Introduction
Lung cancer is the leading cause of cancer death
worldwide, accounting for 19% of total cancer deaths. The ratio of
mortality to incidence is 0.87, reflecting the highly fatal nature
of the disease, despite advances in surgery, chemotherapy and
radiotherapy (1,2). Lung cancer is categorised into two
major groups: small cell lung cancer (SCLC) and non-small cell lung
cancer (NSCLC). NSCLC comprises the majority of lung cancers, and
is more often diagnosed at an advanced stage with poor survival
(3). More intensive and effective
studies are required, to identify new diagnostic, prognostic and
therapeutic markers, such that improvements can be made in early
diagnosis and targeted treatments.
From primary tumour to metastasis, cancer cells rely
on diverse signalling pathways to promote their proliferation,
survival, invasion and subsequent dissemination through lymphatic
and blood vessels. Receptor tyrosine kinases (RTKs) contain an
extracellular domain for ligand binding, a trans-membrane helix and
a cytoplasmic tyrosine kinase domain, and an intracellular
juxtamembrane regulatory region (4). RTKs play a pivotal role in regulation
of cellular processes including cell survival, cell proliferation,
cell differentiation, cell migration and cell cycle control
(5,6). Dysregulation of RTKs, including
autocrine activation, chromosomal translocations, overexpression of
RTKs or gain-of-function mutations, occurs in cancer (4).
The downstream of kinase (DOK) protein family,
comprising seven members, mediates intracellular signal
transduction downstream of RTKs (7–9). DOK
proteins share a structural topology characterised by an
NH2-terminal pleckstrin homology (PH) domain, a central
phosphotyrosine binding (PTB) domain, followed by SH2 target motifs
in the carboxyl-terminal (7,10). The
DOK proteins are divided into three subgroups; DOK 1–3 are
primarily expressed in haematopoietic tissues (11), DOK 4–6 are predominantly expressed
within the nervous system (12,13),
and DOK7 is mainly expressed in skeletal muscle and the heart, and
plays a distinct role in comparison with the other members
(14).
Deregulation of DOK proteins have been observed in
various malignancies. DOK1, DOK2, and DOK3 have been shown to
cooperatively suppress aggressive histiocytic sarcoma in a mouse
model (11). DOK2 has been
suggested as a predictive factor for poor prognosis of gastric
cancer (15,16). In lung cancer, loss of DOK1, DOK2
and DOK3 resulted in aberrant proliferation of alveolar type II
cells and bronchoalveolar stem cells, with subsequent development
of lung cancer in a mouse model (7). Reduced expression of DOK2 was also
seen in human lung cancers, and a forced overexpression of DOK2
inhibited the proliferation of lung cancer cells (7).
DOK7 is a cytoplasmic adaptor protein that is
necessary for muscle specific kinase (MuSK) activation and
Acetylcholine receptor (AChR) clustering, which are indispensible
for neuromuscular synapse function (17,18).
DOK7 interacts with the juxtamembrane region of MuSK via its PTB
domain, although both PH and PTB domains are required for MuSK
activation (18–20). DOK7 is not only a substrate of MuSK,
but can also activate MuSK kinase activity (21). Agrin requires DOK7 to activate MuSK
and DOK7 is also necessary for the correct localisation of MuSK in
muscle (22). Overexpression of
DOK7 was associated with increased activation of MuSK and
neuromuscular junction formation (22). On the other hand, DOK7 expression
enhanced MuSK activation and restored neuromuscular junction
formation in agrin-deficient mice (23,24).
In addition to its profound function at neuromuscular junction, a
recent study has revealed a hypermethylation of DOK7 promoter in
breast cancer (25). However, the
expression and biological function of DOK7 in other malignancies,
particularly lung cancer, remains largely unknown.
In the present study, we aimed to evaluate DOK7
expression in lung cancer and the involvement in disease
progression. Our current study revealed predominant expression of
DOK7 protein isoform 1 (DOK7V1) in normal lung tissues, which was
reduced in lung cancers. We further investigated its regulatory
role of cellular functions in lung cancer cell lines using an
overexpression plasmid vector carrying a DOK7V1 coding
sequence.
Materials and methods
Cell culture
Lung cancer cell lines, A549 and H3122 were
purchased from the American Type Culture Collection (ATCC,
Manassas, VA, USA). Cells were routinely cultured in Dulbecco's
modified Eagle's medium (DMEM) with L-glutamine (Thermo Fisher
Scientific, Hudson, NH, USA) supplemented with 10% fetal bovine
serum (ExCellBio, Shanghai, China), in an incubator at 37°C, 5%
CO2 and 95% humidity.
Construction of DOK7 expression vectors and
transfection: the DOK7-Flag and pEnter (vector) plasmids were
purchased from Vigene Biosciences (Rockville, MD, USA). Purified
DOK7V1 transgenes and control plasmid vectors were transfected into
A549 and H3122 cells, respectively, using Neofect™ DNA transfection
reagent (Neofectbiotech Co., Ltd., Beijing, China).
Western blot analysis
Protein concentrations were determined with the BCA
Protein Assay kit and a spectrophotometer (both by Thermo Fisher
Scientific). Equal amounts of protein sample were separated using
10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE) and blotted onto a nitrocellulose membrane. The
membranes were blocked with 5% skimmed milk in TBS buffer for 1 h.
Proteins were then probed with anti-FLAG monoclonal antibody
(Sigma, St. Louis, MO, USA), anti-DOK7 antibody (Abcam, Cambridge,
MA, USA), monoclonal mouse anti-human GAPDH antibody (Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), anti-Akt antibody (Sigma)
and anti-phosphorylated-Akt (Ser473, Cell Signaling Technology,
Danvers, MA, USA) and corresponding peroxidase-conjugated secondary
antibody. Protein bands were visualised and analysed using Vilber
Fusion Fx5 (Vilber, Marne La Vallée, France).
