Introduction
Lung cancer is the leading cause of deaths with the
most rapidly increasing incidence worldwide. Non-small cell lung
cancer (NSCLC) has the highest mortality rate among all solid
tumors with a poor prognosis (1).
More than half of lung carcinomas are detected in a progressed or
already metastasized state with a 5-year survival, for lacking of
characteristic early symptoms (2).
The general therapy treating the majority of patients is
chemotherapy, which often induces resistance (3). It is necessary to develop novel
therapeutic approaches to better understand the lung cancer
progression.
Autophagy is a fundamental cellular homeostatic
process that cells use to degrade and recycle cellular proteins
(4). This process can be induced in
response to either intracellular or extracellular factors, such as
hypoxia, low cellular energy state and organelle damage (5). The microtubule-associated protein 1
light chain 3 (LC3), functions as a structural component in the
formation of autophagosomes. The conversion of the cytosolic form
of LC3 (LC3 I) to lipidated form (LC3 II) indicates autophagosome
formation (6). Beclin 1, is
required for the initiation and in the process of autophagosome
formation (7). p62 acts as a
receptor or adaptor for autophagic degradation of ubiquitinated
proteins, the upregulation of p62 is commonly detected in human
tumors and contributes directly to tumorigenesis (8). Thus, LC3, Beclin 1 and p62 were
considered as autophagy markers in many studies (9,10).
Transforming growth factor-β (TGF-β) has a crucial
role in homeostasis, fibrosis angiogenesis, carcinogenesis and
differentiation of the cell. A report indicated that TGF-β reduced
cell apoptosis via induction of autophagy (11). TGF-β also induces the
transcriptional activation of several autophagy-related genes,
including BECLIN 1, ATG5, ATG7 and death-associated protein kinase
(DAPK) (12,13). Thus hinting that the inhibitor of
TGF-β may control cell autophagy and proliferation. BMP and activin
receptor membrane bound inhibitor (BAMBI) gene is evolutionally
conserved in vertebrates (14).
BAMBI was described as a modulator, with a putative function as a
dominant negative, non-signaling, competitive pseudo-receptor for
members of the TGF-β receptor type 1 (TβR1) family (14). Apart from being suggested as a
competitive receptor antagonist for the TGF-β receptor family,
BAMBI was indicated as a negative regulator for the subsequent Smad
pathway (15), indicating BAMBI may
antagonize autophagy through downregulating TGF-β/Smad pathway.
β-Sitosterol is a natural product isolated from
traditional Chinese herbs, including Trifolium repens,
Houttuynia cordata and Lasia spinosa (16). β-Sitosterol has been applied in
treating many diseases because of the anti-inflammatory,
anti-proliferative and anticancer effects (17,18).
However, the effect of BAMBI together with β-sitosterol on cell
autophagy and proliferation in NSCLC is rarely reported.
In this study, we aimed to explore the effect of
BAMBI overexpression and β-sitosterol in the context of NSCLC. We
found that BAMBI overexpression and β-sitosterol suppressed the
autophagy flux of A549 cells. Besides, BAMBI overexpression and
β-sitosterol restrained tumor growth in vitro and in
vivo through inactivating TGF-β/Smad2/3 pathway. Taken
together, our results suggest BAMBI overexpression together with
β-sitosterol treatment may provide novel insight into the mechanism
and treatment of NSCLC.
Materials and methods
Cell culture
Human NSCLC A549, NCI-H1975 and H1299 cells were
obtained from the American Type Culture Collection (ATCC; Manassas,
VA, USA), and cultivated in modified Eagle's medium (MEM),
supplemented with 20% fetal bovine serum (FBS) and 1%
antibiotic-antimycotic (Invitrogen Life Technologies, Carlsbad, CA,
USA). The cells were incubated at 37°C in a humidified 21%
O2, 5% CO2 atmosphere.
Constructs and transfection
Human BAMBI expression plasmid was constructed by
inserting BAMBI cDNA purchased from Beyotime (Shanghai, China) into
the pcDNA3-EGFP (Invitrogen, Shanghai, China) expressing vector.
The plasmid was amplified with Top 10 bacteria (Invitrogen),
extracted, and purified by Plasmid Midi kit (Qiagen, Shanghai,
China). For transfection, the three NSCLC cells (A549, NCI-H1975
and H1299) were cultured in antibiotic- and serum-free α-MEM medium
(at 50–60% confluence). BAMBI plasmid (2 µg/well) or the vector (2
µg/well) was transfected into NSCLC cells with Lipofectamine™ 2000
(Invitrogen) following the manufacturer's instructions. After
transfection for 48 h, BAMBI expression in transfected cells was
examined by western blotting.
β-Sitosterol treatment
For the treatment of β-sitosterol, 2×104
A549 cells transfected with or without pcDNA-BAMBI were added to
96-well plates. Then, the RPMI-1640 medium was added to make the
volume in each well up to 200 µl. After culturing for 24 h, 10
mg/ml β-sitosterol extracts were added to each well, respectively.
Control group was added with equal volume of 0.01 mol/l
phosphate-buffered saline (PBS). After incubation at 37°C and 5%
CO2 for another 24 h, cells were collected for the
following experiments.
Western blotting
NSCLC cells were lysed in lysis buffer (Beyotime)
supplemented with 1 mM phenylmethanesulfonyl fluoride (PMSF). The
protein concentration was determined using the BCA protein assay
(Tiangen Biotech Co., Ltd., Beijing, China). Twenty micrograms of
protein in each sample was separated by 12% SDS-PAGE and
electrotransferred to polyvinylidene fluoride (PVDF) membranes
(Millipore, Billerica, MA, USA) for immunoblotting. The following
primary antibodies were used: anti-BAMBI (1:500, ab203070),
anti-LC3 (1:500, ab48394), anti-Beclin 1 (1:500, ab55878), anti-p62
(1:200, ab56416), anti-Smad2/3 (1:1,000, ab202445), anti-p-Smad2/3
(1:1,000, ab63399), anti-c-Myc (1:10,000, ab32072), anti-TGF-β
(1:500, ab66043), anti-caspase-3 (1:5,000, ab32351), anti-caspase-8
(1:1,000, ab32397), anti-caspase-9 (1:2,000, ab202068), anti-mTOR
(1:2,000, ab32028) and anti-GAPDH (1:2,500, ab9485) (all from
Abcam, Cambridge, MA, USA), which was used as the internal
reference. After incubation with the appropriate horseradish
peroxidase (HRP)-conjugated secondary antibody (Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA), proteins were detected
using a ChemiDoc XRS imaging system and Quantity One analysis
software (Bio-Rad Laboratories, Inc., San Francisco, CA, USA).
Autophagy measurement using
GFP-LC3
The autophagy measurement was conducted according to
a previous study (19). Briefly,
A549 cells were transfected with a GFP-LC3 expression plasmid
(Sigma-Aldrich, St. Louis, MO, USA) incorporated into the
lentiviral vector using Lipofectamine 2000 reagent. After selection
with puromycin, expression of fluorescence was confirmed by
microscopic evaluation before irradiation. Cells were then observed
for the fluorescence of GFP-LC3 under a fluorescence
microscope.
Autophagic flux measurement
A549 cells were treated with or without 20 µM
chloroquine for 1 h prior to β-sitosterol treatment and BAMBI
transfection. The recovered cell lysates were used to detect the
accumulation of LC3 II, autophagic flux were detected by
immunoblotting according to a previous study (20), briefly, cells were rinsed with PBS
and lysed with an lysis buffer. The proteins were then separated by
SDS-PAGE and transferred onto nitrocellulose membranes. The
membranes were incubated with primary antibodies against LC3 and
GAPDH and probed with an HRP-labeled secondary antibody and
detected using an ECL reagent. Protein expression level was
measured by a ChemiDoc XRS imaging system (Bio-Rad Laboratories,
Inc.).
Flow cytometry analysis of the cell
cycle
A549 cells at 1×106 cells/well were
cultured in 6-well plates and transfected with or without
pcDNA-BAMBI for 48 h. The cells were then treated with or withour
10 mg/ml β-sitosterol extracts, respectively. Cells were harvested
and fixed in 70% ice-cold ethanol for 24 h, followed by staining
with propidium iodide (PI). The different cell cycle phases were
analyzed with the FACSCalibur instrument using CellQuest software
(Becton-Dickinson, Mountain View, CA, USA).
