Introduction
Cancer of the skin is by far the most common of all
cancers. Melanoma accounts for only ~1% of skin cancers but causes
a large majority of skin cancer deaths, due to metastasis to other
areas of the body, such as lymph nodes, lungs, liver, brain or
bone. Though often curable in its early stages, metastatic
malignant melanoma is an extremely aggressive cancer with no
current viable treatment. The American Cancer Society estimates
that 76,380 new melanomas will be diagnosed (~46,870 in men and
29,510 in women) in the United States for 2016. Approximately
10,130 people are expected to die of melanoma (~6,750 men and 3,380
women). The rates of melanoma have been rising for the last 30
years (1).
Thus, any successful treatment for melanoma has to
target metastasis, which is dependent upon degradation of the
extracellular matrix (ECM), which, when intact, acts as a barrier
to block cancer cell invasion (2–4).
Clinical and experimental studies have demonstrated that elevated
levels of matrix metalloproteinases are associated with rapid
progression of metastatic melanoma (5,6). MMP
activity is regulated by and dependent upon environmental
influences from surrounding stroma cells, ECM proteins, systemic
hormones and other factors. Inflammation has been reported to drive
cancer progression (7–9). Inflammatory cytokines such as
interleukin (IL)-1β play significant roles in inflammation driven
melanoma growth and progression (10). In the present study we investigated
the effects of selected cytokines, inducers and inhibitors
affecting cancer cell metabolism on the regulation of MMP-2 and
MMP-9 activities in melanoma A-2058 cell line.
Materials and methods
Materials
Human melanoma A-2058 cells were obtained from the
American Type Culture Collection (ATCC; Rockville, MD, USA).
Antibiotics, penicillin and fetal bovine serum (FBS), were obtained
from Gibco-BRL (Long Island, NY, USA). Twenty-four well tissue
culture plates were obtained from Corning Costar Corp. (Cambrdige,
MA, USA). Gelatinase zymography was performed in 10% Novex pre-cast
SDS polyacrylamide gel (Invitrogen) with 0.1% gelatin in
non-reducing conditions. Interleukin 1β, tumor necrosis factor-α
(TNF-α), phorbol 12-myristate 13-acetate (PMA), lipopolysaccharide
(LPS), doxycycline, epigallocatechin gallate (EGCG), actinomycin-D,
cyclohexamide, retinoic acid and dexamethasone, were purchased from
Sigma-Aldrich (St. Louis, MO, USA). The nutrient mixture (NM),
prepared by VitaTech (Hayward, CA, USA) was composed of the
following ingredients in the relative amounts indicated: Vitamin C
(as ascorbic acid and as Mg, Ca and palmitate ascorbate) 700 mg;
L-lysine 1,000 mg; L-proline, 750 mg; L-arginine, 500 mg; N-acetyl
cysteine, 200 mg; standardized green tea extract (80% polyphenol),
1,000 mg; selenium, 30 µg; copper, 2 mg; manganese, 1 mg. All other
reagents used were of high quality and were obtained from
Sigma-Aldrich, unless otherwise indicated.
Cell cultures
Melanoma cells were grown in Dulbeccos modified
Eagles medium (DMEM), supplemented with 15% FBS, 100 U/ml
penicillin and 100 µg/ml streptomycin in 24-well tissue culture
plates. The cells were plated at a density of 1×105
cells/ml and grown to confluency in a humidified atmosphere at 5%
CO2 at 37°C. Serum-supplemented media were removed and
the cell monolayer was washed once with phosphate-buffered saline
(PBS) and with the recommended serum-free media. The cells were
then incubated in 0.5 ml of serum-free medium with various
cytokines, mitogens, inducers and inhibitors in triplicate, as
indicated: PMA (10, 25, 50 and 100 ng/ml); TNF-α and IL-1β (0.1, 1,
10 and 25 ng/ml); LPS (10, 25, 50 and 100 µg/ml); EGCG (10, 25, 50
and 100 µM) without and with PMA 100 ng/ml; doxycycline (10, 25, 50
and 100 µM) without and with PMA 100 ng/ml; NM (10, 50, 100, 500
and 1,000 µg/ml) without and with PMA 100 ng/ml, retinoic acid (50
µM); dexamethasone (50 µM); actinomycin D and cyclohexamide (2 and
4 µg/ml). The plates were then returned to the incubator. The
conditioned medium from each treatment was collected separately,
pooled and centrifuged at 4°C for 10 min at 3,000 rpm to remove
cells and cell debris. The clear supernatant was collected and used
for gelatinase zymography, as described below.
Gelatinase zymography
Gelatinase zymography was utilized because of its
high sensitivity to gelatinolytic enzymatic activity and ability to
detect both pro and active forms of MMP-2 and MMP-9. Upon
renaturation of the enzyme, the gelatinases digest the gelatin in
the gel and reveal clear bands against an intensely stained
background. Gelatinase zymography was performed in 10% Novex
pre-cast SDS polyacrylamide gel in the presence of 0.1% gelatin
under non-reducing conditions. Culture media (20 µl) were mixed
with sample buffer and loaded for SDS-PAGE with Tris-Glycine SDS
buffer, as suggested by the manufacturer (Novex). Samples were not
boiled before electrophoresis. Following electrophoresis the gels
were washed twice in 2.5% Triton X-100 for 30 min at room
temperature to remove SDS. The gels were then incubated at 37°C
overnight in substrate buffer containing 50 mM Tris-HCl and 10 mM
CaCl2 at pH 8.0 and stained with 0.5% Coomassie Blue
R250 in 50% methanol and 10% glacial acetic acid for 30 min and
destained. Protein standards were run concurrently and approximate
molecular weights were determined by plotting the relative
mobilities of known proteins. Gelatinase zymograms were scanned
using CanoScan 9950F Canon scanner at 300 dpi. The intensity of the
bands was evaluated using the pixel-based densitometer program
Un-Scan-It, version 5.1, 32-bit, by Silk Scientific, Inc., (Orem,
UT, USA), at a resolution of 1 Scanner Unit (1/100 of an inch for
an image that was scanned at 100 dpi).
Statistical analysis
Microsoft Excel 2010 linear trend analysis was
utilized to determine the linear trend analyses of the densitometry
results.
Results
Inducers, mitogens and cytokines
Melanoma A-2058 expressed bands corresponding to
MMP-2 and MMP-9. Table I shows the
quantitative densitometry results from the effects of PMA, TNF-α,
IL-1β and LPS on MMP-2 and MMP-9 expression in A-2058 cells.
 | Table I.Effect of inducers, cytokines and
mitogens on melanoma A-2058 MMP-2 and MMP-9 secretion. |
Table I.
