Introduction
Claudins (CLDNs) are critical transmembrane proteins
in tight junction function primarily as a barrier against
paracellular transport between epithelial cells and the CLDN family
consisting of 27 members that are mostly 20–34 kDa and have four
transmembrane helices with amino- and carboxyl-terminal tail
extending into the cytoplasm and play a crucial role in cellular
adhesion, polarity, permeability and glandular differentiation
(1,2). It is reported that altered expression
and mislocalization of CLDNs such as CLDN6 appear to be tissue
specific in embryo epithelial development and several cancers
(3–5).
CLDN6 gene is located on 16p13.3 and its
expression is mainly found in mouse embryonic stem cells,
epithelial lineage cells during early development and primitive
germ cell tumors such as spermatocytic seminoma, embryonal
carcinoma, mature teratoma and classic seminoma (6). Its expression is very weak or absent
in mouse and tumor tissue (7–9). CLDN6
inhibits cancer cell growth and induces apoptosis (10–12).
It is reported that CLDN6 expression is associated with ERα
expression and MMP-2 and ASK1. Although some functions of CLDN6 are
known, a complete understanding of CLDN6 regulation and function
remains to be studied. Bioinformatic analysis to predict regulatory
mechanism of the gene and protein expression greatly solves these
problems.
Bioinformatics is an interdisciplinary field, which
combines computer science, statistics, mathematics, and engineering
to develop methods and software tools for processing and
understanding biological data (13–15).
In the field of genetics and genomics, it aids in sequencing and
annotating genomes and their observed mutations. Sequence analysis
for DNA elements helps to explain the biological meaning and
functin of the gene. In addition, protein structure prediction is
another important application of bioinformatics. The amino acid
sequence of a protein can be easily determined from the sequence on
the gene that encodes it. This primary structure uniquely
determines a structure in its native environment. Knowledge of the
structural information that is usually classified as one of
secondary, tertiary and quaternary structure, is vital in
understanding the function of the protein (16). Moreover, network analysis seeks to
understand the relationships within biological networks such as
metabolic or protein-protein, small molecular interaction networks.
Therefore, bioinformatics tools can aid in the comparison of
genetic and genomic data and more generally in the understanding of
evolutionary aspects of molecular biology as well as, at a more
integrative level, anlayzing and cataloguing of the biological
pathways and networks that are an important part of systems biology
(16).
In this study, we used bioinformatics tools to
examine the CLDN6 sequence to characterize the gene
TATA-box, GC-box and CAAT-box, promoter, CpG islands, potential
transcriptional factors binding sites (TFBS), encoded protein
structure and its structure, subcellular localization, secondary
and tertiary structures, and even evolutionary relationship. These
characteristics will help define the basis for CLDN6
regulation and differential expression in cancer. These various
bioinformatics tools are among the common tools of molecular
biology helping investigators finding leads to investigate
genes/proteins.
Materials and methods
Bioinformatics databases and online
software
The following were used: NCBI (http://www.ncbi.nlm.nih.gov); Neural network promoter
prediction (http://www.fruitfly.org/seq_tools/promoter.html);
Promoter 2.0 prediction server (http://www.cbs.dtu.dk/services/Promoter/); TFSEARCH
(http://mbs.cbrc.jp/research/db/TFSEARCH.html); EMBOSS
and CpG island searcher (http://www.ebi.ac.uk/Tools/emboss/); expasy
(http://www.expasy.org); Protparam (http://www.expasy.org/tools/protparam.html); compute
pI/mw (http://www.expasy.org/tools/pi_tool.html); ProtScale
(http://www.expasy.org/tools/protscale.html); Clustalx
(http://www.clustal.org/download/current/); treeview
(http://www.taxonomy.zool-ogy.gla.ac.uk/rod/rod.html);
GOR4 (http://npsa-pbil.ibcp.fr/cgi-bin/npsa_automat.pl?page=npsa_gor4.html);
TargetP1.1 (http://www.cbs.dtu.dk/services/TargetP/), SignalP3.0
(http://www.cbs.dtu.dk/services/SignalP/); TMHMM2.0
(http://www.cbs.dtu.dk/services/TMHMM/); Pfam24.0
(http://pfam.sanger.ac.uk/search); SOPMA
(http://npsa-pbil.ibcp.fr/cgi-bin/npsa_automat.pl?page=npsa_sopma.html);
Swiss-model (http://www.expasy.ch/swissmod/SWISS-MODEL.html); KEGG
(http://www.genome.jp/kegg/).
Prediction methods
The following prediction methods were used for CLDN6
regulatory elements, structure and function: promoter (Neural
Network Promoter Prediction), CpG island (EMBOSS and CpG Island
Searcher), TFBS (TFSEARCH), the relatively molecular, amino acid
sequences, protein relatively molecular quality, mass of amino
acids, theoretical isoelectric point, PI, half-life, unstable
factor, the total average hydrophilic (ProtParam); hydrohobicity or
hydrophilicity (Prot Scale); the secondary structure (ExPASy-SOPMA
and GOR4); signal lead peptide (TargetP1.1 Server) and signal
peptide cutting locus (SignalP4.1Server); nuclear localization
signal prediction (NLStradamus); the subcellular localization (WOLF
PSORT and PSORT II); transmembrane area and across the membrane
(TMpred program and TMHMM2.0); structure (SWISS-MODEL); protein
structure and function (InterPro); transmembrane helices (TMpred
program); evolutionary tree and homology analysis (Clustalx program
and BLAST pairwise alignments); the signal pathway analysis
(KEGG).
Results
Properties of the TATA-box, GC-box,
CAAT-box motifs in the 5′ regulatory region of CLDN6
To determine whether there are TATA-box, GC-box,
CAAT-box motifs in the 5′ regulatory region of human CLDN6 to which
the transcription fators, TBP, SP1, and CBF, respectively, can bind
we BLAST searched CLDN6 mRNA (NM_021195.4) and human genomic
sequences between −2000 bp to 200 bp from the transcriptin start
site, for the motifs TATAWAW (where W represents A or T), GGGCGG
and CCAAT. We identified three GC-box fragments, but no TATA- or
CAAT-boxes, suggesting that expression and transcriptional activity
of CLDN6 is regulated by SP1.
Promoter, CpG island and TFBS
prediction in the 5′ regulatory region of CLDN6
The CLDN6 promoter sequence and TFs that bind
to this sequence determine the temporal and spatial expression
pattern of the gene. Therefore, defining of TFBS is important in
the study of gene regulation. We used online programs, such as
neural network promoter prediction, EMBOSS, CpG island searcher and
TFSEARCH to predict promoters, CpG islands and TFBS in 5′
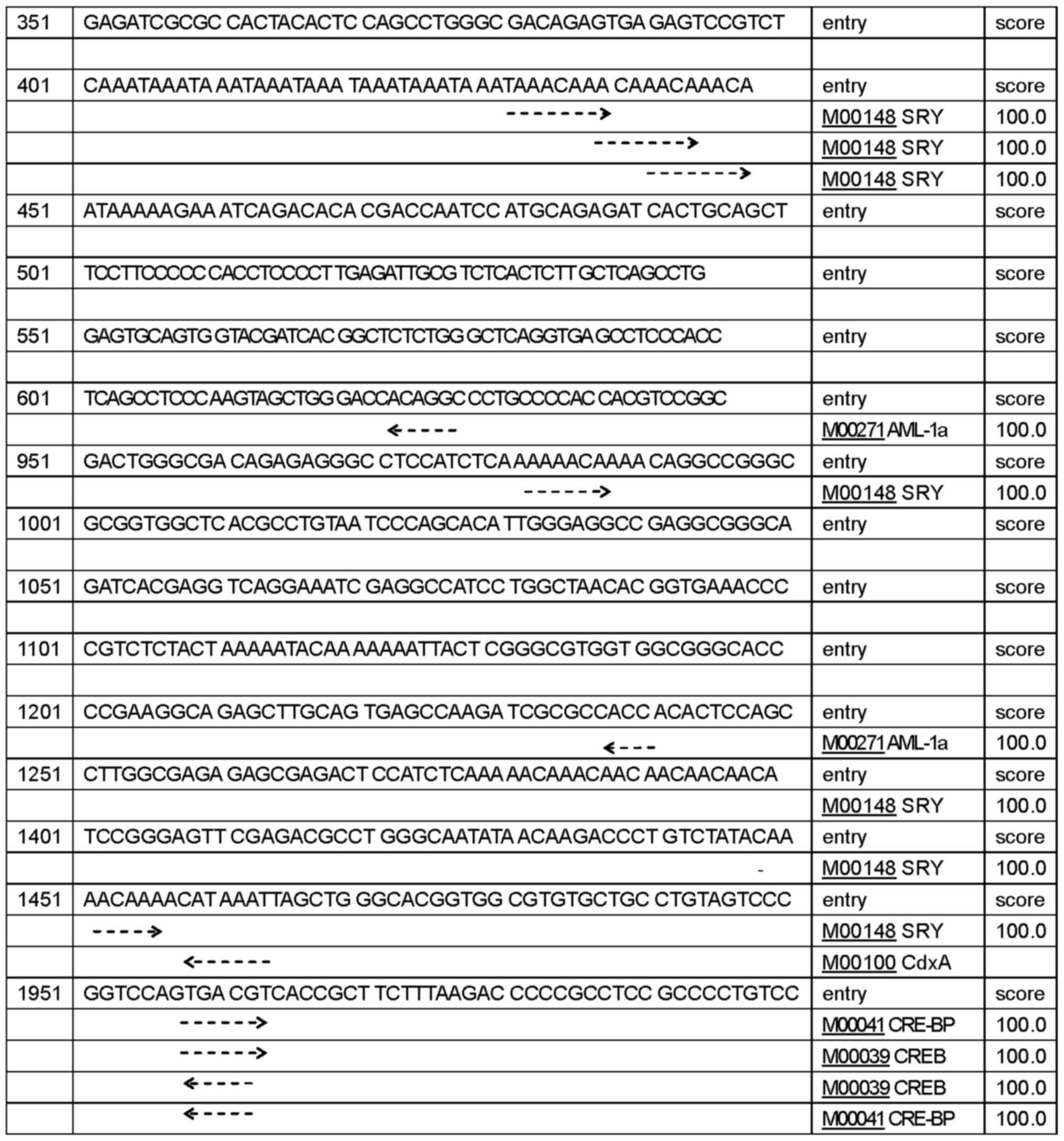
regulatory region sequences of human CLDN6. We identified
five promoters shown in Table I,
and three CpG islands with Obs/Exp ratio >0.60, percent C + G
>50% and length >200. These CpG islands have different length
and location as shown in Fig. 1A.