Detection of apoptosis
For the apoptosis assay, A549 cells with
β-sitosterol treatment and BAMBI transfection were washed with PBS
and stained with PI and Annexin V-FITC (V13241; Invitrogen Life
Technologies) on ice for 10–15 min. The stained cells were analyzed
using a FACScan flow cyto-meter (Becton-Dickinson) and the number
of apoptotic cells was quantified using FlowJo software (Tree Star
Inc., Ashland, OR, USA).
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
The cell viability of A549 cells was assessed using
MTT assay. Shortly afterwards, cells were transfected according to
the above description and were seeded in 96-well plates at
6×103 cells/well. The surviving fractions were
determined at 0, 24, 48, 72, 96 and 120 h. For the detection the
cytotoxicity of autophagy inhibitors on A549 cells, cells were
pretreated with or without 100 nM bafilomycin A1 for 1 h prior to
β-sitosterol treatment and BAMBI transfection. The surviving
fractions were determined after 24 h. Thereafter, the old medium
was discarded and fresh medium containing MTT (5 mg/ml MTT in PBS;
Sangon Biotech Co., Ltd., Shanghai, China) was added and incubated
for an additional 4 h. Then, cell proliferation was measured with a
spectrophotometer (Bio-Rad Laboratories, Inc.) at 470 nm. Each
experiment was performed in triplicate.
TGF-β overexpression
The TGF-β overexpression was achieved by PCR
amplification using TGF-β cDNA as a template, and the hNUDC
expressing vector was constructed by inserting the TGF-β cDNA into
pcDNA3.1 vector. The recombinant plasmid was transfected into
3×106 A549 cells with or without pcDNA-BAMBI
transfection and β-sitosterol treatment using a nucleofector
instrument. Forty-eight hours later, subsequent experiments were
performed on the cells. The experiment was replicated thrice for
data calculations.
NSCLC xenografts
Twenty NOD/SCID mice (Jackson Laboratory, Bar
Harbor, ME, USA) (male; body weight, 20–22 g; age, 8-weeks) were
purchased from the Institute of Zoology, Chinese Academy of Medical
Sciences (Shanghai, China). A549 cells (5×106) were
injected subcutaneously into ten NOD/SCID mice, another ten mice
were injected with equal number of A549 cells transfected with
pcDNA-BAMBI. Half of the mice with or without pcDNA-BAMBI
transfection were then injected with β-sitosterol (1 mg/kg body
weight) every other day. The tumor volumes were measured daily
after the injection, and all the rats were assigned to euthanasia
at the end of measurements on day 25. All animal experiments were
performed according to current prescribed guidelines and under a
protocol approved by the Institutional Animal Care and Use
Committee.
Statistical analysis
All results are presented as mean ± SD from a
minimum of three replicates. Differences between groups were
evaluated by SPSS version 15.0 statistical software (SPSS, Inc.,
Chicago, IL, USA) by one-way analysis of variance (ANOVA) followed
by Bonferroni's test. Differences were considered statistically
significant at P<0.05.
Results
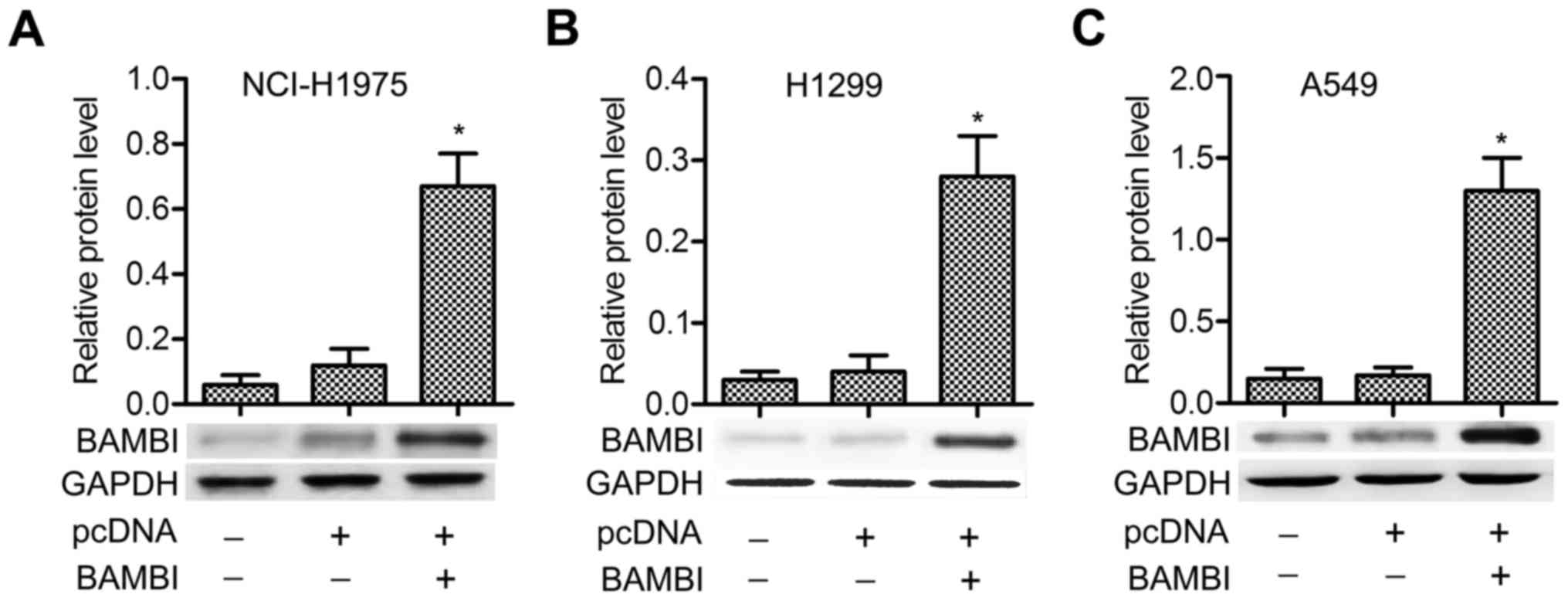
BAMBI is overexpressed by transfection
of the plasmid of pcDNA-BAMBI into NSCLC cells
The pcDNA-BAMBI plasmid was transfected into three
NSCLC cell lines (NCI-H1975, H1299 and A549). No significant
difference of BAMBI level was detected between control and pcDNA
groups, but the expression of BAMBI was strongly promoted in
pcDNA-BAMBI group compared with pcDNA group, especially in A549
cells (P<0.05) (Fig. 1A-C). The
results indicated that BAMBI was overexpressed successfully by
inserting its plasmid into NSCLC cells.
