Introduction
Due to the vast functional multiplicity, microRNAs
(miRNA, miRs) shaped up as the most important class of small
non-coding RNAs (ncRNAs) (1)
playing a crucial role in post-transcriptional regulation of gene
expression and signaling pathways. Mature miRNA is incorporated
into the RNA-induced silencing complex (RISC) which usually leads
to mRNA degradation and translational repression (2,3). Using
this mechanism, miRNAs influence cellular processes of
differentiation, proliferation and apoptosis. Due to their ability
to participate in modulation of oncogenic pathways miRNAs are
important key regulators of tumor suppressor genes and oncogenes
(4–6). A widespread dysregulation of miRNAs is
observed in a variety of human cancers (7–9).
miRNA molecules can be localized intracellularly or,
after secretion, circulate in the extracellular compartment
(10). Release of miRNA in the
extracellular compartment can take place as passive leakage from
cells or can include active export by apoptotic bodies,
high-density lipoproteins, microvesicles, exosomes and protein
complexes (11–13). Argonaute complexes, which are
regular complexes of the RNA silencing machinery, and
Nucleosphosmin-1 play a predominant role in protein-complex
dependent secretion of miRNAs (14). In this context, miRNA packaging and
extracellular export is mediated by Nucleophosmin-1, which also
acts as a protector from RNA degradation (11,15).
For exosomal miRNA secretion, Zhang et al reported
trafficking and sorting mechanisms which play an important role in
cell-cell communication processes (16).
Endometrial cancer (EC) is the most common
gynecologic malignancy, accounting for 7% of all cancers in women
in the United States (17).
Incidence as well as death rate of EC has increased during recent
years (18). EC can be
subclassified into two categories: type I cancers are usually well
to moderately differentiated, estrogen-related endometrial
carcinomas (EEC) with good prognosis, which are associated with
endometrial hyperplasia, whereas type II cancers are non-estrogenic
high grade serous or clear cell carcinomas with poor prognosis,
which are associated with atrophic endometrium. Molecular
characteristics of EC cells do not always correlate with the
traditional classification systems which have been based on
clinical features or on histopathological findings (19–21).
MiRNAs have been shown to participate in regulation
of endometrial growth and differentiation (22–26)
and carcinogenesis of the endometrium has been associated with
alterations in miRNA expression pattern. Furthermore, distinct
miRNA signatures characterizing EC subtypes have been described
(24,27–30).
Since miRNAs have been shown to be stable molecules which are well
preserved in serum and other body fluids (21,31,32)
their use as promising non-invasive biomarkers in EC is under
investigation (21). Jia et
al reported in 2013 the identification of four serum-based
miRNA types as potential non-invasive biomarkers for endometrioid
endometrial cancer (32).
Alterations in miRNA expression patterns were also
found in tumor cells exposed to stressors such as extracellular
hypoxia and acidosis (33,34). However, there are no reports on
miRNA expression under hypoxic or acidic conditions in EC cells. To
our knowledge, to date there are only few studies investigating
miRNA expression aberrations under the influence of hypoxia in
endometriosis (35–37).
Hypoxia is an important regulatory factor in tumor
growth and induces transcriptional cascades that promote a more
aggressive tumor phenotype. In order to survive and proliferate in
a hypoxic microenvironment tumor cells undergo genetic and adaptive
changes and release substances that affect the microenvironment to
promote tumor angiogenesis (38,39).
These processes contribute to a malignant phenotype and aggressive
tumor behavior as well as metastasis (38).
Kulshreshtha et al suggested that hypoxia may
represent a key contributing stress factor for microRNA alterations
in cancer. In colon and breast cancer cell lines, expression of
specific miRNAs was altered under hypoxia (40). Hua et al described a miRNA
directed regulation of VEGF and other angiogenetic factors under
hypoxia in cells from a human nasopharyngeal carcinoma cell line
(41).
Acidosis of the extracellular microenvironment is a
consequence of increased glycolysis due to the adaption of tumor
cells to prolonged periods of hypoxia. Cancer cells suffering
oxygen deprivation use the glycolytic pathways to maintain their
ATP level for survival and proliferation. Acidification of the
peritumoral microenvironment induces necrosis and apoptosis of
normal cells, thus creating space into which the tumor cells may
proliferate (42). Acidosis
promotes tumor invasion by degradation of the extracellular matrix
through the discharge of proteolytic enzymes (43). Lowered pH levels also inhibit
natural killer cell activity and the cytolytic activity of
cytotoxic T-lymphocytes, therefore causing a diminished immune
response to tumor antigens (44).
This way, acidosis facilitates tumor progression and the
development of invasive phenotypes. Furthermore, acidosis and
hypoxia have been associated with resistance to therapeutic
strategies such as multi-drug resistance and poor prognosis
(45).
The present study investigated the quantitative
expression of 24 different miRNAs secreted by EC cells in
vitro in response to the exogenic stimuli hypoxia and acidosis.
The panel of 24 specific miRNA types (let-7a, let-7b, let-7d,
let-7i, miR-10a, −10b, −15a, −15b, −17, −19b, −20a, −20b, −21, −22,
−26.1, 27a, −29c, −30b, −92a, −125b, −128-1, −135b, −200c, −222)
was collocated based on a targeted pre-selection process focusing
on EC-associated circulating miRNAs with tumor biological,
diagnostic and therapeutic relevance. An initial screening
expression analysis attested that all 24 secreted miRNAs were
stably detectable in the supernatant of the selected three EC in
vitro models (Ishikawa, EFE 184, AN3-CA; see Materials and
methods for details). The current data on the tumor biological
relevance of the selected miRNA types is summarized in Table I (1,25,26,28–30,41,46–75).
 | Table I.Tumor-biological relevance of
pre-selected miRNA types. |
Table I.
Tumor-biological relevance of
pre-selected miRNA types.
| Pre-selected
miR | Putative
target | Reported
function | References |
|---|
| Let-7 | BAX | Enhanced survival
and proliferation of cancer cells (promoting EC) | Zhang et al
(70) |
| Let-7a | Aurora B | Inhibition of EC
growth | Liu et al
(57) |
| Let-7b | High mobility group
AT-hook2 | Inhibition of
aggressive phenotypes | Romero-Perez et
al (64) |
| Let-7d | LIN28, C-MYC,
K-RAS, HMGA2 and IMP-1 | Tumor suppressor or
oncogene | Kolenda et
al (54) |
| Let-7i | RAS, HMGA2, c-Myc,
CDC25A, CDK6 and cyclin D2 | Tumor
suppressor | Yang et al
(94) |
| miR-10a | USF2, HOXA1,
HOXD10, HOXB1, HOXB3, RB1CC1 | Invasion,
metastasis (promoting EC) | Dai et al
(50) |
| miR-10b | TBX5m DYRK1A,
PTEN | Oncomir, Migration,
invasion, proliferation | Kim et al
(53) |
| miR-15a | Bck, MCL1, CCND1,
WNT3 | Tumor
suppressor | Aqeilan et
al, Bonci et al (46,47) |
| miR-15b | VEGF, CCND1,
CCNE1 | Tumor suppressor,
antiangiogenic | Zhao et al
(71) |
| miR-17 | VEGF-A, NOR-1,
GALNT3 | Antiangiogenic | Doebele et
al, Hua et al, Lu et al, Ramon et al
(41,51,63,95) |
| miR-19b | PTEN, TGFβ | Oncogene component
of mir-17-92-Cluster | Doebele et
al, Lu et al, Ramon et al, Fuziwara et al
(51,63,73,95) |
| miR-20a | VEGF-A | Reduces
angiogenesis in EC | Doebele et
al, Lu et al, Ramon et al (51,63,95) |
| miR-20b | MMP-2 | Tumor
suppressor | Park et al
(59) |
| miR-21 | Maspin, Pdcd4,
PTEN | Tumorigenesis Cell
proliferation (promoting EC) | Qin et al,
Torres et al (30,62) |
| miR-22 | Cyclin D1, MMP2,
MM9 Erα | Tumor
suppressor | Li et al,
Wang et al (56,69) |
| miR-26a-1 | CCNE1, ERα, FGF9,
MTDH, EZH2, MCL-1 | Antiproliferation,
proapoptotic, metastasis inhibition, growth inhibition | Chen et al
(49) |
| miR-27a | FOXO1 BAX | Myometrial invasion
Inhibition of apoptosis, increased survival of cancer cells
(promoting EC) | Zhang et al,
Mozos et al, Myatt et al (70,22,23) |
| miR-29c | SPARCS (in EC)
PI3K, CD42 | Tumor
suppressor | Castilla et
al, Park et al (24,25) |
| miR-30b | SIX1 | Inhibits migration
and invasion | Zhao et al
(72) |
| miR-92a |
PTEN/PI3K/Akt/mTOR | Member of miR-17-92
cluster, promotes carcinogenesis in endometrial epithelium,
oncogene | Torres et al
(67) |
| miR-125b | TP53INP1 | Proliferation and
migration of cancer cells (promoting EC) | Jiang et al,
Shang et al (28,29) |
|
| ERBB2 | Inhibition of
cancer cell invasion (protecting against EC) |
|
| miR-128-1 | BMI1, E2F3 | Tumor
suppressor | Shan et al
(65) |
| miR-135b | VEGF A, HIF1 A,
HIFAN | Tumor promoting
Upregulated in EC | Tsukamoto et
al (68) |
| miR-200c | TIMP2 RD7 ZEBs,
VEGF-A, FLT1, IKKβ, KLF9, FBLN5 | Promoting EC:
Metastasis of cancer cells Induction of cell survival and
proliferation Reduction of apoptosis Transition into cancerous
states Protective: | Li et al,
Park et al, Snowdon et al (32,33,34) |
|
| FN1, MSN, NTRK2,
LEPR, ARHGAP19 | Downregulation
promotes EMT phenotype and aggressive behavior of cells |
|
| miR-222 | c-kit CD117,
PBX3 | Antiangiogenic
activity | Ramon et al,
Poliseno et al, Yanokura et al (14,35,36) |
Materials and methods
Cell culture conditions and
treatments
Established endometrial cancer cell lines Ishikawa
i) (type I, well differentiated, ER and PR positive) (76). AN3-CA ii) (type II, poorly
differentiated, ER negative) and EFE-184 iii) (type I) were
incubated in humidified atmosphere at 37°C and 5% CO2 in
i) RPMI-1640 or (ii and iii) DMEM/F12 medium supplemented with 10%
newborn calf serum (Gibco™, Thermo Fisher Scientific, Karlsruhe,
Germany), 1% HEPES buffer (Gibco) and 100 U/ml
penicillin/streptomycin (Sigma-Aldrich®, Taufkirchen,
Germany).
