Introduction
Gastric cancer is one of the most common malignant
tumors worldwide (1). Although
improvements in the early diagnosis and treatment have recently
been made, the morbidity and mortality rates are still high
(2). For patients with advanced
gastric cancer, chemotherapy is still the primary therapeutic
regimen as preoperative and postoperative adjuvant therapy
(3,4).
Curcumin, a polyphenol extracted from turmeric
(Curcuma longa), is an agent with anticancer potential
against various types of tumors. As a natural compound, it has been
proven to be effective with minimal toxicity, which selectively
acts on cancer cells over normal cells (5,6).
Hitherto, curcumin and its analog have been reported to play an
anticancer role in several tumor models, including glioblastoma
(7), liver (8), colorectal (9), lung (10), ovarian (11), breast (12) and oral (13) cancer. The underlying mechanisms have
been demonstrated to be associated with the inhibition of
proliferation, angiogenesis, invasion and metastasis of cancer
cells, or apoptosis induction by curcumin (14–16).
However, research on the anticancer effect of curcumin against
gastric cancer and the related molecular mechanism remain to be
elucidated.
Autophagy, a self-degradative process that is highly
conserved among different types of mammalian cells, has been
demonstrated in studies to play a critical, and complicated role in
cancer development and progression as well as therapy resistance
(17–19). Autophagy is an intracellular process
starting with the formation of double membrane vesicles. Then, the
contents of the vesicles, long-lived proteins and damaged
organelles, are delivered to the lysosome to degrade. It has been
found that autophagy exerts a dual effect on cancer (17,18).
On one hand, autophagy may function as a tumor suppressor by
preserving cellular integrity. Autophagy-deficient cells are prone
to develop tumorigenesis in vivo. On the other hand,
autophagy may play an oncogenic role by maintaining tumor cell
survival and preventing apoptotic cell death after anticancer
treatment. Recently, several studies demonstrated that natural
compounds with anticancer abilities could induce autophagy in
different types of tumors (20–22).
However, the precise role of autophagy (tumor suppressor or
promoter) involved in the mechanism of natural compound-induced
cancer cell death needs to be further studied.
In the present study, the anticancer effect of
curcumin on human gastric cancer and the underlying mechanism were
investigated. We designed the study to determine the regulatory
role of curcumin in the survival or apoptosis of three different
gastric cancer cell lines BGC-823, SGC-7901 and MKN-28. Thereafter,
the effect of curcumin-induced autophagy on gastric cancer cells
and the related molecular mechanism were further elucidated.
Materials and methods
Cell culture and reagents
Human gastric cancer cell lines BGC-823, SGC-7901
and MKN-28 were gifts from The First Affiliated Hospital of Soochow
University (Jiangsu, China). The cells were routinely cultured in
RPMI-1640 medium supplemented with 10% (v/v) fetal bovine serum
(FBS) (both from Gibco, Invitrogen Corporation, Carlsbad, CA, USA)
at 37°C in a humidified atmosphere of 5% CO2. The
adherent cells were subcultured every 2–3 days and harvested for
the subsequent experiments.
Reagents of curcumin (C1386), MTT (M2128), acridine
orange (AO; A6014), 3-methyladenine (3-MA; M9281) and dimethyl
sulfoxide (DMSO; D2650) used in the present study, were all
obtained from Sigma-Aldrich (St. Louis, Mo, USA). Curcumin or
autophagy inhibitor 3-MA was dissolved in DMSO as stock solution
and kept at −20°C prior to usage. In addition, MTT or AO was
dissolved in phosphate-buffered saline (PBS) for solution
preparation.
Cell viability assay
The regulatory effect of curcumin on gastric cancer
cell growth was evaluated using an MTT assay. Briefly, cells were
seeded at a density of 1×104 cells/well in a 96-well
culture plate, incubated overnight and treated with or without
increasing amounts of curcumin (0–200 µM) for 24, 48 and 72 h,
respectively. Subsequently, 10 µl of MTT solution (5 mg/ml) was
added into each well and incubated with the cells at 37°C for
another 4 h. Then, the solution was discarded and 150 µl of DMSO
was added into the remaining cells to dissolve the formazan
crystals. The optical density (OD) at 490 nm was measured using a
microplate reader (Benchmark, Bio-Rad Laboratories, Hercules, CA,
USA). The formula used in the present study was: Cell viability (%)
= (OD of the experimental sample/OD of the control group) ×
100%.
TUNEL assay
TUNEL staining was performed in the present study to
determine the apoptosis-promoting effect of curcumin on gastric
cancer cells. In brief, the cells were seeded in a 24-well plate at
a density of 7×104 cells/well. After incubation
overnight, gastric cancer cells were exposed to the indicated
concentrations of curcumin for 48 h. Then, the cells were fixed
with 4% paraformaldehyde and stained for the TUNEL assay. In the
present study, TUNEL staining was performed with a One Step TUNEL
Apoptosis Assay kit (Beyotime Biotechnology, Nantong, China)
according to the manufacturer's instructions. Thereafter, the cells
were observed and photographed under a fluorescence microscope
(Olympus Optical Co., Hamburg, Germany) and the red stained nuclei
were considered as TUNEL-positive.
Flow cytometric analysis
Flow cytometry was used to confirm the
apoptosis-inducing effect of curcumin on gastric cancer cells.
After 12 h of incubation, gastric cancer cells seeded into a 6-well
plate (3×105 cells/well) were treated with the indicated
amounts of curcumin for 48 h. Then, the cells were harvested,
washed with ice-cold PBS and re-suspended with the complete culture
medium. The cell suspension was incubated with a Muse™ Annexin V
& Dead Cell Assay kit (EMD Millipore, Hayward, CA, USA)
according to the manufacturer's instructions, and analyzed on a
Muse™ Cell Analyzer system (EMD Millipore). Data were analyzed
using Muse™ Analysis software (EMD Millipore).