Effect of inducers, cytokines and
mitogens on melanoma A-2058 MMP-2 and MMP-9 secretion.
| Treatment | MMP-2 (%) | MMP-9 (%) |
|---|
| PMA (ng/ml) |
|
|
|
Control | 100 | 100 |
| 10 | 92.2 | 100.9 |
| 25 | 89.7 | 146.8 |
| 50 | 94.6 | 288.5 |
| 100 | 97.6 | 399.9 |
| TNF-α (ng/ml) |
|
|
|
Control | 100 | 100 |
| 0.1 | 99.7 | 118.4 |
| 1 | 99.7 | 118.4 |
| 10 | 98.9 | 145.1 |
| 25 | 97.8 | 204.2 |
| IL-1β (ng/ml) |
|
|
|
Control | 100 | 100 |
| 0.1 | 118.3 | 99.8 |
| 1 | 117.4 | 99.6 |
| 10 | 122.1 | 99.7 |
| 25 | 64.6 | 51.2 |
| LPS (µg/ml) |
|
|
|
Control | 100 | 100 |
| 10 | 93.3 | 172 |
| 25 | 103 | 228 |
| 50 | 106 | 200 |
|
100 | 103 | 161 |
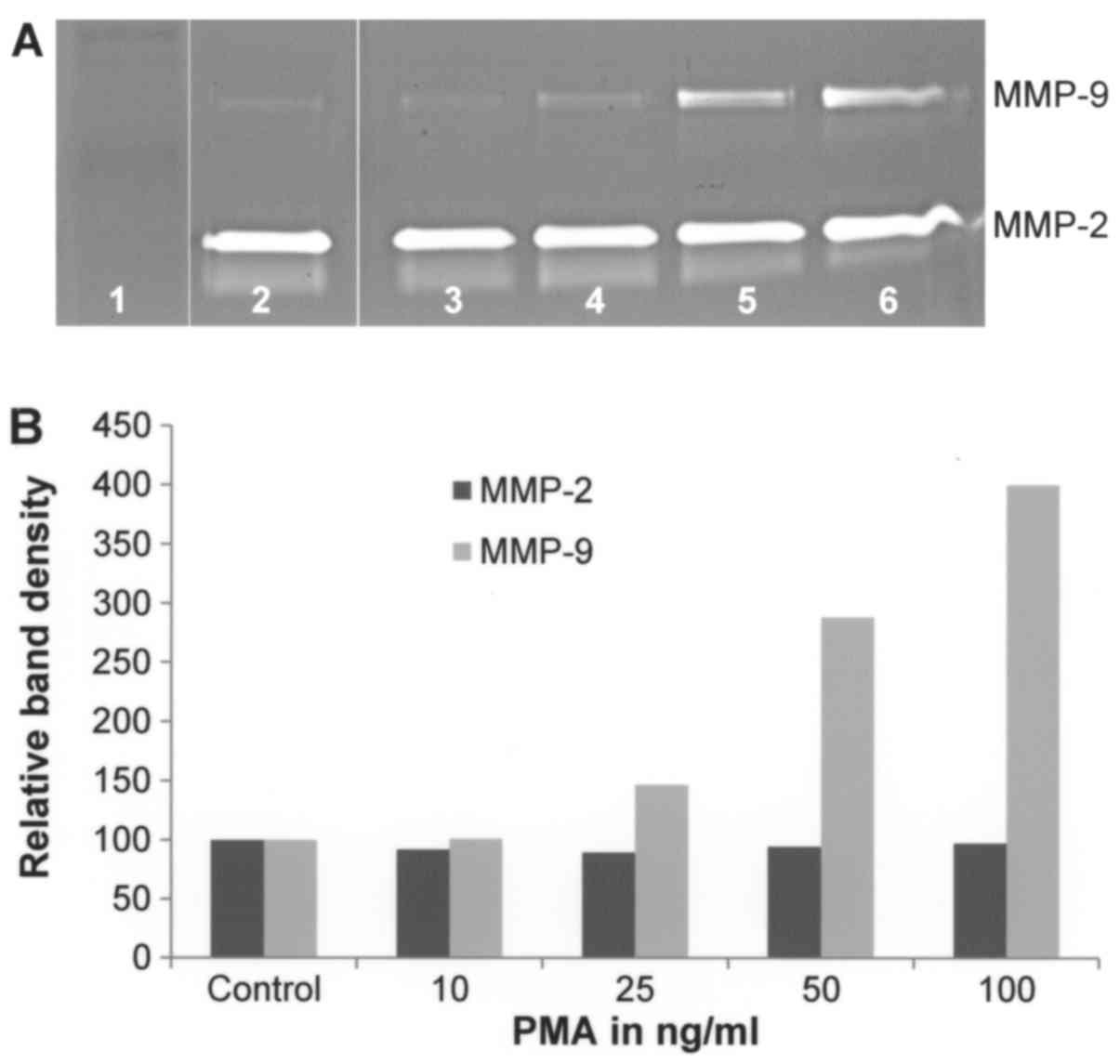
Effect of PMA on A-2058 cell secretion
of MMPs
On gelatinase zymography, A-2058 demonstrated strong
expression of MMP-2 and slight expression of MMP-9. PMA treatment
ranging from concentrations of 10–100 ng/ml showed no significant
effect on MMP-2 secretion (linear trend R2=0.009) but
significant dose-dependent upregulation of MMP-9 secretion with
400% that of control at 100 ng/ml (linear trend
R2=0.883) (Fig. 1).
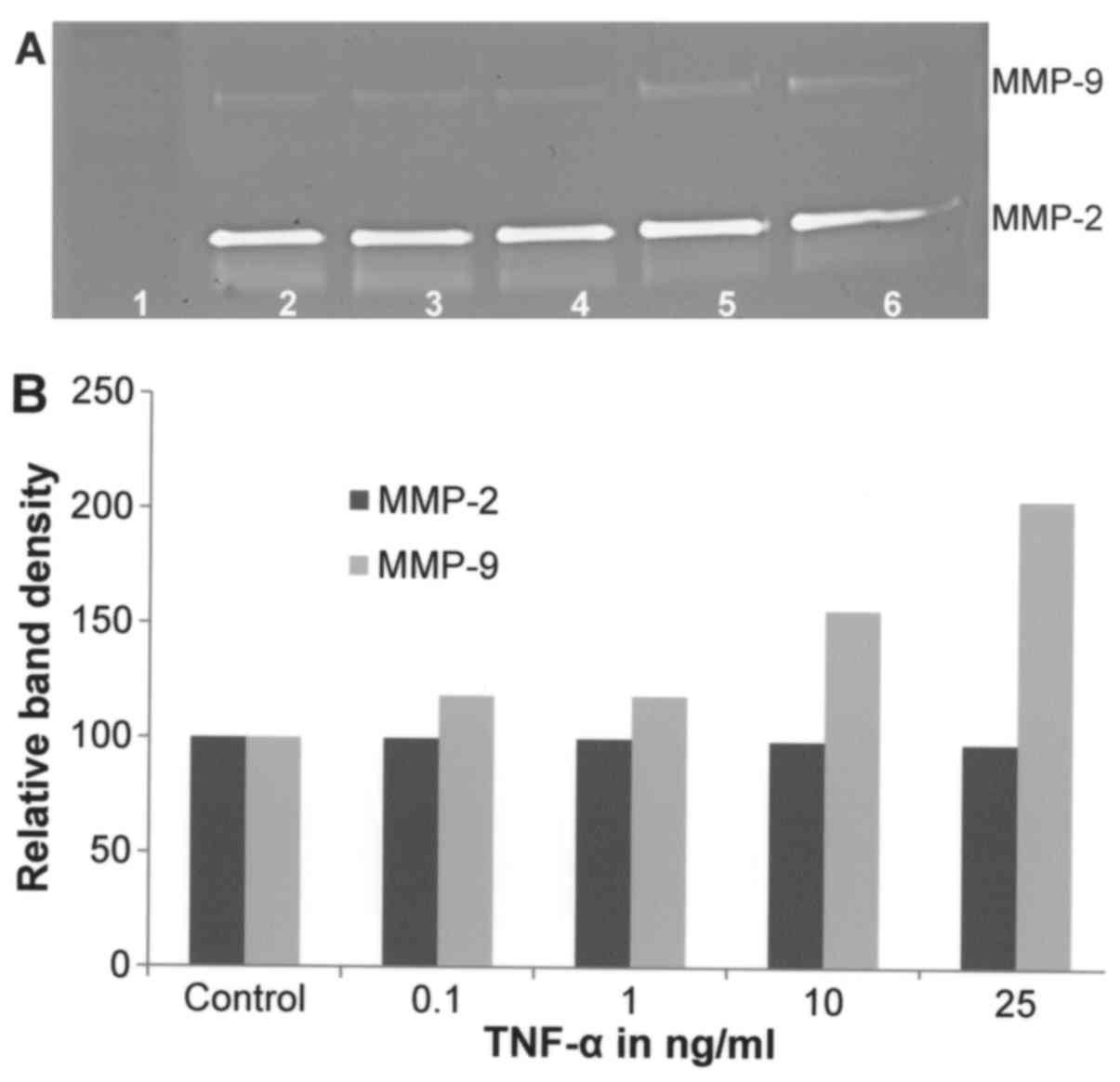
Effect of TNF-α on A-2058 cell
secretion of MMPs
On gelatinase zymography, A-2058 demonstrated strong
expression of MMP-2 and slight expression of MMP-9. TNF-α, used
between 0.1–25 ng/ml, showed no significant overall effect on
expression of MMP-2 (linear trend R2=0.037). TNF-α
showed dose-dependent increased MMP-9 secretion (linear trend
R2=0.876) with 200% of control at 25 ng/ml (Fig. 2).
