Introduction
Currently, quality of life is one of several
important indicators used to evaluate the efficacy of treatment; it
is not only based on clinical objective indices as evaluation
standards but also emphasizes the subjective conditions of cancer
patients (1). The incidence of
gastric cancer (GC) has decreased in the past decade, but it
remains the leading cause of cancer-related mortality in China
(2,3). Despite recent developments, surgery,
chemotherapy and radiotherapy are not effective in completely
eradicating GC cells (4,5). Systemic chemotherapy is widely
accepted as a palliative treatment, leading to good responses,
improved life quality, and increased survival rates. Palliative
treatment is designed to relieve the symptoms and improve quality
of life. Today, a great challenge of cancer chemotherapy is to
eliminate cancer cells and improve the quality of life of
patients.
Chemotherapy has been vigorously investigated in GC,
but single-drug chemotherapy has not increased the survival rates.
However, several combination treatments have been established with
potent anticancer activity. For example, oxaliplatin + cisplatin
(L-OHP/CDDP) combination therapy is frequently used for the
pre-treatment of ovarian cancer (6). GEM (gemcitabine) is used in
combination with 5-fluorouracil (5-FU)/oxaliplatin (L-OHP) for the
treatment of advanced pancreatic cancer (7). This therapy improves the quality of
survival, prolongs the survival time, and shows lower toxicity.
Hydroxycamptothecin (HCPT) has been used in combination with
5-FU/mitomycin C (MMC) for the treatment of advanced pancreatic
cancer (8). Rebucci and Michiels
observed several common drug resistance mechanisms in GC, including
failure of DNA repair or apoptosis, changes in drug export and in
drug metabolism (9). Although
combination treatments are encouraging, high (and non-specific)
toxicity and drug resistance discourage the continuation of
treatment. Therefore, great effort has been directed toward
enhancing drug specificity, reducing toxic side-effects, and
preventing drug resistance. Development of novel therapeutic
anticancer agents is important (10,11),
especially agents without the non-specific toxicity of current
drugs. L-OHP, a third-generation platinum compound, after cisplatin
and carboplatin, is used to treat digestive system tumors with high
clinical activity and low mammalian toxicity. L-OHP is a new
1,2-diaminocyclohexane (DACH) platinum complex with antitumor
activity similar to that of cisplatin against several transplanted
murine tumors (12). L-OHP differs
from cisplatin and carboplatin, particularly in its toxicity
profile due to the absence of renal and auditory toxicities and
minimal myelosuppression (13).
However, L-OHP is toxic to the blood and nervous system, which
inhibits its broader application for cancer therapy (14).
Induced anticancer bioactive peptide (ACBP) is a
low-molecular-weight active substance extracted from goat spleen
via induced immunity and was produced by the Clinical Medicine
Research Center, The Affiliated Hospital at Inner Mongolia Medical
University. While normal ACBPs are also extracted from goat spleen,
they are not induced during the immunity process. Normal and/or
induced ACBPs show superior antineoplastic activity and a lack of
toxicity and side-effects (15). In
culture, ACBPs were found to inhibit tumor growth in nude mice
bearing Dutch gallbladder carcinoma GBS-SD, Dutch GC BGS-823, and
GC MGS-803 cells via a sustained medication mode every few days
(15,16). Previously, we demonstrated that ACBP
combined with lower cisplatin doses could achieve antitumor
efficacy similar to the efficacy of higher doses of cisplatin alone
via a sustained medication mode (17). However, the potential antitumor
efficacy of short-term intermittent ACBP (induced or normal)
medication modes and the combination with chemotherapeutics has not
been previously demonstrated.
Here, we investigated the anticancer effects of
short-term intermittent application of ACBPs (induced or normal) in
combination with L-OHP in a dose-dependent manner in MKN-45 GC
cells. We also investigated the antitumor efficacy of induced ACBP
treatment together with L-OHP using discontinuous short-term
applications by examining cell apoptosis signaling in vitro
and whether combined treatment of induced ACBP and L-OHP could
enhance tumor regression in a mouse xenograft model in vivo,
improve the qualities of life of nude mice with MKN-45 cell Dutch
GC xenografts and cause overexpression of cytochrome c,
caspase-3, −8, and −9 in the tumor cells. We found that induced
ACBP enhanced L-OHP-stimulated apoptosis, reduced the toxic
side-effects of L-OHP, and inhibited tumor cell growth. These
results provide novel ideas and strategies for comprehensive tumor
treatment in the future.
Materials and methods
Materials
ACBP and MKN-45 cells were provided by the Clinical
Medicine Research Center, The Affiliated Hospital, Inner Mongolia
Medical University. L-OHP was produced by Jiangsu Aosaikang
Pharmaceutical Co., Ltd. by Share Ltd. (Chinese Medicine, license
no. H20064296).
MTT assay for cell viability
evaluation
Cell viability was evaluated after treatment with an
MTT assay. GC MKN-45 (1×105) cells were seeded in 100 µl
RPMI-1640 medium supplemented with 10% FBS (both from Gibco, Grand
Island, NY, USA) in 96-well plates and cultured overnight. The
medium was replaced with fresh RPMI-1640 and ACBP (induced or
normal), L-OHP alone or the combination of anticancer bioactive
peptides and oxaliplatin (A+L) at different concentrations
(Fig. 1A). After a further
incubation for 24 h, 20 µl of 5 mg/ml MTT was added to each well
for 4 h. The medium was discarded, 150 µl of dimethyl sulfoxide was
added for 30 min, and the absorbance of the solution was measured
at 490 nm (OD). The tumor inhibition rate (IR) was calculated as
follows: %IR = [100 - (ODtest -
ODblack)/(ODcontrol - ODblack)]
×100, where ODtest is the absorbance value of the test
sample, ODcontrol is the absorbance value of the control
group, and ODblack is the absorbance value of the blank
sample. All experiments were performed in triplicate (18).
Dose-dependent expression of
cytochrome c and caspase-9 in MKN-45 cells
Total RNA was extracted from MKN-45 cells using
TRIzol reagent according to the manufacturer's instructions (Takara
Biomedical Technology Co., Ltd., Beijing, China). After
spectrophotometric quantification, 1 mg of total RNA was used to
synthesize first-strand cDNA with the Revert Aid H Minus First
Strand cDNA synthesis kit (Fermentas, Glen Burnie, MD, USA).
Real-time RT-PCR reactions were carried out on an Mx3000P real-time
PCR system (Stratagene, La Jolla, CA, USA). To correct for
experimental variations between samples, glyceraldehyde-3-phosphate
dehydrogenase (GAPDH) was used as the internal control. Primers
were designed and synthesized by Sangon Biotech Co., Ltd.
(Shanghai, China), as shown in Table
I. The expression of caspase-9 and cytochrome c in the
MKN-45 cells was detected. The PCR program was as follows: i)
initial denaturation at 95°C for 5 min; ⅱ) 35 cycles of
denaturation at 95°C, annealing at 60°C, and elongation at 60°C for
30 sec; and ⅲ) a final extension at 60°C for 40 sec. RT-PCR was
performed using the Taq-Man Gene Expression Assay protocol (Applied
Biosystems, Waltham, MA, USA). The relative quantification and
expression of caspase-9 and cytochrome c in the cell lines
were normalized using GAPDH by the 2−ΔΔCT method
(19).
 | Table I.Primer sequences and amplification
fragment of caspase-3, −8 and −9, cytochrome c and
GAPDH. |
Table I.
Primer sequences and amplification
fragment of caspase-3, −8 and −9, cytochrome c and
GAPDH.
| Target gene | Primer
sequences | AF (kbp) |
|---|
| Caspase-3 | F
5′-AGAACTGGACTGTGGCATTGA | 191 |
|
| R
5′-GCTTGTCGGCATACTGTTTCA |
|
| Caspase-8 | F
5′-GGATGGCCACTGTGAATAACTG | 101 |
|
| R
5′-TCGAGGACATCGCTCTCTCA |
|
| Caspase-9 | F
5′-GTCCTACTCTACTTTCCCAGGTTTTG | 148 |
|
| R
5′-gTgAgCCCACTgCTCAAAgAT |
|
| Cyt c | F
5′-GAGCGGGAGTGTTCGTTGT | 165 |
|
| R
5′-CTTCCGCCCAAAGAGACCAT |
|
| GAPDH | F
5′-TCCACCACCCTGTTGCTGTA | 452 |
|
| R
5′-ACCACAGTCCATGCCATCAC |
|
Hematoxylin and eosin (H&E)
staining of MKN-45 cells in a dose-dependent manner
H&E was used to stain MKN-45 cells after culture
in RPMI-1640 medium following each treatment. The sterile
coverslips were placed in 6-well plates, and each coverslip was
treated with drops of 4% polylysine 100 µl at 37°C. The 6-well
plates were removed after 6 h, and then human GC MKN-45 cells
(1×105 cells/ml) were seeded in a 6-well plate and
incubated at 37°C. After 8 h of incubation, the cell culture
solution was discarded, and 1 ml of the cell culture solution
containing different drugs was added (A, negative control (NS); B,
20 µg/ml induced ACBP; C, 30 µg/ml induced ACBP; D, 20 µg/ml L-OHP;
E, 20 µg/ml induced ACBP + 20 µg/ml L-OHP; F, 30 µg/ml induced ACBP
+ 20 µg/ml L-OHP) and cultured for 24 h. Each well was washed three
times with phosphate-buffered saline (PBS) and were fixed in 95%
ethanol for 30 min, and washed with PBS one time for 2 min. After
incubation, the cells were fixed and stained with H&E for 15
min. Random selected fields were photographed under a light
microscope. For cell viability measurements, H&E-stained cells
displaying nuclear condensation and fragmentation were counted as
apoptotic cells. Observations were obtained from at least three
independent experiments.
Tumor modeling in vivo
Human MKN-45 GC cells were cultured with RPMI-1640
medium containing 10% heat-inactivated FBS, 100 mg/ml streptomycin,
and 100 U/ml of penicillin at 37°C in a 5% CO2
incubator. The MKN-45 cells were harvested with 0.05% trypsin-EDTA
and washed with 1 ml of PBS. Cells (1×107 cells) were
injected subcutaneously into the right ventral flanks of 6-week-old
male BALB/c nude mice (Shizuoka Laboratory Animal Center,
Hamamatsu, Japan) that were maintained under specific pathogen-free
conditions. After 15 days of inoculation, the tumor diameter
reached 1.5 cm, and was used as the formal experimental tumor
strain for tumor tissue block transplantation.