For the induction of stress conditions hypoxia and
acidosis cells were subcultured in 25 cm2 cell culture
flasks and grown for 18 h. Hypoxia was induced by incubating cells
in hypoxic chamber (<3% CO2) for a period of 18 h
overnight. For acidosis exposure, cells were treated with 0.2%
lactic acid in cell culture media and incubated for 48 h at 37°C.
Cells incubated under standard conditions in parallel served as
controls. Following treatment, 2 ml of culture media (supernatant)
were harvested and immediately processed. All experiments were
performed in triplicates.
Total RNA isolation
Total RNAs from supernatant of cultured cells were
isolated by using the innuPREP Micro RNA Kit (Analytic Jena AG,
Jena, Germany) according to the manufacturer's protocol. Assessment
of RNA quantity was done by UV-spectrometry (NanoDrop ND1000
(PEQLAB, Erlangen, Germany). Until further processing RNA samples
were stored at −80°C.
Reverse transcription (RT) and
quantitative PCR
The reaction mixture of RT consists of 4 µl Maxima
RT-buffer (Thermo), 1 µl 5 µM poly(T) adaptor primer (Biomers,
Konstanz, Germany), 1 µl 5 mM dNTPs (Jena Bioscience, Jena,
Germany), 0.25 µl Maxima reverse transcriptase (Thermo), 0.25 µl
SUPERase in RNase inhibitor (Thermo) and 500 ng of the total RNA
sample. The reaction was carried out at 42°C for 30 min and by 85°C
for 10 min in a Nexus Thermal Cycler (Eppendorf, Hamburg, Germany).
Until further analysis, processed cDNA was stored at −20°C.
Relative expression levels of specific microRNAs
were assessed by quantitative PCR applying a SYBR Green assay in a
duplicate analysis. cDNA (1 µl) with concentration of 5 ng/µl was
mixed up with 9 µl master mix containing 1 µl 10 Xq PCR buffer, 0.5
µl 5 mM dNTPs (Jena Bioscience), 0.5 µl miRNA specific qPCR primer
(Biomers), 0.5 µl SYBR Green (Roche Diagnostics GmbH, Mannheim
Germany), 0.05 µl HotStart Taq (Jena Bioscience) and 6.54 µl
nuclease-free water (Analytic Jena). Primers consisted of a
specific primer pair for each miRNA type and for the housekeeping
genes.
Quantitative PCRs were performed on a LightCycler96
(Roche Diagnostics GmbH) at 95°C for 2 min, followed by 40 cycles
at 95°C, for 5 sec and for 30 sec at 60°C.
Data were analyzed in Microsoft Excel using ∆Ct
method based on reference value (geometric mean of housekeeping
genes, RNU48, miR-26b, miR-16 and miR-103 defined via ‘BestKeeper’
software tool (77) to determine
single microRNA expression levels.
Pre-selection of miRNA specimen
A comprehensive survey was performed applying Pubmed
interface for original research (http://www.ncbi.nlm.nih.gov/) and the miRTarBase
platform (http://mirtarbase.mbc.nctu.edu.tw/) (78), specific miRNA information registry,
to determine potential study-relevant miRNA types. These sources
were screened for relevant specimen in regard to ‘endometrial
cancer’, typical endometrial cancer target genes and pathways as
well as literature. A methodological pre-screening of >40 miRNA
types was performed, which was based on the acquired information on
potential miRNA candidates, to evaluate the experimental in
vitro setting focusing on secreted miRNA specimen in cell
culture media. The pre-set of miRNAs was filtered to collocate the
final set of miRNAs which were reliably detectable in culture
media. The hereby identified miRNA types and their functional
implications are summarized in Table
I.
Statistical analysis
Observed miRNA expression levels were graphically
visualized in box plots, showing median and quartiles in cell lines
Ishikawa, EFE-184 and AN3-CA under hypoxia, acidosis and normoxic
conditions. The influence of treatment on miRNA expression levels
of the different BC cell lines was investigated using a linear
model with factor treatment (hypoxia and acidosis compared to
normoxic conditions) and cell line, including an interaction
term.
Results
In the present study we investigated the regulatory
effect of hypoxia and acidosis on the expression levels of 24
different EC associated microRNA types (let-7a, let-7b, let-7d,
let-7i, miR-10a, −10b, −15a, −15b, −17, −19b, −20a, −20b, −21, −22,
−26-1, 27a, −29c, −30b, −92a, −125b, −128-1, −135b, −200c, −222)
that are actively secreted into the tissue culture supernatant by
EC cells. The characteristics of the investigated EC cell lines
Ishikawa, EFE-184 and AN3-CA are summarized in Table II.
 | Table II.Molecular classification of
investigated endometrial cancer cell lines. |
Table II.
Molecular classification of
investigated endometrial cancer cell lines.
| Cell line | Classification | Immunoprofile | Primary tumor | Origin |
|---|
| Ishikawa | Type I | ER+,
PR+, | Endometrial
adenocarcinoma | Uterus |
| EFE-184 | Type I | ER+,
PR− | Endometrium
carcinoma | Ascitic fluid |
| AN3-CA | Type II | ER−,
PR+ | Endometrial
adenocarcinoma | Lymph node
metastasis |
All 24 examined microRNAs were stably detectable in
the supernatants of all three in vitro models. In order to
examine potential regulatory effects triggered by typical
microenvironmental alterations occurring in solid tumors during
tumor growth, progression and metastasis on miRNA expression
(79–82) hypoxia and acidosis were induced in
the three cell lines. Expression levels of all EC associated miRNA
types were determined under normoxic, hypoxic and acidotic
conditions as mean ∆Ct values of the distinct miRNA type normalized
against the geometric mean of the four housekeepers RNU48, miR-16,
miR-26b and miR-103.
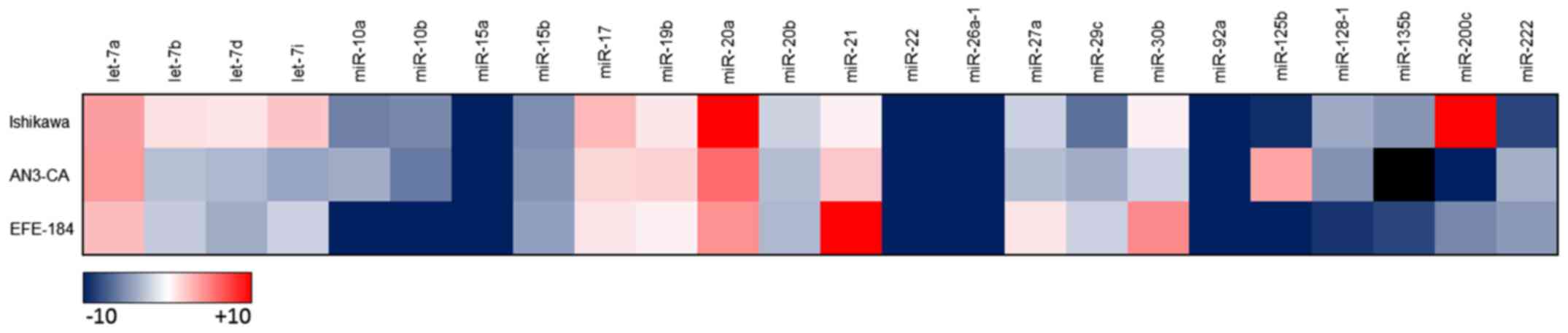
Under standard culture conditions each of the EC
cell lines displayed a unique miRNA expression profile (Fig. 1). In response to hypoxia and
acidosis, singular marked alterations in secreted miRNA expression
levels were identified predominantly in Ishikawa cells.
