Introduction
Worldwide, cervical cancer is the second most common
malignancy after breast cancer, and remains a leading cause of
cancer-related death among females in developing countries. There
were an estimated 527,600 new cervical cancer cases and 265,700
deaths worldwide in 2012 (1).
Currently, surgery, platinum based chemotherapy and radiotherapy
play important roles for cervical cancer treatment. Nevertheless,
acquired resistance to platinum and radiation is considered a main
element for tumor relapse and metastasis. Therefore, to develop
more effective therapeutic strategies and in-depth research into
the molecular and biologic mechanisms of oncogenesis for cervical
cancer is critical. Although the development of cervical cancer is
intimately associated with high-risk human papillomavirus (HPV)
infection (2), not all patients
infected with HPV ultimately develop cervical cancer. Thus, various
molecular dysfunction, including the activation of oncogenes and
inactivation of suppressor genes, are also essential for cervical
cancer development (3,4).
The RAD51 gene is homologous to the E.
coli RecA and yeast RAD51 genes, which are involved in
the repair of DNA double-strand breaks and also play important
roles in recombination repair and various SOS responses to DNA
damage by γ-irradiation and alkylating reagents (5). RAD51 plays a role in several
cellular processes, including genomic integrity, cell cycle
regulation, apoptosis and tumor formation. RAD51 is overexpressed
in a variety types of tumors, including cervical cancer (6), non-small cell lung cancer (7), breast cancer (8), ovarian cancers (9), pancreatic cancer (10), melanoma and glioblastoma (11). The overexpression of RAD51 causes
improper and hyper-recombination, namely contributing to genomic
instability and genetic diversity, which might drive regular cells
towards neoplastic transformation or further contribute to cancer
progression and metastasis (12,13).
Additionally, in Fanconi anemia-like patients, phenotype-derived
mutation in RAD51 plays a role in protection of DNA during
the course of ICL repair, which is independent of RAD51's ability
to maintain genomic integrity (14). Accumulating evidence has indicated
that RAD51 not only is involved in the progression of
carcinogenesis, but also plays a part in resistance to anti-cancer
treatments (16,17). The RAD51 protein is a specific
HR-related target for cancer therapy. Treatment of leukemia,
prostate cancer, pancreatic adenocarcinoma, lung carcinoma and
glioma cells with imatinib can decrease RAD51 expression and
sensitize them to experimental chemotherapy and radiotherapy in
vitro and in vivo (17,18).
The molecular mechanisms of RAD51 in cervical
carcinoma are largely unclear. In the present study, we
demonstrated that RAD51 was overexpressed in cervical carcinoma and
examined the effects of the RAD51 inhibitor RI-1 on the
proliferation, cell cycle and sensitivity of cervical cancer cells.
Budke et al revealed that RI-1 made RAD51 inactive by
directly binding covalently to human RAD51 protein at cysteine 319.
Accordingly, RI-1 inhibited the formation of subnuclear RAD51 foci
in cells in response to DNA damage, without affecting formation of
the replication protein A focus (15). Our study suggested that RI-1 largely
inhibits the growth of cervical cancer cells in vitro and
in vivo by arresting the cell cycle. Moreover, RI-1
decreased resistance to platinum and ionizing radiation. Therefore,
in addition to classical function in HR, RAD51 can also regulate
the progression of the cell cycle, and thus RI-1 might have
promising therapeutic effects against cervical carcinoma.
Materials and methods
Cell lines and cell culture
Human cervical cancer cell lines (CaSki, C33A, HeLa
and SiHa) were purchased from the American Type Culture Collection
(ATCC; Manassas, VA, USA). C33A, HeLa and SiHa cells were cultured
in Dulbecco's Modified Eagle's Medium (DMEM; Sigma-Aldrich, St.
Louis, MO, USA), and CaSki was cultured in RPMI-1640
(Sigma-Aldrich), supplemented with 10% heat-inactivated fetal
bovine serum (FBS; Invitrogen, Carlsbad, CA, USA). All cell lines
were maintained at 37°C in a humidified 5% CO2
incubator.
Tissue specimens
The human specimens (n=107) were collected from
patients at the Second Affiliated Hospital of Xi'an Jiaotong
University from 2010 to 2015. Of the 107 samples 43 were normal
cervical (NC) tissues and 64 were squamous cervical cancer (SCC)
tissues. All of the procedures followed approved medical ethics
practices. None of the patients had received chemotherapy,
immunotherapy or radiotherapy before specimen collection. The
histological classifications and clinical staging were done in
accordance with the International Federation of Gynecology and
Obstetrics classification system.
Immunohistochemistry and RAD51
inhibitor
Formalin-fixed, paraffin-embedded tissue sections
were analyzed in an immunohistochemical study. After being placed
in a 60°C incubator overnight, four micrometer-thick sections were
deparaffinized with xylene and rehydrated in a series of ethanols.
Then the sections were incubated in heat-induced epitope retrieval
in citric acid buffer (pH 7.0) for 2 min. Endogenous peroxidase was
blocked at room temperature using 3% hydrogen peroxide in methanol
for 10 min. Then slides were incubated with mouse monoclonal
anti-RAD51 (1:100; Bioscience, Boston, MA, USA) at 4°C overnight,
followed with inclubation of goat anti-mouse immunoglobulin at room
temperature for 30 min. The slides were counterstained stained with
hematoxylin (7). RAD51
immunostaining was presented by a semi-quantitative
immunoreactivity score (IRS), which (negative 0–3, weak 4–7, strong
8–12) was evaluated by multiplying the values for staining
intensity (scored as 0, no staining; 1, light brown; 2, brown; 3,
dark brown) and the values for percentage of positive cells (scored
as 0, <10%; 1, 10–25%; 2, 25–50%; 3, 50–75%; 4, >75%) in each
sample. An overall score of ≤3 was defined as negative and a score
of >3 as positive. All specimens were evaluated by two
pathologists in a blinded manner.
