUGCCUGCAUUTT-3 and antisense, 5-AAUGCAGGCAAUCUGUUGCTT-3;
and control siRNA sense, 5-UUCUCCGAACGUGUCACGUTT-3 and antisense,
5-AGGUGACACGUUCGGAGAATT-3.
Western blot analysis
EC-1 and Eca-109 cells were treated with or without
paclitaxel for 24 h, then trypsinized and collected in 1.5 ml
Eppendorf tubes, lysed in RIPA buffer (50 mM Tris-HCl, pH 7.4, 1%
Triton X-100, 1% sodium deoxycholate, 0.1% SDS, 150 mM NaCl)
supplemented with protease inhibitor cocktail tablet, NaF (1 mM)
and Na3VO4 (1 mM) for 15 min on ice and
centrifuged at 18,000 × g for 20 min at 4°C. Equal amount of
protein from total lysates were resolved by 15% SDS-PAGE, the
separated proteins were transferred onto nitrocellulose membrane
(Bio-Rad Laboratories, Inc., Hercules, CA, USA) in Tris-glycine
buffer. Blots were blocked at room temperature for 1.5 h in
blocking buffer (5% non-fat milk in TBST) on a shaker and then
incubated overnight at 4°C with primary antibodies as indicated in
the figures.After washing, membranes were incubated for 1 h with an
anti-mouse or anti-rabbit Ig-HRP and detected with the ECL system.
The optical density was quantified by the ImageJ software (National
Institutes of Health, Bethesda, MA, USA).
OXPHOS assay
The Seahorse XF96 Extracellular Flux Analyzer
(Seahorse Bioscience, North Billerica, MA, USA) was used to assay