Tumor xenograft experiments
The tumor tissues were obtained from the donor mice,
put into physiological saline to remove necrotic tissue and then
gently cut into pieces of ~2–3 mm3 on ice. A total of 75
male BALB/c mice (4–5 weeks; average weight, 16 g) were used for
the subcutaneous transplantation of human GC MKN-45 tumors. The
mice were maintained under specific pathogen-free conditions and
were provided with food and water until tumor growth. Next, we
determined the antitumor effect of induced ACBP and A+L on BALB/c
nude mice in the following experiment. We choose 24 male BALB/c
nude mice. The induced ACBP and the combination of induced ACBP and
L-OHP inhibited cancer cell growth at high concentrations (30 µg/ml
ACBP + 20 µg/ml L-OHP). This suggested that at these concentrations
the induced ACBP acts on the MKN-45 cells and also leads to
apoptosis. Therefore, the induced ACBP was used for the following
analysis. Therefore, a total of 24 BALB/c mice (4–5 weeks of age;
average weight, 16 g) were randomized into four groups (n=6 per
group, named R1, R2, R3, R4, R5 and R6, respectively): negative
control with an intraperitoneal injection of 0.5 ml normal saline
(0.9% sodium chloride solution); injection of 0.5 ml, 30 µg/ml ACBP
(induced) alone; 0.5 ml, 20 µg/ml L-OHP alone; or total 0.5 ml
(VACBP:VL-OHP = 1:1), 30 µg/ml ACBP (induced)
+ 20 µg/ml L-OHP (A+L), respectively, into the right anterior flank
via the tail vein each week for two times. All treatments were
administered on day 3, 7, 10, 14. The dose of L-OHP (10 mg/kg) was
chosen by our experiment screening from 1 to 30 µg/ml, which was
chosen based on a similar previous study from 75 to 130
mg/m2 (6,13), while the dose of ACBP was chosen
based on our own previous study (17). The tumor volume (V) was measured
every 3 days by a vernier caliper after the tumors formed. The
minimum width (a) and ribbon width (b) of the tumor were recorded,
and the tumor volume was calculated as V = ab2/2.
Three weeks after inoculation, the blood was
obtained from the eyeball; thereafter, the nude mice were
sacrificed by cervical vertebra dislocation, and the tumor, liver,
and spleen tissues were immediately weighed and fixed by
paraformaldehyde. The blood and tumor sections were analyzed by
flow cytometry. Liver and spleen tissue slices were analyzed by
H&E staining, and additional tumor sections were analyzed by
immunohistochemistry. Normal nude mice (non-tumor xenograft nude
mice) were used as a control group. No side-effects, including
weight loss, skin reaction, or infection, were observed throughout
the treatment periods. The inhibitory tumor rates were calculated
as (Wcontrol -
Wexperiment)/Wcontrol ×100%. All animal
experiments were performed with the approval of the Ethics
Committee for Animal Experiments of Inner Mongolia Medical
University.
H&E staining of liver and spleen
tissues
The fixed tissues were dehydrated through a graded
ethanol series (30, 50, 70, 80, 90, 95, and 100%), immersed in
liquid wax, and embedded in epoxy resin. Tissue blocks were
sectioned (5-µm thickness) using a Leica RM 2016 diamond saw
microtome (Leica Biosystems Nussloch GmbH, Nussloch, Germany) and
collected on coated slides. The samples were prepared for
histopathological analysis using H&E staining. The samples were
then rinsed three times in PBS and incubated in 4% diaminobenzidine
at room temperature. Images were acquired using an optical
microscope connected to a CCD camera (DP25; Olympus, Tokyo,
Japan).
Propidium iodide (PI) staining and
flow cytometry for cell cycle analysis and apoptotic rate
Briefly, tumor cell suspensions (1×106
cells) were prepared by cutting the tumor tissue with scissors,
filtering it through 500-mesh copper mesh, fixing in 70% ethanol,
centrifugation, and washing overnight. An appropriate amount of PBS
and PI containing RNase was added. Cells (1×106) were
washed twice with HEPES-buffered saline, treated with trypsin, and
fixed in cold 70% ethanol at 4°C for 30 min. Cell pellets were
collected and incubated in 1 ml 50 mg/ml PI. Flow cytometric
evaluation was conducted within 5 min using an EPICS XL-MCL FACScan
(Becton-Dickinson, Mountain View, CA, USA). The DNA contents were
analyzed with the MultiCycle software for Windows (Phoenix Flow
Systems, San Diego, CA, USA) (20–22).
Immunohistochemistry
Subcutaneous tumors formed after injection of tumor
cells into the male nude mice were removed and fixed in 10%
buffered formalin for 24 h. Formalin-fixed and paraffin-embedded
sections (4 µm) were used for immunohistochemistry (23,24).
The sections were stored dry until use. After pre-incubation with
0.3% hydrogen peroxide in methanol for 30 min to block endogenous
peroxidase activity, the sections were rinsed in PBS. Caspase-3,
−8, −9, or cytochrome c were visualized
immunohistochemically by the MaxVision method using 1:100 rabbit
anti-caspase-9 or -cytochrome c antibody (Leica Biosystems
Nussloch GmbH) or 1:50 rabbit anti-caspase-3 or −8 antibody (Cell
Signaling Technology, Inc.) and the immunohistochemical MaxVision
kit (product no. KIT-5010/5020/5030), antigen repair fluid (product
no. MVS-0098/0099), and DAB color reagent box (product no.
DAB-0031/1031) according to the manufacturer's protocol (China).
The sections were rinsed and incubated with the secondary antibody
(goat biotinylated anti-rabbit IgG antibody). Sections were washed
with PBS, and the peroxidase reaction was developed by incubating
the sections in diaminobenzidine solution containing 0.003%
hydrogen peroxide and 10 mM sodium azide followed by a Mayer's
hematoxylin counterstain. Sections were then dehydrated through
graded ethanol solutions (70–100%). For quantification, the number
of positive cells in each randomly selected field (~100,000
µm2) was counted at ×400 magnification. Only cells
stained precisely were counted as positive to avoid counting
non-specific staining. Standards of grading are according to the
conventional semi-quantitative scoring method (25).
RNA isolation and RT-PCR
Total RNA was extracted from tumor tissue using the
TRIzol reagent according to the manufacturer's instructions. After
spectrophotometric quantification, 1 mg of total RNA was used to
synthesize first-strand cDNA with the Revert Aid H Minus First
Strand cDNA synthesis kit (Fermentas). Real-time RT-PCR reactions
were carried out on an Mx3000P real-time PCR system (Stratagene).
To correct for the experimental variations between samples, GAPDH
was used as the internal control. Primers were designed and
synthesized by the Sangon Biotech Co., Ltd., as shown in Table I. The expression of caspase-3 p20
(N-19) (sc-1226), caspase-8 p18 (D-8) (sc-5263), caspase-9 (9CSP03)
(sc-73548), and cytochrome c (A-8) (sc-13156) (Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) in the tumor tissue was
detected. The PCR program was as follows: i) initial denaturation
at 95°C for 5 min; ⅱ) 35 cycles of denaturation at 95°C, annealing
at 60°C, and elongation at 60°C for 30 sec; and ⅲ) a final
extension at 60°C for 40 sec. RT-PCR was performed using the
Taq-Man Gene Expression Assay protocol (Applied Biosystems). The
mRNA from tumor tissue was typed with BstUI, and the
digestion products were resolved by 2% agarose gel electrophoresis
with ethidium bromide staining and UV light detection. The results
were documented by digital camera and stored as computer files in
BioCapt software (Vilbert-Lourmant, Marne LaValle, France).
Statistical analysis
The data are expressed as the mean ± standard
deviation (SD). Student's t-tests and one-way analysis of variance
(ANOVA) were performed for determination of P-values with SPSS
version 18.0 software (SPSS, Inc., Chicago, IL, USA). All
experiments were performed at least three times unless otherwise
indicated. P<0.05 was considered statistically significant.