The RAD51 inhibitor RI-1 was purchased from Selleck
Chemicals (Houston, TX, USA), according to the manufacturer's
instructions. RI-1 was dissolved in DMSO at a stock concentration
of 1 mM.
Western blot analysis
Exponentially growing cells were harvested and
resuspended in PBS pH 7.4 (Gibco). Then the cells were lysed by
lysis buffer mixed with protease inhibitor cocktail (Roche,
Mannheim, Germany). The lysate was mixed with Laemmli sample buffer
containing 5% 2-mercaptoethanol and boiled for 5 min. Equal amounts
of protein extracts were separated on a 10% SDS/PAGE, blotted onto
an activated polyvinylidene difluoride membrane (Millipore,
Billerica, MA, USA) and blocked in TBST with 5% dried milk.
Membranes were probed with anti-RAD51 (1:1000, Bioscience),
anti-GAPDH (1:1000, Santa Cruz Biotechnology, Santa Cruz, CA, USA),
anti-cyclin D1 (1:500, Santa Cruz Biotechnology), anti-p21 (1:500,
Santa Cruz Biotechnology) at 4°C overnight. Then, they were probed
with goat anti-mouse secondary antibodies (Thermo Fisher
Scientific, Grand Island, NY, USA) for 1 h.
Cell growth and cell viability
assays
Cells were seeded in triplicates at a density of
5×104 cells with 2 ml of media into 35-mm tissue culture
dishes for 7 days. The numbers of cells were manually counted after
harvesting using a hemocyto-meter under light microscopy every two
days. Cell viability assays were performed by applying
3-(4,5-dimethylthiazol-yl)-2,5-diphenyl tetrazolium bromide (MTT;
Sigma-Aldrich) dye to cells that were seeded in 96-well plates with
800 cells in each well, as described in a standard protocol. Then,
the number of live cells was determined by the absorbance at 490 nm
(Bio-Rad, Hercules, CA, USA).
Tumor xenograft experiment
To assess the tumorigenicity in vivo, cells
(1×107) in the exponential growth phase were collected
and were suspended in 200-µl phosphate-buffered saline and then
injected subcutaneously into the posterior side of 4- to 6-week-old
female BALB/c-nude mice purchased from Shanghai SLAC Laboratory
Animal Co., Ltd. (Shanghai, China). Xenograft tumor volume (V) was
calculated using the length (a) and width (b) by
V=ab2/2. To study the function of the RAD51 inhibitor
RI-1, 5 mg/kg in 200-µl phosphate-buffered saline was injected
intraperitoneally every other day into BALB/c nude mice (n=6) when
the tumor volume reached 120 mm3. In this analysis, the
negative control group (n=6) received saline. The animal
experimental protocols were evaluated and approved by the Animal
Care and Use Committee of the Medical College of Xi'an Jiaotong
University. The mice were sacrificed and tumors were dissected and
weighed at the end of the experiment.
Flow cytometry analysis
Cell cycle analysis was performed using flow
cytometry (FACScan; Becton Dickinson, Franklin Lakes, NJ, USA)
according to the manufacturer's instructions. Cells were harvested
and fixed in 70% ice-cold ethanol overnight at 4°C. Thirty minutes
before FACS analysis, the samples were washed with PBS, treated
with 1 mg/ml RNase A and then stained with 20 µg/ml propidium
iodide (Sigma-Aldrich). Cell cycle distribution was analyzed using
a FACSCalibur flow cytometer with Mod-Fit LT software.
Quantitative real-time PCR
Total RNA was extracted from exponentially growing
cells with the TRIzol reagent (Invitrogen). Total cDNA was used as
a template for PCR amplification. Quantitative real-time PCR was
performed using the IQ5 Real-time PCR Detection System (Bio-Rad) in
triplicates and the following primers: GAPDH (GCACCGT CAAGGCTGAGAAC
and TGGTGAAGACGCCAGTGGA); Cyclin D1 (AAACAGATCATCCGCAAACAC and GTT
GGGGCTCCTCAGGTTC) and p21 (GCAGACCAGCATG ACAGATTTC and
CGGATTAGGGCTTCCTCTTG). The protocol was 95°C for 30 sec, 40 cycles
of 95°C for 5 sec and 60°C for 30 sec, and then a dissociation
stage. The results were analyzed via the ∆∆Ct method using GAPDH as
the housekeeping gene.
Cell survival assay
Cells were treated with cisplatin (Sigma-Aldrich;
0–32 nmol/l) for 24 h. In other experiments, the cells were
irradiated with doses between 0 and 18 Gy under aerobic conditions,
at room temperature, using a 137Cs unit at a dose rate
of 2.5 Gy/min.
Statistical analysis
All of the statistical analyses were performed using
GraphPad Prism 5.0 software (GraphPad Software, La Jolla, CA, USA).
All data are expressed as mean ± standard deviation (SD). Student's
t-test was used for comparisons between two groups and one-way or
two-way analysis of variance (ANOVA) test was used to analyze
statistical differences between groups under different conditions.
P<0.05 was considered to indicate a statistically significant
difference.
