Introduction
Cutaneous squamous cell carcinoma (cSCC) is the
second most common cutaneous malignant tumor, following cutaneous
basal cell carcinoma (cBCC) (1,2).
Pathogenesis of cSCC is related with sun exposure, genodermatosis
and immunosuppression (3,4). Compared with cBCC, cSCC shows more
potential to metastasize to lymph nodes or distant organs (5), and 5-year survival rate of the
patients with local metastasis is <30% (6,7).
Currently, the main treatment is surgery, supplemented with
radiotherapy and/or chemotherapy, however, this may leave serious
sequelae (8). Therefore, it is of
important clinical significance to study the pathogenesis of cSCC
and to develop more effective diagnoses and treatments.
Mammary serine protease inhibitor (Maspin) belongs
to serpin family, and its encoding gene is located in 18q21 in
Homo sapiens (9). Since the
subtle differences between Maspin and other members of serpin
family in secondary structure, there is no protease inhibited by
Maspin via the classical serpin pathway to date (10–13).
Initially Maspin is expressed in human mammary epithelial tissue
and breast carcinoma tissue with an antitumor function (14). Subsequently, a large number of
studies reported that Maspin was expressed in multiple normal
tissues, including breast, prostate, placenta and epidermal
keratinocytes, while downregulated or could not be detected in
various cancers. Maspin inhibits epidermal growth factor
(EGF)-induced epithelial-mesenchymal transition (EMT) of esophageal
carcinoma cells, suppresses proliferation in soft agar, migration
and invasion, even induces transition of tumor cells into benign
cells (15). Maspin inhibits
vasculogenic mimicry in non-small cell lung cancer (NSCLC) cells,
and its expression level is positively correlated with prognostic
implication in NSCLC patients (16). Maspin promotes tumor cells apoptosis
in breast and prostate cancer (17,18).
Maspin suppresses prostate tumor growth, invasion and metastasis by
inhibiting histone deacetylization, and activates host neutrophil-
and B cell-dependent antitumor immune response (19). Therefore, Maspin could be an
effective marker for diagnosis and therapy of cancers. Recently,
some reports found that Maspin is expressed in detected normal skin
tissue, but partly expressed in cSCC and cBCC (20,21),
and the expression level of Maspin is positively correlated with
prognosis of cSCC patients (16),
suggesting that Maspin may participate in cSCC progress. However,
the role of Maspin in cSCC carcinogenesis and development is not
clear.
In this study, we examined the expression level of
Maspin in cSCC tissue, and found that it was obviously
downregulated compared with normal cutaneous tissues. Subsequently,
by establishing Maspin stably expressed cell line, we demonstrated
that Maspin inhibited growth, proliferation, invasion by delaying
cell cycle transition and enhancing apoptosis in cSCC cells. These
findings may provide new insights into carcinogenesis, diagnosis
and therapy of cSCC.
Materials and methods
Human tissue specimens
Seventeen pairs of clinical cSCC samples were
collected from cSCC patients operated from September 2012 to April
2015 in Department of Dermatology, The First Affiliated Hospital of
China Medical University. Each sample pair contains a cSCC tissue,
an adjacent tissue 0.5 cm from cSCC tissue and an adjacent tissue 1
cm from cSCC tissue. The patients had not been treated with
radiotherapy or chemotherapy. Informed consent was obtained from
the patients, and our collection and treatment procedures were in
line with standards of the ethics committee of The First Affiliated
Hospital of China Medical University.
RNA extraction, reverse transcription
and real-time PCR
The total RNA was extracted by total RNA rapid
extraction kit (BioTeke, Beijing, China) from tissues or cells, and
detailed procedure referred to the manufacturer's protocol. The
obtained RNA samples were reverse transcribed into cDNA by Super
M-MLV reverse transcriptase (BioTeke), with the oligo(dT) and
random primers. All instruments in this section were treated by
RNase Erasol (Tiandz, Beijing, China), and all reagents were
RNase-free.
The cDNA was used to perform real-time PCR with 2X
power Taq PCR MasterMix (BioTeke) and SYBR Green (Solarbio,
Beijing, China) to test Maspin mRNA level, with β-actin as the
internal control. The PCR procedure was set as follows: 95°C for 10
min, 40 cycles of 95°C for 10 sec, 60°C for 20 sec and 72°C for 30
sec, and finally 4°C for 5 min. The data were analyzed by
Exicycler™ 96 (Bioneer, Daejeon, Korea), calculated by
2−∆∆Ct method (cells) or 2−∆Ct method
(tissues) (22). Sequences of
real-time PCR primers (Sangon, Shanghai, China) are shown in
Table I.
 | Table I.The real-time PCR data in 17 pairs of
clinical cutaneous squamous cell carcinoma samples. |
Table I.
The real-time PCR data in 17 pairs of
clinical cutaneous squamous cell carcinoma samples.
| Sample no. | 2−∆Ct
mean | Sample no. | 2−∆Ct
mean | Sample no. | 2−∆Ct
mean |
|---|
| 1A | 0.000598 | 1B | 0.000448 | 1C | 0.000799 |
| 2A | 0.001075 | 2B | 0.000848 | 2C | 0.001307 |
| 3A | 0.000963 | 3B | 0.000776 | 3C | 0.001453 |
| 4A | 0.002595 | 4B | 0.002737 | 4C | 0.004600 |
| 5A | 0.000446 | 5B | 0.000537 | 5C | 0.000865 |
| 6A | 0.001572 | 6B | 0.001942 | 6C | 0.002049 |
| 7A | 0.002300 | 7B | 0.002198 | 7C | 0.002537 |
| 8A | 0.002489 | 8B | 0.004308 | 8C | 0.006680 |
| 9A | 0.000755 | 9B | 0.000957 | 9C | 0.001874 |
| 10A | 0.000842 | 10B | 0.001102 | 10C | 0.001078 |
| 11A | 0.003457 | 11B | 0.003549 | 11C | 0.009217 |
| 12A | 0.001512 | 12B | 0.001421 | 12C | 0.002616 |
| 13A | 0.000944 | 13B | 0.001162 | 13C | 0.003862 |
| 14A | 0.001632 | 14B | 0.002303 | 14C | 0.006408 |
| 15A | 0.000317 | 15B | 0.000313 | 15C | 0.000820 |
| 16A | 0.000431 | 16B | 0.001106 | 16C | 0.001471 |
| 17A | 0.000451 | 17B | 0.000665 | 17C | 0.001570 |
Western blotting
The total protein was extracted from cells or
tissues with RIPA lysis buffer (Beyotime, Haimen, Jiangsu, China).