Western blotting
A western blotting assay was performed to evaluate
the expression of apoptosis- or autophagy-related proteins in human
gastric cancer cells. After exposure to the indicated amounts of
curcumin for 48 h, the cells were harvested and solubilized in cold
RIPA buffer supplemented with complete protease inhibitors. Equal
amounts of cellular lysates were separated on SDS-PAGE and
electrophoretically transferred to polyvinylidine fluoride (PVDF)
membranes. The membranes were then blocked with 5% (w/v) blotting
grade milk for 1 h and then incubated with the primary antibodies
overnight at 4°C. The primary antibodies anti-Bcl-2, anti-Bax,
anti-caspase-3 and anti-caspase-9 were purchased from
Merck-Millipore (Billerica, MA, USA); anti-LC3 was obtained from
Abcam (Cambridge, MA, USA); anti-Beclin1 and anti-Atg7 were
purchased from Abgent (San Diego, CA, USA); anti-Atg12-Atg5,
anti-p-Akt, anti-Akt, anti-p-mTOR, anti-mTOR, anti-p-p70S6K and
anti-p70S6K were obtained from Cell Signaling Technology (Beverly,
MA, USA). Anti-β-actin (Santa Cruz Biotechnology, Santa Cruz, CA,
USA) was used as an internal control. Subsequently, the membranes
were washed and treated with the appropriate HRP-conjugated
secondary antibodies for 2 h, and the bound antibodies were
visualized by an enhanced chemiluminescence (ECL) reagent (Beyotime
Biotechnology).
Detection of acidic vesicular
organelles (AVOs)
Acridine orange (AO) staining was used to determine
the vacuolar acidification of autophagosomes, which is a
characteristic of efficient autophagy. Briefly, gastric cancer
cells were seeded in a 6-well plate at a density of
3×105 cells/well and incubated overnight. Following
exposure to the indicated concentrations of curcumin for 48 h, the
cells were treated with AO (1 µg/ml) for 15 min, washed with PBS
and visualized under a fluorescence microscope (Olympus Optical
Co.). The orange-red fluorescence in the cytoplasm was recognized
as AO-positive staining.
Statistical analysis
All quantitative data are expressed as the mean ±
SEM and plotted with the GraphPad Prism software 6.0 (GraphPad
Software, La Jolla, CA, USA). Statistical analysis was performed by
non-parametric Mann-Whitney U test using SPSS 16.0 statistical
software and a P-value <0.05 was considered as statistically
significant.
Results
Gastric cancer cell proliferation is
regulated by curcumin
To confirm the potential role of curcumin in gastric
cancer cell growth, we performed an MTT assay to assess the cell
viability after treatment with curcumin over a wide range of
concentrations (0–200 µM). As shown in Fig. 1A, curcumin inhibited the
proliferation of three different gastric cancer cell lines BGC-823,
SGC-7901 and MKN-28 in both a time- and dose-dependent manner,
albeit to varying degrees. The IC50 values of curcumin
against gastric cancer cells are presented in Table I. After 24 h of culture, MKN-28
cells were found to be the most sensitive to curcumin
(IC50=16.17 µM) and SGC-7901 cells were the most
resistant (IC50=50.45 µM), whereas BGC-823 cells
provided an intermediate sensitivity (IC50=37.58 µM). At
48 h after treatment, the IC50 values of each cell line
decreased and were similar. Thus, the regulatory effects of
curcumin on gastric cancer cells were all observed after 48 h of
exposure in our following studies, at a range of concentrations
between 0 and 20 µM.
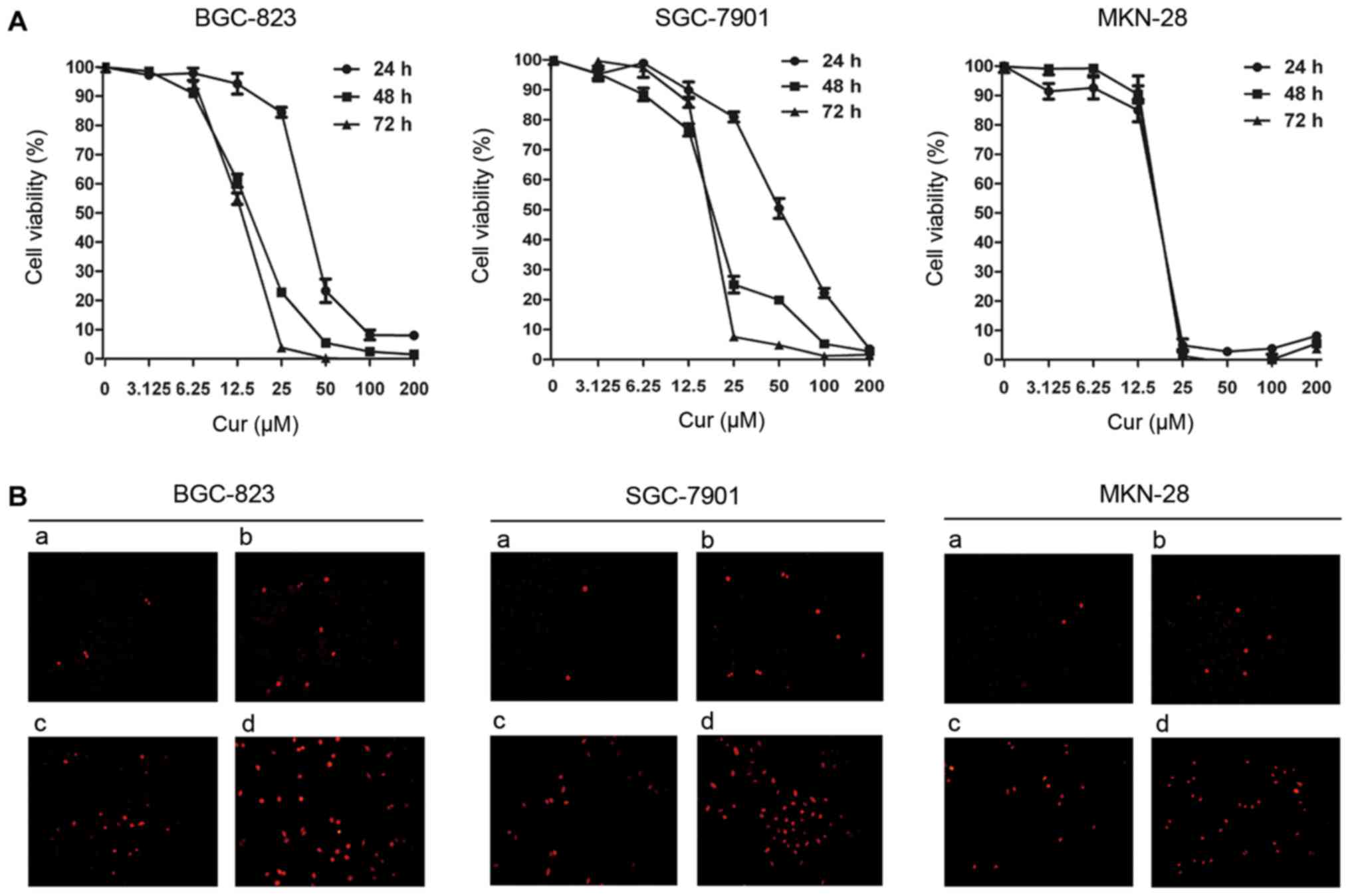 | Figure 1.Viability and apoptosis in human
gastric cancer cells after curcumin treatment. (A) BGC-823,
SGC-7901 and MKN-28 cells were treated with curcumin at the
indicated concentrations for 24, 48 and 72 h, and cell viability
was assessed using an MTT assay. (B) BGC-823, SGC-7901 and MKN-28
cells were treated with curcumin at increasing concentrations for
48 h and apoptotic cell death was determined by TUNEL staining
(magnification, ×200). BGC-823: a, DMSO; b, 5 µM curcumin; c, 10 µM
curcumin; d, 15 µM curcumin. SGC-7901 and MKN-28: a, DMSO; b, 5 µM
curcumin; c, 10 µM curcumin; d, 20 µM curcumin. Cur, curcumin. |
 | Table I.The IC50 values (µM) of
curcumin against gastric cancer cells. |
Table I.
