Introduction
As one of the most common malignancies around the
world, gastric cancer is a highly fatal disease and often diagnosed
in an advanced state with scarce effective therapies. Despite the
improvements in medical and surgical therapy during the past
decades, the mortality and average 5-year survival rate have not
obviously changed after successful surgical intervention (1–3).
Therefore, an efficient therapy of this disease is extremely
urgent, and new approaches, including gene therapy, are requied to
improve treatment results (4).
Midkine (MK) is a heparin-binding growth factor,
first discovered as a highly expressed gene (Mdk) during
mouse embryogenesis (5). Although
prominently expressed during embryogenesis, MK is downregulated to
neglible levels in healthy adults. Accumulating evidence indicates
that MK is involved in numerous pathologies, including ischaemia,
inflammation, autoimmunity and, most notably, in many cancers
(6–11). As a soluble cytokine, the elevated
MK is readily apparent in the blood and other body fluids, which
makes it a relatively convenient, accessible, non-invasive and
inexpensive biomarker for population screening and early disease
detection. Several biological functions of MK are thought to
contribute to tumorigenesis and tumor progression, which promotes
the proliferation, survival, and tumorigenicity of different cells
and stimulates angiogenesis (12,13).
MK is also thought to participate in the pathogenesis of gastric
cancer and positively regulates the proliferation of human gastric
cancer (14). However, the possible
mechanisms of MK in the development of gastric cancer are still not
fully clarified.
In this study, the therapeutic effect of MK
inhibition in gastric cancer in vivo and in vitro was
investigated, by knock-down of MK expression with a small
interfering RNA (siRNA).
Materials and methods
Patients and tissue specimens
A total of 17 patients, who received surgery for
gastric adenocarcinoma at the Department of General Surgery, the
First Affiliated Hospital of Soochow University in 2015, were
enrolled in the study. Complete clinical data and paraffin-embedded
gastric cancer specimens were available for all patients. Gastric
cancer patients were staged using the International Union Against
Cancer (UICC) 1997 TNM staging criteria, and histological typing of
the primary tumor was performed using the World Health Organization
(WHO) criteria. Poorly differentiated (n=5), moderately
differentiated (n=6) and well differentiated (n=6) gastric
adenocarcinoma was diagnosed. Five patients were diagnosed as
poorly differentiated gastric adenocarcinoma, 6 as moderately
differentiated and 6 as well differentiated. Prior to surgery, no
patient received radiotherapy or chemotherapy. The use of the
tissue samples was approved by the local Ethics Committee of the
First Affiliated Hospital of Soochow University and the informed
consent of the patients was obtained according to the institutional
regulations.
Cell culture and transfection
Human pancreatic cancer cell lines GES-1, 803,
SGC7901, MKN4 and AGS were kindly gifted by Laboratory of Cellular
and Molecular Tumor Immunology of Soochow University, and were
cultured in RPMI-1640 (Gibco, USA) supplemented with 10% fetal
bovine serum (FBS), 100 U/ml penicillin and 100 µg/ml streptomycin
in a humidified incubator at 37°C in 5% CO2. The
transfection procedure was as previously reported (14). The pLXSN or pLXSN-MK plasmid was
transfected into packed GP293 cells with Lipofectamine™ 2000
reagent (Invitrogen, CA, USA). After 48 h, 1.5 ml of virus
supernatant from various plasmids was added to 80% confluent AGS
cells, which were incubated at 37°C for 24 h, and then screened
with G418 (400 mg/l). Monoclonal cells were selected and cultured
further. The clones were screened for MK expression with western
blot analysis. The nucleotide sequences of MK siRNA were
5′-GGAGCCGACUGCAAGUACATT-3′ and 5′-UGUACUUGCAGUCGGCUCCAA-3′. The
negative control siRNA sequences were 5′-UUCUCCGAACGUGUCACGUTT-3′
and 5′-ACGUGACACGUUCGGAGAATT-3′.
Cell viability assay
AGS cells were plated in 100 µl medium per well in
96-well plates, blank and zero wells were set. One day after
seeding, cell viability was measured with Cell Counting Kit-8
(Peptide Institute Inc., Osaka, Japan) at 48 h after transfection
for 2-h culture at 37°C, and the surival rate and inhibition rate
were calculated. The OD value at the wavelength of 490 nm was
detected using an enzyme-labeled analyzer. The cell survival rate
was calculated based on the formula: the survival rate = (the OD
value of the experimental group/the OD value of the blank group) ×
100%. For in vitro study, two experiments were carried out.
In experiment 1, the effect of recombinant human midkine (rhMK)
(Abcam, UK) was tested. AGS cells were treated with negative
control group, rhMK group (5 µg/ml), cisplatin group (50 µg/ml),
cisplatin (50 µg/ml) + rhMK group (5 µg/ml), cisplatin (50 µg/ml) +
γ-secretase inhibitor I (GSI; 1 µM), group and cisplatin (50 µg/ml)
+ rhMK (5 µg/ml)+GSI I (1 µM) group. In experiment 2, the effect of
MK siRNA was tested. AGS cells were treated with negative control
group, non-targeted siRNA group, MK siRNA group, cisplatin group
(50 µg/ml), cisplatin group (50 µg/ml) + non-targeted siRNA group,
and cisplatin group (50 µg/ml) + MK siRNA group.
Annexin V/PI assay
AGS cells were plated in 6-well plate and treated as
indicated above. After 48-h incubation cells were collected, washed
in cold PBS twice and then mixed in 100 µl of 1X binding buffer and
incubated with an Annexin V/PI double staining solution (5 µl FITC
Annexin V and 5 µ1 PI) (Sigma-Aldrich, St. Louis, MO, USA) at room
temperature for 15 min (the cell density was adjusted to
1×106/ml). The stained cells were analyzed by flow
cytometry and the percentage of apoptotic cells were calculated
with ModFitLT software (Verity Software House, Topsham, ME, USA).
The percentage of apoptotic cells was calculated.
Western blot assay
Total proteins were prepared by standard procedures
and quantified by the BCA method and loaded onto a 10%
SDS-polyacrylamide gel. After electrophoresis, proteins were
transferred onto a PVDF membrane by electroelution. The membrane
was incubated with primary antibody (MK, Notch 1 and Notch 2)
(Santa Cruz Biotechnology, Santa Cruz, CA, USA) overnight at 4°C.
The next day, the membrane was washed and incubated with
HRP-conjugated secondary antibodies (Santa Cruz Biotechnology) for
1 h at room temperature. After washing, the membrane was developed
using the ECL-detection system, quickly dried, and exposed to ECL
film. We also used anti-β-actin antibody (Santa Cruz Biotechnology)
as an internal standard.
Immunohistochemical staining
The primary antibodies (MK, Delta-like 1 and PCNA)
(Santa Cruz Biotechnology) were utilized for immunohistochemistry.
After deparaffinization, endogenous peroxidase activity was blocked
by incubating the 5-µm tissue sections for 30 min with 0.3%
hydrogen peroxide in methanol. The tissue sections were incubated
with the primary antibodies in phosphate-buffered saline (PBS)
containing 5% bovine serum albumin overnight at 4°C. After washing,
the sections were stained with biotin-conjugated secondary
antibodies and followed by horseradish peroxidase-conjugated
streptavidin (Boster Biological Technology, Wuhan, China) for 30
min. Diaminobenzidine (DAB) was used as the immunodetection
substrate. The in situ cell death detection kit for TUNEL
assay (Beyotime, China) was applied for analyzing cell death.
Immunofluorescence staining
Paraffin-embedded samples were deparaffinized and
rehydrated. Sections were microwave treated (565 min) in EDTA
buffer (pH 9.0), allowed to cool for 30 min, and washed in PBS.
After being blocked for 20 min with 5% bovine serum albumin, slides
were incubated overnight at 4°C with antibodies against Delta-like
1 (1:200 dilution) and against Jagged 1 (1:200 dilution). Sections
were then rinsed in PBST (PBS+0.1% Tween-20) and immunoreactive
protein was detected using a secondary antibody (1:400 dilution)
conjugated with fluorochrome Cy3 (Jackson ImmunoResearch
Laboratory, USA) and a secondary antibody (1:400 dilution)
conjugated with fluorochrome Alexa FluorH 488 (Jackson
ImmunoResearch Laboratory, USA) for 1 h in the dark. After being
rinsed in PBST, slides were mounted with Fluoromount™ mounting
medium (Sigma-Aldrich) with 4′,6-diamidino-2-phenylindole (DAPI)
(1:1,000 dilution). Fluorescence analysis was performed by using a
confocal laser scanning microscope (LSM 710; Zeiss, Germany) and
Zen 2009 software (Carl-Zeiss, Jena, Germany).