After measuring the concentration, the protein sample was denatured
by boiling, separated by SDS-PAGE (density of gel dependent on the
protein size), and transferred onto polyvinylidene fluoride (PVDF)
membranes (Millipore, Boston, MA, USA). After blocking with 5% skim
milk (YILI, Hohhot, Inner Mongolia, China) for 1 h, the PVDF
membrane was incubated with the following antibodies at 4°C
overnight: polyclonal rabbit anti-Maspin (Abcam, Cambrige, UK)
(1:1,000), polyclonal rabbit anti-cleaved caspase-3 (Abcam)
(1:1,000), monoclonal rabbit anti-cleaved poly(ADP-ribose)
polymerase (PARP) (Abcam) (1:1,000), polyclonal rabbit anti-Bcl-2
(Boster, Wuhan, Hubei, China) (1:400), and polyclonal rabbit
anti-Bax (Boster) (1:400). After rinsing with TBST, the PVDF
membrane was incubated with goat anti-rabbit IgG-HRP (Beyotime)
(1:5,000) at room temperature for 45 min, and exposed with ECL
reagent (7 sea, Shanghai, China). After antibodies were removed by
stripping buffer (Beyotime), the PVDF membrane was incubated with
monoclonal mouse anti-β-actin (Santa Cruz, CA, USA) (1:1,000) and
goat anti-mouse IgG-HRP (Beyotime) (1:5,000) to test the internal
control, β-actin.
Plasmid construction
To construct Maspin overexpression plasmid, Maspin
coding sequence (CDS) was obtained by PCR from human cDNA, and
amplified by TA cloning using UltraPower pUM-T rapid cloning kit
(BioTeke). After sequencing, the Maspin CDS fragment was inserted
into pcDNA3.1 vector (XhoI+HindIII), and the
overexpression plasmid pcDNA3.1-Maspin was gained. The sequences of
PCR primers are shown in Table
II.
 | Table II.Sequences of primers used in this
study. |
Table II.
Sequences of primers used in this
study.
| Name | Sequence
(5′-3′) |
|---|
| Maspin F |
5′-TTGTGGTTAATGCTGCCTAC-3′ |
| Maspin R |
5′-CCAAGCCTGTGGACTCATC-3′ |
| β-actin F |
5′-CTTAGTTGCGTTACACCCTTTCTTG-3′ |
| β-actin R |
5′-CTGTCACCTTCACCGTTCCAGTTT-3′ |
| Maspin-CDS F |
5′-CTCAAAGCTTATGGATGCCCTGCAACTA-3′ |
| Maspin-CDS R |
5′-CGACCTCGAGCACTTAAGGAGAACAGAAT-3′ |
Cell culture, transfection and
monoclonal cell line
Three cSCC cell lines A431, SCL-1 and SCC12, were
preserved in our laboratory. A431 cells and SCL-1 cells were
cultured in DMEM (Hyclone, Logan, UT, USA) supplemented with 10%
fetal bovine serum (FBS) (Hyclone) (SCC12 cells with 20% FBS), 100
U/ml penicillin, 100 µg/ml streptomycin (Beyotime) at 37°C
incubator (Lishen, Tianjin, China) with 5% CO2. The
overexpression plasmid pcDNA3.1-Maspin and the control pcDNA3.1
were transfected into A431 cells by Lipofectamine 2000 Reagent
(Invitrogen, Carlsbad, CA, USA) with serum-free medium, 24 h later,
G418 (Invitrogen) was added into medium with 100 µg/ml to screen
the integrated cells, and the medium was refreshed every 2–3 days
until the monoclonal cell lines were obtained.
CCK-8 assay
Cell counting kit-8 (CCK-8) assay was performed to
test the cell viability. A431 cells were seeded into 96-well plates
with 3×103 per pore beforehand. After cells adhered,
CCK-8 reagent (Beyotime) was added into the 96-well plate with 10
µl per pore to incubate for 1 h, and the optical density of the
solution was tested at 450 nm with a microplate reader (BioTek, VT,
USA). This OD450 value was the datum at 0 h, and the
data in 24 h, 48 h, 72 h and 96 h were detected later,
respectively.
Colony formation assay
Colony formation assay was performed to detect the
colony formation ability of A431 cells. A431 cells were seeded into
a 35-mm petri dish at 400/well, and cultured in 5% CO2
at 37°C, medium was refreshed every 3 days. Approximately 14 days
later, most of the clones had formed. The cells were fixed with 4%
paraformaldehyde (Sinopharm, Beijing, China) for 20 min, stained
with Wrings-Giemsa stain (Jiancheng, Nanjing, Jiangsu, China) for
5–8 min, and the clone number was counted with an inverted phase
contrast microscope (Motic, Xiamen, Fujian, China). The clones with
≥50 cells were considered positive.
In vitro Transwell assay
To measure the invasion potential of A431 cells, the
Transwell assay in the presence of Matrigel (BD, Franklin Lakers,
NJ, USA) was performed in Transwell chambers with 8-µm aperture
polycarbonate membrane (Corning, NY, USA). Beforehand 40 µl of 2.5
mg/ml Matrigel was added into Transwell upper chamber to coat the
polycarbonate membrane, and preheated at 37°C for 2 h to solidify.
Then 200 µm serum-free cell suspension (2×104 cells)
were added into Transwell upper chamber, and 800 µm DMEM with 20%
FBS was added in the lower chamber. After culturing for 24 h, the
Transwell chamber was fixed in 4% paraformaldehyde (Sinopharm) for
20 min, and stained by 0.5% crystal violet for 5 min. Then the
cells in upper chamber were wiped off, and the cells on reverse
side of polycarbonate membrane were counted with an inverted phase
contrast microscope (Motic) at ×200 magnification.
