Introduction
Hepatitis B virus (HBV) infection is closely
associated with liver cirrhosis and hepatocellular carcinoma (HCC)
occurrence and development, and may lead to acute and chronic
hepatitis (1). Worldwide, ~2
billion people are infected with HBV. China has a particularly high
incidence of hepatitis B, accounting for ~93 million of the 350
million global HBV carriers (2).
Approximately 25 million patients develop chronic hepatitis B
(3). Annually, ~1 million people
die of hepatic failure, liver cirrhosis or primary HCC caused by
HBV infection (4).
Primary HCC is one of the most common types of
cancer in China. Approximately 90% of primary liver cancer cases
are HCC and 5% are cholangiocarcinoma (cancer of the bile duct
cells) (5). However, mixed liver
cancer is extremely rare. The etiology and pathogenesis of the
disease has not yet been fully determined (6). In China, ~90% of liver cancer patients
have a background of HBV infection. Primary HCC has a high
mortality rate (7). The number
patients who die from liver cancer exceeds 110,000 annually
(8). Liver cancer ranks as the
third most common cause of cancer-associated mortality, after
gastric cancer and esophageal cancer.
MicroRNAs (miRNAs) are non-protein coding gene
regulators that have important regulatory effects on gene
expression in eukaryotes (9). By
complementary pairing in the 3′ untranslated region of the
protein-coding mRNA, the seed sequence of miRNAs alters the
expression of the target gene; expression is primarily regulated
via degradation of the target mRNA or via inhibition of its
translation (10). Research has
shown that miRNAs are important contributors to tumor development,
migration and propagation, and may act as oncogenes or tumor
suppressor genes (11). In
addition, recent studies demonstrated that miRNAs play key roles in
the occurrence and development of liver cancer; miRNAs can act to
inhibit the gene expression of numerous important coding proteins
in this process (12).
There are 8 members of the Smad protein family in
mammals, and Smads play an important role in transforming growth
factor (TGF)-β signaling (13). The
primary function of the Smad proteins is to transduce TGF-β-induced
signals within cells (14). Smad
proteins, which are the direct substrates of TGF-β receptors
(TβRs), transfer the TGF-β signal from the cytoplasm to the
nucleus, and regulate corresponding target gene transcription along
with other transcription factors. Recent research revealed that the
TGF-β/Smad pathway plays an important role in the development of
liver cancer (15). In the present
study, the association between microRNA-15a (miR-15a)/Smad-7/TGF-β
signaling and HBV-associated liver cancer was analyzed, and the
effects of miR-15a/Smad-7/TGF-β on cell proliferation and apoptosis
in HBV-associated liver cancer were evaluated.
Materials and methods
Patient samples
A total of 16 patients with HBV-associated HCC
treated at the Binzhou Tuberculosis Prevention and Control Hospital
(Shandong, China) were included in the present study. HCC and
adjacent liver tissue samples were obtained from these patients
following the acquisition of informed consent.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was extracted from tissues or cells using
Invitrogen TRIzol reagent (Thermo Fisher Scientific, Inc., Waltham,
MA, USA) according to the manufacturer's instructions. Total RNA (2
µg) was used to synthesize cDNA using an All-in-One™ miRNA
First-Strand cDNA Synthesis kit (GeneCopoeia, Inc., Rockville, MD,
USA). qPCR was performed on an Applied Biosystems 7500 Real-Time
PCR System (Thermo Fisher Scientific, Inc.) using the following
conditions: 95°C for 10 min, followed by 40 cycles of 95°C for 15
sec, 60°C for 30 sec, and 72°C for 40 sec. Relative expression was
quantified by using the comparative quantification cycle
(2−ΔΔCq) method.
Western blot analysis
Tissue samples were incubated with 100 µl of lysis
buffer, and cell lines were lysed with 100 ml of pre-cooled cell
lysis buffer (both from Beyotime Institute of Biotechnology,
Shanghai, China) for 30 min on ice. The protein concentration was
determined by the bicinchoninic acid method (Beyotime Institute of
Biotechnology) and 50 µg of total protein from each sample was
separated by SDS-PAGE prior to transfer onto polyvinylidene
difluoride membranes (EMD Millipore, Billerica, MA, USA). Membranes
were blocked with 5% skim milk in TBS-Tween (20 mM Tris-HCl, 150 mM
NaCl and 0.05% Tween-20), followed by incubation with the following
mouse anti-human primary antibodies from Santa Cruz Biotechnology,
Inc. (Dallas, TX, USA): anti-Smad-7 (dilution, 1:4,000),
anti-TGF-β1 (dilution, 1:2,000), anti-Smad-2 (dilution, 1:3,000),
anti-phosphorylated (p)-Smad-2 (dilution, 1:3,000), anti-Smad-4
(dilution, 1:4,000), anti-fibroblast-specific protein 1 (FSP1)
(dilution, 1:2,000) and anti-β-actin (dilution, 1:5,000). Membranes
were then incubated with goat anti-mouse secondary antibodies
conjugated to horseradish peroxidase (dilution, 1:5,000; Santa Cruz
Biotechnology, Inc.) and visualized by enhanced chemiluminescence
(Amersham ECL Prime Western Blotting Detection Reagent; GE
Healthcare Life Sciences, Piscataway, NJ, USA).
Cell culture and transfection
HepG2 and SMMC-7721 human HCC cell lines were
integrated with the HBV genome. L-02, HepG2 and SMMC-7721 cells
were cultured in Gibco RPMI-1640 medium (Thermo Fisher Scientific,
Inc.) containing 10% fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc.), 100 U/ml penicillin and 100 µg/ml streptomycin,
at 37°C in an atmosphere of 5% CO2. Anti-miR-15a plasmid
(50 nM) or a negative control plasmid (50 nM) were transfected into
cells using Invitrogen Lipofectamine 2000 reagent (Thermo Fisher
Scientific, Inc.).
Cell viability analysis
HepG2 cells were seeded into 96-well plates and
incubated for the indicated times. Following incubation, 50 µl of
MTT (0.5 mg/ml; Invitrogen; Thermo Fisher Scientific, Inc.) was
added, and the plates were incubated at 37°C for 2 h. Subsequently,
100 µl of dimethyl sulfoxide (Invitrogen; Thermo Fisher Scientific,
Inc.) was added to each well. Absorbance at 550 nm was assessed
using a luminometer plate-reader.
Caspase-3/-7 activity
HepG2 cells were seeded into 96-well plates and
incubated for the indicated times. The cell lysates were harvested
and analyzed using a Caspase-3/-7 Assay kit (Promega Corporation,
Madison, WI, USA). Caspase-3/-7 activity was assessed with a
luminometer plate-reader at a wavelength of 490 nm.
Statistical analysis
Differences between groups were determined using
Student's t-test. All data are presented as the mean ± standard
deviation. P<0.05 was considered to indicate a statistically
significant difference.
Results
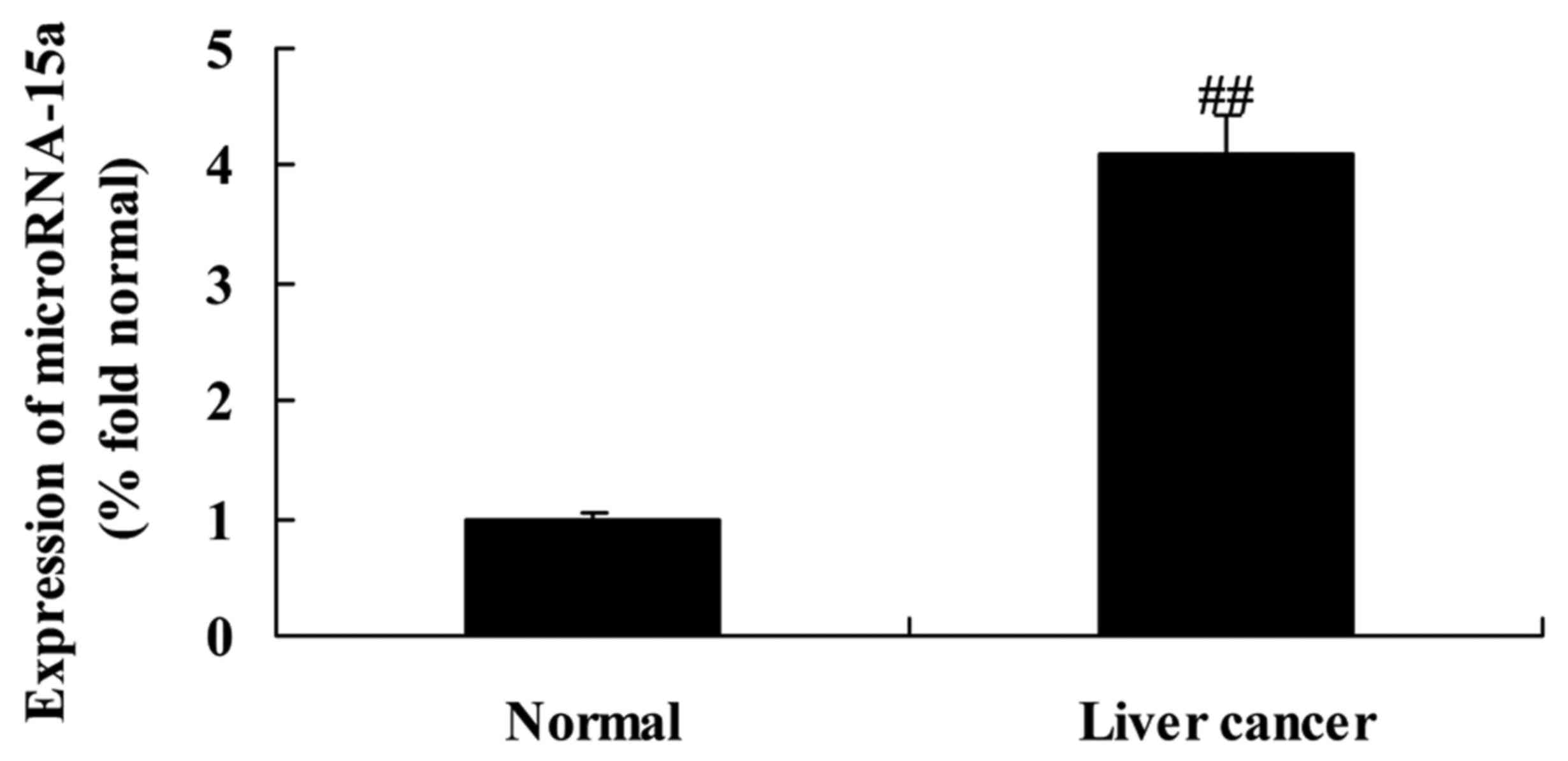
Expression of miR-15a in HBV-induced
liver cancer tissues
Our present study demonstrated that miR-15a
expression in normal tissue samples (adjacent liver tissue samples)
was lower than that in HBV-induced HCC tissue samples (Fig. 1).