CpG Island Searcher program also identified three different CpG
islands (Fig. 1B). TFSEARCH program
predicted 432 potential TFBS with a score higher than 85 points,
156 potential TFBS with a score higher than 90 points, 66 potential
TFBS with a score higher than 95 points, 24 potential TFBS,
including SPR, AML-1a, CdxA, CRE-BP and CREB with a score over 99
points (Fig. 2). Together, these
findings suggest that CLDN6 expression is associated with
the levels of methylation of CpG islands in its promoters.
Different transcription start sites makes CLDN6
transcription in a different way, then produce a variety of
different biological functions of a transcription product.
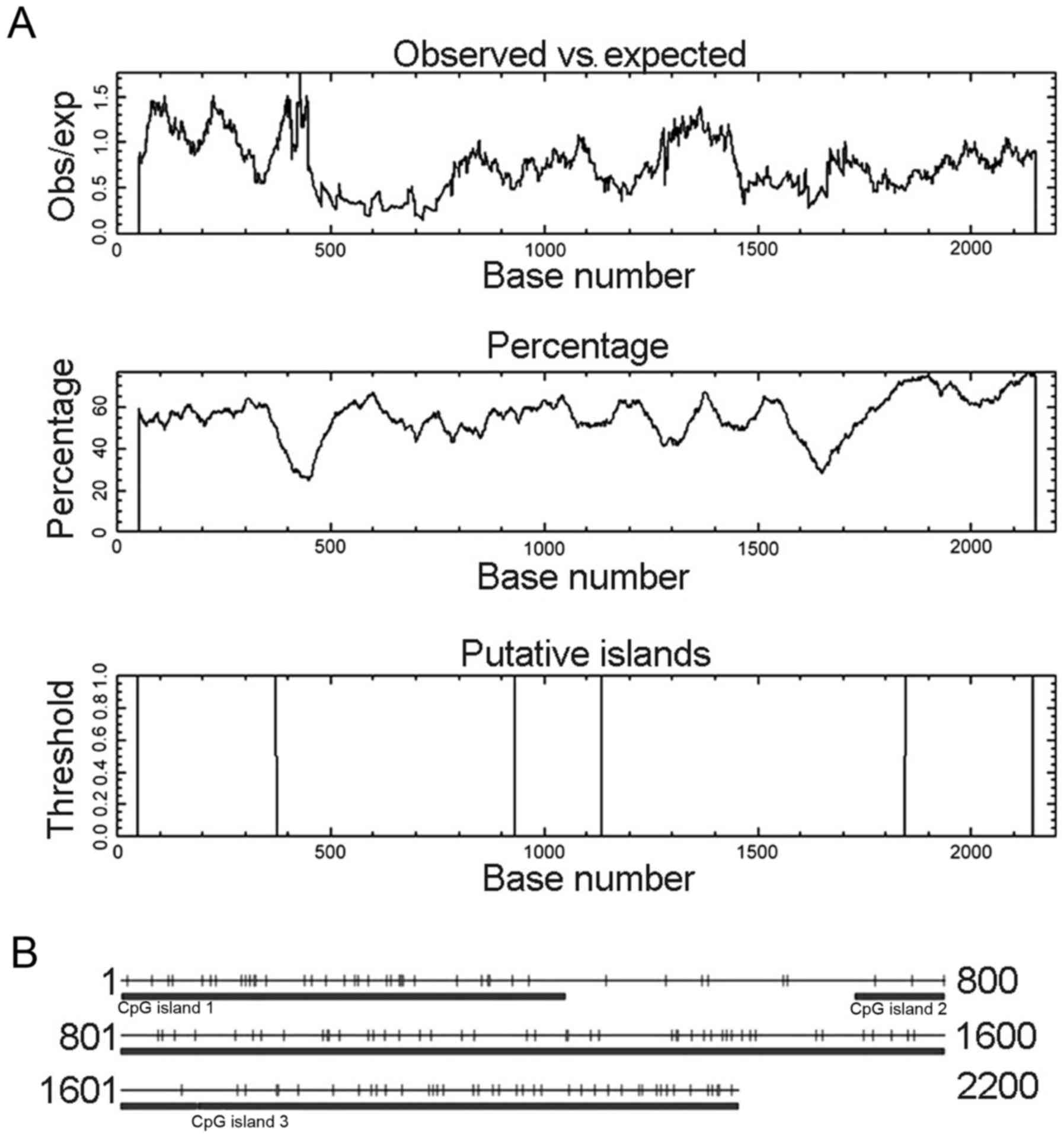 | Figure 1.CpG island prediction for
CLDN6 using two prediction programs. (A) CpG island
prediction using online EMBOSS. These CpG islands were 325 bp in
length (located at 49–373 bp), 204 bp (932–1135 bp) and 299 bp
(1847–2145 bp). (B) CpG island prediction using CpG Island Searcher
program. CpG Island Searcher program identified three different CpG
islands of 432 bp (1–432 bp), 962 bp (713–1674 bp) and 526 bp
(1675–2200 bp). Select lower limits: % GC=50, obsCpG/expCpG = 0.60,
length = 200, distance = 100. CpG island 1 star = 1, end = 432, %
GC=53.9, obsCpG/expCpG = 1.024, length = 432. CpG island 2 star =
713, end = 1674, % GC=52.8, obsCpG/expCpG = 0.735, length = 962.
CpG island 3 star = 1675, end = 2200, % GC=65.4, obsCpG/expCpG =
0.711, length = 526. |
 | Table I.CLDN6 promoter site prediction using
online Neural Network Promoter Prediction program. |
Table I.
CLDN6 promoter site prediction using
online Neural Network Promoter Prediction program.