mitochondrial bioenergetics by measuring the oxygen consumption
rate (OCR). The optimum number of cells was determined to be
20,000/well and incubated in a 37°C 5% CO2 incubator,
and the calibrator plate was equilibrated in a non-CO2
incubator overnight. Next day, the medium was changed to Dulbeccos
modified Eagles medium (unbuffered DMEM, 25 mM glucose, 1 mM
glutamine, 1 mM sodium pyruvate) and equilibrated in a 37°C
CO2-free prep station for 40 min. Then, oligomycin (1 µM
final concentration), an ATP synthase inhibitor, was injected
followed by exposure of carbonylcyanide
p-trifluoromethoxyphenylhydrazone (FCCP) (1 µM final
concentration), an ETC accelerator which causes maximal respiration
and finally rotenone plus antimycin A (1 µM final concentration of
each) which are mitochondrial complex I and III inhibitors into the
cell plate, respectively. EC-1 and Eca-109 cells treated with or
without paclitaxel included three replicates and the results were
obtained by performing three independent experiments.
Statistical analysis
All statistical analyses were carried out using SPSS
version 16.0 statistical software package (SPSS Inc., Chicago, IL,
USA) and presented with GraphPad Prism version 5.0 software
(GraphPad Software, Inc., La Jolla, CA, USA). All P-values are
two-sided, and p<0.05 was considered a statistically significant