Results
RAD51 expression in normal cervix and
squamous cervical carcinoma
To explore the RAD51 expression levels and its
association with normal or neoplastic cervical tissues, we first
conducted the immunohistochemistry assay using paraffin-embedded
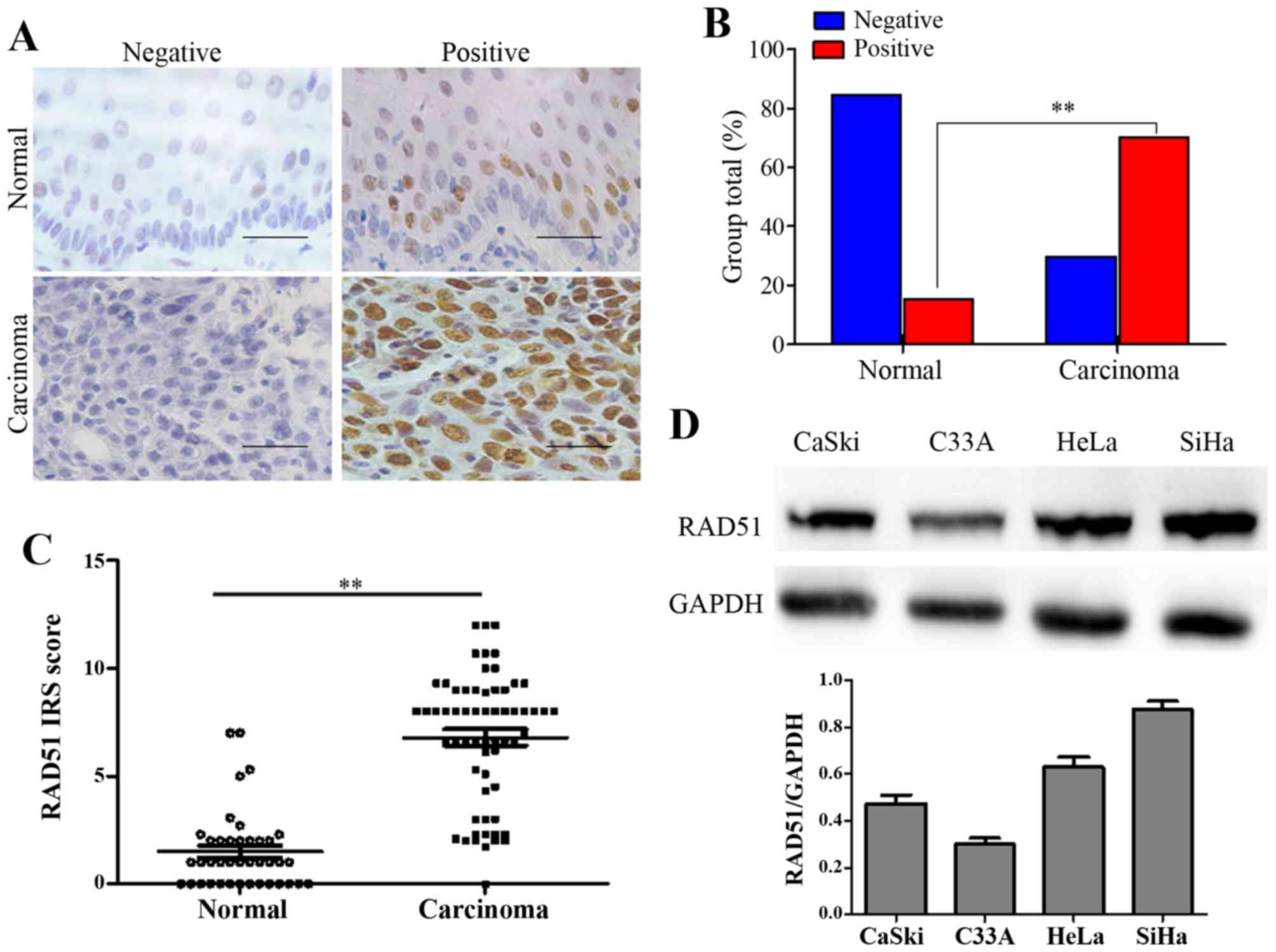
normal cervix and squamous cervical cancer tissues. RAD51 staining
was observed in the nuclei of positive cells in different cervical
tissues (Fig. 1A). The number of
specimens with positive RAD51 staining gradually increased from
13.95% (6/43) in the normal cervical tissues to 73.44% (47/64) in
the cervical cancer tissues (Table
I and Fig. 1B, P<0.01). IHC
score results revealed that the immunoreactivity score (IRS) of
RAD51 staining was 1.6 for the normal cervical tissues and 6.8 for
the cervical cancer tissues (P<0.01, Fig. 1C).
 | Table I.Rad51 expression in tissue
specimens. |
Table I.
Rad51 expression in tissue
specimens.
|
|
| Rad51 staining |
|
|---|
|
|
|
|
|
|---|
| Specimens | Total | Negative, no.
(%) | Positive, no.
(%) | P-value |
|---|
| Normal | 43 | 37 (86.05) | 6
(13.95) |
|
| Carcinoma | 64 | 17 (26.56) | 47 (73.44) | <0.01 |
Next, using western blot assay, we found that RAD51
showed different expression levels in CaSki, C33-A, HeLa and SiHa
cells (Fig. 1D). In particular,
higher levels of the RAD51 protein appeared in the HeLa and Siha
cells.