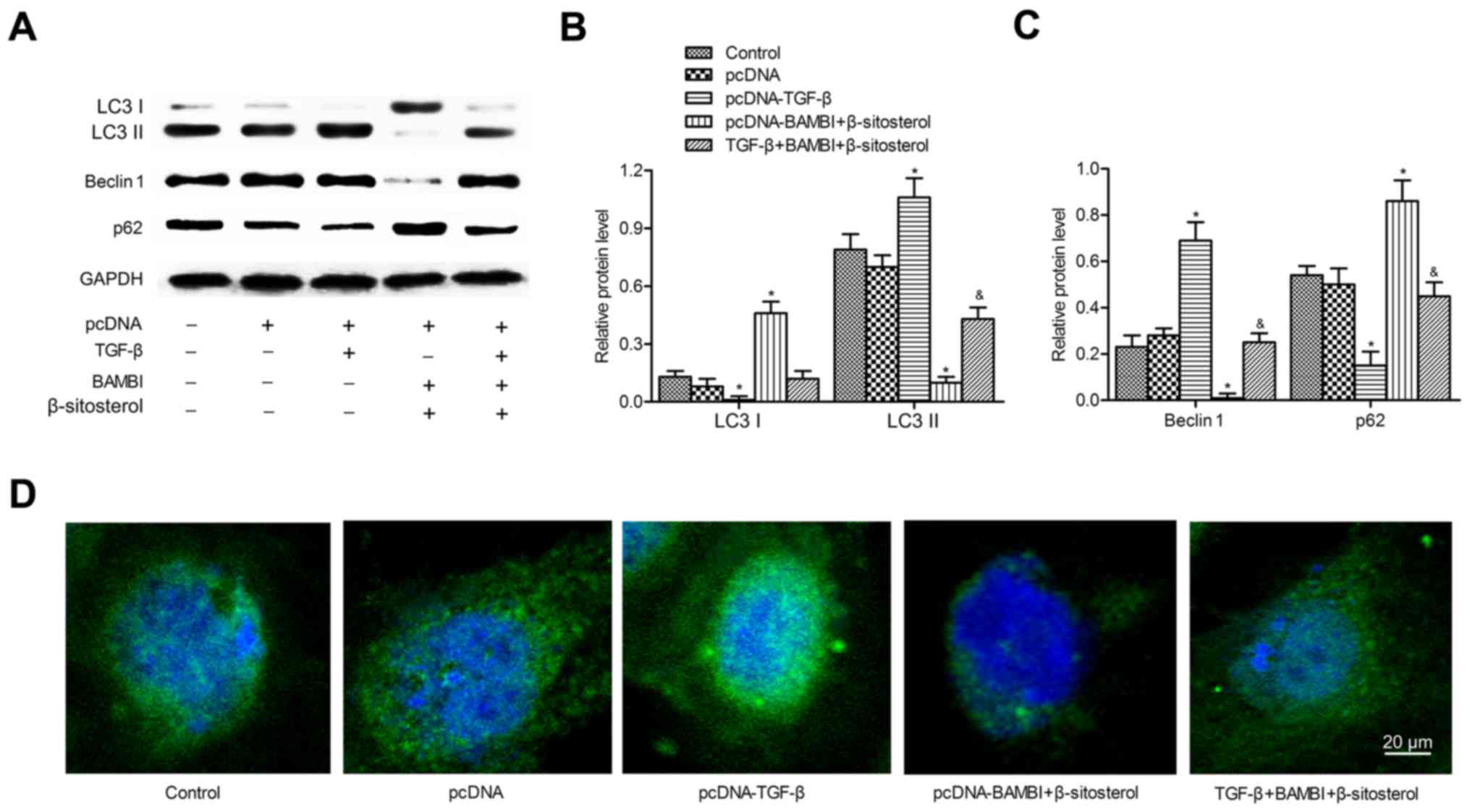
BAMBI overexpression and β-sitosterol
suppress autophagy of A549 cells
We next explored whether BAMBI overexpression played
a functional role in autophagy. A549 cells were chosen for the
following experiments. β-Sitosterol was added alone or with
pcDNA-BAMBI transfection. Western blotting indicated that LC3 II
and Beclin 1 expression levels declined significantly in
pcDNA-BAMBI and β-sitosterol groups compared with control group
(P<0.05). In comparison with β-sitosterol function alone, the
levels of LC3 II and Beclin 1 were particularly decreased when
adding β-sitosterol in pcDNA-BAMBI group (P<0.05). Conversely,
the levels of LC3 I and autophagy substrate p62 were increased
significantly under the treatment of pcDNA-BAMBI and β-sitosterol
(P<0.05). The expression of LC3 I and p62 were strongly promoted
under treatment of pcDNA-BAMBI together with β-sitosterol
(P<0.05) (Fig. 2A-C). In
addition, immunofluorescent assay displayed reduced punctate
accumulations of GFP-LC3 in pcDNA-BAMBI and β-sitosterol groups,
especially in pcDNA-BAMBI + β-sitosterol group compared with
control group (Fig. 2D). In
addition, lipidated LC3 II degradation were used to monitor
autophagic flux as the LC3 II is degraded by autolysosome. Thus,
LC3 II in cells with or without chloroquine was used to examine the
effects of BAMBI overexpression and β-sitosterol on autophagic
flux. Compared to the control group, the level of LC3 II was higher
in A549 cells with BAMBI overexpression and β-sitosterol treatment
(P<0.05). However, no significant difference was observed in
cells pretreated with or without chloroquine and treated with
β-sitosterol with BAMBI transfection (P>0.05) (Fig. 2E). These results suggested that
BAMBI overexpression and β-sitosterol suppressed autophagy of A549
cells.
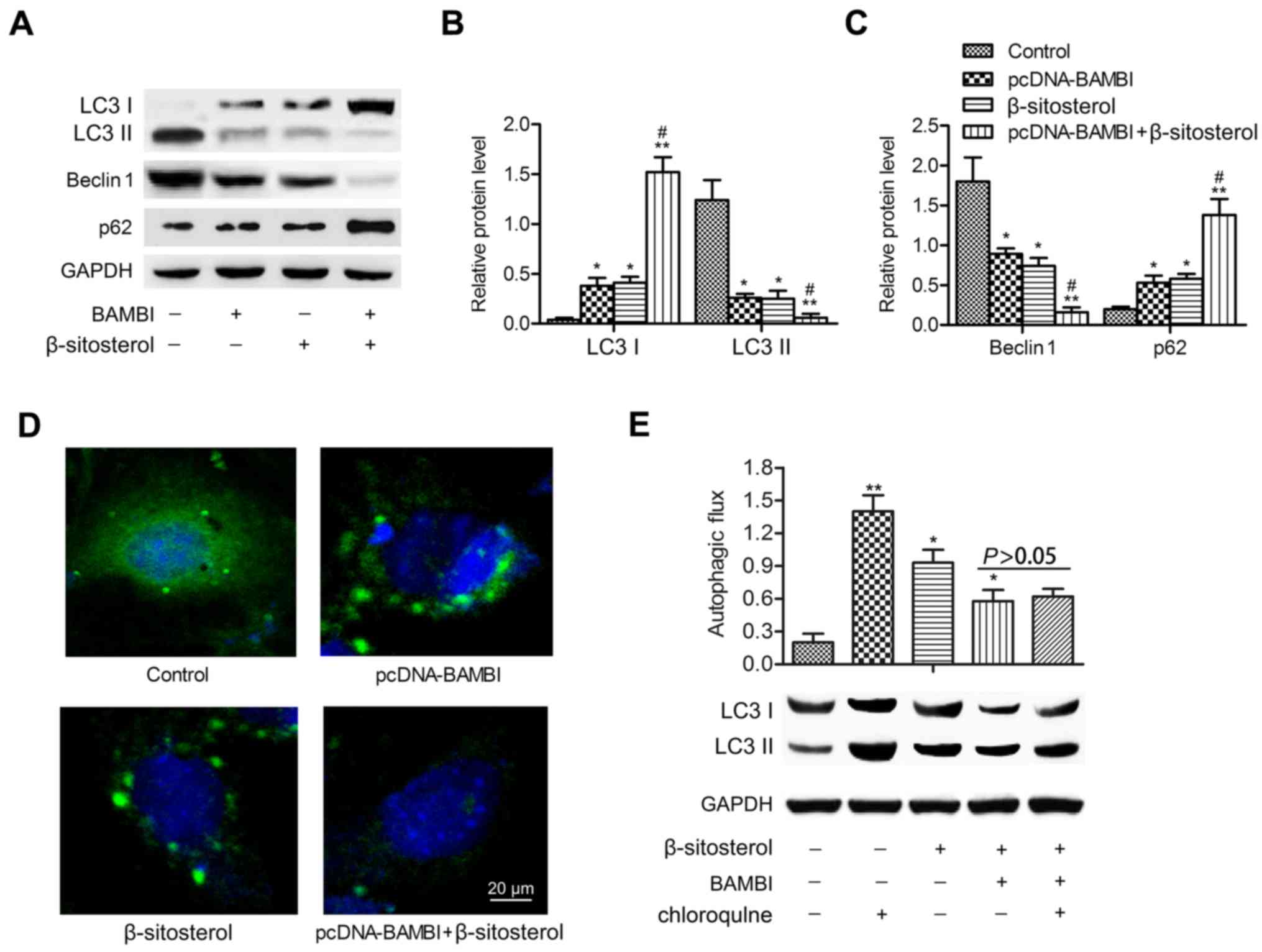 | Figure 2.BAMBI overexpression and β-sitosterol
suppress autophagy of A549 cells. A549 cells were divided into four
groups: control group, A549 cells without any treatment;
pcDNA-BAMBI group, cells transfected with pcDNA-BAMBI plamid;
β-sitosterol group, cells were incubated in 10 mg/ml β-sitosterol
for 24 h; pcDNA-BAMBI + β-sitosterol group, cells were incubated in
10 mg/ml β-sitosterol for another 24 h after pcDNA-BAMBI
transfection for 24 h. (A) The levels of autophagy markers LC3 I,
LC3 II, Beclin 1 and p62 were detected by western blotting. (B) The
relative protein expression of LC3 I and LC3 II. (C) Beclin 1 and
p62 was quantified using Image-Pro Plus 6.0 software and normalized
to GAPDH. Data are represented as the mean ± SD of three
experiments. (D) GFP-LC3 staining was examined by