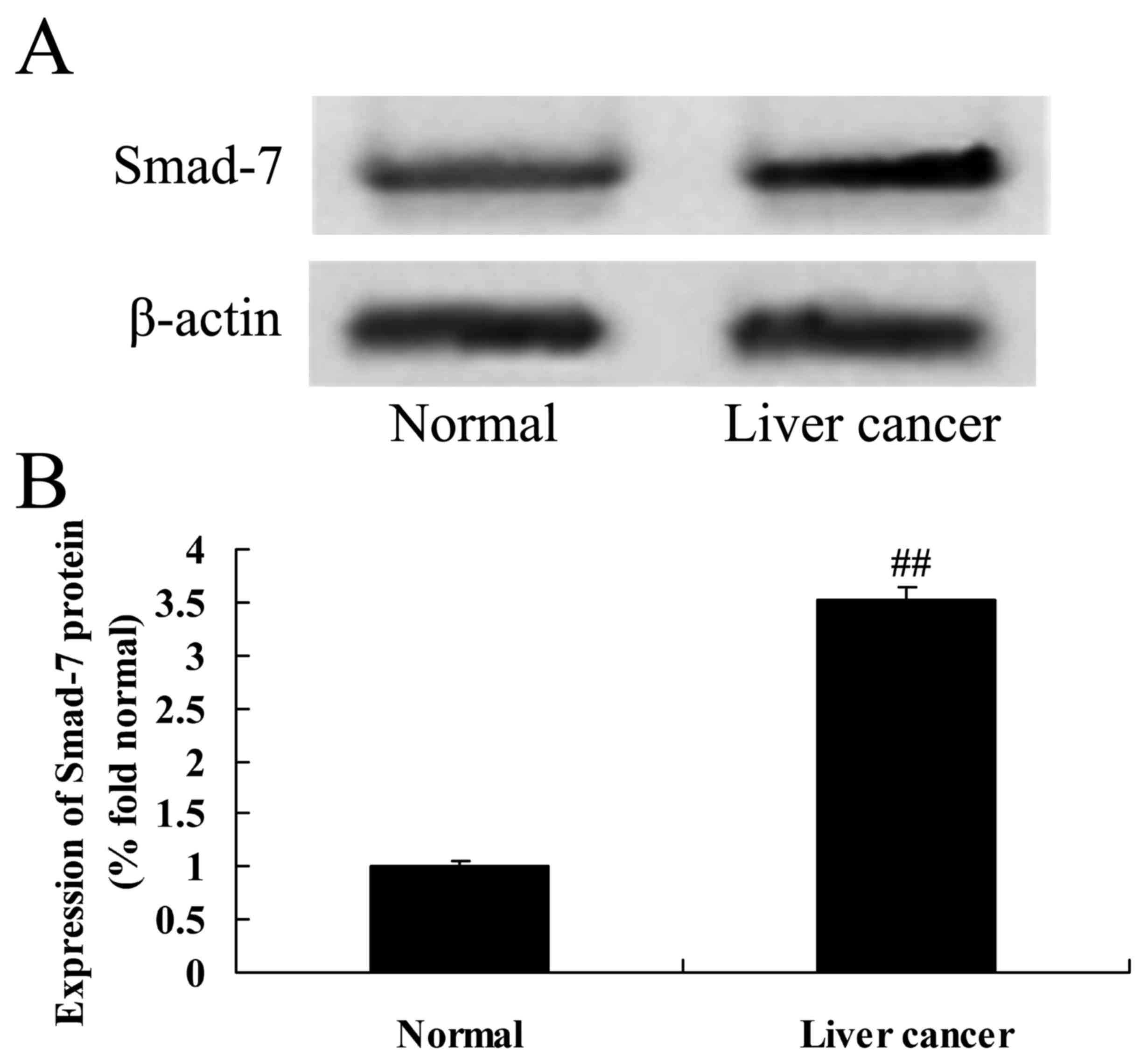
Expression of Smad-7 protein in
HBV-induced liver cancer tissues
The expression of Smad-7 protein was analyzed to
determine the importance of the HBV/miR-15a/Smad-7 pathway. As
demonstrated in Fig. 2, the protein
expression of Smad-7 in adjacent normal liver tissue samples was
lower than in HBV-induced HCC tissue samples.
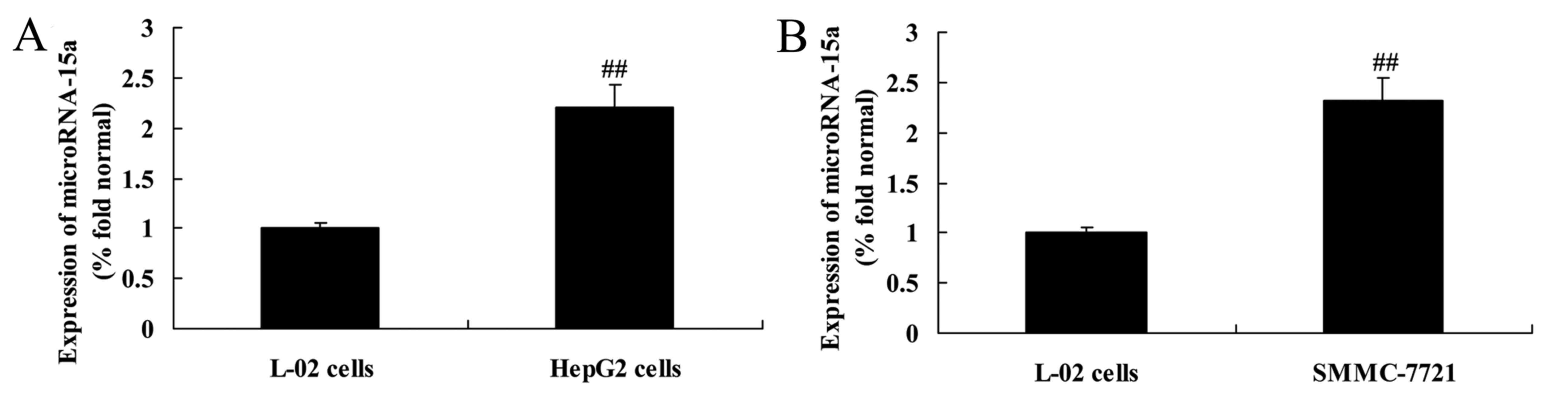
Expression of miR-15a in HepG2,
SMMC-7721 and L-02 cells
RT-qPCR was used to analyze the expression levels of
miR-15a in HepG2, SMMC-7721 and L-02 cells. The expression levels
of miR-15a in HBV-infected HepG2 and SMMC-7721 cells were higher
than that in the L-02 cells (Fig.
3).