In vivo experiment
Six-week-old female athymic nude mice were used in
the experiment in vivo. AGS cells were injected
subcutaneously into the right anterior armpit of nude mice to
establish an animal model of transplanted tumors. AGS cells were
resuspended in serum-free RPMI-1640. The cell suspension was then
injected subcutaneously (5×107 cells; total volume 0.5
ml) into the nude mice. Then mice were divided into six groups:
group 1 was injected with AGS cells; group 2 was injected with AGS
cells stably transfected with pLXSN-non-targeted siRNA; group 3 was
injected with AGS cells stably transfected with pLXSN-MKsiRNA;
group 4 was injected with AGS cells treated with cisplatin; group 5
was injected with AGS cells stably transfected with
pLXSN-non-targeted siRNA, then treated with cisplatin; group 6 was
injected with AGS cells stably transfected with pLXSN-MKsiRNA, then
treated with cisplatin (ten mice in each group). Five days after
the transplantation, cisplatin (2 mg/kg) was administered
peritoneally each day until the end of the study (15). Five weeks after the transplantation,
mice were sacrificed and the tumor tissues were collected for
histological examination and stored at −80°C for protein
extraction. The tumor size was measured every 7 days with calipers.
The tumor volume was calculated with the formula:
(LxW2)/2, where L is the length and W the width of the
tumor. After the mice were sacrificed, weights of the tumors were
measured.
Statistical analysis
Data are expressed as mean ± standard error (SE) and
were analyzed using SPSS PC version 18.0 (SPSS Inc., Chicago, IL,
USA). Statistical analysis was performed using one-analysis of
variance (ANOVA) followed by SNK tests as post hoc test.
Kruskal-Wallis test was used to evaluate the differences of
categorical values followed by Mann-Whitney U tests as post hoc
test. The criterion of significance was set as p-value of
