Introduction
Bladder cancer is the second most common urological
malignancy in the US and is by far the most frequently diagnosed
urological malignancy in China. Epidemiological data provided by
the International Agency for Research on Cancer (IARC) in 2012
reported 55,486 cases and 26,820 deaths of bladder cancer in China
(1). A total of 740,000 new cases
and 16,000 deaths caused by bladder cancer were recorded in 2015 in
the US (2). Genetic polymorphisms,
chromosomal anomalies and genetic and epigenetic alterations are
responsible for the formation and progression of tumors in bladder
cancer (3). The most common type of
bladder cancer is urothelial transitional cell carcinoma, which
accounts for 92–99% of the total cases diagnosed in North America,
Europe and Australia, 70–80% in Southeast Asia and less than 50% in
different parts of Africa (4). The
main therapeutic method for bladder cancer without metastasis is
surgery followed by postoperative intravesical instillation
(5). Despite the significant
advances in surgical techniques and adjuvant chemotherapy, bladder
cancer remains a highly prevalent and lethal malignancy (6). Therefore, new sensitive and reliable
biomarkers and new therapeutic targets and approaches must be
established to treat bladder cancer.
MicroRNAs (miRNAs) are a large group of
single-strand, endogenous, non-coding, short RNAs containing 22
nucleotides (7). miRNAs make up a
novel class of post-transcriptional gene regulators through
interaction with partially complementary target sites at the 3′
untranslated regions (3′ UTRs) of their target genes either by
inducing their degradation or impairing their translation (8,9). More
than 1,000 miRNAs have been identified in the human genome
(http://www.mirbase.org/), and these miRNAs can
regulate ~60% of all human genes (10,11).
miRNAs play a crucial role in cell proliferation, cycle, apoptosis,
angiogenesis, tumor progression, metastasis and many other
physiological and pathologic processes (12,13). A
number of studies have reported that abnormally expressed miRNAs
are involved in the initiation and progression of several human
diseases, such as atherosclerosis, diabetes, migraine and cancer
(14–16). Recently, miRNAs have been identified
as tumour suppressors or oncogenes in tumor onset and development
(17). Therefore, miRNAs can be
utilized to treat malignant conditions.
miR-379-5p has been studied in several types of
human cancer (18–20), but information concerning miR-379-5p
in bladder cancer is unavailable. In the present study, the
expression of miR-379-5p in bladder cancer tissues and cell lines
was evaluated. The effects of miR-379-5p transfection on bladder
cancer cells were also evaluated in vitro to elucidate the
functions and mechanisms of miR-379-5p in bladder cancer initiation
and progression. The results of the present study may contribute
towards identifying a novel therapeutic target for the treatment of
bladder cancer.
Materials and methods
Clinical specimens
The present study was approved by the Ethics
Committee of The First Affiliated Hospital of Nanjing Medical
University and performed in accordance with the ethical standards
of the Declaration of Helsinki. Informed consent was obtained from
all patients prior to the collection of specimens. Twenty-seven
paired bladder cancer and adjacent normal tissues were collected
from patients who had undergone radical cystectomy at The First
Affiliated Hospital of Nanjing Medical University (Nanjing, China)
between January 2011 and August 2014. None of the patients were
treated with chemotherapy or radiotherapy prior to surgery. Fresh
tissues were immediately snap-frozen in liquid nitrogen and stored
at −80°C.
Cell lines and culture conditions
Human bladder cancer cell lines (T24, EJ and TCCSUP)
and the human bladder epithelial immortalized SV-HUC-1 cell line
were purchased from Shanghai Institute of Biochemistry and Cell
Biology (Shanghai, China). All cells were cultured in Dulbecco's
modified Eagle's medium (DMEM) or F12 medium with 10% fetal bovine
serum (FBS), 100 U/ml penicillin and 100 mg/ml streptomycin (all
from Gibco, Grand Island, NY, USA) in a humidified incubator at
37°C with 5% CO2.
Quantitative reverse-transcription
polymerase chain reaction (RT-qPCR)
Total RNA was harvested from tissues and cells with
TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the
manufacturer's instructions. Reverse transcription was conducted
with the PrimeScript RT reagent kit (Takara, Dalian, China)
following the manufacturer's protocol. miR-379-5p expression was
quantified with the TaqMan miRNA assay kit (Applied Biosystems,
Foster City, CA, USA) with U6 as an internal control. SYBR Premix
Ex Taq™ kits (Takara, Tokyo, Japan) were used to detect MDM2 mRNA
expression with β-action as an internal control. RT-qPCR was
performed with the Applied Biosystems® 7900HT Real-Time
PCR system (Thermo Fisher Scientific, Waltham, MA, USA). The
relative expression was analyzed by the 2−ΔΔCt method
(21). The primers used in the
present study were as follows: miR-379-5p,
5′-GCGCTGGTAGACTATGGAA-3′ and 5′-GTGCAGGGTCCGAGGT-3′; U6,
5′-CTCGCTTCGGCAGCACATATACT-3′ and 5′-ACGCTTCACGAATTTGCGTGTC-3′;
MDM2, 5′-CAGGCAAATGTGCAATACCAA-3′ and
5′-GGTTACAGCACCATCAGTAGGTACAG-3′; β-action,
5′-GGAGAATGGCCCAGTCCTC-3′ and 5′-GGGCACGAAGGCTCATCAT-3′.
Transfection
miR-379-5p mimics and miRNA negative control
(miR-NC) were obtained from GenePharma Co., Ltd., (Shanghai,
China). Small interfering RNA (siRNA) for mouse double minute 2
(MDM2) (si-MDM2), negative control siRNA (si-NC), pcDNA3.1-MDM2 and
pcDNA3.1 were synthesized by RiboBio (Guangzhou, China). For
transfection, cells were seeded in each well of a 6-well plate at a
density of 60% confluence and transfected with miR-379-5p mimics
(50 pmol/ml), miR-NC (50 pmol/ml), si-MDM2 (50 pmol/ml), si-NC (50
pmol/ml), pcDNA3.1-MDM2 (2 µg) or pcDNA3.1 (2 µg) using
Lipofectamine 2000 (Invitrogen, Grand Island, NY, USA) following
the manufacturer's protocol. After transfection at 48 h,
transfection efficiency was determined through RT-qPCR.