In vitro cell function assays
Cells were detached from the culture dish and cell
density (per millilitre) was counted. Cells were then seeded onto a
96-well plate at a density of 3000 cells in 200 µl of culture
medium. Five plates were set up to obtain a cell density reading,
following incubation up to 4 days. After the incubation, culture
medium was removed and replaced with 100 µl of 10% CCK-8 (Cell
Counting Kit-8, Dojindo, Japan). Absorbance at 450 nm was then
determined after an incubation at 37°C, 5% CO2 for 1 h
using a spectrophotometer (Thermo Fisher Scientific).
In vitro tumour cell Matrigel adhesion
assay
Matrigel (5 µg) was added to 100 µl of serum-free
medium to coat the culture surface of each well of a 96-well plate
and dried in an oven to form an artificial basement membrane. This
membrane was then rehydrated in 100 µl of sterile distilled water
for 40 min before seeding 20,000 cells/200 µl culture medium into
each well. After an incubation of 40 min, the medium was removed
and non-adherent cells were washed off with 150 µl of PBS buffer.
Adherent cells were then fixed in 4% formaldehyde (v/v) in PBS for
30 min before being stained in 0.5% crystal violet solution (w/v)
in distilled water. Crystal violet staining was dissolved with 10%
acetic acid and the absorbance at 570 nm was then determined using
the spectrophotometer.
In vitro tumour cell migration
assay
Cells (1×106/well) were seeded to a
6-well plate and cultured in the incubator overnight, then
scratched with a 200 µl pipette tip to create a wound and washed
twice with PBS to remove floating cells. The cells were
photographed every 6 h using an inverted microscope over a period
up to 24 h. The size of the wounds were subsequently measured with
ImageJ software.
Results
Expression of DOK7 in lung cancer
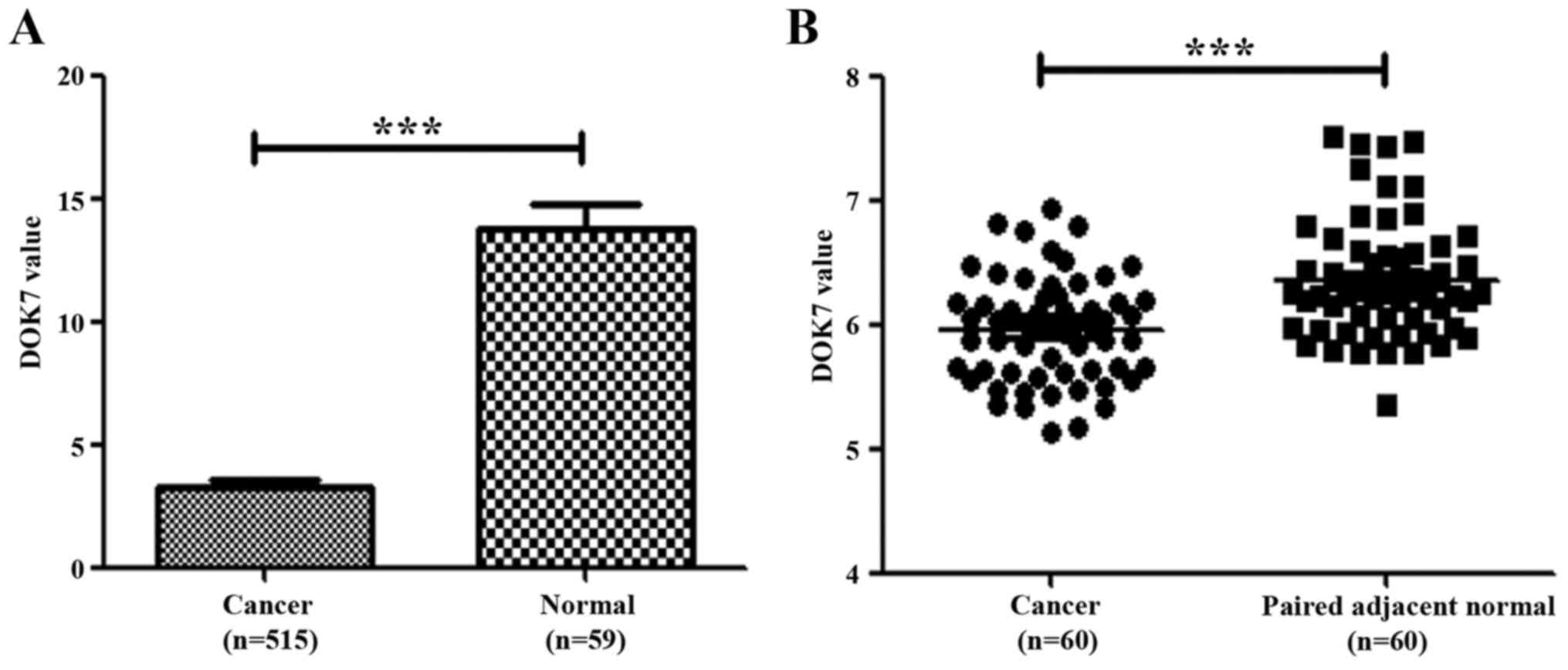
We initially evaluated the expression of DOK7 in
lung cancer by analysing its levels in a cohort of lung cancer
tissue deposited at The Cancer Genome Atlas (TCGA) adenocarcinoma
database. Reduced transcript levels of DOK7 were seen in the lung
cancer compared with normal lung tissues (Fig. 1A). We also analysed DOK7 expression
levels in another cohort of lung cancer which had paired adjacent
normal lung tissues (GSE19804, 240633_at) (26). The reduced expression of DOK7 was
also seen in these lung cancer samples in comparison with the
paired adjacent normal lung tissues, p<0.001 (Fig. 1B).