| Software | Start sites | End sites | Score | Sequence |
|---|
| Neural network
promoter prediction |
237 |
287 | 0.98 |
ACTCGAAATACAAAAATTAGCCGGGCGTGGTGGCGCGCGCCTGCAATCCG |
|
|
975 | 1025 | 0.84 |
ATCTCAAAAAACAAAACAGGCCGGGCGCGGTGGCTCACGCCTGTAATCCC |
|
| 1414 | 1464 | 0.82 |
GACGCCTGGGCAATATAACAAGACCCTGTCTATACAAAACAAAACATAAA |
|
| 1450 | 1500 | 0.93 |
AAACAAAACATAAATTAGCTGGGCACGGTGGCGTGTGCTGCCTGTAGTCC |
|
| 1965 | 2015 | 0.99 |
ACCGCTTCTTTAAGACCCCCGCCTCCGCCCCTGTCCCGACACTCGGCCTA |
The amino acid sequence and
physicochemical properties of CLDN6 protein
To predict the protein structure of CLDN6, we used
ProtParam online software (http://au.expasy.org/tools/) to analyze amino acid
composition, molecular formula, molecular weight and isoelectric
point. CLDN6 consists of 220 amino acids with 20 different amino
acids, including alanine (10.90%), glycine (9.50%), leucine
(14.50%), serine (8.20%) and valine (10.90%) (Table IIA). CLDN6 has the following
properties: protein formula
C1054H1682N268O291S16,
molecular weight 23,2775 kDa, theoretical PI 8.32, estimated
half-life in vitro 30 h, instability index 42.03 and the
hydrophilic residue ratio is lower than that of the hydrophobic
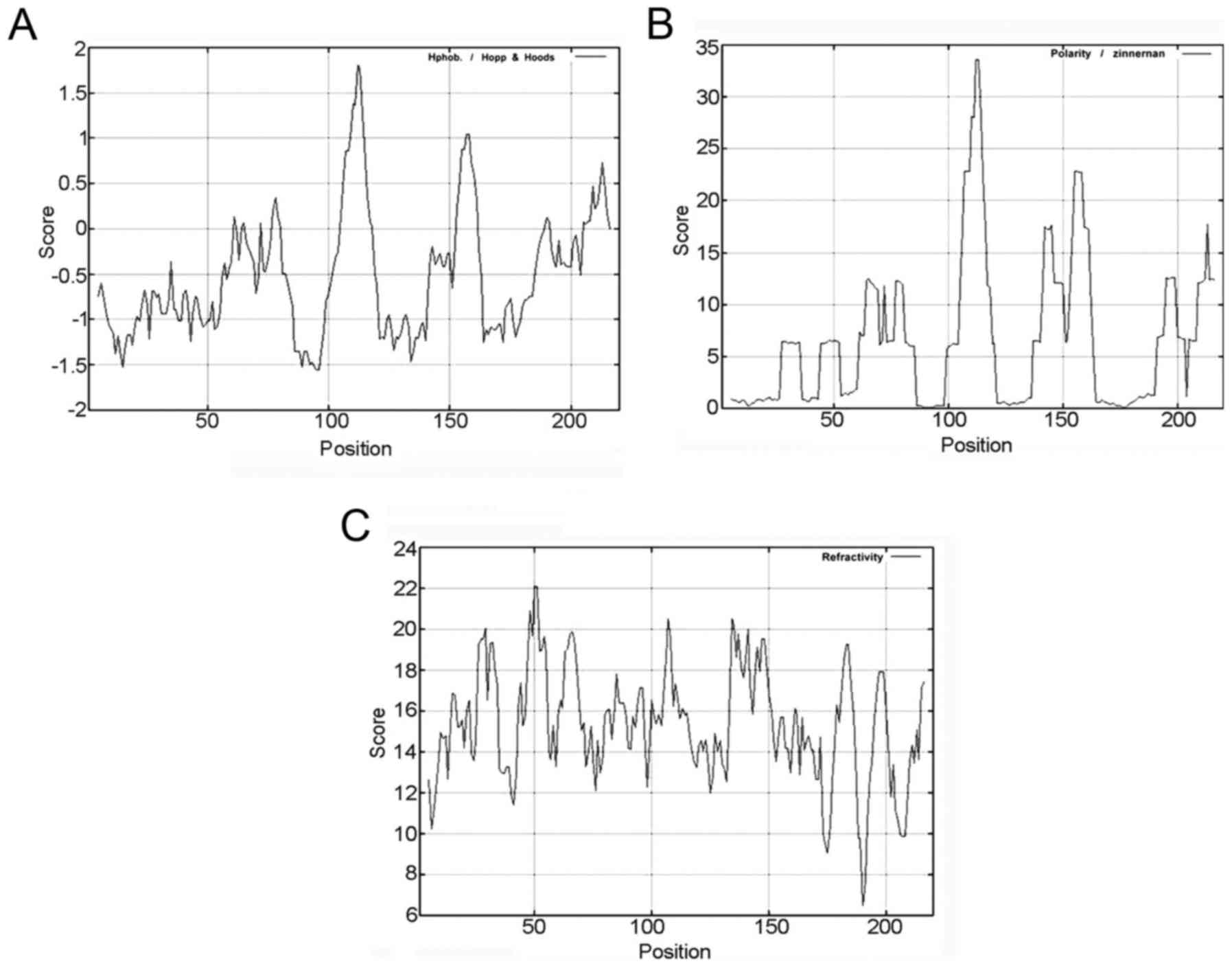
residues. The threshold value of CLDN6 hydrophilicity is highest
at: 105–118 bp, 153–161 and 205–216 bp (Fig. 3A and Table IIB), and the threshold value of
CLDN6 polarity is highest at: 106–116, 142–145 and 154–161 bp
(Fig. 3B and Table IIB), the threshold value of
turn-back coefficient is highest at 14–22, 26–34, 43–55, 60–71,
80–89, 92–97, 100–115, 133–151, 154–156, 179–186, 195–200 and
213–216 bp (Fig. 3C and Table IIB). The overlapping range of these
three parameters is shown in Table
IIB at 106–115 and 154–156 bp. Taken together, these data
suggest that CLDN6 mainly contains hydrophilic amino acid and polar
amino acids, functional overlapping structure ranges from 106 to
156 bp, and that CLDN6 protein maybe a hydrophobic and unstable
protein.
 | Table II.The basic properties of CLDN6
analyzed by using ProtParam online software. |
Table II.
The basic properties of CLDN6
analyzed by using ProtParam online software.
| A, Amino acid
composition of CLDN6. |
|---|
|
| Amino acid | Abbreviations | Number | Composition
(%) |
|---|
| Alanine | Ala(A) | 24 | 10.90 |
| Arginine | Arg(R) | 6 |
2.70 |
| Asparagine | Asn(N) | 4 |
1.80 |
| Aspartate | Asp(D) | 4 |
1.80 |
| Cystine | Cys(C) | 10 |
4.50 |
| Glutamine | Gln(Q) | 9 |
4.10 |
| Glutamate | Glu(E) | 6 |
2.70 |
| Glycine | Gly(G) | 21 |
9.50 |
| Histidine | His(H) | 2 |
0.90 |
| Isoleucine | Ile(I) | 9 |
4.10 |
| Leucine | Leu(L) | 32 | 14.50 |
| Lysine | Lys(K) | 7 |
3.20 |
| Methionine | Met(M) | 6 |
2.70 |
| Phenylalanine | Phe(F) | 4 |
1.80 |
| Proline | Pro(P) | 9 |
4.10 |
| Serine | Ser(S) | 18 |
8.20 |
| Threonine | Thr(T) | 11 |
5.00 |
| Tryptophan | Trp(W) | 6 |
2.70 |
| Tyrosine | Tyr(Y) | 8 |
3.60 |
| Valine | Val(V) | 24 | 10.90 |
|
| B, Molecular
formula, molecular weight, isoelectric point and other basic
properties of CLDN6. |
|
| Parameters | Prediction
results |
|
| Formula |
C1054H1682N268O291S16 |
| Molecular
weight | 23277.5 |
| Theoretical pI | 8.32 |
| Total number of
atoms | 3311 |
| Number of amino
acids | 220 |
| Total number of