<0.05.
Results
Expression of MK in gastric cancer
tissues and cancer cells
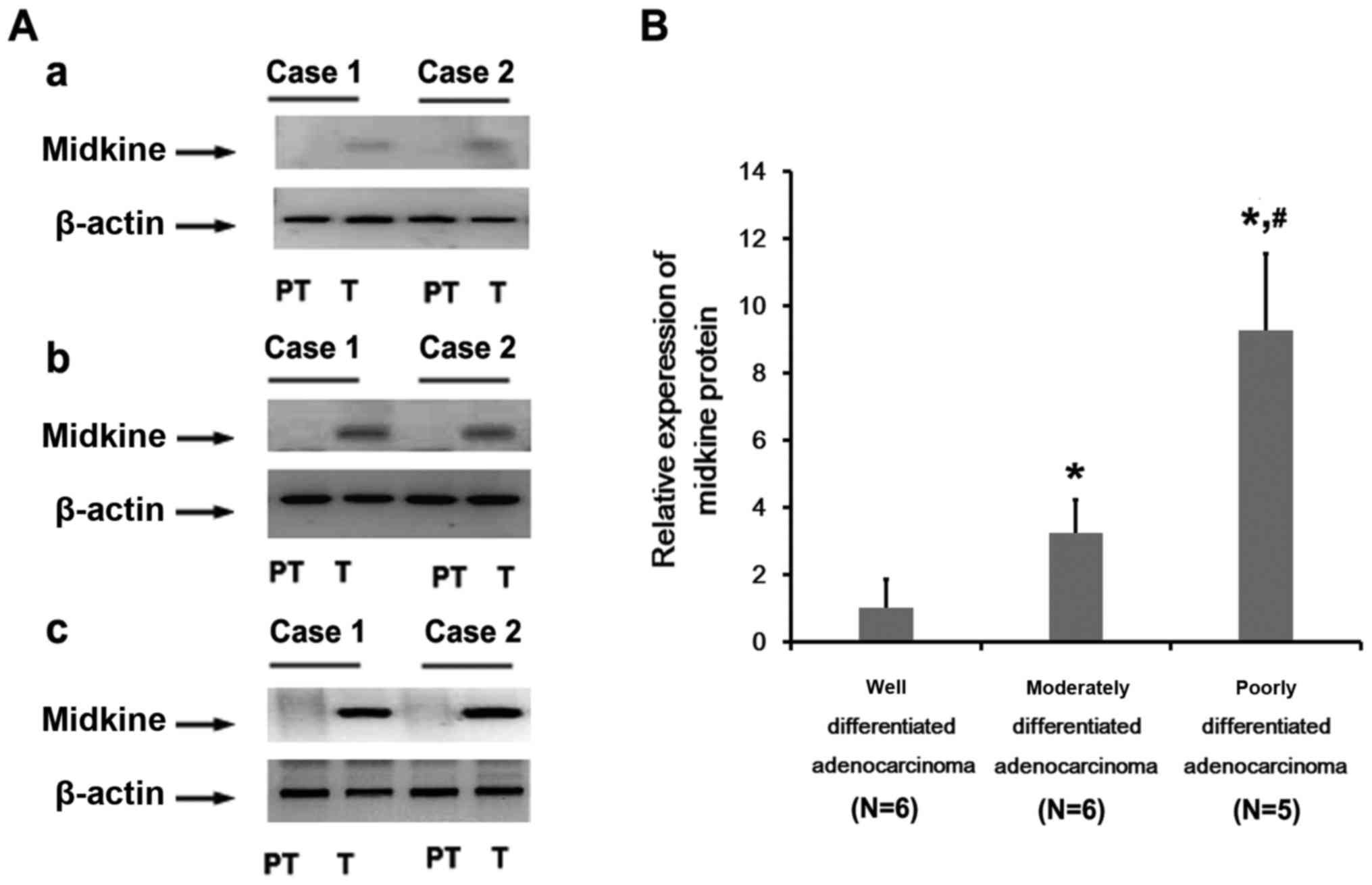
Seventeen patients were enrolled and gastric cancer
tissues were collected. Of the patients 6 were diagnosed as well
differentiated gastric adenocarcinoma, 6 as moderately
differentiated and 5 as poorly differentiated. In well
differentiated gastric adenocarcinoma, western blot analysis showed
that MK protein was detectable in 4 patients, comapared with
non-cancerous tissues adjacent to the cancer. The high expression
of MK protein was found both in moderately differentiated and
poorly differentiated adenocarcinoma. The relative expression of MK
protein was upregulated in poorly differentiated tissues, nearly
3-fold higher than that in the moderately differentiated tissues
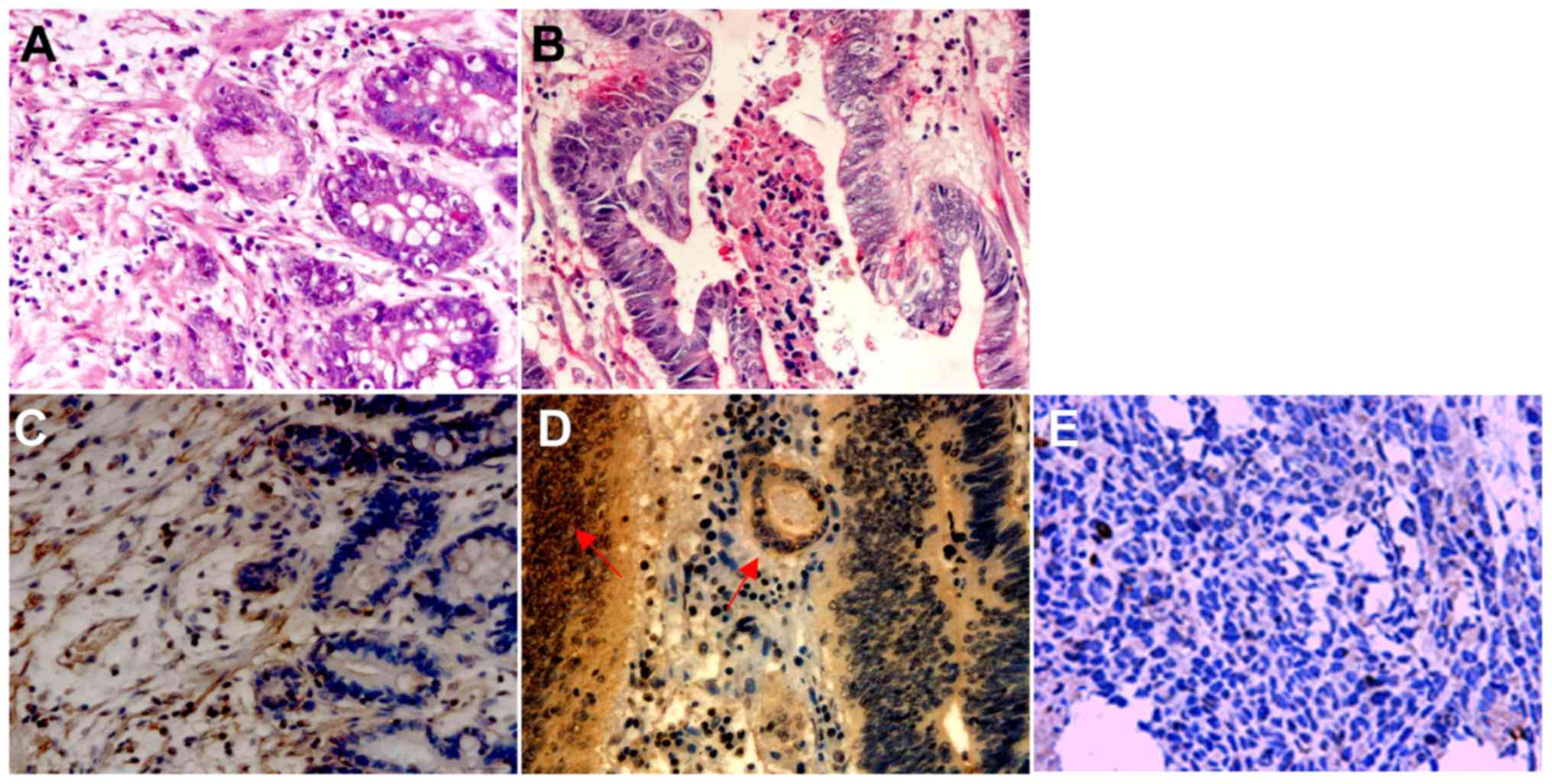
(Fig. 1). Next, we analyzed the
location of MK in gastric cancer tissues. At the cellular level,
the cytoplasm of the cancer cells was immunoreactive with anti-MK
antibodies, as well as nucleolus immunoreactivity partly in poorly
differentiated adenocarcinoma cases. However, fewer immunopositive
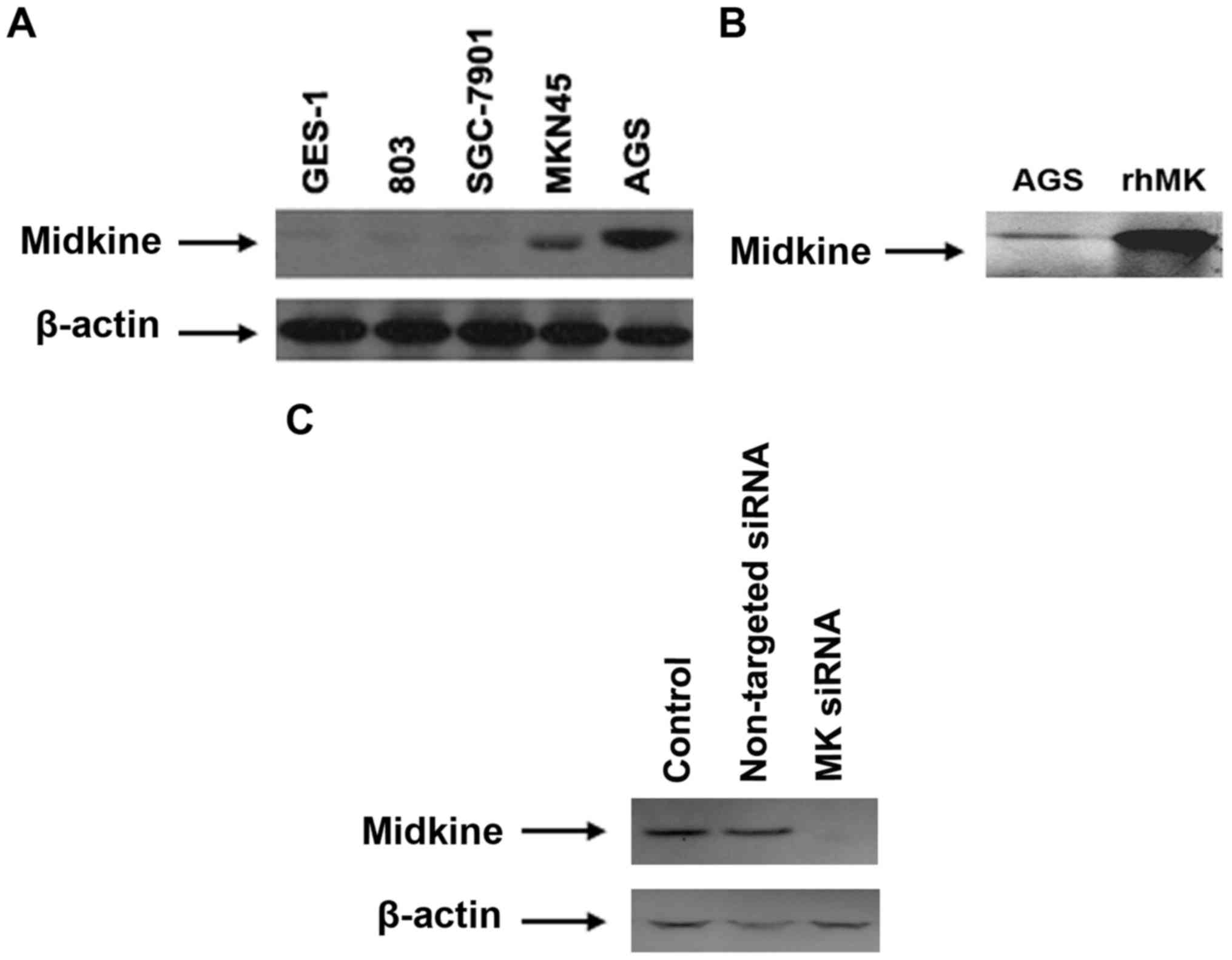
cells were found in well differentiated tissues (Fig. 2). The expression of MK protein was
also detected in gastric cell lines. The protein expression could
be barely found in GES-1, 803 and SGC-7901. Higher expression of MK
protein was detected in AGS cells, compared with MKN45 cells
(Fig. 3). Therefore, AGS cells were
used for further experiments.
RhMK attenuates the cytotoxic effect
of cisplatin on AGS cells in vitro
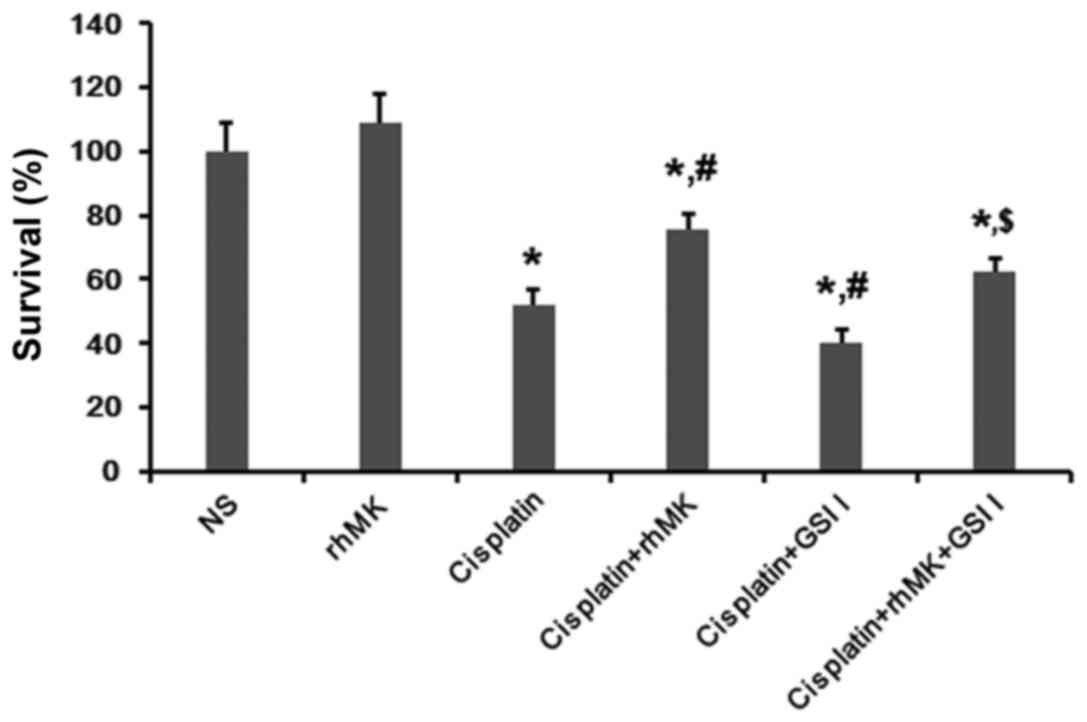
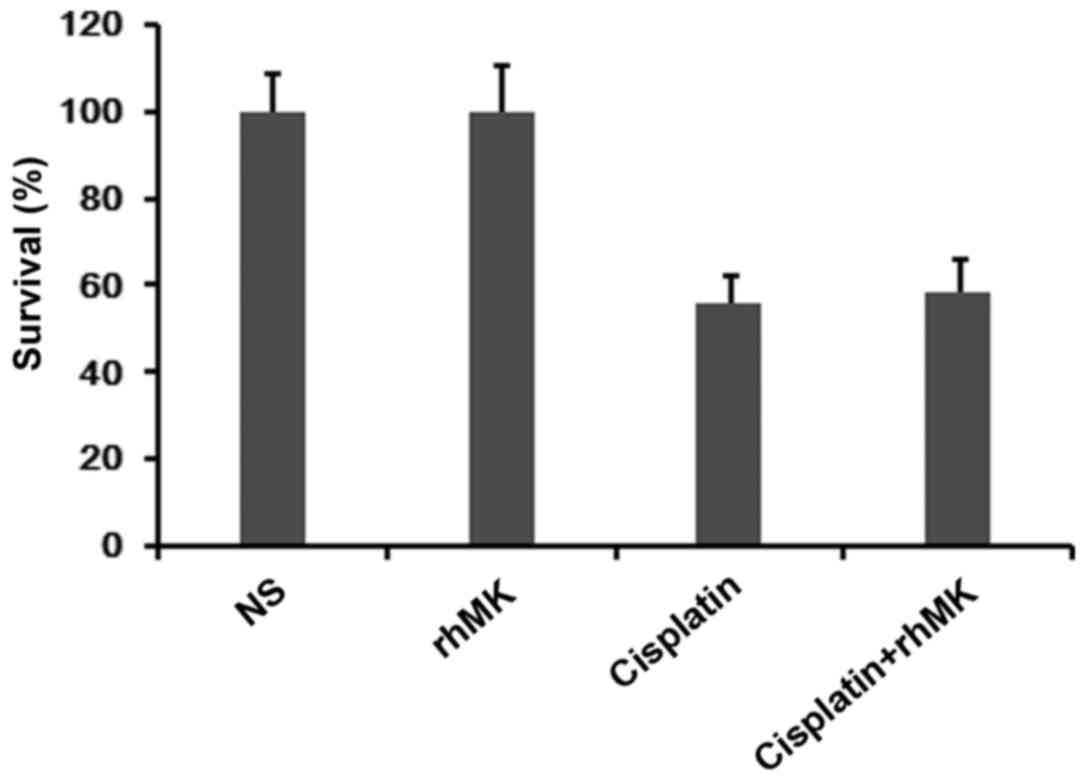
We measured the survival and growth of AGS cells
with or without rhMK treatment, using the CCK-8 kit assay. Compared
with the NS control, rhMK could not affect the growth of AGS cells.
