Introduction
Osteosarcoma is a malignant tumor of mesenchymal
origin and primarily occurs in children, adolescents, and young
adults. This pleiomorphic tumor of the bone, based on animal model
systems (1), depends on new blood
vessel development, also known as angiogenesis, for tumor growth
and metastasis. Although modern multimodality treatment has
significantly improved tumor resectability and the long-term
outcome of these patients, 25–35% of patients with initially
non-metastatic disease subsequently develop metastasis and this
remains the major cause of death (2). At the same time, axial skeletal
osteosarcoma preliminarily responds poorly to chemotherapy and has
been proven to have an even more dismal prognosis (3). From the review of van Maldegem et
al (2) and Lagmay et al
(4), we concluded that in the past
two decades, published phase I/II clinical trials on chemotherapy
for osteosarcoma failed to make significant progress in refractory
cases. With the study of oncogenesis and pathobiological behavior
of osteosarcoma (1), we know that
new blood vessel formation (angiogenesis) is fundamental to tumor
growth, invasion, and metastatic dissemination.
Several groups have evaluated tumor micro-vessel
density and outcome in osteosarcoma (5–7).
Expression of VEGF has been suggested as a means of evaluating the
prognostic importance of angiogenesis in osteosarcoma (8). Monotherapy with second-generation
broad-spectrum VEGF receptor tyrosine kinase inhibitors (TKIs) in
sarcoma has now become an area of active research and application
beyond gastrointestinal stromal tumors (GISTs). Within all of those
preclinical experiments and clinical trials (6, 9–13), the
milestone of the treatment on advanced osteosarcoma should count on
the application of anti-angiogenesis TKIs sorafenib on refractory
cases from the Italian Sarcoma Group (13), which officially raised the 4-month
progression-free survival (PFS) from <30–46% for the first time.
However, things had seemed not to change as dramatically as was
expected since then. The main hurdle that researchers need to get
over should be sensitivity and drug-resistance (14).
The goals of this review are: a) to review
representative agents in in vitro and in vivo
experiments that showed promise for osteosarcoma based on
anti-angiogenesis therapy; b) to summarize the current phase I and
II trials of anti-angiogenensis therapies that have been explored
in advanced osteosarcoma patients; and c) to focus on targeting the
action towards VEGFR and to discuss current hurdles and future
perspectives.
Tumor angiogenesis and anti-angiogenesis
therapy in osteosarcoma
Tumor angiogenesis and optional
treatments
Angiogenesis is the process of new blood vessel
development, which is critical in both physiological development
and pathological processes, such as tumor progression, wound
healing, and cardiovascular, inflammatory, ischemic, and infectious
diseases (15). In response to
hypoxia, tumor tissues produce and release angiogenic growth
factors, such as vasculo-endothelial growth factor (VEGF), the
acidic and basic fibroblast growth factors (aFGF and bFGF), and the
platelet-derived endothelial cell growth factor (PD-ECGF) to
recruit new blood vessels by angiogenesis and vasculogenesis
(16). It is now widely accepted
that both mutations of oncogenes and tumor suppressor genes lead to
the switch into an angiogenic tumor. According to Gorlick et
al (1), osteosarcoma has
complex unbalanced karotypes and with alterations of the p53 and
retinoblastoma pathways in most cases, thus the vasculature playing
an intimate role in the progression of the pathologic development
of osteosarcoma.
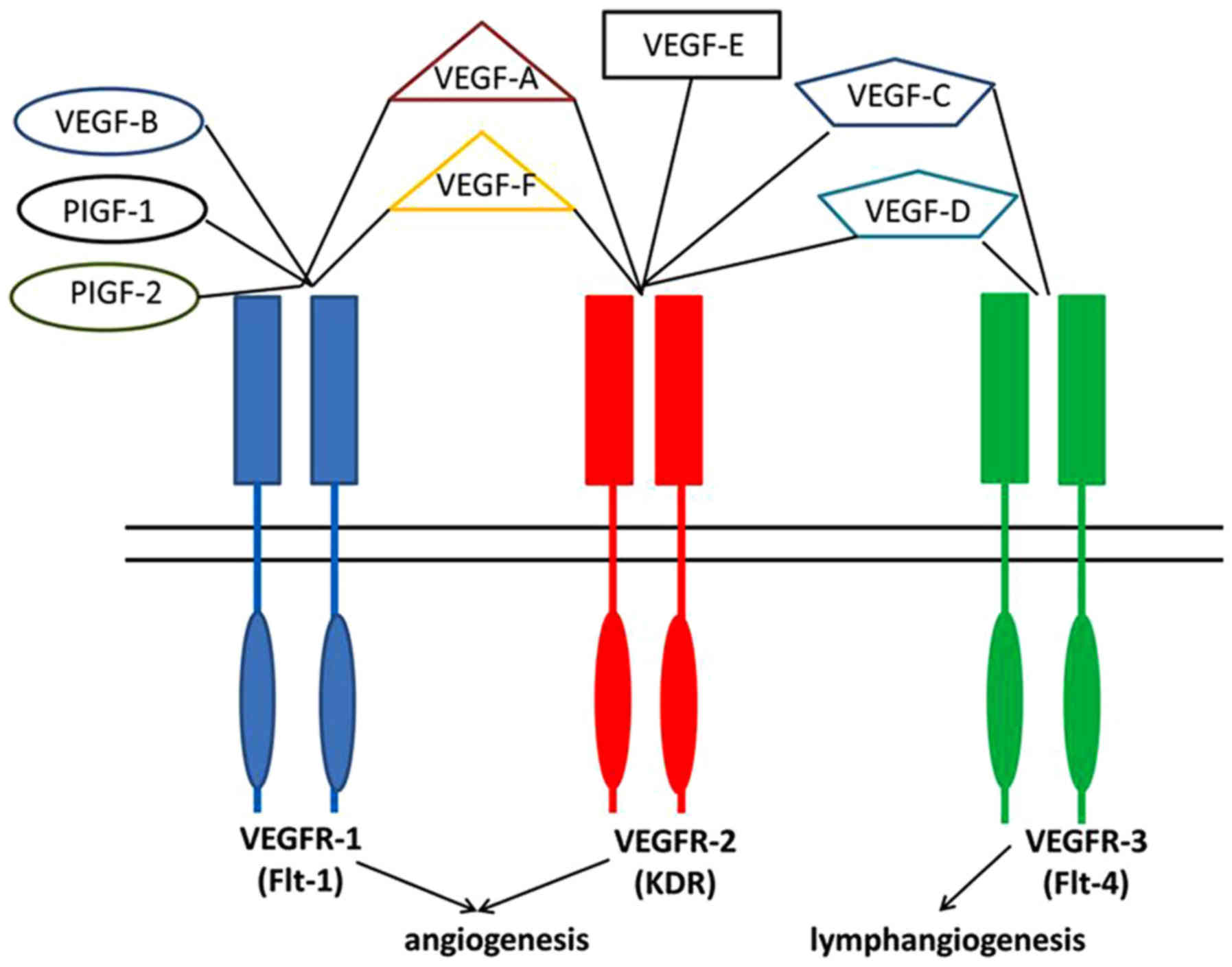
VEGF is a key tumor-derived angiogenic factor that
has multiple functions, including stimulation of angiogenesis,
vasculogenesis, inflammation, and vascular permeability, which
constitutes the most important signaling pathways in tumor
angiogenesis (7). According to Niu
et al (16), the whole VEGF
family has been identified to comprise 8 members with a common VEGF
homology domain: VEGF-A, VEGF-B, VEGF-C, VEGF-D, VEGF-E, VEGF-F,
and placenta growth factor (PIGF)-1 and −2. As shown in Fig. 1, VEGFs signal through 3 tyrosine
kinase receptors, known as Flt-1 (VEGFR-1), Flk-1/KDR (VEGFR-2),
and VEGFR-3 (17), which were
previously thought to be predominantly expressed by endothelial
cells, but in actual fact are also in sarcoma cell lines with
limited study (18–21). It has been reported that both
VEGFR-1 and −2 can promote angiogenesis and VEGFR-3 stimulation
leads to lymphangiogenesis (22).
There is a general consensus that VEGFR-2 is the
dominant receptor in mediating the pro-angiogenic functions of
VEGF-A and this pathway has been prioritized for the development of
antiangiogenic therapies (16,
23). Though VEGFR-1 has a 10-fold
higher binding affinity for VEGF-A, its activation has less impact
on the activation of intracellular signaling intermediates than
VEGFR-2 (23).
Recognition of the VEGF pathway as a key regulator
of angiogenesis has led to the development of several VEGF-targeted
agents, including agents that prevent VEGF-A binding to its
receptors (24), antibodies that
directly block VEGFR-2 (25), and
small molecules that inhibit the kinase activity of VEGFR-2 thereby
block growth factor signaling (26). Some of them have been approved by
the FDA of the US for clinical applications (16). Previous representative
anti-angiogenic compounds (10,27–39)
are summarized in Table I with
median inhibition concentration (IC50) noted for
comparison.
 | Table I.Summary of the mechanisms of action
of the anti-angiogenic compounds in preclinical experiments of
osteosarcoma. |
Table I.