negatively charged residues | 10 |
| Total number of
positively charged residues | 13 |
| Estimated half-life
(mammalian reticulocytes, in vitro) | 30 h |
| Instability
index | 42.03 |
| Hydrophilic | 105–118, 153–161,
205–216 |
| Polarity | 106–116, 142–145,
154–161 |
| Turn-back
coefficient | 14–22, 26–34,
43–55, 60–71, 80–89, 92–97, 100–115, 133–151, 154–156, 179–186,
195–200, 213–216 |
| The predicted
results of the overlapping area | 106–115,
154–156 |
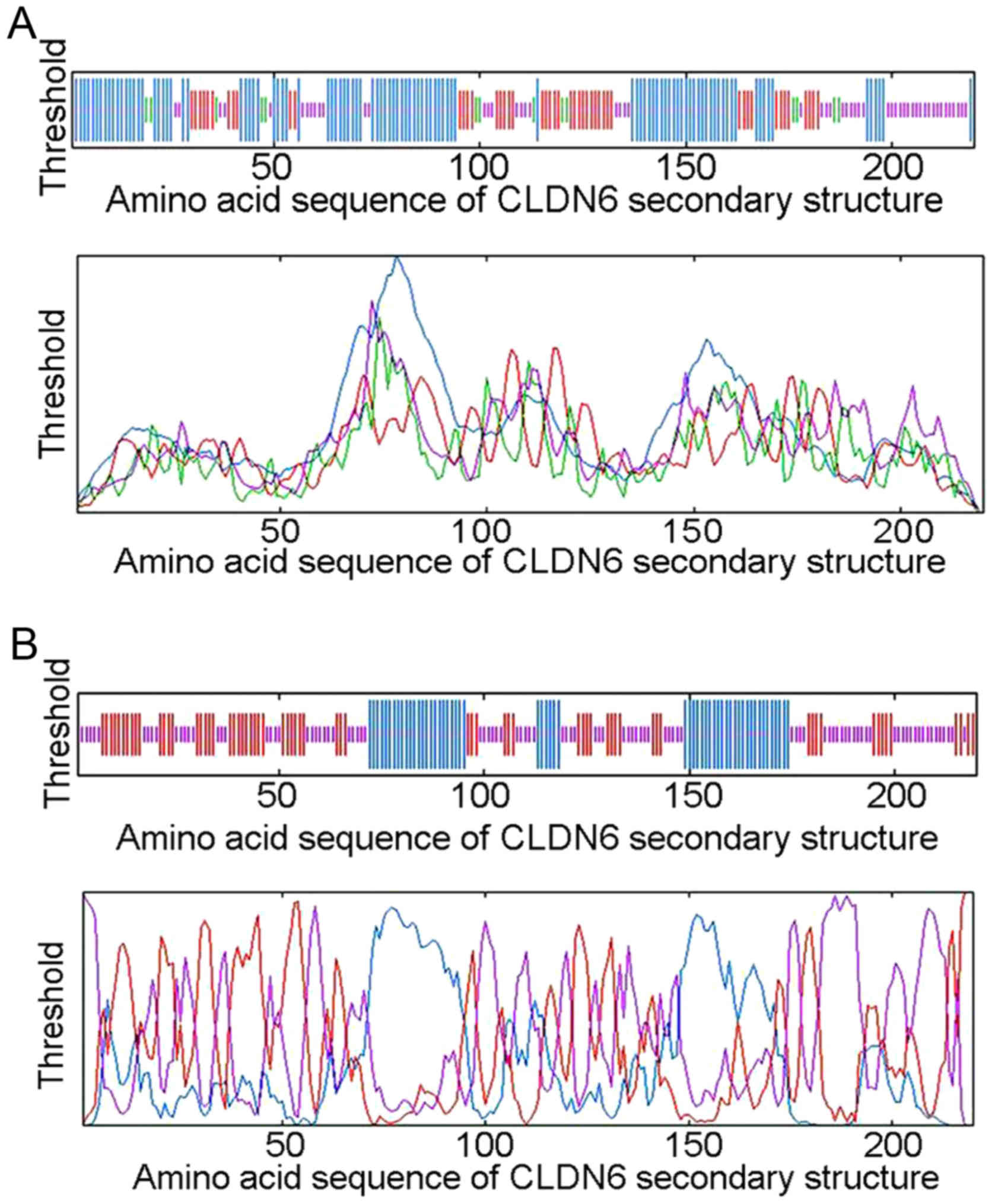
Secondary structure prediction for
CLDN6 protein
The arrangement of atoms in space of the main
polypeptide chain (α helix, extended strand, β turn and random
coil) determines basic protein secondary structure. Determination
of this arrangement can help predict functions, and protein
modifications. We used ExPASy-SOPMA and GOR4 secondary structure
prediction module to calculate CLDN6 secondary structure, and to
draw the structure model. Module output prediction results can be
shown as a peak figure or the diagram can be simplified to show as
the random of coiled and folded areas. The SOPMA method identified
104 (47.27%) α helixs, 48 (21.82%) extended strands, 14 (6.36%) β
turns and 54 (24.55%) random coils and irregular coiled and folded
structures located mainly at 1–70, 96–114, 120–150 and 176–220 bp
between the peak figure and simplified diagram (Fig. 4A and Table III). The GOR4 method identified 56
(25.45%) α helixs, 67 (30.45%) extended strands, and 97 (44.09%)
random coils and irregular coiled and folded structures located
mainly at 26–42, 102–112, 131–139, 147–156, 173–194 and 196–220 bp
(Fig. 4B and Table III). The comparison of two
secondary structure prediction results is shown in Table III. CLDN6 secondary structure
mainly consists of the irregular curl overlapping areas at 26–42,
102–112, 131–139, 147–150, 222–238, 176–194 and 196–220 bp,
suggesting that these areas are mainly composed of α helix,
extended strand and random coil structure. Taken together, the
functional domain of CLDN6 protein is likely to be limited to these
overlapped areas.
 | Table III.Comparison of CLDN6 secondary
structure prediction results between SOPMA and GOR4 methods. |
Table III.
Comparison of CLDN6 secondary
structure prediction results between SOPMA and GOR4 methods.
| Secondary structure
prediction methods | Prediction
results |
|---|
| SOPMA | 1–70, 96–114,
120–150, 176–220 |
| GOR4 | 26–42, 102–112,
131–139, 147–156, 173–194, 196–220 |
| The predicted
results of the overlapping area | 26–42, 102–112,
131–139, 147–150, 222–238, 176–194, 196–220 |
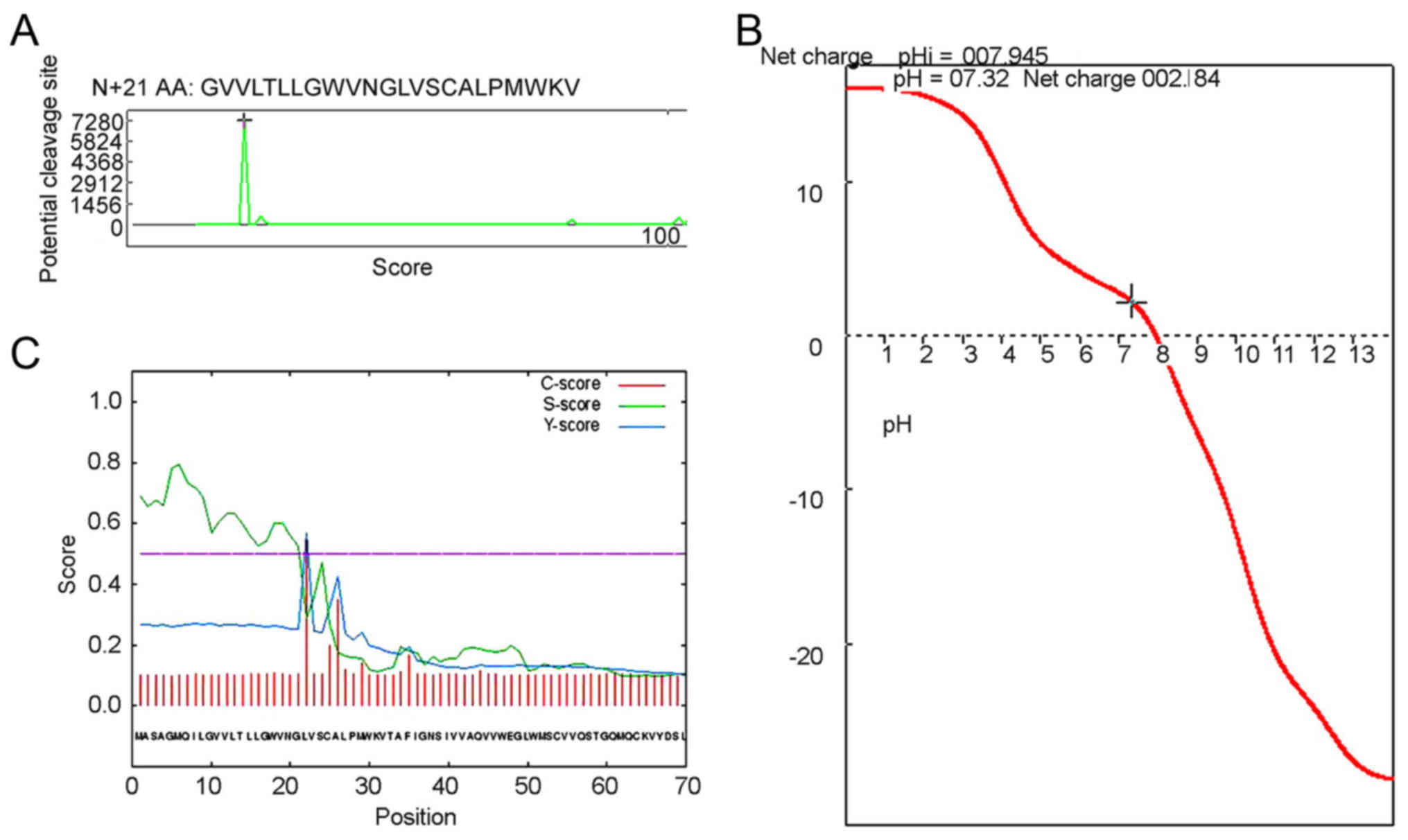
Analysis of signal peptide cleavage
site, subcellular location, transmembrane domains in the CLDN6
protein
Signal peptides direct protein localization in cells
and usually consists of 15–30 N-terminal amino acid residues. To
analyze the CLDN6 signal peptide, we first used the Anthprot signal
peptide cutting locus analysis module and the result showed CLDN6
has a short signal peptide composed of 21 amino acid residues, and
its sequence is shown in Figs. 5A
and 6. Consistent with previous
results, the CLDN6 isoelectric point is approximately near 8.0, and
the physiological state of CLDN6 molecules is closest to pH 7.3,
with a positive charge of 2.184, as shown in Fig. 5B.