Cisplatin obviously inhibited the proliferation and survival of AGS
cells, while this inhibition was attenuated by rhMK treatment
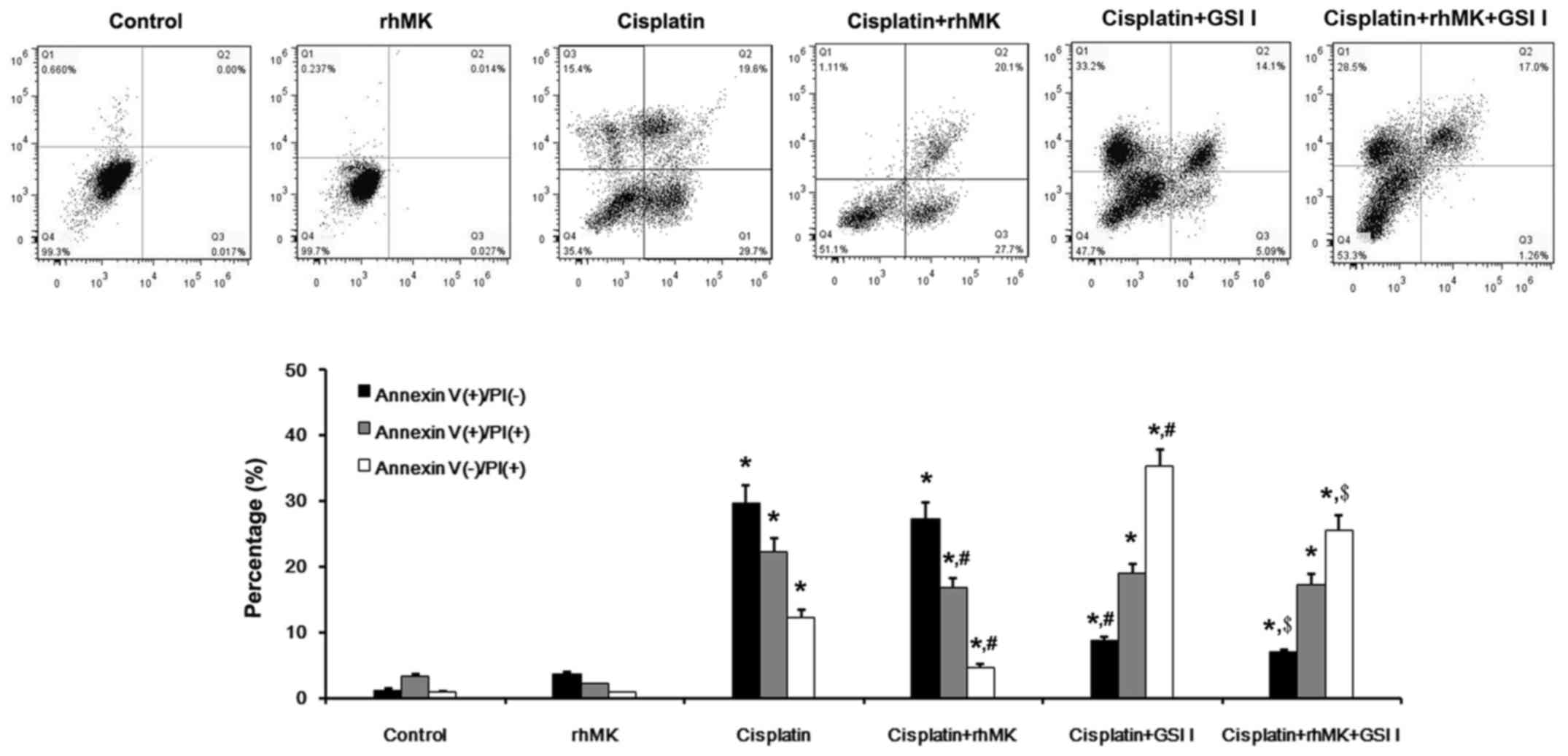
(Fig. 4). Annexin V/PI assay was
applied to detect the cell death in vitro [Annexin V (+)/PI
(−), early apoptosis; Annexin V (−)/PI (+), late apoptosis; Annexin
V (−)/PI (+), necrosis]. Results of Annexin V/PI assay showed that
no obvious apoptosis or necrosis were found both in normal saline
and rhMK-treated cells. In cisplatin-treated cells, significant
apoptotic and necrotic cells were detected, and the necrosis was
attenuated by rhMK treatment (Fig.
5). However, the anti-proliferative effect of cisplatin on
GES-1 cells was not influenced by rhMK treatment (Fig. 6). The expression of Delta and Notch
can be affected by other cytotoxic drugs, according to previous
reports, such as 5-FU, adriamycin, and doxorubicin (16,17).
By using γ-secretase inhibitor, the effects of cisplatin and rhMK
were cancelled.