Results
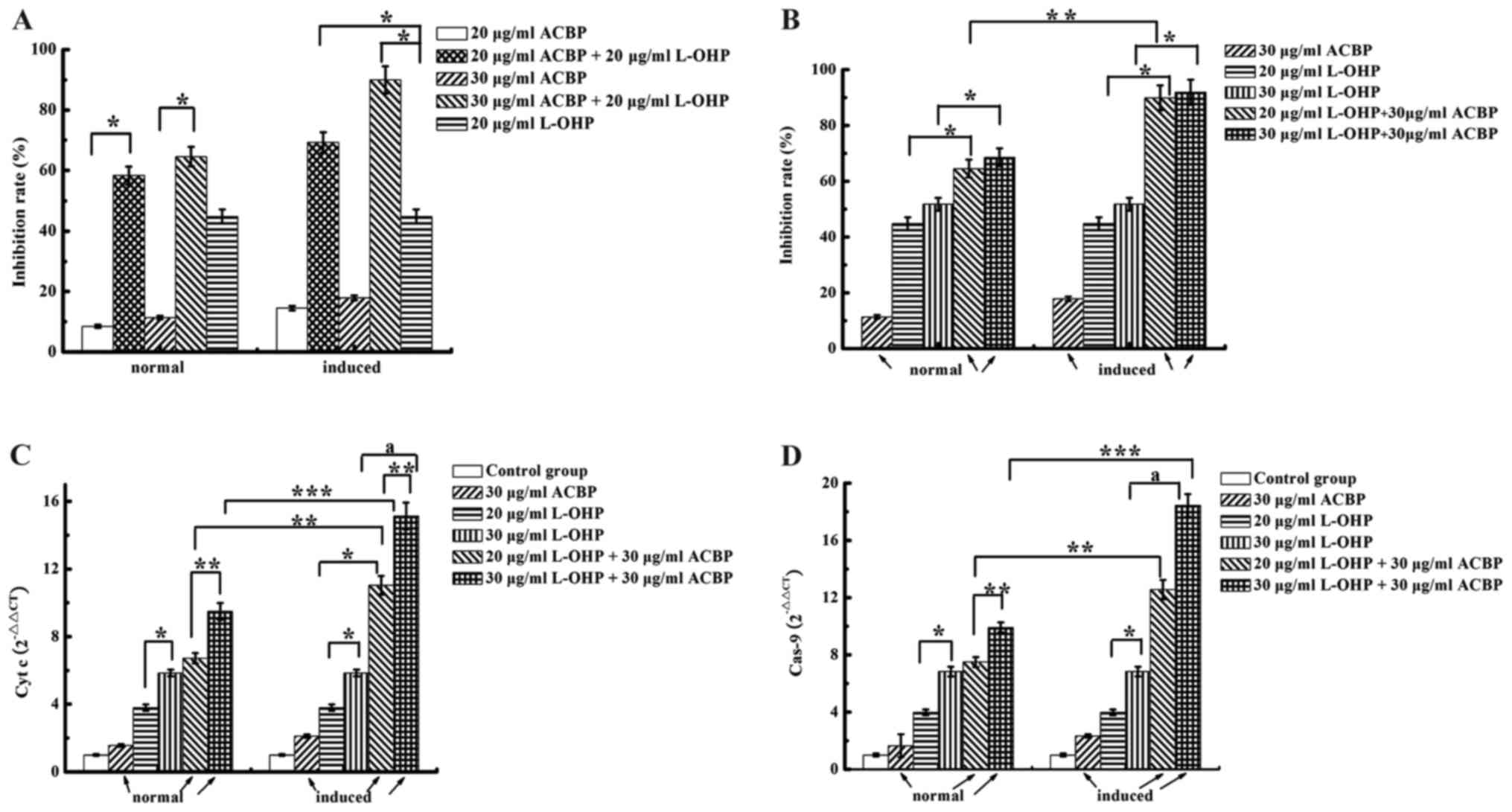
High levels of induced ACBP and L-OHP
increase the IR in MKN-45 cells
The effects of ACBP or induced ACBP and L-OHP on
cytotoxicity and apoptosis in MKN-45 cells are shown in Fig. 1A. Comparison of the single-drug
treatments indicated significantly different growth IRs. The direct
inhibitory effects on MKN-45 cells were as follows: 20 µg/ml L-OHP
> induced ACBP > normal ACBP > control group. Growth IRs
were dose-dependent; 30 µg/ml normal or induced ACBP had a
significantly greater inhibitory effect than 20 µg/ml normal or
induced ACBP (P<0.05). Furthermore, normal and induced ACBP +
L-OHP each had a significantly higher IR than L-OHP alone
(P<0.05). As shown in Fig. 1B
induced ACBP combined with L-OHP had a significantly higher IR than
normal ACBP combined with L-OHP (P<0.05).
Induced ACBP in combination with L-OHP
increases caspase-9 and cytochrome c expression in MKN-45
cells
Combining ACBP and L-OHP is a major strategy for GC
successful treatment. To investigate the mechanism by which A+L
enhances the apoptosis of GC cells, we examined the expression
levels of several related proteins (cytochrome c and
caspase-9) by qRT-PCR (Fig. 1C and
D). The mRNA expression levels of caspase-9 and cytochrome
c in the ACBP-treated MKN-45 cells were significantly higher
than that noted in the control-treated cells. Compared with normal
and induced ACBP, 20 or 30 µg/ml L-OHP upregulated caspase-9 and
cytochrome c expression. The combination of ACBP with
different concentrations of L-OHP showed statistically significant
differences for caspase-9 and cytochrome c expression. For
example, treatment with 30 µg/ml ACBP + 30 µg/ml L-OHP upregulated
caspase-9 and cytochrome c compared to treatment with 30
µg/ml ACBP + 20 µg/ml L-OHP (P=0.000). The MKN-45 cells treated
with induced ACBP in combination with L-OHP exhibited higher
caspase-9 and cytochrome c expression than the normal ACBP
in the combination treatment; the differences were statistically
significant (P<0.05). Therefore, except for the negative
control, the test group and positive control could induce the
expression of caspase-9 and cytochrome c, and the expression
levels were as follows: 30 µg/ml ACBP + 30 µg/ml L-OHP > 30
µg/ml ACBP + 20 µg/ml L-OHP > 30 µg/ml L-OHP > 20 µg/ml L-OHP
> induced ACBP > normal ACBP. These results indicate that A+L
enhances apoptosis. These results demonstrate that not only do ACBP
and L-OHP induce apoptosis by activating the caspase-9 and
cytochrome c pathway but also that A+L has coordinated
effects.
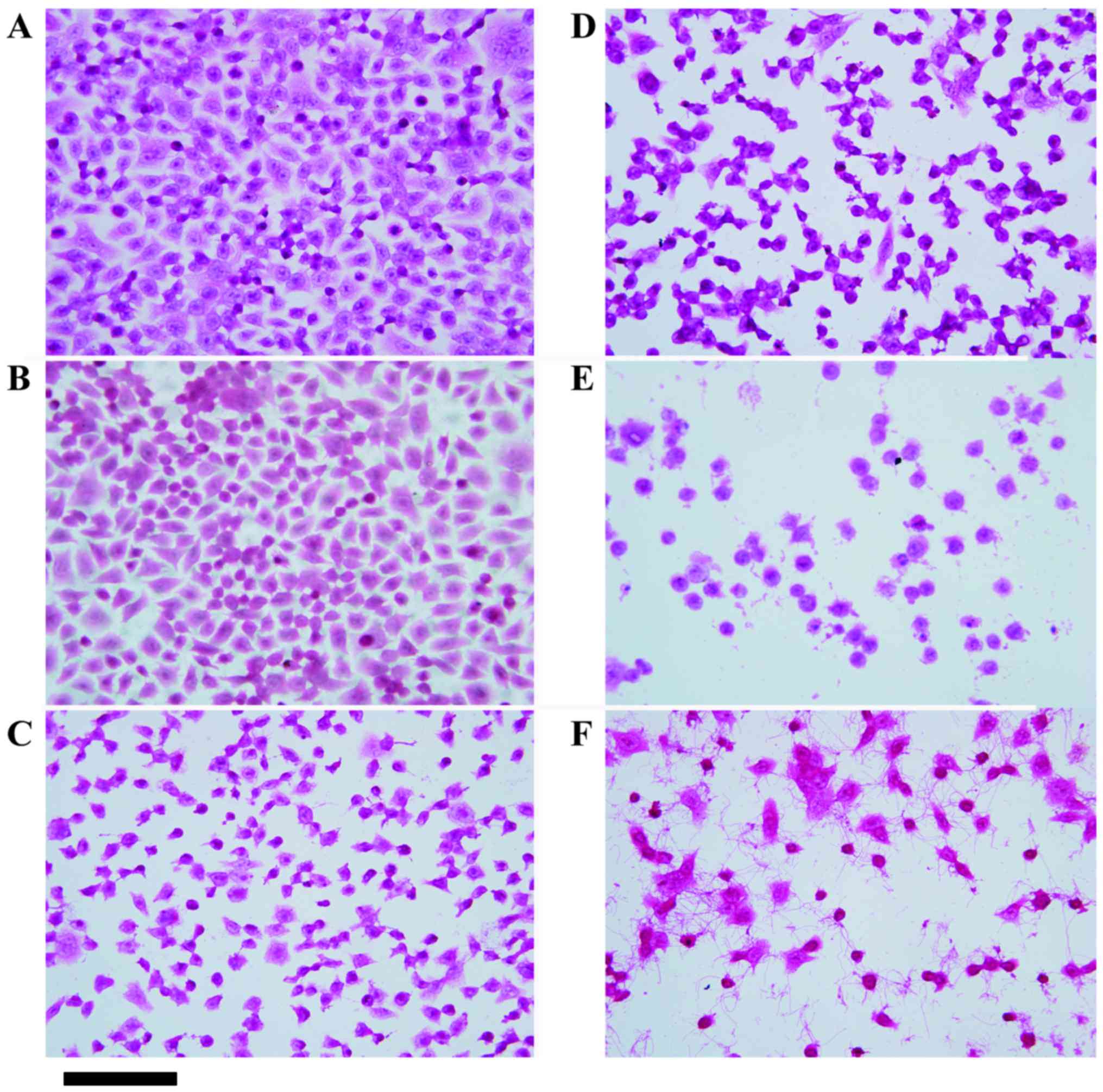
Induced ACBP combined with L-OHP
promotes apoptosis in MKN-45 cells
Based on the above analysis, induced ACBP or induced
ACBP in combination with L-OHP caused higher caspase-9 and
cytochrome c expression than normal ACBP or combination
therapy with normal ACBP. Therefore, we further examined the dose
dependence of apoptosis by the induced ACBP or combination therapy
compared to L-OHP and NS in the MKN-45 cell lines using H&E
staining. H&E-stained MKN-45 cells are shown in Fig. 2. Cells treated with normal saline
exhibited adherent growth and uniform morphology with clear cell
contours. In contrast, treatment with 20 µg/ml induced ACBP, 30
µg/ml induced ACBP, or 20 µg/ml L-OHP led to inhomogeneous cell
sizes and shapes, poor adhesion, and unclear outlines. Furthermore,
the higher doses of induced ACBP were more likely to induce
apoptosis in the MKN-45 cells, such as MKN-45 cell treatment with
30 µg/ml of induced ACBP, caused poor cell adhesion and increased
the number of suspended cells. However, 20 µg/ml ACBP + 20 µg/ml
L-OHP and 30 µg/ml ACBP + 20 µg/ml L-OHP reduced cell adherence to
the wall, increased suspended cells, and exhibited nuclear
condensation and unclear contours. Therefore, the combination of
induced ACBP and L-OHP induced the apoptosis in MKN-45 cells more
effectively than induced ACBP or L-OHP alone.
Antitumor efficacy and quality of life
induced by ACBP and combination therapy with induced ACBP and L-OHP
in a xenograft nude mouse model
A xenograft nude mouse model was established by
subcutaneous inoculation of human gastric MKN-45 cancer cells. The
morphology of the tumor and tumor weight were measured, calculated
at the end of treatment, and the statistical significance of the
tumor weight was examined (Fig.
3A). The measured tumor weights are shown in Fig. 3B. Due to the differences between the
inhibition tumor rate and the IR in MKN-45 cells after treatment
with ACBP or L-OHP in vivo and in vitro, the tumor
weights decreased in the L-OHP group compared with the NS and ACBP
groups; the changes were not statistically significant (P>0.05).