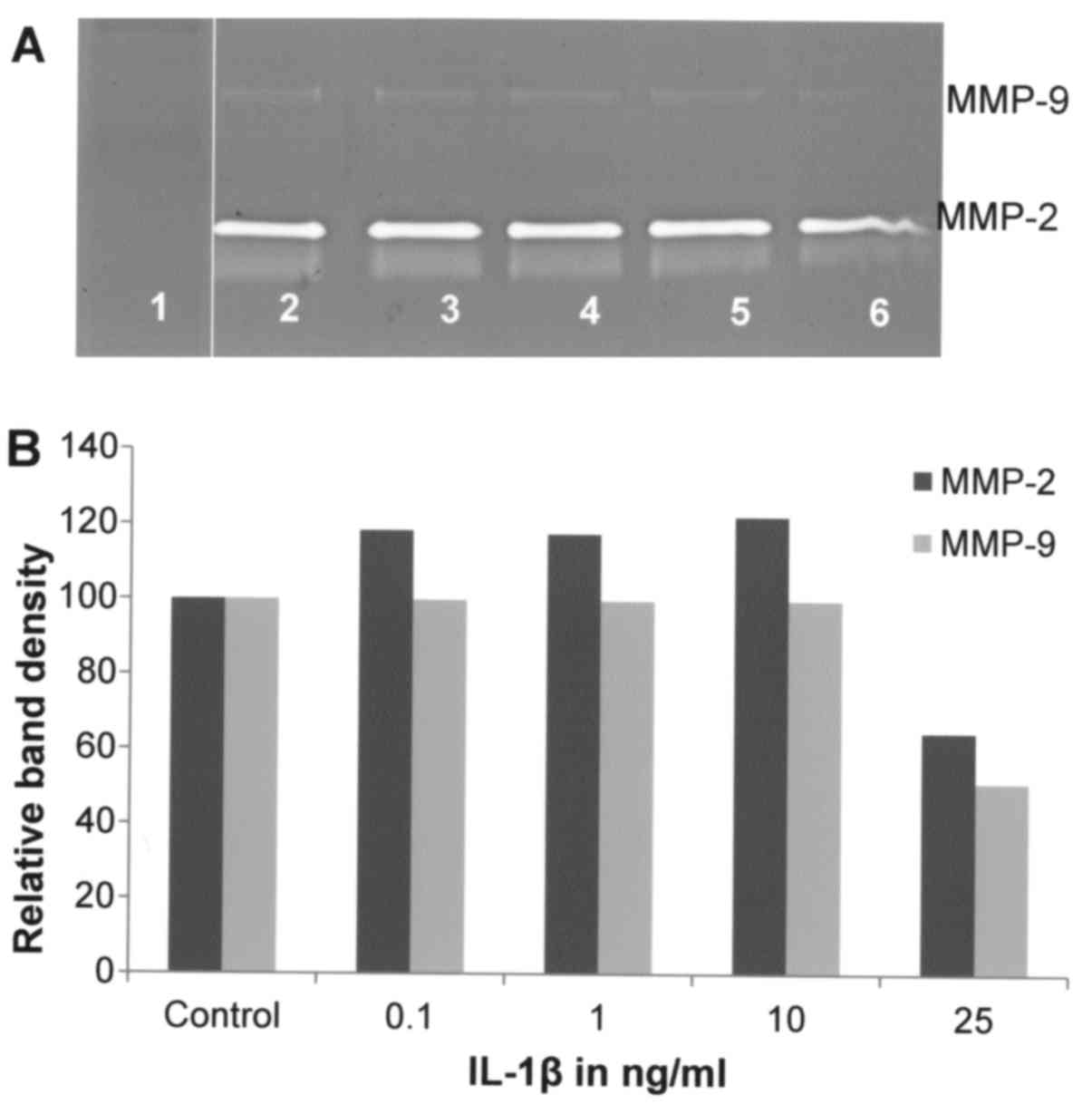
Effect of IL-1β on A-2058 cell
secretion of MMPs
Gelatinase zymography demonstrated strong expression
of MMP-2 by A-2058 cells and slight expression of MMP-9. IL-1β at a
concentration range of 0.1–25 ng/ml did not have significant effect
on MMP-2 secretion by A-2058 cells, except at 25 ng/ml where MMP-2
level was reduced by ~40% (linear trend R2=0.529). Also,
MMP-9 secretion was not modified by IL-1β except for its ~50%
reduction at 25 ng/ml (Fig. 3).