The IC50 values (µM) of
curcumin against gastric cancer cells.
| Cell lines | 24 h | 48 h | 72 h |
|---|
| BGC-823 | 37.58 | 15.18 | 13.00 |
| SGC-7901 | 50.45 | 18.53 | 16.72 |
| MKN-28 | 16.17 | 15.84 | 14.04 |
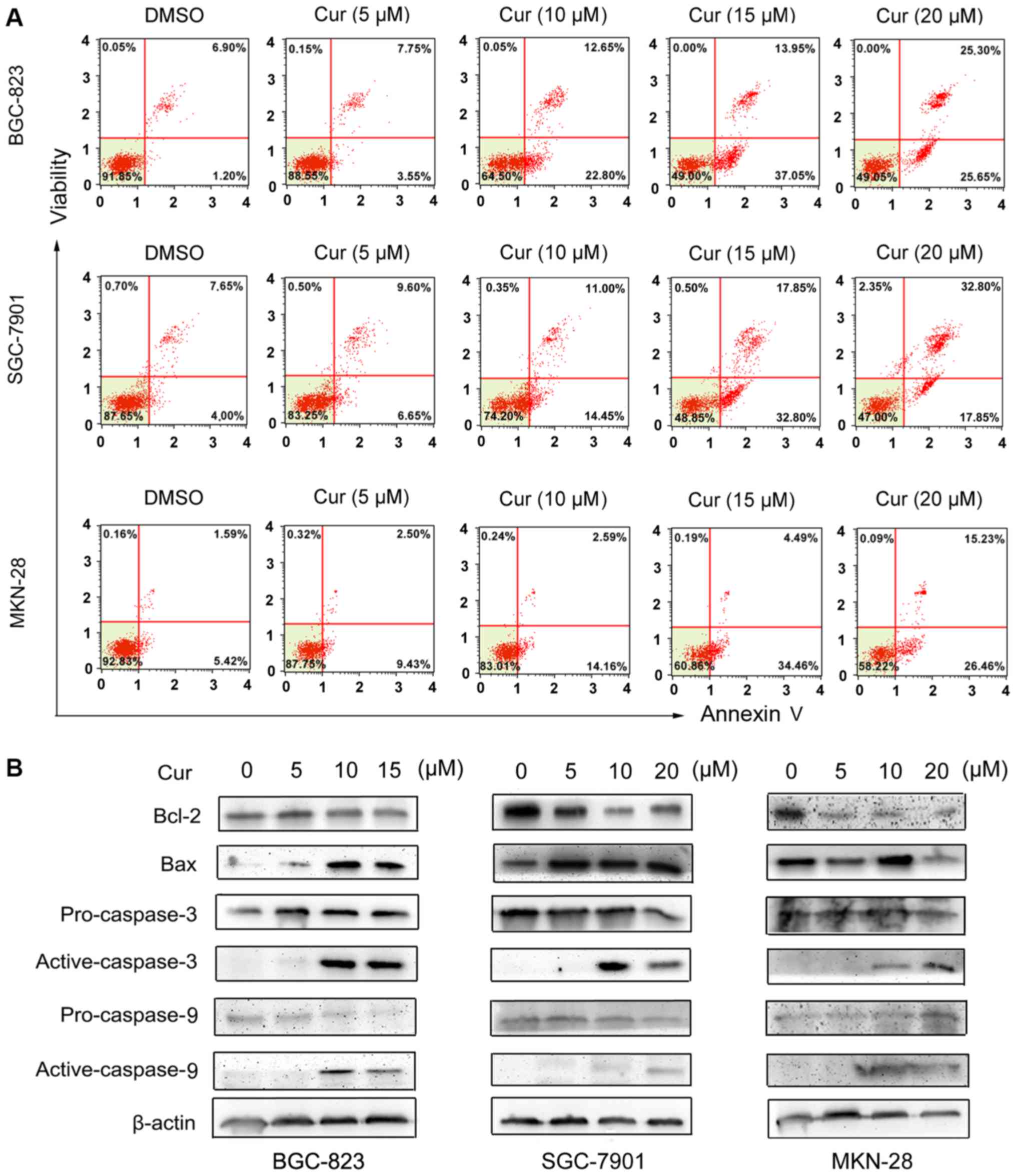
Apoptotic cell death in gastric cancer
cells is induced by curcumin
To explore the effect of curcumin on gastric cancer
cell apoptosis, a combination of TUNEL staining, flow cytometry and
western blotting assays was performed in our investigation. As
shown in Fig. 1B, the nuclei of
curcumin-treated gastric cancer cells were condensed and exhibited
bright red fluorescence at 48 h post-treatment in a dose-dependent
manner. Consistent with this observation, the results of flow
cytometric analysis indicated a significant increase in the
apoptotic population (early apoptosis plus late apoptosis) of
gastric cancer cells treated with the increasing concentrations of
curcumin, compared with the DMSO-control group (Fig. 2A). Furthermore, western blotting was
used to detect the expression of apoptosis-related proteins.