The cytotoxic effect of cisplatin on
AGS cells is promoted by suppressing MK expression in vitro
To determine whether the suppression of MK
expression influenced the cell growth or not, MK-targeted siRNA was
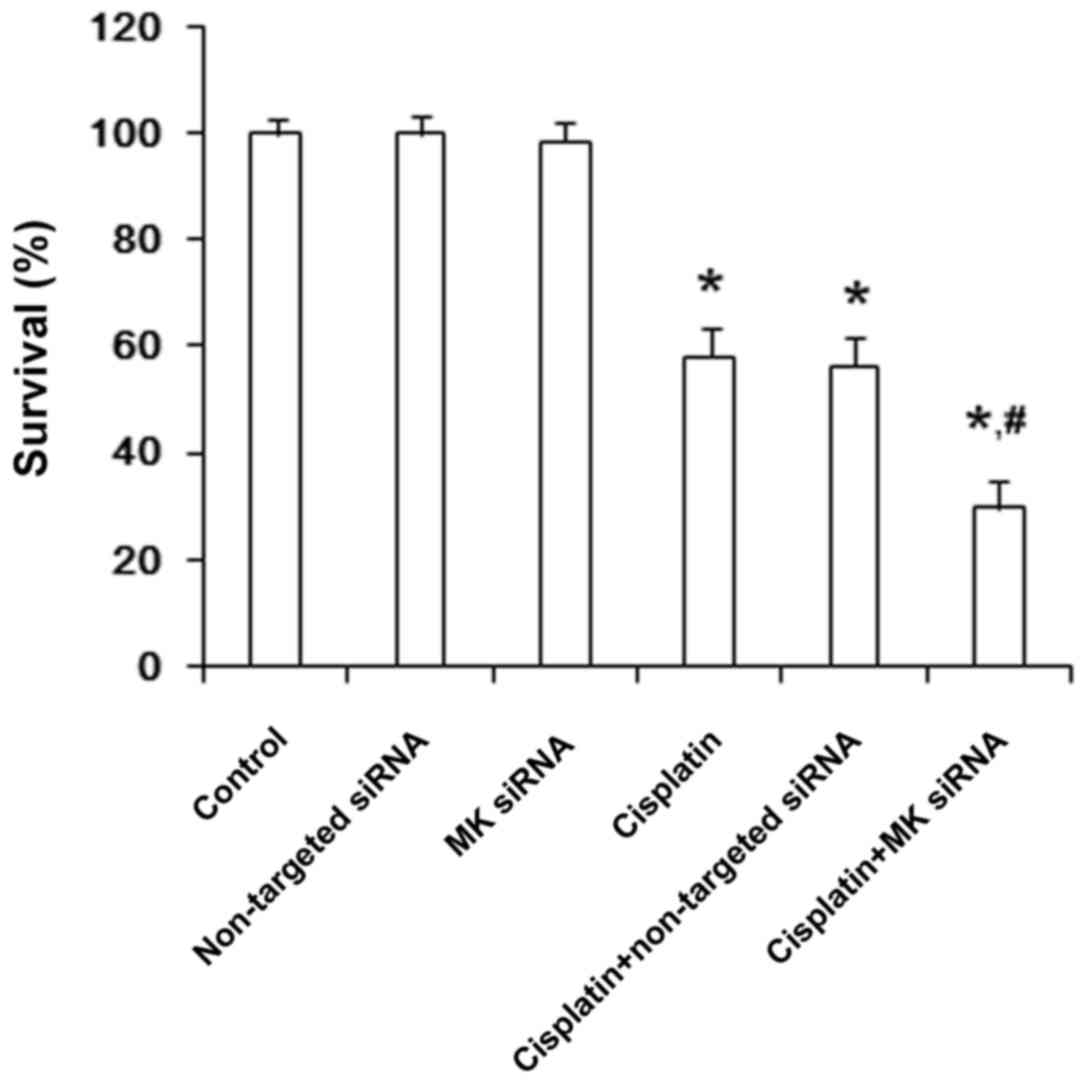
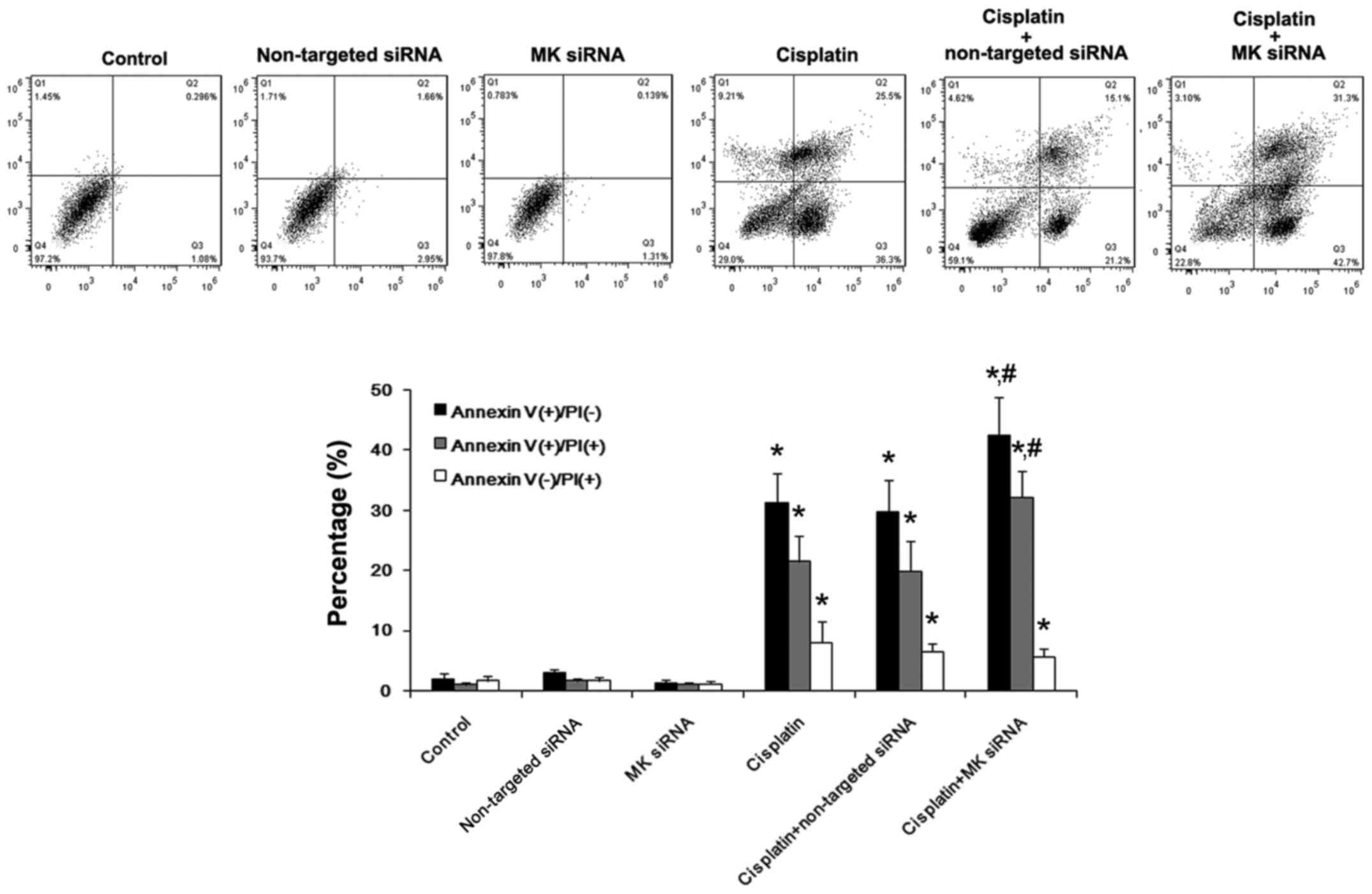
applied in this study. CCK-8 kit assay showed that, without
cisplatin treatment, the proliferation and survival were not
affected either by MK-siRNA or non-targeted siRNA, nor the
percentage of apoptosis and necrosis in AGS cells by Annexin V/PI.
In cisplatin-treated cells, the growth was inhibited and the
percentage of apoptosis and necrosis were obviously increased, and
the apoptosis tended to be further increased by MK-siRNA, while
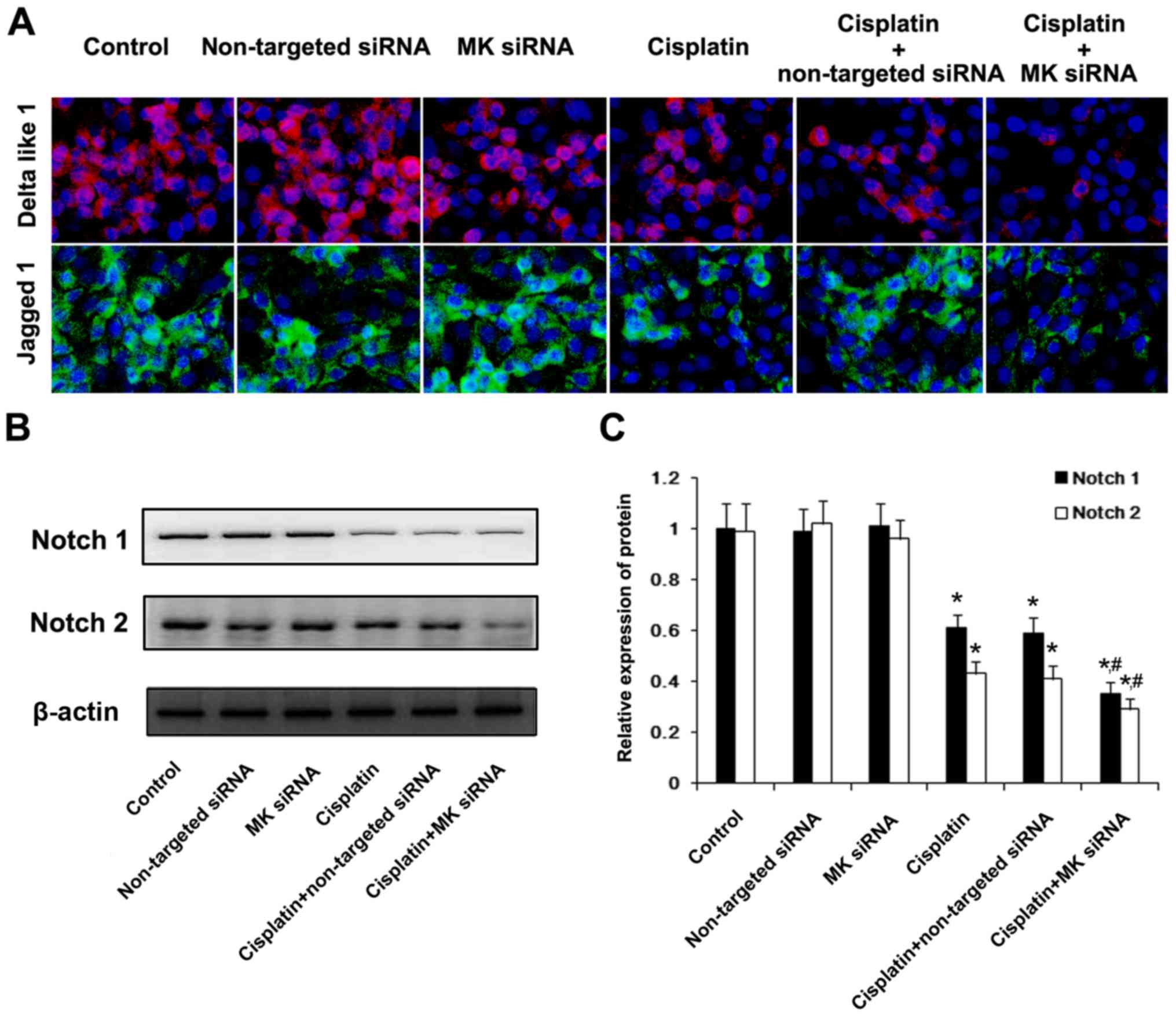
non-targeted siRNA showed no such effect (Figs. 7 and 8). Immunofluorescent staining of
Delta-like 1 and Jagged 1 was conducted to analyze the influence of
the inhibition of MK expression upon the regulation of Notch
signaling pathway related ligands. Without cisplatin intervention,
non-targeted siRNA and MK-siRNA did not affect the expression of
Delta-like 1 and Jagged 1, compared with NS-treated cells. After
receiving cisplatin, the downregulation of Delta-like 1 and Jagged
1 protein expression could be clearly observed, and this change was
enhanced by MK-siRNA. Western blot analysis showed that the
expression of Notch 1 and Notch 2 was also reduced by MK-siRNA
(Fig. 9).
