Introduction
Malignant tumors are the primary cause of death from
disease in developed nations (1).
The main treatment options for tumors are surgery and chemo- and
radiation therapy (2), which yield
good results (3). Nonetheless,
these treatments also have various side effects (4). Therefore, there is a need for novel
antitumor therapies that are not associated with side effects or
complications.
Carbon dioxide (CO2) has been mainly used
as a treatment for peripheral vascular disorders (5). The benefits of a carbonated spa have
long been known in Europe (6), and
are still enjoyed in many countries (7). Bathing in artificial
CO2-enriched water has been shown to improve ischemic
limb symptoms (8). CO2
exerts therapeutic effects by stimulating blood flow and
microcirculation (9) to increase
partial O2 pressure in local tissue, which is known as
the Bohr effect (10). We
previously investigated whether the Bohr effect can be induced by
transcutaneous CO2 application using 100% CO2
gas and CO2 absorption-enhancing hydrogel in humans
(11). We showed that
transcutaneous application of CO2 to the lower limbs in
rats for three months activated the expression of peroxisome
proliferator-activated receptor gamma co-activator 1α in the
tibialis anterior muscle, and increased the number of mitochondria
in skeletal muscles, even in malignant tumor tissues (12–14).
We also found that this CO2 treatment could induce
mitochondrial apoptosis in human malignant fibrous
histiocytoma/undifferentiated pleomorphic sarcoma (MFH/UPS)
(13), murine osteosarcoma
(15), and human oral squamous cell
carcinoma (14) without any side
effects such as loss of body weight and induction of
metastasis.
These results suggest that percutaneous
CO2 treatment can be used as an antitumor therapy.
However, before initiating clinical trials, the optimal conditions,
including the duration and frequency of transcutaneous
CO2 application, must be established to decrease tumor
volume and induce apoptosis in tumor cells. The present study
represents a preclinical test to investigate the antitumor effects
of transcutaneously applied CO2, against three types of
human tumors, with regard to treatment conditions including
duration, frequency, and site of CO2 exposure, in mouse
xenograft models.
Materials and methods
Cell lines
MDA-MB-231 human breast cancer cells (American Type
Culture Collection (ATCC), Rockville, MD, USA) (16), MG63 human osteosarcoma cells (ATCC)
(17), and Nara-H human MFH/UPS
cells (ScienStuff Co., Nara, Japan) (13,18)
were maintained in Dulbecco's modified Eagle's medium supplemented
with 10% (v/v) fetal bovine serum and 100 U/ml
penicillin/streptomycin solution (all from Sigma-Aldrich, St.
Louis, MO, USA) at 37°C in a humidified atmosphere of 5%
CO2.
Animal models
Male, athymic BALB/c nude mice (5–8 weeks old) were
obtained from CLEA Japan (Tokyo, Japan). The animals were
maintained under pathogen-free conditions; experiments were
performed in accordance with the Guidelines for Animal
Experimentation of Kobe University Graduate School of Medicine and
Kobe University Animal Experimentation Regulations (permission nos.
P110905-R1 and P-101203) and were approved by the Institutional
Animal Care and Use Committee. To create human tumor xenograft
models, MDA-MB-231 (3.0×106), MG63 (5.0×106),
and Nara-H (5.0×106) cells in 500 µl of
phosphate-buffered saline (PBS) were injected into the dorsal
subcutaneous area of mice, as previously described (13–15).
CO2 treatment was initiated after cell implantation when
the tumors were of a measurable size. Tumor volume and body weight
were monitored twice weekly until the end of the treatment. Tumor
volume was calculated as described previously (13,18),
using the formula: V = π/6 × a2 x b, where a and b
represent the shorter and longer dimensions of the tumor,
respectively.