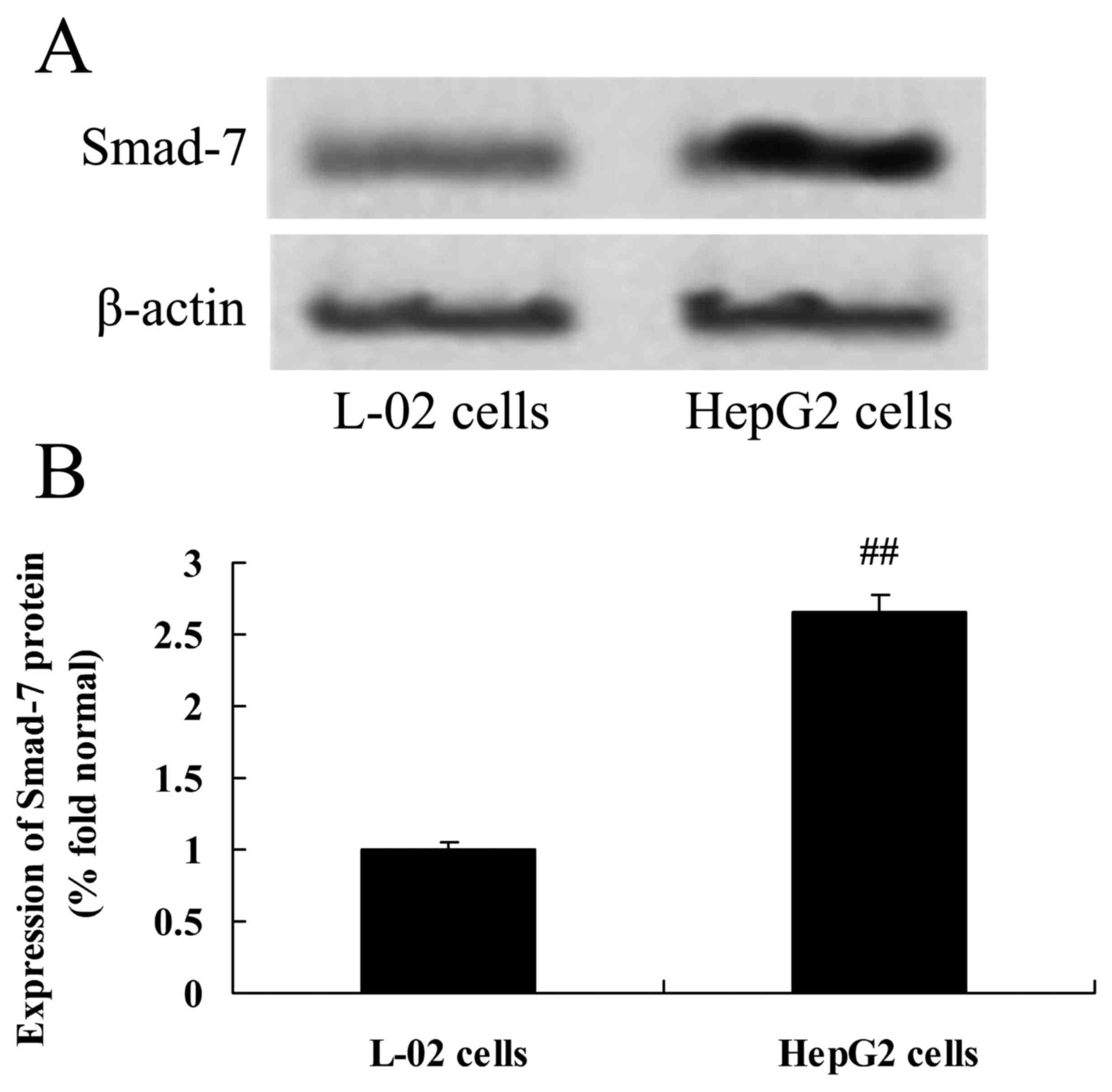
Expression of Smad-7 protein in HepG2
cells
Expression of Smad-7 protein in HepG2 cells was
examined using western blotting. The results indicated that,
compared with the control group (L-02 cells), HBV infection was
associated with significantly increased expression of Smad-7
protein in HepG2 cells (Fig.
4).
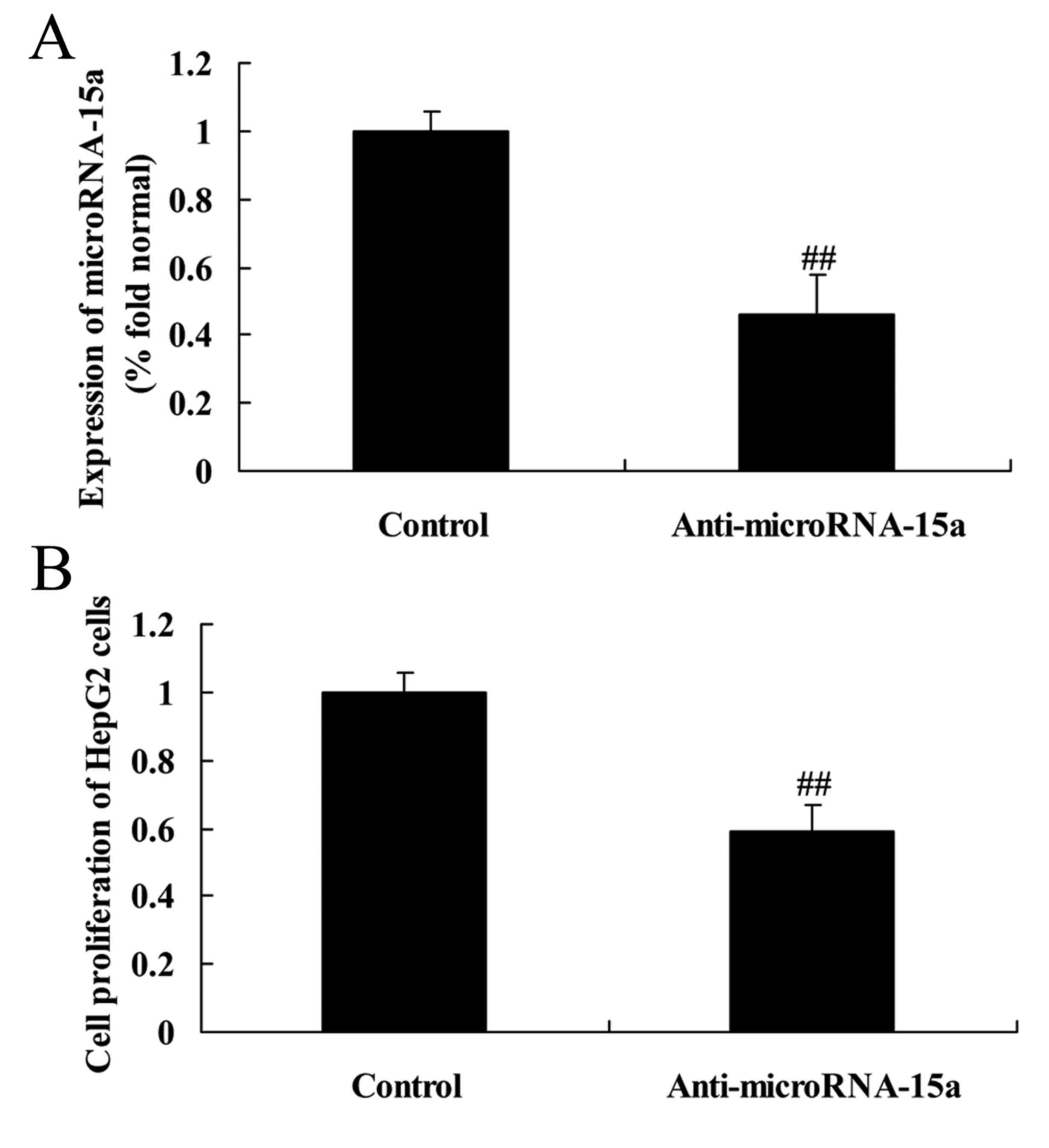
Effects of miR-15a on the viability of
HepG2 cells
To further understand the effects of miR-15a on the
viability of HepG2 cells, the cells were transfected with
anti-miR-15a or negative control plasmids, and the viability of
HepG2 cells was examined by MTT assay. The results revealed that
anti-miR-15a significantly decreased miR-15a expression compared
with the control group (cells transfected with negative control
plasmids) and was associated with decreased viability in HepG2
cells (Fig. 5).