We also used SignalP4.1 to predict the signal
peptide and its cleavage site. As shown in Fig. 5C and Table IV the cleavage site is between
amino acids 21 and 22: VNG-LV. We also used NLStradamus, a simple
Hidden Markov Model for nuclear localization signal prediction, to
show that there were no nuclear localization signal sequences,
suggesting that CLDN6 does not enter the nucleus. TargetP1.1
predicts CLDN6 to be located in the secretory pathway (98.7%)
(Table V), while WoLF PSORT
predicts CLDN6 to be a multi-pass membrane protein with subcellular
localization to tight junction or the cell membrane. However, PSORT
II Prediction indicated that CLDN6 may locate to the endoplasmic
reticulum (ER, 66.7%) and mitochondria (33.3%) (Table VI). The review by Koval on
differential pathways of claudin oligomerization and integration
into tight junctions described the three potential models for
claudin oligomerization (cis interactions) occurring in the
endoplasmic reticulum (ER) (17). A
requirement for CLDN quality control early in the secretory pathway
and mutant CLDNs which are misfolded accumulate in the ER (18,19).
CLDN oligomerization is more likely to happen in the TGN or another
late secretory pathway and formation of stable claudin-claudin
intermediates, however, given the structural diversity of different
claudin family members, it is unlikely that all claudins will
oligomerize via the same pathway. There is some evidence that
CLDN16, CLDN3 and CLDN4 tagged with an ER retentin signal,
His-Lys-Lys-Ser-Leu (HKKSL) are retained in the ER (20,21),
while there is no reported for CLDN6 retaining in ER. We considered
that CLDN6, as common protein functional maturation steps,
oligomerizes in ER and is transported via the secretory pathway and
integrates into tight junction.
 | Table IV.Results of CLDN6 protein signal
peptide using SignalP-4.1 euk predictions. |
Table IV.
Results of CLDN6 protein signal
peptide using SignalP-4.1 euk predictions.
| # Measure | Position | Value | Cut off | Signal peptide |
|---|
| Max. C | 22 | 0.545 |
| No |
| Max. Y | 22 | 0.570 |
| No |
| Max. S |
6 | 0.795 |
| No |
| Mean S | 1–21 | 0.635 |
| No |
| D | 1–21 | 0.596 | 0.500 | Yes |
 | Table V.Subcellular localization prediction
of CLDN6 using TargetP1.1. |
Table V.
Subcellular localization prediction
of CLDN6 using TargetP1.1.
| Name | Len | mTP | SP | Other | Loc | RC |
|---|
| Sequence | 220 | 0.022 | 0.987 | 0.023 | S | 1 |
| Cut off |
| 0.000 | 0.000 | 0.000 |
|
|
 | Table VI.CLDN6 protein may locate in the
endoplasmic reticulum (ER, 66.7%) and mitochondria (33.3%) using
PSORT II Prediction. |
Table VI.
CLDN6 protein may locate in the
endoplasmic reticulum (ER, 66.7%) and mitochondria (33.3%) using
PSORT II Prediction.
| K=9/23 |
|---|
| 66.7%: Endoplasmic
reticulum |
| 33.3%:
Mitochondrial |
| >> Prediction
for QUERY is end (k=9) |
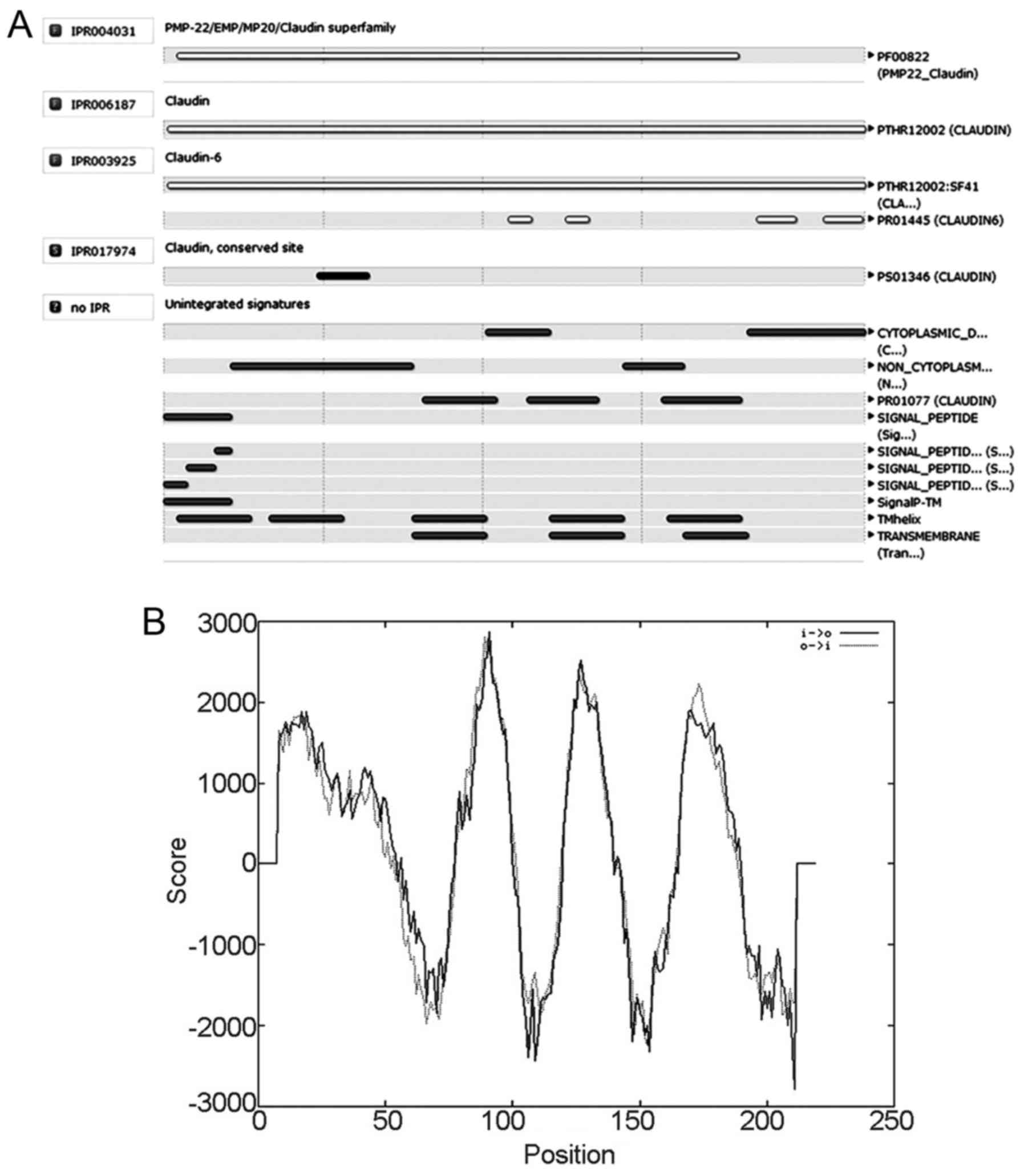
Prediction of CLDN6 protein domain and
function site
To predict CLDN6 structural domain and important
functional sites, we used InterPro software. The results show that
CLDN6 belongs to the PMP-22/EMP/CLDN superfamily (IPR004031) and
CLDN (IPR006187) as shown in Fig.
7A. Its function is associated with structural molecular
activity (GO: 0005198) and that the action contributes to cellular
component in bicellular tight junction (GO: 0005923) and the
structure integrity of a complex or assembly within or outside a
cell (GO: 0016021).
CLDN6 has a four-element fingerprint that provides a
signature for CLDNs. The fingerprint was derived from an initial
alignment of two sequences. The motifs were drawn from conserved
regions within the C-terminal half of the alignment, focusing on
those sections that characterize CLDN6 and distinguish it from
other family members: motif 1 lies in the intracellular loop; motif
2 resides within TM domain 3; and motifs 3 and 4 lie within the
cytoplasmic C-terminal region. A single iteration on SPTR39_14f was
required to reach convergence, no further sequences were identified
beyond the starting set. CLDN6 possibly has four strong
transmembrane helices according to the strongly preferred model
(Fig. 7B). Taken together, CLDN6 is
four-transmembrane tight junction protein.