The RAD51 inhibitor RI-1 suppresses
the proliferation of cervical cancer cells in vitro
Having shown that RAD51 expression was increased in
cervical cancer progression, the role of RAD51 in cervical cancer
was functionally evaluated. We conducted experiments using the
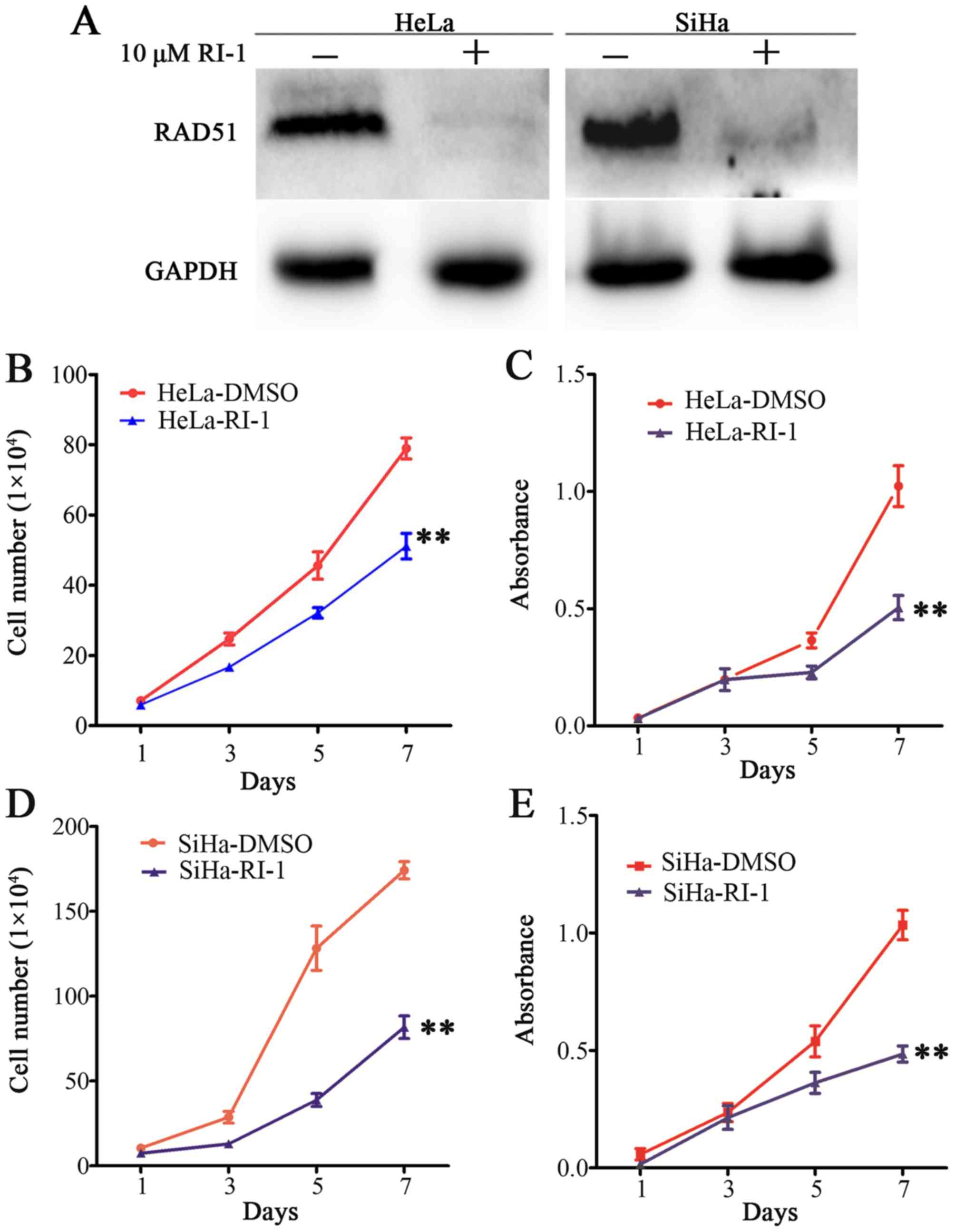
RAD51 inhibitor RI-1. We found that RAD51 was almost eliminated
using 10 µM RI-1 in HeLa and SiHa cells that expressed higher
levels of RAD51 protein by western blotting (Fig. 2A). Next, we tested whether the
inhibition of RAD51 activity would suppress cervical cancer cell
proliferation. Cell growth curve and MTT assays showed that
compared to controls, RI-1 significantly reduced cell growth and
viability in HeLa (Fig. 2B and C,
P<0.01) and SiHa (Fig. 2D and E,
P<0.01). These results demonstrated that RI-1 suppressed the
proliferation of cervical cancer cells in vitro.
The RAD51 inhibitor RI-1 suppresses
the growth of cervical cancer xenografts in vivo
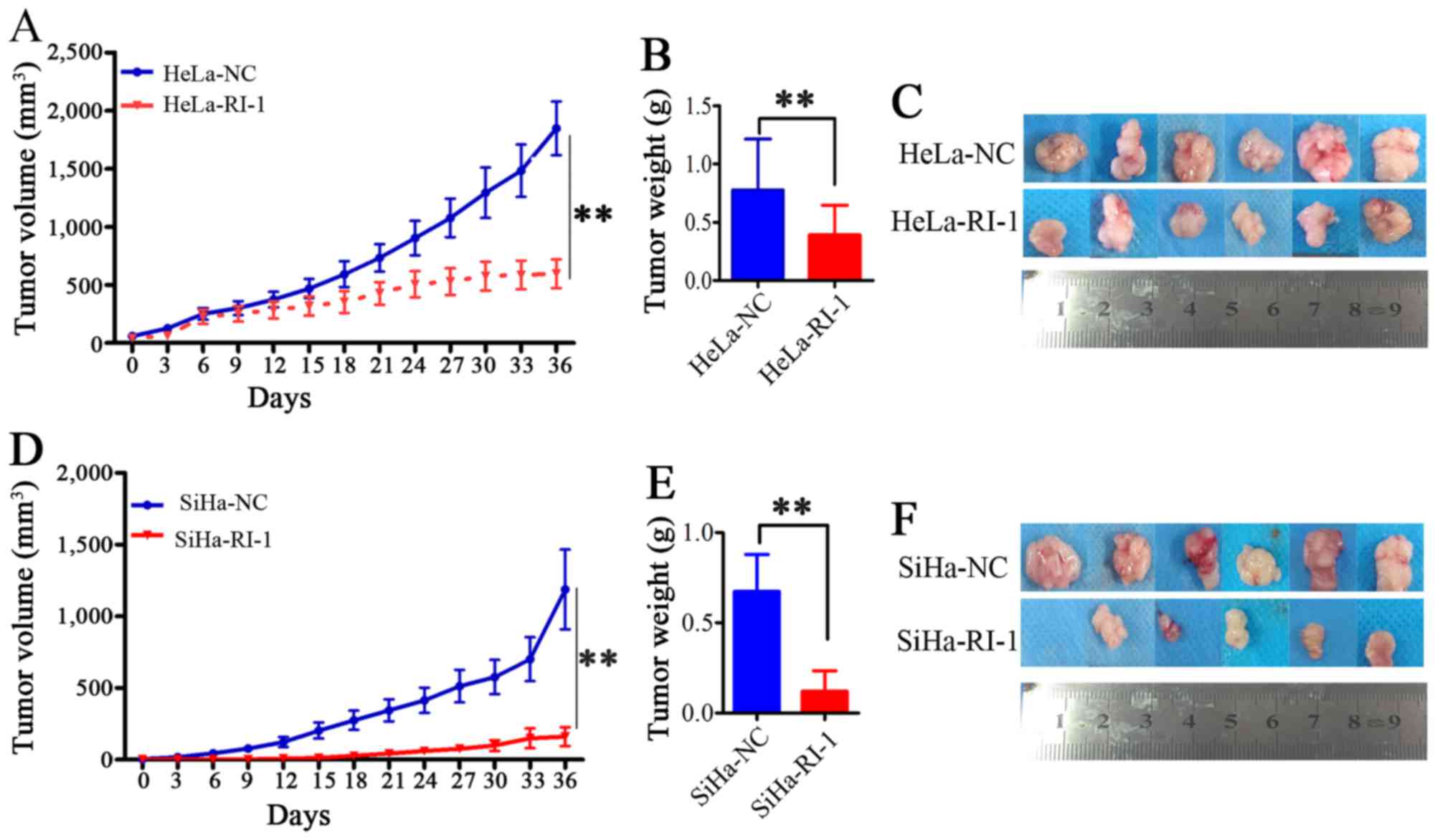
To investigate whether RI-1 has similar inhibitory
effect on tumorigenicity in vivo, female athymic nude mice
(6 mice per group) were injected subcutaneously with HeLa and SiHa
cells. When tumor formation reached 120 mm3, the volume
of tumors was measured every three days, and we intervened with an
intraperitoneal injection of RI-1 (5 mg/kg in 200 µl PBS) or normal