Suppression of MK gene promotes the
inhibitory effect of cisplatin on AGS cells in vivo
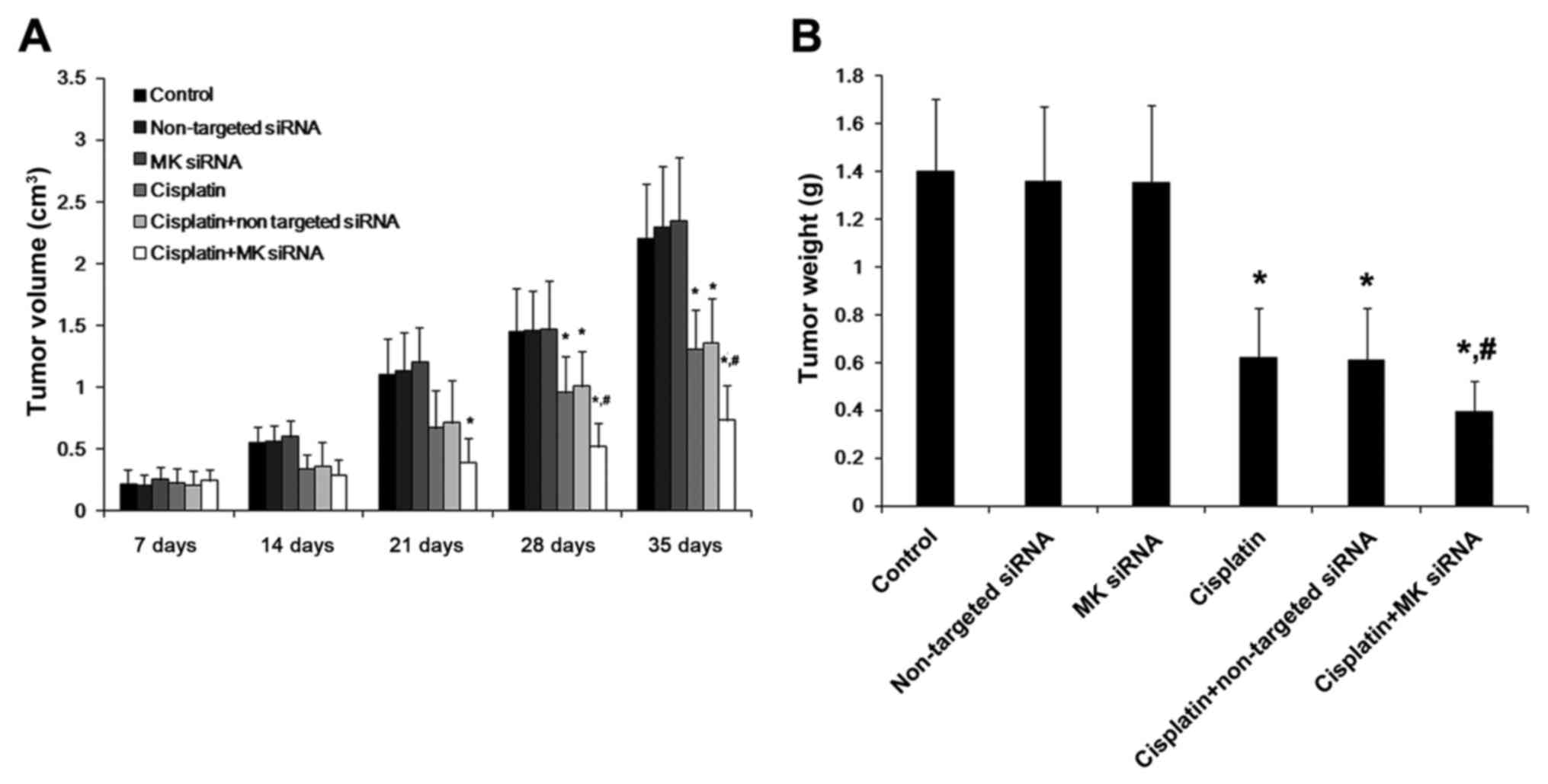
According to the results of the in vivo
experiment, the treatment of cisplain alone exerted a beneficial
effect on the gastric cancer in terms of the decreased tumor volume
and weight, compared with the control groups. Furthermore, the
tumor volume and weight were decreased more significantly after the
combined treatment with cisplatin and MK-siRNA, during the whole
observation period (Fig. 10).