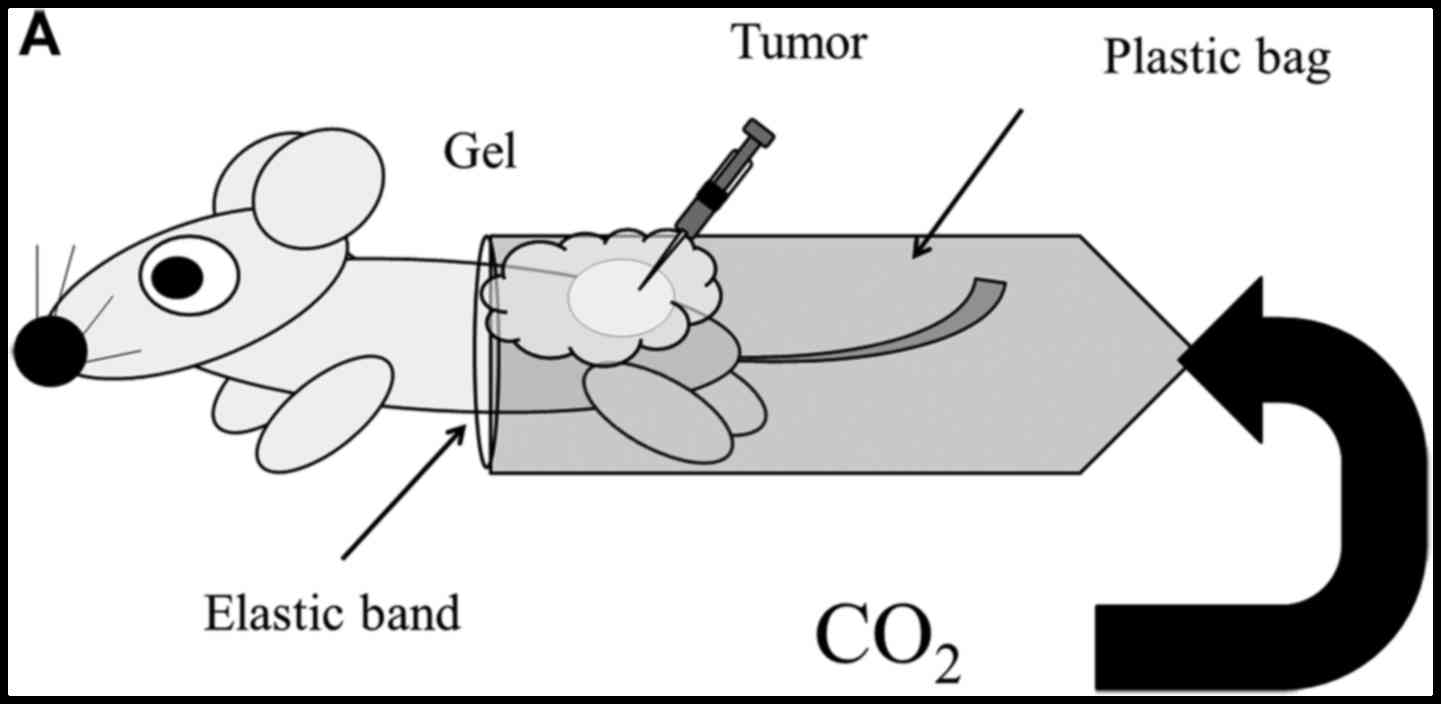
Transcutaneous CO2
application
For treatment, CO2 was administered
transcutaneously, as described previously (12–15).
Briefly, the area of skin around the implanted tumor was treated
with a CO2 hydrogel. The area was then sealed with a
polyethylene bag, and 100% CO2 gas was delivered into
the bag.
Effect of treatment duration on
different tumor types
Based on the average tumor volume after the tumors
reached a measurable size, 24 mice for each cell line were randomly
divided into four groups of six mice each; these groups received
the treatment for 0, 5, 10, or 20 min per application, with the
applications performed twice weekly for 2 weeks (12–15).
Results are shown as the ratio of the final tumor volume to the
corresponding pre-treatment value.
Effect of treatment frequency and
interval
In total, 18 mice with Nara-H cell implantation were
randomly divided into three groups of six mice each; one group was
a control, whereas the other groups received the treatment twice
per week or five times per week. Mice were treated with
CO2 for 10 min at the specified frequency for 1 week
starting 4 days after cell implantation (Fig. 1A). At the completion of treatment,
the ratio of tumor volume after the treatment compared to that
before the treatment was calculated.
To assess the effect of treatment interval,
CO2-treatment was performed twice per week for 2 weeks,
using two different treatment intervals: 2 and 5 day intervals (2 +
5 interval group) or 3 and 4 day intervals (3 + 4 interval group),
as shown in Fig. 1B. For each
treatment interval, 18 mice with Nara-H cell implantation were
divided into two groups of nine mice each as follows: control group
for the 2 + 5 interval (n=9), CO2-treatment group for
the 2 + 5 interval (n=9), control group for the 3 + 4 interval
(n=9), and CO2-treatment group for the 3 + 4 interval
(n=9). At the completion of treatment, the ratio of tumor volume
relative to that of the control was calculated.
Effect of treatment application
site
We also investigated whether CO2
application had antitumor effects at distant sites. Nara-H cells
were implanted into the upper back of 12 mice, and the mice were
randomly divided into two groups of six mice each: the control and
CO2 groups. After 4 days of cell implantation, treatment
with CO2 or air (as control) was applied to the
abdominal region, which was a location completely different from
that of the implanted tumor (Fig.
1C). CO2-treatment was performed transcutaneously
for 10 min each, twice weekly for 2 weeks.
DNA fragmentation assay
DNA fragmentation was evaluated using the APO-Direct
kit (BD Biosciences, Franklin Lakes, NJ, USA) according to the
manufacturer's protocol. Briefly, upon completion of the
treatments, the implanted tumors were excised, minced, and filtered
through a cell strainer (BD Biosciences) to obtain single-cell
suspensions. Erythrocytes were lysed in lysis buffer (BD
Biosciences), and the remaining cells were pelleted and
re-suspended in PBS. Single-cell suspensions were fixed with 1%
(v/v) paraformaldehyde and re-suspended in 70% (v/v) ice-cold
ethanol at a concentration of 1×106 cells/ml. Each cell
pellet was re-suspended in 51 µl of DNA labeling solution, and was
incubated for 60 min at 37°C. FITC dUTP-labeled cells were analyzed
using a FACS Calibur flow cytometer (BD Biosciences) with a 488 nm
argon laser (12,13,18).