Cell Counting Kit-8 (CCK-8) assay
Transfected cells were collected, suspended and
re-seeded at 3,000/well on 96-well plates. The cells were then
incubated at 37°C in a humidified incubator with 5% CO2.
Cell proliferation was evaluated 1, 2, 3 and 4 days after seeding
by the CCK-8 assay (Dojindo, Kumamoto, Japan). Briefly, 10 µl of
CCK-8 solution was added to each well. After 2 h of incubation at
37°C, an ELISA reader (Bio-Rad, Richmond, CA, USA) was used to
measure optical density (OD) at the wavelength of 450 nm. Each
experiment was performed in triplicate.
Cell migration and invasion
assays
Cell migration and invasion assays were performed in
Transwell chambers with an 8-µm pore polycarbonate membrane (BD
Biosciences, Franklin Lakes, NJ, USA). For the cell migration
assay, transfected cells were collected 48 h post-transfection and
suspended in an FBS-free culture medium. A total of
5×104 cells were seeded into the upper chambers, whereas
the lower chambers were filled with 500 µl of DMEM containing 20%
FBS. Cells were incubated at 37°C in a humidified incubator with 5%
CO2 for 48 h. The cells that did not migrate through the
pores in the membranes were scraped and washed away. The cells that
migrated were fixed with 100% methanol, stained with 0.5% crystal
violet, washed with phosphate-buffered saline and dried in air. The
cells were counted with an inverted microscope (magnification of
×200; Olympus, Tokyo, Japan). Cell invasion assays were performed
in a similar manner, but the cells were allowed to migrate through
Transwell chambers coated with Matrigel (BD Biosciences, San Jose,
CA, USA).
Bioinformatic analysis and luciferase
reporter assay
TargetScan Human 7.0 (http://www.targetscan.org/) and miRanda (http://www.microrna.org/microrna/) were employed
to identify the potential target genes of miR-379-5p.
For the luciferase reporter assay, the reporter
plasmids were synthesized and purified with GenePharma. The 3′ UTR
sequence of MDM2, which was predicted to interact with miR-379-5p
or a mutated sequence within the predicted target sites, was
produced and inserted into the pmirGLO vector. Twenty-four hours
before transfection, the cells were seeded on a 24-well plate at a
density of 30–40% confluence. Lipofectamine 2000 was used to
co-transfect the cells with pmirGLO-MDM2-3′ UTR Wt or
pmirGLO-MDM2-3′ UTR Mut and miR-379-5p mimics or miR-NC.
Forty-eight hours after transfection, the cells were harvested for
reporter assay. Luciferase activities were detected through
dual-luciferase reporter assays (Promega, Manheim, Germany)
according to the manufacturer's instructions. Firefly luciferase
activities were normalized to Renilla luciferase
activities.
Western blotting
Total protein was isolated from tissues and cells
with a radioimmunoprecipitation assay lysis buffer [150 mM of NaCl,
1% NP-40, 0.5% deoxycholate and 1% sodium dodecyl sulphate (SDS)]
containing protease and phosphatase inhibitors (Thermo Fisher
Scientific, Franklin Lakes, MA, USA). A bicinchoninic acid assay
kit (Beyotime Institute of Biotechnology, Haimen, China) was used
to determine the concentration of total protein. Equal amounts of
proteins were separated through 10% SDS-polyacrylamide gel
(SDS-PAGE) electrophoresis and then transferred to polyvinylidine
flouride membranes (Millipore, Billerica, MA, USA). Subsequently,
the membranes were blocked in Tris-buffered saline with 0.1%
Tween-20 (TBST) containing 5% skimmed milk and incubated with
primary antibodies, namely, mouse anti-human MDM2 antibody (1:1,000
dilution; sc-965) and mouse anti-human monoclonal GADPH antibody
(1:1,000 dilution; sc-365062) (both from Santa Cruz Biotechnology,
Santa Cruz, CA, USA), at 4°C overnight. After being washed in TBST,
the membranes were incubated with goat anti-mouse (HRP)-conjugated
secondary antibody (1:5,000 dilution, sc-2005; Santa Cruz
Biotechnology) at room temperature for 1 h. The protein bands were
visualized with ECL substrates (Millipore). Glyceraldehyde
3-phosphate dehydrogenase was used as a loading control.
Statistical analysis
Data are presented as means ± standard deviation and
were compared with the Student's t-test or ANOVA using SPSS version
13.0 software (SPSS, Inc., Chicago, IL, USA). A P-value of <0.05
was considered statistically significant.
Results
miR-379-5p expression is downregulated
in bladder cancer tissues and cell lines
To determine whether miR-379-5p contributes to the
progression of bladder cancer, we determined the miR-379-5p
expression in bladder cancer and adjacent normal tissues by
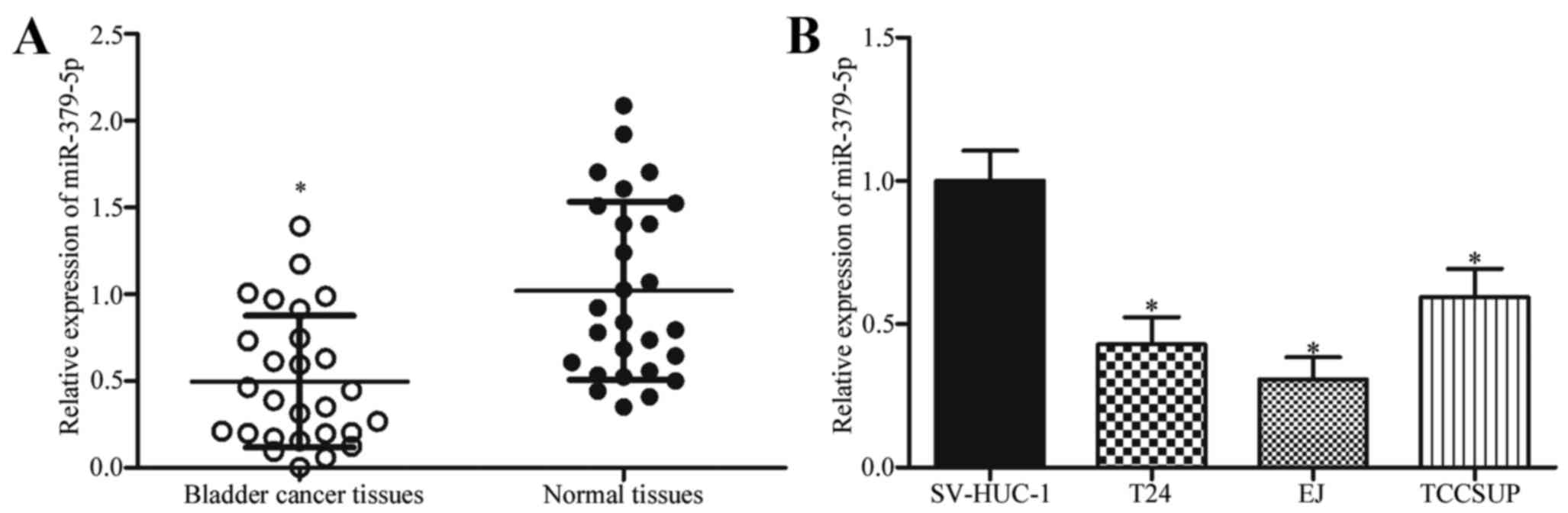
RT-qPCR. As shown in Fig. 1A,
miR-379-5p in bladder cancer tissues was underexpressed compared
with that in adjacent normal tissues (P<0.05). Subsequently, the
expression levels of miR-379-5p in 3 bladder cancer cell lines and
human bladder epithelial immortalized SV-HUC-1 cell line were
determined. The results showed that miR-379-5p in bladder cancer
cell lines was underexpressed compared with that in the SV-HUC-1
cell line (Fig. 1B; P<0.05).