immunofluorescence, scale bar, 20 µm. (E) Autophagic flux was
detected by immunoblotting. A549 cells were treated with or without
20 µM chloroquine for 1 h prior to β-sitosterol treatment and BAMBI
transfection. *P<0.05, **P<0.01 vs. control group;
#P<0.05 vs. β-sitosterol group. BAMBI, BMP and
activin receptor membrane bound inhibitor; LC3, light chain 3. |
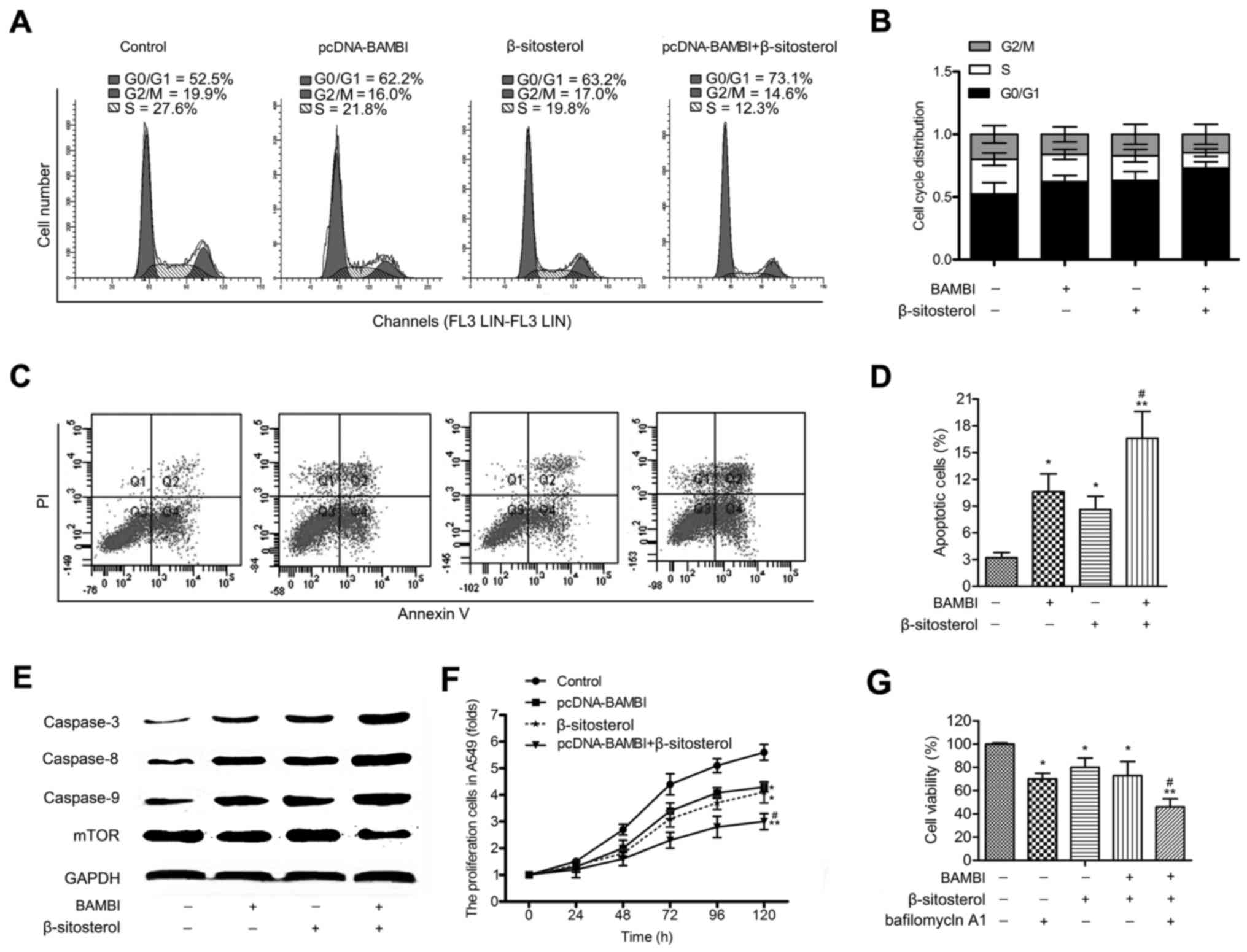
BAMBI overexpression and β-sitosterol
inhibit cell progression and proliferation of A549 cells
Flow cytometric analysis exhibited that G0/G1 phase
arrest in A549 cells was observed in pcDNA-BAMBI and β-sitosterol
groups. Besides, a large accumulation of G0/G1 phase was measured
in pcDNA-BAMBI + β-sitosterol group (Fig. 3A and B). These results suggested an
inactivation of G0/G1 progression in A549 cells following
incubation with pcDNA-BAMBI and β-sitosterol. Besides, cell
apoptosis assay indicated that BAMBI transfection strengthened the
apoptosis-inducing effect of β-sitosterol in A549 cells. The number
of apoptotic cells was increased in pcDNA-BAMBI + β-sitosterol
group compared with β-sitosterol group (P<0.05) (Fig. 3C and D). Accordingly, the expression
level of caspase-3, −8 and −9 was increased, while the level of
mTOR was decreased in cells treated with β-sitosterol together with
BAMBI overexpression compared with β-sitosterol group (P<0.05)
(Fig. 3E). Further, the cell
proliferation assay was performed in A549 cells. The transfection
of pcDNA-BAMBI and β-sitosterol treatment strongly decreased cell
growth compared with control group (P<0.01). The inhibitory
effect was stronger in pcDNA-BAMBI + β-sitosterol group compared
β-sitosterol group (P<0.05) (Fig.
3F). The cytotoxicity of autophagy inhibitor bafilomycin A1 on
A549 cells was measured, the results displayed that pcDNA-BAMBI and
β-sitosterol decreased the viability of A549 cells, the adding of
bafilomycin A1 enhanced this effect (Fig. 3G). These findings suggest that BAMBI
overexpression and β-sitosterol inhibited the viabilityof A549
cells.
The TGF-β/Smad2/3 pathway is
restrained by BAMBI overexpression and β-sitosterol
BAMBI is a member of TGF-β family, we further
explored the effect of pcDNA-BAMBI and β-sitosterol on the
expression of TGF-β pathway proteins. The results indicated that
the level of Smad2/3 exhibited no significant difference, while the
expression of TGF-β, p-Smad2/3 and c-Myc were decreased by
pcDNA-BAMBI and β-sitosterol compared with control group
(P<0.05). Moreover, the protein expression was strongly
suppressed under the treatment of pcDNA-BAMBI together with
β-sitosterol (Fig. 4A-E). These
results suggested that BAMBI overexpression and β-sitosterol
restrained the TGF-β/Smad2/3/c-Myc pathway.