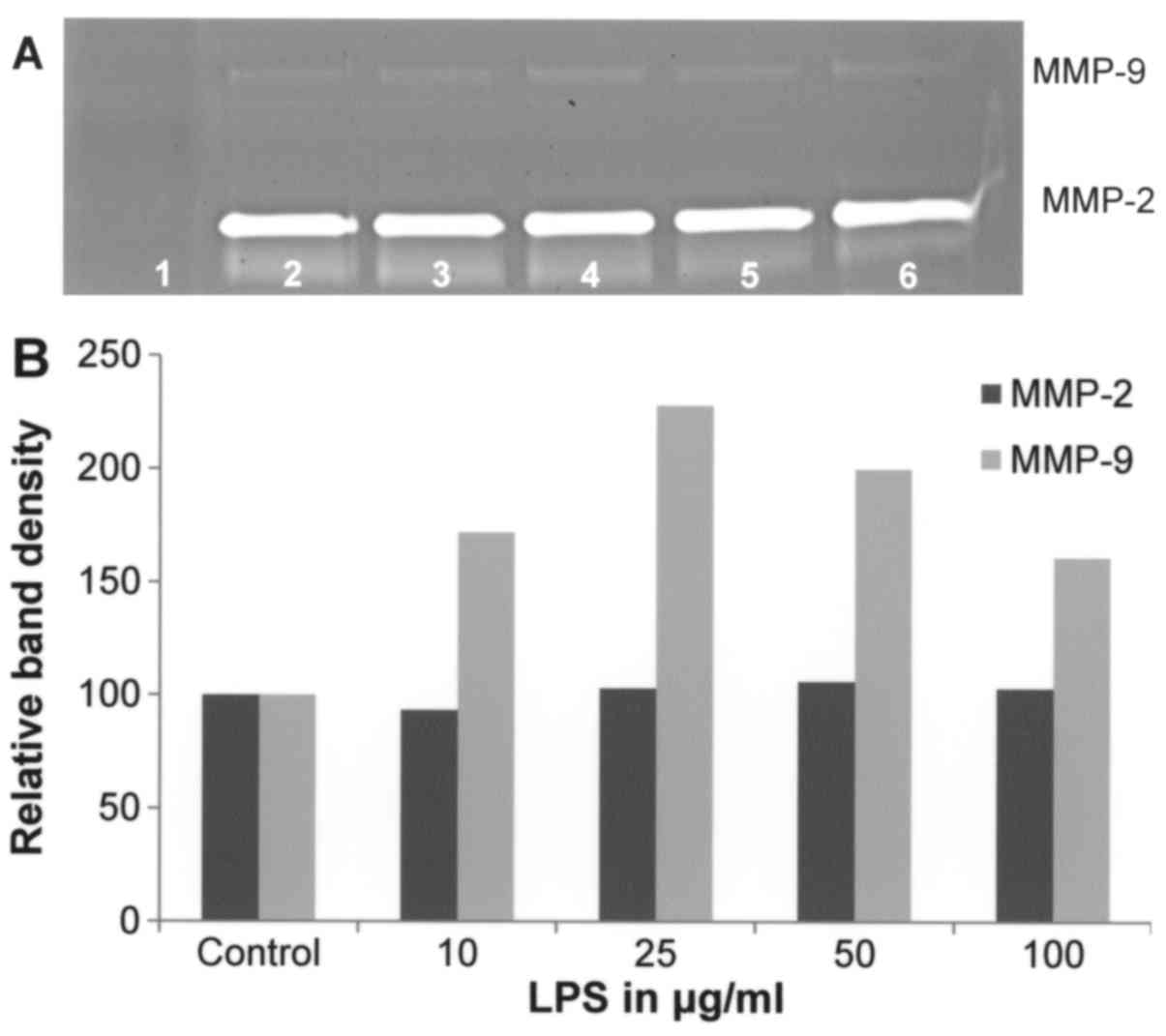
Effect of LPS on A-2058 cell secretion
of MMPs
Gelatinase zymography demonstrated strong expression
of MMP-2 and slight expression of MMP-9 by A-2058 melanoma cells.
LPS treatment between 10–100 µg/ml showed no significant effect on
MMP-2 secretion (linear trend R2=0.375). However,
enhanced MMP-9 secretion was observed at LPS up to 25 µg/ml that
was followed by a decreased MMP-9 level (linear trend
R2=0.244) (Fig. 4).
Chemical inhibitors
Table II shows the
quantitative densitometry results from the effects of select
chemical inhibitors doxycycline, dexamethasone, actinomycin D and
cyclohexamide on MMP-2 and MMP-9 expression in melanoma A-2058 cell
line.
 | Table II.Effect of inhibitors on melanoma
A-2058 MMP-2 and MMP-9 secretion. |
Table II.
Effect of inhibitors on melanoma
A-2058 MMP-2 and MMP-9 secretion.
|
| Untreated | PMA 100
ng/ml-treated |
|---|
|
|
|
|
|---|
| Treatment | MMP-2 (%) | MMP-9 (%) | MMP-2 (%) | MMP-9 (%) |
|---|
| Doxycycline
(µM) |
|
|
|
|
|
Control | 100 | 100 | 100 | 100 |
| 10 | 89.8 | 99.3 | 102 | 122.6 |
| 25 | 89.2 | 105.5 | 95 | 111.2 |
| 50 | 66.5 | 97.9 | 69.8 | 68.7 |
|
100 | 28.3 | 70.1 | 33.2 | 29.5 |
| EGCG (µM) |
|
|
|
|
|
Control | 100 | 100 | 100 | 100 |
| 10 | 98.8 | 97.8 | 95.3 | 87.8 |
| 25 | 101.9 | 104.4 | 88.6 | 70.5 |
| 50 | 81.3 | 95.5 | 56.2 | 43.5 |
|
100 | 42.6 | 26.8 | 35 | 1 |