Reduced expression of DOK7 was
associated with the survival of patients with lung cancer
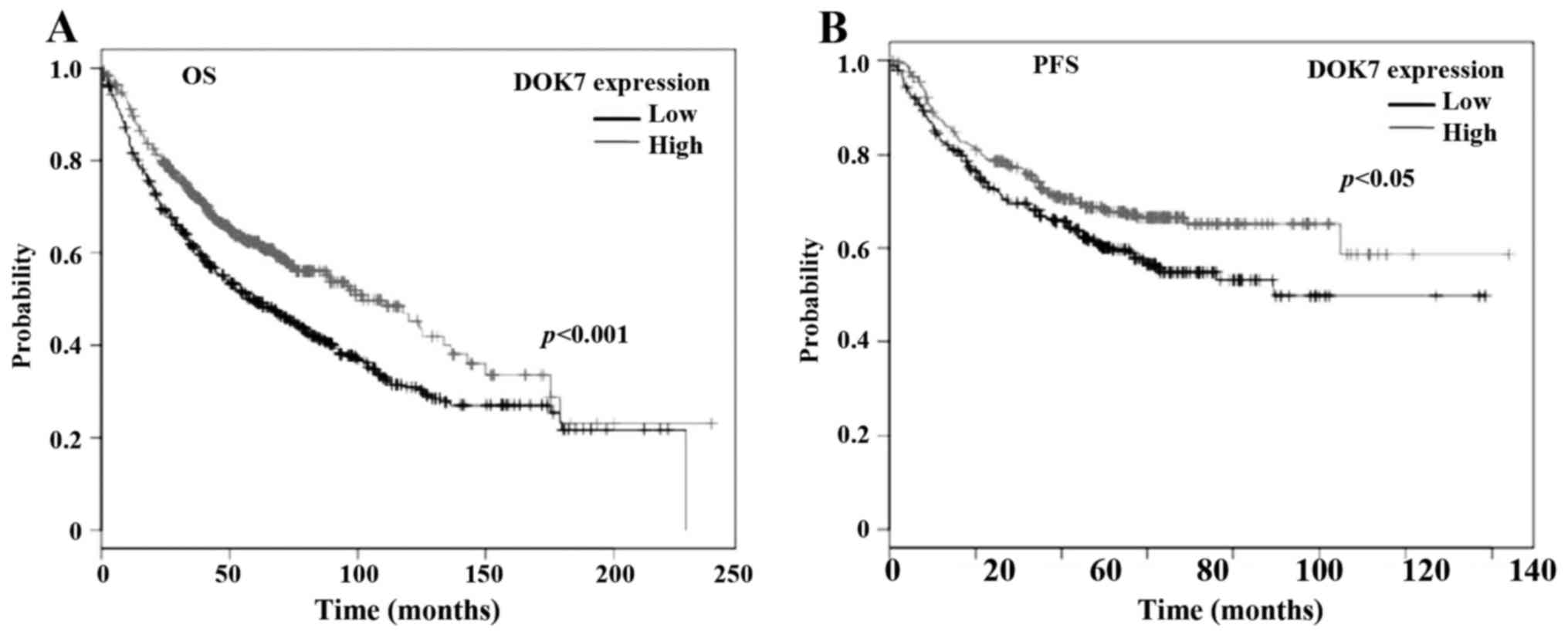
To further clarify the role played by this molecule
in lung cancer, we analysed the association between DOK7 expression
(Affy ID 240633_at) and survival of patients with lung cancer using
an online Kaplan-Meier survival analysis tool (KMplot, http://kmplot.com/analysis/) (27). The Kaplan-Meier analyses showed that
the reduced expression of DOK7 was significantly associated with
poorer overall survival (OS), p<0.001 (Fig. 2A), in a cohort of 1145 cases of lung
cancer. The reduced DOK7 expression was also associated with poor
progression-free survival (PFS) of a cohort of 596 patients with
lung cancer, p=0.019 (Fig. 2B).
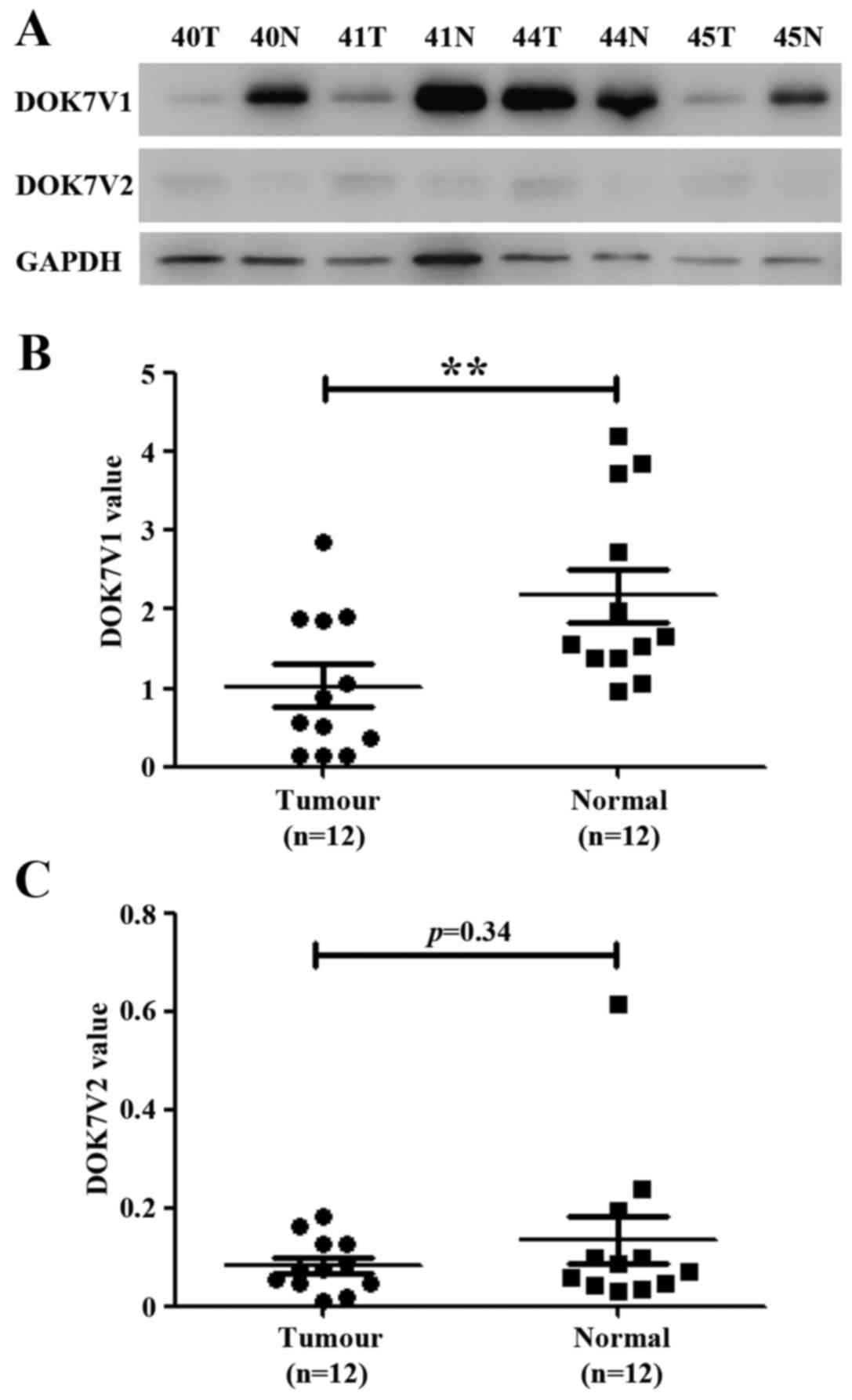
Expression of DOK7 protein isoforms in
human lung cancer tissues
The expression of DOK7 protein isoforms was examined
in a cohort of lung cancer tissues using western blot analysis.
DOK7 isoform 1 (DOK7V1) was predominantly expressed in normal lung