Summary of the mechanisms of action
of the anti-angiogenic compounds in preclinical experiments of
osteosarcoma.
| Compound | Mechanism | Target
(IC50, nM) | Refs. |
|---|
| Endostatin | Internal fragment
of the carboxy-terminus of collagen XVIII | A broad-spectrum
endogenous antiangiogenic molecule | (62,74) |
| MMPs | A family of enzymes
that proteolytically degrade various components of the ECM | Non-specific | (74) |
| PEDF | A secreted
glycoprotein that is a non-inhibitory member of the serine protease
inhibitor | Non-specific | (47–52,74) |
| Bevacizumab | A humanized
anti-VEGF antibody |
VEGF-A(ED50 = 50 ng/ml) | (31,35,40,98,114–116) |
| Pegaptanib | An anti-VEGF RNA
aptamer | VEGF165
(0.75–1.4) | (66) |
| VEGF Trap
(Aflibercept) | A soluble receptor
to VEGF | VEGF-A and VEGF-B
(0.001), placental growth factor (0.045) | (67) |
| Sorafenib | TKIs | VEGFR-2 (90), Raf-1 (6), B-Raf (22), c-kit (68), FGFR-1 (580), FLT-3 (58) | (15,16,35,70,71,86) |
| Sunitinib | TKIs | VEGFR-1 (2), VEGFR-2 (80), VEGFR-3 (17), PDGFR-β (2) | (43,45,92) |
| Cediranib | TKIs | VEGFR-2 (<1),
VEGFR-1 (5), | (13,42) |
| Pazopanib | TKIs | VEGFR-3 (≤3),
VEGFR-1 (10), VEGFR-2 (30), VEGFR-3 (47), PDGFR-β (84), c-kit (140), FGFR (74), | (37–39,72) |
| Ramucirumab | A fully humanized
MAb targeting to the extracellular VEGF-binding domain of
VEGFR-2 | c-fms (146),
VEGFR-2 (0.05) | (73) |
| Dasatinib | TKIs | BCR/ABL (<1),
c-kit (79), Src (0.8) | (14) |
| Regorafenib | TKIs | VEGFR-1 (13), VEGFR-2 (4.2), VEGFR-3 (46), PDGFR-β (22), c-kit (7), RET (1.5), Raf-1 (2.5) | (74) |
| Everolimus | mTOR signaling
pathways | FKBP12
(1.6–2.4) | (15,87,124) |
| Imatinib | TKIs | v-Abl (600), c-kit
(100), PDGFR (100) | (54) |
Moreover, Broadhead et al (40–43)
repeatedly reported that pigment epithelium-derived factor (PEDF),
co-localized with VEGF in tumor tissue, was probably important in
the fine-tuning of tumor vasculature and aggression. However, the
clinical application of this agent is under investigation
(NCT00702494).
Fundamental study of angiogenesis in
osteosarcoma and other related cellular signaling pathways
Geller and Gorlick (44) reviewed HER-2 targeted treatment of
osteosarcoma. The results showed that HER-2 expression as a
prognostic factor in osteosarcoma remained controversial and a
comparison of the results is difficult because of variables,
including the handling and preparation of material, tissue
heterogeneity, fixation techniques, storage conditions, antibody
characteristics, scoring scheme, and staining interpretation due to
single-institution, retrospective studies that were limited in
size. Abdeen et al (45)
stated in 2009 that there was a negative correlation between
VEGFR-3 and both overall survival and event-free survival of
osteosarcoma, and VEGF-B was correlated with a poor histologic
response to chemotherapy. In 2011, Yang et al (46) reported that vascular endothelial
growth factor (VEGF) pathway genes collectively were amplified, and
alterations of this pathway were validated by fluorescence in
situ hybridization (FISH) and immunohistochemistry analyses in
58 formalin-fixed, paraffin-embedded osteosarcoma archival tissues
that had clinical follow-up information. Lammli et al
(47) in 2012 demonstrated that
there was a significant positive correlation between VEGF
expression and tumor stages among these cases (P<0.01). The data
also suggested a higher cancer recurrence and more frequent cases
of remote metastasis in the high-VEGF group compared to the
low-VEGF group. The expression of VEGF has been used as a more
objective means of evaluating the prognostic importance of
angiogenesis in osteosarcoma. One group found that 63% of
osteosarcoma samples demonstrated VEGF immunohistochemical staining
in tumor cells (8).
In 2013, Chen et al (48) completed a meta-analysis of published
studies and performed a systematic review to provide a
comprehensive assessment of the prognostic role of VEGF expression.
They included 12 studies with a total of 559 osteosarcoma patients
in the systematic review and meta-analysis. Compared with
osteosarcoma patients with low or negative VEGF expression,
patients with high VEGF expression were obviously associated with
lower disease-free survival (OR=0.25, 95% CI 0.11–0.58, P=0.001,
I2=56.4%). In addition, patients with high VEGF
expression were obviously associated with lower overall survival
(OR=0.22, 95% CI 0.13–0.35, P<0.001, I2=0.0 %).
Therefore, the findings from this systematic review suggested that
VEGF expression was an effective biomarker of prognosis in patients
with osteosarcoma. However, different from soft tissue sarcoma
(49), osteosarcoma has not been
classified by which subtypes of VEGFR expression correlate with
prognosis. Kampmann et al (49) reported in 2015 that the high
expression of VEGFR1-3 and PDGFR-β was significantly correlated
with higher grading (G2 vs. G3, P<0.05), and high VEGFR-2 was
significantly correlated with decreased patient survival
(P<0.001).
According to Aurby et al (50), angiogenesis inhibitors can be
divided into 2 classes: direct inhibitors and indirect inhibitors.
Direct inhibitors target endothelial cells by arresting
proliferation and migration of these cells or by inducing their
apoptosis. Indirect angiogenesis inhibitors act on the signaling
pathways induced by angiogenic stimuli, by sequestering the
angiogenic factors secreted by tumor cells, or by blocking the
signal transduction pathways that are activated when binding
factors meet their receptors on endothelial cells. The first direct
inhibitor was endostatin, which was an internal fragment of the
carboxy-terminus of collagen XVIII (51). It is a paradigm of a broad-spectrum
endogenous anti-angiogenic molecule, through which the results of
in vitro experiments are satisfactory. However, methods of
resolubilization gave very low yields of active proteins, which
makes it hard to be a mature pharmaceutical and obstructs its
further development. Bevacizumab (52) neutralizes all isoforms of human VEGF
and inhibits VEGF-induced proliferation of endothelial cells in
vitro with an ED50 of approximately 50 ng/ml. It was
tested in combination with several chemotherapeutic drugs, such as
doxorubicin, topotecan, paclitaxel, and docetaxel, showing an
additive antitumor effect (28,53,54).
However, as for osteosarcoma, clinical application did not prove as
effective as the experiments (53).
With innovation from the VEGF-A aptamer (55) to VEGF trap (56), more focus has been given to the
VEGFR tyrosine kinase inhibitors (TKIs) (7,16).
Protein kinases are key enzymes in the regulation of
various cellular processes that catalyse transfer of a phosphate
group from ATP to a hydroxyl group of a serine or a threonine.
Among the 90 identified genes encoding proteins with tyrosine
kinase activity, 58 encode receptors divided into 20 subfamilies
(57). Of these, EGFR/ErbB (class
I), the receptor for insulin (class II), PDGF (class III), FGF
(class IV), VEGF (class V), and HGF (MET, class VI) are strongly
associated with oncological diseases (58). Unlike bevacizumab, VEGF Trap, and
pegaptanib, which target extracellular VEGF, TKIs target the
intracellular signaling pathways of VEGF receptors as well as a
variety of receptors that rely on a tyrosine kinase component to
function properly, including PDGF receptor, FMS-like tyrosine
kinase 3 (FLT3), RAF, and c-KIT receptors (16). In Table
I, we summarize the classic TKIs compounds in preclinical
experiments for osteosarcoma and their main targeted region.
Sunitinib and sorafenib share a similar mechanism of action and
primarily target tumor angiogenesis by inhibiting a variety of
tyrosine kinases (36,59,60).
Pazopanib is an oral, second-generation multi-targeted tyrosine
kinase inhibitor targeting VEGF-1, −2, and −3 receptors, PDGF-α
and-β receptors, and c-kit, which exhibited good potency against
all of the human VEGFRs and closely related tyrosine receptor
kinases in vitro (30,31,61).
Besides TKIs, antibodies blocking VEGFR2 have also been developed
(62).
In addition to anti-angiogenesis drugs, there are
some other cellular signaling pathways that should be mentioned as
they are always used in combination with anti-angiogenesis target
drugs in clinical trials of osteosarcoma. A signal transduction
pathway through insulin-like growth factor (IGF) receptor
signaling, which is also an attractive therapeutic target for the
treatment of osteosarcoma, is the mammalian target of the rapamycin
(mTOR) pathway (63). mTOR
technically does not belong to anti-angiogenesis therapy according
to Hanahan et al (64).
Under conditions favorable for cell growth, mTOR activates
ribosomal protein translation (via S6K1) and cap-dependent
translation (via eIF4E), allowing G1 to S phase cell
cycle progression. This signal pathway was not originally activated
in most sarcoma patients (65), but
after using anti-angiogenesis TKIs for a while, many sarcomas show
secondary activated pathway, which makes this target as a
supplement to TKIs for long-term use (66). At the same time, the involvement of
the IGF/IGF1-R axis in tumorigenesis makes it an attractive target
for anticancer therapeutics, especially in Ewing's sarcoma
(65). A human IgG1 type monoclonal
antibody directed against the human IGF-IR, has been developed to
antagonize IGF-IR signaling (67,68).
Clinical trials of anti-angiogenesis of
osteosarcoma
Current status of second-line
chemotherapy for osteosarcoma
After failing standard first-line chemotherapy for
osteosarcoma, patients who relapse present a more challenging
treatment dilemma. In general, recurrence portends an extremely
poor long-term prognosis (1). In
some cases, through aggressive surgical resection of all gross
disease, patients can still acquire long-term survival (69,70).
