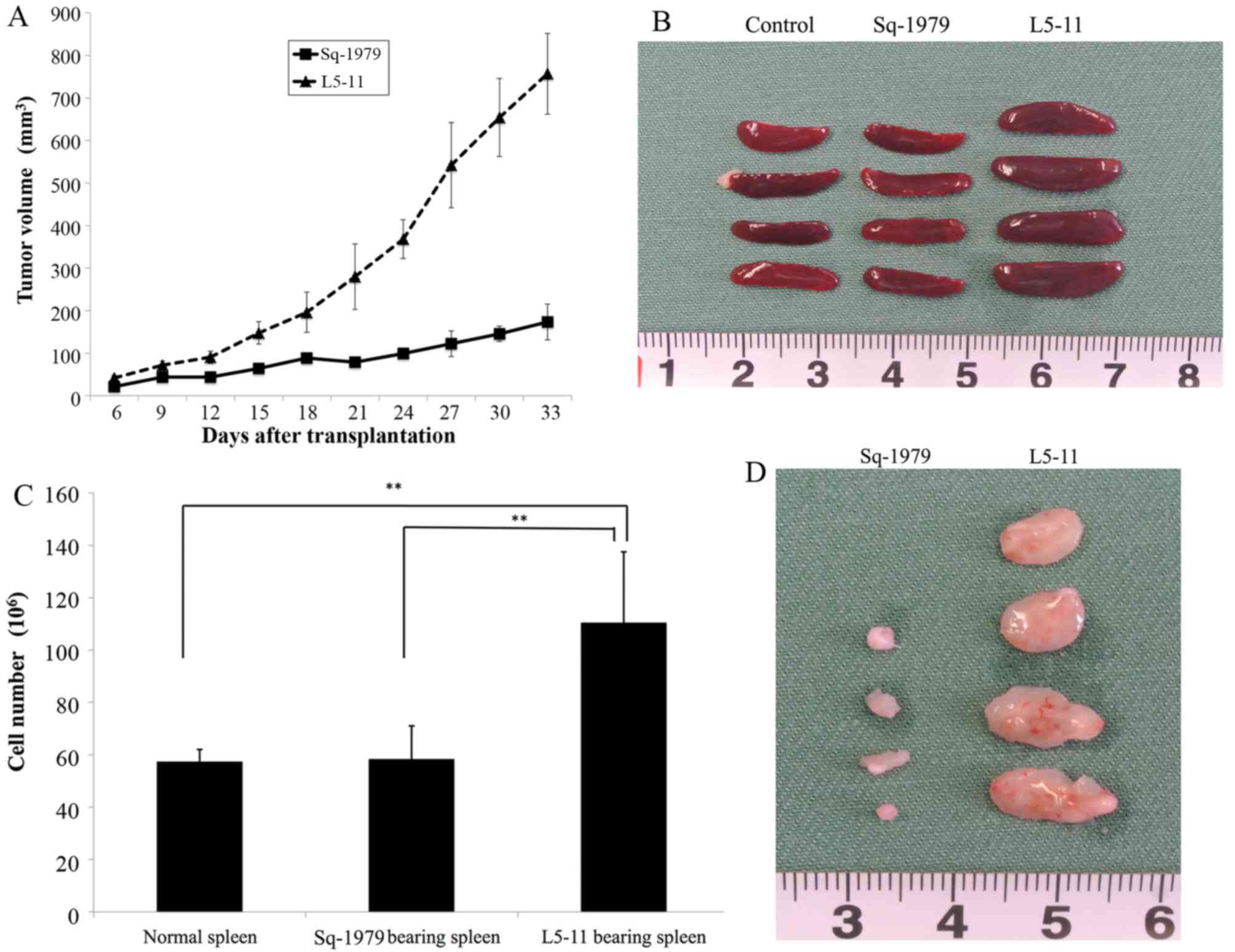
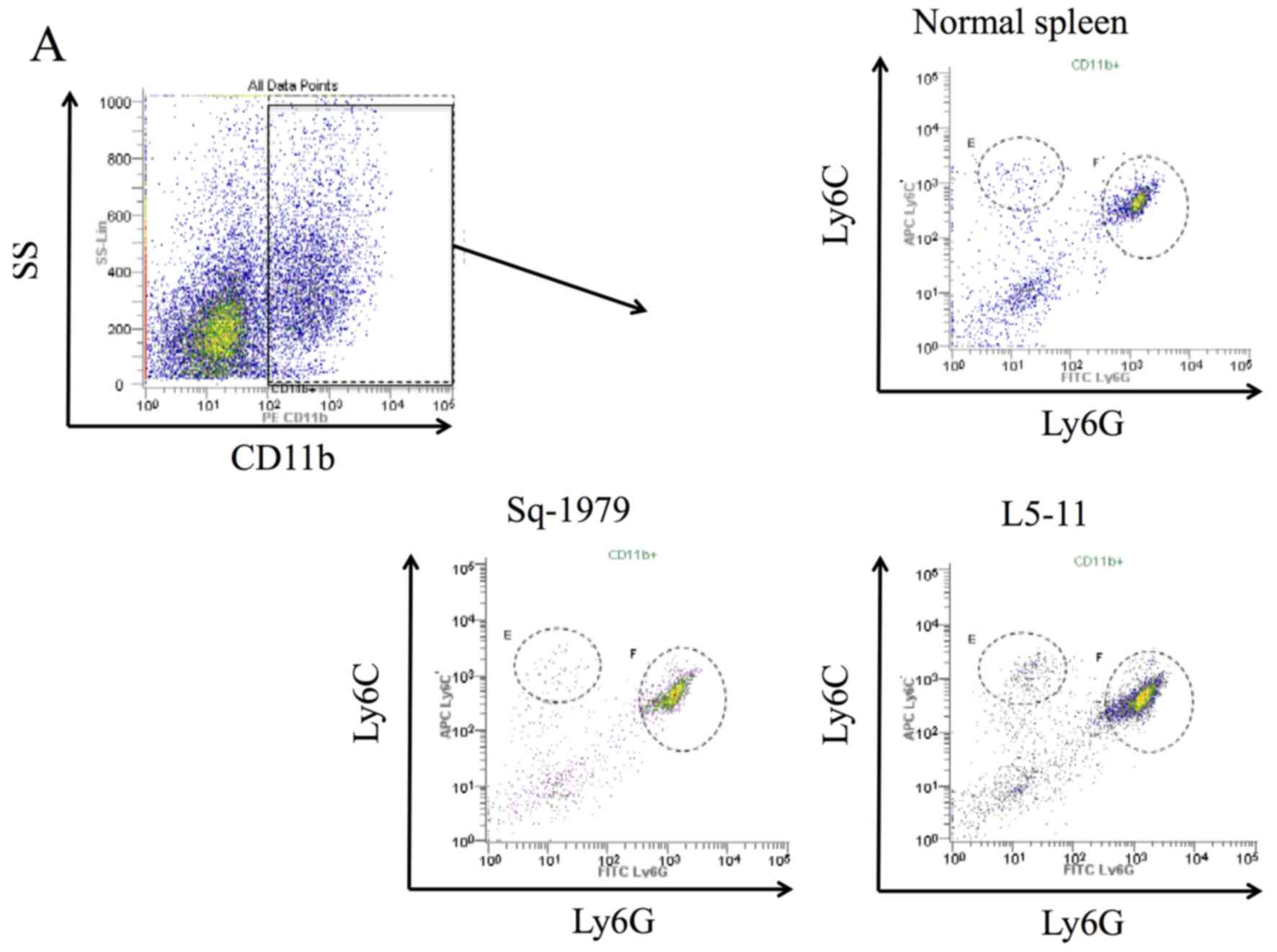
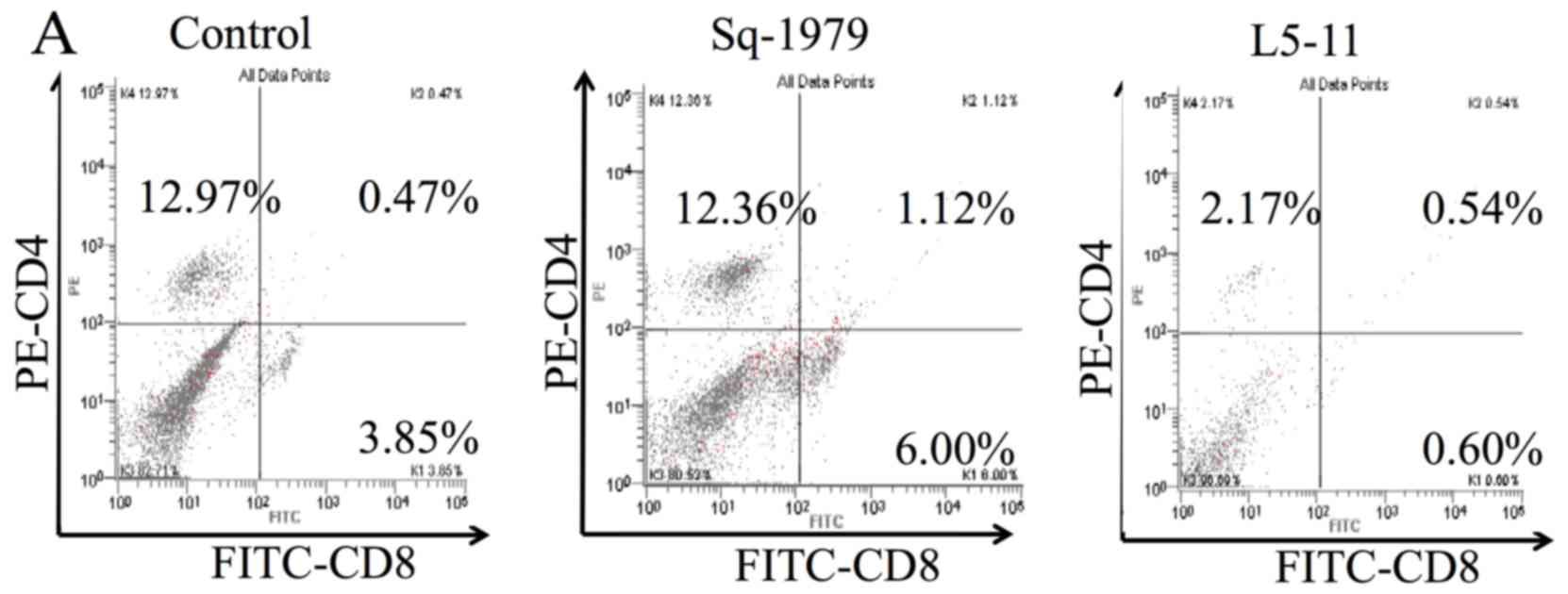
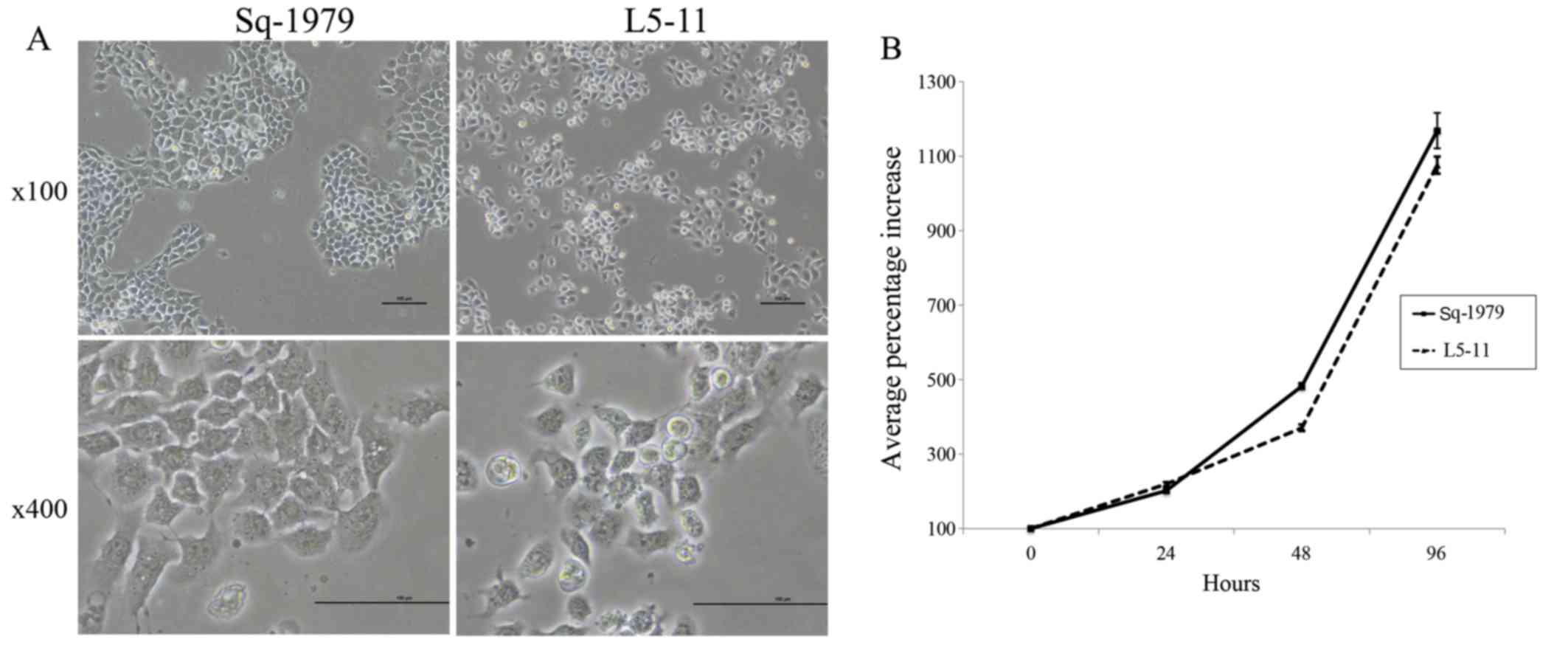
|
1
|
Kalnins IK, Leonard AG, Sako K, Razack MS
and Shedd DP: Correlation between prognosis and degree of lymph
node involvement in carcinoma of the oral cavity. Am J Surg.
134:450–454. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Grandi C, Alloisio M, Moglia D, Podrecca
S, Sala L, Salvatori P and Molinari R: Prognostic significance of
lymphatic spread in head and neck carcinomas: Therapeutic
implications. Head Neck Surg. 8:67–73. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yu CC, Chen PN, Peng CY, Yu CH and Chou
MY: Suppression of miR-204 enables oral squamous cell carcinomas to
promote cancer stemness, EMT traits, and lymph node metastasis.