Fig. 2B revealed that curcumin
significantly induced the downregulation of Bcl-2 and the
upregulation of Bax expression in all of the three gastric cancer
cell lines. Moreover, curcumin clearly cleaved pro-caspase-3 and −9
to their active forms in each gastric cancer cell line at a
concentration over 10 µM. Therefore, the aforementioned
observations reveal that curcumin could induce apoptotic cell death
in human gastric cancer cells in a dose-dependent manner.
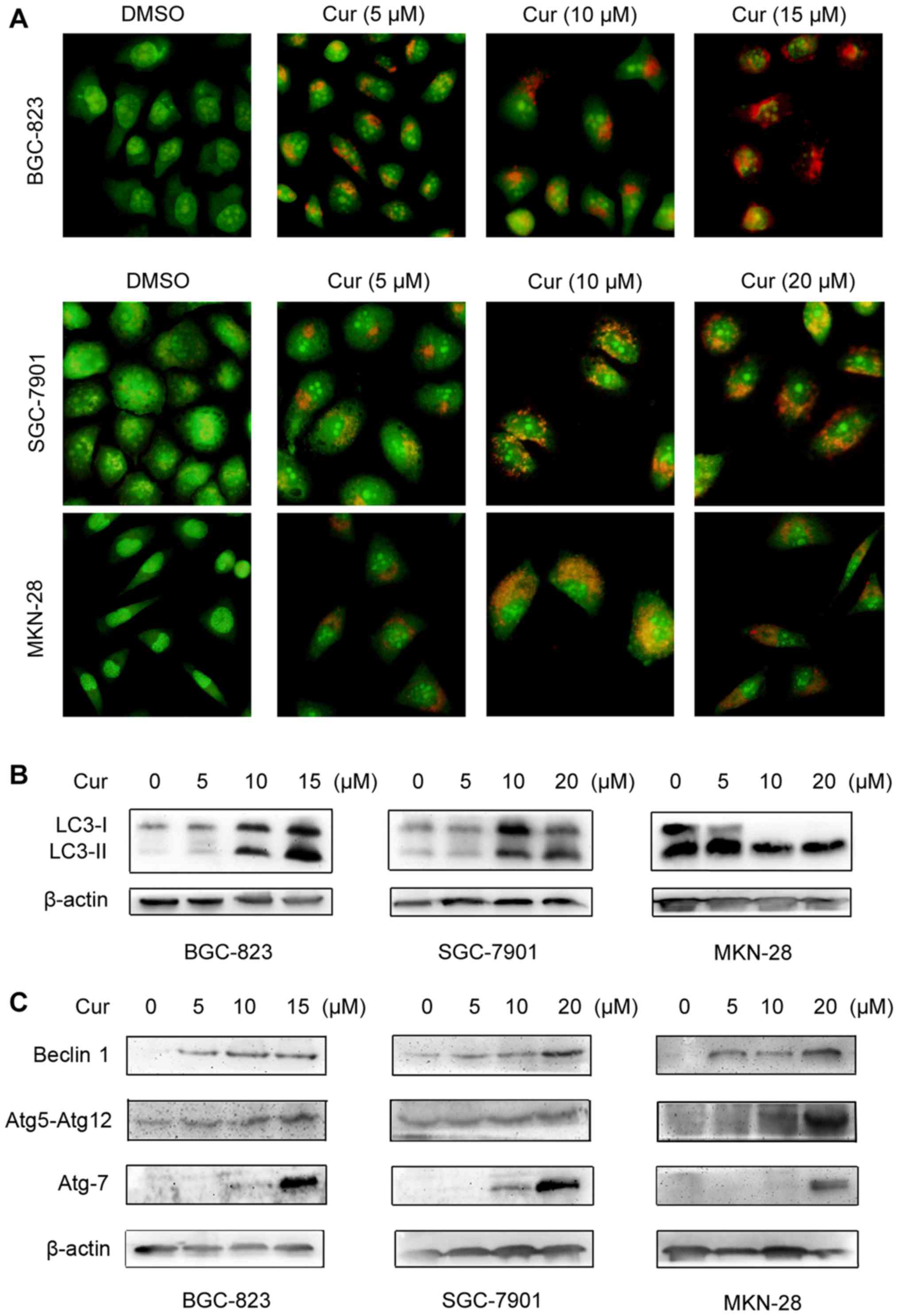
Autophagy in gastric cancer cells is
induced by curcumin
It has been reported that apoptosis and autophagy
have a complex relationship in the process of cancer cell death
(23,24). Therefore, we wondered whether the
activation of autophagy is also involved in the anticancer effect
of curcumin. To confirm the ability of curcumin to trigger
autophagy in human gastric cancer cells, we firstly performed AO
staining to assess the formation of AVOs, a characteristic of
autophagolysosomes, in curcumin-treated cancer cells. As shown in
Fig. 3A, curcumin treatment
resulted in pronounced accumulation of orange-red autophagic
vacuoles in all of the three cell lines (BGC-823, SGC-7901 and
MKN-28) in a dose-dependent manner, whereas the DMSO-control group
displayed green fluorescence, indicating the absence of AVO
formation in the cytoplasm.
Furthermore, we detected conversion of
microtubule-associated protein 1 light chain 3 (LC3) from LC3-I to
LC3-II by western blotting assay. When autophagy is activated,
LC3-I residing in the cytosol is cleaved to LC3-II and aggregated
on the autophagosomal membranes. Transformation of LC3-I to LC3-II
is thus a good marker for autophagy introduction. Our data showed
LC3 turnover in all of the three different gastric cancer cell
lines after exposure to curcumin and the accumulation of LC3-II was
more significant following the increase of curcumin concentration
(Fig. 3B). Thereafter, we
investigated the regulatory effect of curcumin on the expression of
autophagy-related (Atg) proteins in gastric cancer cells using
western blotting assay. As shown in Fig. 3C, the levels of Beclin1, Atg7 and
Atg5-Atg12-conjugate were all upregulated in BGC-823, SGC-7901 and
MKN-28 cells by curcumin treatment in a dose-dependent manner,
suggesting that curcumin has a potent ability to induce the
activation of Atg protein expression in human gastric cancer cells,
which is a critical characteristic for autophagy. Therefore, these
data suggest that curcumin could induce autophagy activation as
well as apoptotic cell death in human gastric cancer cells.