The differences, which mainly reflect nerve immune regulation,
individual differences in the nude mice and regulating the function
of ACBP in vivo, identified the inhibitory role of L-OHP on
the quality of life of the nude mice. Because the tumor sizes were
unregulated, the differences in the tumor volume (V) (V =
ab2/2; a is the minimum width and b is the ribbon width)
in the L-OHP and ACBP alone groups were not significant
(P>0.05); this volume was significantly decreased in the A+L
group (P<0.05) (Fig. 3C).
Fig. 3D shows the rate of tumor
growth with different drug treatments. Compared with the negative
control, ACBP, L-OHP, and A+L significantly inhibited tumor growth.
The percentages of growth inhibition for ACBP and L-OHP were 5.69
and 30.61%, respectively, whereas inhibition increased to 47.06%
for A+L, indicating that GC cells were highly inhibited by the
chemotherapeutic agents. When we examined ACBP and A+L antitumor
activity in vivo, we also observed the quality of life of
the tumor-bearing nude mice. Changes in body weight and diet of the
nude mice were in response to the quality of life as affected by
ACBP and combination treatments. The results showed that ACBP alone
increased the food intake and body weight of the nude mice compared
with the NS group; the changes were not statistically significant
(P>0.05). Because L-OHP displayed toxicity and side-effects, the
food intake and body weights of the nude mice were decreased
compared with the ACBP group; the difference was significant
(P<0.05). In contrast, the food intake and body weights of the
nude mice treated with A+L increased compared with the L-OHP group;
the difference was significant (P<0.05) (Fig. 3E). These results identified that
ACBP has an important role in reducing the side-effects of
chemotherapy, especially by significantly increasing the
sensitizing effects of L-OHP, which is an innovative point of this
article. The nude mice in the ACBP or A+L group were more active,
had good appetite and appearance, and their body weights were
closer to that of a normal mouse. The L-OHP group showed little
changes in feeding quantity and activity or weight reduction
compared to the control group (P>0.05). Therefore, short-term
intermittent use of ACBP alone inhibited tumor growth and improved
the quality of life of nude mice. Short-term intermittent use of
A+L significantly increased the sensitizing effects of L-OHP and
improved the quality of life of the nude mice.
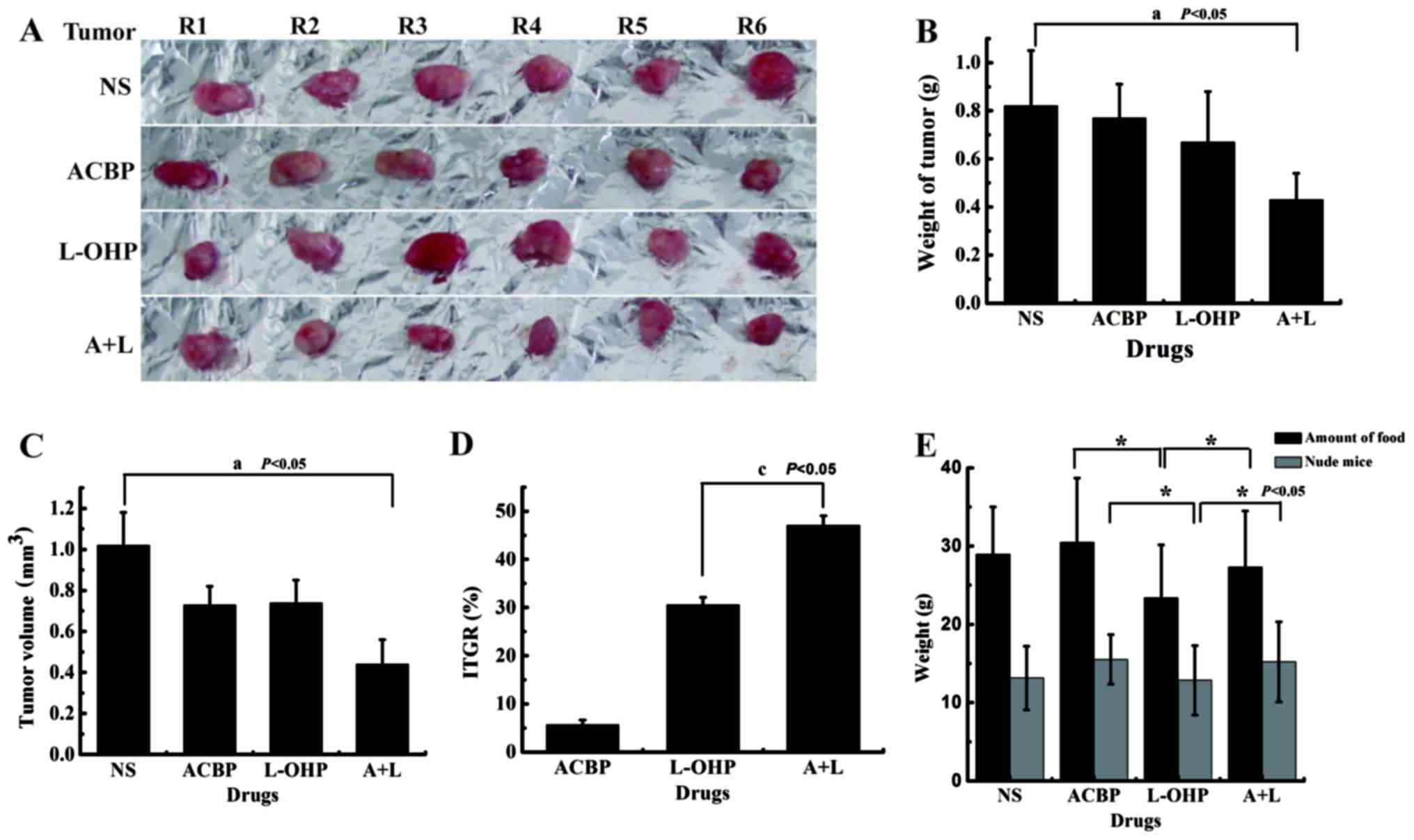 | Figure 3.A+L treatment suppresses gastric
tumor growth and improves quality of life in the xenograft tumor
model. The in vivo tumor growth experiment was established
by subcutaneous injection of 1×107 MKN-45 GC cells.
After tumors were palpable and when the sizes of tumors were ~2-3
mm3, the tumor-bearing mice were randomized with six
mice (n=6) each into four groups: control with intraperitoneal
injection of saline, injection ACBP alone (30 µg/ml), L-OHP alone
(20 µg/ml), and A+L (30 and 20 µg/ml) via every week for two times,
all given the drug at day 3, 7, 10, 14. The final (A) tumor
morphology, (B) tumor weight, (C) tumor volume, (D) ITGR, and (E)
the diet and weight of nude mice were calculated before the end of
the experiment. The data were calculated and are presented as the
mean ± SD. Statistical significance was determined by Student's
t-test (statistical difference was indicated as P<0.05, when
compared with the control group (aP<0.05), L-OHP
(cP<0.05) and ACBP or A+L (*P<0.05). A+L,
combination of anticancer bioactive peptide and oxaliplatin; GC,
gastric cancer; ACBP, anticancer bioactive peptide; L-OHP,
oxaliplatin; ITGR, inhibition tumor growth rate; SD, standard
deviation. |
A+L treatment decreases liver weights
in xenograft tumor models
The liver and spleen coefficients are directly
proportional to the degree of structural destruction. The liver and
spleen coefficients are defined as the ratio of the weight of
liver/spleen to the weight of the nude mouse. Due to the different
weights of the nude mice treated with various drugs (NS, ACBP,
L-OHP and A+L), the values of the liver and spleen coefficients are
relative. This method is adequate for evaluating the effect of
treatment on GCs. Fig. 4A and B
shows the effects of the ACBP, L-OHP, and A+L treatments on the
livers of tumor-bearing nude mice. H&E staining has been used
as a standard in vivo method to characterize the stem
cell-like behaviors (Fig. 4C-F).
Black normal saline treatment displayed hepatocellular injury,
whereas both free ACBP and L-OHP treatments drastically reduced
damage to the hepatocytes with milder effects on hepatic steatosis,
loose cytoplasm, eosinophilic change, hepatocellular necrosis,
inflammatory cell infiltration, hepatic vein dilation, liver cell
damage, and other cable structures. Notably, treatment with A+L
completely inhibited hepatocellular injury compared with L-OHP but
had milder effects on the liver steatosis coefficient, loss of
cytoplasm, eosinophilic change, hepatocellular necrosis,
inflammatory cell infiltration, hepatic vein dilation, and hepatic
cord structural changes in liver cells. The results suggest that
A+L selectively inhibits the growth of GC (Fig. 4B). This result is important as the
differences in the liver coefficient are insignificant and the
major cause of hepatocellular injury is not related to the
drugs.