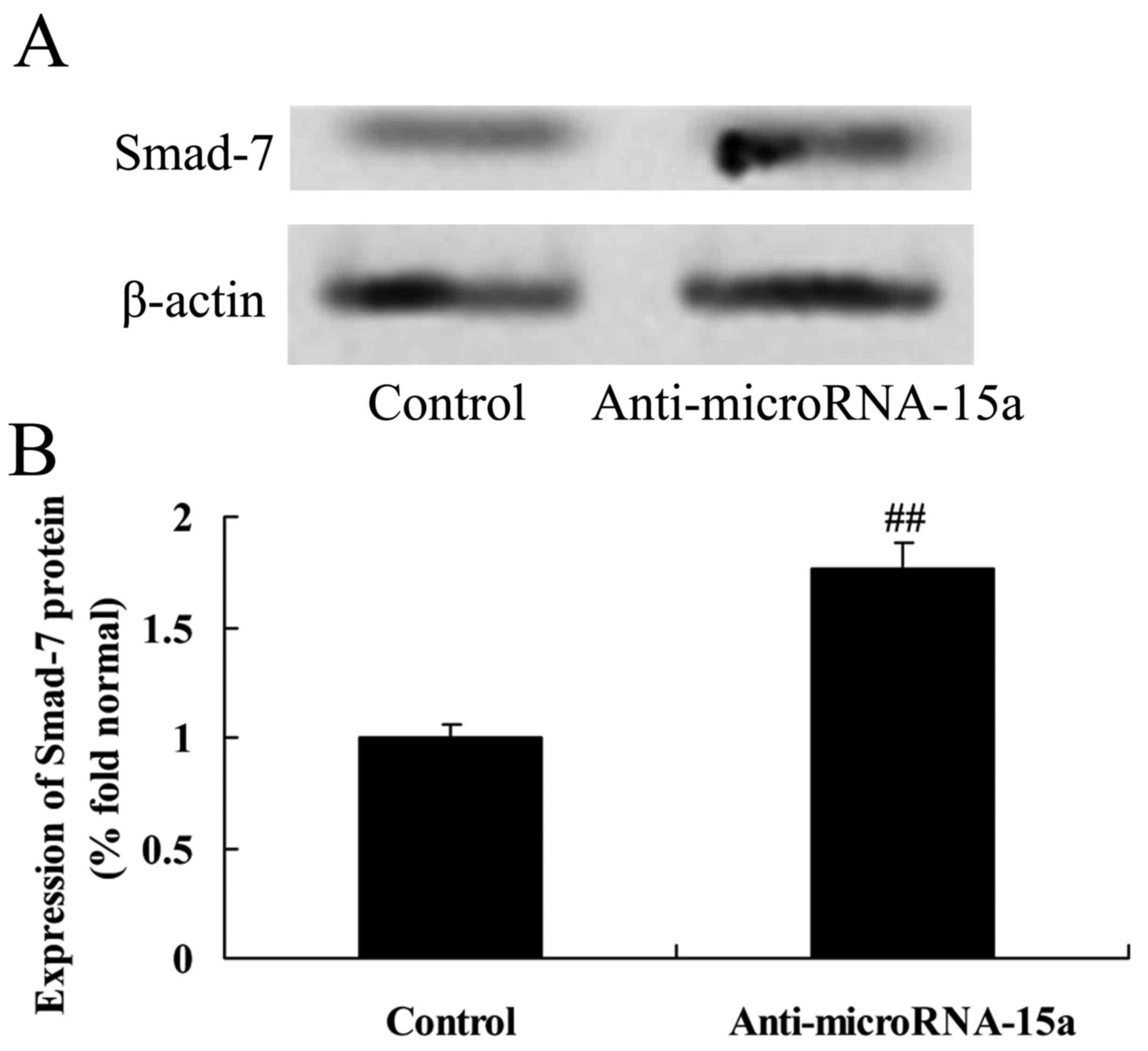
Effects of miR-15a on the expression
of Smad-7 protein in HepG2 cells
miR-15a was selected for further study since it is
an important regulator of Smad-7 signaling. As demonstrated in
Fig. 6, compared with control group
(cells transfected with negative control plasmids), anti-miR-15a
markedly upregulated the protein expression of Smad-7 in
HBV-infected HepG2 cells (Fig.
6).
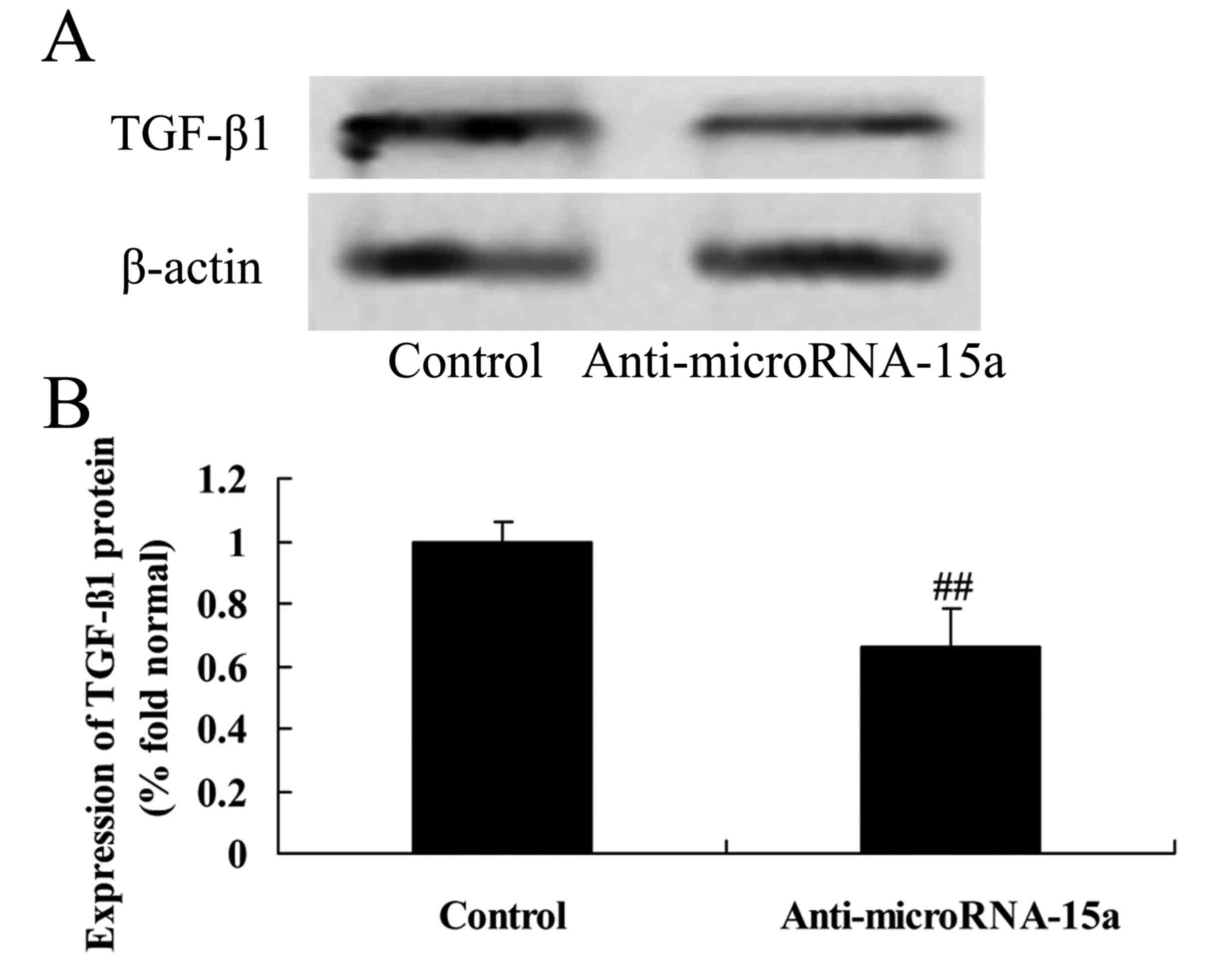
Effects of miR-15a on expression of
TGF-β1 protein in HepG2 cells
Subsequently, whether TGF-β1 is a potential target
of miR-15a was investigated using western blotting. The
anti-miR-15a plasmid was transfected into HBV-infected HepG2 cells,
and the results indicated that anti-miR-15a significantly
suppressed the expression of TGF-β1 protein in HepG2 cells relative
to the control group (cells transfected with negative control
plasmids; Fig. 7).
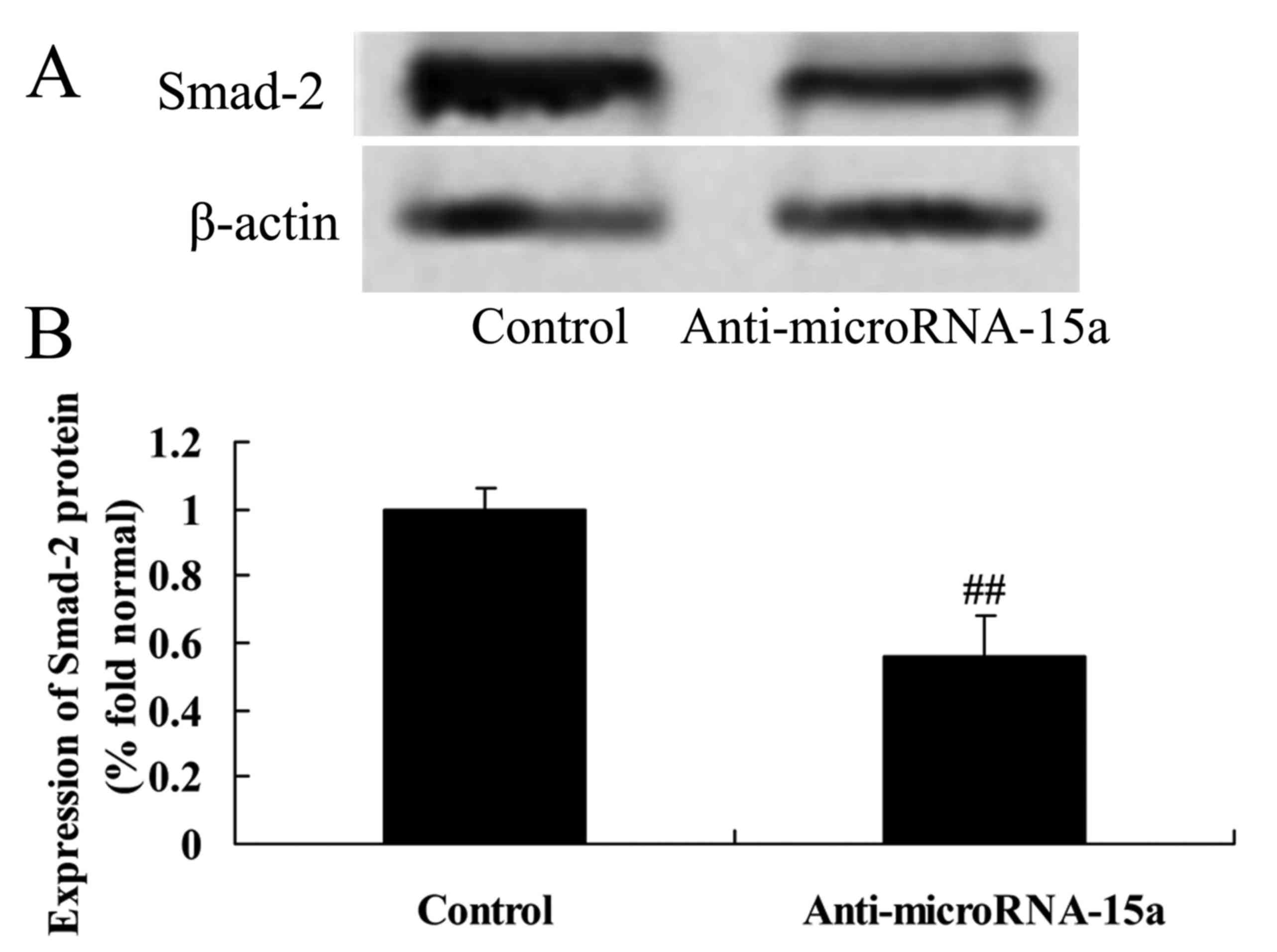
Effects of miR-15a on the expression
of Smad-2 and p-Smad-2 proteins in HepG2 cells
We confirmed whether miR-15a affected the level of
Smad-2 and p-Smad-2 proteins in HepG2 cells. As demonstrated in
Fig. 8, the protein expression
levels of Smad-2 and p-Smad-2 in HepG2 cells transfected with
anti-miR-15a was lower than that in the control group (cells
transfected with negative control plasmids).