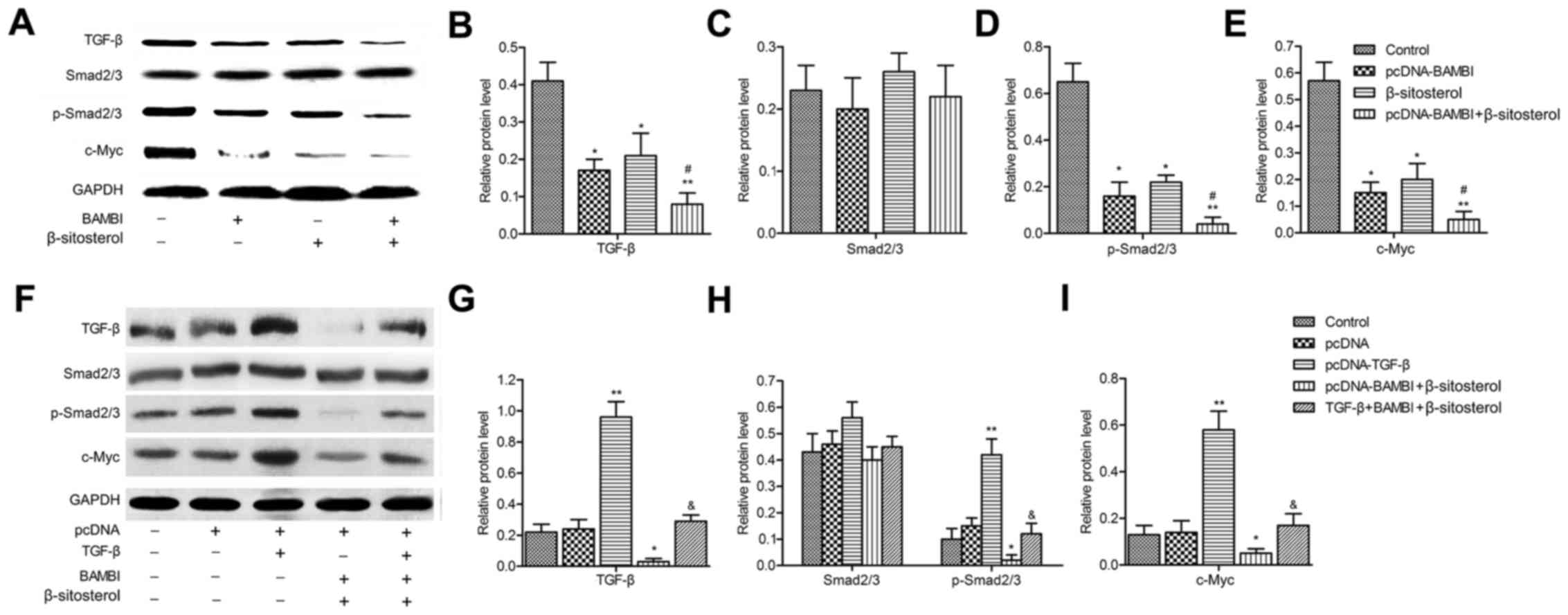 | Figure 4.BAMBI overexpression and β-sitosterol
inhibit the TGF-β/Smad2/3/c-Myc pathway. (A) The expressions of
TGF-β, Smad2/3, p-Smad2/3 and c-Myc were detected by western
blotting and quantified using Image-Pro Plus 6.0 software and
normalized to GAPDH (B-E). TGF-β was then overexpressed by
inserting the plasmid of pcDNA-TGF-β into A549 cells. Cells were
divided into five groups: control, pcDNA, pcDNA-TGF-β, pcDNA-BAMBI
+ β-sitosterol and TGF-β + BAMBI + β-sitosterol groups, cells were
co-transfected with pcDNA-TGF-β and pcDNA-BAMBI and incubated in 10
mg/ml β-sitosterol for 24 h. (F) The expressions of TGF-β, Smad2/3,
p-Smad2/3 and c-Myc were detected by western blotting and
quantified using Image-Pro Plus 6.0 software and normalized to
GAPDH (G-I). Data are represented as the mean ± SD of three
experiments. *P<0.05, **P<0.01 vs. control group;
#P<0.05 vs. β-sitosterol group;
&P<0.05 vs. pcDNA-BAMBI + β-sitosterol group.
BAMBI, BMP and activin receptor membrane bound inhibitor. |
The inhibitory effect of BAMBI and
β-sitosterol on autophagy and viability of A549 cells is through
TGF-β/Smad2/3 pathway
To confirm the TGF-β pathway was involved in the
inhibition effect of BAMBI and β-sitosterol on the viability of
A549. Firstly, the plasmid of pcDNA-TGF-β was constructed and
transfected into A549 cells. Western blotting revealed that the
level of TGF-β, p-Smad2/3 and c-Myc was promoted in pcDNA-TGF-β
group in comparison with control group (P<0.01). The
downregulation of their expression was partially counteracted when
cells in pcDNA-BAMBI + β-sitosterol group were transfected with
pcDNA-TGF-β (P<0.05) (Fig.
4F-I).
We measured the levels of autophagy markers, the
results displayed that A549 cells transfected pcDNA-TGF-β exhibited
enhanced expression of LC3 II and Beclin 1 with decreased LC3 I and
p62 levels compared with control group (P<0.05). The expression
of LC3 II and Beclin 1 was strongly restrained in pcDNA-BAMBI +
β-sitosterol group in comparison with control group (P<0.05),
and their levels were increased adding pcDNA-TGF-β in pcDNA-BAMBI +
β-sitosterol group (P<0.05). The opposite behavior was observed
in the expression of LC3 I and p62 (Fig. 5A and B). In addition, a large
accumulation of GFP-LC3 was measured by pcDNA-TGF-β transfection.
The GFP-LC3 II punctate accumulation was significantly reduced in
pcDNA-BAMBI + β-sitosterol, but increased adding pcDNA-TGF-β in
pcDNA-BAMBI + β-sitosterol groups (Fig.
5C).
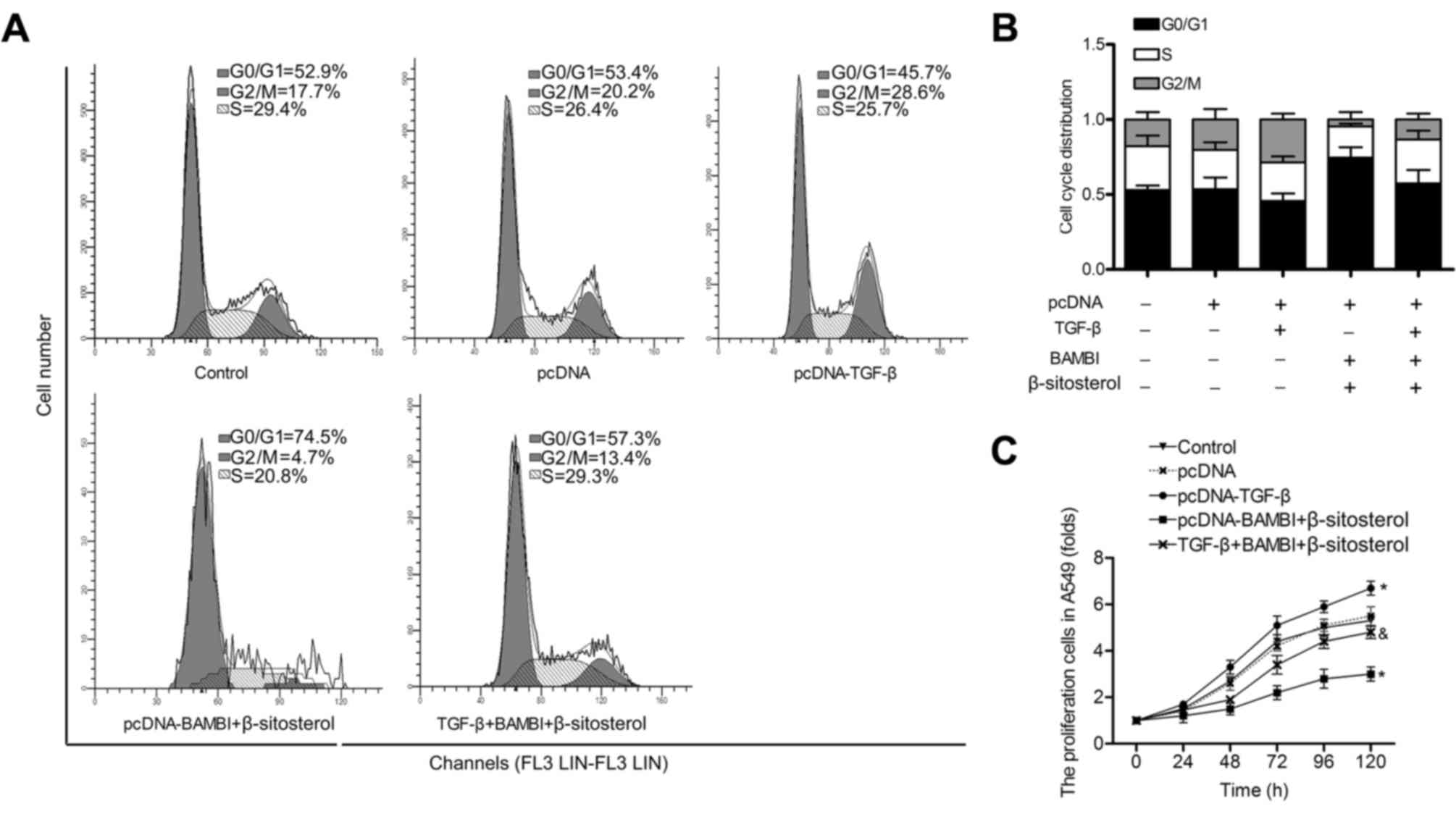
In accordance with these results, the G0/G1 phase
and cell proliferation were accelerated in pcDNA-TGF-β group
compared with control group. In addition, the cell cycle arrest and
the inhibition of cell growth were partially offset when cells in
pcDNA-BAMBI + β-sitosterol group were transfected with pcDNA-TGF-β
(P<0.05) (Fig. 6A-C). These
results indicated that the inhibitory effect of BAMBI
overexpression and β-sitosterol on the viability of A549 is through
the TGF-β/Smad2/3/c-Myc pathway.