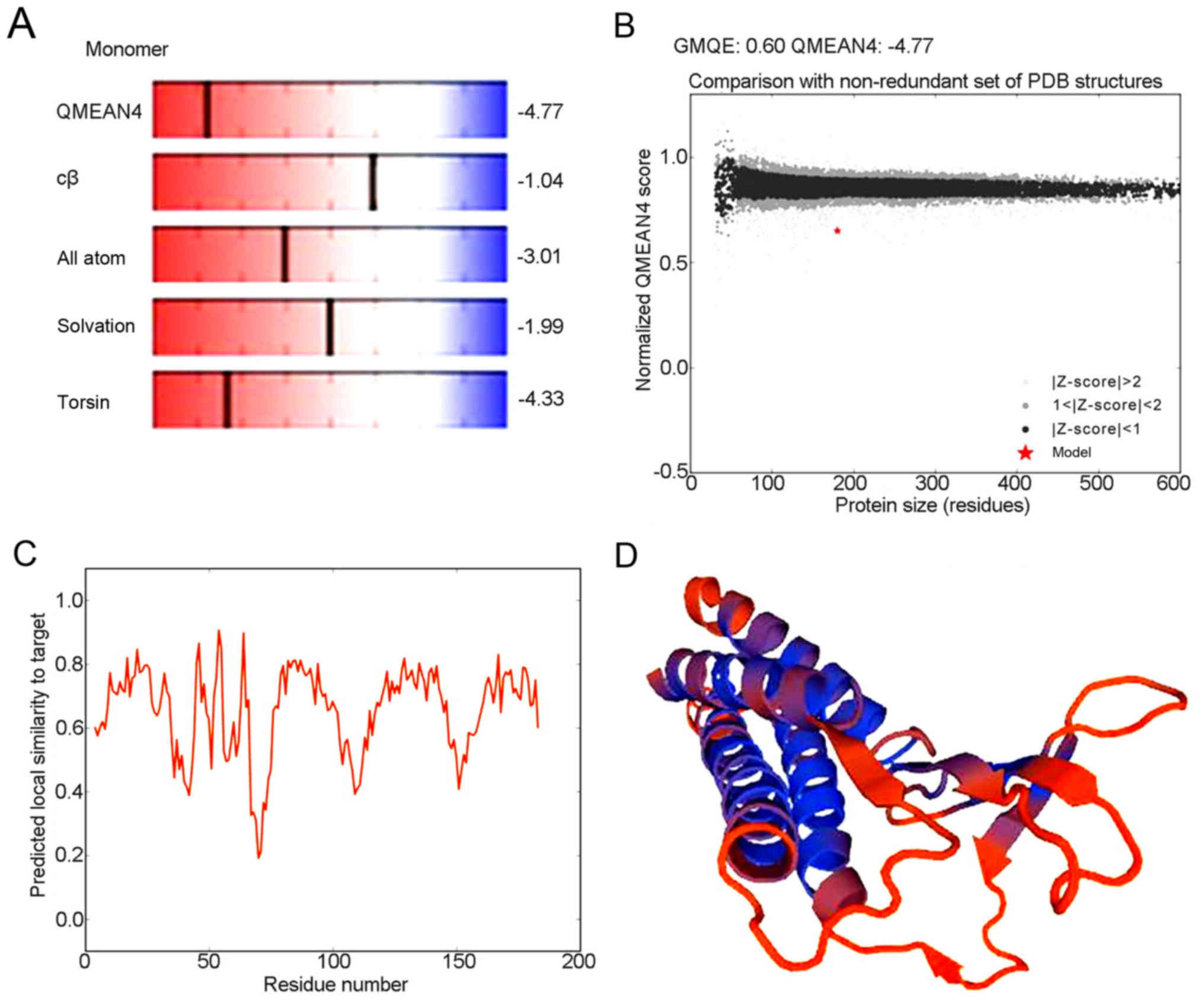
Advanced structure of human CLDN6
protein
Analysis of the detailed structures of CLDN6 will
further our understanding of its biological role. To obtain a high
level protein structure simulation map, the amino acid sequence was
analyzed using Swissmodel server. CLDN6 protein and its structure
database template 3×29.1.A, has 42.62% amino acid sequence, which
is derived from the template CLDN19 that GMQE is 0.60 and QMEAN4 is
−4.77, which 3D structure as shown in Fig. 8A-D.
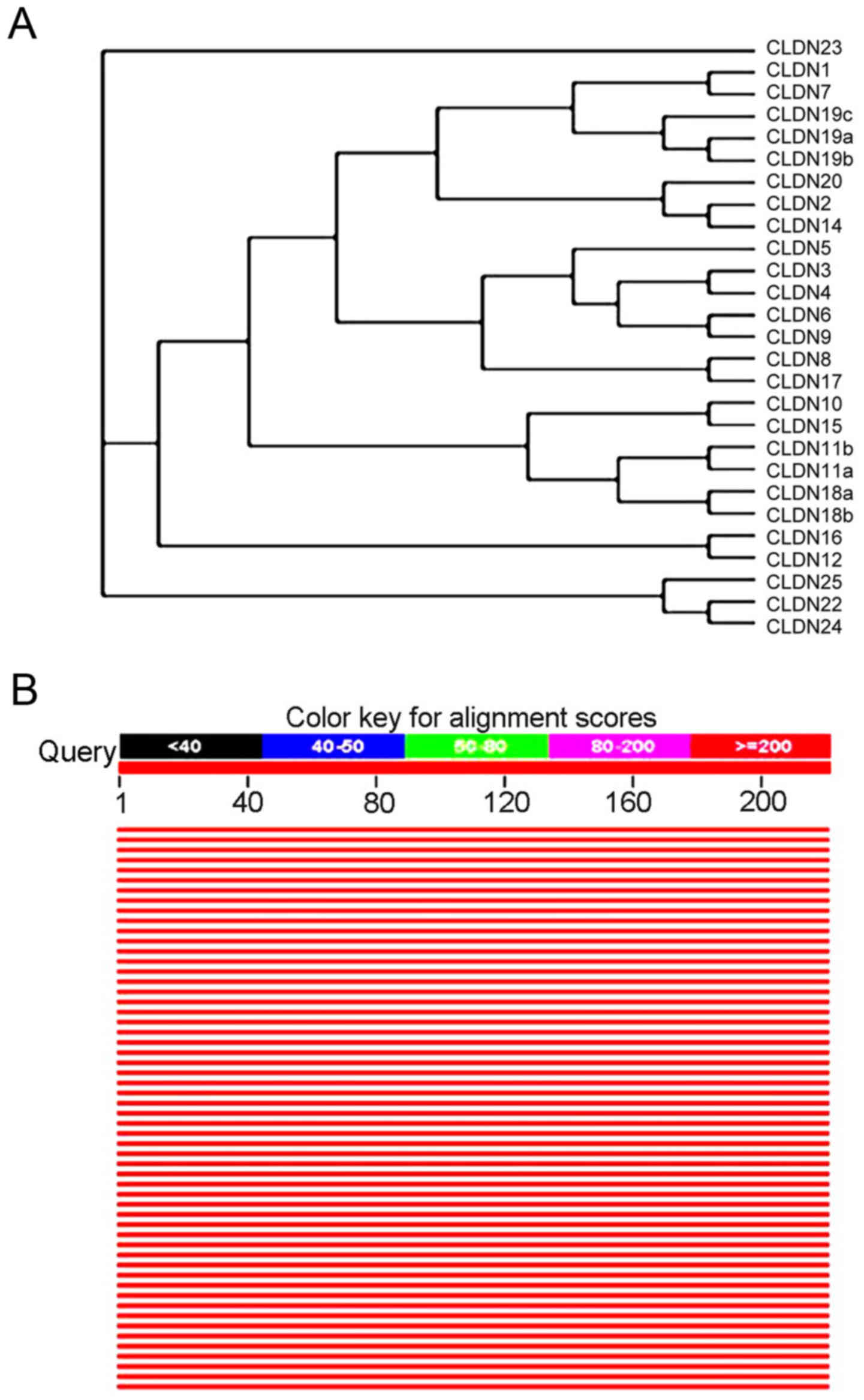
The evolutionary tree of CLDN family
amino acid sequence rendering system and homology analysis of human
CLDN6 protein sequences
CLDN6 was identified by searching expressed
sequence tag (EST) databases for sequences similar to CLDN1
and CLDN2 (22). It was
subsequently cloned and expressed in cells, where it was shown to
concentrate at tight junctions (22) and human and mouse isoforms have been
identified. With Clustalx program construct phylogenetic tree, and
with the Treeview software on the system of evolutionary tree edit
and comparison, the family system evolutionary tree of CLDNs
protein amino acid sequence was drawn. All members of CLDNs are
clustered closely except CLDN23, and except CLDN5, CLDN19c, CLDN20
and CLDN25, 22 members of the others exist in pairs and CLDN6 in
particular is paired with CLDN9, suggesting they are the closest
(Fig. 9A). Comparison of the amino
acid seuqences showed that CLDN6 shares 25–70% overall similarity
with other CLDNs at the amino acid level with highest similarity to
CLDN9. Through the relevant data in the library collection download
sequence similarity information encoding protein >50% of the
species to build the system tree, as shown in Fig. 9B, the CLDN6 protein with human and
rodent coding product has high homology of other animals in the
phylogenetic tree perimeter, they may play the same biological
functions.