Effects of miR-15a on the expression
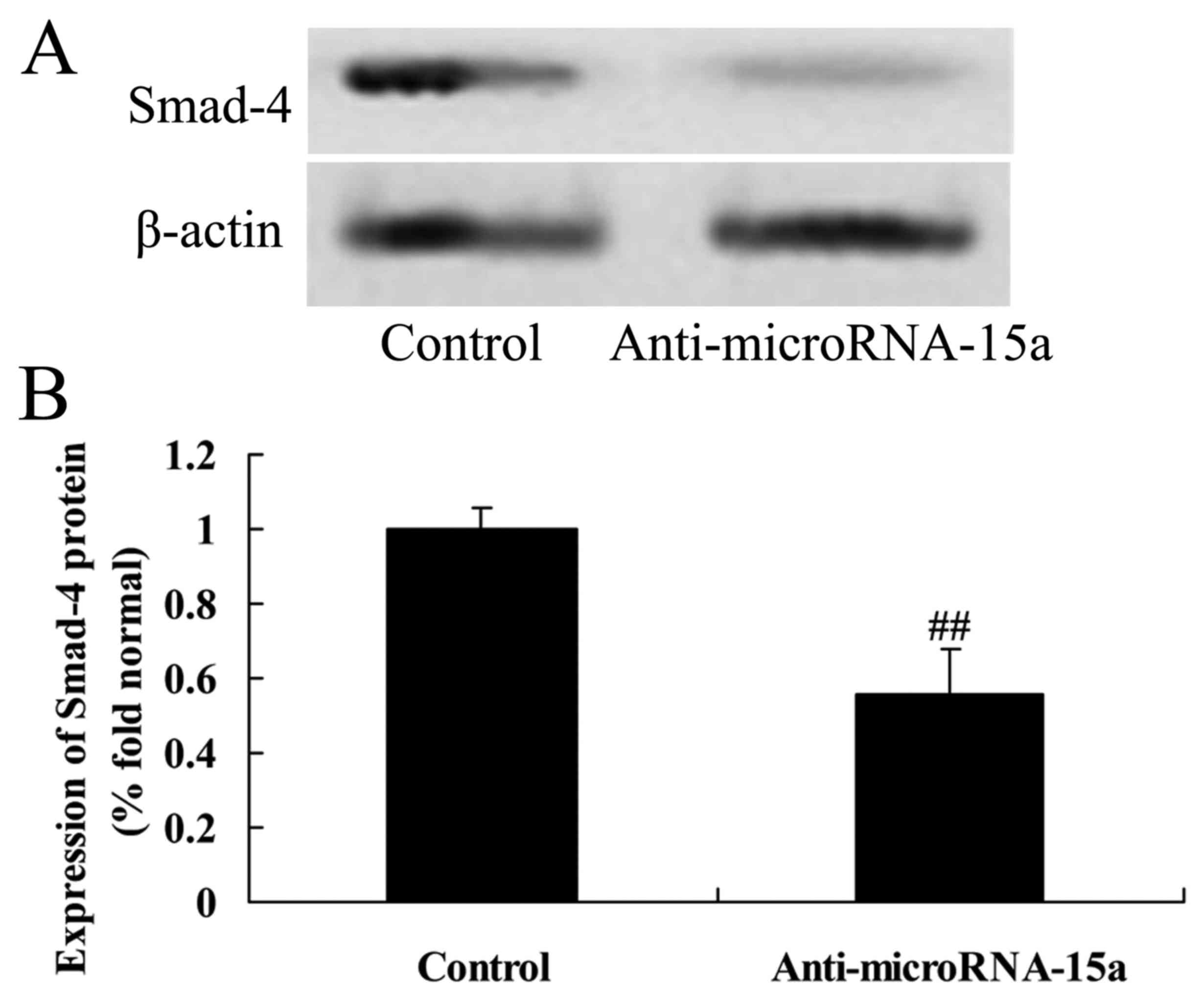
of Smad-4 protein in HepG2 cells
The association between miR-15a and Smad-4
expression in HBV-infected HepG2 cells was examined. As
demonstrated in Fig. 9, Smad-4
protein expression was markedly inhibited in HepG2 cells
transfected with anti-miR-15a compared with the control group
(cells transfected with negative control plasmids).
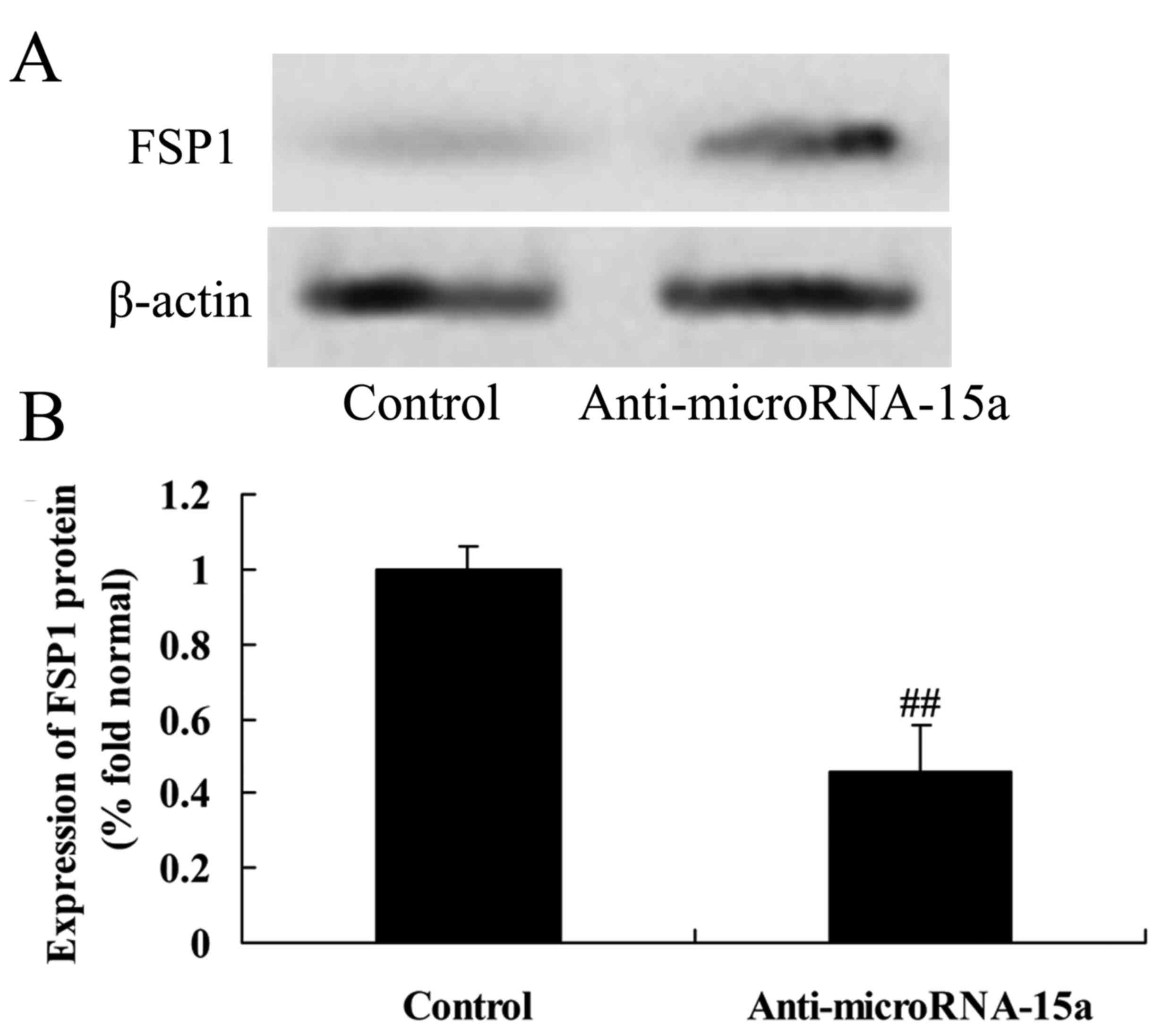
Effects of miR-15a on the expression
of FSP1 protein in HepG2 cells
HepG2 cells were transfected with anti-miR-15a, so,
as to analyze the expression of FSP1 protein in HBV-infected HepG2
cells. The results indicated that anti-miR-15a significantly
suppressed FSP1 protein expression in HBV-infected HepG2 cells
compared with the control group (cells transfected with negative
control plasmids; Fig. 10).