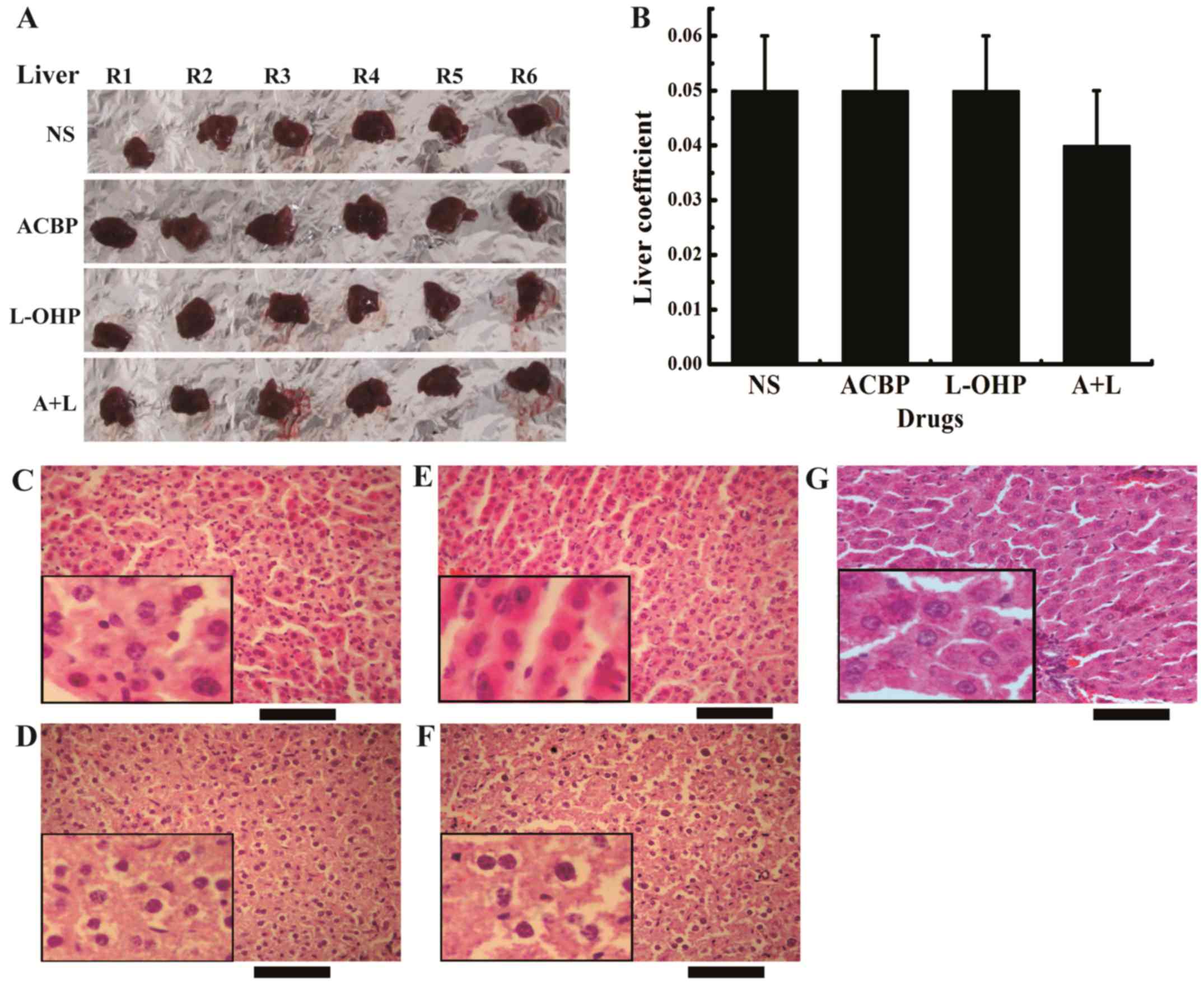 | Figure 4.A+L treatment decreases liver weight
in the xenograft tumor model. (A) The liver morphology and (B)
liver coefficient were calculated before the end of the experiment.
The data were calculated and are presented as the mean ± SD.
H&E staining (x400) of the liver of tumor-bearing nude mice
treated with (C) NS, (D) ACBP, (E) L-OHP, (F) A+L and (G) the
normal control morphology images of the liver. Scale bar, 200 µm.
A+L, combination of anticancer bioactive peptide and oxaliplatin;
SD, standard deviation; H&E, hematoxylin and eosin; NS, normal
saline; ACBP, anticancer bioactive peptide; L-OHP, oxaliplatin. |
A+L decreases the spleen coefficient
in the xenograft tumor model
The spleen coefficient was measured as an indication
of L-OHP systemic toxicity. Fig. 5A
shows the spleen damage after treatment with ACBP, L-OHP, and A+L.
The results were different from the liver damage results. The
spleen coefficient after ACBP treatment decreased significantly
(P<0.05) in comparison with the control (Fig. 5B); the spleen structure showed
decreased damage to the malpignian corpuscle and the splenic cords,
and the sinus of the red pulp region displayed more sinus cells
(Fig. 5C-F). The spleen coefficient
after treatment with A+L showed a significant reduction compared to
L-OHP; expansion of the spleen was not obvious, and the damage to
the spleen was significantly decreased (Fig. 5E and F), indicating that the
toxicity of L-OHP was decreased by the combination treatment with
ACBP. Therefore, short-term intermittent use of A+L can
significantly reduce the side-effects of chemotherapy.
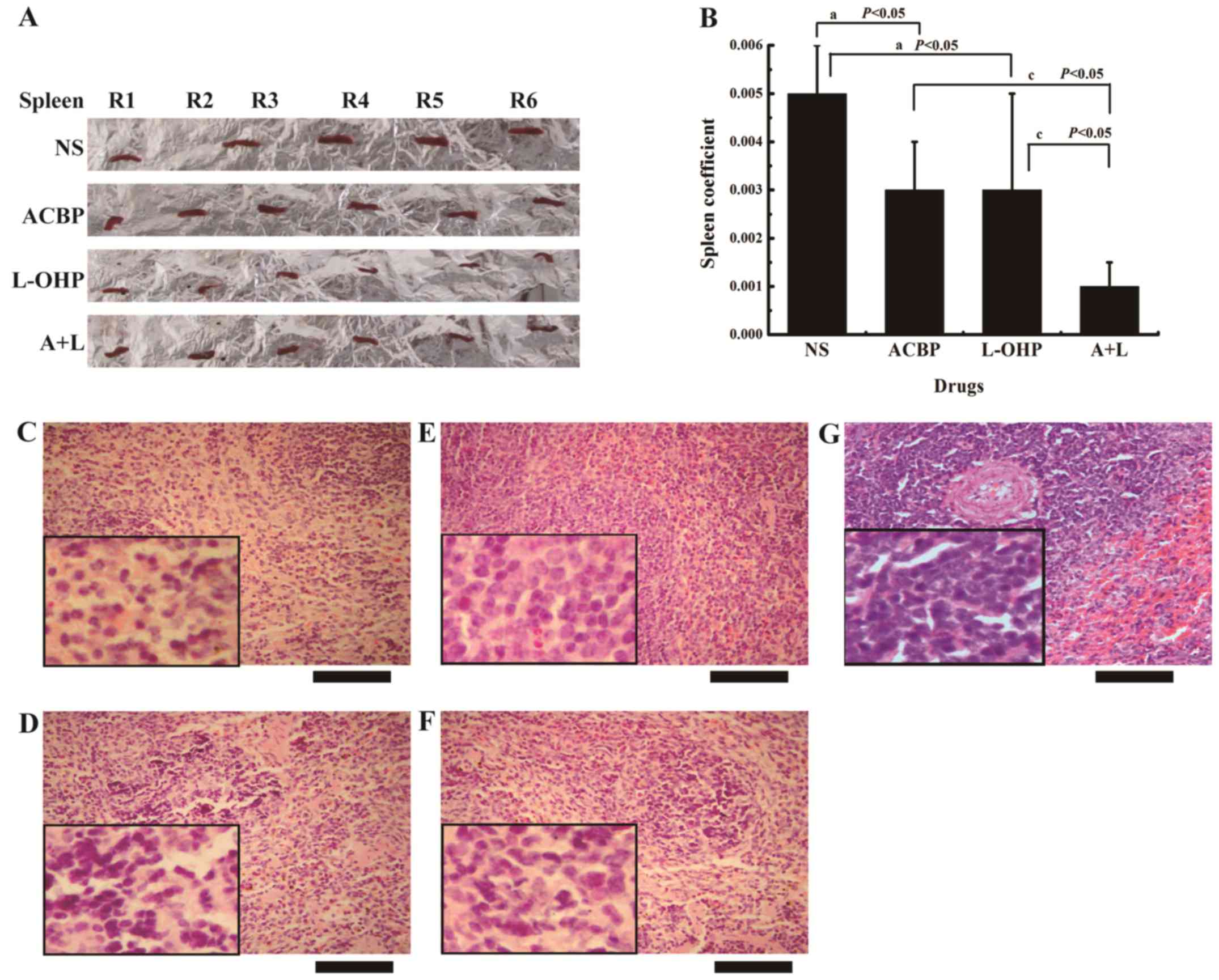 | Figure 5.A+L treatment decreases the spleen
coefficient in the xenograft tumor model. (A) The spleen morphology
and (B) spleen coefficient were calculated before the end of the
experiment. The data were calculated and are presented as the mean
± SD. Statistical significance was determined by Student's t-test
[statistical difference was indicated as P<0.05, when compared
with (a) the control group, (c) L-OHP or (c) ACBP, P<0.05].
H&E staining (x400) of the spleen of tumor-bearing nude mice
treated with (C) NS, (D) ACBP, (E) L-OHP, (F) A+L and (G) the
normal control morphology images of the spleen. Scale bar, 200 µm.
A+L, combination of anticancer bioactive peptide and oxaliplatin;
SD, standard deviation; L-OHP, oxaliplatin; ACBP, anticancer
bioactive peptide; H&E, hematoxylin and eosin; NS, normal
saline. |
A+L increases apoptosis and arrests
the cell cycle
Because GC forms solid tumors without capillaries
between the cells, which prevents drug penetration to the center of
the tumor, a tumor model was established to evaluate the tumor cell
cycle effect on apoptosis (Fig.
6A-D). Cytological assays that measure cell cycle revealed that
increased amounts of the drugs (A+L, L-OHP, and ACBP) reversed the
inhibitory effect of GC cells on the cell cycle. Compared with the
control, A+L, L-OHP, and ACBP significantly enhanced the apoptotic
rate of the tumor cells (P<0.05) by increasing the proportion of
cells in the S phase (P<0.05) (Fig.
6E and F). Based on our flow cytometric analysis, A+L
significantly increased the proportion of cells in the G2/M phase
(P<0.05) relative to ACBP or L-OHP alone. Next, we examined the
apoptotic rate of the MKN-45 GC cells. A+L and ACBP alone caused
23.15±3.18 and 21.56±5.18% of the cells to undergo apoptosis,
respectively. After treatment with ACBP and A+L, MKN-45 cells
displayed higher apoptotic levels compared with the control
(3.24±0.57%) or L-OHP (10.75±3.05%), indicating the statistically
significant increase in apoptosis (P<0.01). Compared with the
control, ACBP, L-OHP and A+L all resulted in statistically
significant increases in apoptosis (P<0.01). Therefore, A+L
treatment suppressed the cell cycle in the S and G2/M phase and
enhanced cellular apoptosis.