BAMBI overexpression and β-sitosterol
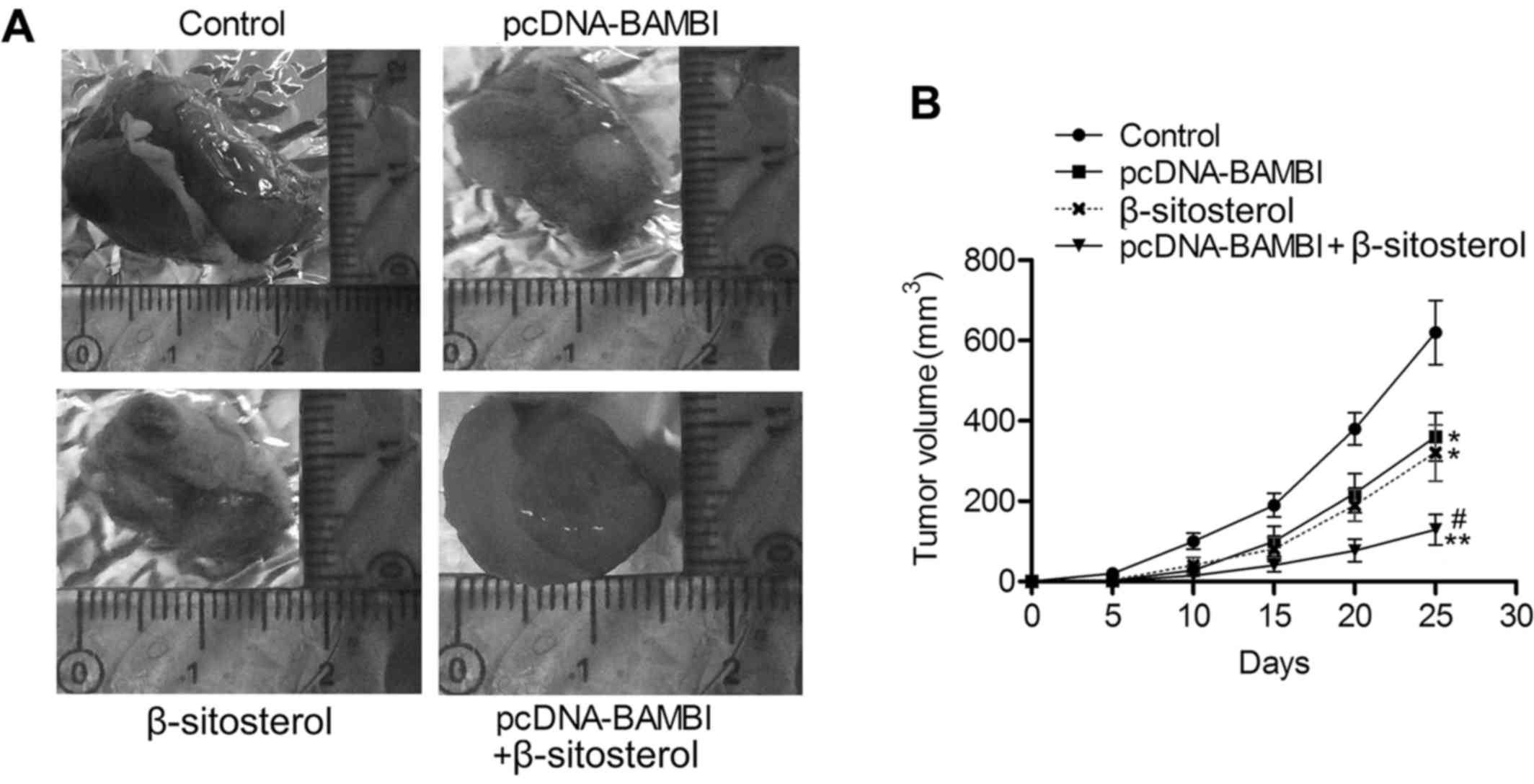
suppress the proliferation of NSCLC in vivo
To further explore the tumor suppression effect of
BAMBI overexpression and β-sitosterol, we assessed tumor growth of
NSCLC xenografts under treatment of pcDNA-BAMBI and/or
β-sitosterol. As shown in Fig. 7A and
B, tumor growth was suppressed in pcDNA-BAMBI and β-sitosterol
groups compared with control group (P<0.05), and the minimum
tumor volume was detected in pcDNA-BAMBI + β-sitosterol group. The
in vivo experiments were convincing that BAMBI
overexpression and β-sitosterol suppressed the proliferation of
NSCLC.
Discussion
Chemotherapy is common in treatment of NSCLC, but
the development of chemoresistance in NSCLC is a major obstacle in
treating patients (21). A study
indicated that human lung cancer tissues that experienced
chemotherapy showed increase of autophagy (22). Thus, a strategy that is specific to
anti-autophagy with tumor-suppressive effect would be beneficial in
treating NSCLC. BAMBI is a type I TGF-β receptor antagonist, the
epigenetic silencing of BAMBI was identified as a hallmark of NSCLC
(3). The methanol extract from
β-sitosterol possesses antitumor potentiality. The mechanism of
BAMBI and β-sitosterol on cell autophagy and growth was explored in
this study.
The physiological function of BAMBI remains unclear,
high expression of BAMBI has been detected in colorectal cancer
(11) and ovarian cancer (12). On the contrary, BAMBI is
epigenetically silenced in high-grade bladder cancer (14) and absent from breast cancer
(15). In the context of lung
diseases, BAMBI was almost completely absent from the lung cancer
tissues compared with the tumor-free lung tissues. Besides, BAMBI
downregulation drives the invasiveness of NSCLC (3), indicating that the upregulation of
BAMBI could reduce the severity of NSCLC. In this study, BAMBI was
overexpressed successfully by inserting its plasmid into three
NSCLC cell lines (NCI-H1975, H1299 and A549). Recent studies
revealed the paradoxical nature of autophagy in deciding cell-fate
machinery. Autophagy induces cell death, suppresses inflammation
and enhances genomic stability; on the contrary, autophagy also
renders cells viable in stressful conditions and is considered a
pro-survival mechanism (23,24).
Studies indicated that TGF-β controls autophagic responses during
angiogenesis, and fibrogenesis in many human cellular systems, such
as atrial myofibroblasts (25),
renal tubular epithelial (26) and
endothelial cells (27). Research
also indicated that the TGF-β-activated fibroblasts increased
autophagy and greatly sustained the growth of breast cancer cells
(28). These findings suggested
that the inhibitor of TGF-β may prevent tumorigenesis by regulating
autophagy. As the antagonist of TGF-β receptor, the transfection of
pcDNA-BAMBI together with β-sitosterol treatment in this study
significantly reduced the levels of LC3 II and Beclin 1 with
decreased GFP-LC3 punctuate structures, whereas the levels of LC3 I
and p62 were strongly promoted. Besides, no significant increase of
autophagy flux was observed in cells pretreated with or without
chloroquine and treated with β-sitosterol and BAMBI transfection.