difference. Error bars for the experiments represent the standard
deviation of the mean value (mean ± SD) from three separate
experiments.
Results
Paclitaxel inhibits EC-1 and Eca-109
cell growth and increases intracellular ROS in a time-dependent
manner
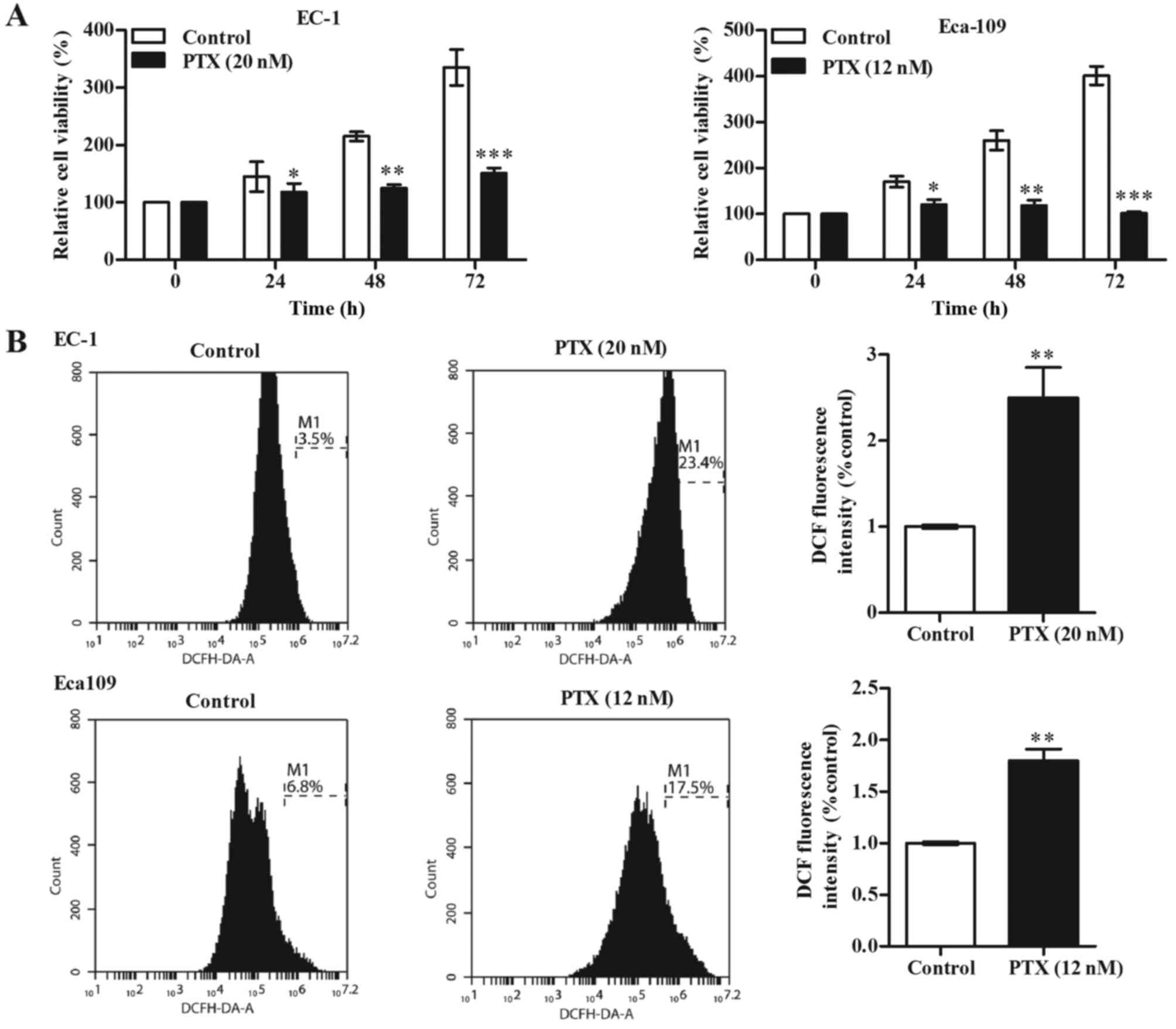
The anti-proliferative effect of paclitaxel on EC-1
and Eca-109 cells was determined by performing an MTT assay. We
found that paclitaxel treatment reduced the ESCC cell viability in
a time-dependent manner (Fig. 1A).
In addition, we determined the intracellular ROS production induced
by paclitaxel treatment, and our results showed that paclitaxel
treatment significantly promoted ROS generation in EC-1 and Eca-109
cells (Fig. 1B).
Paclitaxel induces apoptosis in EC-1
and Eca-109 cells
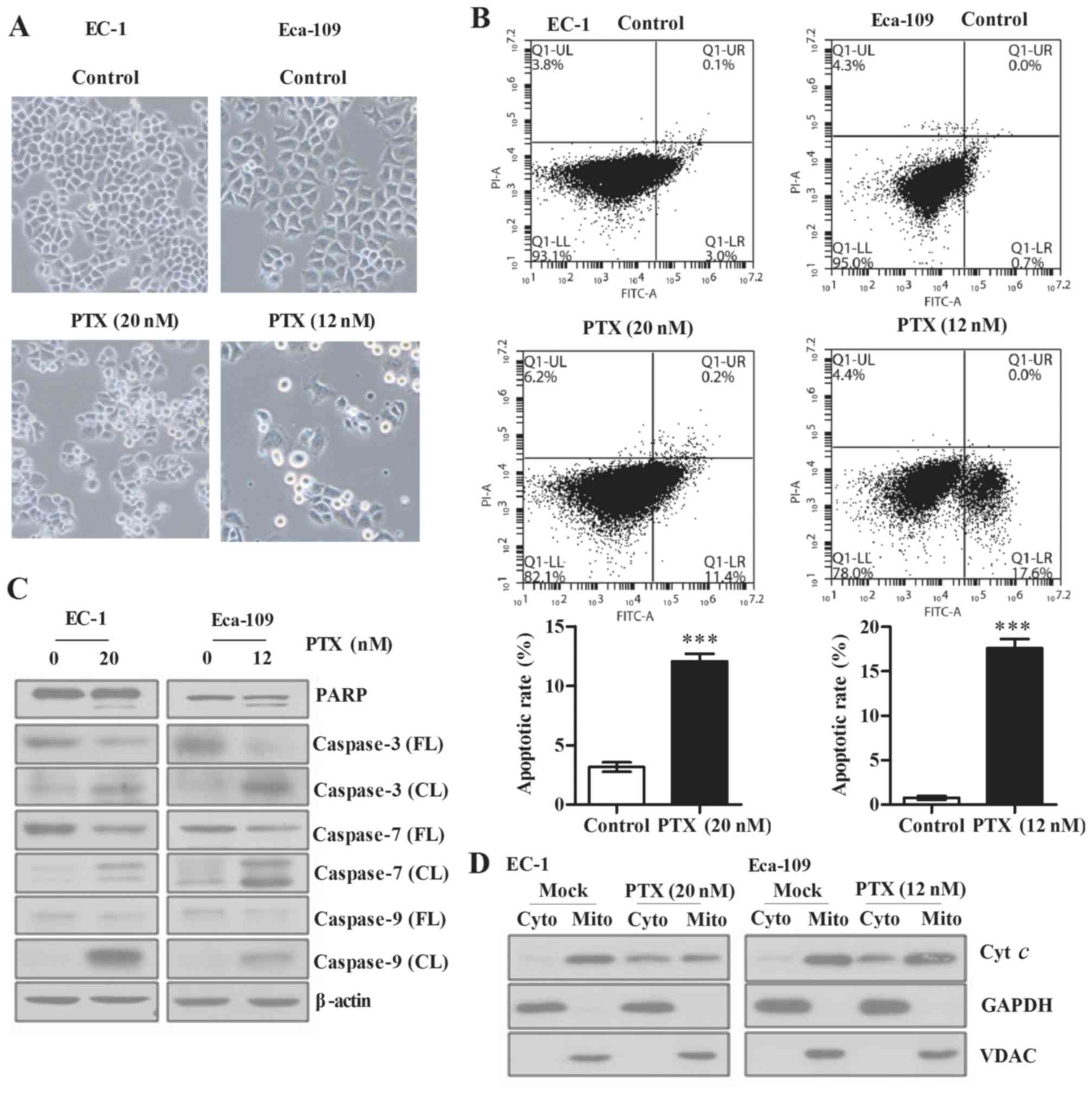
We next examined the effect of paclitaxel on
apoptosis of ESCC cells. Optical microscopy revealed a significant
increase in cell apoptosis (Fig.
2A) and the flow cyto-metry results showed that apoptotic rates
were increased from 2.6 and 0.3% (DMSO control) to 23.5%
(p<0.01) and 16.4% (p<0.001) when treated with 12 and 20 nM
paclitaxel for 24 h in Eca-109 and EC-1 cells, respectively
(Fig. 2B). Additionally, we found
that cleaved PARP, cleaved caspase-3, cleaved caspase-7 and cleaved