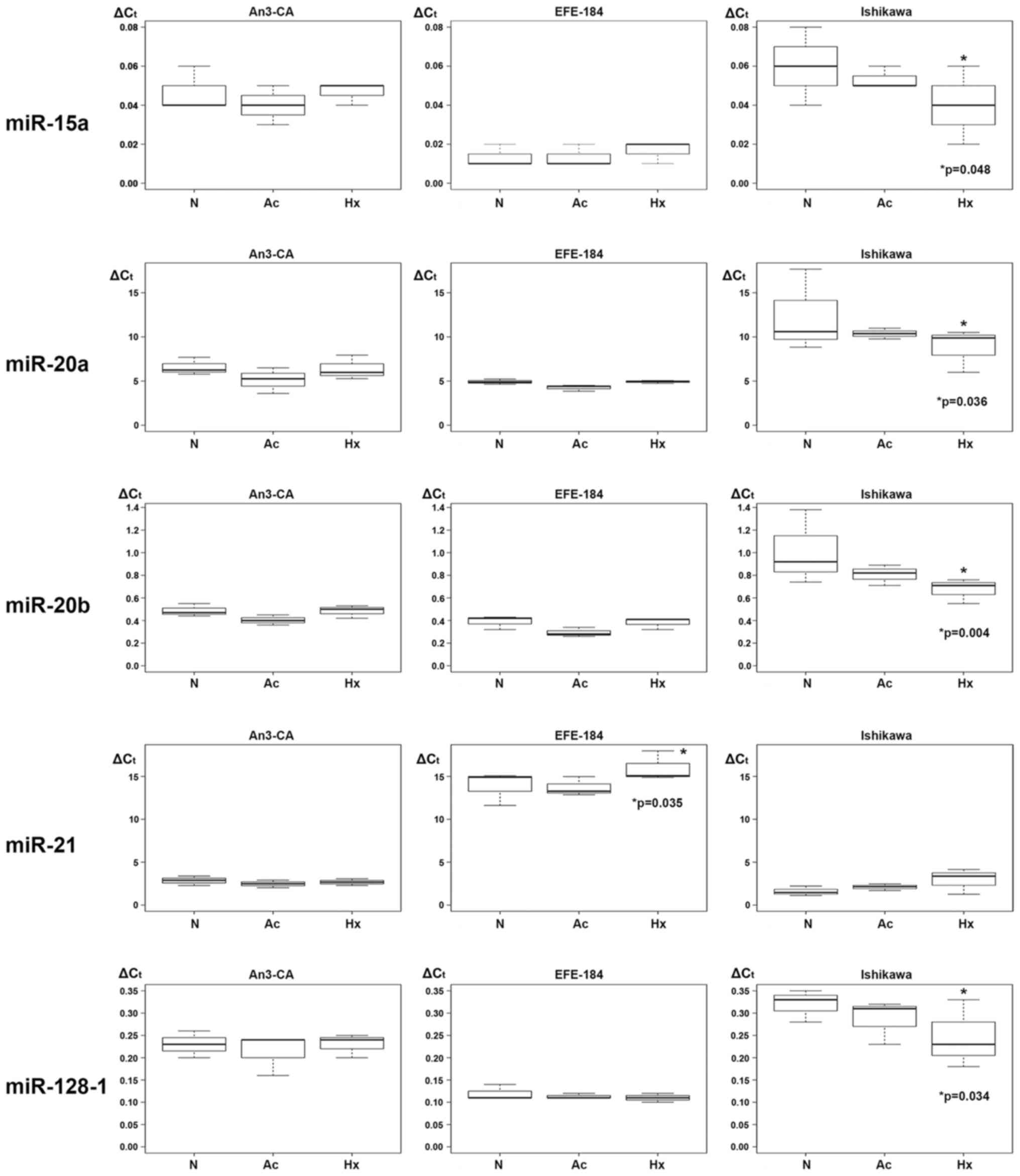
Hypoxia triggered regulatory effects on the
expression levels of miR-15a, miR-20a, miR-20b, miR-21 and
miR-128-1. In Ishikawa cells, multivariable regression analysis
identified a significant hypoxia-dependent downregulation of
miR-15a, miR-20a, miR-20b and miR-128-1 expression. In detail, the
expression level of miR-15a was decreased by a mean value of 0.020
(CI: −0.038 - −0.002; p=0.048) (Table
III, Fig. 2), of miR-20a by
3.577 (CI: −6.663 - −0.491; p=0.036) (Table IV, Fig.
2), miR-20b by 0.340 (−0.544 - −0.136; p=0.004) (Table V, Fig.
2) and of miR-128-1 by 0.073 (−0.136 - −0.011; p=0.034)
(Table VI, Fig. 2). In EFE-184 and AN3-CA cells a
comparable hypoxia-driven downregulatory effect on miRNA expression
could not be detected. In EFE-184 cells a hypoxia-induced
upregulation of miR-21 expression was observed. The expression
levels of secreted miR-21 levels in hypoxia-conditioned EFE-184
cells were increased by a mean value of 2.103 (CI: 0.300 - 3.907;
p=0.035) (Table VII, Fig. 2). A hypoxia-dependent upregulatory
effect on miRNA expression of AN3-CA and Ishikawa cells was not
observed.
 | Table III.Two-way analysis of variance for
levels of secreted miR-15a under control, acidotic and hypoxic
conditions in three different endometrial cancer cell lines. |
Table III.
Two-way analysis of variance for
levels of secreted miR-15a under control, acidotic and hypoxic
conditions in three different endometrial cancer cell lines.
|
| Estimate | Standard error | 95% CI | p-value |
|---|
| Intercept | 0.047 | 0.007 | 0.034, 0.060 | 0.000 |
| Control |
|
EFE-184 | −0.033 | 0.009 | −0.052, −0.015 | 0.002 |
|
Ishikawa | 0.023 | 0.009 | −0.005, 0.032 | 0.174 |
| Acidosis |
|
AN3-CA | −0.007 | 0.009 | −0.025, 0.012 | 0.489 |
|
EFE-184 | 0.000 | 0.009 | −0.018, 0.018 | 1.000 |
|
Ishikawa | −0.007 | 0.009 | −0.025, 0.012 | 0.489 |
| Hypoxia |
|
AN3-CA | 0.000 | 0.009 | −0.018, 0.018 | 1.000 |
|
EFE-184 | 0.003 | 0.009 | −0.015, 0.022 | 0.728 |
|
Ishikawa | −0.020 | 0.009 | −0.038, −0.002 | 0.048 |
 | Table IV.Two-way analysis of variance for
levels of secreted miR-20a under control, acidotic and hypoxic
conditions in three different endometrial cancer cell lines. |
Table IV.
Two-way analysis of variance for
levels of secreted miR-20a under control, acidotic and hypoxic
conditions in three different endometrial cancer cell lines.
|
| Estimate | Standard error | 95% CI | p-value |
|---|
| Intercept | 6.573 | 1.113 | 4.391, 8.756 | 0.000 |
| Control |
|
EFE-184 | −1.657 | 1.575 | −4.743, 1.429 | 0.307 |
|
Ishikawa | 5.797 | 1.575 | 2.711, 8.883 | 0.002 |
| Acidosis |
|
AN3-CA | −1.453 | 1.575 | −4.539, 1.633 | 0.368 |
|
EFE-184 | −0.660 | 1.575 | −3.746, 2.426 | 0.680 |
|
Ishikawa | −1.993 | 1.575 | −5.079, 1.093 | 0.222 |
| Hypoxia |
|
AN3-CA | −0.177 | 1.575 | −3.263, 2.909 | 0.912 |
|
EFE-184 | 0.007 | 1.575 | −3.079, 3.093 | 0.997 |
|
Ishikawa | −3.577 | 1.575 | −6.663, −0.491 | 0.036 |
 | Table V.Two-way analysis of variance for
levels of secreted miR-20b under control, acidotic and hypoxic
conditions in three different endometrial cancer cell lines. |
Table V.