These results suggested that BAMBI overexpression and β-sitosterol
suppressed autophagy in NSCLC cells.
Apart from the effect in cell autophagy, BAMBI gene
suppression was indicated as one of the epigenetic events affecting
the invasiveness or aggressiveness of bladder cancers (29) and NSCLC (3). BAMBI overexpression significantly
increased the number of apoptotic cells in T24 line (29). Research also indicated that BAMBI
was stimulated by fibroblast growth factor 18, which increased
apoptosis in ovarian granulosa cells (30). These studies suggested a cell
apoptosis-inducing role of BAMBI. As component in active fractions
of plants, β-sitosterol has a protective effect against colon,
prostate, stomach, ovarian and breast cancers via interfering with
multiple cell signaling pathways, including cell cycle, apoptosis,
proliferation, invasion, angiogenesis and carcinogenesis (31–33).
Animal studies have shown that β-sitosterol, extracted from
Aristolochia mollissima Hance, has an inhibitory effect on
the proliferation of osteosarcoma HOS cells (34). β-Sitosterol has also been reported
to affect cell cycle progression by inducing sub-G1 arrest in human
colon cancer cells (HT-29) (35)
and U266 multiple myeloma cells (36). Consistent with these studies,
pcDNA-BAMBI and β-sitosterol applied in this study suppressed cell
proliferation and induced accumulation of G0/G1 arrest and cell
apoptosis in A549 cells. The inhibitory effect was stronger when
cells were under pcDNA-BAMBI transfection plus β-sitosterol
treatment. These findings revealed an anticancer effect of BAMBI
overexpression and β-sitosterol in NSCLC cells.
The TGF-β signaling pathway is believed to
contribute to carcinoma development by increasing cancer cell
motility, invasiveness and metastasis (37). TGF-β has immunosuppressive
properties and may enable cancer cells to evade the immune
responses (38). Upon binding of
the ligand to TGF-β receptors type 2 (TGF-βR2), TGF-βR1 is
phosphorylated and recruits SMAD2 and SMAD3, migrates to the
nucleus, and stimulates target gene expression (39). In lung cancer, high TGF-β serum
levels is associated with a poor prognosis, lymph node metastasis,
and tumor progression (40,41). In addition, TGF-β was suggested as
an independent risk factor for the occurrence of pulmonary
metastasis in NSCLC (42). A study
also revealed that the presence of p-SMAD2 and 3 was significantly
higher in the NSCLC cancer tissues compared with the tumor-free
lung tissues (3). In this study,
the level of TGF-β, p-Smad2/3 and c-Myc was decreased by
pcDNA-BAMBI transfection and β-sitosterol treatment. The
transfection of pcDNA-TGF-β further confirmed the inhibitory effect
in this pathway. In addition, the inhibition of cell autophagy and
cell survival were partially offset when cells in pcDNA-BAMBI +
β-sitosterol group were transfected with pcDNA-TGF-β. These results
confirmed that BAMBI overexpression and β-sitosterol suppression of
cell autophagy and viability of NSCLC is through the
TGF-β/Smad2/3/c-Myc pathway.
In vivo experiment showed that BAMBI
transduction abolished protumor effects of an orthotopic breast
cancer xenograft model (43) and
modulated the effects of diabetes via inhibiting TGF-β signaling
(44). Our study found that BAMBI
overexpression and β-sitosterol suppressed tumor growth in NSCLC
xenografts, revealing the antitumor effect of BAMBI overexpression
and β-sitosterol.
In conclusion, this study explored pcDNA-BAMBI
transfection and β-sitosterol treatment on autophagy and
progression of NSCLC cells. We found that BAMBI overexpression and
β-sitosterol suppressed cell autophagy, induced G0/G1 cell cycle
arrest and inhibited cell proliferation in vitro and in
vivo possibly through inactivating the TGF-β/Smad2/3/c-Myc
pathway. Moreover, the inhibitory effect was stronger when
pcDNA-BAMBI and β-sitosterol functioned together. These results
indicate that BAMBI overexpression and β-sitosterol may serve as
novel targets for the treatment of NSCLC.
Acknowledgements
The authors would like to thank the members of
Internal Medicine, the Cancer Hospital of Linyi, for providing
technical support and helpful discussions concerning the present
study.
Glossary
Abbreviations
Abbreviations:
|
NSCLC
|
non-small cell lung cancer
|
|
BAMBI
|
BMP and activin receptor membrane
bound inhibitor
|
|
LC3
|
light chain 3
|
|
TGF-β
|
transforming growth factor-β
|
References
|
1
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J,
Murray T and Thun MJ: Cancer statistics, 2008. CA Cancer J Clin.
58:71–96. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Marwitz S, Depner S, Dvornikov D, Merkle
R, Szczygieł M, Müller-Decker K, Lucarelli P, Wäsch M, Mairbäurl H,
Rabe KF, et al: Downregulation of the TGFβ pseudoreceptor BAMBI in
non-small cell lung cancer enhances TGFβ signaling and invasion.
Cancer Res. 76:3785–3801. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xie Z and Klionsky DJ: Autophagosome
formation: core machinery and adaptations. Nat Cell Biol.
9:1102–1109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mizushima N: Physiological functions of
autophagy. Curr Top Microbiol Immunol. 335:71–84. 2009.PubMed/NCBI
|
|
6
|
Suzuki K, Kirisako T, Kamada Y, Mizushima
N, Noda T and Ohsumi Y: The pre-autophagosomal structure organized
by concerted functions of APG genes is essential for autophagosome
formation. EMBO J. 20:5971–5981. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yue Z, Jin S, Yang C, Levine AJ and Heintz
N: Beclin 1, an autophagy gene essential for early embryonic
development, is a haploinsufficient tumor suppressor. Proc Natl
Acad Sci USA. 100:15077–15082. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Son YO, Pratheeshkumar P, Roy RV, Hitron
JA, Wang L, Zhang Z and Shi X: Nrf2/p62 signaling in apoptosis
resistance and its role in cadmium-induced carcinogenesis. J Biol
Chem. 289:28660–28675. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Masuda GO, Yashiro M, Kitayama K, Miki Y,
Kasashima H, Kinoshita H, Morisaki T, Fukuoka T, Hasegawa T,
Sakurai K, et al: Clinicopathological correlations of
autophagy-related proteins LC3, Beclin 1 and p62 in gastric cancer.
Anticancer Res. 36:129–136. 2016.PubMed/NCBI
|
|
10
|
Deng Q, Wang Z, Wang L, Zhang L, Xiang X,
Wang Z and Chong T: Lower mRNA and protein expression levels of LC3
and Beclin1, markers of autophagy, were correlated with progression
of renal clear cell carcinoma. Jpn J Clin Oncol. 43:1261–1268.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ding Y, Kim JK, Kim SI, Na HJ, Jun SY, Lee
SJ and Choi ME: TGF-{beta}1 protects against mesangial cell
apoptosis via induction of autophagy. J Biol Chem. 285:37909–37919.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Korah J, Canaff L and Lebrun JJ: The
retinoblastoma tumor suppressor protein (pRb)/E2 promoter binding
factor 1 (E2F1) pathway as a novel mediator of TGFβ-induced
autophagy. J Biol Chem. 291:2043–2054. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kiyono K, Suzuki HI, Matsuyama H,
Morishita Y, Komuro A, Kano MR, Sugimoto K and Miyazono K:
Autophagy is activated by TGF-beta and potentiates
TGF-beta-mediated growth inhibition in human hepatocellular
carcinoma cells. Cancer Res. 69:8844–8852. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Onichtchouk D, Chen YG, Dosch R, Gawantka
V, Delius H, Massagué J and Niehrs C: Silencing of TGF-beta
signalling by the pseudoreceptor BAMBI. Nature. 401:480–485. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Seki E, De Minicis S, Osterreicher CH,
Kluwe J, Osawa Y, Brenner DA and Schwabe RF: TLR4 enhances TGF-beta
signaling and hepatic fibrosis. Nat Med. 13:1324–1332. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vijaya. Yadav AK: In vitro anthelmintic
assessment of selected phytochemicals against Hymenolepis
diminuta, a zoonotic tapeworm. J Parasit Dis. 40:1082–1086.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dey YN, Sharma G, Wanjari MM, Kumar D,
Lomash V and Jadhav AD: Beneficial effect of Amorphophallus
paeoniifolius tuber on experimental ulcerative colitis in rats.