caspase-9 were all markedly increased when Eca-109 and EC-1 cells
were treated with paclitaxel (Fig.
2C). Moreover, our results showed that paclitaxel treatment led
to increased release of cytochrome c from mitochondria to
cytoplasm (Fig. 2D).
Paclitaxel reduces MMP and STAT3
phosphorylation at Ser727 in EC-1 and Eca-109 cells
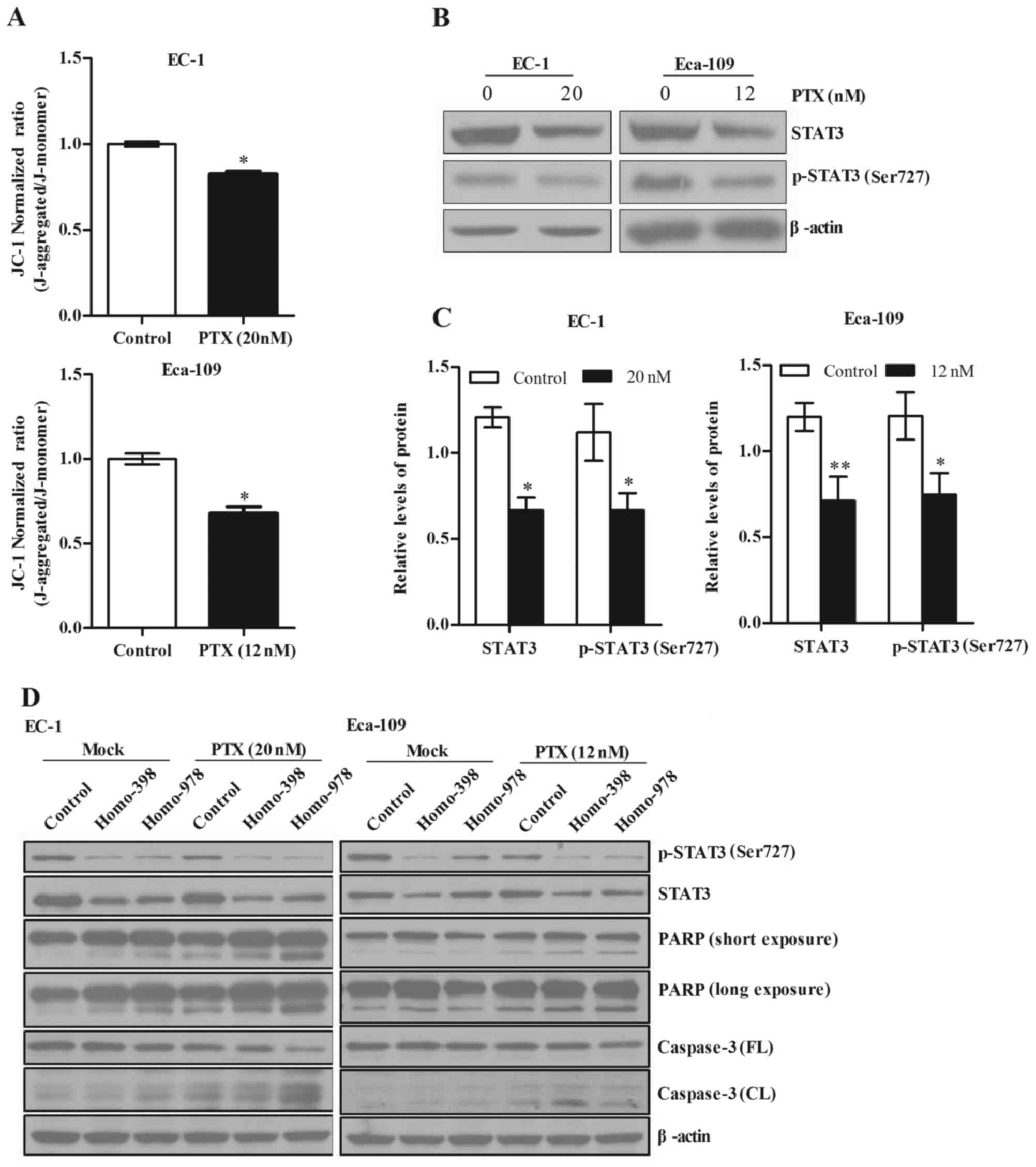
JC-1 is a membrane-permeable lipophilic dye that
exists as J-aggregates in the mitochondrial matrix (red
fluorescence) and as monomers in the cytoplasm (green
fluorescence). During mitochondrial depolarization, the red
J-aggregates form green monomers due to a change in MMP. Thus,
depolarization can be measured as an increasing green
fluorescent/red fluorescent intensity ratio. Our results showed
that paclitaxel causes a significant loss of MMP in EC-1 and
Eca-109 cells (Fig. 3A; p<0.1).
In addition, we analyzed the protein levels of total and
phosphorylated (Ser727) STAT3 in EC-1 and Eca-109 cells by western
blot analysis, and our results showed that paclitaxel treatment led
to significantly decreased total and phosphorylated (Ser727) STAT3
protein levels (Fig. 3B and C;
p<0.05). Moreover, the knockdown of STAT3 in Eca-109 and EC-1
cells enhanced paclitaxel induced PARP and caspase-3 cleavage in
Eca-109 and EC-1 cells (Fig.
3D).