Statistical analysis
Analysis of variance with a post-hoc test was
performed to compare continuous values. Differences were considered
significant at P<0.05. Data are presented as the mean ± standard
error (SE). For normally distributed data, the two-tailed t-test
was used for comparisons between groups.
Results
CO2 administration for at
least 10 min reduces tumor volume
We investigated the optimal CO2
administration time for inhibiting breast cancer, osteosarcoma, and
MFH/UPS growth in vivo, using murine xenograft models.
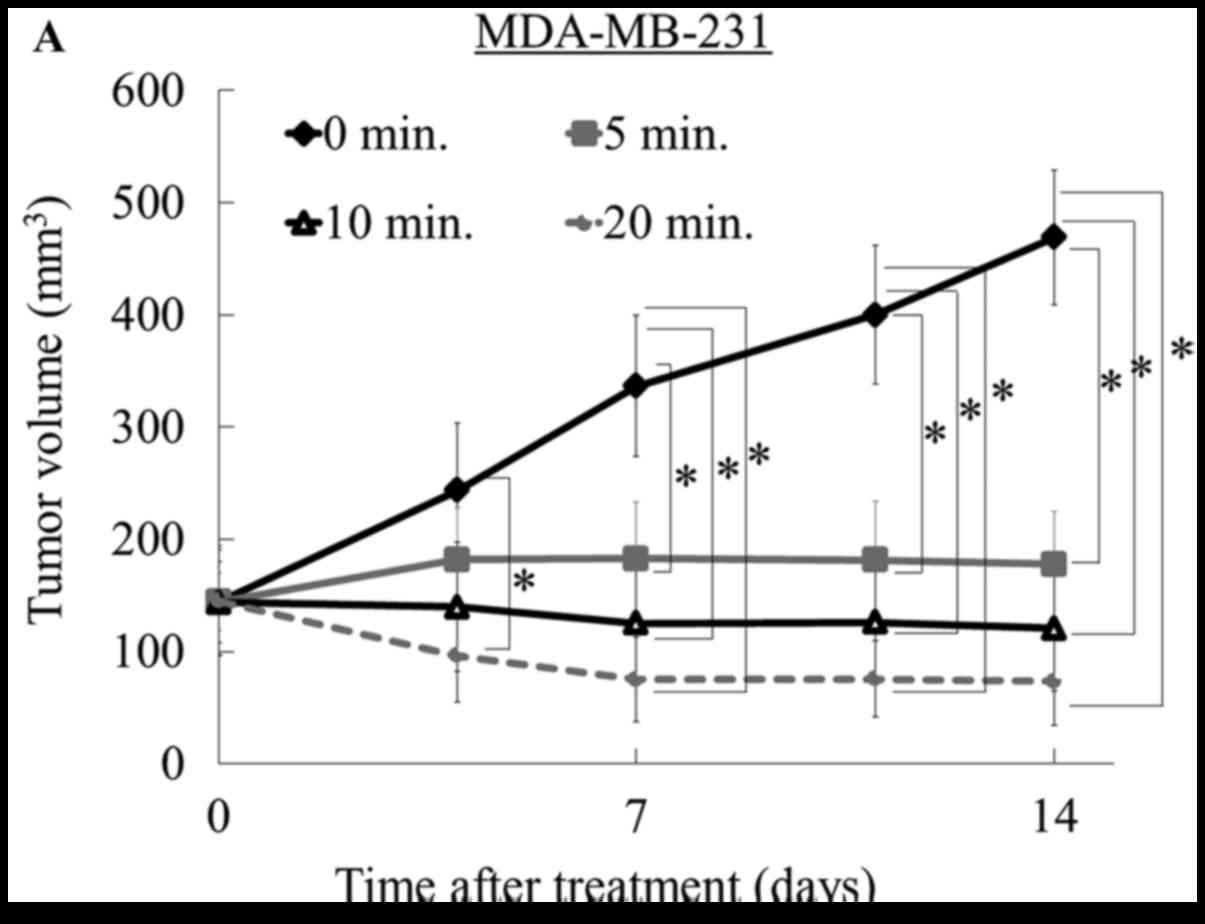
CO2-treatment times of 5, 10, or 20 min reduced tumor
volume relative to the control group (0 min) in MDA-MB-231 breast
cancer and MG63 osteosarcoma mice (P<0.05); however, there were
no differences among the three treatment groups using the
MDA-MB-231 model (Fig. 2A) or
between the 10- and 20-min groups using the MG63 model (Fig. 2B). In the Nara-H MFH/UPS model, a
significant difference compared to the control was observed in the
5-, 10-, and 20-min treatment groups, but only at the end of
treatment (P<0.05), and there was no significant difference
among the groups during the treatments (Fig. 2C). Body weight did not significantly
change during the treatments in all mice implanted with any of the
cell lines, and there was no evidence of pulmonary metastasis or
symptoms of kidney damage such as hematuria (data not shown). These
results indicate that CO2 administration has a
time-dependent inhibitory effect on breast cancer, osteosarcoma,
and MFH/UPS growth in vivo without obvious side effects.