Two-way analysis of variance for
levels of secreted miR-20b under control, acidotic and hypoxic
conditions in three different endometrial cancer cell lines.
|
| Estimate | Standard error | 95% CI | p-value |
|---|
| Intercept | 0.487 | 0.073 | 0.343, 0.631 | 0.000 |
| Control |
|
EFE-184 | −0.097 | 0.104 | −0.300, 0.107 | 0.365 |
|
Ishikawa | 0.527 | 0.104 | 0.323, 0.730 | 0.000 |
| Acidosis |
|
AN3-CA | −0.083 | 0.104 | −0.287, 0.120 | 0.433 |
|
EFE-184 | −0.097 | 0.104 | −0.300, 0.107 | 0.365 |
|
Ishikawa | −0.207 | 0.104 | −0.410, −0.003 | 0.062 |
| Hypoxia |
|
AN3-CA | −0.003 | 0.104 | −0.207, 0.200 | 0.975 |
|
EFE-184 | −0.010 | 0.104 | −0.214, 0.194 | 0.924 |
|
Ishikawa | −0.340 | 0.104 | −0.544, −0.136 | 0.004 |
 | Table VI.Two-way analysis of variance for
levels of secreted miR-128-1 under control, acidotic and hypoxic
conditions in three different endometrial cancer cell lines. |
Table VI.
Two-way analysis of variance for
levels of secreted miR-128-1 under control, acidotic and hypoxic
conditions in three different endometrial cancer cell lines.
|
| Estimate | Standard error | 95% CI | p-value |
|---|
| Intercept | 0.230 | 0.023 | 0.186, 0.274 | 0.000 |
| Control |
|
EFE-184 | −0.110 | 0.032 | −0.173, −0.047 | 0.003 |
|
Ishikawa | 0.090 | 0.032 | 0.027, 0.153 | 0.011 |
| Acidosis |
|
AN3-CA | −0.017 | 0.032 | −0.079, 0.046 | 0.608 |
|
EFE-184 | −0.007 | 0.032 | −0.069, 0.056 | 0.837 |
|
Ishikawa | −0.033 | 0.032 | −0.096, 0.029 | 0.310 |
| Hypoxia |
|
AN3-CA | 0.000 | 0.032 | −0.063, 0.063 | 1.000 |
|
EFE-184 | −0.010 | 0.032 | −0.073, 0.053 | 0.757 |
|
Ishikawa | −0.073 | 0.032 | −0.136, −0.011 | 0.034 |
 | Table VII.Two-way analysis of variance for
levels of secreted miR-21 under control, acidotic and hypoxic
conditions in three different endometrial cancer cell lines. |
Table VII.
Two-way analysis of variance for
levels of secreted miR-21 under control, acidotic and hypoxic
conditions in three different endometrial cancer cell lines.
|
| Estimate | Standard error | 95% CI | p-value |
|---|
| Intercept | 2.853 | 0.651 | 1.578 4.128 | 0.000 |
| Control |
|
EFE-184 | 11.013 | 0.920 | 9.210 12.817 | 0.000 |
|
Ishikawa | −1.253 | 0.920 | −3.057 0.550 | 0.190 |
| Acidosis |
|
AN3-CA | −0.377 | 0.920 | −2.180 1.427 | 0.687 |
|
EFE-184 | −0.167 | 0.920 | −1.970 1.637 | 0.858 |
|
Ishikawa | 0.510 | 0.920 | −1.293 2.313 | 0.586 |
| Hypoxia |
|
AN3-CA | −0.177 | 0.920 | −1.980 1.627 | 0.850 |
|
EFE-184 | 2.103 | 0.920 | 0.300 3.907 | 0.035 |
|
Ishikawa | 1.330 | 0.920 | −0.473 3.133 | 0.165 |
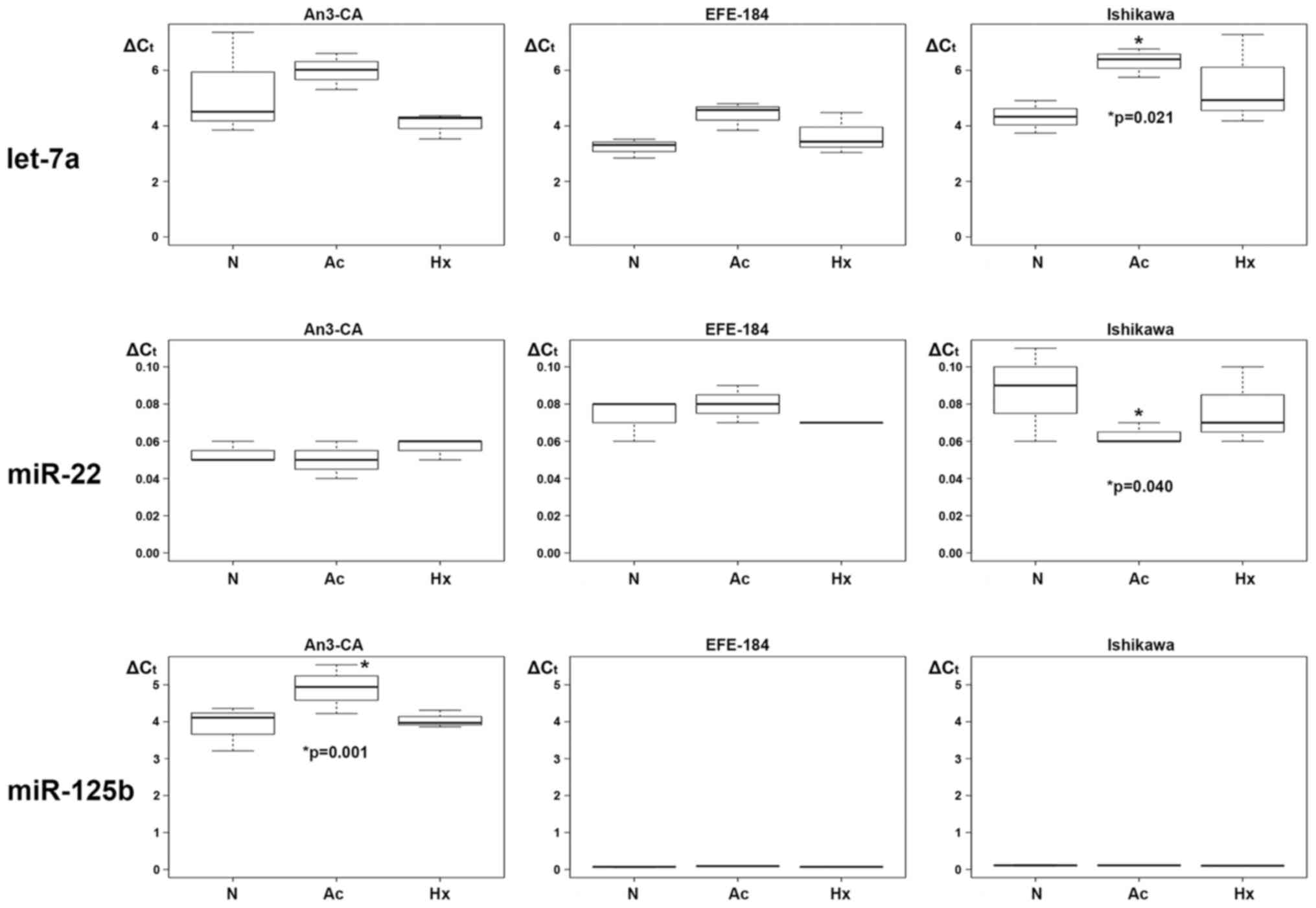
In response to acidosis, single marked alterations
in secreted expression for let-7a, miR-22 and miR-125b were
observed. In Ishikawa cells, ∆Ct expression levels of let-7a were
increased by a mean value of 1.980 (−0.442 - 3.518; p=0.021)
(Table VIII, Fig. 3) and ∆Ct expression levels of miR-22
were decreased by a mean value of 0.023 (−0.044 - −0.003; p=0.040)
(Table IX, Fig. 3). In AN3-CA cells a specific
acidosis-dependent upregulation was observed for miR-125b. In
detail, a mean increase of secreted miR-125b ∆Ct level by 1.007
(CI: 0.512 - 1.501; p=0.001) became evident (Table X, Fig.
3). No significant acidosis-dependent miRNA expression level
alterations were detected in EFE-184 cells.
 | Table VIII.Two-way analysis of variance for
levels of secreted let7a under control, acidotic and hypoxic
conditions in three different endometrial cancer cell lines. |
Table VIII.
Two-way analysis of variance for
levels of secreted let7a under control, acidotic and hypoxic
conditions in three different endometrial cancer cell lines.
|
| Estimate | Standard error | 95% CI | p-value |
|---|
| Intercept | 5.243 | 0.555 | 4.156, 6.331 | 0.000 |
| Control |
|
EFE-184 | −2.020 | 0.785 | −3.558, −0.482 | 0.019 |
|
Ishikawa | −0.917 | 0.785 | −2.454, 0.621 | 0.258 |
| Acidosis |
|
AN3-CA | −0.737 | 0.785 | −0.801, 2.274 | 0.360 |
|
EFE-184 | 1.180 | 0.785 | −0.358, 2.718 | 0.150 |
|
Ishikawa | 1.980 | 0.785 | 0.442, 3.518 | 0.021 |
| Hypoxia |
|
AN3-CA | −1.183 | 0.785 | −2.721, 0.354 | 0.149 |
|
EFE-184 | 0.427 | 0.785 | −1.111, 1.964 | 0.593 |
|
Ishikawa | 1.140 | 0.785 | −0.398, 2.678 | 0.163 |
 | Table IX.Two-way analysis of variance for
levels of secreted miR-22 under control, acidotic and hypoxic
conditions in three different endometrial cancer cell lines. |
Table IX.