These results suggest that miR-379-5p is frequently downregulated
in bladder cancer and may play important roles in the progression
of bladder cancer.
miR-379-5p suppresses bladder cancer
cell proliferation, migration and invasion
To determine the function of miR-379-5p in bladder
cancer, miR-379-5p mimics were introduced into T24 and EJ cells to
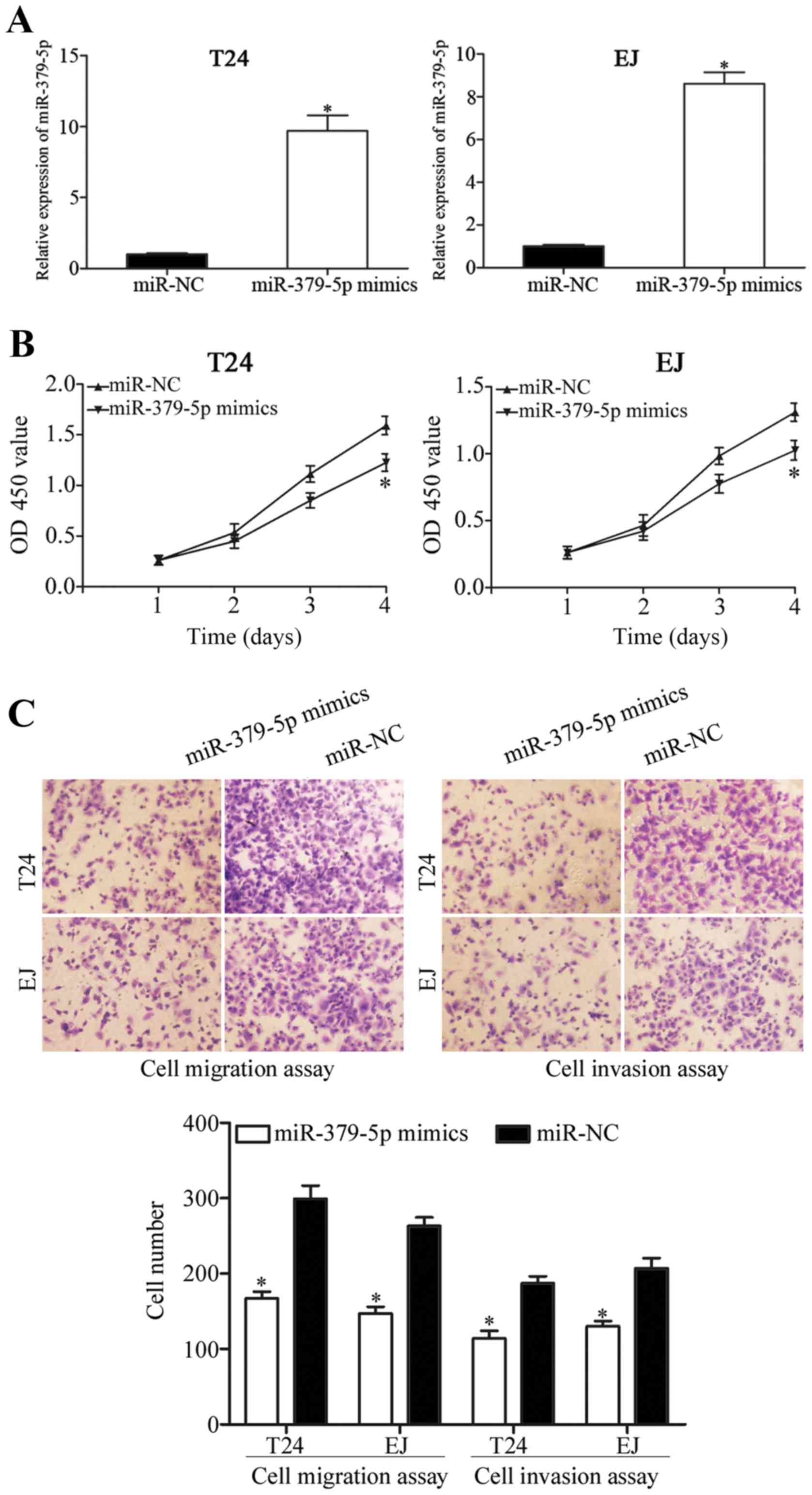
increase the expression level of miR-379-5p. Transfection
efficiency was evaluated through RT-qPCR 48 h post-transfection. As
shown in Fig. 2A, miR-379-5p
expression was markedly increased in the T24 and EJ cells
transfected with the miR-379-5p mimics (P<0.05). CCK-8 assay was
then performed to evaluate the effect of miR-379-5p on cell
proliferation in bladder cancer. The results showed that the
upregulation of miR-379-5p inhibited T24 and EJ cell proliferation
(Fig. 2B; P<0.05). Cell
migration and invasion assays were performed to further investigate
the function of miR-379-5p in the regulation of cell migration and
invasion of bladder cancer cells. As shown in Fig. 2C, ectopic expression of miR-379-5p
led to a significant decrease in the migration and invasion
capacities of the T24 and EJ cells (P<0.05). These results
suggest that miR-379-5p plays a tumor-suppressive role in bladder
cancer growth and metastasis.