The choice of second-line chemotherapy and the use of
investigational drugs are not standardized and the outcomes are
dismal (4). van Maldegem et
al (2) carried out a
comprehensive analysis of published phase I/II clinical trials
between 1990–2010 in osteosarcoma and Ewing's sarcoma, and it
turned out that the results were not convincing for benefit and
most of the time disappointing. From osteosarcoma trials they found
only 8% CR, 2.8% PR, and 4% SD. The phase II trials were mainly on
second-line chemo-drugs, which contained high-intensity
ifosfamide-based therapy with or without autologous peripheral
blood stem cell support transplantation, etoposide, topotecan,
epirubicin, and even cyclophosphamide. Lagmay et al
(4) reviewed the outcome of
patients with recurrent osteosarcoma enrolled in 7 phase II trials
through Children's Cancer Group, Pediatric Oncology Group, and
Children's Oncology Group and found that in each included trial,
the drugs tested were determined to be inactive on the basis of
radiographic response rates. The event-free survival for 96
patients with osteosarcoma with measurable disease was 12% at 4
months (95% CI, 6% to 19%), with treatments that included drugs
such as docetaxel, topotecan, irinotecan, rebeccamycin,
oxaliplatin, xabepilone, and even imatinib, aerosolized
granulocyte-macrophage colony-stimulating factor (GM-CSF) from 1997
to 2007 (4). From these data, we
can establish a baseline of the expected time for disease
progression in patients with relapsed osteosarcoma.
Phase I and II trials of target
therapy for osteosarcoma
In actual fact, it is difficult to carry out trials
in advanced osteosarcoma, mainly because of the problems of
recruiting enough eligible patients for the trials. In search of
phase I and II anti-angiogenesis trials of osteosarcoma, we
identified 35 phase I/II trials for osteogenic sarcoma that were
published between 2005 and 2016. Unfortunately, the phase I trials
for general sarcoma may not be complete because many trials do not
distinguish between different subtypes of osteogenic sarcoma.
However, different kinds of sarcomas have totally different
biological behaviors, such as osteosarcoma, Ewing's sarcoma, and
chondrosarcoma, which all originate from bone but show totally
different sensitivity to chemo-drugs. Thus, we will just focus on
the results of clinical trials that specialized in osteosarcoma so
as to make the comparison more meaningful. With an internet search
of MEDLINE, the Embase database, the Cochrane Central Register of
Controlled Trials database, the American Society of Clinical
Oncology (ASCO), and the European Society for Medical Oncology
(ESMO), we summarize the list of phase I and II trials in Tables II and III, which only include the data of
osteosarcoma and the clinical results (11–13,27–33,35–39,53,61,67,68,71–78).
 | Table II.Clinical results of phase I trial
with currently available anti-angiogenesis therapy on
osteosarcoma. |
Table II.
Clinical results of phase I trial
with currently available anti-angiogenesis therapy on
osteosarcoma.
| Drug | Targets | Combined with
chemotherapy | The first author's
surname | Year of
publication | Trial sponsor | Clinical
results | Refs. |
|---|
| Gefitinib | EGFR | No | Daw | 2005 | COG | 6/6 PD | (44) |
| Everolimus;
Figitumumab | mTOR; IGF-IR | No | Quek | 2010 | Novartis and
Pfizer | 3/3 SD | (87) |
| Cediranib | VEGFR1-3 | No | Fox | 2010 | NIH, NCI | 1/4 PR | (42) |
| R1507 | IGF-IR | No | Bagatell | 2010 | NIH | 2/3 SD | (46) |
| Sunitinib | VEGFR; PDGFR;
c-kit; Flt3, CSF-1 receptor, and RET | No | Dubios | 2011 | COG | 1/2 SD | (43) |
| Cixutumumab | IGF-IR | No | Malempati | 2012 | COG | 3/3 PD | (36) |
| Pazopanib | VEGFR1-3;
PDGFR | No | Bender | 2013 | COG | 1/4 SD | (39) |
| Sorafenib;
Bevacizumab | VEGFR-2, Raf-1,
B-Raf, c-kit, FGFR-1, FLT-3; VEGF-A | Low-dose
cyclophosphamide | Navid | 2013 | Novartis and
Pfizer | 2/2 SD | (35) |
 | Table III.Summary of Phase II trial results of
currently available anti-angiogenesis therapy for osteosarcoma. |
Table III.
Summary of Phase II trial results of
currently available anti-angiogenesis therapy for osteosarcoma.
| Drug | Combined with
chemotherapy | Stage | The first author's
last name | Year of
publication | Trial sponsor | No. of
patients | Clinical
outcome | Refs. |
|---|
| Sorafenib | No | Advanced | Grignani | 2011 | Italian Sarcoma
Group | 35 | 4 months-PFS 46%;
DR 4 months; ORR 14% | (16) |
| Trastuzumab | Cytotoxic
chemotherapy | Newly diagnosed,
high-grade metastatic | Ebb | 2012 | COG | 41 | 30 months-EFS 32%;
30 months-OS 50%; without significant difference comparing with
control group | (64) |
| Sirolimus |
Cyclo-phosphamide | Advanced | Schuetze | 2012 | Michigan
University | 5 | ORR 0%; 4
months-PFS 30% (combined with other sarcoma) | (82) |
| Cixutumumab and
temsirolimus | No | Advanced | Schwartz | 2013 | MSKCC fund | 24 | ORR 13%; median PFS
6 weeks | (85) |
| Cixutumumab | No | Advanced | Weigel | 2014 | COG | 11 | ORR 0%; 1/11 SD for
140 days | (36) |
| R1507 | No | Advanced | Pappo | 2014 | SARC | 38 | ORR 2.5%; DR: 12
weeks; 12 weeks-PFS 17% | (88) |
| Sorafenib;
Everolimus | No | Advanced | Grignani | 2015 | Italian Sarcoma
Group | 38 | 6 months-PFS 45%;
DR 5 months; ORR 10% | (15) |
| Cixutumumab;
Temsirolimus | No | Advanced | Wagner | 2015 | COG | 11 | ORR 0% | (79) |
| Dasatinib | No | Advanced | Schuetze | 2016 | SARC | 46 | ORR 6.5%; DR 5.7
months; 2 years-OS 15% | (14) |
Abundant active agents had been tested for
osteosarcoma patients in a small number for their availability.
Geller and Gorlick (44) proposed
the use of HER-2 directed therapy for a subset of patients with
osteosarcoma, which in theory was an appealing idea assuming HER-2
expression was in fact associated with poor prognosis, since
expression can be accurately and reproducibly identified. However,
a phase II clinical trial initiated by Children's Oncology Group of
trastuzumab, in addition to standard chemotherapy for patients with
newly diagnosed metastatic osteosarcoma (Table III), has proven to be without
significant difference when compared with the control group (30
months-EFS 32%; 30 months-OS 50%) (53). From the list of phase I trials
(Table II), although there is a
small number of patients, it seems to us that the EGFR inhibitor
and the antibody against type 1 insulin-like growth factor receptor
(IGF-IR) did not show similar activity in osteosarcoma as in
Ewing's sarcoma (67,68,74).
Most anti-angiogenesis TKIs can only keep the tumor
stable rather than make it obviously shrunken, while only
cediranib, which is a TKIs targeted particularly towards VEGFR2
(IC50 especially low, shown in Table I), had 1 refractory osteosarcoma
partial response (35). Another
phase I study that combined cediranib with gefitinib showed
antitumor activity in patients with advanced solid tumors,
including one osteosarcoma patient (37). Since we could not get detailed
information for this osteosarcoma patient, it was not included in
Table II. The authors also
demonstrated changes in VEGF and soluble VEGFR-2 levels following
treatment. These initial subgroup results, which were not as
responsive as we expected, might lead to many pharmaceutical
companies stopping further investigation of their phase II trials
for osteosarcoma. This may be why we seem to stagnate after the
sorafenib trial reported by Grignani et al (12,13).
As for phase II trials, the greatest progress
belonged to Italian Sarcoma Group, which held 2 cohort phase II
trials with advanced osteosarcoma patients with an object response
rate (ORR) of 14 and 10%, respectively (12,13).
Although the addition of everolimus did not obviously change the
response rate, the combination of sorafenib and everolimus
significantly prolonged the duration of response from 4 months in
sorafenib alone to 5 months, which has been stated to be the best
treatment results in the second-line drug therapy history of
osteosarcoma. However, this 45% 6-month PFS (combination therapy)
was less than the pre-specified threshold of activity (6 month PFS
of 50% or greater) to be deemed worthy of a phase III trial. In
addition, the toxic effects seemed to be more severe than sorafenib
alone (13). Children's Oncology
Group (COG) has conducted several phase II trials with cixutumumab
(68), which is the insulin-like
growth factor-I receptor (IGF-IR), since preclinical data suggested
that inhibition of the IGF-IR might constitute an important
therapeutic target in a variety of pediatric solid tumors,
including rhabdomyosarcoma, neuroblastoma and Wilms tumor. For
refractory solid tumors, there was only a sub-group analysis for
osteosarcoma with the number of patients 11 in both the cixutumumab
single-drug trial (67), and in
combination with cixutumumab and temsirolimus in a trial (68). However, for pediatric advanced
osteosarcoma, the ORR was 0% for both of the trials. With PFS and
OS data unextractable in both of these trials, only 1 patient using
cixutumumab alone had stable disease for 140 days (67). In 2013, Memorial Sloan-Kettering
Cancer Center (MSKCC) proceeded with a similar trial with a
combination of cixutumumab and temsirolimus on refractory
osteosarcoma, which showed an ORR of 13% with a median PFS of 6
weeks (74). The number of patients
was 24, which showed a little more than the same trial conducted by
COG of 11 (68). MSKCC included
patients older than 16 years while COG included all solid tumor
patients aged from 1–30 years. Due to the small sample size, we
could not speculate why their results were so different. This
combination therapy may need further study with a larger sample
size in a randomized controlled trial to identify its
effectiveness. The Sarcoma Alliance for Research Through
Collaboration Study has also completed 2 trials on advanced
osteosarcoma, which were respectively IGF-IR R1507 and TKI
dasatinib (11,77). R1507 did not seem to be effective
with an ORR of 2.5% and 12-week-PFS of 17%, while dasatinib showed
an ORR of 6.5% and duration of response of 5.7 months, which
indicated that BCR/ABL, c-kit, and src might not be the target for
osteosarcoma. The Bayesian design allowed for the early termination
of accrual in osteosarcoma subtypes because of the lack of drug
activity (11).