| NM (µg/ml) |
|
|
|
|
|
Control | 100 |
| 100 | 100% |
| 10 | 112.8 |
| 119.9 | 87.7 |
| 50 | 92.4 |
| 82.8 | 17.4 |
|
100 | 26.6 |
| 42.3 | 14.4 |
|
500 | 12.3 |
| 13.1 | 2 |
|
1,000 | 0 |
| 4.1 | 1 |
| Actinomycin D
(µg/ml) |
|
|
|
|
|
Control | 100 |
|
|
|
| 2 | 74.3 |
|
|
|
| 4 | 72.6 |
|
|
|
| Cyclohexamide
(µg/ml) |
|
|
|
|
|
Control | 100 |
|
|
|
| 2 | 17.4 |
|
|
|
| 4 | 19.9 |
|
|
|
| Dexamethasone
(µM) |
|
|
|
|
|
Control | 100 |
|
|
|
| 50 | 107 |
|
|
|
| Retinoic acid
(µM) |
|
|
|
|
|
Control | 100 |
|
|
|
| 50 | 0 |
|
|
|
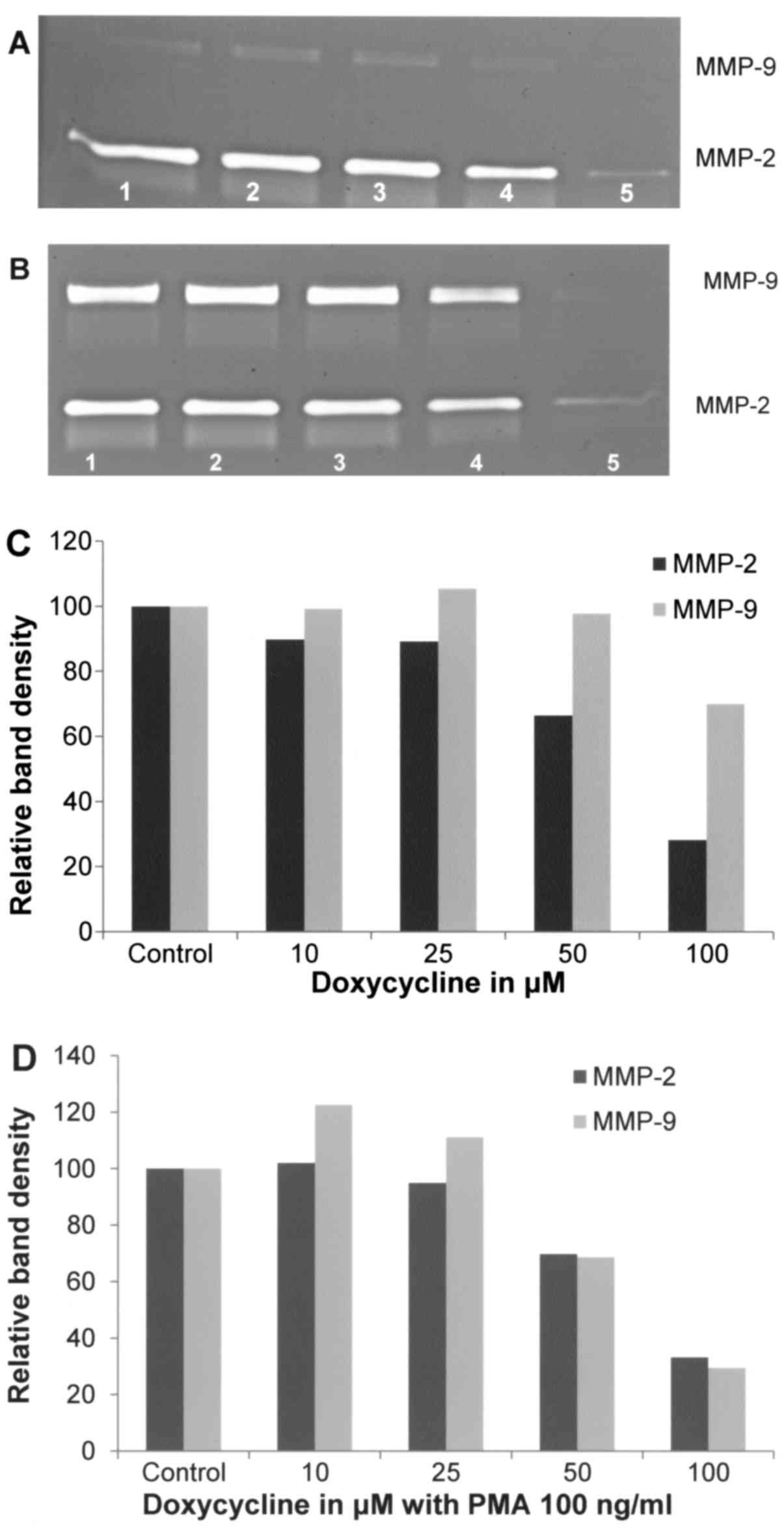
Effect of doxycycline on A-2058 cell
secretion of MMPs
Doxycycline inhibited MMP-2 secretion by A-2058
cells in a dose-dependent manner with 72% blockage at 100 µM
(linear trend R2=0.843). MMP-9 secretion was not
affected by doxycycline up to 50 µM, and at 100 µM it was decreased
by 30% (linear trend R2=0.480). In the presence of PMA
at 100 ng/ml, doxycycline downregulated the expression of MMP-2 in
a dose-dependent manner, with 67% block (R2=0.8072) at
100 µM and 70% block (linear trend R2=0.6711) of MMP-9
at 100 µM (Fig. 5).
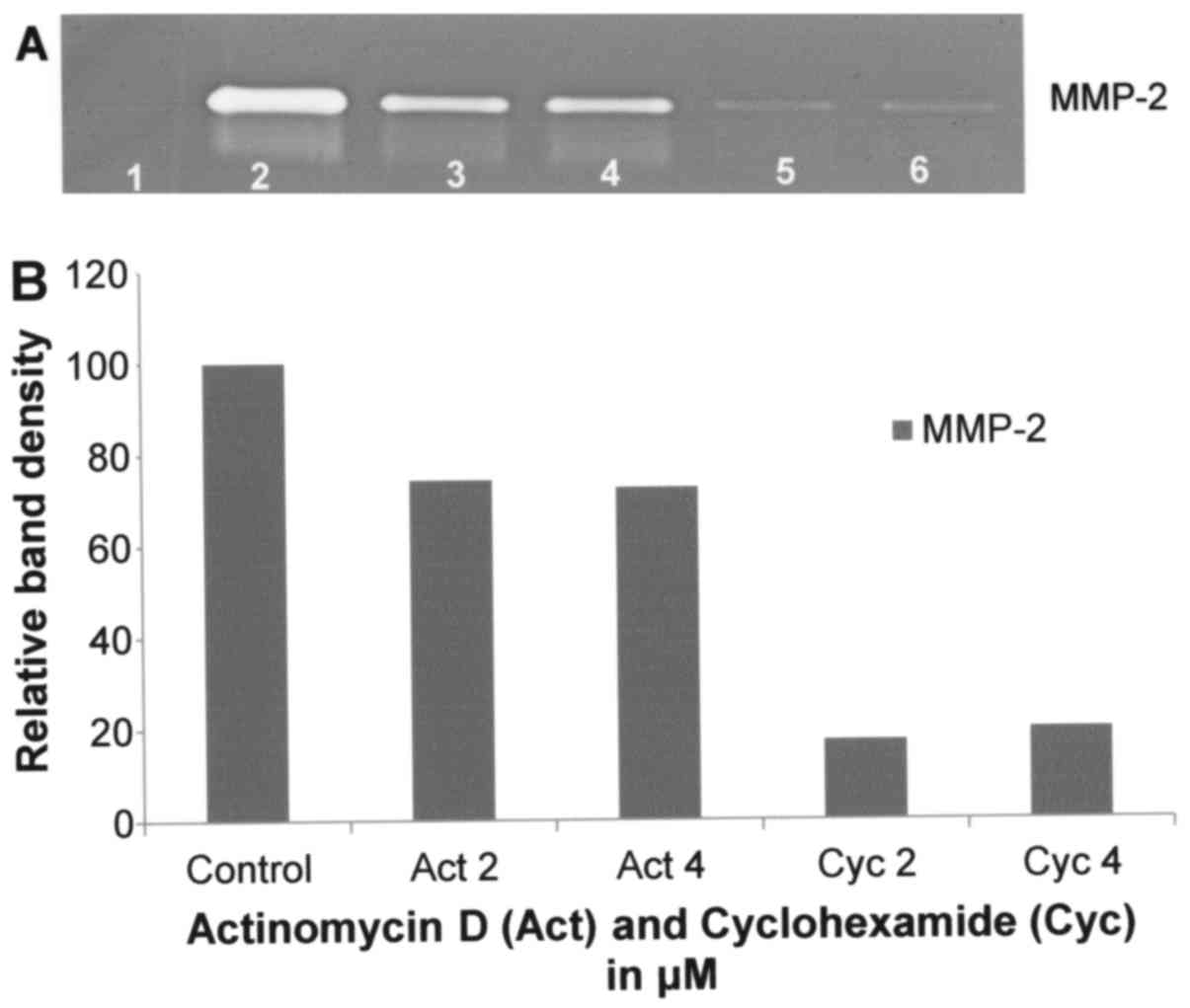
Effect of actinomycin D on A-2058 cell
secretion of MMPs
Actinomycin D had moderate inhibitory effect on
A-2058 MMP-2 secretion (R2=0.797) with 26 and 27%
inhibition at 2 and 4 µM, respectively, as shown in Fig. 6.