Paclitaxel mainly decreases mtSTAT3
and phosphorylated (Ser727) STAT3 in EC-1 and Eca-109 cells
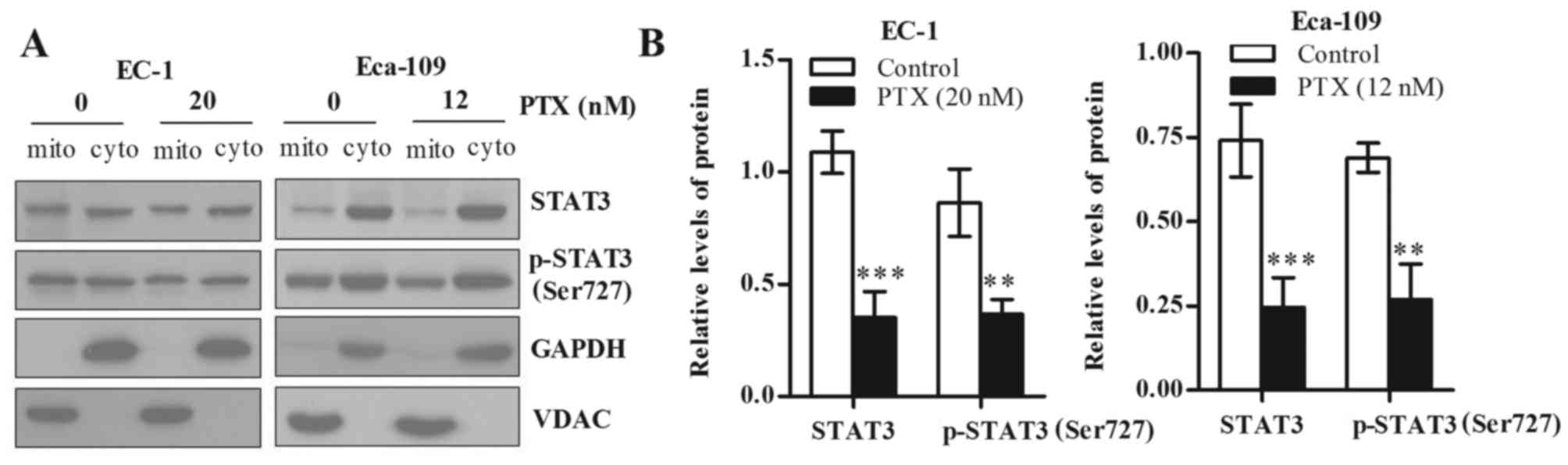
STAT3 is also detected in the mitochondria and has
functions in regulating glycolysis and OXPHOS of cancer cells. To
explore which part of total and phosphorylated (Ser727) STAT3 of
EC-1 and Eca-109 cells was reduced after paclitaxel treatment,
mitochondrial and cytoplasmic fraction were isolated, and the
proteins levels of both total and phosphorylated (Ser727) STAT3
were detected by western blot analysis. Our results showed that
paclitaxel treatment reduced total and phosphorylated (Ser727)
STAT3 in the mitochondria, but did not decrease the total and
phosphorylated (Ser727) STAT3 in the cytosol. GAPDH was used as a
cytoplasmic loading control and VDAC was used as a mitochondrial
loading control (Fig. 4A). To
further clarify the extent of reduction of total and phosphorylated
(Ser727) STAT3, we used loading control to normalize total and
phosphorylated (Ser727) STAT3 protein level, and the results are
statistically significant (Fig. 4B;
p<0.05).
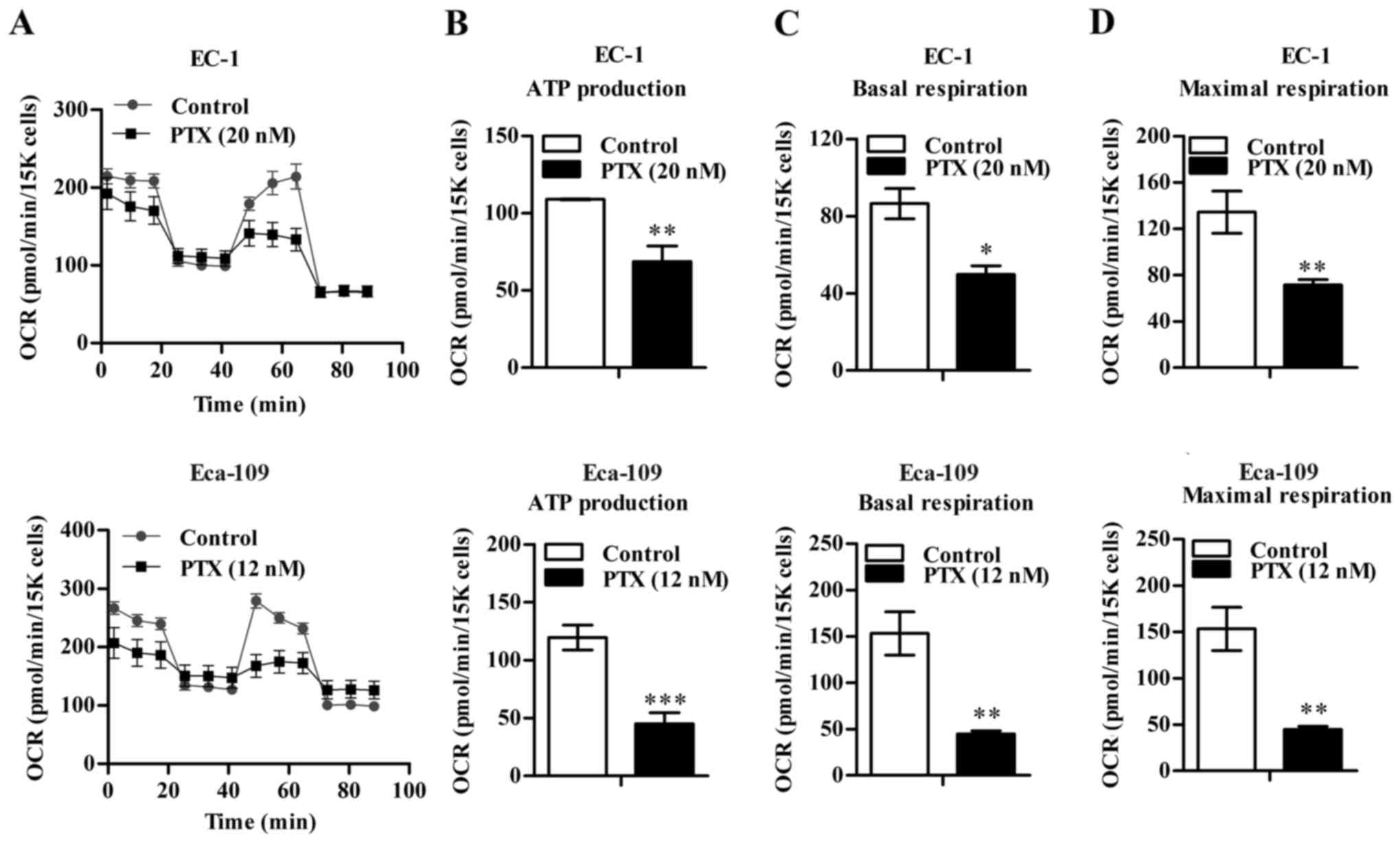
Paclitaxel inhibits mitochondrial
respiration in EC-1 and Eca-109 cells
Next, we detected the OCR in EC-1 and Eca-109 cells
by Seahorse XF96 extracellular flux analyzer. Paclitaxel treatment
decreased OCR of EC-1 and Eca-109 cells markedly (Fig. 5A-C). Furthermore, the ATP production
was strongly reduced in response to paclitaxel treatment (Fig. 5D), which indicates that paclitaxel
treatment may impair the OXPHOS of cancer cells.
Discussion
Paclitaxel is widely used as a common clinical
medicine in the treatment of solid tumors (23,24).
In this study, we demonstrated that paclitaxel inhibits the cell
viability significantly in both Eca-109 and EC-1 cells through
inducing apoptosis of ESCC cells by downregulation of STAT3 Ser727
phosphorylation.
Understanding the mechanism of ROS generation may
provide a new method for the development of therapeutic agents that
are capable of selectively inducing apoptosis of cancer cells. ROS
generation has been shown to be a common cellular mechanism for
multiple cell death pathways, including gene activation, cell cycle
arrest and apoptosis (21). ROS may