MDM2 is a direct target of miR-379-5p
in bladder cancer
miRNAs mainly function by negatively regulating
their target genes; therefore, we investigated the direct target of
miR-379-5p. Bioinformatic algorithms (TargetScan and miRanda) were
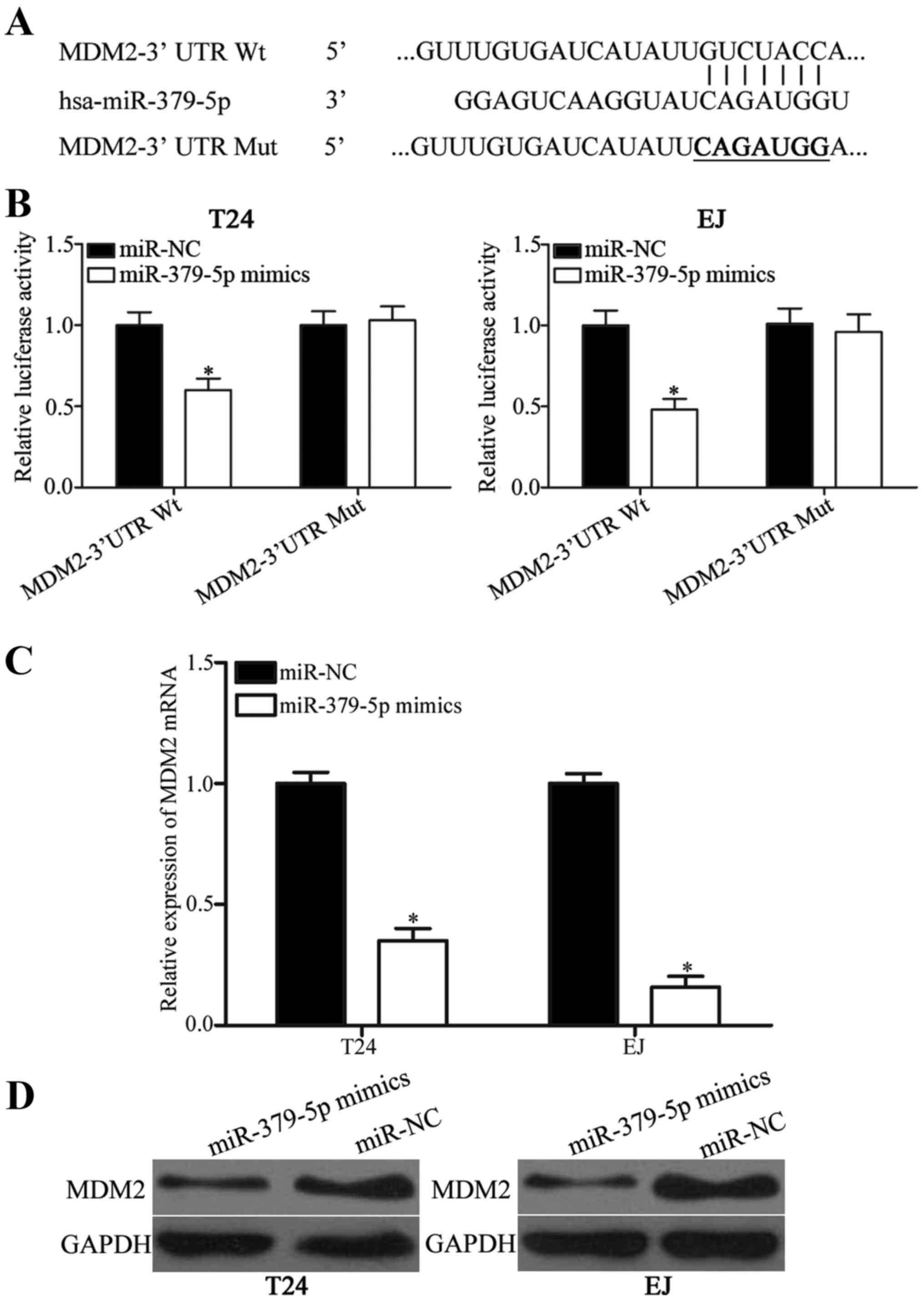
used to predict the potential targets of miR-379-5p. As shown in
Fig. 3A, the 3′ UTR of MDM2
contains a target sequence for miR-379-5p. To confirm whether MDM2
is a direct target of miR-379-5p, luciferase reporter assay was
conducted on T24 and EJ cells. Luciferase activities were evidently
decreased in the T24 and EJ cells co-transfected with the
miR-379-5p mimics and pmirGLO-MDM2-3′ UTR Wt (Fig. 3B; P<0.05). However, the
luciferase activities of pmirGLO-MDM2-3′ UTR Mut were
unaffected.
To further confirm this hypothesis, RT-qPCR and
western blotting were employed to evaluate the regulatory effects
of miR-379-5p on endogenous MDM2 expression in the T24 and EJ
cells. As shown in Fig. 3C and D,
MDM2 mRNA and protein expression were significantly reduced in the
T24 and EJ cells transfected with miR-379-5p mimics compared with
cells transfected with miR-NC (both P<0.05). Collectively, these
findings suggest that miR-379-5p negatively regulates MDM2
expression by directly binding to the 3′ UTR of its mRNA in bladder
cancer.