Moving forward, what stops us
Resistance
For advanced osteosarcoma patients, drug sensitivity
is pivotal at the beginning of therapy to help patients to
establish the confidence to continue using it; however, from the
observation through phase I trials, these TKIs hardly seemed to
reduce the tumor size, which usually stopped investigators to open
a phase II trial to explore the activity towards osteosarcoma. From
the perspective of Versleijen-Jonkers et al (6), unlike chemotherapeutic agents,
angiogenesis inhibitors slow or stop tumor growth rather than cause
tumor shrinkage.
Up to now there was no direct evidence on which kind
of TKIs have more potency to be sensitive, but we may boldly
speculate that cediranib (35),
which is the only drug that made refractory osteosarcoma smaller in
size in a phase I trial, might be more sensitive than other TKIs
for its low IC50 value towards VEGFR, especially
VEGFR-2. There is a general consensus that VEGFR-2 is the dominant
receptor in mediating the pro-angiogenic functions of VEGF-A, and
this pathway has been prioritized for the development of
anti-angiogenic therapies (16).
Clinical trial expression analysis of different subtypes of
tyrosine kinases as predictive biomarkers is still not a standard
approach. Furthermore, only limited studies have investigated the
expression of different subtypes of tyrosine kinase receptors on
the protein level, especially on osteosarcoma (45–47,79–82).
We do not know what kinds of TKIs showed more sensitivity and what
kind of TKIs have more long-term lasting effectiveness based on
those clinical trials. We may need to focus more on the
IC50 values of specific subtypes of VEGFR and carry out
more detailed work to choose appropriate targets for clinical
use.
Twenty-five TKIs are currently FDA-approved and
>130 are being evaluated in clinical trials (14). Increasing evidence suggests that
drug exposure of TKIs may significantly contribute to drug
resistance independently of somatic variation of TKI target genes.
Membrane transport proteins may limit the amount of TKI reaching
the target cells. In the early study of sunitinib on solid tumors,
a decrease in the expression level of soluble VEGFR has been
consistently reported (83).
Conversely, an increased level of VEGF seems to occur and may have
a role in the flare-up of tumor growth that may occur after
sunitinib discontinuation (83). In
addition, activation of alternative signaling pathways may overcome
VEGFR inhibition. According to Loges et al (84), this reality depends on the
mechanisms of refractoriness and evasive escape and the lack of
well-validated biomarkers to monitor efficacy as well as optimal
dosing or predict toxicity or resistance to VEGF-targeted therapy.
Mutation of VEGFR/PDGFR or altered receptors or polymorphisms may
also have a role in the resistance to anti-VEGF/VEGFR therapy
(85). The resistance of this
peripheral rim of viable tumor cells may be overcome by combination
TKIs with targeted agents directed against kinases, such as mTOR,
mitogen-activated protein kinases (MAPKs), and protein kinase C
(PKC) or the addition of cytotoxic drugs to destroy sub-clones
evading multi-targeted agents (65).
In addition to morphological differences, tumor
endothelial cells have distinct gene expression profiles, which may
also contribute to the resistance to antiangiogenic treatment
strategies (6). Furthermore, the
heterogeneity of cancer has often been a subject of interest and
concern. DNA sequencing in a single tumor biopsy of 1 patient was
not uniformly detectable throughout the sampling region (86). Such dynamic genomic changes in
cancer cells are also expected to induce resistance in response to
anti-angiogenesis drugs (87). The
above explains why sorafenib only has the duration of response of 4
months while a combination with everolimus makes it 5 months
(12,13), which does not yet seems to be
satisfactory for advanced patients. From these mechanisms, we can
expect that combination therapy or sequencing therapy may benefit
more people, which will be discussed later.
Toxicity
Compared with conventional chemotherapy, the
toxicity and side effects of anti-angiogenesis TKIs are mild and
can be tolerated by most heavily pre-treated advanced osteosarcoma
patients. The most common toxicities are hypertension (grade 3 in
approximately 10% patients), hand and foot syndrome, fatigue,
proteinuria (usually grade 1–2), hemorrhage, arterial and venous
thrombotic events (88), impaired
wound healing, and occasionally gastrointestinal perforation
(89), which was mostly observed in
the initial phase II bevacizumab trials in ovarian cancer. VEGFR
TKIs have also been associated with clinical hypothyroidism, which
could be caused by the inhibition of iodine uptake in the thyroid
(90). Some of those syndromes were
reported to be related with better response and can be relieved
gradually after months of therapy (88). However, combination therapy with
multiple anti-angiogensis agents were considered to have more
severe toxicity than some patients could tolerate (12). How to manage toxicity and efficiency
is still a problem.
Evaluation systems
In addition to the challenge of identifying the most
promising agents for clinical trials in osteosarcoma, obstacles
inherent to this disease further complicate the successful design
and completion of trials. In evaluating the efficacy of all the
trials we have mentioned, the standard approach is to use imaging
response criteria, such as response evaluation criteria in solid
tumors 1.1 (RECIST 1.1) to compare the size and/or volume of lesion
pretreatment and at regular intervals post-treatment (91). For a patient eligible for a trial
using this approach, he or she must have measurable disease.
However, as we mentioned before, angiogenesis inhibitors slowed or
stopped tumor growth rather than causing the tumor's shrinkage
(16).
Referring to most successful TKI therapy examples,
such as renal cell carcinoma, GIST, or even soft tissue sarcoma,
and considering with the characteristics of anti-angiogenesis TKIs,
various new clinical evaluation systems have turned up, such as
Choi (2009) (92), mChoi (2010)
(93), SACT (2010) (94), and MASS (2010) (94). Nevertheless we cannot
indiscriminately copy this evaluation. Because for unresectable
primary osteosarcoma, which is located at the axial skeleton, for
example, lesions mainly manifest as a bone lesion combined with or
without soft tissue mass, which may not be evaluable according to
these criteria. The lesion's shrinkage is originally not so obvious
as other solid tumors. Besides, osteosarcoma is an osteogenic tumor
that should not be evaluated by the M.D. Anderson system (95), which was developed to evaluate
metastatic osteolytic lesions. All the above makes it even more
complicated to assess the condition.
There are a few potential biomarkers in the blood
that can be used to determine in vivo efficacy of
anti-angiogenic treatment, i.e., VEGF-A, VEGF-B, and PIGF (96), circulating endothelial cells
(97,98), and even neutrophil-to-lymphocyte
ratio (99). However, these
biomarkers need to be studied further before they can be used in
the clinic. In addition, functional imaging might be beneficial for
evaluation, such as dynamic contrast-enhanced magnetic resonance
imaging (DCE-MRI) (100), positron
emission computed tomography (PET/CT) (101), and so on, which are under study
and could make the prediction and monitoring of response more
sensitive and ultimately lead to personalized anti-angiogenic
treatment.
Strategy for advanced osteosarcoma in the
era of targeted therapy
With the development of precision medicine,
tremendous improvement has happened to traditional pathology. From
the linking genomic and immunotherapy approaches to molecular
subtype theory of Lim et al (102), we got to know that osteosarcoma
has a more increased mutation burden than Ewing's sarcoma or
synovial sarcoma, which is why it has not benefited from
comprehensive molecular profiling (103,104). Gerlinger et al (86) proposed the theory of intratumor
heterogeneity and branched evolution of tumor cells in 2012, which
made it even more difficult for the targeted therapy to maintain a
long-term effect. Facing the intratumor heterogeneity at the
genomic, epigenomic, and micro-environmental levels, the question
is what is the optimal therapeutic option for these refractory
groups.
Combination therapy, what we can
do
The combination of anti-angiogenesis drugs with
chemotherapy has been proposed for quite a while and has been
verified in clinical trials of osteosarcoma with unfavorable
results (53,71) (Table
III). It is a sound deduction that a treatment aimed at
reducing the blood supply of a tumor would also reduce the delivery
of any other therapy, such as chemotherapy, which is also important
for radiotherapy for anti-angiogenesis agents and may reduce the
oxygen supply necessary for a response to radiotherapy (16). However, synergism of
anti-angiogenetics and chemotherapeutics has been observed in
patients with colon cancers (105), non-small cell lung cancers
(106), and breast cancers
(107,108). One explanation is that with the
blockage of VEGF signaling, anti-angiogenetics induces a
normalization of newly formed vessels, and thus, reduces the
interstitial tissue pressure (ITP) within tumors, allowing enhanced
delivery of chemotherapy to the tumors (7). However, advanced osteosarcoma usually
has shown resistance to conventional chemotherapy. Second-line
chemotherapy did not have much of an effect on these tumors, which
makes the combination therapy not reasonable.