tissues in comparison to the expression of its isoform 2 (DOK7V2)
(Fig. 3). DOKV1 exhibited low
expression in lung cancer tissues compared with the paired adjacent
normal lung tissues (p<0.01, n=12). DOK7V2 protein was either
weakly expressed or absent from both lung cancer tissues and
adjacent normal lung tissues (Fig.
3).
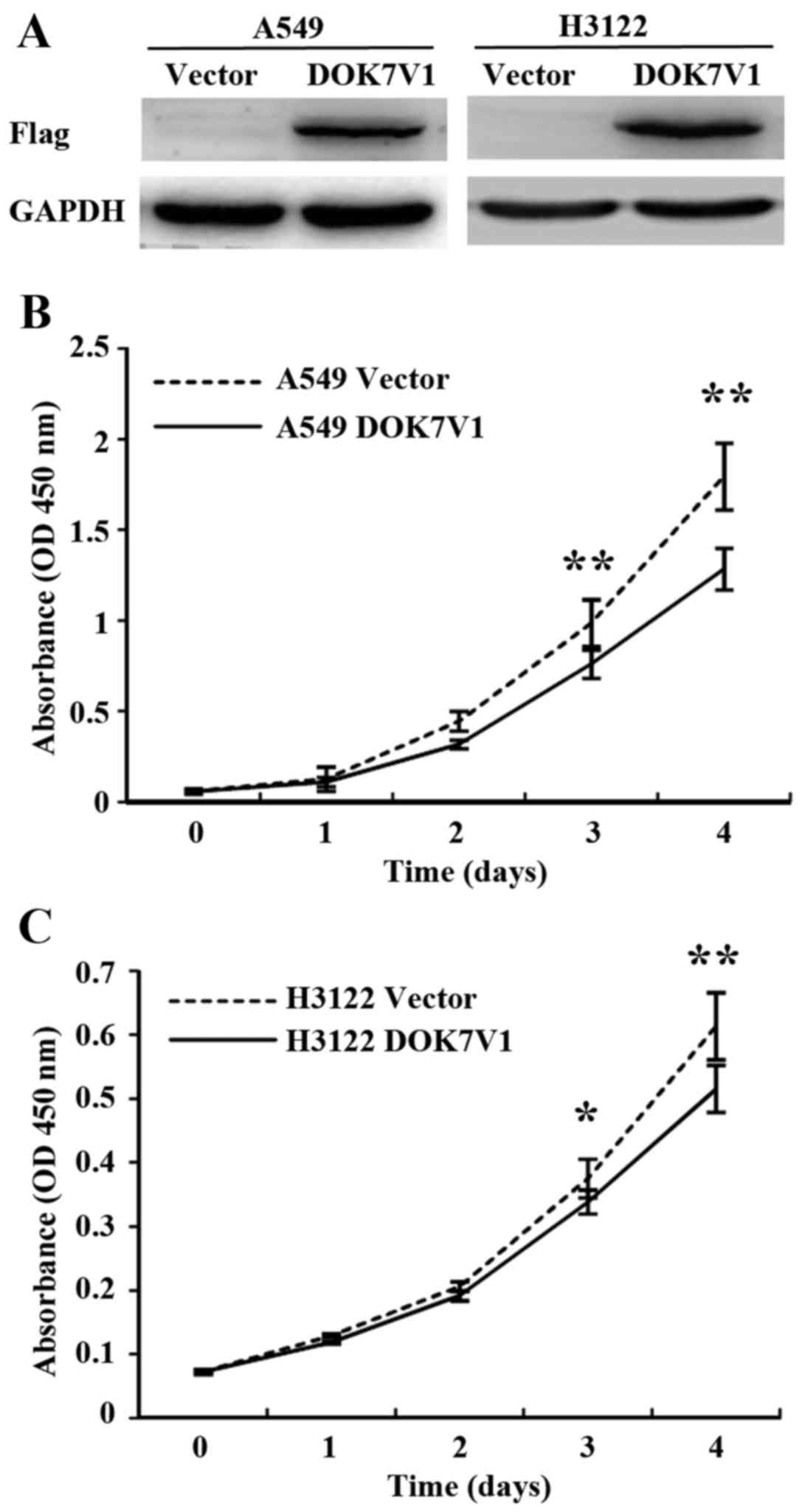
Overexpression of DOK7V1 exhibited an
inhibitory effect on proliferation of lung cancer cell lines
To further study the role of DOK7 in lung cancer, we
constructed an overexpression plasmid vector which carried the
coding sequence of DOK7V1. Overexpression of DOK7V1 was performed
in A549 and H3122 lung cancer cell lines. Following transfection
and selection, transfected cell lines were verified for the
expression of DOK7V1 using western blotting (Fig. 4A). The overexpression of DOK7V1
resulted in a reduction of in vitro cell proliferation of
A549 cells compared with the control cells which were transfected
with the empty plasmid vector (Fig.