Inhibition of MDM2 exerts similar
effects as miR-379-5p overexpression in bladder cancer
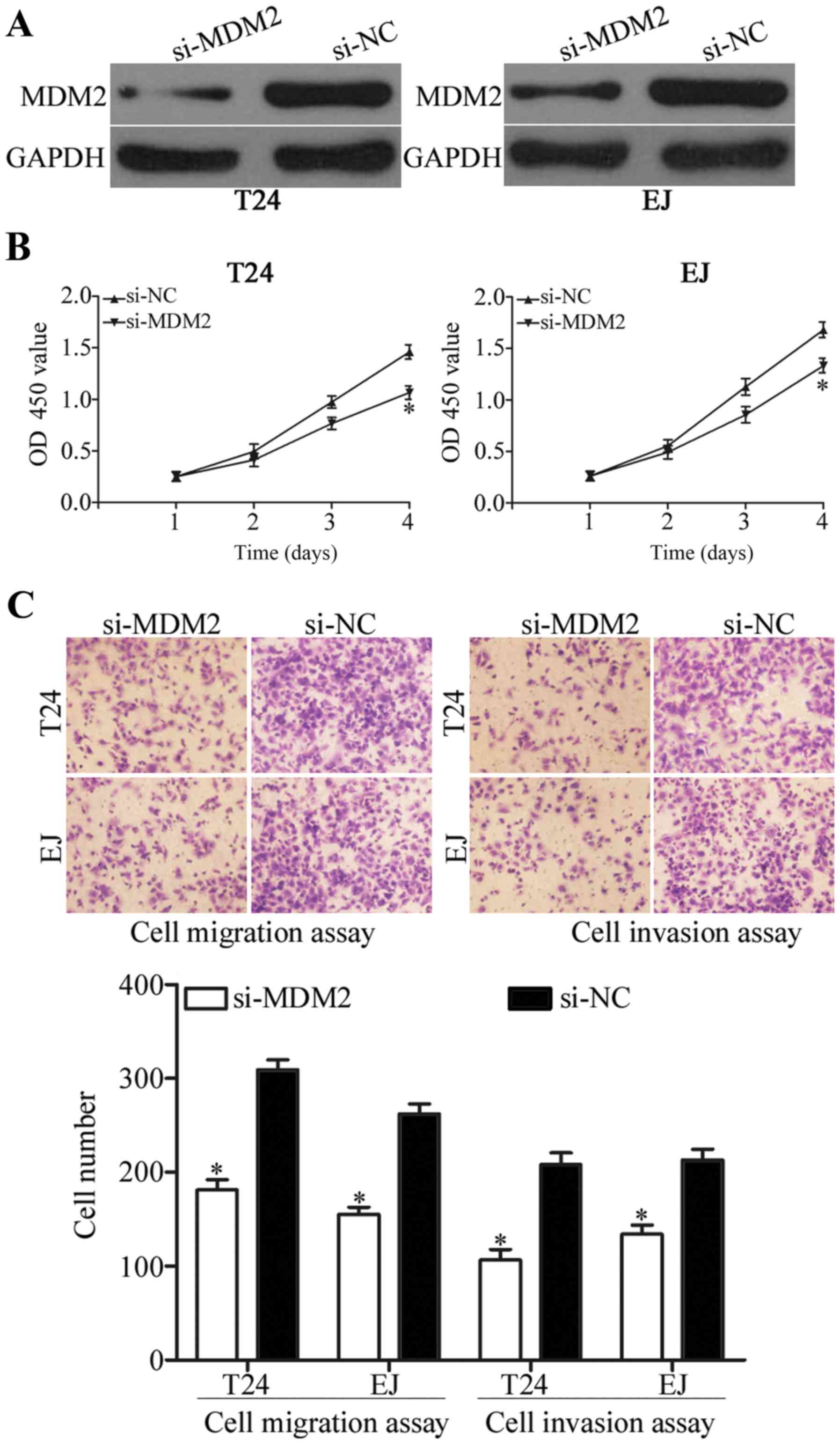
To determine the involvement of MDM2 in bladder
cancer, the biological role of MDM2 in cell proliferation,
migration and invasion were examined. As shown in Fig. 4A, MDM2 protein was underexpressed in
the T24 and EJ cells after transfection with si-MDM2 (P<0.05).
CCK-8 assay and cell migration and invasion assays showed that
inhibition of MDM2 expression suppressed the growth (Fig. 4B; P<0.05) and metastasis
(Fig. 4C; P<0.05) of T24 and EJ
cells. These results suggest that MDM2 knockdown exerts a
suppressive effect similar to miR-379-5p overexpression in bladder
cancer and further confirm that MDM2 is a functional target of
miR-379-5p.
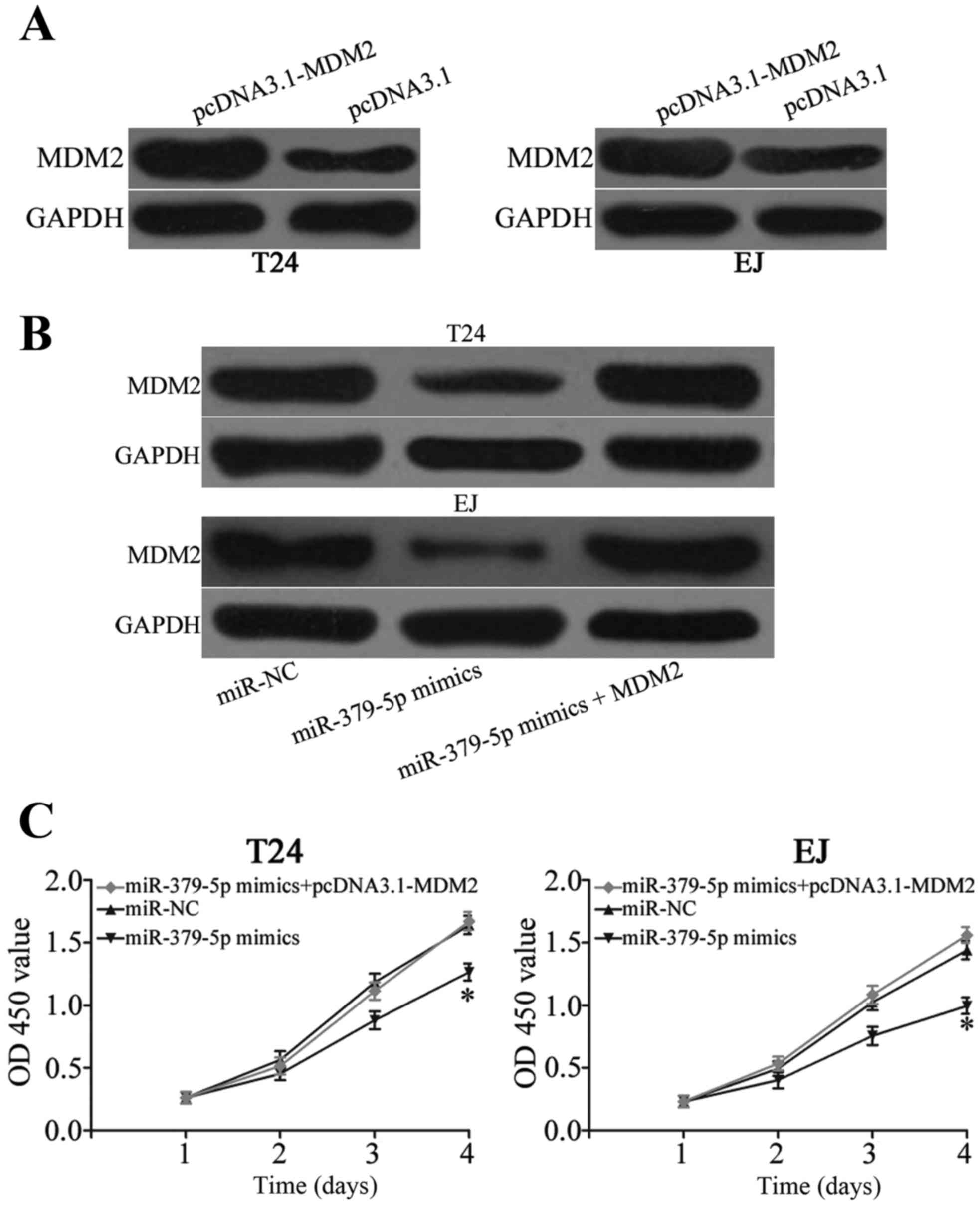
Enforced expression of MDM2 reverses
miR-379-5p-induced effects on cell proliferation, migration and
invasion in bladder cancer
A ‘rescue experiment was performed to determine