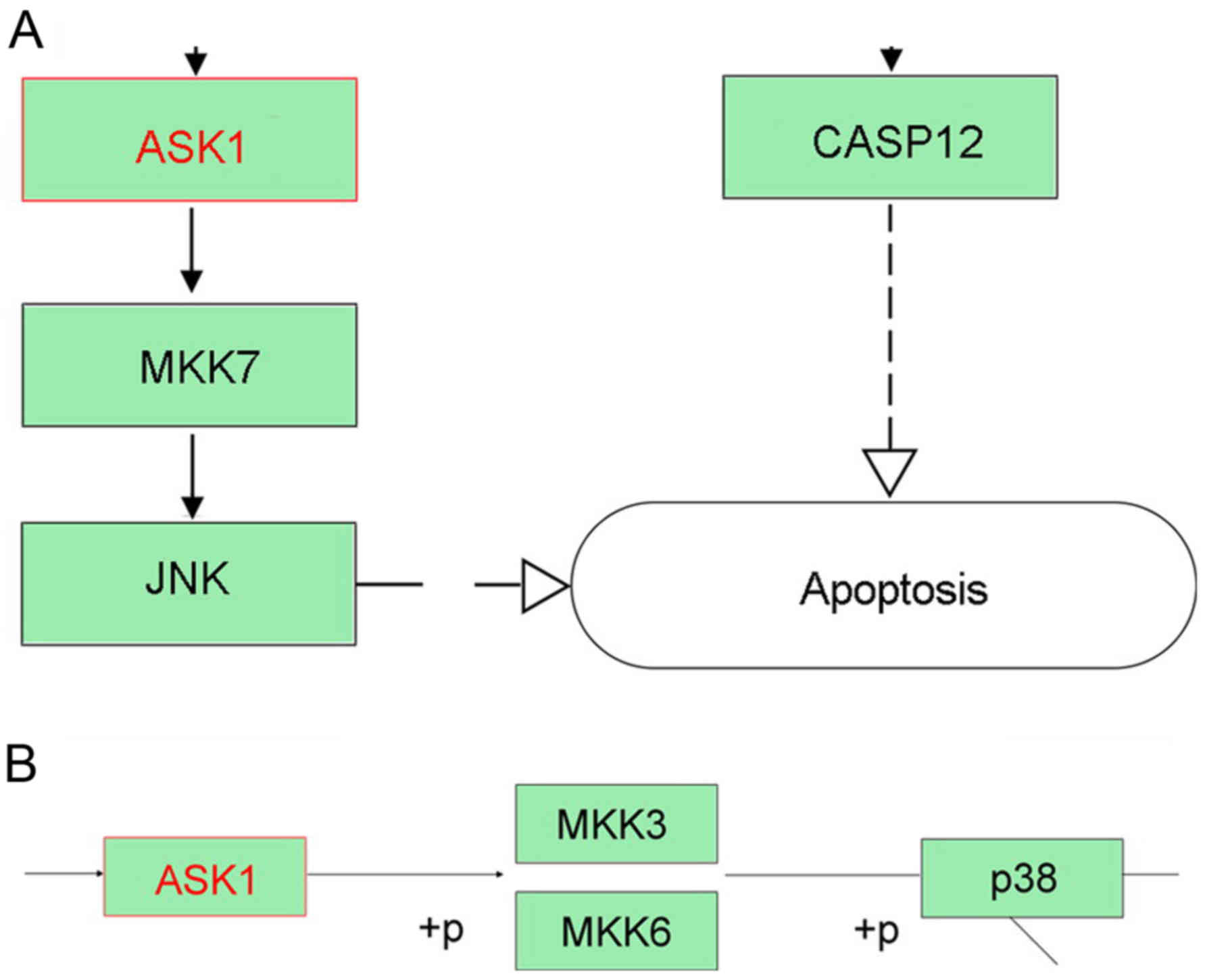
CLDN6 related signaling pathway
analysis
To identify CLDN6-related signaling pathway, We
focused on CLDN6-related molecular network information in KEGG
pathway maps. CLDN6 is related to four signaling pathways including
hsa04514; hsa04530; hsa04670; hsa05160 and has been identified as
exosomal proteins of cancer cells such as ovarian cancer, and
colorectal cancer, are closely related to CLDN6. CLDNs activated by
miR-122 can activate RIP1, TRAF6, p38 and JNK in hsa05160, and
apoptosis signal-regulating kinase 1 (ASK1) can activate downstream
p38 and JNK-induced apoptosis (Fig.
10A and B). However, the other signaling pathways are not
involved in apoptosis. ASK1, a member of the mitogen-activated
protein kinase (MAPK) kinase family has been proved to positively
correlate with the level of CLDN6 and associated with its
pro-apoptosis effect in cervical carcinoma and breast cancer
(12,23,24).
Taken together, CLDN6 may induce cancer cell apoptosis via
ASK1-p38/JNK MAPK pathway.
Discussion
CLDN6 on chromosome 16p13.3 encodes a 23 kDa
four-transmembrane protein. CLDN6 has been shown to be expressed
differentially in various tumor tissues and cells (25–27),
and to a certain degree its alteration can inhibit or pomote tumor
cell growth, apoptosis, invastion, migration and EMT in
vitro, and methylation of the gene may be involved in
tumorigenesis (7,26,28).
Although we have clarified some functions of CLDN6, more questions
remain to be answered, e.g. why it is differentially expressed in
different cells, which signal pathway relates to it and how it is
regulated, suggesting alternative ways are needed to answer the
questions. Bioinformics may be a way complementary to experimental
biology in understanding CLDN6 regulation and function, which
necessarily must be validated by experiments in vitro or
in vivo.
We studied the 5′ regulatory region of CLDN6
using bioinformatics tools and found that it contained three
GC-boxes, three CpG islands and in the 5′ regulatory region
sequence 432 and 24 potential TFBS were predicted with a score of
85–99 and diverse TFs such as SP1 are predicted to bind to these
TFBS. These results are supported by published literatures, e.g.
Anelli and Sitia documented that the associations between CLDN6
expression and mRNA levels of SP1 in ovarian carcinoma effusions
(18), as well as transcription
factors such as CRE-BP and CREB (17), the expression of CLDN6 is
associated with methylating CpG islands; treatment with TSA and/or
5-aza or DMSO induced marked decreases in the levels of methylation
of CpG islands in the promoters of CLDN6. The above are also
supported by our experiments, which shows that DNA
methyltransferase (DNMT1) inhibits the expression of CLDN6 to
affect the function of tight junction in breast cancer MCF-7 cells
in our unpublished work. In addition, it is reported that some
TFBS, including those for AP-1, c-Jun, ATF-2, HNF-4α and COUP-TF
have been shown to contribute to CLDN6 regulation (29–34).
The amino acid sequence of CLDN6 was also analyzed
by bioinformatics methods. The results showed that the isoelectric
point was 8.32; leucine, valine, glycine, serine and alanine were
the most abundant amino acids; CLDN6 was an unstable protein
because of hydrophobic amino acids. Further, the investigation also
found that CLDN6 was a trans-membrane protein with leader peptides
and a signal peptide at the N-terminus that was hydrophilic. CLDN
conformation in the plane of the membrane shows the four
transmembrane α-helical domain as a tightly packed complex, classes
of claudin-claudin interactions within a tight junction strand and
they can interact via head-to-head binding in the extracellular
environment between adjacent cells and within the plane of the
plasma membrane in the same cell (21). The main predicted secondary
structures of CLDN6 are α helices, extended strands and random
coils in our results. CLDN6 encodes four-transmembrane
domain protein components of tight junction strands (22). Our CLDN evolutionary tree shows
CLDN6 and CLDN9 are most closely related, which is similar to a
report by Lal-Nag et al (35) and it is reported that both CLDN6 and
CLDN9 function as additional coreceptors for hepatitis C virus
(25), suggesting they have common
functions. CLDN6 related signaling pathway analysis results
obtained from these tools are used to support CLDN6-induced
apoptosis via regulating ASK1-p38/JNK signaling in breast cancer
MCF-7 cells (36).
In summary, these results reveal that CLDN6
gene may have diverse transcription start sites and its
transcription is regulated by DNA methylation and transcription
factors such as SP1. Additionally, CLDN6 may be oligomerized in ER
and transported to cell membrane via secretory pathway and finally
integrated into tight junctions.
Acknowledgements
This study was supported by National Natural Science
Foundation of China (code: 81172499) and Jilin Province Science and
Technology Development Projects (20140414036GH) This study is
funded by Key Laboratory of Natural Resources of Changbai Mountain
& Functional Molecules (Yanbian University), Ministry of
Education.
Glossary
Abbreviations
Abbreviations:
|
CLDN
|
claudin
|
|
GC
|
cytosine and guanine
|
|
CpG
|
cytosine-phosphoric acid-guanine
|
|
ASK1
|
Apoptosis signal regulating kinase
1
|
|
JNK
|
c-Jun NH2-terminal kinase
|
|
ERα
|
estrogen receptor α
|
|
MMP-2
|
matrix metalloproteinase-2
|
|
TFBS
|
transcriptional factor binding
sites
|
|
TBP
|
TATA binding protein
|
|
SP1
|
specificity protein 1
|
|
CBF
|
C-repeat binding factor
|
|
AML-1a
|
acute myeloid leukemia 1a
|
|
CREB
|
cAMP-response element bingding
protein
|
|
CRE-BP
|
CREB-binding protein
|
|
ER
|
endoplasmic reticulum
|
|
EST
|
expressed sequence tag
|
References
|
1
|
Günzel D and Yu AS: Claudins and the
modulation of tight junction permeability. Physiol Rev. 93:525–569.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lu S, Singh K, Mangray S, Tavares R, Noble
L, Resnick MB and Yakirevich E: Claudin expression in high-grade
invasive ductal carcinoma of the breast: Correlation with the
molecular subtype. Mod Pathol. 26:485–495. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mineta K, Yamamoto Y, Yamazaki Y, Tanaka
H, Tada Y, Saito K, Tamura A, Igarashi M, Endo T, Takeuchi K, et
al: Predicted expansion of the claudin multigene family. FEBS Lett.