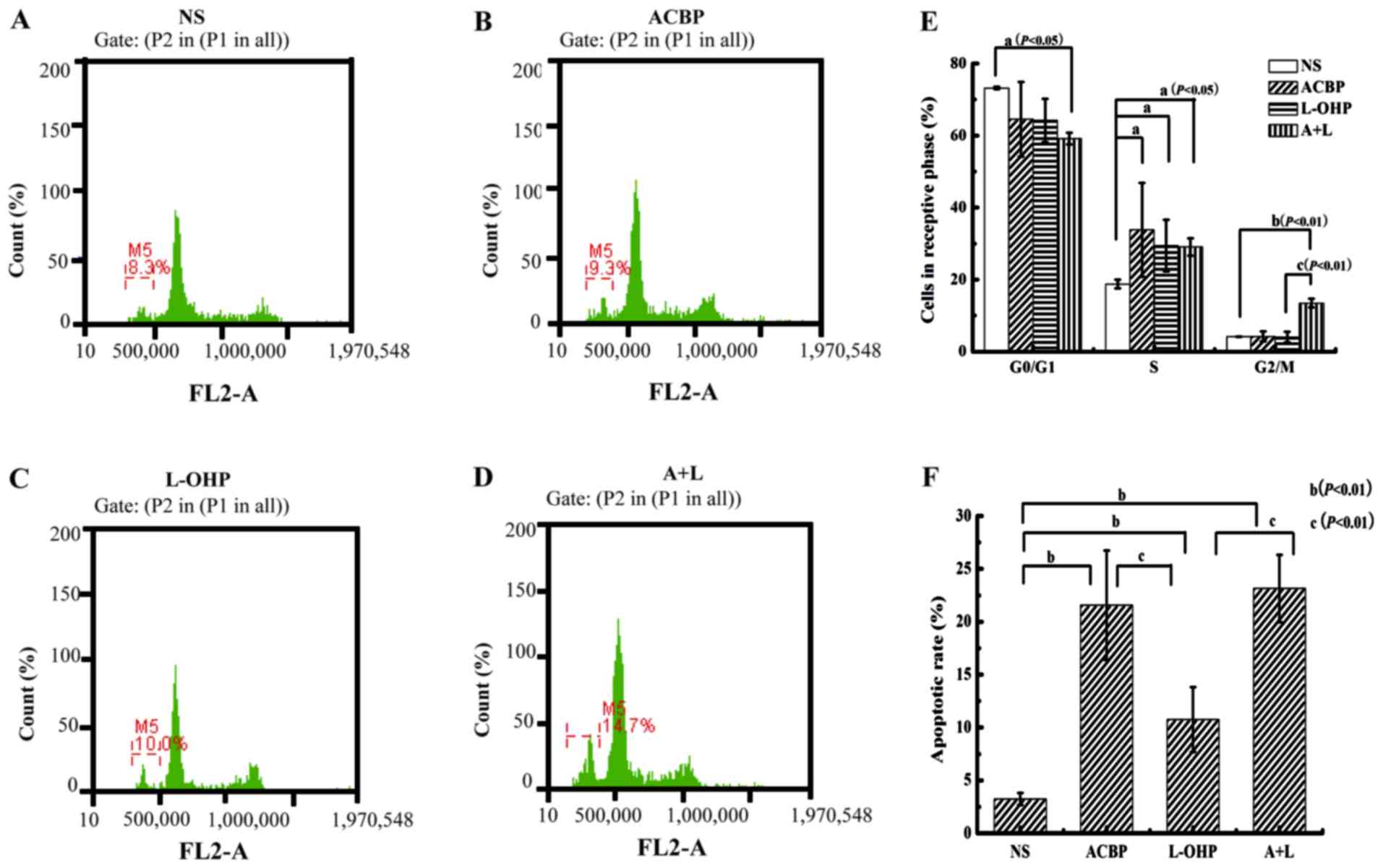 | Figure 6.PI staining and flow cytometry for
cell cycle analysis and apoptotic rate. (A) The control NS, (B)
ACBP alone, (C) L-OHP alone, and (D) A+L effects on MKN-45 cell
tumors. (E) Cells in respective cell cycle phase and (F) apoptotic
rate were calculated and are presented as the mean ± SD.
Statistical significance was determined by Student's t-test
[statistical difference was indicated as P<0.05, when compared
with the control group (aP<0.05,
bP<0.01), and L-OHP (cP<0.01)]. PI,
propidium iodide; NS, normal saline; ACBP, anticancer bioactive
peptide; L-OHP, oxaliplatin; A+L, combination of anticancer
bioactive peptide and oxaliplatin; SD, standard deviation. |
Protein expression of cytochrome c,
caspase-3, −8, and −9 in GC tissue
Initially, we tested the expression of cytochrome
c, caspase-3, −8, and −9 in GC tissues treated with ACBP,
L-OHP, or A+L and a matched control (NS control) using
immunohistochemical staining (Fig.
7). Observation under an optical microscope showed positive
staining for cytochrome c, caspase-3, −8, and −9 as well as
yellow, pale brown or chocolate brown colors. The positive staining
for cytochrome c, caspase-3, −8, and −9 was higher in the GC
cells. Protein expression was examined via conventional
semi-quantitative score methods (Fig.
8A). Cytochrome c, caspase-3, −8, and −9 were positively
expressed in the GC cells. The immunohistochemical scores for
cytochrome c, caspase-3, −8, and −9 protein expression
changed from 12 to 17, 15 to 18, 5 to 10, and 3 to 5, respectively
in the drug-treated (ACBP to A+L) tumor tissues, indicating that
(A+L)-treated cells displayed statistically significant (P<0.05)
expression compared to scores (10, 12, 1, and 2, respectively) from
the matched control. Quantitative analysis indicated that the
levels of caspase-8 in the GC tissues treated with L-OHP were
significantly higher than in the NS control (P<0.05).
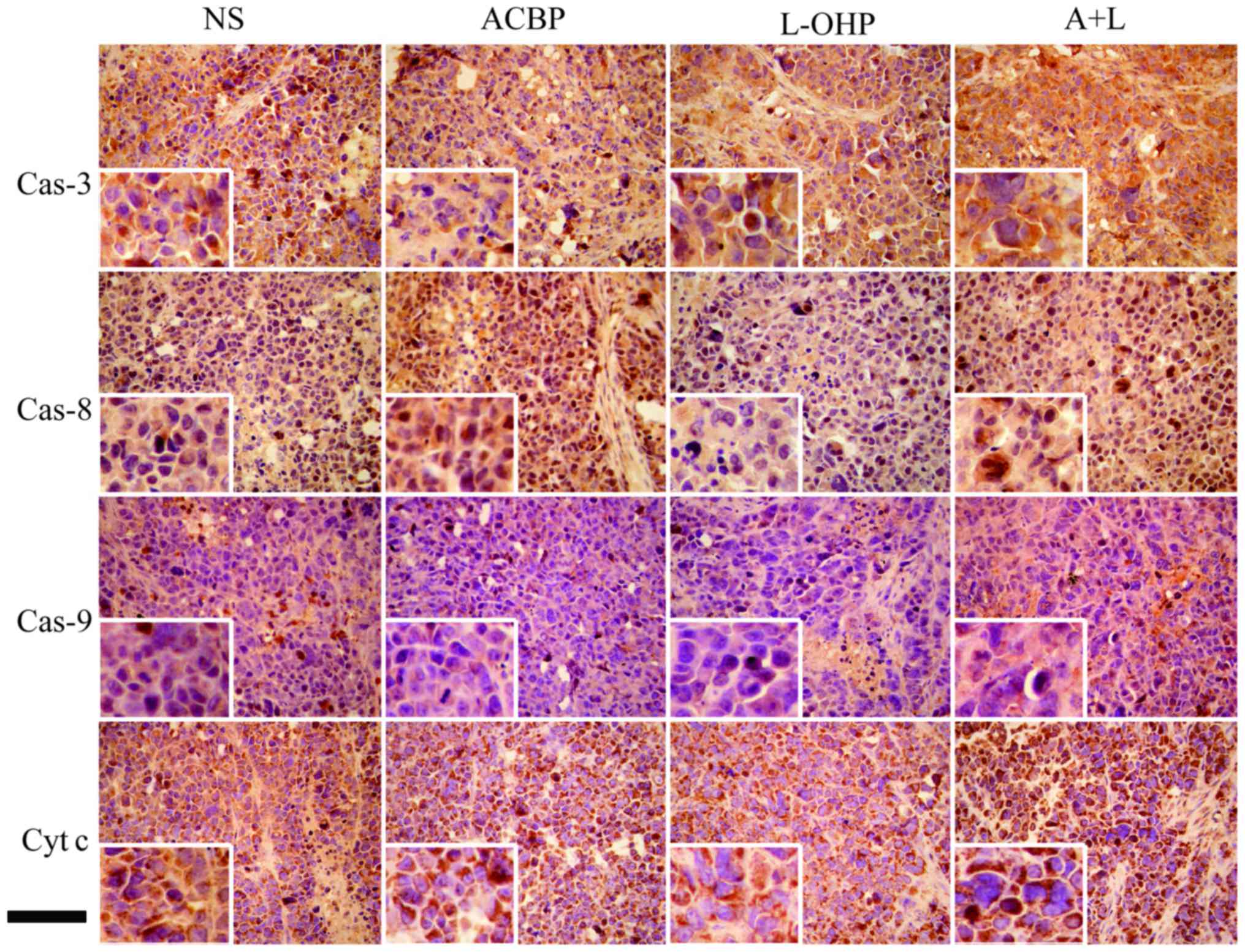 | Figure 7.Immunohistochemical staining for
caspase-3, caspase-8, caspase-9 and cytochrome c protein
expression. Representative images of Cas-3 (first row), Cas-8
(second row), Cas-9 (third row), and Cyt c (fourth row)
protein expression in GC tumor tissues treated with NS (first
column), ACBP (second column), L-OHP (third column), and A+L
(fourth column) by immunohistochemical analysis (x400). Scale bar,
100 µm. Cas-3, caspase-3; Cas-8, caspase-8; Cas-9, caspase-9; Cyt
c, cytochrome c; GC, gastric cancer; NS, normal
saline; ACBP, anticancer bioactive peptide; L-OHP, oxaliplatin;
A+L, combination of anticancer bioactive peptide and
oxaliplatin. |
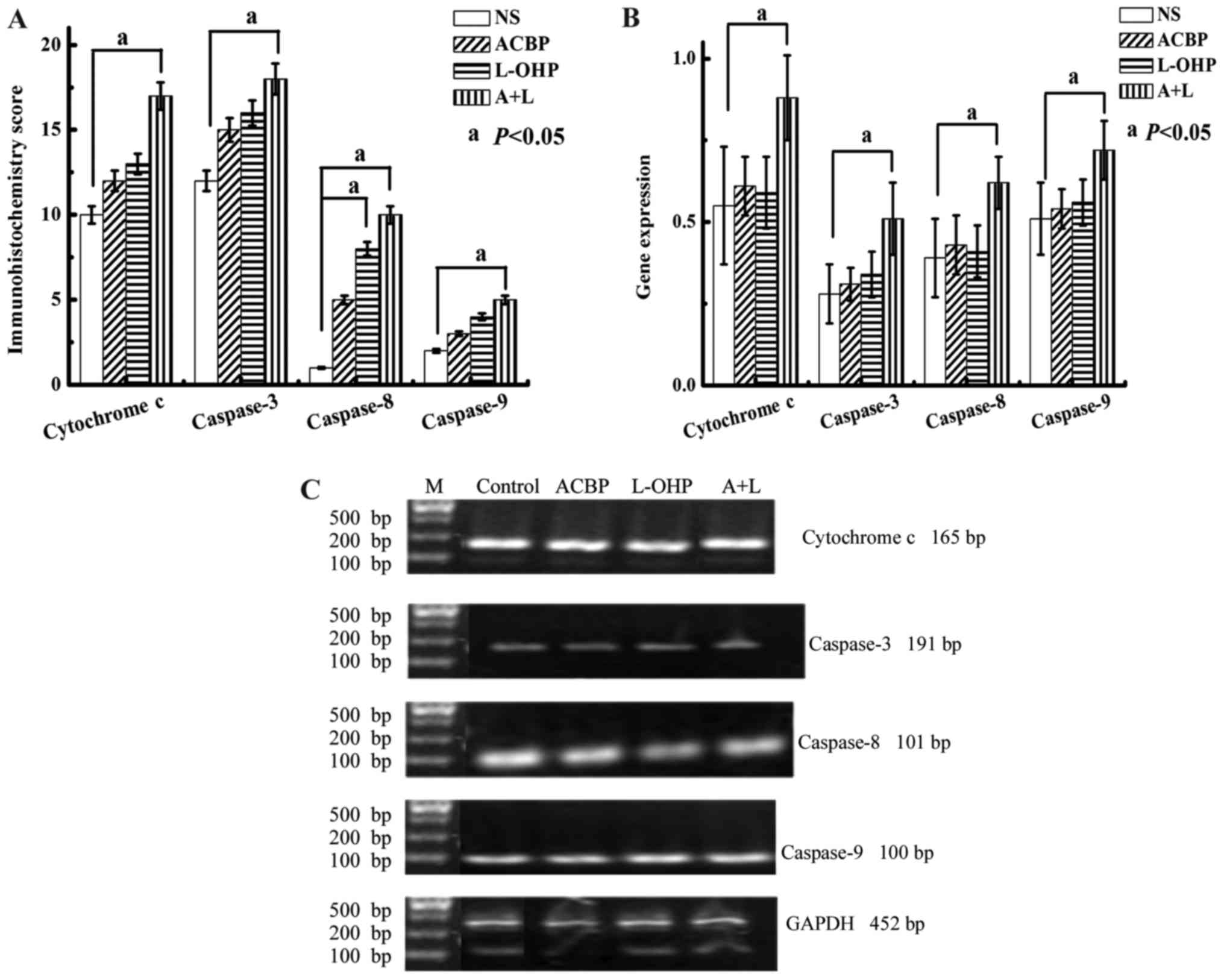 | Figure 8.Immunohistochemical score of
cytochrome c, caspase-3, caspase-8 and caspase-9 protein
expression in tumor tissues. (A) The immunohistochemical score was
calculated according to the conventional semi-quantitative scoring
method. Statistical significance was determined by Student's t-test
[statistical difference was indicated as P<0.05, when compared
with (a) the control group]. (B) RT-PCR and 2% agarose gel
electrophoresis for Cas-3, Cas-8, Cas-9 and Cyt c gene
expressions. The MKN-45 GC cells in the xenograft tumor model were
treated with NS (control), ACBP, L-OHP, and A+L for 48 h for RT-PCR
analysis. (C) Cas-3, Cas-8, Cas-9, and Cyt c gene expression
in human MKN-45 GC cells was typed with BstUI and resolved
by 2% agarose gel electrophoresis. Gene expression levels were
calculated and are presented as the mean ± SD. Statistical
significance was determined by Student's t-test [statistical
difference was indicated as P<0.05, when compared with (a) the
control group]. Cyt c, cytochrome c; Cas-3,
caspase-3; Cas-8, caspase-8; Cas-9, caspase-9; GC, gastric cancer;
NS, normal saline; ACBP, anticancer bioactive peptide; L-OHP,
oxaliplatin; A+L, combination of anticancer bioactive peptide and
oxaliplatin; SD, standard deviation. |
Gene expression of cytochrome c,
caspase-3, −8, and −9 in GC tissue
RT-PCR and electrophoresis were used to analyze the
expression of cytochrome c, caspase-3, −8, and −9 in GC
tissues (Fig. 8B and C). Gene
expression of cytochrome c, caspase-3, −8, and −9 was
increased following the A+L, L-OHP, and ACBP treatments relative to
the NS control, although the differences were not significant
(P>0.05), except for that between the A+L and NS treatments
(P<0.05).
Discussion
ACBPs are a leading prospect for the treatment of
many diseases, as they were found to inhibit the growth of Dutch
GCMGC-803 and BGC-823 cells and gallbladder carcinoma GBC-SD cells.
Daily ACBP in combination with continuous cisplatin improves the
efficacy of cisplatin, reduces its use, and decreases the toxicity
of chemotherapy drugs (26,27). In the present study, ACBP (normal
and/or induced) and L-OHP treatment of MKN-45 GC cells showed
significant inhibition of cell proliferation in a dose-dependent
manner after 24 h. A+L was more effective than either drug alone at
inhibiting cell growth (P<0.05). The growth IR was better in the
A+L group compared to the L-OHP alone group; the difference was
significant (P<0.05). For each experimental group compared with