In the current study, the most significant antitumor
effect was observed in the MDA-MB-231 breast cancer model. At the
end of treatment, all treated tumors in the MDA-MB-231 model were
smaller than pre-treated tumors, and notably, two of the six tumors
in the 20-min group had disappeared. Similarly, in the osteosarcoma
model, tumor volumes in both the 10- and 20-min treatment groups
decreased compared to the pre-treatment volume. In contrast, when
using the MFH/UPS cell model, the tumor volume was not reduced
compared to the pre-treatment volume, although tumor growth was
suppressed at all three treatment times relative to that of the
control (Fig. 2D and Table I). We assessed the effect of
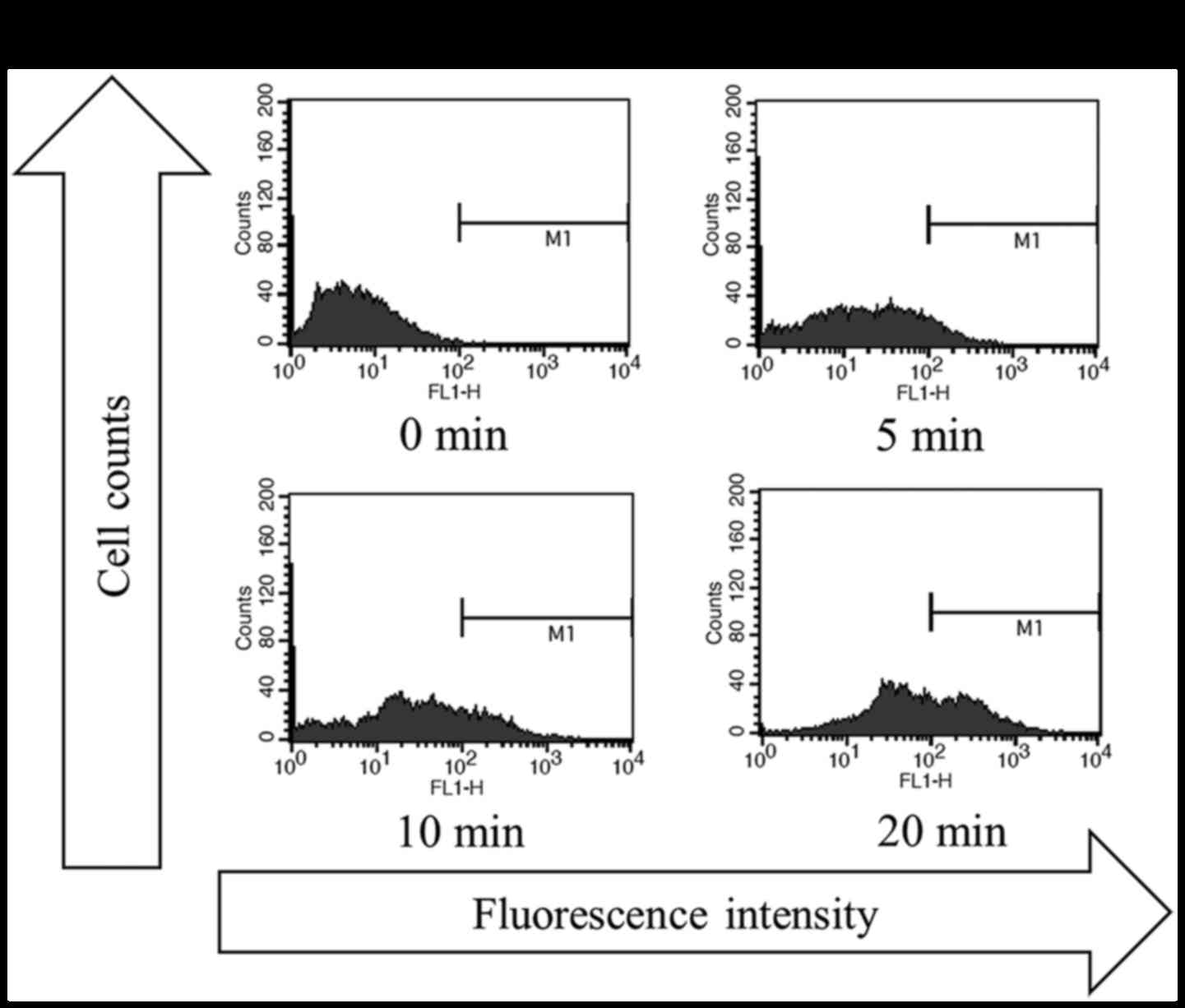
transcutaneous CO2 application on apoptotic activity in
the MFH/UPS model and found that CO2-treatment, with all
three treatment durations (5, 10, and 20 min), strongly increased
the rate of apoptosis, with a greater increase observed with longer
treatment durations (Fig. 3A and
B).
 | Table I.Antitumor effect after CO2
application with various treatment durations in each mouse. |
Table I.