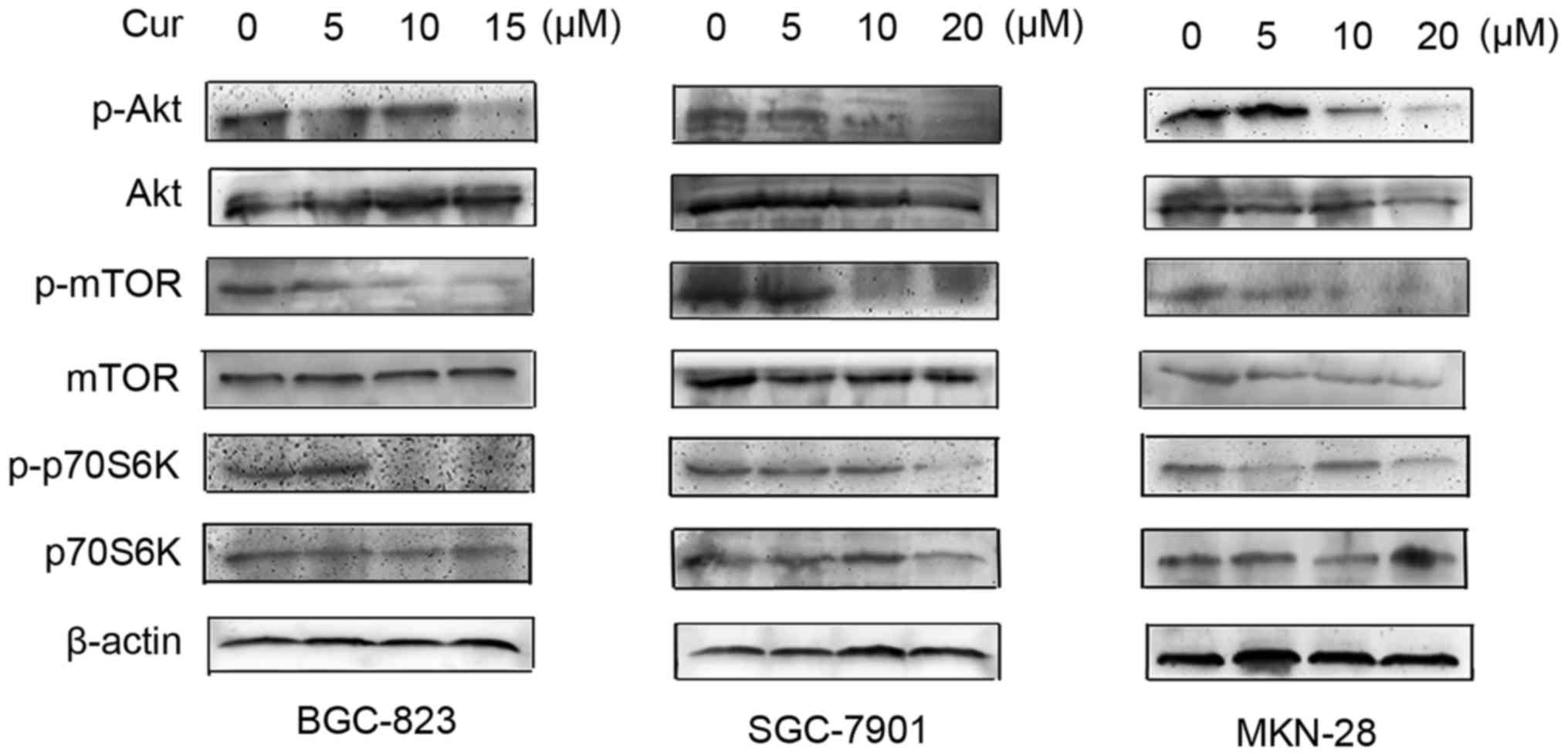
The PI3K/Akt/mTOR signaling pathway in
gastric cancer cells is regulated by curcumin
Since the PI3K/Akt/mTOR signaling pathway has been
confirmed to play an important role in the process of both
apoptosis and autophagy, we further studied the effect of curcumin
on the phosphorylation of Akt, mTOR and p70S6K in human gastric
cancer cells. Results of western blotting assay conveyed that the
expression levels of phospho-Akt in the three cell lines (BGC-823,
SGC-7901 and MKN-28) were all obviously downregulated by curcumin
treatment in a dose-dependent manner, followed by the
downregulation of downstream phospho-mTOR and phospho-p70S6K
(Fig. 4). This observation
indicates that curcumin could inhibit the Akt/mTOR signaling
pathway in gastric cancer cells, which may contribute to its
induction of both apoptosis and autophagy in human gastric cancer
cells.