Two-way analysis of variance for
levels of secreted miR-22 under control, acidotic and hypoxic
conditions in three different endometrial cancer cell lines.
|
| Estimate | Standard error | 95% CI | p-value |
|---|
| Intercept | 0.053 | 0.007 | 0.039, 0.068 | 0.000 |
| Control |
|
EFE-184 | 0.020 | 0.011 | −0.001, 0.041 | 0.074 |
|
Ishikawa | 0.033 | 0.011 | 0.013, 0.054 | 0.005 |
| Acidosis |
|
AN3-CA | −0.003 | 0.011 | −0.024, 0.017 | 0.755 |
|
EFE-184 | 0.007 | 0.011 | −0.014, 0.027 | 0.535 |
|
Ishikawa | −0.023 | 0.011 | −0.044, −0.003 | 0.040 |
| Hypoxia |
|
AN3-CA | 0.003 | 0.011 | −0.017, 0.024 | 0.755 |
|
EFE-184 | −0.003 | 0.011 | −0.024, 0.017 | 0.755 |
|
Ishikawa | −0.010 | 0.011 | −0.031, 0.011 | 0.355 |
 | Table X.Two-way analysis of variance for
levels of secreted miR-125b under control, acidotic and hypoxic
conditions in three different endometrial cancer cell lines. |
Table X.
Two-way analysis of variance for
levels of secreted miR-125b under control, acidotic and hypoxic
conditions in three different endometrial cancer cell lines.
|
| Estimate | Standard error | 95% CI | p-value |
|---|
| Intercept | 3.893 | 0.178 | 3.544, 4.243 | 0.000 |
| Control |
|
EFE-184 | −3.830 | 0.252 | −4.324, −3.336 | 0.000 |
|
Ishikawa | −3.783 | 0.252 | −4.278, −3.289 | 0.000 |
| Acidosis |
|
AN3-CA | 1.007 | 0.252 | 0.512, 1.501 | 0.001 |
|
EFE-184 | 0.027 | 0.252 | −0.468, 0.521 | 0.917 |
|
Ishikawa | 0.000 | 0.252 | −0.494, 0.494 | 1.000 |
| Hypoxia |
|
AN3-CA | 0.153 | 0.252 | −0.341, 0.648 | 0.551 |
|
EFE-184 | 0.003 | 0.252 | −0.491, 0.498 | 0.990 |
|
Ishikawa | −0.010 | 0.252 | −0.504, 0.484 | 0.969 |
No marked hypoxia- or acidosis-driven alterations in
expression levels of let-7b, let-7d, let-7i, miR-10a, −10b, −15b,
−17, −19b, −26-1, 27a, −29c, −30b, −92a, −135b, −200c and 222 were
observed in the investigated EC cell lines (data not shown).
Discussion
Recent and ongoing studies account for a continually
growing number of miRNA types with regulatory influence on EC
tumorigenesis. While some of these miRNAs play an important role as
tumor suppressors, expression of others is associated with
promotion of tumor growth, invasion and metastasis (oncogenic
miRNAs, oncomiRs). In the present study, we analyzed the expression
of a panel of secreted EC associated microRNAs with impact on
angiogenesis, proliferation, invasion, migration and metastasis
including let-7a, let-7b, let-7d, let-7i, miR-10a, −10b, −15a,
−15b, −17, −19b, −20a, −20b, −21, −22, −26.1, 27a, −29c, −30b,
−92a, −125b, −128-1, −135b, −200c, −222. We were able to reliably
detect all 24 secreted miRNA types in the supernatants of the three
analyzed EC cell lines. Based on these EC in vitro models a
quantitative analysis of secreted miRNA expression levels was
employed to detect potential alterations under the typical tumor
biological epiphenomena hypoxia and acidosis.
Our data clearly account for a hypoxia- and
acidosis-dependent regulatory effect in the expression levels of
eight specific secreted miRNAs (let-7a, miR-15a, miR-20a, miR-20b,
miR-21, miR-22, miR-125b and miR-128-1) among the investigated
miRNA types. The observed alterations in miRNA expression levels
were not consistent in all three EC cell lines. Significant miRNA
deregulation due to hypoxia or acidosis was predominantly observed
in Ishikawa cells. In EFE-184 and AN3-CA cells a regulatory effect
was observed solely for one specific miRNA type. Hypoxia resulted
in downregulation of miR-15a, miR-20a, miR-20b and miR-128-1 in
Ishikawa cells and upregulation of miR-21 in EFE-184 cells.
miR-15a is considered to trigger tumor suppressive
effects by inhibiting several target oncogenes, like BCL2, MCL1,
CCND1 and WNT3A (46,47). Thus, cells react with diminished
proliferation, an increase in apoptosis rates and suppressed
tumorigenicity both in vitro and in vivo. In chronic
lymphocytic lymphoma (CLL), prostate carcinomas and pituitary
adenomas, downregulation of miR-15a expression has been observed
(46). In the present study,
hypoxic conditions resulted in decreased miR15a expression in
Ishikawa cells. This finding accounts for a reduced inhibitory
signaling function on angiogenesis in type I EC cells under hypoxic
stress and is supported by the results of Hua et al reported
that in a tumor cell model VEGF expression is repressed, besides
others, by miRNAs (miR15b, miR16) from the miR15-16 clustered
family (41).
The miR-17-92 cluster, which contains six mature
miRNAs (miR-17, −18a, −19a, −19b, −20a and −92a), conducts a
complex role in angiogenetic signaling cascades (65) In the present analysis, we evaluated
four specific miRNAs of this cluster: miR-17, miR-19b, miR-20a and
miR-92a. Anti-angiogenetic activity of miR-20a by targeting VEGF-A
has been reported (41,65). Inconsistent results have been
reported for the role of miR-17 so far. While some investigatory
approaches hypothesize an anti-angiogenetic activity (41,51),
other studies account for either pro-angiogenetic activity
(83,84) or no association with VEGF-A
expression (65). Our data
identified a significant hypoxia-driven expression reduction of
miR20a in Ishikawa cells. For miR-17, miR-19b and miR-92a no
significant changes in expression levels were observed. Our results
are in line with the findings of Ramon et al and support the
proposition that miR-20a, but not miR-17 and miR-19b, displays an
anti-angiogenetic activity, which is reduced under hypoxic stress
conditions in EC tumor cells (65).
MiR-20b has a tumor suppressive function by impeding
MMP-2 expression leading to cell cycle arrest (61) as well as regulative function on
oxygen balance of cells. Lei et al described that tumor
cells are able to adapt to alterations of oxygen concentration by
miR-20b triggered regulation of HIF-1α and VEGF. In their study
inhibition of miR-20b increased protein levels of VEGF and HIF-1α
in normoxic tumor cells, an increase of miR-20b expression in
hypoxic tumor cells resulted in reduced protein levels of VEGF and
HIF-1α (85). Park et al
showed that miR-20b expression was downregulated in bladder cancer
and an upregulation of this miRNA type lead to inhibition of
proliferation, migration and invasion (61). In gastric cancer, downregulated
miR-20b expression was associated with hypoxia-induced
chemoresistance. In the present study we observed reduced miR-20b
expression levels in Ishikawa cells under hypoxic conditions. These
data are in line with aforementioned reports of Lei et al as
well as of Danza et al in gastric cancer (85,86).
Under hypoxic conditions expression levels of
miR-128-1 were also found reduced in Ishikawa cells. miR-128-1 is
supposed to act as a tumor suppressor inhibiting tumor cell
proliferation, invasion and self-renewal by direct targeting of
BMI1 and E2F3 in glioblastomas (67). Furthermore, it has been associated
with inhibition of the mTOR signaling pathway in glioblastomas.
Decreased miR-128-1 expression levels in glioblastomas could be
strongly associated with poor survival (87). The observed reduced expression
levels of miR-128-1 in Ishikawa cells under hypoxic conditions may
also account for a regulative function of miR-128-1 in EC.
MiR-21 expression levels were increased in EFE-184
cells under oxygen deprivation. This observation is in line with
previous studies showing that miR-21 is an active participant in
EEC malignant transformation by abrogating the regulatory function
of PTEN, which has been found to be upregulated in EEC (24,64).
Furthermore, a regulating role of miR-21 on the expression of the
tumor suppressor gene Pdcd4 (programmed cell death-4) and Maspin,
which is strongly associated with angiogenesis in EC, has been
indicated (88). Whether Maspin
acts as tumor suppressor or tumor promoter, seems to be complex and
dependent on the tissue specific environment (89). In EC high Maspin expression has been
associated with higher FIGO stage, lymph node involvement and the
depth of myometrial invasion as well as poor prognosis (90). Additionally a correlation between
high miR-21 expression and increased Maspin expression has been
reported in EC (89).