Oncotarget. 7:20180–20192. 2016.PubMed/NCBI
|
|
4
|
Ohnishi K, Yamaguchi M, Erdenebaatar C,
Saito F, Tashiro H, Katabuchi H, Takeya M and Komohara Y:
Prognostic significance of CD169-positive lymph node sinus
macrophages in patients with endometrial carcinoma. Cancer Sci.
107:846–852. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jung EJ, Santarpia L, Kim J, Esteva FJ,
Moretti E, Buzdar AU, Di Leo A, Le XF, Bast RC Jr, Park ST, et al:
Plasma microRNA 210 levels correlate with sensitivity to
trastuzumab and tumor presence in breast cancer patients. Cancer.
118:2603–2614. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Condamine T, Ramachandran I, Youn JI and
Gabrilovich DI: Regulation of tumor metastasis by myeloid-derived
suppressor cells. Annu Rev Med. 66:97–110. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Heim CE, Vidlak D, Scherr TD, Kozel JA,
Holzapfel M, Muirhead DE and Kielian T: Myeloid-derived suppressor
cells contribute to Staphylococcus aureus orthopedic biofilm
infection. J Immunol. 192:3778–3792. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gabrilovich DI and Nagaraj S:
Myeloid-derived suppressor cells as regulators of the immune
system. Nat Rev Immunol. 9:162–174. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Youn JI, Kumar V, Collazo M, Nefedova Y,
Condamine T, Cheng P, Villagra A, Antonia S, McCaffrey JC, Fishman
M, et al: Epigenetic silencing of retinoblastoma gene regulates
pathologic differentiation of myeloid cells in cancer. Nat Immunol.
14:211–220. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nagaraj S, Schrum AG, Cho HI, Celis E and
Gabrilovich DI: Mechanism of T cell tolerance induced by
myeloid-derived suppressor cells. J Immunol. 184:3106–3116. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Weiss JM, Subleski JJ, Back T, Chen X,
Watkins SK, Yagita H, Sayers TJ, Murphy WJ and Wiltrout RH:
Regulatory T cells and myeloid-derived suppressor cells in the
tumor microenvironment undergo Fas-dependent cell death during
IL-2/alphaCD40 therapy. J Immunol. 192:5821–5829. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Marvel D and Gabrilovich DI:
Myeloid-derived suppressor cells in the tumor microenvironment:
Expect the unexpected. J Clin Invest. 125:3356–3364. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Corzo CA, Condamine T, Lu L, Cotter MJ,
Youn JI, Cheng P, Cho HI, Celis E, Quiceno DG, Padhya T, et al:
HIF-1α regulates function and differentiation of myeloid-derived
suppressor cells in the tumor microenvironment. J Exp Med.