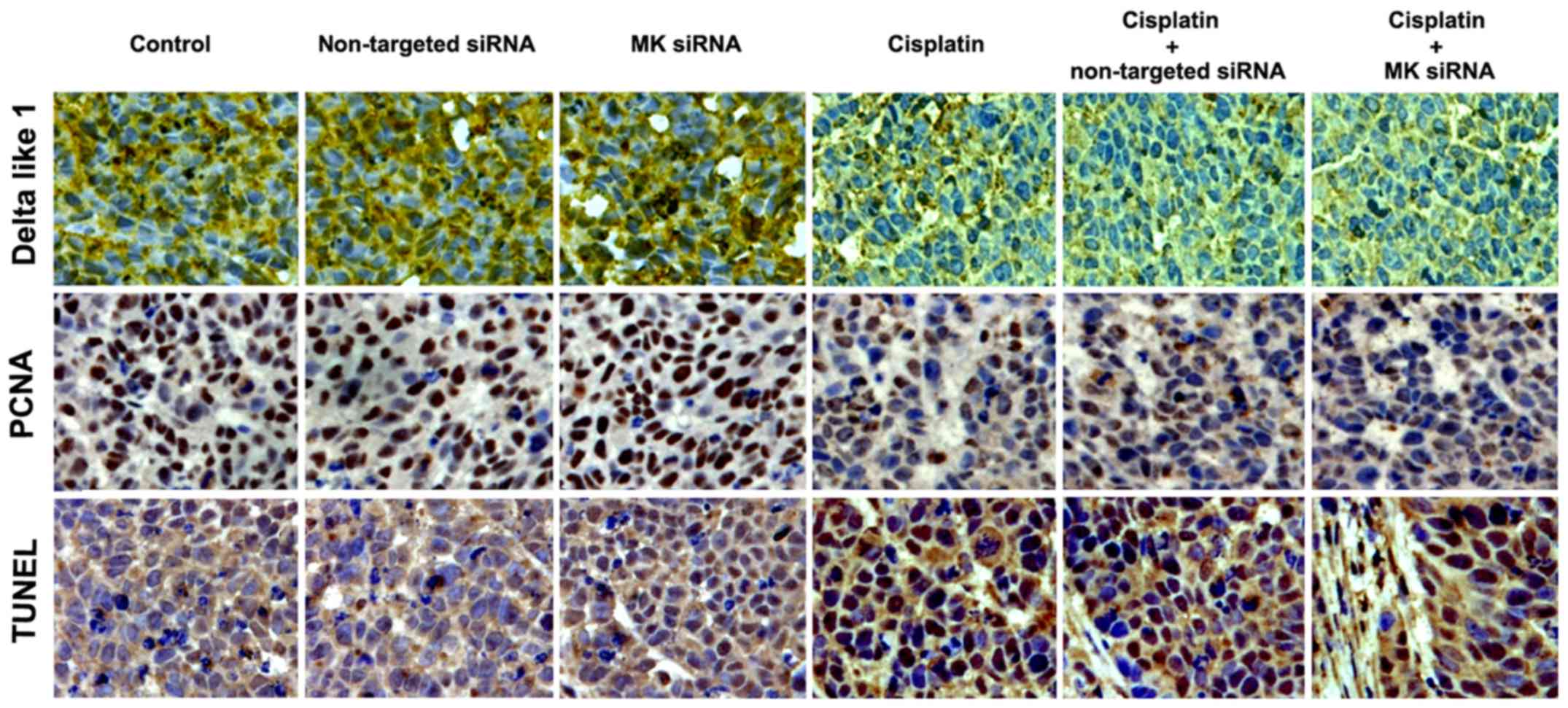
Histochemical staining indicated that the chemical density of
Delta-like 1 and PCNA was reduced by cisplatin, which could be
promoted by MK-siRNA. The TUNEL analysis revealed that cisplatin
caused apoptosis in cancerous tissues was also enhanced by MK-siRNA
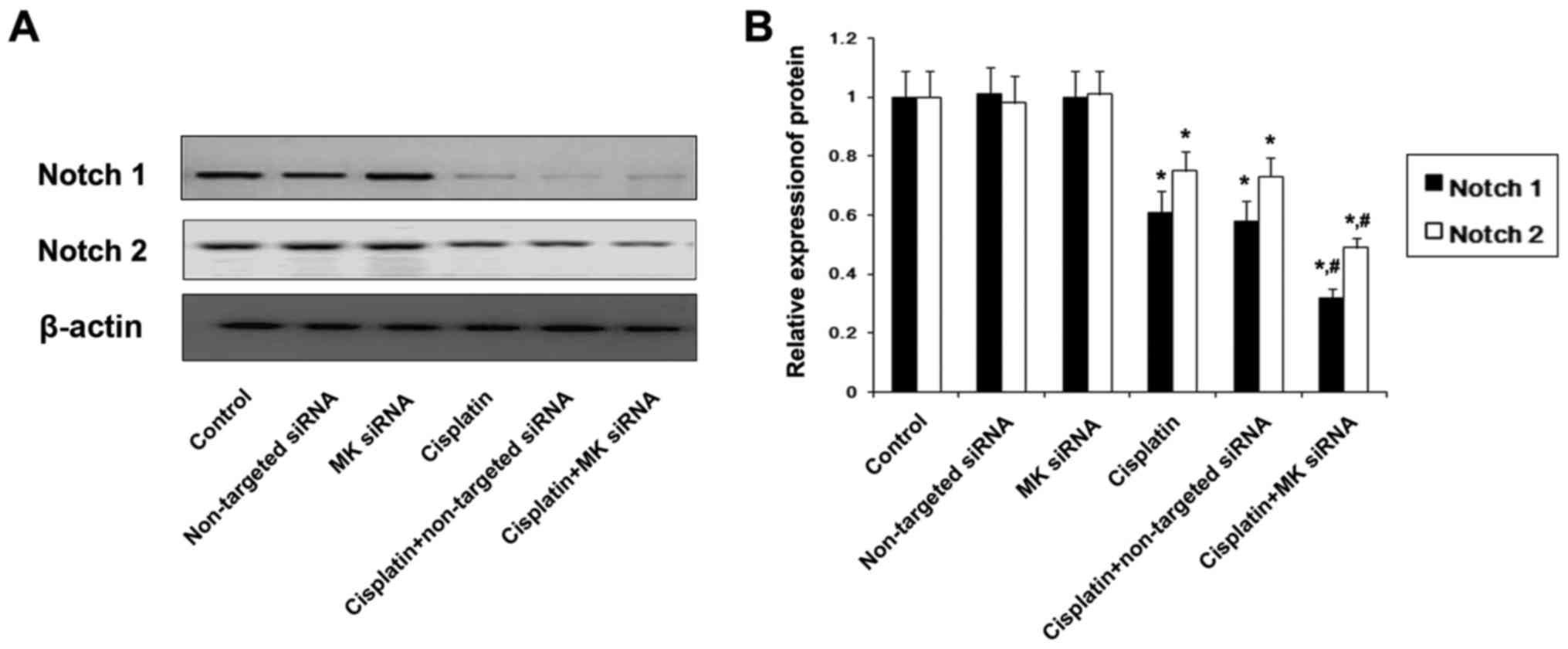
(Fig. 11). The expression of Notch
1 and Notch 2 protein was also found to be reduced in
cisplatin-treated cells, and the reduction was promoted by MK-siRNA
(Fig. 12).
Discussion
Gastric cancer is one of the most commonly seen
malignancies with the highest mortality in China. Despite advances
in its diagnosis and treatment, the prognosis for advanced gastric
cancer is poor, with a 5-year survival rate of <10%. Therefore,
it is crucial to develop new and more effective therapeutic
strategies for this fatal disease. Numerous growth factors and
their downstream signaling systems are involved in the development,
progression, and dissemination of gastric cancer. Increased MK
expression has been reported in various human carcinomas. As a
heparin-binding growth factor, the expression of which is generally
low or undetectable in adults, whereas it is high in various human
cancers, including esophageal, gastric, urinary bladder,
pancreatic, colorectal, breast, and lung carcinomas, neuroblastoma,
and Wilms's tumor. Some reports have shown that urinary MK and
serum MK levels are elevated in cancer patients and are associated
with disease progression. MK mRNA and protein levels are also
reported to be both associated with the clinical stages and distant
metastases in gastric cancer in Chinese patients (12,18,19).
Similarly in our study, MK protein expression was also found in
gastric carcinoma tissues, and the extent of expression was
correlated with the differentiation of tissue specimens. In cancer
cell lines, high expressed MK protein was also obseverd in poorly
differentiated cells. These results further proved that MK may be
involved in the development of human gastric cancer. However, the
possible mechanisms of MK in the pathogenesis and the therapeutic
effect of MK in gastric cancer are still not fully clarified, which
then prompted our further experiments.
As an oncoprotein that functions in gastric
carcinogenesis, MK is reported to positively regulate the
proliferation of human gastric cancer cells (14). Thus, we investigated the therapeutic
effect of the recombinant human MK (rhMK) on AGS cells, the cells
which could detect the highly expressed MK protein. In contrast to
the results of Xu et al (14), rhMK did not show a proliferative
effect on cancer cells in our study. However, the cytotoxic effect
of cisplatin on AGS cells in vitro was attenuated by rhMK
via reducing the cisplatin-induced apoptosis. The different
selection of the cell line may lead to the different result, but on
the other hand, our results would indicate that MK might be
involved in the chemotherapy-resistance in gastric cancer. Then,
MK-targeted siRNA was applied to study the effect of MK inhibition
on AGS cells. Although MK-targeted siRNA treatment did not play a
growth-inhibitory function on AGS cells, it promoted the cytotoxic
effect of cisplatin by inducing more severe apoptosis. Moreover,
the tumor growth in nude mice was inflenced by MK inhibition as
well. Previous study has also shown that the apoptosis of MK siRNA
transfected cells might be mediated by the
mitochondria-apoptosome-mediated pathway. The downregulated
expression of Bcl-2 and upregulated expression of Bax decreased
mitochondrial membrane potential, and led to release of cytochrome
c, and activated caspase-3, −8 and −9, especially caspase-3
and −9, induced apoptosis of the cells (20). These results suggest that MK
inhibition may benefit chemotherapy treatment in gastric
cancer.
Notch signaling is defined as an evolutionarily
conserved local cell interaction mechanism that is involved in a
variety of cellular processes. Recently, emerging evidence suggests
that Notch signaling pathway is one of the most important signaling
pathways in chemotherapeutic drug resistance. Importantly,
interfering Notch signaling by γ-secretase inhibitors (GSIs) or
downregulation of Notch 1 receptor can induce drug sensitivity,
leading to increased inhibition of cancer cell growth and
metastasis (21–25). The role of Notch signaling pathway
in cancer cell drug resistance was widely studied, and studies have
demonstrated that Notch signaling regulate the formation of cancer
stem cells (CSCs) and contributes to epithelial-mesenchymal
transition (EMT), which was found associated with drug resistance
(26–28). It was also reported that inhibition
of Notch signaling downregulates pro-survival pathways and
anti-apoptosis genes. Previous studies have shown that the aberrant
expression of Notch 1 and Notch 2 was related to a growing number
of solid tumors, as well as gastric cancer (29,30).