4B). In comparison with the A549 cells, H3122 exhibited less
response to the inhibitory effect on proliferation by DOK7V1
overexpression (Fig. 4C).
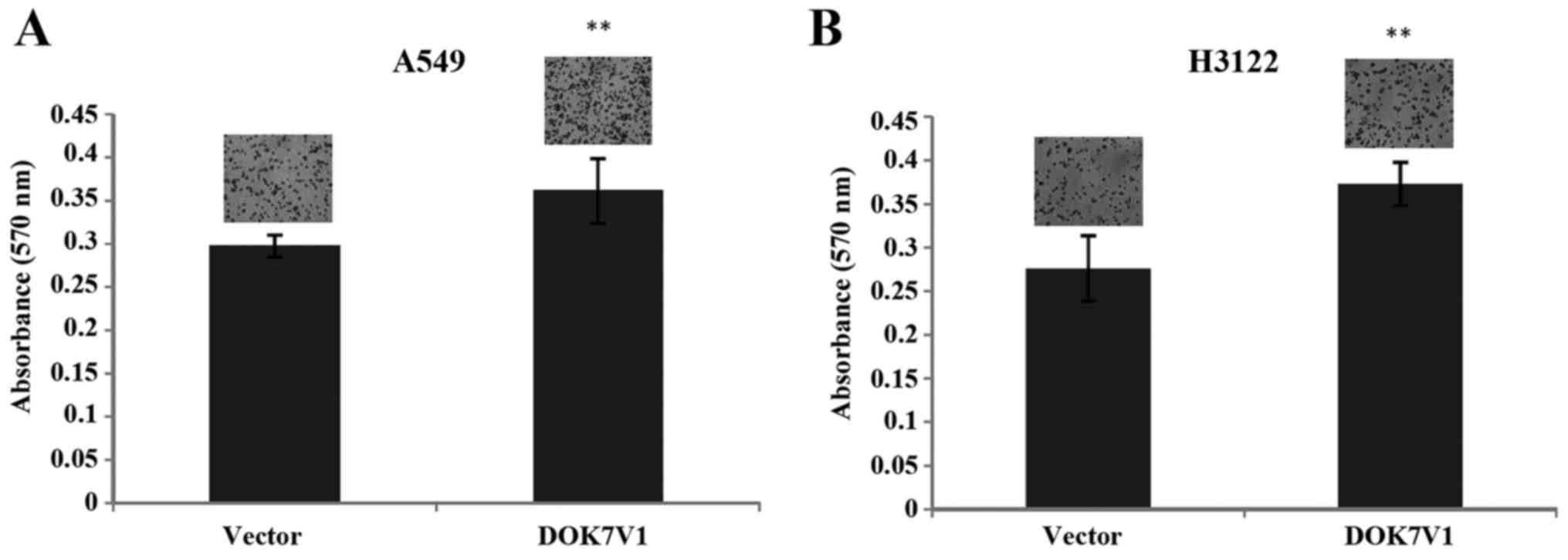
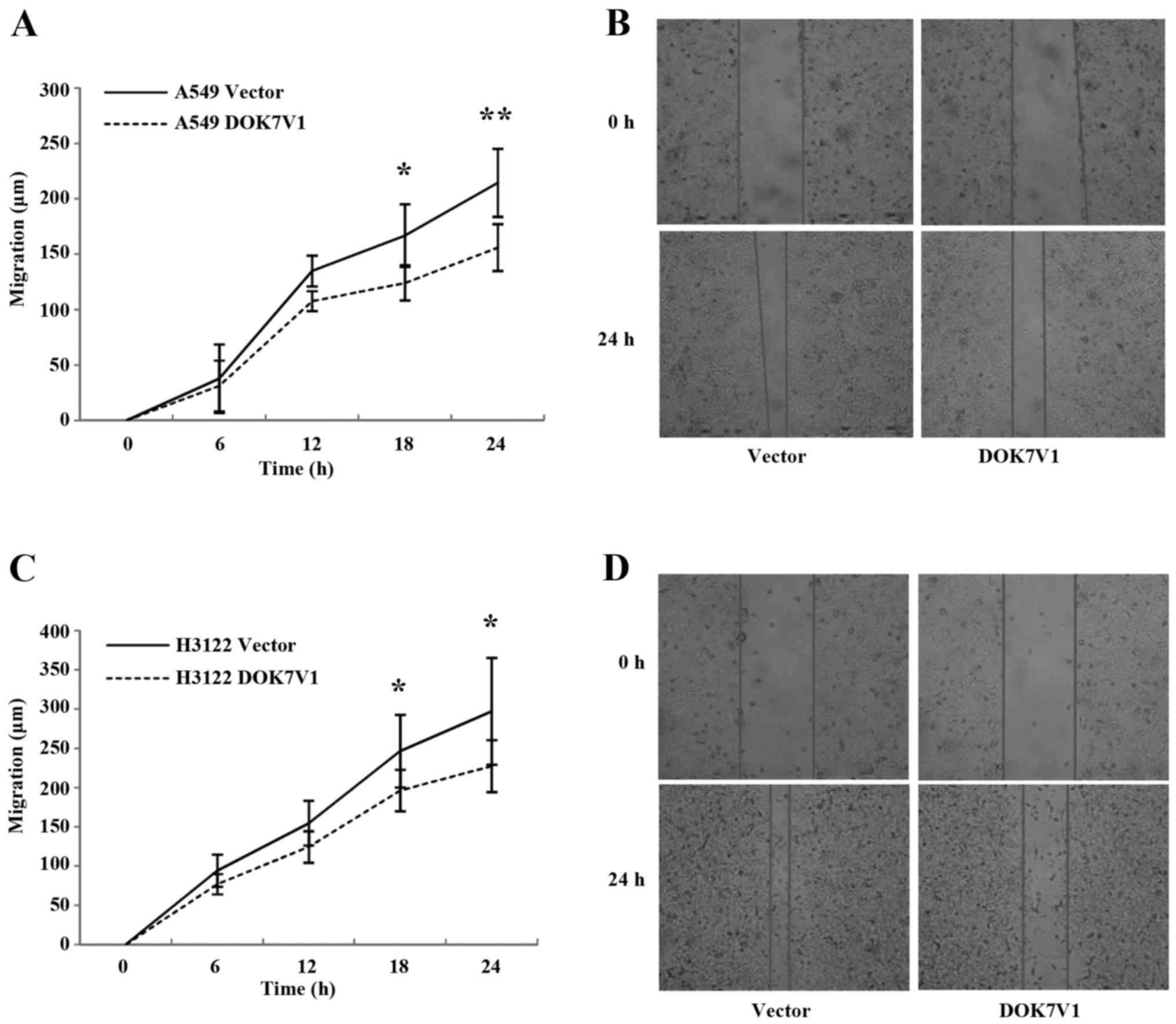
Influence of DOK7V1 on adhesion and
migration of lung cancer cells
The overexpression of DOK7V1 resulted in an increase
in cell adhesion to an artificial matrix gel in both A549 and H3122
lung cancer cell lines (Fig. 5). In
contrast to the promotion of cellular adhesion, a reduction was
seen in the migration of both A549 and H3122 cell lines following