Hoechst staining
In order to observe the nuclear morphology change,
Hoechst staining was performed. A431 cells were seeded onto glass
slides in 12-well plates with 1×105/pore. When the
confluence reached 80%, the cells were treated with Hoechst
Staining kit (Beyotime), observed with a fluorescence microscope
(Olympus, Tokyo, Japan) at ×400 magnification.
Flow cytometry
Flow cytometry was performed to test apoptosis and
cell cycle of A431 cells. A431 cells cultured in 6-well plates were
collected when the confluence reached 90%. After rinsing with PBS
(Double-helix, Shanghai, China) twice, the cells were treated with
Annexin V-FITC/PI cell apoptosis test kit (Wanleibio, Shenyang,
Liaoning, China) and detected using FACSCalibur (BD) according to
the manufacturer's protocol.
For the cell cycle analysis, the collected cells
were treated by Cell Cycle Analysis kit (Beyotime), and detected
using FACSCalibur (BD) according to the specification.
Proliferation index (PI) = (percentage of cells in S phase + G2/M
phase) / percentage of cells in G1 phase (23).
Statistical analysis
The data in this study are presented as mean ±
standard deviation (SD) with three individual experiments, and
analyzed by one-way ANOVA test or paired Student's t-test. It was
considered statistically significant at P<0.05, P<0.01, or
P<0.001; NS, not significant).
Results
Maspin is downregulated in cSCC
tissues
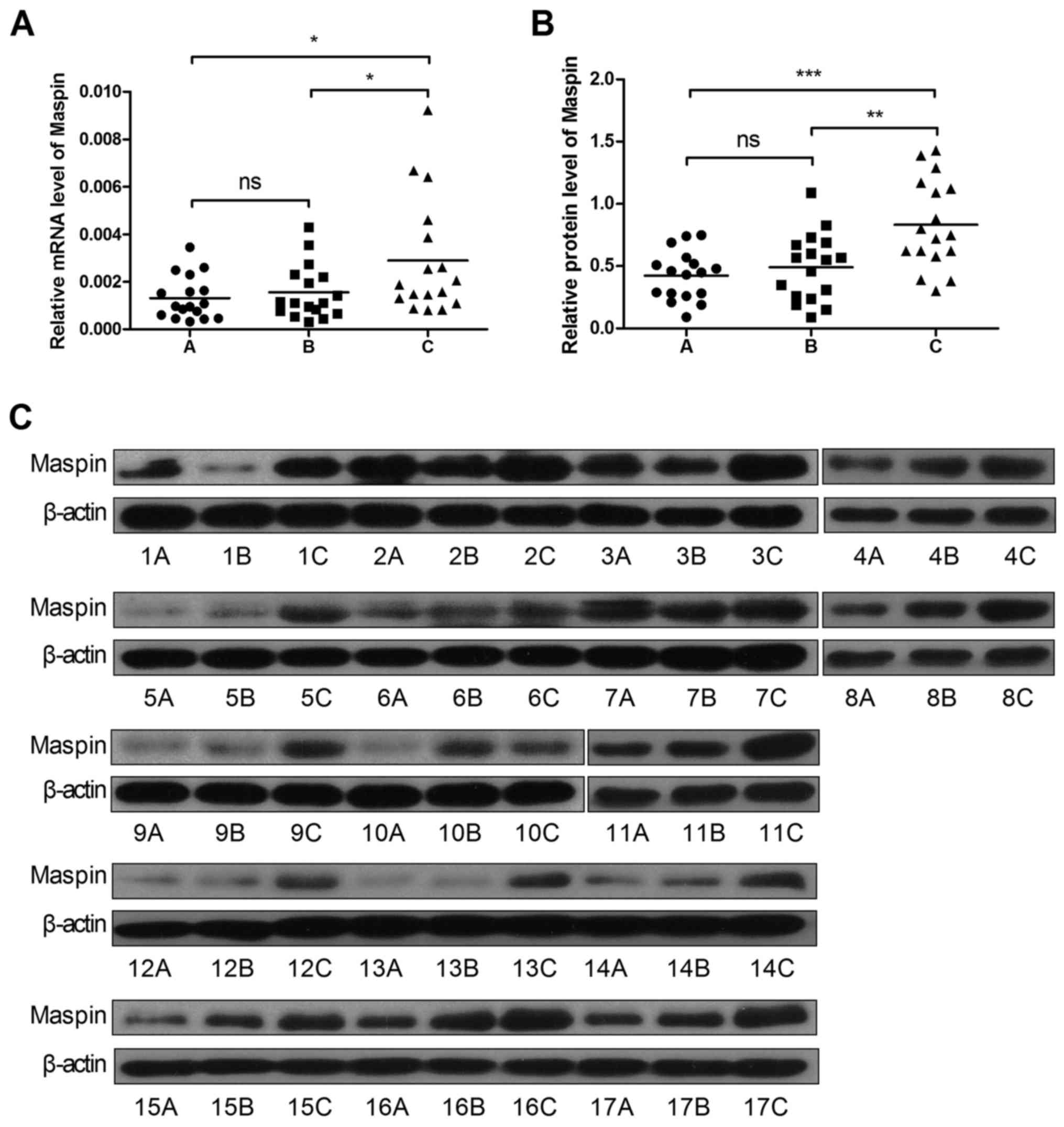
First we detected the expression level of Maspin in
17 pairs of cSCC and adjacent tissues by real-time PCR and western
blotting. As shown in Fig. 1, A, B and
C groups represented the cSCC tissues, the adjacent tissues 0.5
cm from cSCC and the adjacent tissues 1 cm from cSCC, respectively.
The results showed that the mRNA level of Maspin in cSCC tissues
decreased by 55% (Fig. 1A and
Table II), and the protein level
decreased by 49% compared to the adjacent tissue 1 cm from cSCC
tissues (Fig. 1B and C),
respectively.