whether miR-379-5p inhibits the growth and metastasis of bladder
cancer cells by targeting MDM2. pcDNA3.1-MDM2 was transfected into
T24 and EJ cells to increase its expression (Fig. 5A; P<0.05). The expression level
of MDM2 protein in the T24 and EJ cells was recovered after
co-treatment with miR-379-5p mimics and pcDNA3.1-MDM2 (Fig. 5B; P<0.05). Moreover, resumption
of expression of MDM2 restored proliferation (Fig. 5C; P<0.05), migration and invasion
(Fig. 5D; P<0.05) induced by
miR-379-5p overexpression in the T24 and EJ cells. These results
suggest that miR-379-5p suppresses bladder cancer cell
proliferation, migration and invasion partly by targeting MDM2.
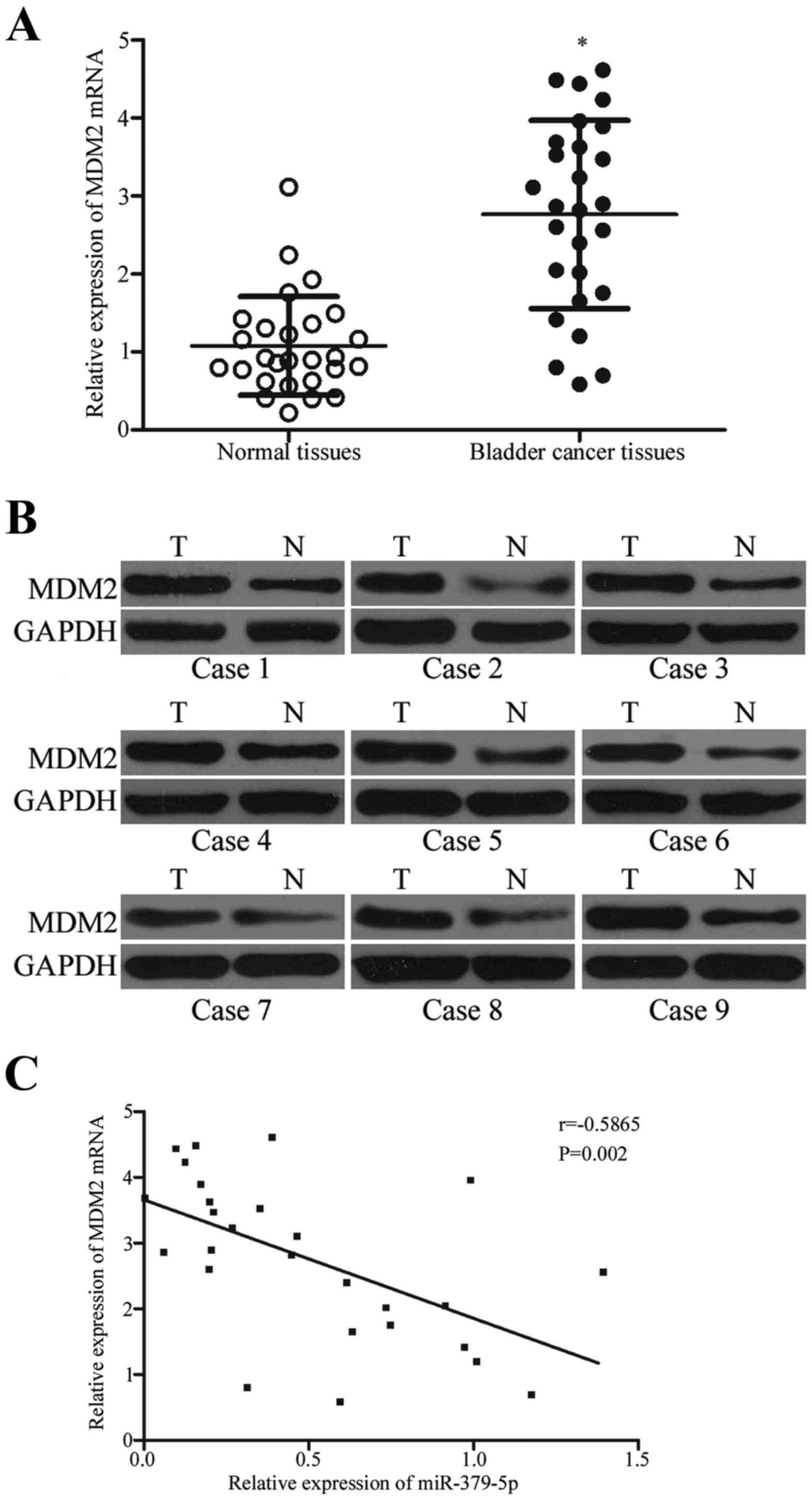
miR-379-5p is negatively correlated
with MDM2 expression in bladder cancer tissues
We analyzed the association between the expression
levels of miR-379-5p and MDM2 in clinical bladder cancer tissues.
MDM2 mRNA was higher in the bladder cancer tissues than that noted
in the adjacent normal tissues (Fig.