the overexpression of DOK7V1 in comparison with the respective
control cells (Fig. 6).
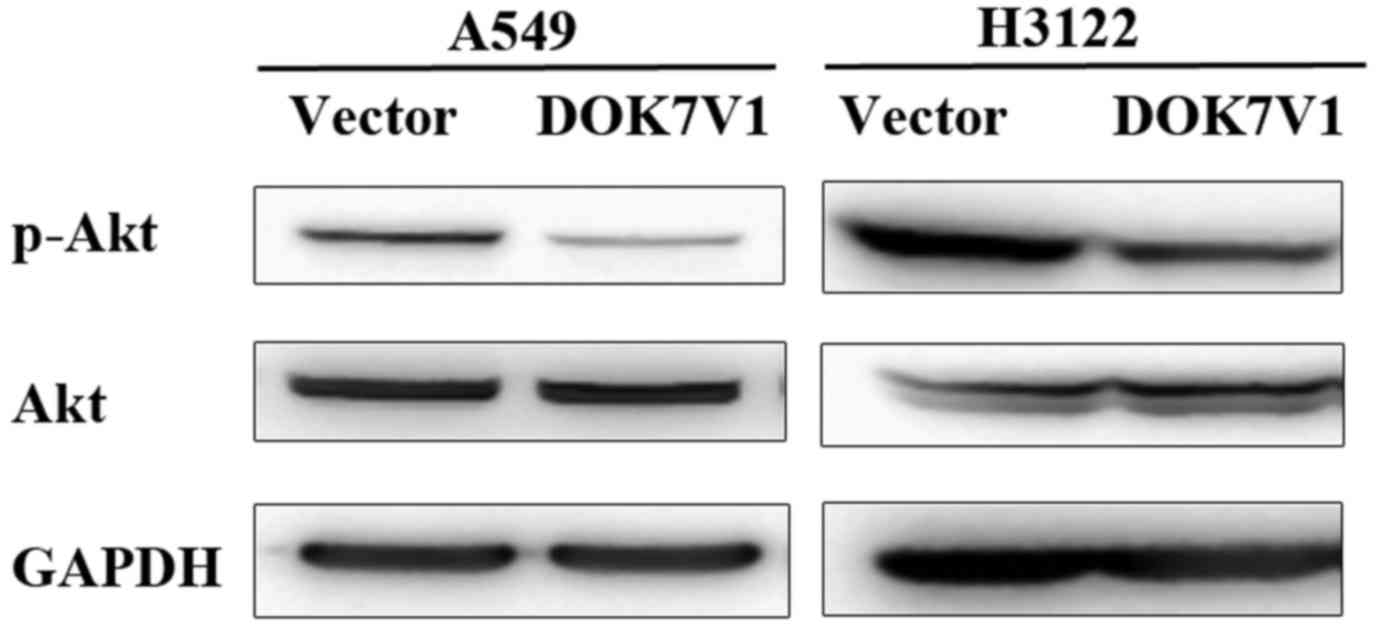
Phosphorylation of Akt was altered in
the DOK7V1 overexpression lung cancer cell lines
Following the functional assays, we performed some
experiments to examine involvement of Akt and ERK pathways which
have shown associated with functions of certain DOK proteins
(7). A decrease of Akt
phosphorylation was seen in both A549 and H3122 cell lines which
overexpressed the DOK7 variant 1 while the total Akt protein levels
remained similar in comparison with their corresponding controls
(Fig. 7).