Effect of cyclohexamide on A-2058 cell
secretion of MMPs
Cyclohexamide had potent dose-dependent inhibitory
effect on MMP-2 secretion, resulting in its inhibition by 80% at 4
µM (linear trend R2=0.727), as shown in Fig. 6.
Effect of dexamethasone on A-2058 cell
secretion of MMPs
Dexamethasone had slight stimulatory effect on
MMP-2, with 107% of control at 50 µM (data not shown).
Natural inhibitors
Table II shows the
quantitative densitometry results, presenting the effects of
natural compounds EGCG alone, the EGCG in a complex with other
natural compounds (NM) and retinoic acid on MMP-2 and MMP-9
expression in melanoma A-2058 cells.
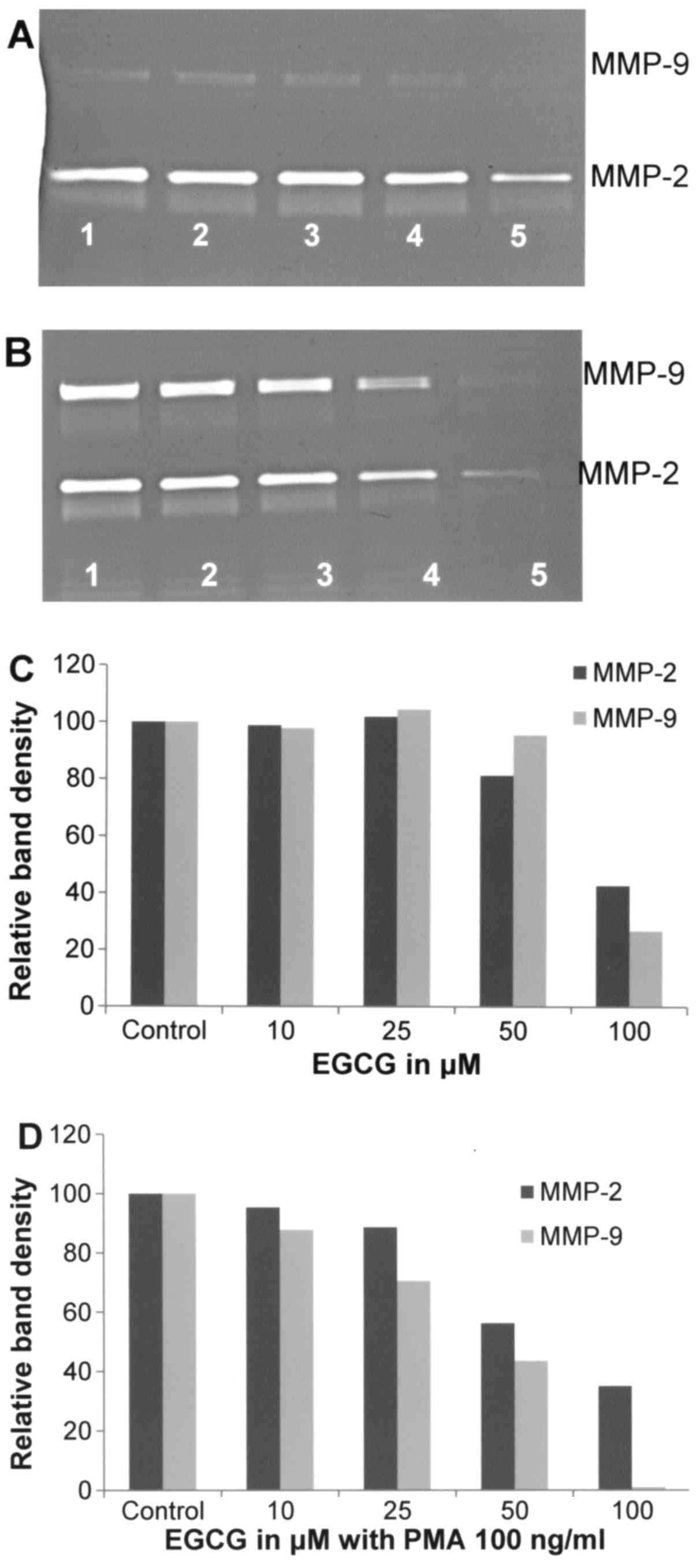
Effect of EGCG on A-2058 cell
secretion of MMPs
Fig. 7 shows the
effects of EGCG at 10, 25, 50 and 100 µM concentrations on MMP-2
and MMP-9 secretions by unstimulated and PMA-stimulated A2058
melanoma cells. EGCG showed inhibitory effects on MMP-2 and −9
secretions by melanoma cells in a dose-dependent manner. In the
presence of 100 µM EGCG, the secretion of MMP-2 decreased by 57.4%
(linear trend R2=0.697) and MMP-9 by 73.2% (linear trend
R2=0.519), as shown in Fig.
7A and C. In the presence of PMA (100 ng/ml) the secretion of
these enzymes was inhibited by EGCG in a dose-dependent manner.
Slight inhibitory effect of EGCG on PMA-induced MMP-9 secretion was
already observed at 25 µM (30% inhibition) and its total block at
100 µM (linear trend R2=0.901), as shown in Fig. 7B and D.
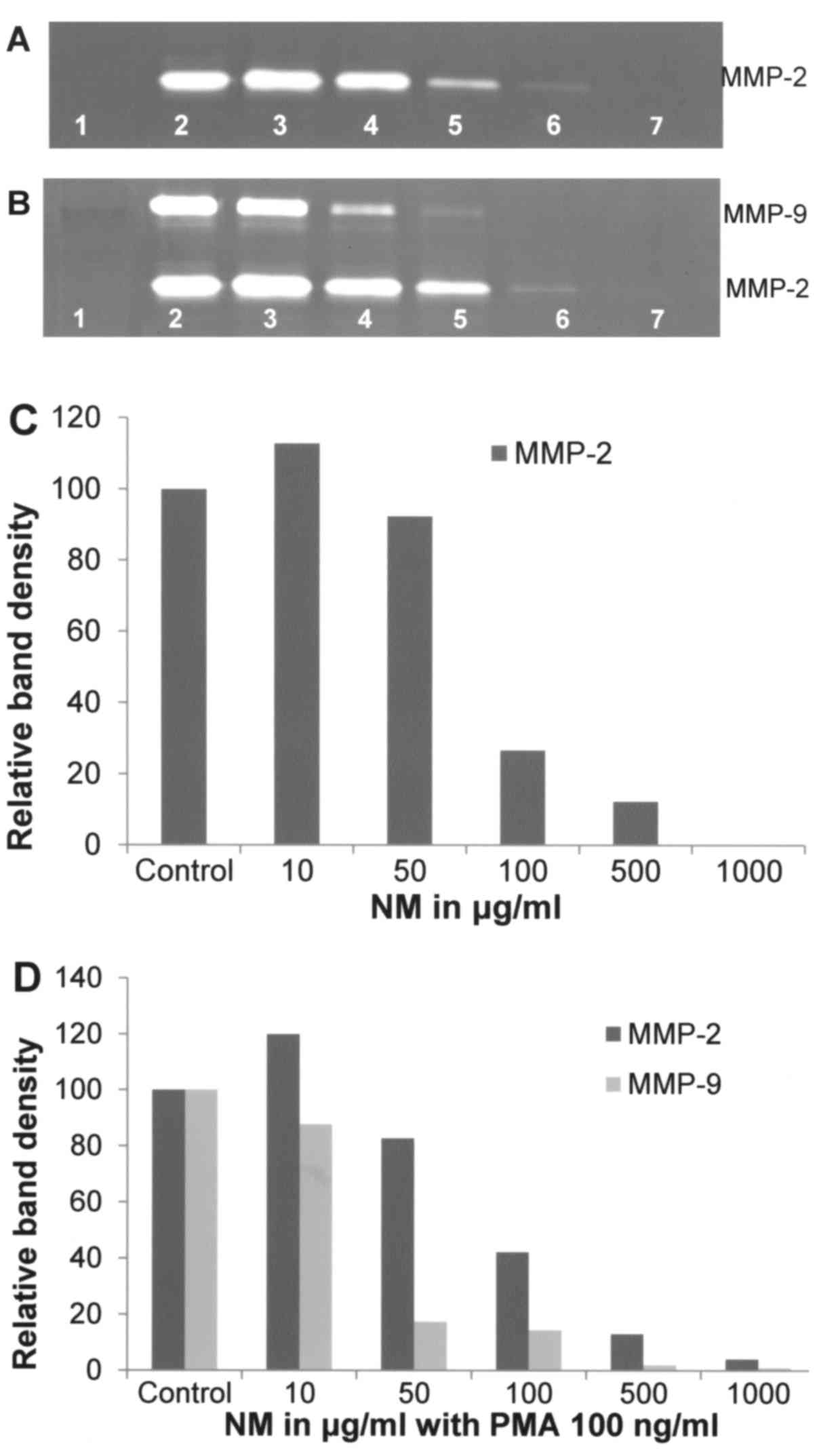
Effect of NM on A-2058 cell secretion
of MMPs
As shown in Fig. 8A and
C, NM was effective in inhibiting secretion of MMP-2 by
uninduced A-2058 cells in a dose-dependent manner when used at 10,
50, 100, 500 and 1,000 µg/ml (linear trend R2=0.868)
with virtual total block at 1,000 µg/ml. NM also showed
dose-dependent inhibition of MMP-2 and −9 expression in PMA-treated
A-2058 cells as presented in Fig. 8B
and D, which showed 96% blockage of MMP-2 secretion at 1,000
µg/ml and total block of MMP-9 at 1,000 µg/ml (linear trends
R2=0.889 and 0.818, respectively).
Effect of retinoic acid on A-2058 cell
secretion of MMPs
Retinoic acid inhibited A-2058 MMP-2 secretion with
total block at 50 µM (data not shown).
Discussion
Elevated MMP levels correlate with melanoma tumor
progression, as documented in clinical studies (5,6). Thus,
knowledge of MMP regulation is of importance for developing
therapeutic strategies for melanoma. Nikkola et al (5) found that melanoma patients with
high-serum levels of MMP-9 had significantly poorer overall
survival than patients with lower serum MMP-9 levels and that high
MMP-9 levels were correlated with visceral or bone metastasis and
presence of liver metastases. Malaponte et al (6) found that plasma levels of MMP-2 and
TGF-β in patients with primary melanoma were significantly higher
than those of healthy controls and those significantly higher
levels were found in patients with metastatic melanoma.
Extracellular factors, such as the inflammatory cytokine IL-1β has
been implicated in facilitating inflammation and melanoma tumor
growth (10).
In the present study, we compared MMP secretion
patterns in the presence of cytokines, PMA and LPS in melanoma
A-2058 cells. In addition, we investigated the effect of inhibitors
doxycycline, EGCG, NM and other compounds, such as dexamethasone,
cyclohexamide, retinoic acid and agents that affect protein
transcription and translation levels, such as actinomycin D. None