the control group, the cell numbers were reduced, the cell
morphology ranged in size, and the cells displayed poor adherence,
cell shrinkage, and cell lysis. The number of adherent cells was
significantly reduced in the 30 µg/ml ACBP + 20 µg/ml L-OHP
combination drug-treated cells. Cell-free zones, suspension cells
and karyopyknosis were visible, although the cell contours were not
obvious. Therefore, short-term application of ACBP and L-OHP
inhibited human GC cell growth. A+L increased the utility and
reduced the toxicity of L-OHP.
We also investigated the short-term intermittent
application of induced ACBP with L-OHP in human gastric carcinoma
xenograft tumors. MKN-45 GC cells were subcutaneously inoculated in
nude mice for xenograft tumor model preparation. We demonstrated
that discontinuous short-term injection of ACBP improved the food
intake, activity, and body weight of the nude mice and inhibited
tumor growth compared with the control mice, although free ACBP
showed no significant differences. The spleen coefficient
significantly decreased with ACBP treatment relative to NS
(P<0.05), and decreased liver and spleen tissue injury indicated
an improved quality of life for the tumor-bearing nude mice.
Furthermore, intraperitoneal injection of A+L increased the
feeding, activity, and body weight of nude mice and decreased liver
and spleen damage and coefficients compared to treatment with free
L-OHP (P<0.05). The results indicate that A+L mitigates L-OHP
toxicity and improves the quality of life of tumor-bearing nude
mice.
Previous studies of the mechanisms of tumor
occurrence suggest that malignant tumors occur and develop not only
from accelerated proliferation and decreased apoptosis but also
from the relationships between inhibition, abnormal proliferation,
and apoptotic regulation. Some scholars believe that the main
driver of malignant tumor development is inhibition of normal cell
apoptosis. Apoptosis is a cellular suicide program, and inducing
apoptosis is a key tactic for eliminating cancer cells without
stimulating an inflammatory reaction. Several conventional drugs
believed to induce cell apoptosis via activation of these elements
are currently used in anticancer chemotherapy (28). Therefore, an increasing
understanding of the inhibitory mechanisms of cell apoptosis is
providing new insights into tumor development.
Current research shows that apoptosis is closely
related to the cell cycle (29),
which has become a new target for cancer therapy. Many anticancer
drugs function primarily to induce apoptosis in cancer cells and
prevent tumor development (30,31).
In many cases, extensive DNA damage leads to activation of cell
cycle check points and results in cell cycle arrest and apoptosis
(32). Previous studies have shown
that ACBP promoted cell proliferation in GC cells and inhibited
MGC-803 cancer cells (17) and
L-OHP-resistant human colon cancer cell lines with increasing dose
of L-OHP in culture (33). In this
study, we found that A+L induced apoptosis in MKN-45 cells, which
was revealed by the PI assay. When the cells were treated with NS,
L-OHP, ACBP and A+L for 48 h, the average proportion of PI
staining-positive cells was significantly increased from 3.24% in
the control to 10.75, 21.56 and 23.15% in the L-OHP, ACBP and A+L
groups, respectively. We also evaluated the effect of NS, L-OHP,
ACBP and A+L on cell cycle phase distribution using flow cytometry.
It was observed that ACBP and L-OHP induced cell cycle arrest in
the S and G2/M phase, respectively.
In recent years, caspases have been used to
determine the apoptotic state, as they play a critical role in the
apoptotic molecular mechanism. Currently, most in-depth studies of
apoptosis in mammalian cells focus on death receptor- and
mitochondrial-mediated apoptosis. Mitochondrial-mediated apoptosis
involves the release of cytochrome c from the mitochondria.
It combines with Apaf-1 and, in the presence of dATP or ATP,
oligomerically activates caspase-9, which starts the caspase
downstream cascade and promotes apoptosis (34). In these two apoptotic pathways,
activation of the caspase cascade is critical; caspase-3
activation, which is downstream, is the central link between the
two apoptotic pathways. It has been shown that caspase-3 expression
was significantly lower in gastric carcinoma than in adjacent
normal gastric mucosa. This suggests that the decrease in caspase-3
expression in GC cells, which inhibits the cells from undergoing
apoptosis, may be involved in the formation of gastric carcinomas.
ACBPs have been confirmed to promote p16, p21, p27, caspase-8, and
Bax mRNA expression (17,26). These results suggest that ACBPs
could inhibit tumor growth through the induction of the
apoptosis-related genes caspase-3, −8 and −9. Existing research has
demonstrated that expression of caspase-3, −8, and −9 in GC tissue
is obviously lower than in adjacent normal gastric mucosa tissue,
suggesting that inhibition of apoptosis may be key to GC
progression (35). In contrast,
expression of cytochrome c, a known pro-apoptotic factor,
decreased with decreasing GC tissue differentiation.