Acidosis resulted in divergent miRNA expression
patterns in the analyzed EC cell lines. While let-7a expression was
found to be upregulated, miR-22 expression levels were
downregulated under acidotic conditions in Ishikawa cells. Let-7a
acts as tumor suppressor due to inhibition of EC growth by
targeting and downregulating Aurora B (1, 21, 24). In EC let-7a
expression levels are usually downregulated (58). miR-22 exerts tumor suppressive
functions by influencing Cyclin D1, MMP2, PTEN and others (71). Li et al reported an
inhibitory function of miR-22 expression on proliferation and
invasion in estrogen receptor α-positive endometrial endometrioid
carcinoma cells (57).
In AN3-CA cells miR-125b was significantly
upregulated under acidotic conditions. These data are in line with
previous findings showing an upregulated miR-125b expression in
type II EC cells. Proliferation and migration of type II EC cancer
cells was promoted by miR-125b in this study by targeting the tumor
suppressor gene TP53INP1 (53).
In summary, our data clearly identify a
hypoxia-dependent downregulation of secreted miRNAs with tumor
suppressive and anti-angiogenetic function (miR-15a, miR-20a,
miR-20b, miR-128-1) as well as upregulation of secreted miRNAs with
tumor and angiogenesis promoting function (miR-21) in type I EC
cell lines (Ishikawa, EFE-184). In contrast, in the analyzed type
II EC cell line (AN3-CA) hypoxia did not show any significant
impact on the expression pattern of the analyzed EC related
miRNAs.
Acidosis triggered upregulation of the tumor
promoting miRNA miR-125b in AN3-CA cells (type II EC). In Ishikawa
cells (type I EC) the tumor promoting or tumor suppressive impact
of acidosis is not clear yet since miRNAs with tumor suppressive
function were found altered in divergent directions, both up-
(let-7a) and down- (miR-22) regulated.
In conclusion, our current findings emphasize the
functional importance of secreted miRNAs in the immediate response
of EC cells to exogenic stress situations such as the typical tumor
epiphenomena hypoxia and acidosis. Focusing on the specific
potential of secreted, thus circulating miRNA molecules, that also
conduct an inter-cellular (hormone-like) signaling function,
alterations in expression levels not only influence intracellular
gene expression and signaling cascades, but also transfer the
induction of (tumor)biological cellular changes to adjacent cells
(91,92) Thus, the fate of whole tissue areas
may undergo transformation in respect to metabolic pathways, cell
cycle control and neo-angiogenesis, that support malignant
progression. To rule out the possibility of in vitro/in
vivo differences of the analyzed secreted miRNA expression
levels as well as to support the transferability of the observed
results to in vivo settings, the data of the present in
vitro study on EC cancer cell lines should be confirmed by
corresponding in vivo analyses based on tumor tissue
specimen and/or body fluids (e.g. blood, urine) of EC patients.
Continuous elucidation of miRNA functions augments
the potential of these regulatory nucleic acid elements to either
be implemented as useful biomarkers in disease/malignancy
diagnosis, prognosis and therapy monitoring, as well as novel
therapeutic targets (‘AntagomiRs’) in clinical cancer management
(1,21,93).
The potential applicability of circulating miRNAs as non-invasive
biomarkers for diagnosis (32) or
as therapeutic targets (94,95) in
EC is currently subject of various studies. In this context this
in vitro model may serve as initial step to elucidate the
influence of microenvironmental changes on expression profiles of
circulating microRNAs in EC.
References
|
1
|
Yanokura M, Banno K, Iida M, Irie H, Umene
K, Masuda K, Kobayashi Y, Tominaga E and Aoki D: MicroRNAS in
endometrial cancer: Recent advances and potential clinical
applications. EXCLI J. 14:190–198. 2015.PubMed/NCBI
|
|
2
|
Iorio MV, Visone R, Di Leva G, Donati V,
Petrocca F, Casalini P, Taccioli C, Volinia S, Liu CG, Alder H, et
al: MicroRNA signatures in human ovarian cancer. Cancer Res.
67:8699–8707. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liz J and Esteller M: lncRNAs and
microRNAs with a role in cancer development. Biochim Biophys Acta.
1859:169–176. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lewis BP, Burge CB and Bartel DP:
Conserved seed pairing, often flanked by adenosines, indicates that
thousands of human genes are microRNA targets. Cell. 120:15–20.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sandhu S and Garzon R: Potential
applications of microRNAs in cancer diagnosis, prognosis, and
treatment. Semin Oncol. 38:781–787. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Thorsen SB, Obad S, Jensen NF, Stenvang J
and Kauppinen S: The therapeutic potential of microRNAs in cancer.
Cancer J. 18:275–284. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Boren T, Xiong Y, Hakam A, Wenham R, Apte
S, Wei Z, Kamath S, Chen DT, Dressman H and Lancaster JM: MicroRNAs
and their target messenger RNAs associated with endometrial
carcinogenesis. Gynecol Oncol. 110:206–215. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Le XF, Merchant O, Bast RC and Calin GA:
The roles of microRNAs in the cancer invasion-metastasis cascade.
Cancer Microenviron. 3:137–147. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ratner ES, Tuck D, Richter C, Nallur S,
Patel RM, Schultz V, Hui P, Schwartz PE, Rutherford TJ and Weidhaas
JB: MicroRNA signatures differentiate uterine cancer tumor
subtypes. Gynecol Oncol. 118:251–257. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kosaka N, Iguchi H and Ochiya T:
Circulating microRNA in body fluid: A new potential biomarker for
cancer diagnosis and prognosis. Cancer Sci. 101:2087–2092. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huang YK and Yu JC: Circulating microRNAs
and long non-coding RNAs in gastric cancer diagnosis: An update and
review. World J Gastroenterol. 21:9863–9886. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kosaka N and Ochiya T: Unraveling the
mystery of cancer by secretory microRNA: Horizontal microRNA
transfer between living cells. Front Genet. 2:972012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Turchinovich A, Weiz L, Langheinz A and
Burwinkel B: Characterization of extracellular circulating
microRNA. Nucleic Acids Res. 39:7223–7233. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Arroyo JD, Chevillet JR, Kroh EM, Ruf IK,
Pritchard CC, Gibson DF, Mitchell PS, Bennett CF,
Pogosova-Agadjanyan EL, Stirewalt DL, et al: Argonaute2 complexes
carry a population of circulating microRNAs independent of vesicles
in human plasma. Proc Natl Acad Sci USA. 108:5003–5008. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang K, Zhang S, Weber J, Baxter D and
Galas DJ: Export of microRNAs and microRNA-protective protein by
mammalian cells. Nucleic Acids Res. 38:7248–7259. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang J, Li S, Li L, Li M, Guo C, Yao J
and Mi S: Exosome and exosomal microRNA: Trafficking, sorting, and
function. Genomics Proteomics Bioinformatics. 13:17–24. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sorosky JI: Endometrial cancer. Obstet
Gynecol. 120:383–397. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hecht JL and Mutter GL: Molecular and
pathologic aspects of endometrial carcinogenesis. J Clin Oncol.
24:4783–4791. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Murali R, Soslow RA and Weigelt B:
Classification of endometrial carcinoma: More than two types.
Lancet Oncol. 15:e268–e278. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sianou A, Galyfos G, Moragianni D,
Andromidas P, Kaparos G, Baka S and Kouskouni E: The role of
microRNAs in the pathogenesis of endometrial cancer: A systematic
review. Arch Gynecol Obstet. 292:271–282. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chung TK, Lau TS, Cheung TH, Yim SF, Lo
KW, Siu NS, Chan LK, Yu MY, Kwong J, Doran G, et al: Dysregulation
of microRNA-204 mediates migration and invasion of endometrial
cancer by regulating FOXC1. Int J Cancer. 130:1036–1045. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Devor EJ, Hovey AM, Goodheart MJ,
Ramachandran S and Leslie KK: microRNA expression profiling of
endometrial endometrioid adenocarcinomas and serous adenocarcinomas
reveals profiles containing shared, unique and differentiating
groups of microRNAs. Oncol Rep. 26:995–1002. 2011.PubMed/NCBI
|
|
24
|
Kontomanolis EN and Koukourakis MI:
MicroRNA: The Potential Regulator of Endometrial Carcinogenesis.
MicroRNA. 4:18–25. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Myatt SS and Lam EW: The emerging roles of
forkhead box (Fox) proteins in cancer. Nat Rev Cancer. 7:847–859.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Myatt SS, Wang J, Monteiro LJ, Christian
M, Ho KK, Fusi L, Dina RE, Brosens JJ, Ghaem-Maghami S and Lam EW:
Definition of microRNAs that repress expression of the tumor
suppressor gene FOXO1 in endometrial cancer. Cancer Res.