585:606–612. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kwon MJ: Emerging roles of claudins in
human cancer. Int J Mol Sci. 14:18148–18180. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li X, Li Y, Qiu H and Wang Y:
Downregulation of claudin 7 potentiates cellular proliferation and
invasion in endometrial cancer. Oncol Lett. 6:101–105.
2013.PubMed/NCBI
|
|
6
|
Rendon-Huerta EP, Torres-Martínez AC and
Montaño L: CLDN6 (claudin 6). Atlas Genet Cytogenet Oncol Haematol.
17:396–399. 2013.
|
|
7
|
Zavala-Zendejas VE, Torres-Martinez AC,
Salas-Morales B, Fortoul TI, Montaño LF and Rendon-Huerta EP:
Claudin-6, 7, or 9 overexpression in the human gastric
adenocarcinoma cell line AGS increases its invasiveness, migration,
and proliferation rate. Cancer Invest. 29:1–11. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang L, Xue Y, Shen Y, Li W, Cheng Y, Yan
X, Shi W, Wang J, Gong Z, Yang G, et al: Claudin 6: A novel surface
marker for characterizing mouse pluripotent stem cells. Cell Res.
22:1082–1085. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang Q, Zhang Y, Zhang T, Han ZG and Shan
L: Low claudin-6 expression correlates with poor prognosis in
patients with non-small cell lung cancer. Onco Targets Ther.
8:1971–1977. 2015.PubMed/NCBI
|
|
10
|
Xu X, Jin H, Liu Y, Liu L, Wu Q, Guo Y, Yu
L, Liu Z, Zhang T, Zhang X, et al: The expression patterns and
correlations of claudin-6, methy-CpG binding protein 2, DNA
methyltransferase 1, histone deacetylase 1, acetyl-histone H3 and
acetyl-histone H4 and their clinicopathological significance in
breast invasive ductal carcinomas. Diagn Pathol. 7:332012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ren Y, Wu Q, Liu Y, Xu X and Quan C: Gene
silencing of claudin-6 enhances cell proliferation and migration
accompanied with increased MMP-2 activity via p38 MAPK signaling
pathway in human breast epithelium cell line HBL-100. Mol Med Rep.
8:1505–1510. 2013.PubMed/NCBI
|
|
12
|
Zhang X, Ruan Y, Li Y, Lin D, Liu Z and
Quan C: Expression of apoptosis signal-regulating kinase 1 is
associated with tight junction protein claudin-6 in cervical
carcinoma. Int J Clin Exp Pathol. 8:55352015.PubMed/NCBI
|
|
13
|
Luscombe NM, Greenbaum D and Gerstein M:
What is bioinformatics? A proposed definition and overview of the
field. Methods Inf Med. 40:346–358. 2001.PubMed/NCBI
|
|
14
|
Ilzins OA, Isea R and Hoebeke J III: Can
bioinformatics be considered as an experimental biological science?
Open Sci J Biosci Bioeng. 2:60–62. 2015.
|
|
15
|
Isea R: The present-day meaning of the
word bioinformatics. Glob J Adv Res. 2:70–73. 2015.
|
|
16
|
Eck RV and Dayhoff MO: Evolution of the
structure of ferredoxin based on living relics of primitive amino
acid sequences. Science. 152:363–366. 1966. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Koval M: Differential pathways of claudin
oligomerization and integration into tight junctions. Tissue
Barriers. 1:e245182013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Anelli T and Sitia R: Protein quality
control in the early secretory pathway. EMBO J. 27:315–327. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Piontek J, Winkler L, Wolburg H, Müller
SL, Zuleger N, Piehl C, Wiesner B, Krause G and Blasig IE:
Formation of tight junction: Determinants of homophilic interaction
between classic claudins. FASEB J. 22:146–158. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hou J, Renigunta A, Konrad M, Gomes AS,
Schneeberger EE, Paul DL, Waldegger S and Goodenough DA: Claudin-16
and claudin-19 interact and form a cation-selective tight junction
complex. J Clin Invest. 118:619–628. 2008.PubMed/NCBI
|
|
21
|
Overgaard CE, Daugherty BL, Mitchell LA
and Koval M: Claudins: control of barrier function and regulation
in response to oxidant stress. Antioxid Redox Signal. 15:1179–1193.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Morita K, Furuse M, Fujimoto K and Tsukita
S: Claudin multigene family encoding four-transmembrane domain
protein components of tight junction strands. Proc Natl Acad Sci
USA. 96:511–516. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Guo Y, Xu X, Liu Z, Zhang T, Zhang X, Wang
L, Wang M, Liu Y, Lu Y, Liu YA, et al: Apoptosis signal-regulating
kinase 1 is associated with the effect of claudin-6 in breast
cancer. Diagn Pathol. 7:1112012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang X, Ruan Y, Li Y, Lin D and Quan C:
Tight junction protein claudin-6 inhibits growth and induces the
apoptosis of cervical carcinoma cells in vitro and in vivo. Med
Oncol. 32:1482015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zheng A, Yuan F, Li Y, Zhu F, Hou P, Li J,
Song X, Ding M and Deng H: Claudin-6 and claudin-9 function as
additional coreceptors for hepatitis C virus. J Virol.
81:12465–12471. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wu Q, Wu X-Y, Zhang H-Y, Liu Y-F, Ren Y,
Qu S-S, Quan C-S and Li Y-L: Expression of tight junctions protein
claudin-6 in breast cancer tissues and cell lines and its
relationship with metastasis of breast cancer. J Jilin Univ Med
Edit. 34:274–279. 2008.(In Chinese).
|
|
27
|
Lin Z, Zhang X, Liu Z, Liu Q, Wang L, Lu
Y, Liu Y, Wang M, Yang M, Jin X, et al: The distinct expression
patterns of claudin-2, −6, and −11 between human gastric neoplasms
and adjacent non-neoplastic tissues. Diagn Pathol. 8:1332013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wu Q, Liu Y, Ren Y, Xu X, Yu L, Li Y and
Quan C: Tight junction protein, claudin-6, downregulates the
malignant phenotype of breast carcinoma. Eur J Cancer Prev.
19:186–194. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu Y, Jin X, Li Y, Ruan Y, Lu Y, Yang M,
Lin D, Song P, Guo Y, Zhao S, et al: DNA methylation of claudin-6
promotes breast cancer cell migration and invasion by recruiting
MeCP2 and deacetylating H3Ac and H4AcJ. J Exp Clin Cancer Res.
35:1202016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ohazama A and Sharpe PT: Expression of
claudins in murine tooth development. Dev Dyn. 236:290–294. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Osanai M, Murata M, Chiba H, Kojima T and
Sawada N: Epigenetic silencing of claudin-6 promotes
anchorage-independent growth of breast carcinoma cells. Cancer Sci.
98:1557–1562. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ribeiro A, Archer A, Le Beyec J, Cattin
AL, Saint-Just S, Pinçon-Raymond M, Chambaz J, Lacasa M and Cardot
P: Hepatic nuclear factor-4, a key transcription factor at the
crossroads between architecture and function of epithelia. Recent
Pat Endocr Metab Immune Drug Discov. 1:166–175. 2007. View Article : Google Scholar
|
|
33
|
Hui PJH: Small proline rich protein-2
expression and regulation in the Caco-2 model of intestinal
epithelial differentiation along the crypt-villus axis. QSPACE
Kingston, Ontario, Canada: Queen's University; 2008, http://hdl.handle.net/1974/1183
|
|
34
|
Nishikiori N, Sawada N and Ohguro H:
Prevention of murine experimental corneal trauma by epigenetic
events regulating claudin 6 and claudin 9. Jpn J Ophthalmol.
52:195–203. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lal-Nag M, Battis M, Santin A and Morin P:
Claudin-6: A novel receptor for CPE-mediated cytotoxicity in
ovarian cancer. Oncogenesis. 1:e332012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Guo Y, Lin D, Zhang M, Zhang X, Li Y, Yang
R, Lu Y, Jin X, Yang M, Wang M, et al: CLDN6-induced apoptosis via
regulating ASK1-p38/JNK signaling in breast cancer MCF-7 cells. Int
J Oncol. 48:2435–2444. 2016.PubMed/NCBI
|