From the experience of Grignani et al
(12), the multi-targeted approach
with TKIs or a combination of different pathway inhibitors seemed
to have advantages in synergistic therapeutic effect and to
overcome drug resistance. A disadvantage of using multi-targeted
agents was that it might increase the toxicity and be difficult to
determine which particular kinase inhibition results in an
antitumor effect. Anyway, the prolonged time seemed not to be long
enough to continue with a phase III trial (12). How to pick the appropriate drugs for
combination therapy is still pending.
Sequencing therapy, timing, and
strategy
Sequencing TKI therapy is a new concept for
osteosarcoma. However, as for renal cell carcinoma (RCC) (109) and non-small cell lung cancer
(110), it has been under
discussion for a long time. At present, there is a strong rationale
for sequencing targeted therapy for metastatic clear cell renal
cancer (111), but the timing of
the switch and the best agent to switch to remains unclear.
Sunitinib and pazopanib are approved treatments in first-line
therapy for patients with favorable or intermediate-risk clear cell
RCC (112). Temsirolimus has been
proven to be beneficial over interferon-α (IFN-α) in patients with
non-clear cell RCC (non-ccRCC) (113). Until recently, with regard to
choosing the second-line treatment after the failure of therapy
with VEGFR-TKIs, the continued inhibition of the VEGF/VEGR pathway
or the switch to a mTOR inhibitor was controversial (114). These two options are characterized
by partly different targets with completely different toxicity, but
a comparable efficacy. This scenario changed dramatically, after
the publication of 2 randomized, controlled, phase III trials, in
which cabozantinib (115) and
nivolumab (116) proved to be
superior compared to everolimus. Regarding third-line treatment,
where a sequence of 2 VEGFR-TKIs has been used beforehand, the
choice is represented by the mTOR inhibitor everolimus, while if a
VEGFR-TKI followed by everolimus is chosen, a return to VEGF
pathway inhibition is suggested (112), which indicates the activation of
different pathways might change during sequencing therapy and using
targeted therapy back and forth may benefit drug-resistance
patients and prolong survival. In the perspective of Maute et
al (117), possible sequences
include TKI-mTOR-TKI or TKI-TKI-mTOR with the upcoming checkpoint
inhibitors in perspective, which might establish a new standard of
care after previous TKI therapy.
However, for GIST, long-term follow-up results of
the B2222 study and updated results of the BFR14 trial demonstrate
that continuous imatinib treatment in patients with advanced GIST
is associated with reduced risk of progression (118). For patients progressing on or
intolerant of imatinib, continuing therapy with TKIs sunitinib
followed by regorafenib is recommended (118), which seems to us a totally
different strategy. For non-small cell lung cancer (NSCLC) therapy,
the reversible epidermal growth factor receptor (EGFR) TKIs
gefitinib and erlotinib have been proven to be the first-line
therapy for NSCLC harboring activating EGFR mutations (119). Acquired resistance to EGFR TKIs is
mainly mediated through 3 pathways: 1) activated EGFR family
proteins and ligands, 2) activated various growth factor receptors,
and 3) activated downstream signaling molecules (110). To explore the various proposed
mechanisms of acquired resistance to EGFR-TKI therapy, agents that
target secondary driving gene mutation as well as signaling
pathways downstream of EGFR are being studied in molecularly
selected advanced NSCLC (110),
which, in a certain sense, formulates a more logical therapeutic
strategy for advanced solid tumors. A degree of cross-resistance
appears to exist between all of these current agents and has
resulted in a drive toward the development of new therapies with
novel modes of action (14).
Conclusion
For advanced osteosarcoma, due to its increased
mutation burden and intratumor heterogeneity, therapy based on
comprehensive molecular profiling has not been successfully proven.
At present, anti-angiogenesis TKIs showed promising initial results
for this group of patients compared to other second-line
chemotherapy, but the results are still not satisfactory. Based on
the limited options of effective agents, the algorithm of choosing
optimal target drugs is still understudied. The anti-angiogenesis
TKIs therapy of other solid tumors may shed light on the treatment
for advanced OS.
Acknowledgements
We thank Dr Carola A.S. Arndt of Mayo Clinic
Pediatric Oncology for her professional advice for the modification
of this study.
References
|
1
|
Gorlick R, Anderson P, Andrulis I, Arndt
C, Beardsley GP, Bernstein M, Bridge J, Cheung NK, Dome JS, Ebb D,
et al: Biology of childhood osteogenic sarcoma and potential
targets for therapeutic development: meeting summary. Clin Cancer
Res. 9:5442–5453. 2003.PubMed/NCBI
|
|
2
|
van Maldegem AM, Bhosale A, Gelderblom HJ,
Hogendoorn PC and Hassan AB: Comprehensive analysis of published
phase I/II clinical trials between 1990–2010 in osteosarcoma and
Ewing sarcoma confirms limited outcomes and need for translational
investment. Clin Sarcoma Res. 2:52012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dickerson ME, Page RL, LaDue TA, Hauck ML,
Thrall DE, Stebbins ME and Price GS: Retrospective analysis of
axial skeleton osteosarcoma in 22 large-breed dogs. J Vet Intern
Med. 15:120–124. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lagmay JP, Krailo MD, Dang H, Kim A,
Hawkins DS, Beaty O III, Widemann BC, Zwerdling T, Bomgaars L,
Langevin AM, et al: Outcome of patients with recurrent osteosarcoma
enrolled in seven phase II trials through children's cancer group,
pediatric oncology group, and children's oncology group: Learning
from the past to move forward. J Clin Oncol. 34:3031–3038. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
MacGabhann F, Qutub AA, Annex BH and Popel
AS: Systems biology of pro-angiogenic therapies targeting the VEGF
system. Wiley Interdiscip Rev Syst Biol Med. 2:694–707. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Versleijen-Jonkers YM, Vlenterie M, van de
Luijtgaarden AC and van der Graaf WT: Anti-angiogenic therapy, a
new player in the field of sarcoma treatment. Crit Rev Oncol
Hematol. 91:172–185. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Quan GM and Choong PF: Anti-angiogenic
therapy for osteosarcoma. Cancer Metastasis Rev. 25:707–713. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
DuBois S and Demetri G: Markers of
angiogenesis and clinical features in patients with sarcoma.
Cancer. 109:813–819. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Segal E, Pan H, Ofek P, Udagawa T,
Kopecková P, Kopecek J and Satchi-Fainaro R: Targeting
angiogenesis-dependent calcified neoplasms using combined polymer
therapeutics. PLoS One. 4:e52332009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
van Cruijsen H, Voest EE, Punt CJ, Hoekman
K, Witteveen PO, Meijerink MR, Puchalski TA, Robertson J, Saunders
O, Jürgensmeier JM, et al: Phase I evaluation of cediranib, a
selective VEGFR signalling inhibitor, in combination with gefitinib
in patients with advanced tumours. Eur J Cancer. 46:901–911. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schuetze SM, Wathen JK, Lucas DR, Choy E,
Samuels BL, Staddon AP, Ganjoo KN, von Mehren M, Chow WA, Loeb DM,
et al: SARC009: Phase 2 study of dasatinib in patients with
previously treated, high-grade, advanced sarcoma. Cancer.
122:868–874. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Grignani G, Palmerini E, Ferraresi V,
D'Ambrosio L, Bertulli R, Asaftei SD, Tamburini A, Pignochino Y,
Sangiolo D, Marchesi E, et al: Italian Sarcoma Group: Sorafenib and
everolimus for patients with unresectable high-grade osteosarcoma
progressing after standard treatment: A non-randomised phase 2
clinical trial. Lancet Oncol. 16:98–107. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Grignani G, Palmerini E, Dileo P, Asaftei
SD, D'Ambrosio L, Pignochino Y, Mercuri M, Picci P, Fagioli F,
Casali PG, et al: A phase II trial of sorafenib in relapsed and
unresectable high-grade osteosarcoma after failure of standard
multimodal therapy: an Italian Sarcoma Group study. Ann Oncol.
23:508–516. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Neul C, Schaeffeler E, Sparreboom A,
Laufer S, Schwab M and Nies AT: Impact of membrane drug
transporters on resistance to small-molecule tyrosine kinase
inhibitors. Trends Pharmacol Sci. 37:904–932. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Carmeliet P: Angiogenesis in life, disease
and medicine. Nature. 438:932–936. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Niu G and Chen X: Vascular endothelial
growth factor as an anti-angiogenic target for cancer therapy. Curr
Drug Targets. 11:1000–1017. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Steeghs N, Nortier JW and Gelderblom H:
Small molecule tyrosine kinase inhibitors in the treatment of solid
tumors: An update of recent developments. Ann Surg Oncol.
14:942–953. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kuhnen C, Lehnhardt M, Tolnay E,
Muehlberger T, Vogt PM and Müller KM: Patterns of expression and
secretion of vascular endothelial growth factor in malignant
soft-tissue tumours. J Cancer Res Clin Oncol. 126:219–225. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Potti A, Ganti AK, Tendulkar K, Sholes K,
Chitajallu S, Koch M and Kargas S: Determination of vascular
endothelial growth factor (VEGF) overexpression in soft tissue
sarcomas and the role of overexpression in leiomyosarcoma. J Cancer
Res Clin Oncol. 130:52–56. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fuchs B, Inwards CY and Janknecht R:
Vascular endothelial growth factor expression is up-regulated by
EWS-ETS oncoproteins and Sp1 and may represent an independent
predictor of survival in Ewing's sarcoma. Clin Cancer Res.