saline. The net weights of the sacrificed mice were recorded upon
termination of the experiment. As shown in Fig. 3A, compared to the negative controls,
the RI-1-treated mice exhibited significantly inhibited tumor
growth in HeLa cells (P<0.01). The average volume and weight of
xenografts of the RI-1-treated groups were markedly reduced
(Fig. 3A-C, P<0.01). The results
were similar in SiHa cells (Fig.
3D-F, P<0.01). These results indicated that RI-1 suppressed
tumor formation of cervical cancer cells in vivo.
RI-1 suppresses the proliferation of
cervical cancer cells by attenuating the cell cycle transition from
G0/G1 to S phase through acting on the expression of cell cycle
associated protein cyclin D1 and p21
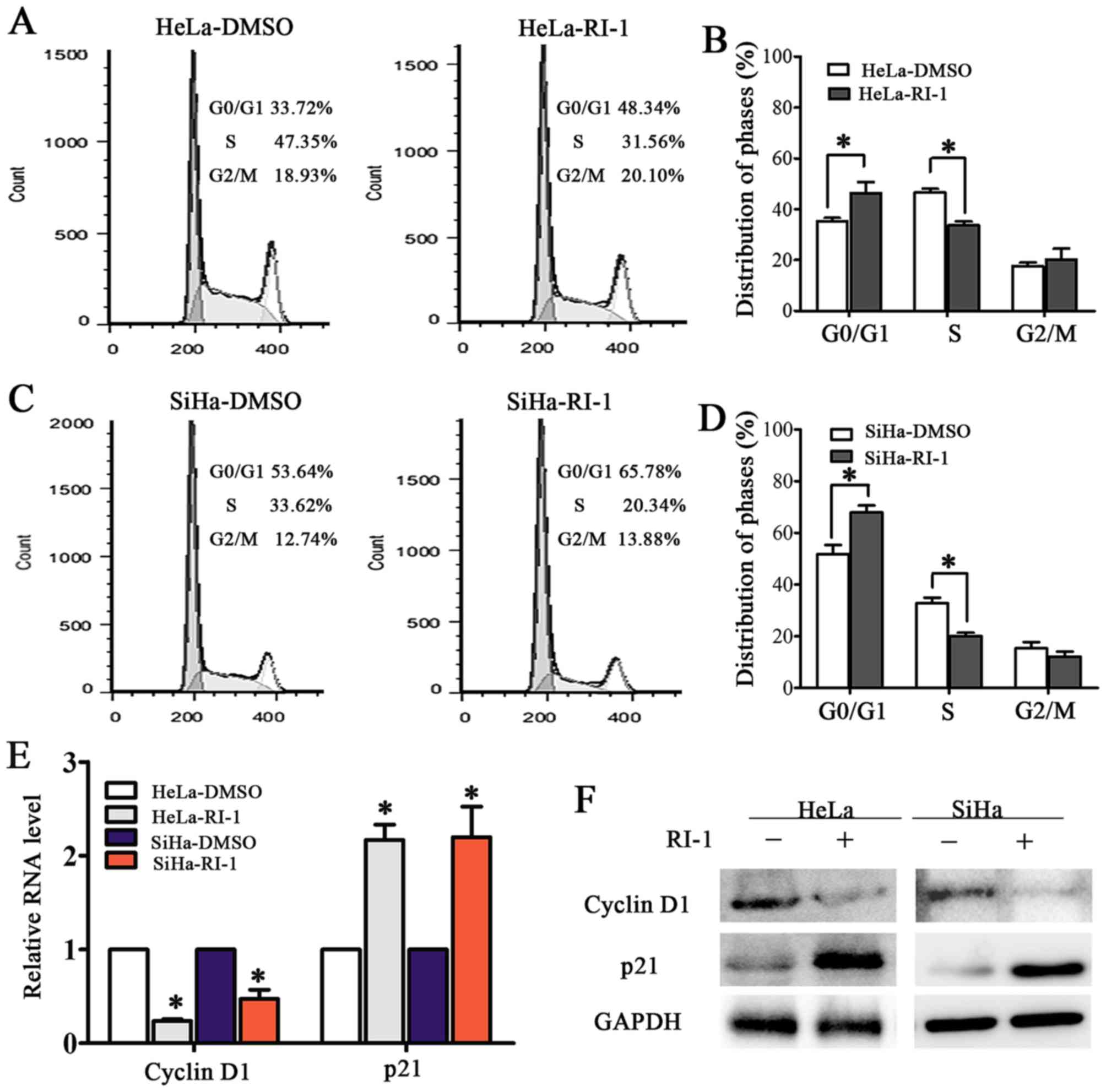
To investigate the mechanism of RI-1-mediated
suppression of cervical cancer cell proliferation, cell cycle
analyses of RI-1-HeLa and SiHa and control cells was performed by
fluorescence-activated cell sorting (FACS). The percentage of cells
in S phase significantly decreased from 47.25% for HeLa-DMSO cells
to 31.56% for HeLa-RI-1 cells (Fig. 4A
and B, P<0.05). A similar effect was observed in SiHa-RI-1
cells, which had 20.34% in the S phase compared to 33.12% of
control cells (Fig. 4C and D,
P<0.05). This result showed that the RAD51 inhibitor RI-1 may
arrest the cell cycle transition at the G1/S phase and further
suppress the proliferation of cervical cancer cells.