also serve as a signal for apoptosis instead of being the
consequence of the cellular changes induced by apoptosis (22). ROS generation may alter the
redox-state of cells and the sensitivity of cells to apoptotic
stimulus and ultimately trigger the subsequent apoptotic events
(25–28). Our results showed that paclitaxel
treatment resulted in the disruption of MMP, increased ROS
generation, and thus promoted apoptosis in Eca-109 and EC-1
cells.
It has been demonstrated that STAT3 also located in
mitochondria (18). In the present
study, we found that paclitaxel treatment significantly decreased
the total and Ser727 phosphorylated STAT3 level in mitochondria of
Eca-109 and EC-1 cells. These results suggest that paclitaxel
treatment could reduce the phosphorylation level of STAT3. The
reason why total STAT3 changed in paclitaxel-induced ESCC cells is
probably associated with the feedback of ESCC cells to paclitaxel
treatment as an integrated system.
STAT3 was able to positively regulate the
mitochondrial respiration in terminally differentiated cells
(29,30) and mtSTAT3 increased the activity of
complex I and II in the ETC in a transcriptional-independent manner
(18). STAT3 was also linked to
carcinogenesis and tumor development shown in previous studies
(31–33). Our results indicated that the OCR
significantly decreased in paclitaxel-treated Eca-109 and EC-1
cells, which may be attributed to the decrease of mitochondrial
respiratory chain enzyme activities. In addition, mitochondrial
cytochrome c, which is a downstream regulation factor of
STAT3, also significantly increased in the cytosol of both Eca-109
and EC-1 cells, which indicates the loss of MMP caused the leakage
of cytochrome c due to the paclitaxel treatment of ESCC cells. It
is well known that the increment of ROS level and the deceasing of
MMP may induce apoptosis through caspase-3 activation and
cytochrome c release (28,34,35),
the increasing cytochrome c protein level in the cytosol of
both Eca-109 and EC-1 cells suggested the paclitaxel-induced
mitochondria-dependent apoptotic pathway. Moreover, depletion of
STAT3 increased the sensitivity of ESCC cells to paclitaxel through
enhancing the cleavage of PARP and caspase-3.