10:1344–1353. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gee MF, Tsuchida R, Eichler-Jonsson C, Das
B, Baruchel S and Malkin D: Vascular endothelial growth factor acts
in an autocrine manner in rhabdomyosarcoma cell lines and can be
inhibited with all-trans-retinoic acid. Oncogene. 24:8025–8037.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Su JL, Yen CJ, Chen PS, Chuang SE, Hong
CC, Kuo IH, Chen HY, Hung MC and Kuo ML: The role of the
VEGF-C/VEGFR-3 axis in cancer progression. Br J Cancer. 96:541–545.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Waltenberger J, Claesson-Welsh L, Siegbahn
A, Shibuya M and Heldin CH: Different signal transduction
properties of KDR and Flt1, two receptors for vascular endothelial
growth factor. J Biol Chem. 269:26988–26995. 1994.PubMed/NCBI
|
|
24
|
Agulnik M, Yarber JL, Okuno SH, von Mehren
M, Jovanovic BD, Brockstein BE, Evens AM and Benjamin RS: An
open-label, multicenter, phase II study of bevacizumab for the
treatment of angiosarcoma and epithelioid hemangioendotheliomas.
Ann Oncol. 24:257–263. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Watanabe H, Mamelak AJ, Wang B, Howell BG,
Freed I, Esche C, Nakayama M, Nagasaki G, Hicklin DJ, Kerbel RS, et
al: Anti-vascular endothelial growth factor receptor-2 (Flk-1/KDR)
antibody suppresses contact hypersensitivity. Exp Dermatol.
13:671–681. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ciardiello F, Caputo R, Damiano V, Caputo
R, Troiani T, Vitagliano D, Carlomagno F, Veneziani BM, Fontanini
G, Bianco AR, et al: Antitumor effects of ZD6474, a small molecule
vascular endothelial growth factor receptor tyrosine kinase
inhibitor, with additional activity against epidermal growth factor
receptor tyrosine kinase. Clin Cancer Res. 9:1546–1556.
2003.PubMed/NCBI
|
|
27
|
Trippett TM, Herzog C, Whitlock JA, Wolff
J, Kuttesch J, Bagatell R, Hunger SP, Boklan J, Smith AA, Arceci
RJ, et al: Phase I and pharmacokinetic study of cetuximab and
irinotecan in children with refractory solid tumors: A study of the
pediatric oncology experimental therapeutic investigators'
consortium. J Clin Oncol. 27:5102–5108. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Navid F, Baker SD, McCarville MB, Stewart
CF, Billups CA, Wu J, Davidoff AM, Spunt SL, Furman WL, McGregor
LM, et al: Phase I and clinical pharmacology study of bevacizumab,
sorafenib, and low-dose cyclophosphamide in children and young
adults with refractory/recurrent solid tumors. Clin Cancer Res.
19:236–246. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Malempati S, Weigel B, Ingle AM, Ahern CH,
Carroll JM, Roberts CT, Reid JM, Schmechel S, Voss SD, Cho SY, et
al: Phase I/II trial and pharmacokinetic study of cixutumumab in
pediatric patients with refractory solid tumors and Ewing sarcoma:
A report from the Children's Oncology Group. J Clin Oncol.
30:256–262. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kerklaan BM, Lolkema MP, Devriese LA,
Voest EE, Nol-Boekel A, Mergui-Roelvink M, Langenberg M, Mykulowycz
K, Stoebenau J, Lane S, et al: Phase I and pharmacological study of
pazopanib in combination with oral topotecan in patients with
advanced solid tumours. Br J Cancer. 113:706–715. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Inada-Inoue M, Ando Y, Kawada K, Mitsuma
A, Sawaki M, Yokoyama T, Sunakawa Y, Ishida H, Araki K, Yamashita
K, et al: Phase 1 study of pazopanib alone or combined with
lapatinib in Japanese patients with solid tumors. Cancer Chemother
Pharmacol. 73:673–683. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bender Glade JL, Lee A, Reid JM, Baruchel
S, Roberts T, Voss SD, Wu B, Ahern CH, Ingle AM, Harris P, et al:
Phase I pharmacokinetic and pharmacodynamic study of pazopanib in
children with soft tissue sarcoma and other refractory solid
tumors: A children's oncology group phase I consortium report. J
Clin Oncol. 31:3034–3043. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bender Glade JL, Adamson PC, Reid JM, Xu
L, Baruchel S, Shaked Y, Kerbel RS, Cooney-Qualter EM, Stempak D,
Chen HX, et al: Children's Oncology Group Study: Phase I trial and
pharmacokinetic study of bevacizumab in pediatric patients with
refractory solid tumors: A Children's Oncology Group Study. J Clin
Oncol. 26:399–405. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Freeman BB III, Daw NC, Geyer JR, Furman
WL and Stewart CF: Evaluation of gefitinib for treatment of
refractory solid tumors and central nervous system malignancies in
pediatric patients. Cancer Invest. 24:310–317. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fox E, Aplenc R, Bagatell R, Chuk MK,
Dombi E, Goodspeed W, Goodwin A, Kromplewski M, Jayaprakash N,
Marotti M, et al: A phase 1 trial and pharmacokinetic study of
cediranib, an orally bioavailable pan-vascular endothelial growth
factor receptor inhibitor, in children and adolescents with
refractory solid tumors. J Clin Oncol. 28:5174–5181. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dubois SG, Shusterman S, Ingle AM, Ahern
CH, Reid JM, Wu B, Baruchel S, Glade-Bender J, Ivy P, Grier HE, et
al: Phase I and pharmacokinetic study of sunitinib in pediatric
patients with refractory solid tumors: a children's oncology group
study. Clin Cancer Res. 17:5113–5122. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Daw NC, Furman WL, Stewart CF, Iacono LC,
Krailo M, Bernstein ML, Dancey JE, Speights RA, Blaney SM, Croop
JM, et al: Children's Oncology Group: Phase I and pharmacokinetic
study of gefitinib in children with refractory solid tumors: A
Children's Oncology Group Study. J Clin Oncol. 23:6172–6180. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Brell JM, Krishnamurthi SS, Rath L, Bokar
JA, Savvides P, Gibbons J, Cooney MM, Meropol NJ, Ivy P and Dowlati
A: Phase I trial of sunitinib and gemcitabine in patients with
advanced solid tumors. Cancer Chemother Pharmacol. 70:547–553.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bagatell R, Herzog CE, Trippett TM, Grippo
JF, Cirrincione-Dall G, Fox E, Macy M, Bish J, Whitcomb P, Aikin A,
et al: Pharmacokinetically guided phase 1 trial of the IGF-1
receptor antagonist RG1507 in children with recurrent or refractory
solid tumors. Clin Cancer Res. 17:611–619. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Broadhead ML, Choong PF and Dass CR:
Efficacy of continuously administered PEDF-derived synthetic
peptides against osteosarcoma growth and metastasis. J Biomed
Biotechnol. 2012:2302982012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Takenaka K, Yamagishi S, Jinnouchi Y,
Nakamura K, Matsui T and Imaizumi T: Pigment epithelium-derived
factor (PEDF)-induced apoptosis and inhibition of vascular
endothelial growth factor (VEGF) expression in MG63 human
osteosarcoma cells. Life Sci. 77:3231–3241. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ek ET, Dass CR, Contreras KG and Choong
PF: PEDF-derived synthetic peptides exhibit antitumor activity in
an orthotopic model of human osteosarcoma. J Orthop Res.
25:1671–1680. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Dass CR, Ek ET and Choong PF: PEDF as an
emerging therapeutic candidate for osteosarcoma. Curr Cancer Drug
Targets. 8:683–690. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Geller DS and Gorlick R: HER-2 targeted
treatment of osteosarcoma: The challenges of developing targeted
therapy and prognostic factors for rare malignancies. Expert Opin
Pharmacother. 11:51–61. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Abdeen A, Chou AJ, Healey JH, Khanna C,
Osborne TS, Hewitt SM, Kim M, Wang D, Moody K and Gorlick R:
Correlation between clinical outcome and growth factor pathway
expression in osteogenic sarcoma. Cancer. 115:5243–5250. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yang J, Yang D, Sun Y, Sun B, Wang G,
Trent JC, Araujo DM, Chen K and Zhang W: Genetic amplification of
the vascular endothelial growth factor (VEGF) pathway genes,
including VEGFA, in human osteosarcoma. Cancer. 117:4925–4938.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lammli J, Fan M, Rosenthal HG, Patni M,
Rinehart E, Vergara G, Ablah E, Wooley PH, Lucas G and Yang SY:
Expression of vascular endothelial growth factor correlates with
the advance of clinical osteosarcoma. Int Orthop. 36:2307–2313.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Chen D, Zhang YJ, Zhu KW and Wang WC: A
systematic review of vascular endothelial growth factor expression
as a biomarker of prognosis in patients with osteosarcoma. Tumour
Biol. 34:1895–1899. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kampmann E, Altendorf-Hofmann A, Gibis S,
Lindner LH, Issels R, Kirchner T and Knösel T: VEGFR2 predicts
decreased patients survival in soft tissue sarcomas. Pathol Res
Pract. 211:726–730. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Aubry K, Barriere G, Chable-Rabinovitch H,
Dutour A, Paraf F, Monteil J and Rigaud M: Molecular mechanisms
regulating the angiogenic phenotype in tumors: Clinical impact in
the future. Anticancer Res. 27:3111–3119. 2007.PubMed/NCBI
|
|
51
|
O'Reilly MS, Boehm T, Shing Y, Fukai N,
Vasios G, Lane WS, Flynn E, Birkhead JR, Olsen BR and Folkman J:
Endostatin: An endogenous inhibitor of angiogenesis and tumor
growth. Cell. 88:277–285. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Nalluri SR, Chu D, Keresztes R, Zhu X and
Wu S: Risk of venous thromboembolism with the angiogenesis
inhibitor bevacizumab in cancer patients: A meta-analysis. JAMA.