Further experiments showed that the mRNA level of
the cell cycle associated protein cyclin D1 was reduced while that
of p21 increased after RI-1 treatment in HeLa and SiHa cells
(Fig. 4E, P<0.05). The
administration of RI-1 for 48 h resulted in a downregulation of
cyclin D1 and upregulation of p21 by western blotting (Fig. 4F, P<0.01). Collectively, these
results suggested that inhibition of RAD51 blocked the cell cycle
transition possibly through interaction with cyclin D1 and p21.
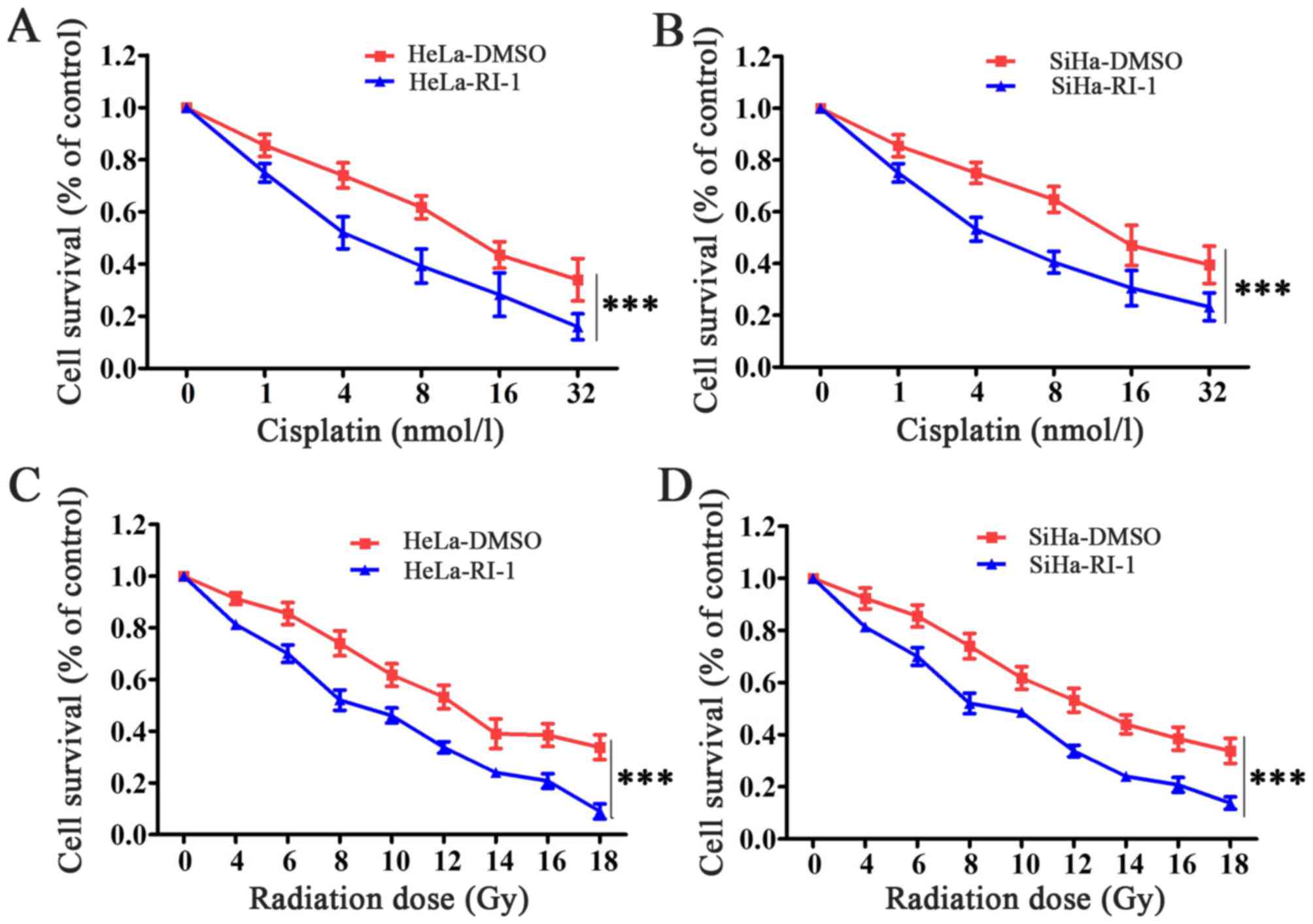
Inhibition of RAD51 enhances the
sensitivity of cervical cancer cells to chemotherapeutic drugs and
irradiation
Given the decreased expression and function of RAD51
in cervical cancer cell lines after using RI-1, we investigated
whether RI-1 could lead to an increase in tumor cell sensitivity to
cisplatin and radiation. We observed that cervical cancer cells,
HeLa and SiHa, when treated with RI-1 had significantly reduced
survival compared to cells treated with DMSO in response to
cisplatin (Fig. 5A and B). In
response to radiotherapy, HeLa and SiHa cells treated with RI-1 had
also significantly reduced survival compared to cells treated with
DMSO (Fig. 5C and D). Thus, we
concluded that RI-1 can sensitize cervical cancer cells to
chemotherapeutic drugs and radiation.