Establishment of Maspin stably
expressed cell line
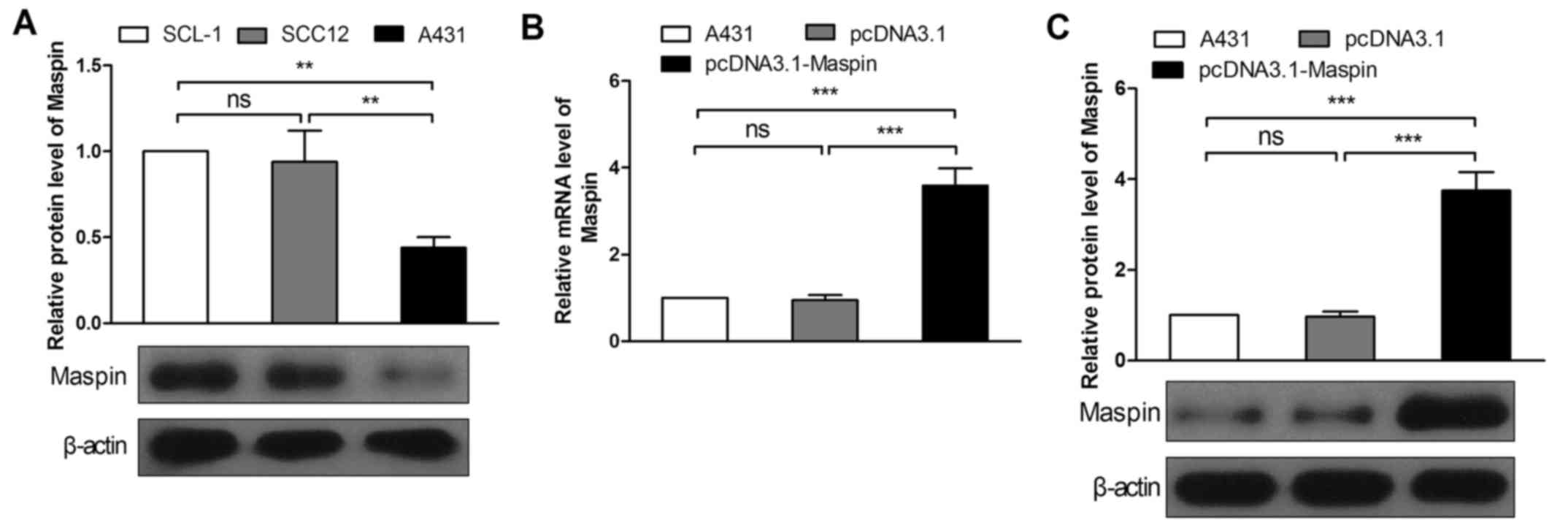
Because of the low expression level of Maspin in
cSCC tissues, among three cSCC cell lines SCL-1, SCC12 and A431, we
selected A431 cells, in which the Maspin expression level was the
lowest (Fig. 2A), to measure the
effect of Maspin on cSCC cells. To stably express Maspin, the
overexpression plasmid pcDNA3.1-Maspin was constructed, and
transfected into A431 cells. By screening with G418, monoclonal
cells of pcDNA3.1-Maspin were obtained, and the pcDNA3.1 vector
monoclonal cells were gained as the control. Real-time PCR and
western blotting were performed to verify the expression efficiency
of pcDNA3.1-Maspin. The results showed that the mRNA level
increased by 3.78-fold (Fig. 2B),
and the protein level increased by 3.86-fold (Fig. 2C) in contrast to the pcDNA3.1 group.
Hence Maspin overexpression cell line was used for the subsequent
experiments.
Maspin inhibits growth, proliferation
and invasion in cSCC cells
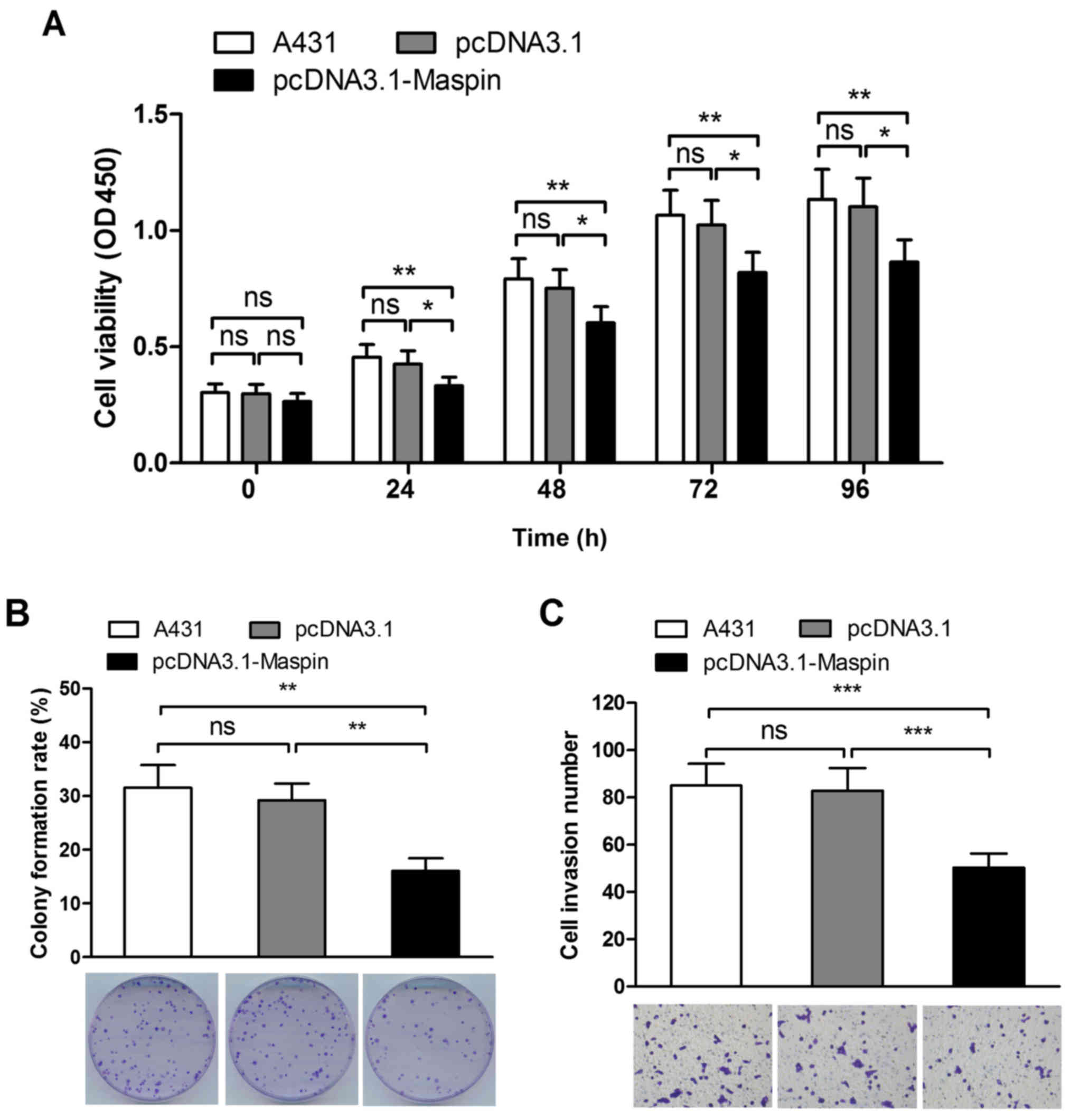
CCK-8 assay, colony formation assay and Transwell