Discussion
This is the first study regarding the downstream of
tyrosine kinase 7 (DOK7) in lung cancer, in which we determined
protein isoform −1 and −2 expression levels in human lung cancer
tissues, compared with paired adjacent normal lung tissues. In the
present study, a reduced transcript level of DOK7 was seen in lung
cancer in comparison to normal lung tissues or paired adjacent
normal lung tissues. Western blot analyses further revealed that
one of the DOK7 protein isoforms, isoform 1 (DOKV1) was
predominantly expressed in the normal lung tissues, and was reduced
in lung cancers.
Recent studies of other DOK proteins in lung cancers
have demonstrated that DOK1, DOK2 and DOK3 are reduced or absent in
lung cancer (7,28). In mouse models, loss of DOK1, DOK2
and DOK3 resulted in an increased proliferation of alveolar type II
cells and bronchoalveolar stem cells leading to a development of
lung cancer particularly in a triple knockout mouse model (7). A reduced expression of DOK2 was also
observed in human lung adenocarcinomas, whilst DOK2 overexpression
resulted in an inhibition of proliferation of lung cancer cells
(7). Chromosome 8p21.3 where DOK2
gene is located, is one of the common regions deleted in human lung
cancer (28). The loss of one copy
of the DOK2 gene was seen in 37% of human lung adenocarcinoma and
33% of human non-small cell lung cancers (7). Reduced expression of DOK2 was seen in
both primary lung adenocarcinomas and lymph node metastases
(7). In contrast to the observation
in mouse models, no differential expression of DOK1 and DOK3 was
seen in human lung cancer in comparison with normal lung tissues,
although a reduced DOK3 was noted in lymph node metastases compared
with its expression in the primary tumours (7).
A recent study of DOK7 in breast cancer has
demonstrated that CpG islands of DOK7 promoter are hypermethylated
leading to a downregulation its expression (25). These studies indicate that an
inhibitory role may be played by DOK proteins in cancer.
Downregulation or loss of DOK proteins may occur in cancer cells
due to gene deletion or hypermethylation. Additionally, mutations
in DOK7 are a common cause of congenital myasthenic syndrome
(29). However, whether the reduced
expression or loss of DOK7 in lung cancer results from these events
is yet to be investigated. The role of DOK7 mutation in cancer also
requires investigation.
Kaplan-Meier survival analyses of DOK7 transcripts
in lung cancer show that the reduced transcript levels of DOK7 were
associated with both poorer OS and PFS. It suggests that DOK7 plays
an inhibitory role during disease progression and relapse of lung
cancer. To further explore this hypothesis, we constructed a
plasmid vector carrying a DOK7V1 coding sequence. Overexpression of
this isoform resulted in a decreased proliferation of both A549 and
H3122 lung cancer cell lines. In keeping with the inhibitory role
of DOK proteins in lung cancer, forced expression of DOK2 in a lung
cancer cell line has been seen to reduce growth rate and both Akt
and Erk phosphorylation (7).
It has been suggested that certain DOK proteins play
an inhibitory role in signal transduction of some RTKs, therefore
the loss of these DOK proteins leads to a promotion of the
RTK-mediated proliferation. Further study will elucidate the role
played by DOK7 in RTK signalling and the disease progression of
lung cancer.
In addition to its effect on cell proliferation, a
similar inhibitory effect was also observed in migration of both
A549 and H3122 cell lines following the overexpression of DOK7V1.
Moreover, a contrasting effect was seen in adhesion of these cell
lines to an artificial basement membrane, with DOK7V1 enhancing the
adhesiveness of these lung cancer cell lines. Our further
investigations showed a reduced activation of Akt in the lung
cancer cell lines following the overexpression of DOK7 variant 1.
However, how DOK7 protein coordinates signalling through Akt
pathway and what implication DOK7-regulated Akt pathway has in lung
cancer are yet to be investigated in future study.
In conclusion, DOK7 transcripts were reduced in lung
cancer and the reduced DOK7 transcript levels were associated with
poorer OS and PFS. DOK7 protein isoform 1 was more abundantly
expressed in normal lung tissues and was reduced in lung cancers.
Overexpression of the DOK7 isoform 1 inhibited proliferation and
migration of lung cancer cells in which a reduced activation of Akt
was observed, but enhanced the adhesion of lung cancer cells to
extracellular matrix. Further investigations are needed to shed
light on the interaction between DOK7 and RTKs, including the
RTK-mediated signalling that enhanced as a result of DOK7
downregulation, thus leading to disease progression and
relapse.
Acknowledgements
The authors wish to thank Cancer Research Wales,
Life Sciences Research Network Wales (Welsh Government's Ser Cymru
program) and Albert Huang Foundation. Mr. Gang Chen is a recipient
of the China Medical Scholarship from Cardiff University. This
study was supported by the National Natural Science Foundation of
China (no. 81672726).
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang M, Lewinska M, Fan X, Zhu J and Yuan
ZM: PRR14 is a novel activator of the PI3K pathway promoting lung
carcinogenesis. Oncogene. 35:5527–5538. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lemmon MASJ and Schlessinger J: Cell
signaling by receptor tyrosine kinases. Cell. 141:1117–1134. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Blume-Jensen P and Hunter T: Oncogenic
kinase signalling. Nature. 411:355–365. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ullrich A and Schlessinger J: Signal
transduction by receptors with tyrosine kinase activity. Cell.
61:203–212. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Berger AH, Niki M, Morotti A, Taylor BS,
Socci ND, Viale A, Brennan C, Szoke J, Motoi N, Rothman PB, et al:
Identification of DOK genes as lung tumor suppressors. Nat Genet.