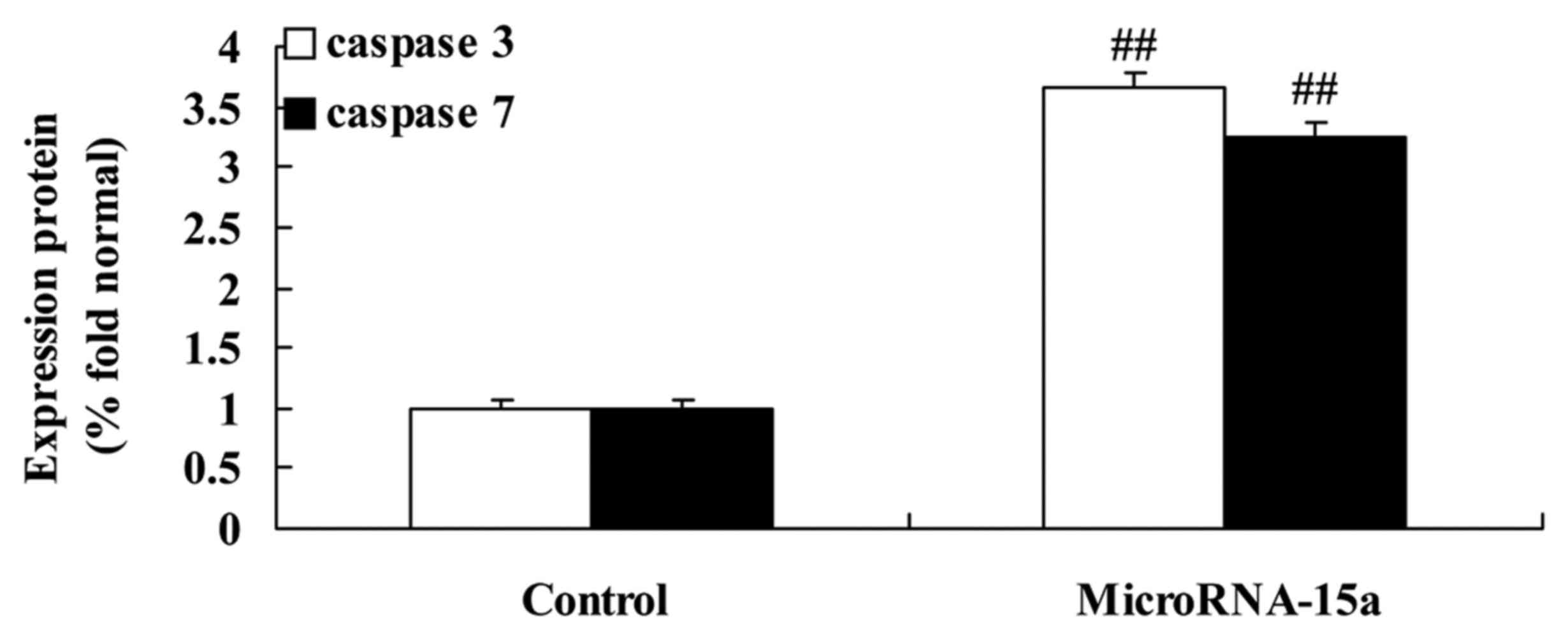
Effects of miR-15a on caspase-3/-7
activities in HepG2 cells
The association between miR-15a expression and
caspase-3/-7 activities in HepG2 cells were analyzed. Consistently,
the activities of caspase-3/-7 in HepG2 cells transfected with
anti-miR-15a were higher than those in the control group (cells
transfected with negative control plasmids), as demonstrated in
Fig. 11.
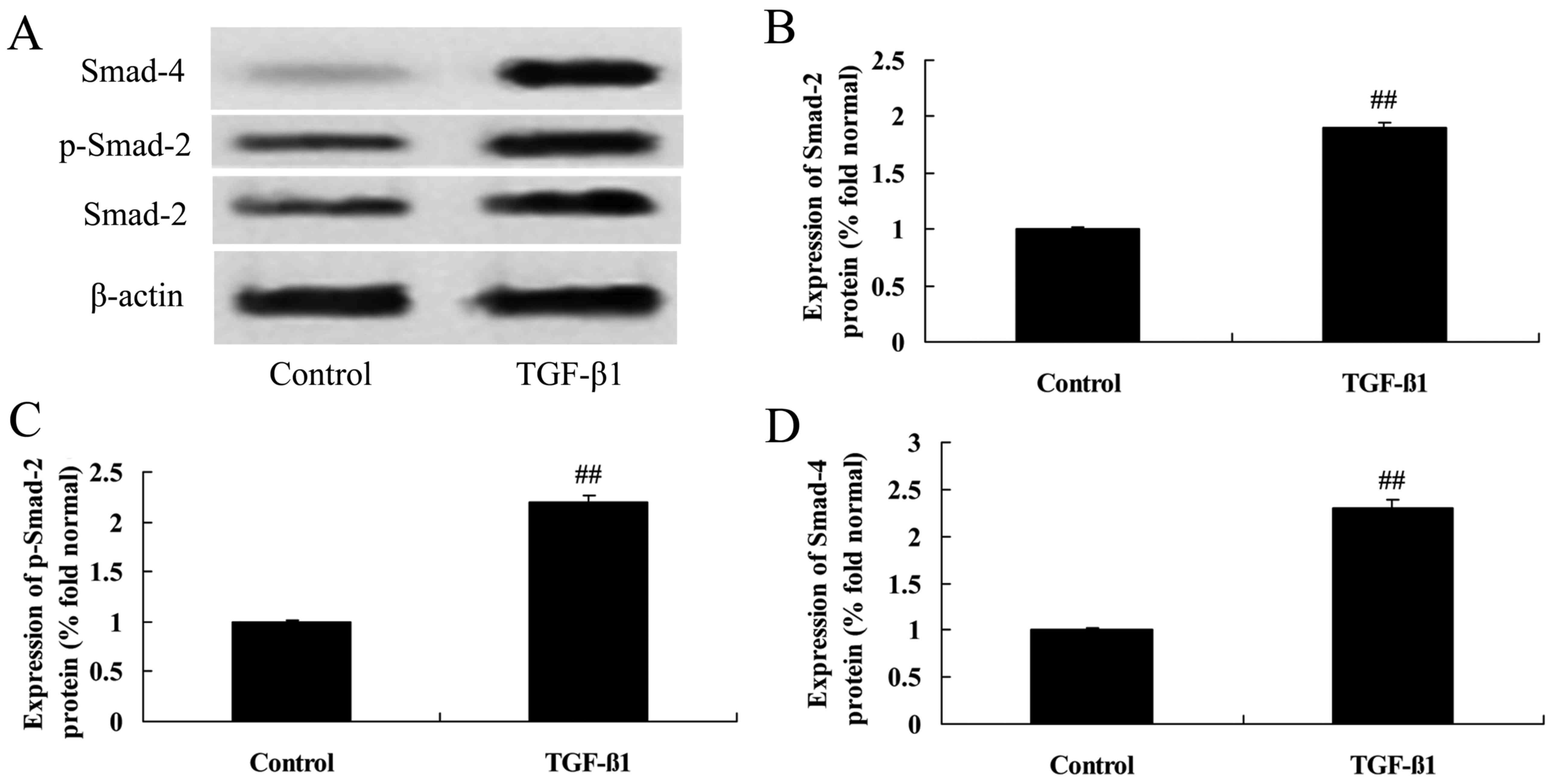
Effects of TGF-β1 treatment on
Smad-2/-4 and p-Smad-2 protein expression in HepG2 cells
Human recombinant TGF-β1 was purchased from
BioLegend (San Diego, CA, USA). TGF-β1 (at concentrations of 0, 2,
4 or 8 ng/ml) was added into each well for 24 h of culture. As
demonstrated in Fig. 12, TGF-β1 at
concentrations of 4 or 8 ng/ml markedly enhanced the levels of
Smad-2, p-Smad-2 and Smad-4 in HepG2 cells.