Pharm Biol. 55:53–62. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cheng D, Guo Z and Zhang S: Effect of
β-sitosterol on the expression of HPV E6 and p53 in cervical
carcinoma cells. Contemp Oncol (Pozn). 19:36–42. 2015.PubMed/NCBI
|
|
19
|
Jo GH, Bögler O, Chwae YJ, Yoo H, Lee SH,
Park JB, Kim YJ, Kim JH and Gwak HS: Radiation-induced autophagy
contributes to cell death and induces apoptosis partly in malignant
glioma cells. Cancer Res Treat. 47:221–241. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shu CW, Chang HT, Wu CS, Chen CH, Wu S,
Chang HW, Kuo SY, Fu E, Liu PF and Hsieh YD: RelA-mediated BECN1
expression is required for reactive oxygen species-induced
autophagy in oral cancer cells exposed to low-power laser
irradiation. PLoS One. 11:e01605862016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Toge M, Yokoyama S, Kato S, Sakurai H,
Senda K, Doki Y, Hayakawa Y, Yoshimura N and Saiki I: Critical
contribution of MCL-1 in EMT-associated chemo-resistance in A549
non-small cell lung cancer. Int J Oncol. 46:1844–1848.
2015.PubMed/NCBI
|
|
22
|
Lee JG, Shin JH, Shim HS, Lee CY, Kim DJ,
Kim YS and Chung KY: Autophagy contributes to the chemo-resistance
of non-small cell lung cancer in hypoxic conditions. Respir Res.
16:1382015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ju LL, Zhao CY, Ye KF, Yang H and Zhang J:
Expression and clinical implication of Beclin1, HMGB1, p62,
survivin, BRCA1 and ERCC1 in epithelial ovarian tumor tissues. Eur
Rev Med Pharmacol Sci. 20:1993–2003. 2016.PubMed/NCBI
|
|
24
|
Jiang K, Liu M, Lin G, Mao B, Cheng W, Liu
H, Gal J, Zhu H, Yuan Z, Deng W, et al: Tumor suppressor Spred2
interaction with LC3 promotes autophagosome maturation and induces
autophagy-dependent cell death. Oncotarget. 7:25652–25667.
2016.PubMed/NCBI
|
|
25
|
Ghavami S, Cunnington RH, Gupta S, Yeganeh
B, Filomeno KL, Freed DH, Chen S, Klonisch T, Halayko AJ, Ambrose
E, et al: Autophagy is a regulator of TGF-β1-induced fibrogenesis
in primary human atrial myofibroblasts. Cell Death Dis.
6:e16962015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ding Y, Kim S, Lee SY, Koo JK, Wang Z and
Choi ME: Autophagy regulates TGF-β expression and suppresses kidney
fibrosis induced by unilateral ureteral obstruction. J Am Soc
Nephrol. 25:2835–2846. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pan CC, Kumar S, Shah N, Bloodworth JC,
Hawinkels LJ, Mythreye K, Hoyt DG and Lee NY: Endoglin regulation
of Smad2 function mediates Beclin1 expression and endothelial
autophagy. J Biol Chem. 290:14884–14892. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Guido C, Whitaker-Menezes D, Capparelli C,
Balliet R, Lin Z, Pestell RG, Howell A, Aquila S, Andò S,
Martinez-Outschoorn U, et al: Metabolic reprogramming of
cancer-associated fibroblasts by TGF-β drives tumor growth:
connecting TGF-β signaling with ‘Warburg-like’ cancer metabolism
and L-lactate production. Cell Cycle. 11:3019–3035. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Khin SS, Kitazawa R, Win N, Aye TT, Mori
K, Kondo T and Kitazawa S: BAMBI gene is epigenetically silenced in
subset of high-grade bladder cancer. Int J Cancer. 125:328–338.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jiang Z, Guerrero-Netro HM, Juengel JL and
Price CA: Divergence of intracellular signaling pathways and early
response genes of two closely related fibroblast growth factors,
FGF8 and FGF18, in bovine ovarian granulosa cells. Mol Cell
Endocrinol. 375:97–105. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shoja MH, Reddy ND, Nayak PG, Srinivasan
KK and Rao CM: Glycosmis pentaphylla (Retz.) DC arrests cell
cycle and induces apoptosis via caspase-3/7 activation in breast
cancer cells. J Ethnopharmacol. 168:50–60. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sayeed Bin MS and Ameen SS:
Beta-sitosterol: a promising but orphan nutraceutical to fight
against cancer. Nutr Cancer. 67:1214–1220. 2015.PubMed/NCBI
|
|
33
|
Baskar AA, Al Numair KS, Gabriel Paulraj
M, Alsaif MA, Muamar MA and Ignacimuthu S: β-Sitosterol prevents
lipid peroxidation and improves antioxidant status and
histoarchitecture in rats with 1,2-dimethylhydrazine-induced colon
cancer. J Med Food. 15:335–343. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yu Y, Bo Z, Chao H and Minghua Z: A study
on the anticancer activity of ethanol extract of Aristolochia
mollissima Hance on osteosarcoma HOS cells. Afr J Tradit
Complement Altern Med. 10:551–554. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li C and Wang MH: Aristolochia
debilis Sieb. et Zucc. induces apoptosis and reactive oxygen
species in the HT-29 human colon cancer cell line. Cancer Biother
Radiopharm. 28:717–724. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sook SH, Lee HJ, Kim JH, Sohn EJ, Jung JH,
Kim B, Kim JH, Jeong SJ and Kim SH: Reactive oxygen
species-mediated activation of AMP-activated protein kinase and
c-Jun N-terminal kinase plays a critical role in
beta-sitosterol-induced apoptosis in multiple myeloma U266 cells.
Phytother Res. 28:387–394. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Akhurst RJ and Derynck R: TGF-beta
signaling in cancer - a double-edged sword. Trends Cell Biol.
11:S44–S51. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kumai T, Oikawa K, Aoki N, Kimura S,
Harabuchi Y, Celis E and Kobayashi H: Tumor-derived TGF-beta and
prostaglandin E2 attenuate anti-tumor immune responses in head and
neck squamous cell carcinoma treated with EGFR inhibitor. J Transl
Med. 12:2652014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Moustakas A and Heldin CH: The regulation
of TGFbeta signal transduction. Development. 136:3699–3714. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chen Y, Zou L, Zhang Y, Chen Y, Xing P,
Yang W, Li F, Ji X, Liu F and Lu X: Transforming growth factor-β1
and α-smooth muscle actin in stromal fibroblasts are associated
with a poor prognosis in patients with clinical stage I–IIIA
nonsmall cell lung cancer after curative resection. Tumour Biol.
35:6707–6713. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yang H, Wang L, Zhao J, Chen Y, Lei Z, Liu
X, Xia W, Guo L and Zhang HT: TGF-β-activated SMAD3/4 complex
transcriptionally upregulates N-cadherin expression in non-small
cell lung cancer. Lung Cancer. 87:249–257. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zu L, Xue Y, Wang J, Fu Y, Wang X, Xiao G,
Hao M, Sun X, Wang Y, Fu G, et al: The feedback loop between
miR-124 and TGF-β pathway plays a significant role in non-small
cell lung cancer metastasis. Carcinogenesis. 37:333–343. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Shangguan L, Ti X, Krause U, Hai B, Zhao
Y, Yang Z and Liu F: Inhibition of TGF-β/Smad signaling by BAMBI
blocks differentiation of human mesenchymal stem cells to
carcinoma-associated fibroblasts and abolishes their protumor
effects. Stem Cells. 30:2810–2819. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Fan Y, Li X, Xiao W, Fu J, Harris RC,
Lindenmeyer M, Cohen CD, Guillot N, Baron MH, Wang N, et al: BAMBI
elimination enhances alternative TGF-β signaling and glomerular
dysfunction in diabetic mice. Diabetes. 64:2220–2233. 2015.
View Article : Google Scholar : PubMed/NCBI
|