Antitumor effect after CO2
application with various treatment durations in each mouse.
| Cell line | Response | 0 min | 5 min | 10 min | 20 min |
|---|
| MDA-MB-231 |
Reduceda | 0/6 | 2/6 | 4/6 | 6/6 |
| (Breast
cancer) | Lost | 0/6 | 0/6 | 1/6 | 2/6 |
| MG63 |
Reduceda | 0/6 | 2/6 | 4/6 | 6/6 |
| (Osteosarcoma) | Lost | 0/6 | 0/6 | 0/6 | 0/6 |
| Nara-H |
Reduceda | 0/6 | 0/6 | 0/6 | 0/6 |
| (MFH/UPS) | Lost | 0/6 | 0/6 | 0/6 | 0/6 |
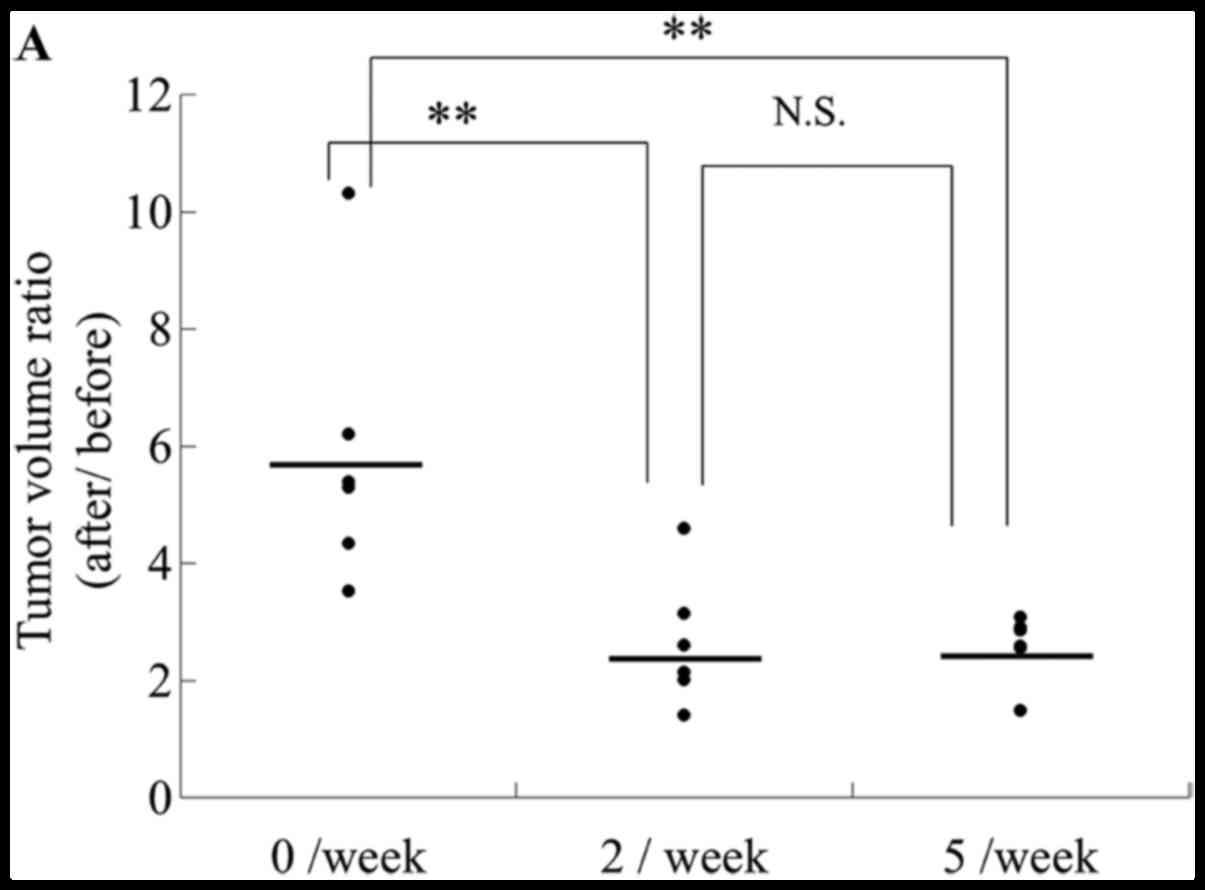
CO2 administration twice
per week at intervals of fewer than 4 days is optimal for
inhibiting tumor growth
To determine the optimal frequency of transcutaneous
CO2 application, mice were treated with CO2
at a frequency of twice or five times per week. The tumor volumes
after treatment twice or five times per week were smaller compared
to those of the control mice, and the antitumor effect was not
affected by treatment frequency; i.e., the effect was the same
regardless of whether the frequency was twice or five times per
week (Fig. 4A). These results
indicate that transcutaneous CO2 application at twice
per week has a significant antitumor effect.