The Notch signaling pathway is a conserved ligand-receptor
signaling pathway present in most multicellular organisms. The
mammalian canonical ligands are designated as Delta-like (Delta
1–4) or Serrate-like ligands known as Jagged 1 and Jagged 2, and
the Notch receptors are single-pass transmembrane receptor proteins
encoded by Notch genes (Notch 1–4) (31,32).
In our study, the expression of Notch 1, Notch 2, Delta 1 and
Jagged 1 was observed in control AGS cells. When interfering with
MK-siRNA, these protein changes were significantly impacted in
cisplatin-treated cells. Many studies have focused on the fact that
MK exerts an anti-apoptotic effect by influencing the Bcl-2 family
proteins and the caspase cascade. In the present study, Notch
signaling related proteins were obviously downregulated in MK
hypoexpressing cells, along with the decrease of apoptotic cells.
This result suggested that Notch signaling pathway could be
suppressed by MK inhibition, which facilitated the chemotherapeutic
effect of cisplatin in gastric cancer. Understanding the molecular
mechanisms, driving MK-induced chemotherapeutic agent resistance,
will provide benefits in developing new therapies for gastric
cancer.
In conclusion, suppression of MK gene promoted the
antitumoral effect of cisplatin on human gastric cell line AGS
in vitro and in vivo. The downregulation of Notch
signaling pathway-related proteins induced by suppressing MK gene
is likely to be involved in modulating the cisplatin-induced
apoptosis in gastric cancer cells.
Acknowledgements
This study was supported by the National Natrual
Science Foundation of China (no. 81300375), Suzhou Municipal
Science and Technology Development (SYS201333 and SYS201461) and
Specialized Clinical Research Project of the Department of Science
and Technology, Jiangsu (BL2014046).
References
|
1
|
Zhao EH, Ling TL and Cao H: Current status
of surgical treatment of gastric cancer in the era of minimally
invasive surgery in China: Opportunity and challenge. Int J Surg.
28:45–50. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yang L: Incidence and mortality of gastric
cancer in China. World J Gastroenterol. 12:17–20. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Takayama S, Wakasugi T, Funahashi H and
Takeyama H: Strategies for gastric cancer in the modern era. World
J Gastrointest Oncol. 2:335–341. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bornschein J, Kandulski A, Selgrad M and
Malfertheiner P: From gastric inflammation to gastric cancer. Dig
Dis. 28:609–614. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kadomatsu K, Tomomura M and Muramatsu T:
cDNA cloning and sequencing of a new gene intensely expressed in
early differentiation stages of embryonal carcinoma cells and in
mid-gestation period of mouse embryogenesis. Biochem Biophys Res
Commun. 151:1312–1318. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Garver RIJ Jr, Radford DM, Donis-Keller H,
Wick MR and Milner PG: Midkine and pleiotrophin expression in
normal and malignant breast tissue. Cancer. 74:1584–1590. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
O'Brien T, Cranston D, Fuggle S, Bicknell
R and Harris AL: The angiogenic factor midkine is expressed in
bladder cancer, and overexpression correlates with a poor outcome
in patients with invasive cancers. Cancer Res. 56:2515–2518.
1996.PubMed/NCBI
|
|
8
|
Koide N, Hada H, Shinji T, Ujike K,
Hirasaki S, Yumoto Y, Hanafusa T, Kadomatsu K, Muramatsu H,
Muramatsu T, et al: Expression of the midkine gene in human
hepatocellular carcinomas. Hepatogastroenterology. 46:3189–3196.
1999.PubMed/NCBI
|
|
9
|
Ye C, Qi M, Fan QW, Ito K, Akiyama S,
Kasai Y, Matsuyama M, Muramatsu T and Kadomatsu K: Expression of
midkine in the early stage of carcinogenesis in human colorectal
cancer. Br J Cancer. 79:179–184. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mishima K, Asai A, Kadomatsu K, Ino Y,
Nomura K, Narita Y, Muramatsu T and Kirino T: Increased expression
of midkine during the progression of human astrocytomas. Neurosci
Lett. 233:29–32. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ikematsu S, Okamoto K, Yoshida Y, Oda M,
Sugano-Nagano H, Ashida K, Kumai H, Kadomatsu K, Muramatsu H,
Takashi Muramatsu and Sakuma S: High levels of urinary midkine in
various cancer patients. Biochem Biophys Res Commun. 306:329–332.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Obata Y, Kikuchi S, Lin Y, Yagyu K,
Muramatsu T and Kumai H: Tokyo Research Group on Prevention of
Gastric Cancer: Serum midkine concentrations and gastric cancer.
Cancer Sci. 96:54–56. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kadomatsu K, Hagihara M, Akhter S, Fan QW,
Muramatsu H and Muramatsu T: Midkine induces the transformation of
NIH3T3 cells. Br J Cancer. 75:354–359. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xu Y, Qu X, Zhang X, Luo Y, Zhang Y, Luo
Y, Hou K and Liu Y: Midkine positively regulates the proliferation
of human gastric cancer cells. Cancer Lett. 279:137–144. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yoon C, Cho SJ, Aksoy BA, Park DJ, Schultz
N, Ryeom SW and Yoon SS: Chemotherapy resistance in diffuse-type
gastric adenocarcinoma is mediated by RhoA activation in cancer
stem-like cells. Clin Cancer Res. 22:971–983. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee HW, Kim SJ, Choi IJ, Song J and Chun
KH: Targeting Notch signaling by γ-secretase inhibitor I enhances
the cytotoxic effect of 5-FU in gastric cancer. Clin Exp
Metastasis. 32:593–603. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mei H, Yu L, Ji P, Yang J, Fang S, Guo W,
Liu Y and Chen X: Doxorubicin activates the Notch signaling pathway
in osteosarcoma. Oncol Lett. 9:2905–2909. 2015.PubMed/NCBI
|
|
18
|
Huang Y, Cao G, Wang H, Wang Q and Hou Y:
The expression and location of midkine in gastric carcinomas of
Chinese patients. Cell Mol Immunol. 4:135–140. 2007.PubMed/NCBI
|
|
19
|
Zhao ZQ, Yang S and Lu HS: Expression of
midkine and vascular endothelial growth factor in gastric cancer
and the association of high levels with poor prognosis and
survival. Mol Med Rep. 5:415–419. 2012.PubMed/NCBI
|
|
20
|
Wang Q, Huang Y, Ni Y, Wang H and Hou Y:
siRNA targeting midkine inhibits gastric cancer cells growth and
induces apoptosis involved caspase-3,8,9 activation and
mitochondrial depolarization. J Biomed Sci. 14:783–795. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Leong KG and Karsan A: Recent insights
into the role of Notch signaling in tumorigenesis. Blood.
107:2223–2233. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
South AP, Cho RJ and Aster JC: The
double-edged sword of Notch signaling in cancer. Semin Cell Dev
Biol. 23:458–464. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Koduru S, Kumar R, Srinivasan S, Evers MB
and Damodaran C: Notch-1 inhibition by Withaferin-A: A therapeutic
target against colon carcinogenesis. Mol Cancer Ther. 9:202–210.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang Z, Li Y, Ahmad A, Azmi AS, Banerjee
S, Kong D and Sarkar FH: Targeting Notch signaling pathway to
overcome drug resistance for cancer therapy. Biochim. Biophys Acta.
1806:258–267. 2010.
|
|
25
|
Miele L: Notch signaling. Clin Cancer Res.
12:1074–1079. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ding Y and Shen Y: Notch increased
vitronection adhesion protects myeloma cells from drug induced
apoptosis. Biochem Biophys Res Commun. 467:717–722. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hang Q, Sun R, Jiang C and Li Y: Notch 1
promotes cisplatin-resistant gastric cancer formation by
upregulating lncRNA AK022798 expression. Anticancer Drugs.
26:632–640. 2015.PubMed/NCBI
|
|
28
|
Zhao J, Nie Y, Wang H and Lin Y: miR-181a
suppresses autophagy and sensitizes gastric cancer cells to
cisplatin. Gene. 576:828–833. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Piazzi G, Fini L, Selgrad M, Garcia M,
Daoud Y, Wex T, Malfertheiner P, Gasbarrini A, Romano M, Meyer RL,
et al: Epigenetic regulation of Delta-Like1 controls Notch1
activation in gastric cancer. Oncotarget. 2:1291–1301. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yeh TS, Wu CW, Hsu KW, Liao WJ, Yang MC,
Li AF, Wang AM, Kuo ML and Chi CW: The activated Notch 1 signal
pathway is associated with gastric cancer progression through
cyclooxygenase-2. Cancer Res. 69:5039–5048. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kopan R and Ilagan MX: The canonical Notch
signaling pathway: Unfolding the activation mechanism. Cell.
137:216–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Artavanis-Tsakonas S, Rand MD and Lake RJ:
Notch signaling: Cell fate control and signal integration in
development. Science. 284:770–776. 1999. View Article : Google Scholar : PubMed/NCBI
|