assay supplemented with Matrigel in vitro were performed to
detect the cell viability, colony formation ability and invasion
potential of A431 cells. The results showed that A431 cell
viability decreased by 22%, 19%, 20% and 22% at 24 h, 48 h, 72 h
and 96 h, respectively (Fig. 3A),
the colony formation rate declined by 45% (Fig. 3B), and the invasion potential
descended by 39% as a result of elevation of Maspin (Fig. 3C). Collectively, the results
demonstrated that Maspin inhibited growth, proliferation and
invasion in A431 cells.
Maspin delays cell cycle transition
and enhances apoptosis in cSCC cells
We confirmed that Maspin expression was low in cSCC
tissues, and inhibited growth, proliferation and invasion in cSCC
cells, hence we were interested in how Maspin affects the phenotype
of cSCC cells. In order to explore the underlying mechanism, we
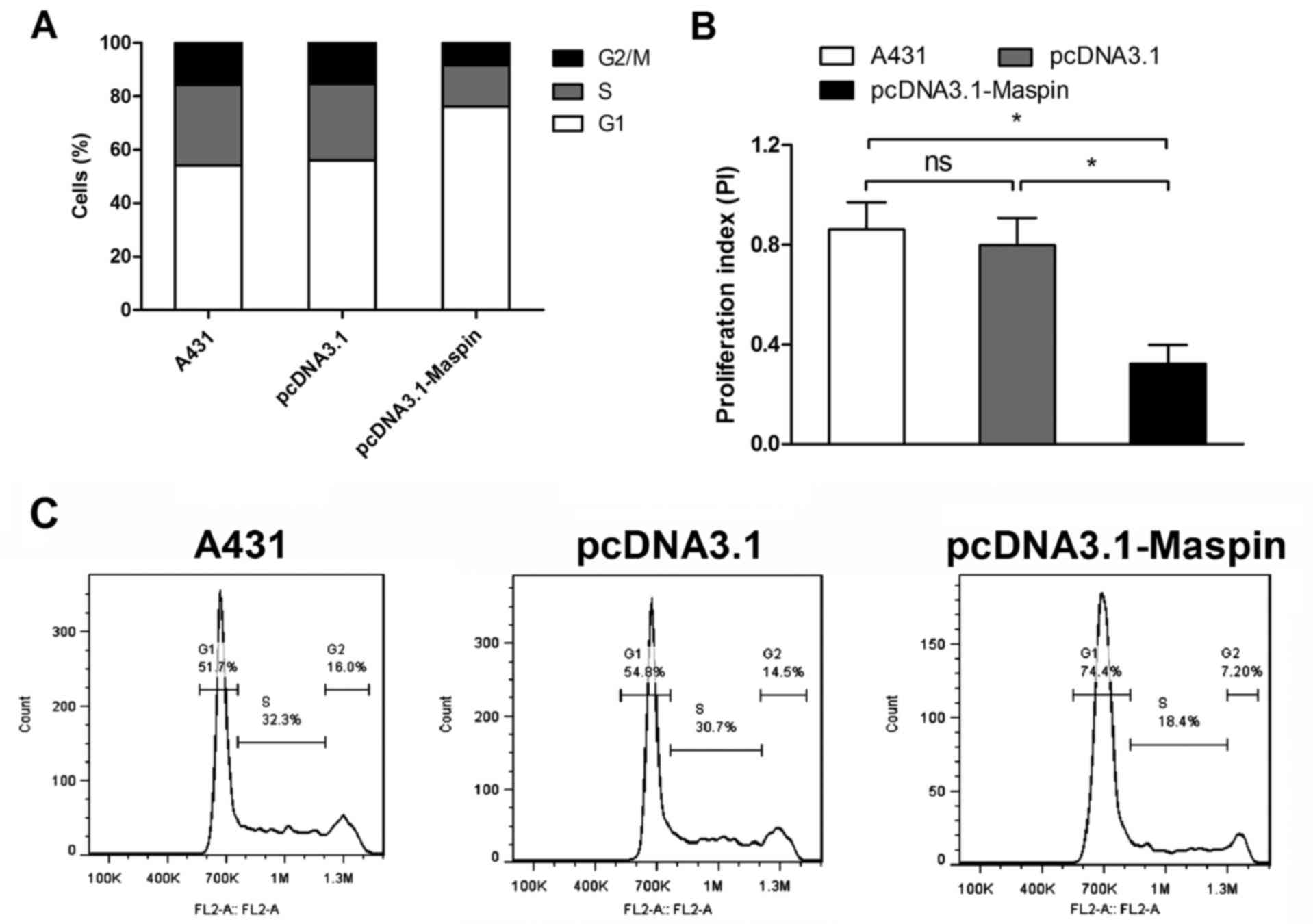
tested whether Maspin affected cell cycle transition and apoptosis
in cSCC cells. Flow cyto-metry results showed that Maspin
overexpression delayed cell cycle G1/S/G2 transition (Fig. 4A), and proliferation index (PI)
decreased by 60% in A431 cells (Fig.