Using a treatment frequency of twice per week, we
also evaluated the differences in the antitumor effect according to
the treatment interval, i.e., an interval of 2 and 5 days (2 + 5
interval) or 3 and 4 days (3 + 4 interval). As shown in Fig. 4B, the tumor volume in the 3 + 4
interval group was significantly lower than that in both the
control and 2 + 5-interval groups, whereas the volume in the 2 + 5
interval group was not significantly different from that of the
control group. These results indicate that the treatment interval
of transcutaneous CO2 application should be fewer than 4
days with a treatment frequency of twice per week.
Transcutaneous CO2 exerts
an antitumor effect at the site of application
To determine whether transcutaneous CO2
application has local or systemic antitumor effects, tumor cells
were injected into the upper back of mice (Fig. 1C), and CO2 was applied to
the abdominal region of the body for 10 min, twice per week for 2
weeks. As shown in Fig. 5, there
was no reduction in tumor volume with this treatment when compared
to that in control mice, indicating that local application of
CO2 at the tumor site can induce antitumor effects, but
application at a site distant from the tumor cannot induce
antitumor effects.
Discussion
To obtain the best effect, new treatments for
diseases including cancer must meet certain criteria; i.e.
administration of the effective drug should be performed at the
appropriate dose and time via the ideal delivery route in each
patient (19). In this study, we
investigated the appropriate use of transcutaneous CO2
application as an antitumor therapy by evaluating its effects on
different tumor types, testing various administration frequencies
and intervals, and assessing whether the treatment effects were
local or systemic. We found that CO2-treatment was most
effective against local tumors when applied transcutaneously for at
least 10 min each, twice per week, with an interval of 3 and 4
days, and these conditions yielded no discernable side effects.
CO2-treatment had little effect when
applied for a short duration (5 min) in each treatment, whereas a
longer treatment time (20 min) markedly increased the rate of
apoptosis in human tumors. Additionally, in a preliminary study, no
further decrease in tumor volume was observed with the 1-h
treatment compared to the 20-min treatment in the Nara-H xenograft
model (data not shown). A DNA fragmentation assay also showed that
the induction of apoptosis by CO2 application was
time-dependent, with a maximal increase observed for the 20-min
treatment. These observations strongly suggest that the optimal
treatment time for transcutaneous CO2 application is 20
min for each treatment, and that a treatment time of at least 10
min should be used. For the CO2 therapy, there was no
difference in effectiveness of frequencies of twice and five times
per week; in addition, a twice-weekly treatment regimen was
sufficient to inhibit in vivo tumor growth, even in MFH/UPS,
which is a high-grade sarcoma cell line. Additionally, using a
treatment frequency of twice per week, tumor volumes were smaller
with a treatment interval of 3 and 4 days than with a treatment
interval of 2 and 5 days. We also found that transcutaneous
CO2 application was ineffective when applied at a
distant site relative to the tumor location. Therefore, the
CO2 therapy should be applied to the body surface close
to the tumor location. We previously developed an alternative
system for CO2 application that can access deep-seated
tumors using intra-arterial infusion of saturated CO2
solution (20). These findings
suggest that continuous treatment, twice per week with an interval
of 3 + 4 days, applied to the local site of the tumor, provides the
best antitumor effect for transcutaneous CO2
application.
Chemo- and radiation therapies are effective for
many types of malignant tumors (4,21).
However, these therapies at high dosages can damage not only
malignant tumor cells, but also healthy cells and tissues (21–22),
often resulting in side effects and complications (21). Specifically, chemotherapeutic drugs
such as doxorubicin, ifosfamide, and cisplatin are known to cause
myelosuppression, cardio- and nephritic toxicity, cystitis
hemorrhage, and nausea (4,22), whereas radiation therapy is
associated with radiodermatitis, myelosuppression, and radiation
disease (21,23–25).
In this study, transcutaneous CO2 application did not
result in any side effects such as body weight loss, cystitis
hemorrhage, dermatitis, cardiac arrest, or metastasis even with
long and/or frequent treatment. This indicates that CO2
therapy is likely safe for treating patients with malignant tumors.
In addition, we previously showed that CO2 had
synergistic effects by reducing hypoxia when used in combination
with chemo- (26) and radiation
therapies (27); however, it
remains unclear exactly how CO2-treatment should be used
in combination with these types of therapy.
This study had several limitations. First, in the
current study, the antitumor effects of transcutaneous
CO2 application were tested in only three cancer types,
with one cell line for each cancer type. Our previous studies
(13–15,26–27)
suggest that transcutaneous CO2 application should be
effective in various types of cancers; however, we need to verify
the optimal conditions using more cancer types and several
different cell lines for each type. Second, we confirmed the
antitumor effects of CO2 therapy on human cancer cell
types; however, the experiments were performed using animal models.
The antitumor effects of transcutaneous CO2 application
in humans should now be evaluated in clinical studies.