300:2277–2285. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ebb D, Meyers P, Grier H, Bernstein M,
Gorlick R, Lipshultz SE, Krailo M, Devidas M, Barkauskas DA, Siegal
GP, et al: Phase II trial of trastuzumab in combination with
cytotoxic chemotherapy for treatment of metastatic osteosarcoma
with human epidermal growth factor receptor 2 overexpression: A
report from the Children's Oncology Group. J Clin Oncol.
30:2545–2551. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Wildiers H, Guetens G, De Boeck G,
Verbeken E, Landuyt B, Landuyt W, de Bruijn EA and van Oosterom AT:
Effect of antivascular endothelial growth factor treatment on the
intratumoral uptake of CPT-11. Br J Cancer. 88:1979–1986. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
White RR, Sullenger BA and Rusconi CP:
Developing aptamers into therapeutics. J Clin Invest. 106:929–934.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Holash J, Davis S, Papadopoulos N, Croll
SD, Ho L, Russell M, Boland P, Leidich R, Hylton D, Burova E, et
al: VEGF-Trap: A VEGF blocker with potent antitumor effects. Proc
Natl Acad Sci USA. 99:11393–11398. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Blume-Jensen P and Hunter T: Oncogenic
kinase signalling. Nature. 411:355–365. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Ségaliny AI, Tellez-Gabriel M, Heymann MF
and Heymann D: Receptor tyrosine kinases: Characterisation,
mechanism of action and therapeutic interests for bone cancers. J
Bone Oncol. 4:1–12. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Pignochino Y, Grignani G, Cavalloni G,
Motta M, Tapparo M, Bruno S, Bottos A, Gammaitoni L, Migliardi G,
Camussi G, et al: Sorafenib blocks tumour growth, angiogenesis and
metastatic potential in preclinical models of osteosarcoma through
a mechanism potentially involving the inhibition of ERK1/2, MCL-1
and ezrin pathways. Mol Cancer. 8:1182009. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Wolfesberger B, Tonar Z, Gerner W,
Skalicky M, Heiduschka G, Egerbacher M, Thalhammer JG and Walter I:
The tyrosine kinase inhibitor sorafenib decreases cell number and
induces apoptosis in a canine osteosarcoma cell line. Res Vet Sci.
88:94–100. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Safwat A, Boysen A, Lücke A and Rossen P:
Pazopanib in metastatic osteosarcoma: Significant clinical response
in three consecutive patients. Acta Oncol. 53:1451–1454. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Lu D, Jimenez X, Zhang H, Bohlen P, Witte
L and Zhu Z: Selection of high affinity human neutralizing
antibodies to VEGFR2 from a large antibody phage display library
for antiangiogenesis therapy. Int J Cancer. 97:393–399. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
O'Day K and Gorlick R: Novel therapeutic
agents for osteosarcoma. Expert Rev Anticancer Ther. 9:511–523.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Chandhanayingyong C, Kim Y, Staples JR,
Hahn C and Lee FY: MAPK/ERK Signaling in osteosarcomas, ewing
sarcomas and chondrosarcomas: Therapeutic implications and future
directions. Sarcoma. 2012:4048102012. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Allen E, Miéville P, Warren CM, Saghafinia
S, Li L, Peng MW and Hanahan D: Metabolic symbiosis enables
adaptive resistance to anti-angiogenic therapy that is dependent on
mTOR signaling. Cell Rep. 15:1144–1160. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Weigel B, Malempati S, Reid JM, Voss SD,
Cho SY, Chen HX, Krailo M, Villaluna D, Adamson PC and Blaney SM:
Phase 2 trial of cixutumumab in children, adolescents, and young
adults with refractory solid tumors: A report from the Children's
Oncology Group. Pediatr Blood Cancer. 61:452–456. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Wagner LM, Fouladi M, Ahmed A, Krailo MD,
Weigel B, DuBois SG, Doyle LA, Chen H and Blaney SM: Phase II study
of cixutumumab in combination with temsirolimus in pediatric
patients and young adults with recurrent or refractory sarcoma: A
report from the Children's Oncology Group. Pediatr Blood Cancer.
62:440–444. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Kempf-Bielack B, Bielack SS, Jürgens H,
Branscheid D, Berdel WE, Exner GU, Göbel U, Helmke K, Jundt G,
Kabisch H, et al: Osteosarcoma relapse after combined modality
therapy: An analysis of unselected patients in the Cooperative
Osteosarcoma Study Group (COSS). J Clin Oncol. 23:559–568. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Steliga M and Vaporciyan A: Surgical
treatment of pulmonary metastases from osteosarcoma in pediatric
and adolescent patients. Cancer Treat Res. 152:185–201. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Schuetze SM, Zhao L, Chugh R, Thomas DG,
Lucas DR, Metko G, Zalupski MM and Baker LH: Results of a phase II
study of sirolimus and cyclophosphamide in patients with advanced
sarcoma. Eur J Cancer. 48:1347–1353. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Demetri GD, Chawla SP, Ray-Coquard I, Le
Cesne A, Staddon AP, Milhem MM, Penel N, Riedel RF, Bui-Nguyen B,
Cranmer LD, et al: Results of an international randomized phase III
trial of the mammalian target of rapamycin inhibitor ridaforolimus
versus placebo to control metastatic sarcomas in patients after
benefit from prior chemotherapy. J Clin Oncol. 31:2485–2492. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Chawla SP, Staddon AP, Baker LH, Schuetze
SM, Tolcher AW, D'Amato GZ, Blay JY, Mita MM, Sankhala KK, Berk L,
et al: Phase II study of the mammalian target of rapamycin
inhibitor ridaforolimus in patients with advanced bone and soft
tissue sarcomas. J Clin Oncol. 30:78–84. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Schwartz GK, Tap WD, Qin LX, Livingston
MB, Undevia SD, Chmielowski B, Agulnik M, Schuetze SM, Reed DR,
Okuno SH, et al: Cixutumumab and temsirolimus for patients with
bone and soft-tissue sarcoma: A multicentre, open-label, phase 2
trial. Lancet Oncol. 14:371–382. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Reed DR, Mascarenhas L, Manning K, Hale
GA, Goldberg J, Gill J, Sandler E, Isakoff MS, Smith T, Caracciolo
J, et al: Pediatric phase I trial of oral sorafenib and topotecan
in refractory or recurrent pediatric solid malignancies. Cancer
Med. 5:294–303. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Quek R, Wang Q, Morgan JA, Shapiro GI,
Butrynski JE, Ramaiya N, Huftalen T, Jederlinic N, Manola J, Wagner
AJ, et al: Combination mTOR and IGF-1R inhibition: phase I trial of
everolimus and figitumumab in patients with advanced sarcomas and
other solid tumors. Clin Cancer Res. 17:871–879. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Pappo AS, Vassal G, Crowley JJ, Bolejack
V, Hogendoorn PC, Chugh R, Ladanyi M, Grippo JF, Dall G, Staddon
AP, et al: A phase 2 trial of R1507, a monoclonal antibody to the
insulin-like growth factor-1 receptor (IGF-1R), in patients with
recurrent or refractory rhabdomyosarcoma, osteosarcoma, synovial
sarcoma, and other soft tissue sarcomas: Results of a Sarcoma
Alliance for Research Through Collaboration study. Cancer.
120:2448–2456. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Olmos D, Postel-Vinay S, Molife LR, Okuno
SH, Schuetze SM, Paccagnella ML, Batzel GN, Yin D, Pritchard-Jones
K, Judson I, et al: Safety, pharmacokinetics, and preliminary
activity of the anti-IGF-1R antibody figitumumab (CP-751,871) in
patients with sarcoma and Ewing's sarcoma: A phase 1 expansion
cohort study. Lancet Oncol. 11:129–135. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Hassan SE, Bekarev M, Kim MY, Lin J,
Piperdi S, Gorlick R and Geller DS: Cell surface receptor
expression patterns in osteosarcoma. Cancer. 118:740–749. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Freeman SS, Allen SW, Ganti R, Wu J, Ma J,
Su X, Neale G, Dome JS, Daw NC and Khoury JD: Copy number gains in
EGFR and copy number losses in PTEN are common events in
osteosarcoma tumors. Cancer. 113:1453–1461. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Roth M, Linkowski M, Tarim J, Piperdi S,
Sowers R, Geller D, Gill J and Gorlick R: Ganglioside GD2 as a
therapeutic target for antibody-mediated therapy in patients with
osteosarcoma. Cancer. 120:548–554. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Kubo T, Piperdi S, Rosenblum J, Antonescu
CR, Chen W, Kim HS, Huvos AG, Sowers R, Meyers PA, Healey JH, et
al: Platelet-derived growth factor receptor as a prognostic marker
and a therapeutic target for imatinib mesylate therapy in
osteosarcoma. Cancer. 112:2119–2129. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Ebos JM, Lee CR, Christensen JG, Mutsaers
AJ and Kerbel RS: Multiple circulating proangiogenic factors
induced by sunitinib malate are tumor-independent and correlate
with antitumor efficacy. Proc Natl Acad Sci USA. 104:17069–17074.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Loges S, Schmidt T and Carmeliet P:
Mechanisms of resistance to anti-angiogenic therapy and development
of third-generation anti-angiogenic drug candidates. Genes Cancer.