6A; P<0.05). Western blot results indicated that MDM2
protein expression in the bladder cancer tissues was upregulated
compared with that in the adjacent normal tissues (Fig. 6B; P<0.05). Moreover, Spearman's
correlation analysis indicated an inverse correlation between
miR-379-5p and MDM2 mRNA expression in the bladder cancer tissues
(Fig. 6C; r=−0.5865; P=0.002).
These results further confirm that MDM2 is a direct target gene of
miR-379-5p in bladder cancer.
Discussion
An increasing amount of evidence indicates that
miRNAs play important roles in many biological processes related to
carcinogenesis and progression, such as cell proliferation,
apoptosis, cell cycle, invasion and metastasis (22–24).
Furthermore, miRNAs represent novel diagnostic and therapeutic
targets for the treatment of human cancer (25). In the present study, miR-379-5p was
downregulated in bladder cancer tissues and cell lines. Restoration
of the expression of miR-379-5p inhibited bladder cancer cell
proliferation, migration and invasion. Moreover, MDM2 was
identified as the direct target gene of miR-379-5p. These results
suggest that miR-379-5p acts as a tumor suppressor in bladder
cancer and may be investigated as an efficient therapeutic target
for clinical application. To the best of our knowledge, the present
study is the first to investigate the expression patterns,
biological functions and underlying mechanism of miR-379-5p in
bladder cancer.
An increasing number of studies have shown that
miR-379-5p, which is located on chromosome 14q32.31, plays
important roles in tumorigenesis and tumor development. For
instance, Khan et al reported that miR-379-5p is
downregulated in breast cancer tissues compared with that in normal
breast tissues. The low expression level of miR-379-5p was found to
be significantly correlated with tumor stage. miR-379-5p
re-expression suppressed cell proliferation by negatively
regulating cyclin B1 (18). Chen
et al found that miR-379-5p expression is lower in
hepatocellular carcinoma tissues and cell lines. Reduced miR-379-5p
was found to be associated with the TNM stage and metastasis of
patients. Restoration of the expression of miR-379-5p inhibited
cell migration, invasion, epithelial-to-mesenchymal transition
(EMT) and metastasis both in vitro and in vivo by
directly targeting focal adhesion kinase (FAK), thus leading to the
suppression of AKT signaling (19).
Chen et al revealed that upregulation of miR-379-5p
decreased cell migration and invasion by downregulation of MMP-2
and MMP-9 (20). Furthermore,
miR-379-5p was highly expressed in bone metastatic prostate cancer
tissues and cell lines and was significantly correlated with
progression-free survival of patients with prostate cancer.
Downregulation of miR-379-5p reduced prostate cancer cell EMT and
invasive ability (26). These
findings suggest that miR-379-5p can be a diagnostic and prognostic
biomarker for human cancers.
Identification of the targets of miRNAs is critical
for understanding its role in tumorigenesis (27). To investigate the mechanisms
underlying the suppression of bladder cancer cell proliferation,
migration and invasion induced by miR-379-5p, we performed
bioinformatic analysis and found that the 3′ UTR of MDM2 contains a
complementary site for the seed region of miR-379-5p. Through a
luciferase reporter assay, we demonstrated that miR-379-5p directly
targeted the 3′ UTR of MDM2. In addition, miR-379-5p overexpression
decreased the expression of endogenous MDM2 both at the mRNA and
protein levels in bladder cancer cells. Our experimental data
further showed that inhibition of MDM2 exerted a suppressive effect
similar to miR-379-5p overexpression in bladder cancer cells. MDM2
overexpression rescued miR-379-5p-induced effects on bladder cancer
cell proliferation, migration and invasion. Moreover, MDM2 was
highly expressed in bladder cancer tissues compared with that in
adjacent normal tissues, which was negatively correlated with
miR-379-5p expression patterns. These results suggest that
miR-379-5p plays a tumor-suppression role in bladder cancer partly
by targeting MDM2.
MDM2, which is located on chromosome 12q13.14, is a
proto-oncogene that was firstly identified in a locus amplified on
double minute chromosomes in a tumorigenic mouse cell line (3T3-DM)
(28). Numerous studies have
reported that the MDM2 gene is amplified or overexpressed in
numerous types of human cancers, such as breast (29), lung (30) and colorectal cancer (31), and testicular germ cell tumors
(32). In bladder cancer, a study
conducted by Lianes et al showed that MDM2 was upregulated
in tumor tissues and displayed a significant correlation with
low-stage, low-grade bladder tumors of bladder cancer patients
(33). Tuna et al revealed
that MDM2 overexpression exhibits a significant association with
tumor grade and recurrence of bladder cancer and may be a valuable
parameter in predicting recurrence (34). Studies have also shown that MDM2
plays important roles during the tumorigenesis and progression of
bladder cancer (35,36). Consistent with previous findings,
our results demonstrated that MDM2 knockdown exerts
anti-proliferative and anti-metastasis effects on bladder cancer.
MDM2 is a potential prognostic and therapeutic target for patients
with bladder cancer.
In conclusion, miR-379-5p was downregulated in
bladder cancer tissues and cell lines and suppressed cell
proliferation, migration and invasion by directly targeting MDM2.
These results suggest that miR-379-5p/MDM2-based targeted therapy
may be a promising therapeutic treatment for bladder cancer in the
future.
Acknowledgements
The present study was supported by a grant from the
National Natural Science Foundation of China (no. 81270685).
References
|
1
|
Zhang Y, Sun Y, Chen T, Hu H, Xie W, Qiao
Z, Ding N, Xie L, Li S, Wang W, et al: Genetic variations
rs11892031 and rs401681 are associated with bladder cancer risk in
a Chinese population. Int J Mol Sci. 15:19330–19341. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Knowles MA: Molecular pathogenesis of
bladder cancer. Int J Clin Oncol. 13:287–297. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Scélo G and Brennan P: The epidemiology of
bladder and kidney cancer. Nat Clin Pract Urol. 4:205–217. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wu D, Zhou Y, Pan H, Zhou J, Fan Y and Qu
P: microRNA-99a inhibiting cell proliferation, migration and
invasion by targeting fibroblast growth factor receptor 3 in
bladder cancer. Oncol Lett. 7:1219–1224. 2014.PubMed/NCBI
|
|
6
|
Kim WJ and Bae SC: Molecular biomarkers in
urothelial bladder cancer. Cancer Sci. 99:646–652. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ambros V: MicroRNA pathways in flies and
worms: Growth, death, fat, stress, and timing. Cell. 113:673–676.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu W, Sun M, Zou GM and Chen J: MicroRNA
and cancer: Current status and prospective. Int J Cancer.