In conclusion, our findings demonstrated that
paclitaxel has significant anti-proliferation effects by inducing
mitochondrial apoptosis of ESCC cells via STAT3 signaling pathways.
Thus, our data provide a novel insight into the mitochondrial
apoptosis mechanism of paclitaxel on the ESCC cells in
vitro. These findings suggested that paclitaxel may be of
therapeutic potential in ESCC treatment through the induction of
mitochondrial apoptosis in ESCC cells.
Acknowledgements
The authors would like to thank Dr Sushil Kumar for
the critical review of the manuscript. This study was supported by
the grant from the National Natural Science Foundation of China
(nos. 31171345 and 31570772) to B.L. and the Natural Science
Foundation of Zhejiang Province (LY17C070005) to Y.L.
References
|
1
|
Kamangar F, Dores GM and Anderson WF:
Patterns of cancer incidence, mortality, and prevalence across five
continents: defining priorities to reduce cancer disparities in
different geographic regions of the world. J Clin Oncol.
24:2137–2150. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tang WR, Fang JY, Wu KS, Shi XJ, Luo JY
and Lin K: Epidemiological characteristics and prediction of
esophageal cancer mortality in China from 1991 to 2012. Asian Pac J
Cancer Prev. 15:6929–6934. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Song QK, Li J, Jiang HD, He YM, Zhou XQ
and Huang CY: Esophageal cancer mortality during 2004–2009 in
Yanting County, China. Asian Pac J Cancer Prev. 13:5003–5006. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xu Y, Yu X, Chen Q and Mao W: Neoadjuvant
versus adjuvant treatment: which one is better for resectable
esophageal squamous cell carcinoma? World J Surg Oncol. 10:1732012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Blot WJ: Esophageal cancer trends and risk
factors. Semin Oncol. 21:403–410. 1994.PubMed/NCBI
|
|
6
|
Brooks-Brunn JA: Esophageal cancer: an
overview. Medsurg Nurs. 9:248–254. 2000.PubMed/NCBI
|
|
7
|
Scripture CD, Figg WD and Sparreboom A:
Paclitaxel chemotherapy: from empiricism to a mechanism-based
formulation strategy. Ther Clin Risk Manag. 1:107–114. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ajani JA, Ilson DH, Daugherty K, Pazdur R,
Lynch PM and Kelsen DP: Activity of taxol in patients with squamous
cell carcinoma and adenocarcinoma of the esophagus. J Natl Cancer
Inst. 86:1086–1091. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ilson DH, Ajani J, Bhalla K, Forastiere A,
Huang Y, Patel P, Martin L, Donegan J, Pazdur R, Reed C, et al:
Phase II trial of paclitaxel, fluorouracil, and cisplatin in
patients with advanced carcinoma of the esophagus. J Clin Oncol.
16:1826–1834. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Adelstein DJ, Rice TW, Rybicki LA, Larto
MA, Ciezki J, Saxton J, DeCamp M, Vargo JJ, Dumot JA and Zuccaro G:
Does paclitaxel improve the chemoradiotherapy of locoregionally
advanced esophageal cancer? A nonrandomized comparison with
fluorouracil-based therapy. J Clin Oncol. 18:2032–2039. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Polee MB, Eskens FA, van der Burg ME,
Splinter TA, Siersema PD, Tilanus HW, Verweij J, Stoter G and van
der Gaast A: Phase II study of bi-weekly administration of
paclitaxel and cisplatin in patients with advanced oesophageal
cancer. Br J Cancer. 86:669–673. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tishler RB, Schiff PB, Geard CR and Hall
EJ: Taxol: a novel radiation sensitizer. Int J Radiat Oncol Biol
Phys. 22:613–617. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Choy H, Rodriguez FF, Koester S,
Hilsenbeck S and Von Hoff DD: Investigation of taxol as a potential
radiation sensitizer. Cancer. 71:3774–3778. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Leonard CE, Chan DC, Chou TC, Kumar R and
Bunn PA: Paclitaxel enhances in vitro radiosensitivity of squamous
carcinoma cell lines of the head and neck. Cancer Res.