of the tested inducers and cytokines was found to enhance MMP-2
secretion. PMA and TNF-α treatment showed potent dose-dependent
upregulation of MMP-9 secretion up to 400% that of control at 100
ng/ml PMA and 200% of control at 25 ng/ml TNF-α. Surprisingly,
IL-1β demonstrated no effect on MMP-9 secretion, except its
decrease at 25 ng/ml and LPS showed no consistent effect, resulting
in an upregulation and downregulation of MMP-9 secretion. Among
tested inhibitors, chemical inhibitor doxycycline and natural
inhibitors EGCG and NM with and without PMA treatment, potently
downregulated the secretion of A-2058 MMP-2 and MMP-9 in a
dose-dependent manner. MMP-2 secretion was potently inhibited by
cyclohexamide and retinoic acid, moderately inhibited by
actinomycin D and slightly stimulated by dexamethasome.
In addition to individual compounds we tested the
effects of a specific nutrient mixture (NM) which has demonstrated
anticancer efficacy in various in vitro and in vivo
studies by affecting various mechanisms, including MMPs secretion
(11). EGCG from green tea is one
of its components, with green tea extract comprising ~23% total NM
weight. Another component is amino acid lysine, which is a natural
inhibitor of plasmin-induced proteolysis, including MMPs (12,13).
Lysine also contributes to 23% of weight in NM. These and other
compounds in NM (i.e. vitamin C, lysine, proline, copper, manganese
and N-acetyl cysteine) are important in supporting the integrity
and stability of connective tissue through various mechanisms,
thereby contributing to tumor encapsulation and curtailing cancer
cells spread (14–22). In addition, NM components N-acetyl
cysteine and selenium have been shown to inhibit tumor cell MMP-9
and invasive activities and migration of endothelial cells through
the ECM (23–25). Ascorbic acid has been documented to
modulate cancer cell and tumor growth as well as to prevent
metastasis (26–31) and low levels of ascorbic acid are
found in cancer patients (32–34).
The inhibitory effects of NM in comparison to EGCG alone on MMP-9
and MMP-2 secretion in unstimulated and PMA-stimulated A-2058 cells
suggest enhancing effectiveness of EGCG when applied in a mixture
with other natural compounds of similar and complementary
mechanisms. The contribution of individual NM components towards
inhibition of MMPs warrants further investigation.
In conclusion, our results showed that various
inducers, cytokines, mitogens and inhibitors tested in this study
modulate MMP-2 and −9 secretions by melanoma A-2058 cells. They
suggest the clinical use of MMP inhibitors, especially potent and
non-toxic ones as the nutrient mixture (NM) and its component EGCG
in management of melanomas.
Acknowledgements
The present study was funded by the Dr. Rath Health
Foundation (Santa Clara, CA), a non-profit organization.
References
|
1
|
ACS: Key statistics for melanoma skin
cancer. http://www.cancer.org/cancer/skincancer-melanoma/detailedguide/melanoma-skin-cancer-key-statisticsLast
revised: 05/20/2016. 12 /12–/16
|
|
2
|
Yurchenco PD and Schittny JC: Molecular
architecture of basement membranes. FASEB J. 4:1577–1590.
1990.PubMed/NCBI
|
|
3
|
Barsky SH, Siegal GP, Jannotta F and
Liotta LA: Loss of basement membrane components by invasive tumors
but not by their benign counterparts. Lab Invest. 49:140–147.
1983.PubMed/NCBI
|
|
4
|
Liotta LA, Tryggvason K, Garbisa S, Hart
I, Foltz CM and Shafie S: Metastatic potential correlates with
enzymatic degradation of basement membrane collagen. Nature.