In conclusion, our findings indicate that local
transcutaneous CO2 application for at least 10 min,
twice per week, with an inter-treatment interval of fewer than 4
days, and with administration to the tumor site can be an effective
therapeutic approach for various types of cancers and sarcomas. The
results strongly suggest that this novel transcutaneous
CO2 application might be useful to treat primary tumors,
with less side effects, and therefore could be safe for clinical
trials.
Acknowledgements
This work was supported by grants from the Division
of Rehabilitation Medicine and Department of Orthopaedic Surgery,
Kobe University Graduate School of Medicine, and Grants-in-Aid for
Scientific Research (C) (no. 26462265) and (B) (no. 24792209) from
the Japan Society for the Promotion of Science. We thank Shiho
Kobayashi for clerical assistance (Division of Rehabilitation
Medicine, Kobe University Graduate School of Medicine), Minako
Nagata, Maya Yasuda, and Kyoko Tanaka for technical assistance
(Department of Orthopaedic Surgery, Kobe University Graduate School
of Medicine), and Editage (www.editage.jp) for English language editing. The
CO2 device used in this study is related to two patent
applications: Carbon Dioxide External Administration Device
(international application no. PCT/JP2003/008381) belonging to
NeoChemir Ltd., and Antitumor Agent Comprising Carbon Dioxide as an
Active Ingredient (international application no. PCT/JP2012/057360)
shared by Kobe University, NeoChemir Ltd., and CO2BE
Medical and Engineering.
References
|
1
|
Bray F and Soerjomataram I: The changing
global burden of cancer: transitions in human development and
implications for cancer prevention and control, disease control
prioritiesCancer: Disease Control Priorities. 3. 3rd. Gelband H,
Jha P, Sankaranarayanan R and Horton S: The International Bank for
Reconstruction and Development/The World Bank; Washington, DC: pp.
23–44. 2015
|
|
2
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
DeSantis CE, Lin CC, Mariotto AB, Siegel
RL, Stein KD, Kramer JL, Alteri R, Robbins AS and Jemal A: Cancer
treatment and survivorship statistics, 2014. CA Cancer J Clin.
64:252–271. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Maurel J, López-Pousa A, de Las Peñas R,
Fra J, Martín J, Cruz J, Casado A, Poveda A, Martínez-Trufero J,
Balañá C, et al: Efficacy of sequential high-dose doxorubicin and
ifosfamide compared with standard-dose doxorubicin in patients with
advanced soft tissue sarcoma: An open-label randomized phase II
study of the Spanish group for research on sarcomas. J Clin Oncol.
27:1893–1898. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dogliotti G, Galliera E, Iorio E, De
Bernardi Di Valserra M, Solimene U and Corsi MM: Effect of
immersion in CO2-enriched water on free radical release
and total antioxidant status in peripheral arterial occlusive
disease. Int Angiol. 30:12–17. 2011.PubMed/NCBI
|
|
6
|
Hartmann BR, Bassenge E, Pittler M and
Hartmann BR: Effect of carbon dioxide-enriched water and fresh
water on the cutaneous microcirculation and oxygen tension in the
skin of the foot. Angiology. 48:337–343. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Joly F, Galoppin L, Bordat P, Cousse H and
Neuzil E: Calcium and bicarbonate ions mediate the inhibition of
mast cell histamine release by Avène spa water. Fundam Clin
Pharmacol. 14:611–613. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Toriyama T, Kumada Y, Matsubara T, Murata
A, Ogino A, Hayashi H, Nakashima H, Takahashi H, Matsuo H and
Kawahara H: Effect of artificial carbon dioxide foot bathing on
critical limb ischemia (Fontaine IV) in peripheral arterial disease
patients. Int Angiol. 21:367–373. 2002.PubMed/NCBI
|
|
9
|
Irie H, Tatsumi T, Takamiya M, Zen K,
Takahashi T, Azuma A, Tateishi K, Nomura T, Hayashi H, Nakajima N,
et al: Carbon dioxide-rich water bathing enhances collateral blood
flow in ischemic hindlimb via mobilization of endothelial
progenitor cells and activation of NO-cGMP system. Circulation.