207:2439–2453. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Khaled YS, Ammori BJ and Elkord E:
Increased levels of granulocytic myeloid-derived suppressor cells
in peripheral blood and tumour tissue of pancreatic cancer
patients. J Immunol Res. 2014:8798972014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Walter S, Weinschenk T, Stenzl A, Zdrojowy
R, Pluzanska A, Szczylik C, Staehler M, Brugger W, Dietrich PY,
Mendrzyk R, et al: Multipeptide immune response to cancer vaccine
IMA901 after single-dose cyclophosphamide associates with longer
patient survival. Nat Med. 18:1254–1261. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Youn JI, Collazo M, Shalova IN, Biswas SK
and Gabrilovich DI: Characterization of the nature of granulocytic
myeloid-derived suppressor cells in tumor-bearing mice. J Leukoc
Biol. 91:167–181. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wynn TA: Myeloid-cell differentiation
redefined in cancer. Nat Immunol. 14:197–199. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Haverkamp JM, Smith AM, Weinlich R, Dillon
CP, Qualls JE, Neale G, Koss B, Kim Y, Bronte V, Herold MJ, et al:
Myeloid-derived suppressor activity is mediated by monocytic
lineages maintained by continuous inhibition of extrinsic and
intrinsic death pathways. Immunity. 41:947–959. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bronte V, Brandau S, Chen SH, Colombo MP,
Frey AB, Greten TF, Mandruzzato S, Murray PJ, Ochoa A,
Ostrand-Rosenberg S, et al: Recommendations for myeloid-derived
suppressor cell nomenclature and characterization standards. Nat
Commun. 7:121502016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schouppe E, Mommer C, Movahedi K, Laoui D,
Morias Y, Gysemans C, Luyckx A, De Baetselier P and Van
Ginderachter JA: Tumor-induced myeloid-derived suppressor cell
subsets exert either inhibitory or stimulatory effects on distinct
CD8+ T-cell activation events. Eur J Immunol.
43:2930–2942. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Luo M, Hou L, Li J, Shao S, Huang S, Meng
D, Liu L, Feng L, Xia P, Qin T, et al: VEGF/NRP-1axis promotes
progression of breast cancer via enhancement of
epithelial-mesenchymal transition and activation of NF-κB and
β-catenin. Cancer Lett. 373:1–11. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Goel HL and Mercurio AM: VEGF targets the
tumour cell. Nat Rev Cancer. 13:871–882. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
De Francesco EM, Lappano R, Santolla MF,
Marsico S, Caruso A and Maggiolini M: HIF-1α/GPER signaling
mediates the expression of VEGF induced by hypoxia in breast cancer
associated fibroblasts (CAFs). Breast Cancer Res. 15:R642013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Noman MZ, Desantis G, Janji B, Hasmim M,
Karray S, Dessen P, Bronte V and Chouaib S: PD-L1 is a novel direct
target of HIF-1α, and its blockade under hypoxia enhanced
MDSC-mediated T cell activation. J Exp Med. 211:781–790. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen X, Eksioglu EA, Zhou J, Zhang L, Djeu
J, Fortenbery N, Epling-Burnette P, Van Bijnen S, Dolstra H, Cannon
J, et al: Induction of myelodysplasia by myeloid-derived suppressor
cells. J Clin Invest. 123:4595–4611. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Miao HQ, Lee P, Lin H, Soker S and
Klagsbrun M: Neuropilin-1 expression by tumor cells promotes tumor
angiogenesis and progression. FASEB J. 14:2532–2539. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Casazza A, Laoui D, Wenes M, Rizzolio S,
Bassani N, Mambretti M, Deschoemaeker S, Van Ginderachter JA,
Tamagnone L and Mazzone M: Impeding macrophage entry into hypoxic
tumor areas by Sema3A/Nrp1 signaling blockade inhibits angiogenesis
and restores antitumor immunity. Cancer Cell. 24:695–709. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hu ZQ, Zhou SL, Zhou ZJ, Luo CB, Chen EB,
Zhan H, Wang PC, Dai Z, Zhou J, Fan J, et al: Overexpression of
semaphorin 3A promotes tumor progression and predicts poor
prognosis in hepatocellular carcinoma after curative resection.
Oncotarget. 7:51733–51746. 2016.PubMed/NCBI
|