42:216–223. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Simister PC and Feller SM: Order and
disorder in large multi-site docking proteins of the Gab family -
implications for signalling complex formation and inhibitor design
strategies. Mol Biosyst. 8:33–46. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jones N and Dumont DJ: Recruitment of
Dok-R to the EGF receptor through its PTB domain is required for
attenuation of Erk MAP kinase activation. Curr Biol. 9:1057–1060.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bedirian A, Baldwin C, Abe J, Takano T and
Lemay S: Pleckstrin homology and phosphotyrosine-binding
domain-dependent membrane association and tyrosine phosphorylation
of Dok-4, an inhibitory adapter molecule expressed in epithelial
cells. J Biol Chem. 279:19335–19349. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mashima R, Honda K, Yang Y, Morita Y,
Inoue A, Arimura S, Nishina H, Ema H, Nakauchi H, Seed B, et al:
Mice lacking Dok-1, Dok-2, and Dok-3 succumb to aggressive
histiocytic sarcoma. Lab Invest. 90:1357–1364. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Crowder RJ, Enomoto H, Yang M, Johnson EM
Jr and Milbrandt J: Dok-6, a Novel p62 Dok family member, promotes
Ret-mediated neurite outgrowth. J Biol Chem. 279:42072–42081. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Grimm J, Sachs M, Britsch S, Di Cesare S,
Schwarz-Romond T, Alitalo K and Birchmeier W: Novel p62dok family
members, dok-4 and dok-5, are substrates of the c-Ret receptor
tyrosine kinase and mediate neuronal differentiation. J Cell Biol.
154:345–354. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cossins J, Liu WW, Belaya K, Maxwell S,
Oldridge M, Lester T, Robb S and Beeson D: The spectrum of
mutations that underlie the neuromuscular junction synaptopathy in
DOK7 congenital myasthenic syndrome. Hum Mol Genet. 21:3765–3775.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
An CH, Kim MS, Yoo NJ and Lee SH:
Mutational and expressional analysis of a haploinsufficient tumor
suppressor gene DOK2 in gastric and colorectal cancers. APMIS.
119:562–564. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Miyagaki H, Yamasaki M, Takahashi T,
Kurokawa Y, Miyata H, Nakajima K, Takiguchi S, Fujiwara Y, Mori M
and Doki Y: DOK2 as a marker of poor prognosis of patients with
gastric adenocarcinoma after curative resection. Ann Surg Oncol.
19:1560–1567. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yamanashi Y, Higuch O and Beeson D:
Dok-7/MuSK signaling and a congenital myasthenic syndrome. Acta
Myol. 27:25–29. 2008.PubMed/NCBI
|
|
18
|
Okada K, Inoue A, Okada M, Murata Y,
Kakuta S, Jigami T, Kubo S, Shiraishi H, Eguchi K, Motomura M, et
al: The muscle protein Dok-7 is essential for neuromuscular
synaptogenesis. Science. 312:1802–1805. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hamuro J, Higuchi O, Okada K, Ueno M,
Iemura S, Natsume T, Spearman H, Beeson D and Yamanashi Y:
Mutations causing DOK7 congenital myasthenia ablate functional
motifs in Dok-7. J Biol Chem. 283:5518–5524. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hallock PTXC, Xu CF, Park TJ, Neubert TA,
Curran T and Burden SJ: Dok-7 regulates neuromuscular synapse
formation by recruiting Crk and Crk-L. Genes Dev. 24:2451–2461.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bergamin E, Hallock PT, Burden SJ and
Hubbard SR: The cytoplasmic adaptor protein Dok7 activates the
receptor tyrosine kinase MuSK via dimerization. Mol Cell.
39:100–109. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Inoue A, Setoguchi K, Matsubara Y, Okada
K, Sato N, Iwakura Y, Higuchi O and Yamanashi Y: Dok-7 activates
the muscle receptor kinase MuSK and shapes synapse formation. Sci
Signal. 2:ra72009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tezuka T, Inoue A, Hoshi T, Weatherbee SD,
Burgess RW, Ueta R and Yamanashi Y: The MuSK activator agrin has a
separate role essential for postnatal maintenance of neuromuscular
synapses. Proc Natl Acad Sci USA. 111:16556–16561. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ohkawara B, Cabrera-Serrano M, Nakata T,
Milone M, Asai N, Ito K, Ito M, Masuda A, Ito Y, Engel AG, et al:
LRP4 third β-propeller domain mutations cause novel congenital
myasthenia by compromising agrin-mediated MuSK signaling in a
position-specific manner. Hum Mol Genet. 23:1856–1868. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Heyn H, Carmona FJ, Gomez A, Ferreira HJ,
Bell JT, Sayols S, Ward K, Stefansson OA, Moran S, Sandoval J, et
al: DNA methylation profiling in breast cancer discordant identical
twins identifies DOK7 as novel epigenetic biomarker.
Carcinogenesis. 34:102–108. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lu TP, Tsai MH, Lee JM, Hsu CP, Chen PC,
Lin CW, Shih JY, Yang PC, Hsiao CK, Lai LC, et al: Identification
of a novel biomarker, SEMA5A, for non-small cell lung carcinoma in
nonsmoking women. Cancer Epidemiol Biomarkers Prev. 19:2590–2597.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Szász AM, Lánczky A, Nagy Á, Förster S,
Hark K, Green JE, Boussioutas A, Busuttil R, Szabó A and Győrffy B:
Cross-validation of survival associated biomarkers in gastric
cancer using transcriptomic data of 1,065 patients. Oncotarget.
7:49322–49333. 2016.PubMed/NCBI
|
|
28
|
Chitale D, Gong Y, Taylor BS, Broderick S,
Brennan C, Somwar R, Golas B, Wang L, Motoi N, Szoke J, et al: An
integrated genomic analysis of lung cancer reveals loss of DUSP4 in
EGFR-mutant tumors. Oncogene. 28:2773–2783. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Müller JS, Herczegfalvi A, Vilchez JJ,
Colomer J, Bachinski LL, Mihaylova V, Santos M, Schara U, Deschauer
M, Shevell M, et al: Phenotypical spectrum of DOK7 mutations in
congenital myasthenic syndromes. Brain. 130:1497–1506. 2007.
View Article : Google Scholar : PubMed/NCBI
|