284:67–68. 1980. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nikkola J, Vihinen P, Vuoristo MS,
Kellokumpu-Lehtinen P, Kähäri VM and Pyrhönen S: High serum levels
of matrix metalloproteinase-9 and matrix metalloproteinase-1 are
associated with rapid progression in patients with metastatic
melanoma. Clin Cancer Res. 11:5158–5166. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Malaponte G, Zacchia A, Bevelacqua Y,
Marconi A, Perrotta R, Mazzarino MC, Cardile V and Stivala F:
Co-regulated expression of matrix metalloproteinase-2 and
transforming growth factor-β in melanoma development and
progression. Oncol Rep. 24:81–87. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
de Visser KE and Coussens LM: The
inflammatory tumor microenvironment and its impact on cancer
development. Contrib Microbiol. 13:118–137. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Coussens LM and Werb Z: Inflammation and
cancer. Nature. 420:860–867. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mantovani A, Allavena P, Sica A and
Balkwill F: Cancer-related inflammation. Nature. 454:436–444. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Qin Y, Ekmekcioglu S, Liu P, Duncan LM,
Lizée G, Poindexter N and Grimm EA: Constitutive aberrant
endogenous interleukin-1 facilitates inflammation and growth in
human melanoma. Mol Cancer Res. 9:1537–1550. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Niedzwiecki A, Roomi MW, Kalinovsky T and
Rath M: Micronutrient synergy: a new tool in effective control of
metastasis and other key mechanisms of cancer. Cancer Metastasis
Rev. 29:529–542. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rath M and Pauling L: Plasmin-induced
proteolysis and the role of apoprotein(a), lysine and synthetic
analogs. Orthomolecular Med. 7:17–23. 1992.
|
|
13
|
Sun Z, Chen YH, Wang P, Zhang J, Gurewich
V, Zhang P and Liu JN: The blockage of the high-affinity lysine
binding sites of plasminogen by EACA significantly inhibits
prourokinase-induced plasminogen activation. Biochim Biophys Acta.
1596:182–192. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mussini E, Hutton JJ Jr and Udenfriend S:
Collagen proline hydroxylase in wound healing, granuloma formation,
scurvy, and growth. Science. 157:927–929. 1967. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kivirikko KI and Myllylä R: Collagen
glycosyltransferases. Int Rev Connect Tissue Res. 8:23–72. 1979.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Valcic S, Timmermann BN, Alberts DS,
Wächter GA, Krutzsch M, Wymer J and Guillén JM: Inhibitory effect
of six green tea catechins and caffeine on the growth of four
selected human tumor cell lines. Anticancer Drugs. 7:461–468. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mukhtar H and Ahmad N: Tea polyphenols:
Prevention of cancer and optimizing health. Am J Clin Nutr. 71
Suppl:1698S–1702S, discussion 1703S-1704S. 2000.PubMed/NCBI
|
|
18
|
Yang GY, Liao J, Kim K, Yurkow EJ and Yang
CS: Inhibition of growth and induction of apoptosis in human cancer
cell lines by tea polyphenols. Carcinogenesis. 19:611–616. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Taniguchi S, Fujiki H, Kobayashi H, Go H,
Miyado K, Sadano H and Shimokawa R: Effect of (−)-epigallocatechin
gallate, the main constituent of green tea, on lung metastasis with
mouse B16 melanoma cell lines. Cancer Lett. 65:51–54. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hara Y: Green tea: Health Benefits and
Applications. Marcel Dekker; New York, Basel: 2001, https://doi.org/10.1201/9780203907993 View Article : Google Scholar
|
|
21
|
Gupta S, Hastak K, Afaq F, Ahmad N and
Mukhtar H: Essential role of caspases in
epigallocatechin-3-gallate-mediated inhibition of nuclear factor
kappa B and induction of apoptosis. Oncogene. 23:2507–2522. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fujiki H, Suganuma M, Okabe S, Sueoka E,
Suga K, Imai K, Nakachi K and Kimura S: Mechanistic findings of
green tea as cancer preventive for humans. Proc Soc Exp Biol Med.
220:pp. 225–228. 1999; View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kawakami S, Kageyama Y, Fujii Y, Kihara K
and Oshima H: Inhibitory effect of N-acetylcysteine on invasion and
MMP-9 production of T24 human bladder cancer cells. Anticancer Res.
21:213–219. 2001.PubMed/NCBI
|
|
24
|
Morini M, Cai T, Aluigi MG, Noonan DM,
Masiello L, De Flora S, DAgostini F, Albini A and Fassina G: The
role of the thiol N-acetylcysteine in the prevention of tumor
invasion and angiogenesis. Int J Biol Markers. 14:268–271.
1999.PubMed/NCBI
|
|
25
|
Yoon SO, Kim MM and Chung AS: Inhibitory
effect of selenite on invasion of HT1080 tumor cells. J Biol Chem.
276:20085–20092. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cha J, Roomi MW, Ivanov V, Kalinovsky T,
Niedzwiecki A and Rath M: Ascorbate supplementation inhibits growth
and metastasis of B16FO melanoma and 4T1 breast cancer cells in
vitamin C-deficient mice. Int J Oncol. 42:55–64. 2013.PubMed/NCBI
|
|
27
|
Naidu KA, Karl RC, Naidu KA and Coppola D:
Antiproliferative and proapoptotic effect of ascorbyl stearate in
human pancreatic cancer cells: Association with decreased
expression of insulin-like growth factor 1 receptor. Dig Dis Sci.
48:230–237. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Anthony HM and Schorah CJ: Severe
hypovitaminosis C in lung-cancer patients: The utilization of
vitamin C in surgical repair and lymphocyte-related host
resistance. Br J Cancer. 46:354–367. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Maramag C, Menon M, Balaji KC, Reddy PG
and Laxmanan S: Effect of vitamin C on prostate cancer cells in
vitro: Effect on cell number, viability, and DNA synthesis.
Prostate. 32:188–195. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Koh WS, Lee SJ, Lee H, Park C, Park MH,
Kim WS, Yoon SS, Park K, Hong SI, Chung MH, et al: Differential
effects and transport kinetics of ascorbate derivatives in leukemic
cell lines. Anticancer Res. 18:2487–2493. 1998.PubMed/NCBI
|
|
31
|
Chen Q, Espey MG, Krishna MC, Mitchell JB,
Corpe CP, Buettner GR, Shacter E and Levine M: Pharmacologic
ascorbic acid concentrations selectively kill cancer cells: Action
as a pro-drug to deliver hydrogen peroxide to tissues. Proc Natl
Acad Sci USA. 102:pp. 13604–13609. 2005; View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Núñez Martín C, de Apodaca y Ruiz A Ortiz
and Ruiz A: Ascorbic acid in the plasma and blood cells of women
with breast cancer. The effect of the consumption of food with an
elevated content of this vitamin. Nutr Hosp. 10:368–372. 1995.(In
Spanish).
|
|
33
|
Kurbacher CM, Wagner U, Kolster B,
Andreotti PE, Krebs D and Bruckner HW: Ascorbic acid (vitamin C)
improves the antineoplastic activity of doxorubicin, cisplatin, and
paclitaxel in human breast carcinoma cells in vitro. Cancer Lett.
103:183–189. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cooke JP and Dzau VJ: Nitric oxide
synthase: Role in the genesis of vascular disease. Annu Rev Med.
48:489–509. 1997. View Article : Google Scholar : PubMed/NCBI
|