4B).
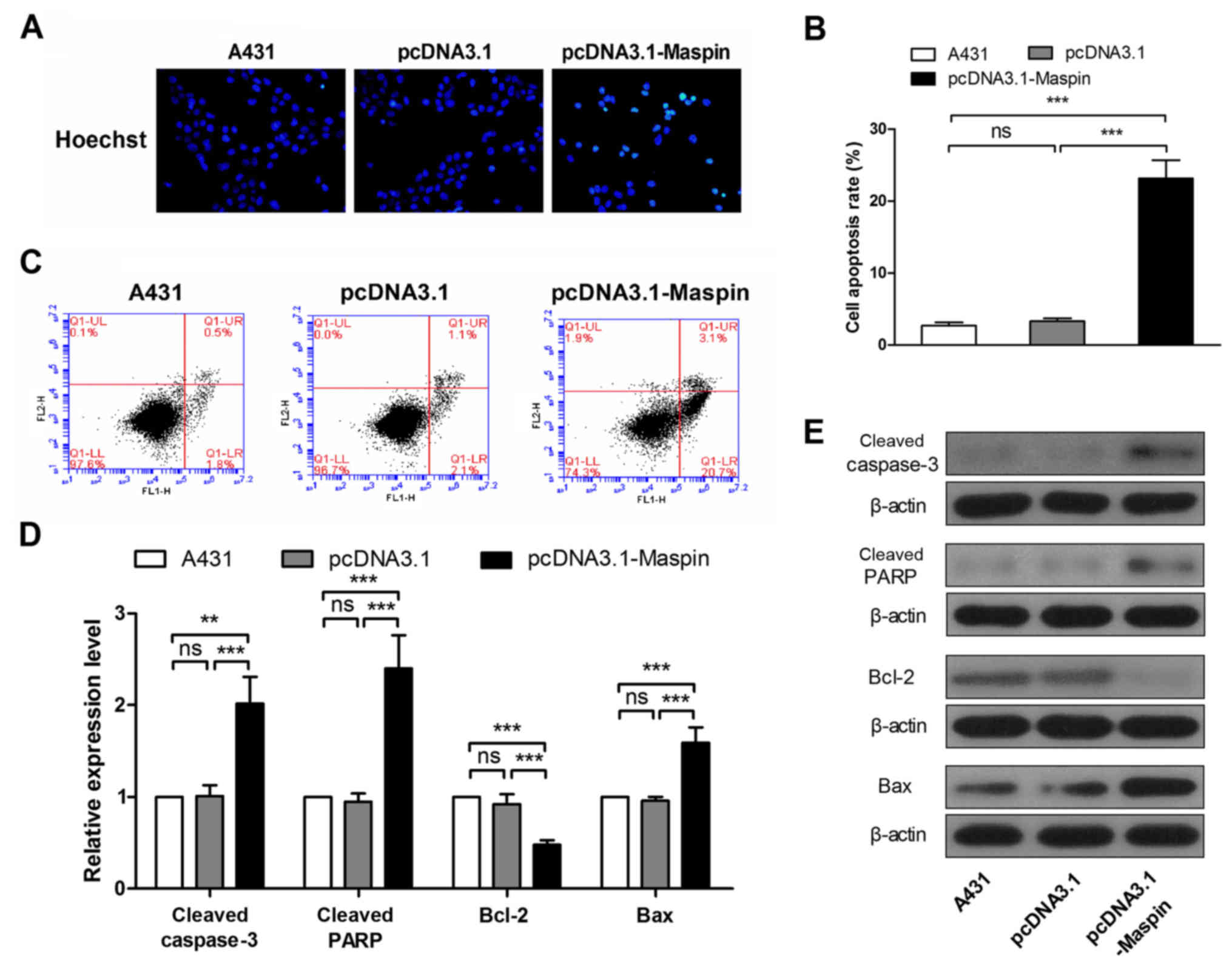
To detect the effect of Maspin on apoptosis of A431
cells, Hoechst staining, flow cytometry and western blotting were
performed. In the Hoechst staining results, Maspin overexpressed
A431 cells showed more intense fluorescence than parental and
pcDNA3.1 groups (Fig. 5A), which is
characteristic of apoptotic cells. Flow cytometry results showed
that Maspin increased cell apoptosis rate of A431 cells 7-fold
(Fig. 5B and C). In addition, we
detected several apoptosis related genes. As shown in Fig. 5, cleaved caspase-3 increased 2-fold,
cleaved PARP was upregulated 2.5-fold, Bcl-2 descended by 48%, and
Bax was elevated 1.6-fold (Fig. 5D and
E). These data suggested that Maspin enhanced apoptosis in A431
cells.
Collectively, Maspin was downregulated in cSCC
tissues, and overexpression of Maspin inhibited growth,
proliferation and invasion by delaying cell cycle transition and
enhancing apoptosis in cSCC cells.
Discussion
With a metastatic rate of 5–10% (24,25),
it is crucial for cSCC to be prevented and diagnosed in the early
stage. Detection of clinical cSCC tissue samples showed that Maspin
was significantly lowly expressed in cSCC tissues, suggesting that
Maspin may be a practicable biomarker. Considering the infiltration
and invasion, we chose the adjacent tissues 1 cm from cSCC tissue
as the control to make the results more reliable.
Proliferation and metastasis of tumor cells are the
crucial aspects of pathogenesis of malignant tumors. Our study
demonstrated that Maspin overexpression inhibited cell growth,
proliferation and invasion by delaying cell cycle transition and
enhancing apoptosis in cSCC cells. Cells in different interphases
could be distinguished by flow cytometry after staining with
propidium iodide due to different amounts of DNA. By counting
number of cells in different interphases and calculating the
proliferation index (PI), we can determine the cell cycle process
and compare the cell division speed among various groups. Apoptosis
involves many aspects, detected by various methods. When apoptosis
occurs, phosphatidylserine (PS) located in the inner cytomembrane
moved to the external cytomembrane, which could be specially
combined by Annexin V, thus early apoptosis could be discovered by
testing PS using flow cytometry (26). With apoptosis further proceeding,
and through staining, condensed chromatin, nuclear fragmentation
and apoptotic bodies in apoptotic cells exhibited intense
fluorescence under a fluorescence microscope (27).