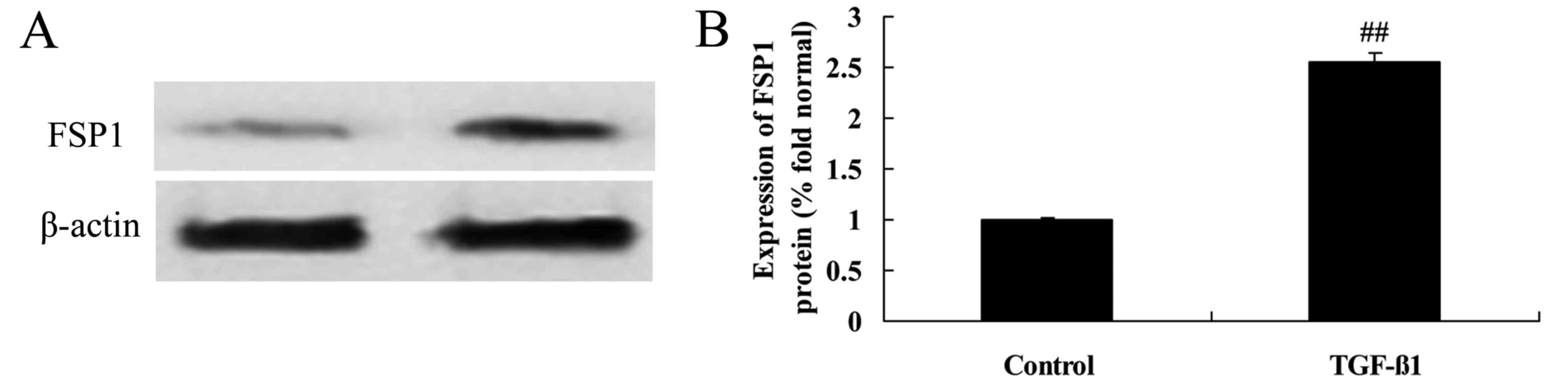
Effects of TGF-β1 treatment on FSP1
protein expression in HepG2 cells
The effects of TGF-β1 treatment on FSP1 protein
expression in HepG2 cells were determined using western blotting.
As demonstrated in Fig. 13, 8
ng/ml of TGF-β1 distinctly promoted FSP1 protein expression in
HepG2 cells.
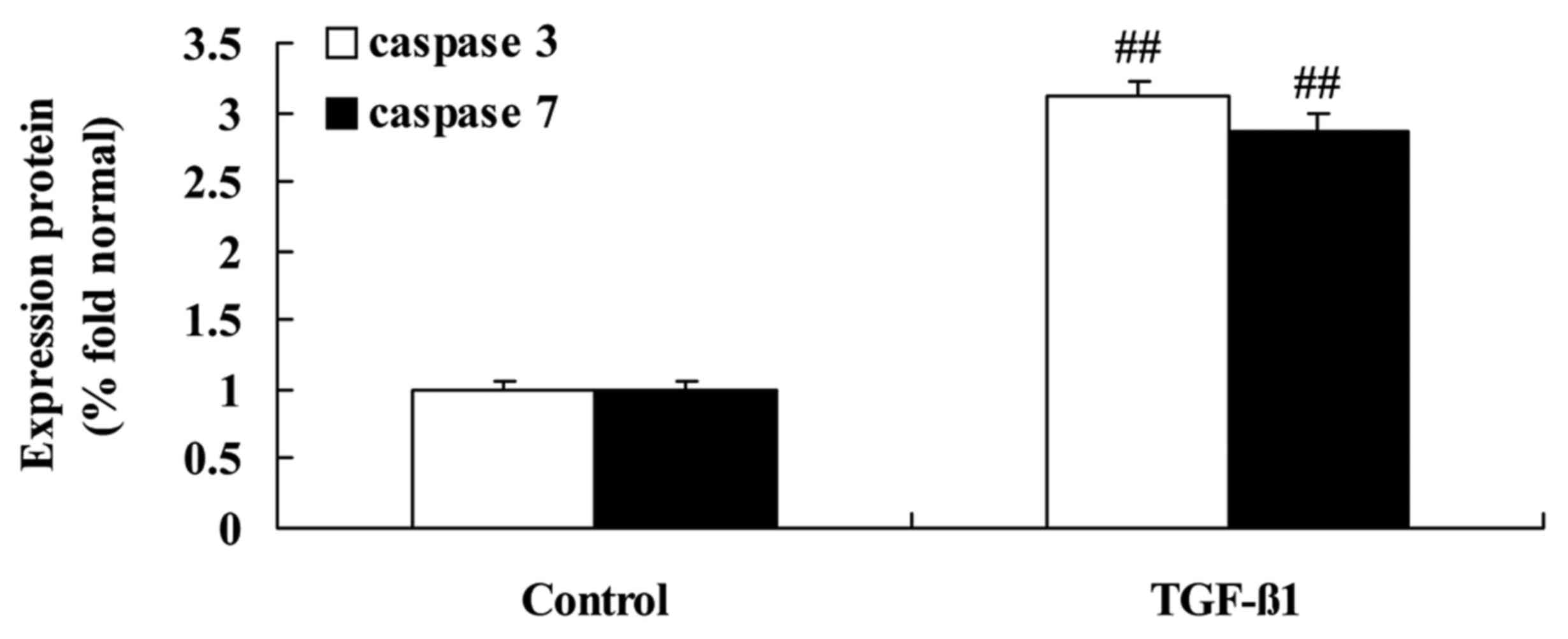
Effects of TGF-β1 treatment on
caspase-3/-7 activities in HepG2 cells
The effect of TGF-β1 treatment on the apoptosis of
HepG2 cells was analyzed by assessing caspase activity. As
demonstrated in Fig. 14, TGF-β1 at
concentrations of 4 or 8 ng/ml activated caspase-3/-7 activities in
HepG2 cells, indicating the induction of apoptosis.
Discussion
Globally, ~2 billion people are infected with HBV,
and HBV infection is particularly prevalent in China (3). The gross population of HBV infection
is estimated to be ~100 million (3). HBV infection leads to repeated damage
and repair of liver tissues and may thus lead to chronic hepatitis
B, cirrhosis and HCC (16). As a
malignant tumor with extremely high rates of morbidity and
mortality, HCC is an important manifestation of poor prognosis in
patients with HBV infection (17).
The majority of patients with HCC are middle-aged males; therefore,
HBV infection and associated HCC can cause significant economic
pressure and mental stress for patients, their families and society
(18). Early diagnosis and early
treatment are the most fundamental measures to improve the
prognosis of patients with HCC. However, the early diagnosis of HCC
remains challenging (19).
The present study assessed HBV-induced miR-15a
expression in patients with HCC. miRNAs have important regulatory
effects on various activities of the body (9), and specific miRNA expression profiles
are observed in different types of cancer. Therefore, miRNAs are
regarded as potential markers for cancer classification and have
important clinical significance (12). Research has demonstrated that miRNA
expression is stable in circulating blood, and may be useful as
prognostic and diagnostic markers for diseases, particularly tumors
(20). In the present study,
anti-miR-15a was demonstrated to significantly decrease HepG2 cell
viability.
Smad-7, an inhibitory Smad, has important effects.
Smad-7 competitively associates with the TβR1-type receptor and
suppresses Smad phosphorylation; thus, Smad-7 regulates TGF-β/Smad
signaling via negative feedback and maintains the balance of the
pathway (13). Dysregulated Smad-7
expression may affect cell responses to TGF-β and promote the
malignant progression of cells (21). TGF-β strongly induces Smad-7
expression. In the TGF-β signaling pathway, Smad-7 is a
self-regulating negative feedback signal, and TGF-β signal strength
and duration is determined by Smad-7 levels in cells (22). Endogenous Smad-7 can inhibit TGF-β
signaling in a number of ways. It can inhibit TGF-β signal
transduction via combining with TβR1 competitively and inhibiting
receptor-regulated Smad phosphorylation (23). Research has also shown that, when
Smad-7 accumulates in the cytoplasm, it combines with activated
TβRI via its MH2 domain to form a stable complex, blocking the
transduction of the TGF-β signal (24). Smad-7 can thus decrease cell
reactivity to TGF-β. The aforementioned processes occur in the
cytoplasm (24). Smad-7 can also
decrease histone acetylation to inhibit transcription. Smad-7
combines with DNA competitively via its MH2 structural domain,
thereby restricting the formation of functional Smad-DNA complexes
induced by TGF-β signaling (15).
This competitive mechanism is widespread in the negative feedback
Smad-7/TGF-β signaling pathway (15). In the present study, the following
two aspects were validated experimentally. Firstly, increased
Smad-7 protein expression was detected in HCC patients with HBV
infection compared with normal tissue samples. Secondly,
transfection with anti-microRNA-15a led to significant activation
of Smad-7 protein expression in HepG2 cells infected with HBV.