70:367–377. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hiroki E, Suzuki F, Akahira J, Nagase S,
Ito K, Sugawara J, Miki Y, Suzuki T, Sasano H and Yaegashi N:
MicroRNA-34b functions as a potential tumor suppressor in
endometrial serous adenocarcinoma. Int J Cancer. 131:E395–E404.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Park YA, Lee JW, Choi JJ, Jeon HK, Cho Y,
Choi C, Kim TJ, Lee NW, Kim BG and Bae DS: The interactions between
MicroRNA-200c and BRD7 in endometrial carcinoma. Gynecol Oncol.
124:125–133. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Snowdon J, Zhang X, Childs T, Tron VA and
Feilotter H: The microRNA-200 family is upregulated in endometrial
carcinoma. PLoS One. 6:e228282011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Torres A, Torres K, Paszkowski T, Radej S,
Staśkiewicz GJ, Ceccaroni M, Pesci A and Maciejewski R: Highly
increased maspin expression corresponds with up-regulation of
miR-21 in endometrial cancer: a preliminary report. Int J Gynecol
Cancer. 21:8–14. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen X, Ba Y, Ma L, Cai X, Yin Y, Wang K,
Guo J, Zhang Y, Chen J, Guo X, et al: Characterization of microRNAs
in serum: A novel class of biomarkers for diagnosis of cancer and
other diseases. Cell Res. 18:997–1006. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jia W, Wu Y, Zhang Q, Gao G, Zhang C and
Xiang Y: Identification of four serum microRNAs from a genome-wide
serum microRNA expression profile as potential non-invasive
biomarkers for endometrioid endometrial cancer. Oncol Lett.
6:261–267. 2013.PubMed/NCBI
|
|
33
|
Blick C, Ramachandran A, McCormick R,
Wigfield S, Cranston D, Catto J and Harris AL: Identification of a
hypoxia-regulated miRNA signature in bladder cancer and a role for
miR-145 in hypoxia-dependent apoptosis. Br J Cancer. 113:634–644.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jung KO, Youn H, Lee CH, Kang KW and Chung
JK: Visualization of exosome-mediated miR-210 transfer from hypoxic
tumor cells. Oncotarget. 8:9899–9910. 2017.PubMed/NCBI
|
|
35
|
Dai L, Lou W, Zhu J, Zhou X and Di W:
MiR-199a inhibits the angiogenic potential of endometrial stromal
cells under hypoxia by targeting HIF-1α/VEGF pathway. Int J Clin
Exp Pathol. 8:4735–4744. 2015.PubMed/NCBI
|
|
36
|
Lin SC, Wang CC, Wu MH, Yang SH, Li YH and
Tsai SJ: Hypoxia-induced microRNA-20a expression increases ERK
phosphorylation and angiogenic gene expression in endometriotic
stromal cells. J Clin Endocrinol Metab. 97:E1515–E1523. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Xu TX, Zhao SZ, Dong M and Yu XR: Hypoxia
responsive miR-210 promotes cell survival and autophagy of
endometriotic cells in hypoxia. Eur Rev Med Pharmacol Sci.
20:399–406. 2016.PubMed/NCBI
|
|
38
|
Harris AL: Hypoxia - a key regulatory
factor in tumour growth. Nat Rev Cancer. 2:38–47. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Umezu T, Tadokoro H, Azuma K, Yoshizawa S,
Ohyashiki K and Ohyashiki JH: Exosomal miR-135b shed from hypoxic
multiple myeloma cells enhances angiogenesis by targeting
factor-inhibiting HIF-1. Blood. 124:3748–3757. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kulshreshtha R, Ferracin M, Wojcik SE,
Garzon R, Alder H, Agosto-Perez FJ, Davuluri R, Liu CG, Croce CM,
Negrini M, et al: A microRNA signature of hypoxia. Mol Cell Biol.
27:1859–1867. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hua Z, Lv Q, Ye W, Wong CK, Cai G, Gu D,
Ji Y, Zhao C, Wang J, Yang BB, et al: MiRNA-directed regulation of
VEGF and other angiogenic factors under hypoxia. PLoS One.
1:e1162006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gatenby RA, Gawlinski ET, Gmitro AF,
Kaylor B and Gillies RJ: Acid-mediated tumor invasion: A
multidisciplinary study. Cancer Res. 66:5216–5223. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chiche J, Brahimi-Horn MC and Pouysségur
J: Tumour hypoxia induces a metabolic shift causing acidosis: A
common feature in cancer. J Cell Mol Med. 14:771–794. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lardner A: The effects of extracellular pH
on immune function. J Leukoc Biol. 69:522–530. 2001.PubMed/NCBI
|
|
45
|
Bristow RG and Hill RP: Hypoxia and
metabolism. Hypoxia, DNA repair and genetic instability. Nat Rev
Cancer. 8:180–192. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Aqeilan RI, Calin GA and Croce CM: miR-15a
and miR-16-1 in cancer: Discovery, function and future
perspectives. Cell Death Differ. 17:215–220. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Bonci D, Coppola V, Musumeci M, Addario A,
Giuffrida R, Memeo L, D'Urso L, Pagliuca A, Biffoni M, Labbaye C,
et al: The miR-15a-miR-16-1 cluster controls prostate cancer by
targeting multiple oncogenic activities. Nat Med. 14:1271–1277.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Castilla MA, Moreno-Bueno G, Romero-Pérez
L, Van De Vijver K, Biscuola M, López-García MÁ, Prat J,
Matías-Guiu X, Cano A, Oliva E, et al: Micro-RNA signature of the
epithelial-mesenchymal transition in endometrial carcinosarcoma. J
Pathol. 223:72–80. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Chen J, Zhang K, Xu Y, Gao Y, Li C, Wang R
and Chen L: The role of microRNA-26a in human cancer progression
and clinical application. Tumour Biol. 37:7095–7108. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Dai Y, Xia W, Song T, Su X, Li J, Li S,
Chen Y, Wang W, Ding H, Liu X, et al: MicroRNA-200b is
overexpressed in endometrial adenocarcinomas and enhances MMP2
activity by downregulating TIMP2 in human endometrial cancer cell
line HEC-1A cells. Nucleic Acid Ther. 23:29–34. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Doebele C, Bonauer A, Fischer A, Scholz A,
Reiss Y, Urbich C, Hofmann WK, Zeiher AM and Dimmeler S: Members of
the microRNA-17-92 cluster exhibit a cell-intrinsic antiangiogenic
function in endothelial cells. Blood. 115:4944–4950. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Fuziwara CS and Kimura ET: Insights into
regulation of the miR-17-92 cluster of miRNAs in cancer. Front Med
(Lausanne). 2:642015.PubMed/NCBI
|
|
53
|
Jiang F, Liu T, He Y, Yan Q, Chen X, Wang
H and Wan X: MiR-125b promotes proliferation and migration of type
II endometrial carcinoma cells through targeting TP53INP1 tumor
suppressor in vitro and in vivo. BMC Cancer. 11:4252011. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Kim J, Siverly AN, Chen D, Wang M, Yuan Y,
Wang Y, Lee H, Zhang J, Muller WJ, Liang H, et al: Ablation of
miR-10b suppresses oncogene-induced mammary tumorigenesis and
metastasis and reactivates tumor-suppressive pathways. Cancer Res.
76:6424–6435. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Kolenda T, Przybyła W, Teresiak A,
Mackiewicz A and Lamperska KM: The mystery of let-7d - a small RNA
with great power. Contemp Oncol (Pozn). 18:293–301. 2014.PubMed/NCBI
|
|
56
|
Li BL, Lu W, Lu C, Qu JJ, Yang TT, Yan Q
and Wan XP: CpG island hypermethylation-associated silencing of
microRNAs promotes human endometrial cancer. Cancer Cell Int.
13:442013. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Li S, Hu R, Wang C, Guo F, Li X and Wang
S: miR-22 inhibits proliferation and invasion in estrogen receptor
α-positive endometrial endometrioid carcinomas cells. Mol Med Rep.
9:2393–2399. 2014.PubMed/NCBI
|
|
58
|
Liu P, Qi M, Ma C, Lao G and Liu Y and Liu
Y and Liu Y: Let7a inhibits the growth of endometrial carcinoma
cells by targeting Aurora-B. FEBS Lett. 587:2523–2529. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Lu J, Zhang X, Zhang R and Ge Q: MicroRNA
heterogeneity in endometrial cancer cell lines revealed by deep
sequencing. Oncol Lett. 10:3457–3465. 2015.PubMed/NCBI
|
|
60
|
Mozos A, Catasús L, D'Angelo E, Serrano E,
Espinosa I, Ferrer I, Pons C and Prat J: The FOXO1-miR27 tandem
regulates myometrial invasion in endometrioid endometrial
adenocarcinoma. Hum Pathol. 45:942–951. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Park SL, Cho TM, Won SY, Song JH, Noh DH,
Kim WJ and Moon SK: MicroRNA-20b inhibits the proliferation,
migration and invasion of bladder cancer EJ cells via the targeting
of cell cycle regulation and Sp-1-mediated MMP-2 expression. Oncol
Rep. 34:1605–1612. 2015.PubMed/NCBI
|
|
62
|
Park SY, Lee JH, Ha M, Nam JW and Kim VN:
miR-29 miRNAs activate p53 by targeting p85 alpha and CDC42. Nat
Struct Mol Biol. 16:23–29. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Poliseno L, Tuccoli A, Mariani L,
Evangelista M, Citti L, Woods K, Mercatanti A, Hammond S and
Rainaldi G: MicroRNAs modulate the angiogenic properties of HUVECs.