In addition, apoptosis involves a variety of
signaling pathways, expression change of genes in these signaling
pathways would suggest the occurrence of apoptosis. Cysteinyl
aspartate specific protease (caspase) is a protease family,
including 11 members, with vital functions in apoptosis (28). Caspase family members are classified
as initiators (caspase-1, −2, −4, −5, −8, −9, −10, −11 and −12) and
executioners (caspase-3, −6, −7 and −14) (29). In normal cells, all caspase members
are present as precursors without protease activity. When the
extrinsic apoptotic signal enters the cell, the initiator (mainly
caspase-8 and −10) is self-cleaved and activated via allosteric
cleavage and activates the executioner (mainly caspase-3) (30,31).
The cleaved caspase-3 shears and activates PARP, causing apoptosis
(32). On the other hand,
executioner caspase also could be actived by intrinsic signals,
such as DNA damage, growth factor deprivation and endoplasmic
reticulum (ER) stress, which are regulated by members of B-cell
lymphoma-2 (Bcl-2) family (33).
Bcl-2 is one of the most important genes in apoptosis. Twenty-five
homologous proteins have been found in Bcl-2 family to date,
divided into two categories: anti-apoptotic proteins, including
Bcl-2, Bcl-XL, Bcl-W, and Mcl-1; pro-apoptotic proteins, including
Bax, Bak, Bim, and Bad (34).
Pro-apoptotic protein receives intrinsic signals and induces
downstream mediators (Bax and Bak), leading to the mitochondrial
membrane permeability changes and the release of apoptogenic
compound such as cytochrome c, which binds APAF-1 and
activates caspase-9, further activating caspase-3 (35,36).
In this study, we demonstrated that Maspin participated in
apoptosis signal pathway to enhance apoptosis, which was consistent
with previous reports in other models (17,18).
Since first reported in 1994 (14), Maspin has been studied widely, and
its antitumor effect has been proved in many cell carcinoma and
adenocarcinoma. Maspin suppresses cell proliferation, invasion and
EMT, enhances apoptosis, repairs DNA damage (37), inhibits angiogenesis and elicits
host antitumor immunity (19).
During these processes, multiple genes play roles, including Bcl-2,
Bax (17), p53, phosphatase and
tensin homolog deleted on chromosome ten (PTEN) (38) and miRNA (39,40),
suggesting that Maspin participates in multiple signaling pathways.
Since secreted by keratinocytes, Maspin is closely related to the
function and fate of epidermal cells and keratinocytes (20). The role of Maspin in skin diseases
has attracted increased attention. It has been reported that the
survival rate of Maspin-positive cSCC patients is much higher than
Maspin-negative patients (16), but
the mechanism is unknown.
Our study found that Maspin was lower expressed in
the clinical cSCC tissues than the adjacent normal tissues. We
overexpressed Maspin in A431 cells, in which Maspin was
downregulated, and demonstrated that the overexpression of Maspin
inhibited cell growth, proliferation and invasion by suppressing
cell cycle transition and enhancing apoptosis in cSCC cells. These
findings may be of significance for the diagnosis and therapy of
cSCC.
References
|
1
|
Lohmann CM and Solomon AR:
Clinicopathologic variants of cutaneous squamous cell carcinoma.
Adv Anat Pathol. 8:27–36. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Alam M and Ratner D: Cutaneous
squamous-cell carcinoma. N Engl J Med. 344:975–983. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bonerandi JJ, Beauvillain C, Caquant L,
Chassagne JF, Chaussade V, Clavère P, Desouches C, Garnier F,
Grolleau JL, Grossin M, et al: French Dermatology Recommendations
Association (aRED): Guidelines for the diagnosis and treatment of
cutaneous squamous cell carcinoma and precursor lesions. J Eur Acad
Dermatol Venereol. 25:(Suppl 5). 1–51. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Aboutalebi S and Strickland FM: Immune
protection, natural products, and skin cancer: Is there anything
new under the sun? J Drugs Dermatol. 5:512–517. 2006.PubMed/NCBI
|
|
5
|
Weinberg AS, Ogle CA and Shim EK:
Metastatic cutaneous squamous cell carcinoma: An update. Dermatol
Surg. 33:885–899. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lippman SM, Parkinson DR, Itri LM, Weber
RS, Schantz SP, Ota DM, Schusterman MA, Krakoff IH, Gutterman JU
and Hong WK: 13-cis-retinoic acid and interferon alpha-2a:
Effective combination therapy for advanced squamous cell carcinoma
of the skin. J Natl Cancer Inst. 84:235–241. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kwa RE, Campana K and Moy RL: Biology of
cutaneous squamous cell carcinoma. J Am Acad Dermatol. 26:1–26.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tanvetyanon T, Padhya T, McCaffrey J, Kish
JA, Deconti RC, Trotti A and Rao NG: Postoperative concurrent
chemotherapy and radiotherapy for high-risk cutaneous squamous cell
carcinoma of the head and neck. Head Neck. 37:840–845. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
El-Maqsoud Abd NM and Tawfiek ER: Loss of
Maspin expression in bladder cancer: Its relationship with p53 and
clinicopathological parameters. J Egypt Natl Canc Inst. 22:1–12.
2010.PubMed/NCBI
|
|
10
|
Hopkins PC and Whisstock J: Function of
maspin. Science. 265:1893–1894. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pemberton PA, Wong DT, Gibson HL, Kiefer
MC, Fitzpatrick PA, Sager R and Barr PJ: The tumor suppressor
maspin does not undergo the stressed to relaxed transition or
inhibit trypsin-like serine proteases. Evidence that maspin is not
a protease inhibitory serpin. J Biol Chem. 270:15832–15837. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Al-Ayyoubi M, Gettins PG and Volz K:
Crystal structure of human maspin, a serpin with antitumor
properties: Reactive center loop of maspin is exposed but
constrained. J Biol Chem. 279:55540–55544. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bodenstine TM, Seftor RE, Khalkhali-Ellis
Z, Seftor EA, Pemberton PA and Hendrix MJ: Maspin: Molecular
mechanisms and therapeutic implications. Cancer Metastasis Rev.