120:953–960. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nelson KM and Weiss GJ: MicroRNAs and
cancer: Past, present, and potential future. Mol Cancer Ther.
7:3655–3660. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Garzon R, Fabbri M, Cimmino A, Calin GA
and Croce CM: MicroRNA expression and function in cancer. Trends
Mol Med. 12:580–587. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Alvarez-Garcia I and Miska EA: MicroRNA
functions in animal development and human disease. Development.
132:4653–4662. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tana C, Giamberardino MA and Cipollone F:
microRNA profiling in atherosclerosis, diabetes and migraine. Ann
Med. 49:93–105. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Piletič K and Kunej T: MicroRNA epigenetic
signatures in human disease. Arch Toxicol. 90:2405–2419. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shang Y, Zang A, Li J, Jia Y, Li X, Zhang
L, Huo R, Yang J, Feng J, Ge K, et al: MicroRNA-383 is a tumor
suppressor and potential prognostic biomarker in human non-small
cell lung caner. Biomed Pharmacother. 83:1175–1181. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Calin GA and Croce CM: MicroRNA signatures
in human cancers. Nat Rev Cancer. 6:857–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Khan S, Brougham CL, Ryan J, Sahrudin A,
O'Neill G, Wall D, Curran C, Newell J, Kerin MJ and Dwyer RM:
miR-379 regulates cyclin B1 expression and is decreased in breast
cancer. PLoS One. 8:e687532013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen JS, Li HS, Huang JQ, Dong SH, Huang
ZJ, Yi W, Zhan GF, Feng JT, Sun JC and Huang XH: MicroRNA-379-5p
inhibits tumor invasion and metastasis by targeting FAK/AKT
signaling in hepatocellular carcinoma. Cancer Lett. 375:73–83.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen JS, Huang JQ, Dong SH and Huang XH:
Effects of microRNA-379-5p on proliferation, migration and invasion
of hepatocellular carcinoma cell line. Zhonghua Yi Xue Za Zhi.
96:1450–1453. 2016.(In Chinese). PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2ΔΔCT method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhong XY, Yu JH, Zhang WG, Wang ZD, Dong
Q, Tai S, Cui YF and Li H: MicroRNA-421 functions as an oncogenic
miRNA in biliary tract cancer through down-regulating farnesoid X
receptor expression. Gene. 493:44–51. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wu D, Niu X, Pan H, Zhou Y, Qu P and Zhou
J: MicroRNA-335 is downregulated in bladder cancer and inhibits
cell growth, migration and invasion via targeting ROCK1. Mol Med
Rep. 13:4379–4385. 2016.PubMed/NCBI
|
|
24
|
Wang X, Liu Y, Liu X, Yang J, Teng G,
Zhang L and Zhou C: MiR-124 inhibits cell proliferation, migration
and invasion by directly targeting SOX9 in lung adenocarcinoma.
Oncol Rep. 35:3115–3121. 2016.PubMed/NCBI
|
|
25
|
Baker M: RNA interference: MicroRNAs as
biomarkers. Nature. 464:12272010. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gururajan M, Josson S, Chu GC, Lu CL, Lu
YT, Haga CL, Zhau HE, Liu C, Lichterman J, Duan P, et al: miR-154*
and miR-379 in the DLK1-DIO3 microRNA mega-cluster regulate
epithelial to mesenchymal transition and bone metastasis of
prostate cancer. Clin Cancer Res. 20:6559–6569. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Osada H and Takahashi T: MicroRNAs in
biological processes and carcinogenesis. Carcinogenesis. 28:2–12.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pichiorri F, Suh SS, Rocci A, De Luca L,
Taccioli C, Santhanam R, Zhou W, Benson DM Jr, Hofmainster C, Alder
H, et al: Downregulation of p53-inducible microRNAs 192, 194, and
215 impairs the p53/MDM2 autoregulatory loop in multiple myeloma
development. Cancer Cell. 18:349–351. 2016. View Article : Google Scholar
|
|
29
|
Marchetti A, Buttitta F, Girlando S, Palma
P Dalla, Pellegrini S, Fina P, Doglioni C, Bevilacqua G and
Barbareschi M: mdm2 gene alterations and mdm2 protein expression in
breast carcinomas. J Pathol. 175:31–38. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Marchetti A, Buttitta F, Pellegrini S,
Merlo G, Chella A, Angeletti CA and Bevilacqua G: mdm2 gene
amplification and overexpression in non-small cell lung carcinomas
with accumulation of the p53 protein in the absence of p53 gene
mutations. Diagn Mol Pathol. 4:93–97. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang C, Liu J, Wang X, Wu R, Lin M,
Laddha SV, Yang Q, Chan CS and Feng Z: MicroRNA-339-5p inhibits
colorectal tumorigenesis through regulation of the MDM2/p53
signaling. Oncotarget. 5:9106–9117. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Riou G, Barrois M, Prost S, Terrier MJ,
Theodore C and Levine AJ: The p53 and mdm-2 genes in human
testicular germ-cell tumors. Mol Carcinog. 12:124–131. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lianes P, Orlow I, Zhang ZF, Oliva MR,
Sarkis AS, Reuter VE and Cordon-Cardo C: Altered patterns of MDM2
and TP53 expression in human bladder cancer. J Natl Cancer Inst.
86:1325–1330. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tuna B, Yörükoğlu K, Tüzel E, Güray M,
Mungan U and Kirkali Z: Expression of p53 and mdm2 and their
significance in recurrence of superficial bladder cancer. Pathol
Res Pract. 199:323–328. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Schmitz-Dräger BJ, Kushima M, Goebell P,
Jax TW, Gerharz CD, Bültel H, Schulz WA, Ebert T and Ackermann R:
p53 and MDM2 in the development and progression of bladder cancer.
Eur Urol. 32:487–493. 1997.PubMed/NCBI
|
|
36
|
Shiina H, Igawa M, Shigeno K, Yamasaki Y,
Urakami S, Yoneda T, Wada Y, Honda S and Nagasaki M: Clinical
significance of mdm2 and p53 expression in bladder cancer. A
comparison with cell proliferation and apoptosis. Oncology.
56:239–247. 1999. View Article : Google Scholar : PubMed/NCBI
|