56:5198–5204. 1996.PubMed/NCBI
|
|
15
|
Catlett-Falcone R, Landowski TH, Oshiro
MM, Turkson J, Levitzki A, Savino R, Ciliberto G, Moscinski L,
Fernández-Luna JL, Nuñez G, et al: constitutive activation of Stat3
signaling confers resistance to apoptosis in human U266 myeloma
cells. Immunity. 10:105–115. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fukada T, Hibi M, Yamanaka Y,
Takahashi-Tezuka M, Fujitani Y, Yamaguchi T, Nakajima K and Hirano
T: Two signals are necessary for cell proliferation induced by a
cytokine receptor gp130: involvement of STAT3 in anti-apoptosis.
Immunity. 5:449–460. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yan S, Zhou C, Zhang W, Zhang G, Zhao X,
Yang S, Wang Y, Lu N, Zhu H and Xu N: Beta-catenin/TCF pathway
upregulates STAT3 expression in human esophageal squamous cell
carcinoma. Cancer Lett. 271:85–97. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wegrzyn J, Potla R, Chwae YJ, Sepuri NB,
Zhang Q, Koeck T, Derecka M, Szczepanek K, Szelag M, Gornicka A, et
al: Function of mitochondrial Stat3 in cellular respiration.
Science. 323:793–797. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gough DJ, Corlett A, Schlessinger K,
Wegrzyn J, Larner AC and Levy DE: Mitochondrial STAT3 supports
Ras-dependent oncogenic transformation. Science. 324:1713–1716.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Blajeski AL, Kottke TJ and Kaufmann SH: A
multistep model for paclitaxel-induced apoptosis in human breast
cancer cell lines. Exp Cell Res. 270:277–288. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ofir R, Seidman R, Rabinski T, Krup M,
Yavelsky V, Weinstein Y and Wolfson M: Taxol-induced apoptosis in
human SKOV3 ovarian and MCF7 breast carcinoma cells is caspase-3
and caspase-9 independent. Cell Death Differ. 9:636–642. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Varbiro G, Veres B, Gallyas F Jr and
Sumegi B: Direct effect of Taxol on free radical formation and
mitochondrial permeability transition. Free Radic Biol Med.
31:548–558. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu X, Kim CN, Yang J, Jemmerson R and
Wang X: Induction of apoptotic program in cell-free extracts:
requirement for dATP and cytochrome c. Cell. 86:147–157.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Susin SA, Lorenzo HK, Zamzami N, Marzo I,
Snow BE, Brothers GM, Mangion J, Jacotot E, Costantini P, Loeffler
M, et al: Molecular characterization of mitochondrial
apoptosis-inducing factor. Nature. 397:441–446. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Johnson TM, Yu ZX, Ferrans VJ, Lowenstein
RA and Finkel T: Reactive oxygen species are downstream mediators
of p53-dependent apoptosis. Proc Natl Acad Sci USA. 93:11848–11852.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cai J and Jones DP: Mitochondrial redox
signaling during apoptosis. J Bioenerg Biomembr. 31:327–334. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Simon HU, Haj-Yehia A and Levi-Schaffer F:
Role of reactive oxygen species (ROS) in apoptosis induction.
Apoptosis. 5:415–418. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Akgul C, Moulding DA and Edwards SW:
Molecular control of neutrophil apoptosis. FEBS Lett. 487:318–322.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zouein FA, Duhé RJ, Arany I, Shirey K,
Hosler JP, Liu H, Saad I, Kurdi M and Booz GW: Loss of STAT3 in
mouse embryonic fibroblasts reveals its janus-like actions on
mitochondrial function and cell viability. Cytokine. 66:7–16. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Carbognin E, Betto RM, Soriano ME, Smith
AG and Martello G: Stat3 promotes mitochondrial transcription and
oxidative respiration during maintenance and induction of naive
pluripotency. EMBO J. 35:618–634. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Boengler K, Hilfiker-Kleiner D, Heusch G
and Schulz R: Inhibition of permeability transition pore opening by
mitochondrial STAT3 and its role in myocardial
ischemia/reperfusion. Basic Res Cardiol. 105:771–785. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nguyen AV, Wu YY, Liu Q, Wang D, Nguyen S,
Loh R, Pang J, Friedman K, Orlofsky A, Augenlicht L, et al: STAT3
in epithelial cells regulates inflammation and tumor progression to
malignant state in colon. Neoplasia. 15:998–1008. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Haricharan S and Li Y: STAT signaling in
mammary gland differentiation, cell survival and tumorigenesis. Mol
Cell Endocrinol. 382:560–569. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ly JD, Grubb DR and Lawen A: The
mitochondrial membrane potential (deltapsi(m)) in apoptosis; an
update. Apoptosis. 8:115–128. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang C and Youle RJ: The role of
mitochondria in apoptosis. Annu Rev Genet. 43:95–118. 2009.
View Article : Google Scholar : PubMed/NCBI
|