111:1523–1529. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bohr C, Hasselbach K and Krogh A: Ueber
emen in biologischen Bezuehung wichtigen Einfluss, den die Kohlen
saurespannung des Blutes anf dessen Samerstoffbinding ubt (In
German). Arch Physiol. 16:402–412. 1904. View Article : Google Scholar
|
|
11
|
Sakai Y, Miwa M, Oe K, Ueha T, Koh A,
Niikura T, Iwakura T, Lee SY, Tanaka M and Kurosaka M: A novel
system for transcutaneous application of carbon dioxide causing an
‘artificial Bohr effect’ in the human body. PLoS One. 6:e241372011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Oe K, Ueha T, Sakai Y, Niikura T, Lee SY,
Koh A, Hasegawa T, Tanaka M, Miwa M and Kurosaka M: The effect of
transcutaneous application of carbon dioxide (CO2) on
skeletal muscle. Biochem Biophys Res Commun. 407:148–152. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Onishi Y, Kawamoto T, Ueha T, Kishimoto K,
Hara H, Fukase N, Toda M, Harada R, Minoda M, Sakai Y, et al:
Transcutaneous application of carbon dioxide (CO2)
induces mitochondrial apoptosis in human malignant fibrous
histiocytoma in vivo. PLoS One. 7:e491892012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Takeda D, Hasegawa T, Ueha T, Imai Y,
Sakakibara A, Minoda M, Kawamoto T, Minamikawa T, Shibuya Y, Akisue
T, et al: Transcutaneous carbon dioxide induces mitochondrial
apoptosis and suppresses metastasis of oral squamous cell carcinoma
in vivo. PLoS One. 9:e1005302014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Harada R, Kawamoto T, Ueha T, Minoda M,
Toda M, Onishi Y, Fukase N, Hara H, Sakai Y, Miwa M, et al:
Reoxygenation using a novel CO2 therapy decreases the
metastatic potential of osteosarcoma cells. Exp Cell Res.
319:1988–1997. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zheng Q, Banaszak L, Fracci S, Basali D,
Dunlap SM, Hursting SD, Rich JN, Hjlemeland AB, Vasanji A, Berger
NA, et al: Leptin receptor maintains cancer stem-like properties in
triple negative breast cancer cells. Endocr Relat Cancer.
20:797–808. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Muñoz M, Berger M, Rosso M,
Gonzalez-Ortega A, Carranza A and Coveñas R: Antitumor activity of
neurokinin-1 receptor antagonists in MG-63 human osteosarcoma
xenografts. Int J Oncol. 44:137–146. 2014.PubMed/NCBI
|
|
18
|
Minoda M, Kawamoto T, Ueha T, Kamata E,
Morishita M, Harada R, Toda M, Onishi Y, Hara H, Kurosaka M, et al:
Antitumor effect of YM155, a novel small-molecule survivin
suppressant, via mitochondrial apoptosis in human MFH/UPS. Int J
Oncol. 47:891–899. 2015.PubMed/NCBI
|
|
19
|
Foote SO and Coleman JR: Medication
administration: The implementation process of bar-coding for
medication administration to enhance medication safety. Nurs Econ.
26:207–210. 2008.PubMed/NCBI
|
|
20
|
Ueshima E, Yamaguchi M, Ueha T, Muradi A,
Okada T, Idoguchi K, Sofue K, Akisue T, Miwa M, Fujii M, et al:
Inhibition of growth in a rabbit VX2 thigh tumor model with
intraarterial infusion of carbon dioxide-saturated solution. J Vasc
Interv Radiol. 25:469–476. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Moding EJ, Kastan MB and Kirsch DG:
Strategies for optimizing the response of cancer and normal tissues
to radiation. Nat Rev Drug Discov. 12:526–542. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Judson I, Verweij J, Gelderblom H,
Hartmann JT, Schöffski P, Blay JY, Kerst JM, Sufliarsky J, Whelan
J, Hohenberger P, et al: European Organisation and Treatment of
Cancer Soft Tissue and Bone Sarcoma Group: Doxorubicin alone versus
intensified doxorubicin plus ifosfamide for first-line treatment of
advanced or metastatic soft-tissue sarcoma: A randomised controlled
phase 3 trial. Lancet Oncol. 15:415–423. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Roila F, Herrstedt J, Aapro M, Gralla RJ,
Einhorn LH, Ballatori E, Bria E, Clark-Snow RA, Espersen BT, Feyer
P, et al: ESMO/MASCC Guidelines Working Group: Guideline update for
MASCC and ESMO in the prevention of chemotherapy- and
radiotherapy-induced nausea and vomiting: Results of the Perugia
consensus conference. Ann Oncol. 21 Suppl 5:v232–v243. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kumar S, Juresic E, Barton M and Shafiq J:
Management of skin toxicity during radiation therapy: A review of
the evidence. J Med Imaging Radiat Oncol. 54:264–279. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Stubbe CE and Valero M: Complementary
strategies for the management of radiation therapy side effects. J
Adv Pract Oncol. 4:219–231. 2013.PubMed/NCBI
|
|
26
|
Onishi Y, Kawamoto T, Ueha T, Hara H,
Fukase N, Toda M, Harada R, Sakai Y, Miwa M, Nishida K, et al:
Transcutaneous application of carbon dioxide (CO2)
enhances chemosensitivity by reducing hypoxic conditions in human
malignant fibrous histiocytoma. J Cancer Sci Ther. 4:174–181. 2012.
View Article : Google Scholar
|
|
27
|
Onishi Y, Akisue T, Kawamoto T, Ueha T,
Hara H, Toda M, Harada R, Minoda M, Morishita M, Sasaki R, et al:
Transcutaneous application of CO2 enhances the antitumor
effect of radiation therapy in human malignant fibrous
histiocytoma. Int J Oncol. 45:732–738. 2014.PubMed/NCBI
|