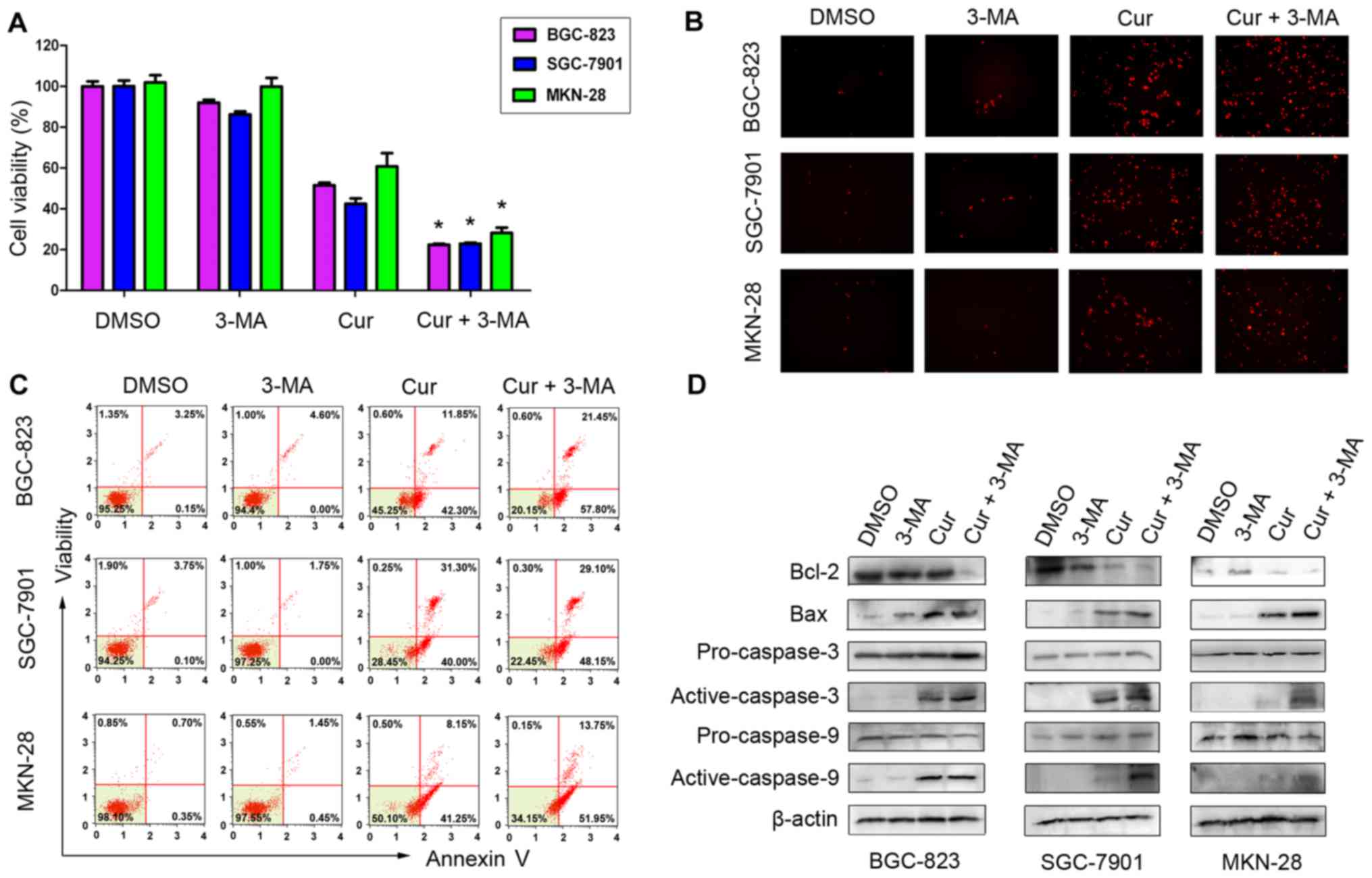
Proliferation and apoptotic cell death
in gastric cancer cells after 3-MA treatment
Autophagy has been reported to have a paradoxical
effect on cancer cell death or survival with various stimuli
(17,25). To study the contribution of
autophagy to curcumin-induced apoptosis in human gastric cancer
cells, we used the autophagy inhibitor 3-MA to prevent completion
of autophagy at the early stage and co-treated the cancer cells
with curcumin and 3-MA for 48 h. As shown in Fig. 5A, curcumin and 3-MA co-treatment
significantly decreased the viability of the three different
gastric cancer cell lines, compared with the control group treated
with curcumin alone. Furthermore, our data indicated that gastric
cancer cells co-treated with curcumin and 3-MA exhibited increased
apoptosis cell death compared with the control group treated with
curcumin alone, which was demonstrated by a combination of TUNEL
staining, flow cytometry and western blotting assays (Fig. 5B-D). Collectively, these results
suggest that curcumin-induced autophagy could play a protective
role in gastric cancer cell death and prevention of autophagy may
enhance the anticancer effect of curcumin in human gastric
cancer.
Discussion
Curcumin, a natural polyphenol derived from the root
of turmeric (Curcuma longa), has been reported to be a
potential traditional remedy in cancer prevention and therapy with
pharmacological safety (16,26).
However, the effect of curcumin on human gastric cancer and the
underlying mechanisms have not been well documented. Previous
studies demonstrated that curcumin attenuates gastric cancer cell
proliferation, induces apoptotic cell death and suppresses
lymphatic vessel density in vivo or in vitro
(27–29). In the present study, we provided the
first evidence that curcumin inhibited cell growth and induced
protective autophagy against apoptotic cell death in human gastric
cancer.
As an anticancer agent, curcumin has been reported
to play a potent role in the regulation of proliferation and
apoptosis in various types of cancer. Bimonte et al revealed
that curcumin treatment inhibited tumor growth and angiogenesis in
human breast cancer both in vivo and in vitro
(14). Koprowski et al
revealed that curcumin could induce growth suppression and
apoptosis of cholangiocarcinoma at low treatment concentrations
(30). Zou et al reported
that an analog of curcumin, WZ35, exhibited the anticancer effect
on gastric cancer via activation of the JNK and ER stress apoptotic
pathways mediated by ROS generation (31).
In the present study, proliferation was obviously
inhibited in both a time- and dose-dependent manner in three
different gastric cancer cell lines (BGC-823, SGC-7901 and MKN-28)
treated with curcumin. Moreover, curcumin was demonstrated to
induce marked apoptosis in human gastric cancer cells in a
dose-dependent manner, which was conveyed by the increase in
TUNEL-positive cells and the apoptosis population by flow
cytometry, as well as the upregulation of apoptosis-related
proteins. Consistent with our findings, studies have majorly
focused on apoptosis to elucidate the mechanisms by which curcumin
exerts its anticancer effect (16,28).
However, the role of curcumin-induced autophagy and the exact
mechanism involved in the interaction between apoptosis and
autophagy with curcumin treatment have not been generally
clarified, particularly in human gastric cancer.
Autophagy, a catabolic degradation process in which
cellular proteins or organelles are degraded in the lysosome and
recycled, is designated as a non-apoptotic form of programmed cell
death called autophagy-induced cell death. It has recently emerged
as a critical player in the development of different diseases,
particularly cancer. Accumulating studies have reported that
medicinal plant-derived compounds or extracts with anticancer
properties, such as curcumin, could induce autophagy in various
types of cancer and it has been established that the role of
autophagy in human cancer is complicated. Kim et al revealed
that curcumin-induced autophagy acts as a pro-death signal, which
contributes to the decreased survival of oral cancer cells
(32). In contrast, Zhou et
al demonstrated that a novel curcumin analog
EF25-(GSH)2 which induced autophagy could be inhibited
by chloroquine, thus leading to significantly enhanced apoptosis
and cytotoxicity in hepatocellular carcinoma (33). Moreover, Wang et al revealed
that quercetin could induce autophagy antagonizing apoptotic cell
death in gastric cancer cells, by modulation of the Akt/mTOR and
HIF-1α signaling pathways (20).