A previous study, demonstrated that the expression
of Smad-7 protein in HCC tissue was markedly higher than that in
carcinoma-adjacent and normal tissues, the anticancer effect of
TGF-β/Smad signaling was suppressed and the development of liver
cancer was promoted (25). In
another study, following the transfection of mouse hepatic cells
with a Smad-7-containing adenovirus, Smad-7 was observed to
decrease the intranuclear accumulation of Smads, including Smad3
induced by activin A, promote DNA synthesis stimulated by
epithelial growth factor and alleviate the growth-inhibitory effect
of activin A on hepatic cells (26). Furthermore, a study by Liu et
al (27) revealed that, after
an exogenous Smad-7 gene was transferred into L-02 hepatic cells,
Smad-7 rescued the TGF-β-induced inhibition of L-02 hepatocellular
proliferation and its apoptosis-inducing effect (27). The present study, reported that HBV
induced apoptosis via the miR-15a/Smad-7/TGF-β pathway (27). The findings of the present study
indicated that miR-15a/Smad-7/TGF-β signaling suppressed the cell
growth of HBV-associated liver cancer via Smad-2/-4/TGF-β/FSP1 in
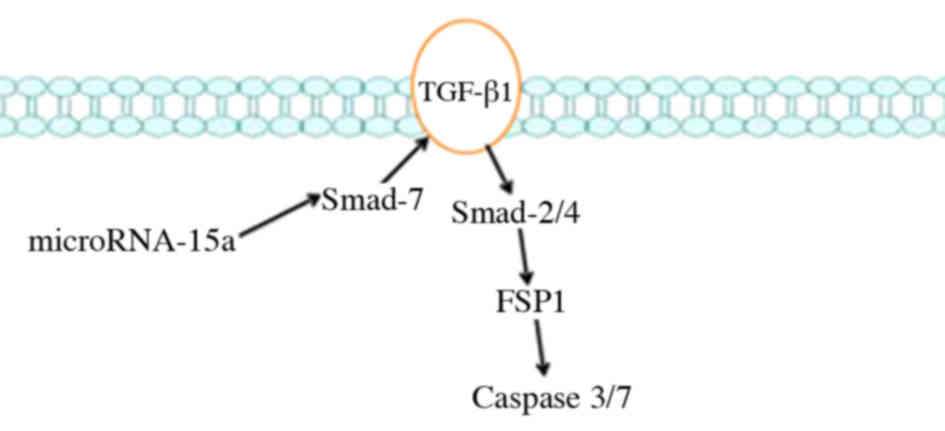
HepG2 cells. In summary, the results indicate an association
between HBV-associated liver cancer and miR-15a, which is a
critical regulator of Smad-7/TGF-β/Smad-2/-4/FSP1/caspase-3/-7
signaling (Fig. 15).
References
|
1
|
Andreotti M, Pirillo MF, Liotta G, Jere H,
Maulidi M, Sagno JB, Luhanga R, Amici R, Mancini MG, Gennaro E, et
al: The impact of HBV or HCV infection in a cohort of HIV-infected
pregnant women receiving a nevirapine-based antiretroviral regimen
in Malawi. BMC Infect Dis. 14:1802014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Celen MK, Mert D, Ay M, Dal T, Kaya S,
Yildirim N, Gulsun S, Barcin T, Kalkanli S, Dal MS, et al: Efficacy
and safety of tenofovir disoproxil fumarate in pregnancy for the
prevention of vertical transmission of HBV infection. World J.
19:9377–9382. 2013.
|
|
3
|
Ive P, MacLeod W, Mkumla N, Orrell C,
Jentsch U, Wallis CL, Stevens W, Wood R, Sanne I and Bhattacharya
D: Low prevalence of liver disease but regional differences in HBV
treatment characteristics mark HIV/HBV co-infection in a South
African HIV clinical trial. PLoS One. 8:e749002013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Amaddeo G, Cao Q, Ladeiro Y, Imbeaud S,
Nault JC, Jaoui D, Mathe Y Gaston, Laurent C, Laurent A,
Bioulac-Sage P, et al: Integration of tumour and viral genomic
characterizations in HBV-related hepatocellular carcinomas. Gut.
64:820–829. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
El-Serag H, McGlynn KA, Graham GN, So S,
Howell CD, Fang T, Anderson JT and Thiel TK: Achieving health
equity to eliminate racial, ethnic, and socioeconomic disparities
in HBV- and HCV-associated liver disease. J Fam Pract. 59 Suppl
4:S37–S42. 2010.PubMed/NCBI
|
|
6
|
Piao CY, Fujioka S, Iwasaki Y, Fujio K,
Kaneyoshi T, Araki Y, Hashimoto K, Senoh T, Terada R, Nishida T, et
al: Lamivudine treatment in patients with HBV-related
hepatocellular carcinoma - using an untreated, matched control
cohort. Acta Med Okayama. 59:217–224. 2005.PubMed/NCBI
|
|
7
|
Zheng J, Zeng Z, Zhang D, Yu Y, Wang F and
Pan CQ: Prevalence and significance of hepatitis B reverse
transcriptase mutants in different disease stages of untreated
patients. Liver Int. 32:1535–1542. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Qu C, Chen T, Fan C, Zhan Q, Wang Y, Lu J,
Lu LL, Ni Z, Huang F, Yao H, et al: Efficacy of neonatal HBV
vaccination on liver cancer and other liver diseases over 30-year
follow-up of the Qidong hepatitis B intervention study: A cluster
randomized controlled trial. PLoS Med. 11:e10017742014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu Q, Qin H, Zhao Q and He XX: Emerging
role of transcription factor-microRNA-target gene feed-forward
loops in cancer. Biomed Rep. 3:611–616. 2015.PubMed/NCBI
|
|
10
|
Gu L, Li H, Chen L, Ma X, Gao Y, Li X,
Zhang Y, Fan Y and Zhang X: MicroRNAs as prognostic molecular
signatures in renal cell carcinoma: A systematic review and
meta-analysis. Oncotarget. 6:32545–32560. 2015.PubMed/NCBI
|
|
11
|
Huang YK and Yu JC: Circulating microRNAs
and long non-coding RNAs in gastric cancer diagnosis: An update and
review. World J Gastroenterol. 21:9863–9886. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Anwar SL and Lehmann U: MicroRNAs:
Emerging novel clinical biomarkers for hepatocellular carcinomas. J
Clin Med. 4:1631–1650. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Macias MJ, Martin-Malpartida P and
Massagué J: Structural determinants of Smad function in TGF-β
signaling. Trends Biochem Sci. 40:296–308. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xu L, Tian D and Zheng Y: Pleiotropic
roles of TGFβ/Smad signaling in the progression of chronic liver
disease. Crit Rev Eukaryot Gene Expr. 23:237–255. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Heldin CH, Landström M and Moustakas A:
Mechanism of TGF-beta signaling to growth arrest, apoptosis, and
epithelial-mesenchymal transition. Curr Opin Cell Biol. 21:166–176.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gordon SC, Lamerato LE, Rupp LB, Li J,
Holmberg SD, Moorman AC, Spradling PR, Teshale EH, Vijayadeva V,
Boscarino JA, et al: CHeCS Investigators: Antiviral therapy for
chronic hepatitis B virus infection and development of
hepatocellular carcinoma in a US population. Clin Gastroenterol
Hepatol. 12:885–893. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Giannini EG, Savarino V, Risso D, Di Nolfo
MA, Del Poggio P, Benvegnù L, Farinati F, Zoli M, Borzio F,
Caturelli E, et al: Italian Liver Cancer (ITA.LI.CA.) group:
Relative decrease in the role played by hepatitis B virus infection
in the aetiology of hepatocellular carcinoma during a 20-year
period: A multicentre Italian study. Liver Int. 31:192–196. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shuqun C, Mengchao W, Han C, Feng S, Jiahe
Y, Wenming C, Zhengfeng Y, Yuxiang Z and Peijun W: Antiviral
therapy using lamivudine and thymosin alpha1 for hepatocellular
carcinoma coexisting with chronic hepatitis B infection.
Hepatogastroenterology. 53:249–252. 2006.PubMed/NCBI
|
|
19
|
Chung YH, Di Bisceglie AM, McMahon BJ,
Lanier AP, Harpster A, Alter MJ, Parkinson AJ and Zanis C:
Hepatocellular carcinoma not related to hepatitis B virus infection
among Alaska natives. Int J Circumpolar Health. 58:208–213.
1999.PubMed/NCBI
|
|
20
|
Greene CM, Varley RB and Lawless MW:
MicroRNAs and liver cancer associated with iron overload:
Therapeutic targets unravelled. World J Gastroenterol.
19:5212–5226. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cohen MM Jr: TGF beta/Smad signaling
system and its pathologic correlates. Am J Med Genet A. 116A:1–10.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kang Y: Pro-metastasis function of TGFbeta
mediated by the Smad pathway. J Cell Biochem. 98:1380–1390. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tang WB, Ling GH, Sun L and Liu FY: Smad
anchor for receptor activation (SARA) in TGF-beta signaling. Front
Biosci. 2:857–860. 2010.
|
|
24
|
Conidi A, van den Berghe V and Huylebroeck
D: Aptamers and their potential to selectively target aspects of
EGF, Wnt/β-catenin and TGFβ-smad family signaling. Int J Mol Sci.
14:6690–6719. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Matsuzaki K, Date M, Furukawa F, Tahashi
Y, Matsushita M, Sugano Y, Yamashiki N, Nakagawa T, Seki T,
Nishizawa M, et al: Regulatory mechanisms for transforming growth
factor beta as an autocrine inhibitor in human hepatocellular
carcinoma: Implications for roles of smads in its growth.
Hepatology. 32:218–227. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jiang Y, Wang C, Li YY, et al: Mistletoe
alkaloid fractions alleviates carbon tetrachloride-induced liver
fibrosis through inhibition of hepatic stellate cell activation via
TGF-beta/Smad interference. J Ethnopharmacol. 158(Pt A): 230–238.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu N, Jiao T, Huang Y, Liu W, Li Z and Ye
X: Hepatitis B virus regulates apoptosis and tumorigenesis through
the microRNA-15a-Smad-7-transforming growth factor beta pathway. J
Virol. 89:2739–2749. 2015. View Article : Google Scholar : PubMed/NCBI
|