31:529–551. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zou Z, Anisowicz A, Hendrix MJ, Thor A,
Neveu M, Sheng S, Rafidi K, Seftor E and Sager R: Maspin, a serpin
with tumor-suppressing activity in human mammary epithelial cells.
Science. 263:526–529. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cai Z, Zhou Y, Lei T, Chiu JF and He QY:
Mammary serine protease inhibitor inhibits epithelial growth
factor-induced epithelial-mesenchymal transition of esophageal
carcinoma cells. Cancer. 115:36–48. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu S, Yu L, Cheng Z, Song W, Zhou L and
Tao Y: Expression of maspin in non-small cell lung cancer and its
relationship to vasculogenic mimicry. J Huazhong Univ Sci Technolog
Med Sci. 32:346–352. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang W, Shi HY and Zhang M: Maspin
overexpression modulates tumor cell apoptosis through the
regulation of Bcl-2 family proteins. BMC Cancer. 5:502005.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li X, Chen D, Yin S, Meng Y, Yang H,
Landis-Piwowar KR, Li Y, Sarkar FH, Reddy GP, Dou QP, et al: Maspin
augments proteasome inhibitor-induced apoptosis in prostate cancer
cells. J Cell Physiol. 212:298–306. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dzinic SH, Chen K, Thakur A, Kaplun A,
Bonfil RD, Li X, Liu J, Bernardo MM, Saliganan A, Back JB, et al:
Maspin expression in prostate tumor elicits host anti-tumor
immunity. Oncotarget. 5:11225–11236. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Reis-Filho JS, Torio B, Albergaria A and
Schmitt FC: Maspin expression in normal skin and usual cutaneous
carcinomas. Virchows Arch. 441:551–558. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Abdou AG, Maraee AH, El-Monaem Shoeib MA
and Abo Saida AM: Maspin expression in epithelial skin tumours: An
immunohistochemical study. J Cutan Aesthet Surg. 4:111–117. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang JH, Zhang L, Ma YW, Xiao J, Zhang Y,
Liu M and Tang H: microRNA-34a-upregulated retinoic acid-inducible
gene-I promotes apoptosis and delays cell cycle transition in
cervical cancer cells. DNA Cell Biol. 35:267–279. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cassarino DS, Derienzo DP and Barr RJ:
Cutaneous squamous cell carcinoma: A comprehensive
clinicopathologic classification. Part one. J Cutan Pathol.
33:191–206. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cassarino DS, Derienzo DP and Barr RJ:
Cutaneous squamous cell carcinoma: A comprehensive
clinicopathologic classification - part two. J Cutan Pathol.
33:261–279. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Demchenko AP: The change of cellular
membranes on apoptosis: Fluorescence detection. Exp Oncol.
34:263–268. 2012.PubMed/NCBI
|
|
27
|
Bounda GA, Zhou W, Wang DD and Yu F: Rhein
elicits in vitro cytotoxicity in primary human liver HL-7702 cells
by inducing apoptosis through mitochondria-mediated pathway. Evid
Based Complement Alternat Med. 2015:3298312015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nicholson DW and Thornberry NA: Caspases:
Killer proteases. Trends Biochem Sci. 22:299–306. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yi CH and Yuan J: The Jekyll and Hyde
functions of caspases. Dev Cell. 16:21–34. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sarvothaman S, Undi RB, Pasupuleti SR,
Gutti U and Gutti RK: Apoptosis: Role in myeloid cell development.
Blood Res. 50:73–79. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Snigdha S, Smith ED, Prieto GA and Cotman
CW: Caspase-3 activation as a bifurcation point between plasticity
and cell death. Neurosci Bull. 28:14–24. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gerö D and Szabó C: Poly(ADP-ribose)
polymerase: A new therapeutic target? Curr Opin Anaesthesiol.
21:111–121. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wei MC, Zong WX, Cheng EH, Lindsten T,
Panoutsakopoulou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB and
Korsmeyer SJ: Proapoptotic BAX and BAK: A requisite gateway to
mitochondrial dysfunction and death. Science. 292:727–730. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Besbes S, Mirshahi M, Pocard M and Billard
C: New dimension in therapeutic targeting of BCL-2 family proteins.
Oncotarget. 6:12862–12871. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Danial NN and Korsmeyer SJ: Cell death:
Critical control points. Cell. 116:205–219. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Nagata Y, Nagahisa H, Aida Y, Okutomi K,
Nagasawa T and Todokoro K: Thrombopoietin induces megakaryocyte
differentiation in hematopoietic progenitor FDC-P2 cells. J Biol
Chem. 270:19673–19675. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zou Z, Gao C, Nagaich AK, Connell T, Saito
S, Moul JW, Seth P, Appella E and Srivastava S: p53 regulates the
expression of the tumor suppressor gene maspin. J Biol Chem.
275:6051–6054. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Eitel JA, Bijangi-Vishehsaraei K,
Saadatzadeh MR, Bhavsar JR, Murphy MP, Pollok KE and Mayo LD: PTEN
and p53 are required for hypoxia induced expression of maspin in
glioblastoma cells. Cell Cycle. 8:896–901. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chen WS, Yen CJ, Chen YJ, Chen JY, Wang
LY, Chiu SJ, Shih WL, Ho CY, Wei TT, Pan HL, et al: miRNA-7/21/107
contribute to HBx-induced hepatocellular carcinoma progression
through suppression of maspin. Oncotarget. 6:25962–25974. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yuan K, Xie K, Fox J, Zeng H, Gao H, Huang
C and Wu M: Decreased levels of miR-224 and the passenger strand of
miR-221 increase MBD2, suppressing maspin and promoting colorectal
tumor growth and metastasis in mice. Gastroenterology. 145:853–864,
e859. 2013. View Article : Google Scholar : PubMed/NCBI
|