In the present study, curcumin treatment caused a
dose-dependent formation of acidic vesicular organelles (AVOs) in
human gastric cancer cells. Western blotting also demonstrated a
conversion from LC3-I to LC3-II and the upregulated expression of
autophagy-related proteins including Beclin1, Atg7 and Atg5-Atg12
conjugate in gastric cancer cells exposed to curcumin. These
characteristic changes firstly demonstrated that curcumin could
trigger the autophagic process in human gastric cancer cells in
vitro. Furthermore, whether curcumin-induced autophagy plays a
protective role or promotes cell death in gastric cancer was
confirmed later in our investigation.
The PI3K/Akt/mTOR signaling pathway has been
reported to be the major negative regulator of both apoptosis and
autophagy. Numerous signaling molecules of the PI3K/Akt/mTOR
pathway have oncogenic properties and constitutive activation of
this signaling pathway is often involved in the progression of
various human cancers (34,35). Contrary to tumor suppressor genes
such as PTEN and p53 which can stimulate autophagy, oncogenes of
PI3K and Akt have been confirmed to play an inhibiting role in
autophagy activation. The results of our present study revealed
that curcumin treatment obviously inhibited the phosphorylation of
Akt and downstream mTOR as well as p70S6K in all of the three
different human gastric cancer cell lines in vitro. This
finding indicated that the inhibition of the PI3K/Akt/mTOR
signaling pathway may play an important role in the autophagy of
gastric cancer cells treated with curcumin. However, since other
signaling pathways such as Erk1/2, AMPK and JNK may be also
responsible for autophagy activation, further research is still
warranted in the future.
In addition, autophagy inhibitor 3-MA was used in
the present study, to prevent autophagy in order to further
investigate the dual role of curcumin-induced autophagy in gastric
cancer cells. Consequently, our results demonstrated that autophagy
inhibition significantly decreased cell viability and enhanced
apoptotic cell death in human gastric cancer cells treated with
curcumin. These findings revealed that the prevention of autophagy
significantly promoted the anticancer effect and the toxicity of
curcumin in gastric cancer. In other words, curcumin-induced
autophagy plays a protective role in human gastric cancer, which
may lead to drug resistance by curcumin in cancer treatment.
Therefore, inhibition of this protective autophagy with the
application of an autophagy inhibitor may improve the therapeutic
efficacy of curcumin in human gastric cancer.
In summary, the present study demonstrated for the
first time that the anticancer effect of curcumin was associated
with its inhibition of proliferation and promotion of apoptotic
cell death in gastric cancer cells. Moreover, the present study
provides the first evidence that curcumin induced a protective
autophagy antagonizing apoptotic cell death in human gastric cancer
cells and combination of curcumin with an autophagy inhibitor could
enhance the anticancer toxicity of this chemotherapeutic drug.
Thus, it can be concluded that our findings provide a valuable
strategy for improving therapeutic treatment with curcumin or its
analog in human gastric cancer.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81402280), the
Foundation from Jiangsu Key Laboratory of Medical Science and
Laboratory Medicine (JSKLM-2014-005), and the Doctor Foundation
from the First People's Hospital of Lianyungang (grant no.
BS1503).
References
|
1
|
Kim MJ and Kim H: Anticancer effect of
lycopene in gastric carcinogenesis. J Cancer Prev. 20:92–96. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jeong SH, Kim YW, Yu W, Lee SH, Park YK,
Park SH, Jeong IH, Lee SE, Park Y and Lee YJ: High morbidity in
myocardial infarction and heart failure patients after gastric
cancer surgery. World J Gastroenterol. 21:6631–6638. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ford H and Gounaris I: Docetaxel and its
potential in the treatment of refractory esophagogastric
adenocarcinoma. Therap Adv Gastroenterol. 8:189–205. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Du W, Li C, Wang H, Zhao A, Shen J, Yong F
and Jia H: Effect of neoadjuvant chemotherapy on sevoflurane
MAC-BAR value of patients undergoing radical stomach carcinoma
surgery. Int J Clin Exp Med. 8:5649–5657. 2015.PubMed/NCBI
|
|
5
|
Alizadeh AM, Sadeghizadeh M, Najafi F,
Ardestani SK, Erfani-Moghadam V, Khaniki M, Rezaei A, Zamani M,
Khodayari S, Khodayari H, et al: Encapsulation of curcumin in
diblock copolymer micelles for cancer therapy. Biomed Res Int.
824746:20152015.
|
|
6
|
Chang R, Sun L and Webster TJ: Short
communication: Selective cytotoxicity of curcumin on osteosarcoma
cells compared to healthy osteoblasts. Int J Nanomedicine.
9:461–465. 2014.PubMed/NCBI
|
|
7
|
Zanotto-Filho A, Braganhol E, Klafke K,
Figueiró F, Terra SR, Paludo FJ, Morrone M, Bristot IJ, Battastini
AM, Forcelini CM, et al: Autophagy inhibition improves the efficacy
of curcumin/temozolomide combination therapy in glioblastomas.
Cancer Lett. 358:220–231. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Marquardt JU, Gomez-Quiroz L, Camacho LO
Arreguin, Pinna F, Lee YH, Kitade M, Domínguez MP, Castven D,
Breuhahn K, Conner EA, et al: Curcumin effectively inhibits
oncogenic NF-κB signaling and restrains stemness features in liver
cancer. J Hepatol. 63:661–669. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shakibaei M, Kraehe P, Popper B, Shayan P,
Goel A and Buhrmann C: Curcumin potentiates antitumor activity of
5-fluorouracil in a 3D alginate tumor microenvironment of
colorectal cancer. BMC Cancer. 15:2502015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu J, Cai Z, Wei X, Chen M, Ying S, Shi L,
Xu RA, He F, Liang G and Zhang X: Anti-lung cancer activity of the
curcumin analog JZ534 in vitro. Biomed Res Int.