Discussion
Elevated expression of RAD51 is associated with
tumor aggressiveness and is known to confer treatment resistance in
a variety of tumors, including those in ovarian cancer (19), breast cancer (16), lung tumors (20), pancreatic adenocarcinoma (13) and malignant gliomas (21). Furthermore, downregulation of RAD51
protein by RAD51 antisense oligonucleotides, RNA
interference (22), aptamers
(23) or small molecule inhibitors
against RAD51 could be used to sensitize tumors to chemotherapy or
radiation. Recent studies have identified that impaired replication
and intra-S mediated CHK1 signaling by RAD51 led to higher genomic
instability and thus drove tumorigenesis (24). The 3′-untranslated region of RAD51
was directly bound by tumor-suppressing miR-34a and thus regulation
of homologous recombination and double-strand break repair was
inhibited in NSCLC cells (20).
In our study, RAD51 expression was found to be
significantly elevated in cervical cancer tissues compared to
normal cervical tissues (Fig. 1),
consistent with results from previous studies of non-small cell
lung cancer (7), breast carcinoma
(8), prostate cancer (25), chronic myeloid leukaemia (CML),
melanoma and glioblastoma (11).
Subsequently, RI-1 was applied to further explore the function of
RAD51 in cervical carcinogenesis. RI-1 could directly and
specifically disrupt HsRAD51, and inhibit sub-nuclear accumulation
of HsRAD51 protein at sites of DNA damage, thereby, this inhibitory
activity sensitizes tumor cells to cross-linking chemotherapy
(15). We further performed western
blotting to evaluate the inhibitory effect of RI-1 on RAD51. A
previous study indicated that the combination of minocycline and
MMC in NSCLC can synergistically inhibit cell proliferation and
reduce cell viability in vitro, while overexpression of
RAD51 expression can restore cell viability upon minocycline and
MMC cotreatment (26). In our
study, the results showed that inhibition of RAD51 significantly
suppressed the proliferation of cervical cancer cells in
vitro and tumor growth in vivo (Figs. 2 and 3). In a previous study, p21 was
investigated because it was well-known as a major mediator of G1
arrest and an inhibitor of G1 Cdks after DNA damage (27). Thus, the RAD51 foci-positive cells
could also be arrested during the G1 phase. This would be
consistent with the observation that G0/G1 arrest upon serum
starvation induced by overexpressed of RAD51 protein. However,
endogenous Rad51 foci were not observed in G1 phase cells.
Overexpression of RAD51 could increase its function, which improved
DNA recombination in S/G2 phase (28). Our cell cycle analyses suggested
that RI-1 prevented the transition from G0/G1 to S phase in
cervical cancer cells. In addition, RI-1 treatment decreased cyclin
D1 and increased p21 mRNA and protein levels (Fig. 4). Nevertheless, the particular
mechanism between RAD51 and cyclin D1 and p21 should be further
investigated. Recent studies have revealed that the enhanced RAD51
protein level in tumor cells is associated with high DNA repair
capacity, elevated recombination rates and increased resistance
against radiotherapy and chemotherapy (10). Our study showed that downregulation
of RAD51 protein could indeed sensitize cervical cancer cells to
chemotherapy and radiation (Fig.
5).
In summary, our findings demonstrate that inhibition
of RAD51 attenuates cell proliferation and tumor formation in
cervical cancer cells by arresting the cell cycle via cyclin D1 and
p21. Therefore, RAD51 might be a promising therapeutic strategy for
the treatment of cervical cancer.
Acknowledgements
This work was supported by a grant from the Science
and Technology Project of Shaanxi Province (2014K11-01-01-18).
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Walboomers JM, Jacobs MV, Manos MM, Bosch
FX, Kummer JA, Shah KV, Snijders PJ, Peto J, Meijer CJ and Muñoz N:
Human papillomavirus is a necessary cause of invasive cervical
cancer worldwide. J Pathol. 189:12–19. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang L, Wu J, Ling MT, Zhao L and Zhao
KN: The role of the PI3K/Akt/mTOR signalling pathway in human
cancers induced by infection with human papillomaviruses. Mol
Cancer. 14:872015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Qureshi R, Arora H and Rizvi MA: EMT in
cervical cancer: Its role in tumour progression and response to
therapy. Cancer Lett. 356:321–331. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kowalczykowski SC: Biochemistry of genetic
recombination: Energetics and mechanism of DNA strand exchange.
Annu Rev Biophys Biophys Chem. 20:539–575. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Paulíková S, Chmelařová M, Petera J,
Palička V and Paulík A: Hypermethylation of RAD51L3 and XRCC2 genes
to predict late toxicity in chemoradiotherapy-treated cervical
cancer patients. Folia Biol (Praha). 59:240–245. 2013.PubMed/NCBI
|
|
7
|
Takenaka T, Yoshino I, Kouso H, Ohba T,
Yohena T, Osoegawa A, Shoji F and Maehara Y: Combined evaluation of
Rad51 and ERCC1 expressions for sensitivity to platinum agents in
non-small cell lung cancer. Int J Cancer. 121:895–900. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Maacke H, Opitz S, Jost K, Hamdorf W,
Henning W, Krüger S, Feller AC, Lopens A, Diedrich K, Schwinger E,
et al: Over-expression of wild-type Rad51 correlates with
histological grading of invasive ductal breast cancer. Int J
Cancer. 88:907–913. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu G, Yang D, Rupaimoole R, Pecot CV, Sun
Y, Mangala LS, Li X, Ji P, Cogdell D, Hu L, et al: Augmentation of
response to chemotherapy by microRNA-506 through regulation of
RAD51 in serous ovarian cancers. J Natl Cancer Inst. 107:1072015.