We found that A+L significantly decreased tumor
weight (P<0.05), whereas there was no significant difference
between the L-OHP group (P>0.05) and the NS group. The gene
expression results indicated that short-term intraperitoneal
injection of A+L could increase the antitumor effect of L-OHP.
Overall, ACBP inhibited tumor growth via a
short-term application and improved the quality of life of
tumor-bearing nude mice after MKN-45 cell transplantation compared
to control. Short-term application of A+L also inhibited tumor
growth and improved the quality of life of nude mice after MKN-45
cell transplantation compared to free L-OHP. In addition, A+L
increased the utility of L-OHP and reduced the toxicity of
chemotherapeutics. These results support a new approach for
exploring the comprehensive treatment of GC.
In summary, we demonstrated novel anticancer
activity for peptides (normal or induced ACBP) and showed that they
could be used in a strategy to reduce the toxicity of L-OHP. Our
results indicated that combination therapy with induced ACBP and
L-OHP inhibited cancer cell growth at high concentrations (30 µg/ml
ACBP + 20 µg/ml L-OHP), suggesting that at these concentrations
ACBP plays a role in MKN-45 cells and also lead to apoptosis. In
addition, our findings highlight the importance of L-OHP in the
anticancer activity of the peptide. By decreasing the toxicity of
L-OHP, we increased its antitumor activity, providing useful
information for the design of conjugate anticancer peptides and
chemotherapeutics with selective target properties. We validated
that the combination of induced ACBP and L-OHP was useful in GC
therapy in a human gastric carcinoma xenograft tumor model. We
observed that the expression of caspase-3, −8, −9 and cytochrome
c was higher in cells treated with induced ACBP, L-OHP and
A+L than in the NS control. Short-term intermittent treatment with
ACBP alone has a certain role in inhibiting tumor growth, while
short-term intermittent A+L treatment mainly increases the L-OHP
sensitizing effect, reduces the side-effects associated with
chemotherapy, and improves the quality of life of nude mice. This
study provides not only further evidence of the remarkable
antitumor effects of ACBP (normal or induced) but also a new
strategy of combination therapy for clinical investigation.
Acknowledgements
We are grateful to all participants in this study.
This study was supported by a grant from the National Natural
Science Foundation of China (#81260058 and #81450047).
Glossary
Abbreviations
Abbreviations:
|
ACBP
|
anticancer bioactive peptide
|
|
L-OHP
|
oxaliplatin
|
|
NS
|
normal saline
|
|
A+L
|
combination of anticancer bioactive
peptide and oxaliplatin
|
References
|
1
|
Kassam Z, Mackay H, Buckley CA, Fung S,
Pintile M, Kim J and Ringash J: Evaluating the impact on quality of
life of chemoradiation in gastric cancer. Curr Oncol. 17:77–84.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen XZ, Zhang WH and Hu JK: A difficulty
in improving population survival outcome of gastric cancer in
mainland China: Low proportion of early diseases. Med Oncol.
31:3152014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ye L, Zhang ZY, Du WD, Schneider ME, Qiu
Y, Zhou Y, Zhou FS, Zuo XB, Chen G, Ma XL, et al: Genetic analysis
of ADIPOQ variants and gastric cancer risk: A hospital-based
case-control study in China. Med Oncol. 30:6582013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fock KM: Review article: The epidemiology
and prevention of gastric cancer. Aliment Pharmacol Ther.
40:250–260. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Soulié P, Bensmaïne A, Garrino C, Chollet
P, Brain E, Fereres M, Jasmin C, Musset M, Misset JL and Cvitkovic
E: Oxaliplatin/cisplatin (L-OHP/CDDP) combination in heavily
pretreated ovarian cancer. Eur J Cancer. 33:1400–1406. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dong HL and Chen M: GEM combination
5-Fu/L-OHP treatment of advanced pancreatic cancer, effect
observation of 47 cases. Mod Prev Med. 35:3872–3873. 2008.
|
|
8
|
Zhang C, Ren Z and Li C: Recent effect of
combination chemotherapy of hydroxycamptothecin and 5-FU, MMC for
advanced pancreatic cancer. Guangzhou Med J. 34:38–39. 2003.
|
|
9
|
Rebucci M and Michiels C: Molecular
aspects of cancer cell resistance to chemotherapy. Biochem
Pharmacol. 85:1219–1226. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cervantes A, Roselló S, Roda D and
Rodríguez-Braun E: The treatment of advanced gastric cancer:
Current strategies and future perspectives. Ann Oncol. 19 Suppl
5:v103–v107. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yoong J, Michael M and Leong T: Targeted
therapies for gastric cancer: Current status. Drugs. 71:1367–1384.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mathé G, Kidani Y, Noji M, Maral R, Bourut
C and Chenu E: Antitumor activity of l-OHP in mice. Cancer Lett.
27:135–143. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Degardin M, Cappelaere P, Krakowski I,
Fargeot P, Cupissol D and Brienza S: Phase II trial of oxaliplatin
(L-OHP) in advanced, recurrent and/or metastatic squamous cell
carcinoma of the head and neck. Eur J Cancer B Oral Oncol.
32B:278–279. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fiteni F, Nguyen T, Vernerey D, Paillard
MJ, Kim S, Demarchi M, Fein F, Borg C, Bonnetain F and Pivot X:
Cisplatin/gemcitabine or oxaliplatin/gemcitabine in the treatment
of advanced biliary tract cancer: A systematic review. Cancer Med.
3:1502–1511. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang C, Jia S and Su X: Effect of
anticancer bioactive peptide on the gene expression of human
gastric cancer BGC-823 cells. J Clin Oncol. 37:1021–1023. 2010.
|
|
16
|
Wen ZH and Su XL: The effect of
anti-cancer bioactive peptide on the expression of p53 and Bcl-2 of
tumor tissues of experimental nude mice. Med Rev. 17:1557–1559.
2011.
|
|
17
|
Su X, Dong C, Zhang J, Su L, Wang X, Cui H
and Chen Z: Combination therapy of anti-cancer bioactive peptide
with Cisplatin decreases chemotherapy dosing and toxicity to
improve the quality of life in xenograft nude mice bearing human
gastric cancer. Cell Biosci. 4:72014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sui CG, Meng FD, Li Y and Jiang YH:
Antiproliferative activity of rosamultic acid is associated with
induction of apoptosis, cell cycle arrest, inhibition of cell
migration and caspase activation in human gastric cancer (SGC-7901)
cells. Phytomedicine. 22:796–806. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−ΔΔC(T)) method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tu K, Zheng X, Zhou Z, Li C, Zhang J, Gao
J, Yao Y and Liu Q: Recombinant human adenovirus-p53 injection
induced apoptosis in hepatocellular carcinoma cell lines mediated
by p53-Fbxw7 pathway, which controls c-Myc and cyclin E. PLoS One.
8:e685742013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yao HJ, Zhang YG, Sun L and Liu Y: The
effect of hyaluronic acid functionalized carbon nanotubes loaded
with salinomycin on gastric cancer stem cells. Biomaterials.
35:9208–9223. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang J, Chen X, Su L, Li P, Cai Q, Liu B,
Wu W and Zhu Z: MicroRNA-126 inhibits cell proliferation in gastric
cancer by targeting LAT-1. Biomed Pharmacother. 72:66–73. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tu K, Zheng X, Zan X, Han S, Yao Y and Liu
Q: Evaluation of Fbxw7 expression and its correlation with the
expression of c-Myc, cyclin E and p53 in human hepatocellular
carcinoma. Hepatol Res. 42:904–910. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yin T, Lu L, Xiong Z, Wei S and Cui D:
ATPase inhibitory factor 1 is a prognostic marker and contributes
to proliferation and invasion of human gastric cancer cells. Biomed
Pharmacother. 70:90–96. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Stacul F, Bertolotto M, De Gobbis F,
Calderan L, Cioffi V, Romano A, Zanconati F and Cova MA: US,
colour-Doppler US and fine-needle aspiration biopsy in the
diagnosis of thyroid nodules. Radiol Med (Torino). 112:751–762.
2007. View Article : Google Scholar
|
|
26
|
Yu L, Yang L, An W and Su X: Anticancer
bioactive peptide-3 inhibits human gastric cancer growth by
suppressing gastric cancer stem cells. J Cell Biochem. 115:697–711.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Su L, Xu G, Shen J, Tuo Y, Zhang X, Jia S,
Chen Z and Su X: Anticancer bioactive peptide suppresses human
gastric cancer growth through modulation of apoptosis and the cell
cycle. Oncol Rep. 23:3–9. 2010.PubMed/NCBI
|
|
28
|
Kundu T, Dey S, Roy M, Siddiqi M and
Bhattacharya RK: Induction of apoptosis in human leukemia cells by
black tea and its polyphenol theaflavin. Cancer Lett. 230:111–121.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Alshatwi AA, Athinarayanan J and Periasamy
VS: Green synthesis of bimetallic Au@Pt nanostructures and their
application for proliferation inhibition and apoptosis induction in
human cervical cancer cell. J Mater Sci Mater Med. 26:1482015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Khoo BY, Chua SL and Balaram P: Apoptotic
effects of chrysin in human cancer cell lines. Int J Mol Sci.
11:2188–2199. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li W, Wang J, Jiang HR, Xu XL, Zhang J,
Liu ML and Zhai LY: Combined effects of cyclooxygenase-1 and
cyclooxygenase-2 selective inhibitors on ovarian carcinoma in vivo.
Int J Mol Sci. 12:668–681. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pietenpol JA and Stewart ZA: Cell cycle
checkpoint signaling: Cell cycle arrest versus apoptosis.
Toxicology. 181–182. 2002.PubMed/NCBI
|
|
33
|
Xiang Z, Kang QJ and Xiang X: Gene and
protein expression in the oxaliplatin-resistant HT29/L-OHP human
colon cancer cell line. Genet Mol Res. 14:11013–11022. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Reubold TF and Eschenburg S: A molecular
view on signal transduction by the apoptosome. Cell Signal.
24:1420–1425. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Huang L, Zhang T, Li S, Duan J, Ye F, Li
H, She Z, Gao G and Yang X: Anthraquinone G503 induces apoptosis in
gastric cancer cells through the mitochondrial pathway. PLoS One.
9:e1082862014. View Article : Google Scholar : PubMed/NCBI
|