2015:5045292015.PubMed/NCBI
|
|
11
|
Terlikowska KM, Witkowska AM, Zujko ME,
Dobrzycka B and Terlikowski SJ: Potential application of curcumin
and its analogues in the treatment strategy of patients with
primary epithelial ovarian cancer. Int J Mol Sci. 15:21703–21722.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Coleman DT, Soung YH, Surh YJ, Cardelli JA
and Chung J: Curcumin prevents palmitoylation of integrin β4 in
breast cancer cells. PLoS One. 10:e01253992015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mishra A, Kumar R, Tyagi A, Kohaar I,
Hedau S, Bharti AC, Sarker S, Dey D, Saluja D and Das B: Curcumin
modulates cellular AP-1, NF-kB, and HPV16 E6 proteins in oral
cancer. Ecancermedicalscience. 9:5252015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bimonte S, Barbieri A, Palma G, Rea D,
Luciano A, DAiuto M, Arra C and Izzo F: Dissecting the role of
curcumin in tumour growth and angiogenesis in mouse model of human
breast cancer. Biomed Res Int. 878134:20152015.
|
|
15
|
Wu J, Lu WY and Cui LL: Inhibitory effect
of curcumin on invasion of skin squamous cell carcinoma A431 cells.
Asian Pac J Cancer Prev. 16:2813–2818. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Amin AR, Haque A, Rahman MA, Chen ZG,
Khuri FR and Shin DM: Curcumin induces apoptosis of upper
aerodigestive tract cancer cells by targeting multiple pathways.
PLoS One. 10:e01242182015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhi X and Zhong Q: Autophagy in cancer.
F1000Prime Rep. 7:182015. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Galluzzi L, Pietrocola F, Bravo-San Pedro
JM, Amaravadi RK, Baehrecke EH, Cecconi F, Codogno P, Debnath J,
Gewirtz DA, Karantza V, et al: Autophagy in malignant
transformation and cancer progression. EMBO J. 34:856–880. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zarzynska JM: The importance of autophagy
regulation in breast cancer development and treatment. Biomed Res
Int. 2014:7103452014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang K, Liu R, Li J, Mao J, Lei Y, Wu J,
Zeng J, Zhang T, Wu H, Chen L, et al: Quercetin induces protective
autophagy in gastric cancer cells: Involvement of Akt-mTOR- and
hypoxia-induced factor 1α-mediated signaling. Autophagy. 7:966–978.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lin Y, Wang K, Hu C, Lin L, Qin S and Cai
X: Elemene injection induced autophagy protects human hepatoma
cancer cells from starvation and undergoing apoptosis. Evid Based
Complement Alternat Med. 2014:6375282014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang SF, Wang XL, Yang XQ and Chen N:
Autophagy-associated targeting pathways of natural products during
cancer treatment. Asian Pac J Cancer Prev. 15:10557–10563. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang MS, Niu FW and Li K: Proflavin
suppresses the growth of human osteosarcoma MG63 cells through
apoptosis and autophagy. Oncol Lett. 10:463–468. 2015.PubMed/NCBI
|
|
24
|
Liu D, Gao M, Yang Y, Qi YU, Wu K and Zhao
S: Inhibition of autophagy promotes cell apoptosis induced by the
proteasome inhibitor MG-132 in human esophageal squamous cell
carcinoma EC9706 cells. Oncol Lett. 9:2278–2282. 2015.PubMed/NCBI
|
|
25
|
Wang X, Qi W, Li Y, Zhang N, Dong L, Sun
M, Cun J, Zhang Y, Lv S and Yang Q: Huaier extract induces
autophagic cell death by inhibiting the mTOR/S6K pathway in breast
cancer cells. PLoS One. 10:e01317712015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rahmani AH, Al Zohairy MA, Aly SM and Khan
MA: Curcumin: A potential candidate in prevention of cancer via
modulation of molecular pathways. Biomed Res Int. 2014:7616082014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu X, Sun K, Song A, Zhang X, Zhang X, He
X and Xiaodong H: Curcumin inhibits proliferation of gastric cancer
cells by impairing ATP-sensitive potassium channel opening. World J
Surg Oncol. 12:3892014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liang T, Zhang X, Xue W, Zhao S, Zhang X
and Pei J: Curcumin induced human gastric cancer BGC-823 cells
apoptosis by ROS-mediated ASK1-MKK4-JNK stress signaling pathway.
Int J Mol Sci. 15:15754–15765. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Da W, Zhu J, Wang L and Sun Q: Curcumin
suppresses lymphatic vessel density in an in vivo human gastric
cancer model. Tumour Biol. 36:5215–5223. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Koprowski S, Sokolowski K, Kunnimalaiyaan
S, Gamblin TC and Kunnimalaiyaan M: Curcumin-mediated regulation of
Notch1/hairy and enhancer of split-1/survivin: Molecular targeting
in cholangiocarcinoma. J Surg Res. 198:434–440. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zou P, Zhang J, Xia Y, Kanchana K, Guo G,
Chen W, Huang Y, Wang Z, Yang S and Liang G: ROS generation
mediates the anti-cancer effects of WZ35 via activating JNK and ER
stress apoptotic pathways in gastric cancer. Oncotarget.
6:5860–5876. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kim JY, Cho TJ, Woo BH, Choi KU, Lee CH,
Ryu MH and Park HR: Curcumin-induced autophagy contributes to the
decreased survival of oral cancer cells. Arch Oral Biol.
57:1018–1025. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhou T, Ye L, Bai Y, Sun A, Cox B, Liu D,
Li Y, Liotta D, Snyder JP, Fu H, et al: Autophagy and apoptosis in
hepatocellular carcinoma induced by EF25-(GSH)2 A novel
curcumin analog. PLoS One. 9:e1078762014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yip PY: Phosphatidylinositol
3-kinase-AKT-mammalian target of rapamycin (PI3K-Akt-mTOR)
signaling pathway in non-small cell lung cancer. Transl Lung Cancer
Res. 4:165–176. 2015.PubMed/NCBI
|
|
35
|
Cheaib B, Auguste A and Leary A: The
PI3K/Akt/mTOR pathway in ovarian cancer: Therapeutic opportunities
and challenges. Chin J Cancer. 34:4–16. 2015. View Article : Google Scholar : PubMed/NCBI
|