View Article : Google Scholar
|
|
10
|
Maacke H, Jost K, Opitz S, Miska S, Yuan
Y, Hasselbach L, Lüttges J, Kalthoff H and Stürzbecher HW: DNA
repair and recombination factor Rad51 is over-expressed in human
pancreatic adenocarcinoma. Oncogene. 19:2791–2795. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Raderschall E, Stout K, Freier S, Suckow
V, Schweiger S and Haaf T: Elevated levels of Rad51 recombination
protein in tumor cells. Cancer Res. 62:219–225. 2002.PubMed/NCBI
|
|
12
|
Hine CM, Seluanov A and Gorbunova V: Use
of the Rad51 promoter for targeted anti-cancer therapy. Proc Natl
Acad Sci USA. 105:20810–20815. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nagathihalli NS and Nagaraju G: RAD51 as a
potential biomarker and therapeutic target for pancreatic cancer.
Biochim Biophys Acta. 1816:209–218. 2011.PubMed/NCBI
|
|
14
|
Wang AT, Kim T, Wagner JE, Conti BA, Lach
FP, Huang AL, Molina H, Sanborn EM, Zierhut H, Cornes BK, et al: A
dominant mutation in human RAD51 reveals its function in DNA
interstrand crosslink repair independent of homologous
recombination. Mol Cell. 59:478–490. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Budke B, Logan HL, Kalin JH, Zelivianskaia
AS, McGuire Cameron W, Miller LL, Stark JM, Kozikowski AP, Bishop
DK and Connell PP: RI-1: A chemical inhibitor of RAD51 that
disrupts homologous recombination in human cells. Nucleic Acids
Res. 40:7347–7357. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hong KJ, Hsu MC and Hung WC: RECK impedes
DNA repair by inhibiting the erbB/JAB1/Rad51 signaling axis and
enhances chemosensitivity of breast cancer cells. Am J Cancer Res.
5:2422–2430. 2015.PubMed/NCBI
|
|
17
|
Choudhury A, Zhao H, Jalali F, Al Rashid
S, Ran J, Supiot S, Kiltie AE and Bristow RG: Targeting homologous
recombination using imatinib results in enhanced tumor cell
chemosensitivity and radiosensitivity. Mol Cancer Ther. 8:203–213.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kübler HR, van Randenborgh H, Treiber U,
Wutzler S, Battistel C, Lehmer A, Wagenpfeil S, Hartung R and Paul
R: In vitro cytotoxic effects of imatinib in combination with
anticancer drugs in human prostate cancer cell lines. Prostate.
63:385–394. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang B, Hou D, Liu Q, Wu T, Guo H, Zhang
X, Zou Y, Liu Z, Liu J, Wei J, et al: Artesunate sensitizes ovarian
cancer cells to cisplatin by downregulating RAD51. Cancer Biol
Ther. 16:1548–1556. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cortez MA, Valdecanas D, Niknam S, Peltier
HJ, Diao L, Giri U, Komaki R, Calin GA, Gomez DR, Chang JY, et al:
In vivo delivery of miR-34a sensitizes lung tumors to radiation
through RAD51 regulation. Mol Ther Nucleic Acids. 4:e2702015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ohnishi T, Taki T, Hiraga S, Arita N and
Morita T: In vitro and in vivo potentiation of radiosensitivity of
malignant gliomas by antisense inhibition of the RAD51 gene.
Biochem Biophys Res Commun. 245:319–324. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mueller AC, Sun D and Dutta A: The miR-99
family regulates the DNA damage response through its target SNF2H.
Oncogene. 32:1164–1172. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Martinez SF, Renodon-Cornière A, Nomme J,
Eveillard D, Fleury F, Takahashi M and Weigel P: Targeting human
Rad51 by specific DNA aptamers induces inhibition of homologous
recombination. Biochimie. 92:1832–1838. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Parplys AC, Seelbach JI, Becker S, Behr M,
Wrona A, Jend C, Mansour WY, Joosse SA, Stuerzbecher HW, Pospiech
H, et al: High levels of RAD51 perturb DNA replication elongation
and cause unscheduled origin firing due to impaired CHK1
activation. Cell Cycle. 14:3190–3202. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mitra A, Jameson C, Barbachano Y, Sanchez
L, Kote-Jarai Z, Peock S, Sodha N, Bancroft E, Fletcher A, Cooper
C, et al: IMPACT Steering Committee and IMPACT and EMBRACE
Collaborators: Overexpression of RAD51 occurs in aggressive
prostatic cancer. Histopathology. 55:696–704. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ko JC, Wang TJ, Chang PY, Syu JJ, Chen JC,
Chen CY, Jian YT, Jian YJ, Zheng HY, Chen WC, et al: Minocycline
enhances mitomycin C-induced cytotoxicity through down-regulating
ERK1/2-mediated Rad51 expression in human non-small cell lung
cancer cells. Biochem Pharmacol. 97:331–340. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Deng C, Zhang P, Harper JW, Elledge SJ and
Leder P: Mice lacking p21CIP1/WAF1 undergo normal development, but
are defective in G1 checkpoint control. Cell. 82:675–684. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tashiro S, Walter J, Shinohara A, Kamada N
and Cremer T: Rad51 accumulation at sites of DNA damage and in
postreplicative chromatin. J Cell Biol. 150:283–291. 2000.
View Article : Google Scholar : PubMed/NCBI
|