1:12–25. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Casanovas O, Hicklin DJ, Bergers G and
Hanahan D: Drug resistance by evasion of antiangiogenic targeting
of VEGF signaling in late-stage pancreatic islet tumors. Cancer
Cell. 8:299–309. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Gerlinger M, Rowan AJ, Horswell S, Larkin
J, Endesfelder D, Gronroos E, Martinez P, Matthews N, Stewart A,
Tarpey P, et al: Intratumor heterogeneity and branched evolution
revealed by multiregion sequencing. N Engl J Med. 366:883–892.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Seoane J and De Mattos-Arruda L: The
challenge of intratumour heterogeneity in precision medicine. J
Intern Med. 276:41–51. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Massey PR, Okman JS, Wilkerson J and Cowen
EW: Tyrosine kinase inhibitors directed against the vascular
endothelial growth factor receptor (VEGFR) have distinct cutaneous
toxicity profiles: A meta-analysis and review of the literature.
Support Care Cancer. 23:1827–1835. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Hurwitz H: Integrating the anti-VEGF-A
humanized monoclonal antibody bevacizumab with chemotherapy in
advanced colorectal cancer. Clin Colorectal Cancer. 4:(Suppl 2).
S62–S68. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Eskens FA and Verweij J: The clinical
toxicity profile of vascular endothelial growth factor (VEGF) and
vascular endothelial growth factor receptor (VEGFR) targeting
angiogenesis inhibitors; a review. Eur J Cancer. 42:3127–3139.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Costelloe CM, Chuang HH, Madewell JE and
Ueno NT: Cancer Response Criteria and Bone Metastases: RECIST 1.1,
MDA and PERCIST. J Cancer. 1:80–92. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Stacchiotti S, Collini P, Messina A,
Morosi C, Barisella M, Bertulli R, Piovesan C, Dileo P, Torri V,
Gronchi A, et al: High-grade soft-tissue sarcomas: Tumor response
assessment - pilot study to assess the correlation between
radiologic and pathologic response by using RECIST and Choi
criteria. Radiology. 251:447–456. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Nathan PD, Vinayan A, Stott D, Juttla J
and Goh V: CT response assessment combining reduction in both size
and arterial phase density correlates with time to progression in
metastatic renal cancer patients treated with targeted therapies.
Cancer Biol Ther. 9:15–19. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Fournier L, Ammari S, Thiam R and Cuénod
CA: Imaging criteria for assessing tumour response: RECIST,
mRECIST, Cheson. Diagn Interv Imaging. 95:689–703. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Hamaoka T, Madewell JE, Podoloff DA,
Hortobagyi GN and Ueno NT: Bone imaging in metastatic breast
cancer. J Clin Oncol. 22:2942–2953. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Pakos EE and Ioannidis JP: The association
of P-glycoprotein with response to chemotherapy and clinical
outcome in patients with osteosarcoma. A meta-analysis. Cancer.
98:581–589. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Jakubowska A, Rozkrut D, Antoniou A,
Hamann U, Scott RJ, McGuffog L, Healy S, Sinilnikova OM, Rennert G,
Lejbkowicz F, et al: OCGN; SWE-BRCA; HEBON; EMBRACE; GEMO Study
Collaborators; KConFab; CIMBA, the Consortium of Investigators of
Modifiers of BRCA1/2-Related Cancer: Association of PHB 1630 C>T
and MTHFR 677 C>T polymorphisms with breast and ovarian cancer
risk in BRCA1/2 mutation carriers: Results from a multicenter
study. Br J Cancer. 106:2016–2024. 2012.PubMed/NCBI
|
|
98
|
Zurita AJ, Jonasch E, Wu HK, Tran HT and
Heymach JV: Circulating biomarkers for vascular endothelial growth
factor inhibitors in renal cell carcinoma. Cancer. 115:(Suppl 10).
2346–2354. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Ahbap E, Sakaci T, Kara E, Sahutoglu T,
Koc Y, Basturk T, Sevinc M, Akgol C, Kayalar AO, Ucar ZA, et al:
Neutrophil-to-lymphocyte ratio and platelet-tolymphocyte ratio in
evaluation of inflammation in end-stage renal disease. Clin
Nephrol. 85:199–208. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Jaffe N, Keifer R III, Robertson R, Cangir
A and Wang A: Renal toxicity with cumulative doses of
cis-diamminedichloroplatinum-II in pediatric patients with
osteosarcoma. Effect on creatinine clearance and methotrexate
excretion. Cancer. 59:1577–1581. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Aras M, Erdil TY, Dane F, Gungor S, Ones
T, Dede F, Inanir S and Turoglu HT: Comparison of WHO, RECIST 1.1,
EORTC, and PERCIST criteria in the evaluation of treatment response
in malignant solid tumors. Nucl Med Commun. 37:9–15.
2016.PubMed/NCBI
|
|
102
|
Lim J, Poulin NM and Nielsen TO: New
strategies in sarcoma: linking genomic and immunotherapy approaches
to molecular subtype. Clin Cancer Res. 21:4753–4759. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Subbiah V, Wagner MJ, McGuire MF, Sarwari
NM, Devarajan E, Lewis VO, Westin S, Kato S, Brown RE and Anderson
P: Personalized comprehensive molecular profiling of high risk
osteosarcoma: Implications and limitations for precision medicine.
Oncotarget. 6:40642–40654. 2015.PubMed/NCBI
|
|
104
|
Egas-Bejar D, Anderson PM, Agarwal R,
Corrales-Medina F, Devarajan E, Huh WW, Brown RE and Subbiah V:
Theranostic profiling for actionable aberrations in advanced high
risk osteosarcoma with aggressive biology reveals high molecular
diversity: The human fingerprint hypothesis. Oncoscience.
1:167–179. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Hurwitz H, Fehrenbacher L, Novotny W,
Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S,
Holmgren E, et al: Bevacizumab plus irinotecan, fluorouracil, and
leucovorin for metastatic colorectal cancer. N Engl J Med.
350:2335–2342. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Sandler A, Gray R, Perry MC, Brahmer J,
Schiller JH, Dowlati A, Lilenbaum R and Johnson DH:
Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell
lung cancer. N Engl J Med. 355:2542–2550. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Nerich V, Chelly J, Montcuquet P,
Chaigneau L, Villanueva C, Fiteni F, Meneveau N, Perrin S, Voidey
A, Monnot T, et al: First-line trastuzumab plus taxane-based
chemotherapy for metastatic breast cancer: Cost-minimization
analysis. J Oncol Pharm Pract. 20:362–368. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Cheng YC, Rondón G, Anderlini P, Khouri
IF, Champlin RE and Ueno NT: Paclitaxel and trastuzumab as
maintenance therapy in patients with HER2-positive metastatic
breast cancer who underwent high-dose chemotherapy and autologous
hematopoietic stem cell transplantation. J Cancer. 4:679–685. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Powles T and Crusz SM: Sequencing systemic
therapies in advanced RCC: Is there a best strategy? Am Soc Clin
Oncol Educ Book. 33:e172–e174. 2013. View Article : Google Scholar
|
|
110
|
Kuwano M, Sonoda K, Murakami Y, Watari K
and Ono M: Overcoming drug resistance to receptor tyrosine kinase
inhibitors: Learning from lung cancer. Pharmacol Ther. 161:97–110.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Porta C, Giglione P and Paglino C:
Targeted therapy for renal cell carcinoma: Focus on 2nd and 3rd
line. Expert Opin Pharmacother. 17:643–655. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Kim SH, Park WS, Kim SH, Joung JY, Seo HK,
Lee KH and Chung J: Systemic treatments for metastatic renal cell
carcinoma: 10-Year experience of immunotherapy and targeted
therapy. Cancer Res Treat. 48:1092–1101. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Kramer MW, Steffens S, von Klot C,
Merseburger AS and Kuczyk MA: Systemic therapy for metastatic renal
cell carcinoma. Aktuelle Urol. 43:265–268. 2012.(In German).
PubMed/NCBI
|
|
114
|
Buti S, Leonetti A, Dallatomasina A and
Bersanelli M: Everolimus in the management of metastatic renal cell
carcinoma: An evidence-based review of its place in therapy. Core
Evid. 11:23–36. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Choueiri TK, Escudier B, Powles T, Tannir
NM, Mainwaring PN, Rini BI, Hammers HJ, Donskov F, Roth BJ, Peltola
K, et al: METEOR investigators: Cabozantinib versus everolimus in
advanced renal cell carcinoma (METEOR): Final results from a
randomised, open-label, phase 3 trial. Lancet Oncol. 17:917–927.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Cella D, Grünwald V, Nathan P, Doan J,
Dastani H, Taylor F, Bennett B, DeRosa M, Berry S, Broglio K, et
al: Quality of life in patients with advanced renal cell carcinoma
given nivolumab versus everolimus in CheckMate 025: A randomised,
open-label, phase 3 trial. Lancet Oncol. 17:994–1003. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Maute L and Bergmann L: Systemic therapy
of metastatic renal cell carcinoma. Dtsch Med Wochenschr.
141:466–469. 2016.(In German). PubMed/NCBI
|
|
118
|
Le Cesne A, Blay JY, Reichardt P and
Joensuu H: Optimizing tyrosine kinase inhibitor therapy in
gastrointestinal stromal tumors: Exploring the benefits of
continuous kinase suppression. Oncologist. 18:1192–1199. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Shien K, Yamamoto H, Soh J, Miyoshi S and
Toyooka S: Drug resistance to EGFR tyrosine kinase inhibitors for
non-small cell lung cancer. Acta Med Okayama. 68:191–200.
2014.PubMed/NCBI
|