Blood. 108:3068–3071. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Qin X, Yan L, Zhao X, Li C and Fu Y:
microRNA-21 overexpression contributes to cell proliferation by
targeting PTEN in endometrioid endometrial cancer. Oncol Lett.
4:1290–1296. 2012.PubMed/NCBI
|
|
65
|
Ramón LA, Braza-Boïls A, Gilabert J,
Chirivella M, España F, Estellés A and Gilabert-Estellés J:
microRNAs related to angiogenesis are dysregulated in endometrioid
endometrial cancer. Hum Reprod. 27:3036–3045. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Romero-Pérez L, Castilla MA, López-García
MA, Díaz-Martín J, Biscuola M, Ramiro-Fuentes S, Oliva E,
Matias-Guiu X, Prat J, Cano A, et al: Molecular events in
endometrial carcinosarcomas and the role of high mobility group
AT-hook 2 in endometrial carcinogenesis. Hum Pathol. 44:244–254.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Shan ZN, Tian R, Zhang M, Gui ZH, Wu J,
Ding M, Zhou XF and He J: miR128-1 inhibits the growth of
glioblastoma multiforme and glioma stem-like cells via targeting
BMI1 and E2F3. Oncotarget. 7:78813–78826. 2016.PubMed/NCBI
|
|
68
|
Shang C, Lu YM and Meng LR: MicroRNA-125b
down-regulation mediates endometrial cancer invasion by targeting
ERBB2. Med Sci Monit. 18:BR149–BR155. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Torres A, Kozak J, Korolczuk A, Wdowiak P,
Domańska-Glonek E, Maciejewski R and Torres K: In vitro and in vivo
activity of miR-92a-Locked Nucleic Acid (LNA)-inhibitor against
endometrial cancer. BMC Cancer. 16:8222016. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Tsukamoto O, Miura K, Mishima H, Abe S,
Kaneuchi M, Higashijima A, Miura S, Kinoshita A, Yoshiura K and
Masuzaki H: Identification of endometrioid endometrial
carcinoma-associated microRNAs in tissue and plasma. Gynecol Oncol.
132:715–721. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Wang J, Li Y, Ding M, Zhang H, Xu X and
Tang J: Molecular mechanisms and clinical applications of miR-22 in
regulating malignant progression in human cancer (Review). Int J
Oncol. 50:345–355. 2017.(Review). PubMed/NCBI
|
|
72
|
Yang N, Kaur S, Volinia S, Greshock J,
Lassus H, Hasegawa K, Liang S, Leminen A, Deng S, Smith L, et al:
MicroRNA microarray identifies Let-7i as a novel biomarker and
therapeutic target in human epithelial ovarian cancer. Cancer Res.
68:10307–10314. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Zhang R, He Y, Zhang X, Xing B, Sheng Y,
Lu H and Wei Z: Estrogen receptor-regulated microRNAs contribute to
the BCL2/BAX imbalance in endometrial adenocarcinoma and
precancerous lesions. Cancer Lett. 314:155–165. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Zhao C, Wang G, Zhu Y, Li X, Yan F, Zhang
C, Huang X and Zhang Y: Aberrant regulation of miR-15b in human
malignant tumors and its effects on the hallmarks of cancer. Tumour
Biol. 37:177–183. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Zhao H, Xu Z, Qin H, Gao Z and Gao L:
miR-30b regulates migration and invasion of human colorectal cancer
via SIX1. Biochem J. 460:117–125. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Nishida M: The Ishikawa cells from birth
to the present. Hum Cell. 15:104–117. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Pfaffl MW, Tichopad A, Prgomet C and
Neuvians TP: Determination of stable housekeeping genes,
differentially regulated target genes and sample integrity:
BestKeeper-Excel-based tool using pair-wise correlations.
Biotechnol Lett. 26:509–515. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Chou CH, Chang NW, Shrestha S, Hsu SD, Lin
YL, Lee WH, Yang CD, Hong HC, Wei TY, Tu SJ, et al: miRTarBase
2016: Updates to the experimentally validated miRNA-target
interactions database. Nucleic Acids Res. 44:D239–D247. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Fang JS, Gillies RD and Gatenby RA:
Adaptation to hypoxia and acidosis in carcinogenesis and tumor
progression. Semin Cancer Biol. 18:330–337. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Gatenby RA and Gillies RJ: Why do cancers
have high aerobic glycolysis? Nat Rev Cancer. 4:891–899. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Gatenby RA, Smallbone K, Maini PK, Rose F,
Averill J, Nagle RB, Worrall L and Gillies RJ: Cellular adaptations
to hypoxia and acidosis during somatic evolution of breast cancer.
Br J Cancer. 97:646–653. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Sameni M, Mullins S, Moin K, Sloane B and
Osuala K: Importance of the tumor microenvironment. Breast Cancer
Metastasis and Drug Resistance: Progress and Prospects. Ahmad A:
New York: Springer; pp. 178–179. 2013
|
|
83
|
Dews M, Homayouni A, Yu D, Murphy D,
Sevignani C, Wentzel E, Furth EE, Lee WM, Enders GH, Mendell JT, et
al: Augmentation of tumor angiogenesis by a Myc-activated microRNA
cluster. Nat Genet. 38:1060–1065. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Suárez Y, Fernández-Hernando C, Yu J,
Gerber SA, Harrison KD, Pober JS, Iruela-Arispe ML, Merkenschlager
M and Sessa WC: Dicer-dependent endothelial microRNAs are necessary
for postnatal angiogenesis. Proc Natl Acad Sci USA.
105:14082–14087. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Lei Z, Li B, Yang Z, Fang H, Zhang GM,
Feng ZH and Huang B: Regulation of HIF-1alpha and VEGF by miR-20b
tunes tumor cells to adapt to the alteration of oxygen
concentration. PLoS One. 4:e76292009. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Danza K, Silvestris N, Simone G, Signorile
M, Saragoni L, Brunetti O, Monti M, Mazzotta A, De Summa S, Mangia
A, et al: Role of miR-27a, miR-181a and miR-20b in gastric cancer
hypoxia-induced chemoresistance. Cancer Biol Ther. 17:400–406.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Chen PH, Cheng CH, Shih CM, Ho KH, Lin CW,
Lee CC, Liu AJ, Chang CK and Chen KC: The inhibition of
microRNA-128 on IGF-1-activating mTOR signaling involves in
Temozolomide-induced glioma cell apoptotic death. PLoS One.
11:e01670962016. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Stefani G and Slack FJ: Small non-coding
RNAs in animal development. Nat Rev Mol Cell Biol. 9:219–230. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Takamizawa J, Konishi H, Yanagisawa K,
Tomida S, Osada H, Endoh H, Harano T, Yatabe Y, Nagino M, Nimura Y,
et al: Reduced expression of the let-7 microRNAs in human lung
cancers in association with shortened postoperative survival.
Cancer Res. 64:3753–3756. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Tsuji T, Umekita Y, Ohi Y, Kamio M, Douchi
T and Yoshida H: Maspin expression is up-regulated during the
progression of endometrioid endometrial carcinoma. Histopathology.
51:871–874. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Mirra P, Raciti GA, Nigro C, Fiory F,
D'Esposito V, Formisano P, Beguinot F and Miele C: Circulating
miRNAs as intercellular messengers, potential biomarkers and
therapeutic targets for Type 2 diabetes. Epigenomics. 7:653–667.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Turchinovich A, Samatov TR, Tonevitsky AG
and Burwinkel B: Circulating miRNAs: Cell-cell communication
function? Front Genet. 4:1192013. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Gambari R, Brognara E, Spandidos DA and
Fabbri E: Targeting oncomiRNAs and mimicking tumor suppressor
miRNAs: Νew trends in the development of miRNA therapeutic
strategies in oncology (Review). Int J Oncol. 49:5–32.
2016.PubMed/NCBI
|
|
94
|
Bai JX, Yan B, Zhao ZN, Xiao X, Qin WW,
Zhang R, Jia LT, Meng YL, Jin BQ, Fan DM, et al: Tamoxifen
represses miR-200 microRNAs and promotes epithelial-to-mesenchymal
transition by up-regulating c-Myc in endometrial carcinoma cell
lines. Endocrinology. 154:635–645. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Shen Y, Lu L, Xu J, Meng W, Qing Y, Liu Y,
Zhang B and Hu H: Bortezomib induces apoptosis of endometrial
cancer cells through microRNA-17-5p by targeting p21. Cell Biol
Int. 37:1114–1121. 2013. View Article : Google Scholar : PubMed/NCBI
|